Innovative medicine development
Abstract
BACKGROUND:
In recent years the global landscape of pharmaceutical development has evolved drastically in order to adapt to innovative products such as immunotherapy, regenerative medicines and cell and gene therapy, all offering the hope to cure numerous untreated diseases. The global COVID-19 pandemic also led competent authorities in charge of approval of new medicines to implement adapted regulatory pathways allowing early access to innovative treatments and vaccines. New challenges are to be overcome, it is the short development time, or the small patient populations, or again drug product features requiring complete new production, testing and distribution strategies.
OBJECTIVE:
This paper provides a short overview of the different adaptations required to allow the development of such innovative medicines.
METHOD:
Several drug development strategies for products under clinical development or recently approved were assessed.
RESULTS:
Fully integrated strategies encompassing research, non-clinical, clinical and product manufacturing development along with adapted regulatory pathways, anticipation of market access and supported by risk based are important. In such fast pace, the development of relevant manufacturing processes and test methods assessing quality, safety and efficacy of new drugs falls on the critical path and requires particular attention, anticipation and detailed planning as early as possible in order to ensure successful filing and approval.
CONCLUSION:
Insights within innovative products approved recently reveal the importance of defining a solid chemistry manufacturing and control strategy early on as part of clinical development. Interactions with agencies at key milestones of development are also essential to reduce risks, prioritize development to support safety and allow early access to patients.
1.Introduction
Progress in science of the past decades allowed the development of more and more innovative drugs. They are in the form of Conjugated Antibodies allowing potent cytotoxic compounds to be targeted to specific cancer cell receptor and enhancing efficacy while minimizing detrimental effects on healthy cells. They are Cell and Gene Therapy Products which have shown high rates of responses in cancer or monogenic disease treatments. Genetically modified bacteria are also currently in development targeting the human microbiome to treat a wide range of diseases. We see the development of 3D printed tissues emerging with the potential to allow repair of damaged tissues and open the possibility to prepare artificial organs. Finally, the extraordinary response of the biopharma industry to the global COVID-19 pandemic confirmed how translation of science into innovative human saving treatments or vaccines can be accelerated.
Innovation requires the interactions and synchronization of different fields, different skillsets, different philosophy and the inevitable requirement to thoroughly manage risks. It is research stepping into the world of regulated development. It is regulation adapting to the new discoveries, but it is foremost the integration of risk-based approaches to support a lean and agile development.
2.Adapted pharmaceutical development
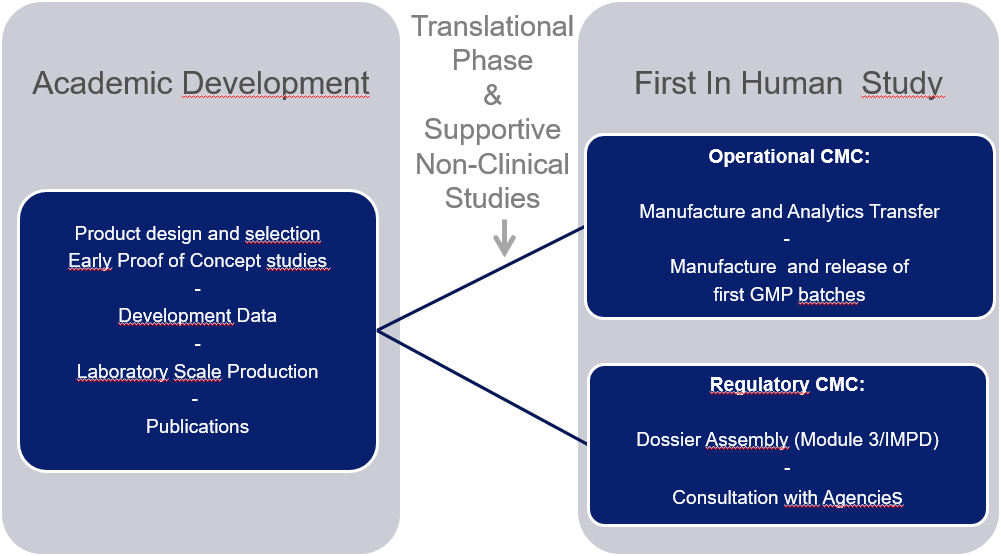
Bringing innovative medicines from the R&D bench to the initiation of the first clinical trials and all the way to marketing authorization requires careful anticipation of the specific challenges linked to pharmaceutical development. The notebooks of the research laboratories and preclinical proof of concept studies provide the foundation of the regulated Chemistry Manufacturing and Control (CMC), the design of safe and consistent manufacturing processes, and the development of relevant tests to demonstrate the quality of each batch of drugs produced as shown in Fig. 1. CMC constitutes an important part of the submissions to regulatory authorities for clinical trial and marketing authorization with specific mandatory milestones to address throughout development.
Figure 1.
CMC development from R&D to first in human clinical studies.
3.Adapted regulatory environment
In recent years, new regulatory pathways have been developed for drugs addressing unmet medical needs and have allowed accelerated approval so that patients may access these therapies as early as possible. Priority Medicine scheme in Europe (PRIME), Breakthrough Therapies Designation in the US, but also Conditional Marketing Authorizations all provide possibilities for accelerated development and approval. More recently, the FDA “Coronavirus Treatment Acceleration Program” (CTAP) [1] and the EMA recently published initiatives for COVID-19 treatment and vaccines [2] also provide adapted regulatory assessment to expedite development.
These approaches cannot however compromise safety and quality of the drugs under development. It is therefore increasingly critical to consider streamlining CMC development and set up an incremental approach, allowing CMC elements to be prioritized and address the relevant compliance and regulatory needs on time. It is important to address biosafety concerns when human stem cells are used as starting material or address new environmental issues when Genetically Modified Organism are used to develop of Gene Therapy or a vaccine. The complexity of the products also requires adapted process and method development, innovative control and release testing strategies, adapted stability considerations, storage and distribution as illustrated recently for mRNA vaccines.
Such approach requires tight integration and communication between R&D, technical operations, regulatory departments as early as possible in order to successfully reach the different milestones to approval and agree on the most relevant accelerated strategy. The integration of health economics considerations, with early identification of opportunities to optimize cost of production while adapting to industrial and GMP environment, is also an important step to support price and reimbursement negotiations upon approval.
4.Adapted guidelines
The agencies acknowledged the risk of potential bottleneck related to CMC and have endorsed in recent years measured compliance and regulatory flexibility in order to accommodate new types of therapy as well as accelerated development. The Good Manufacturing Practice (GMP) guide adapted to Advanced Therapy Manufacturing Products (ATMP) [3] that came into operation in 2018 and more recently the Question and Answers [4] related to the principle of GMP of starting materials used for manufacturing of ATMP offer a certain level of flexibility for investigational products using a risk-based approach (RBA), especially in early phases of clinical trials as product knowledge is often not fully complete.
More recently, and in order to address the difficulties encountered by applicants in completing quality and manufacturing development in the framework of accelerated development, the EMA published a new draft guidance in 2021 [5]. It focuses on the pharmaceutical development and flexibility that could be applied to the type of data provided in the context of a Marketing Authorization Application Application (MAA) taking into consideration the overall benefit/risk of the product. Topics such as product characterisation, specification setting, process and method validation and stability testing as well as early identification of quality attributes are addressed with specific examples. It is presented in a “toolbox approach” to illustrate possible strategies for adapted completion of the quality data packages of the MAA so called Module 3 and Module 2.3. For example, the guidance describes the possibility to depart from traditional requirement of performing a minimum of 3 Process Performance Qualification batches at times of filing if enough supportive data are available. It also provides the opportunity to file with limited stability data, or the possibility to submit reduced comparability along with supportive data based on prior knowledge gathered through development or with similar products, separate assessment of specific changes, and introducing risk-based approaches.
5.Adapted analytical development strategy
Product knowledge sits at the cornerstone of successful pharmaceutical development and comes as a key element to strengthen the accelerated development approaches discussed above. Upon submission for clinical trials or marketing authorization, major objections and questions received from the Agencies often focus on CMC topics. Relevance of the methods used, of the specifications, proper product characterization, potency tests fully aligned with the Mode of Actions, impurity profiles are often subjects of additional work and the cause of delays throughout development and approval. The European Public Assessment Reports (EPAR) of recently approved COVID-19 vaccines also reveal some weaknesses of the analytical package and control strategy, requiring additional development efforts as post approval measures to reinforce product characterization, stability, control strategy and ensure consistent product quality [6, 7].
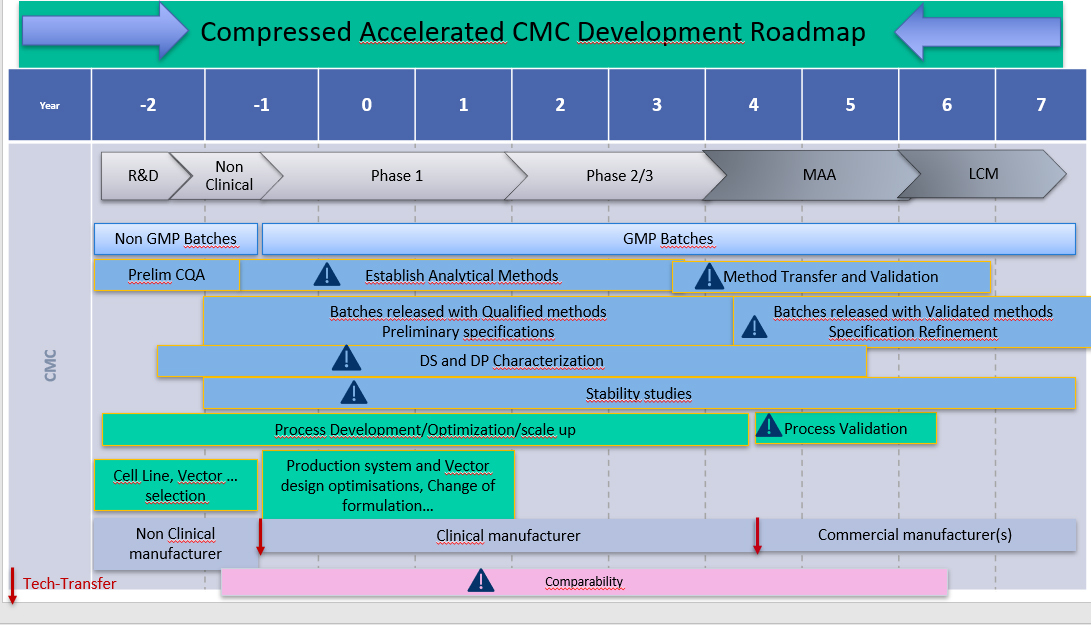
As time for CMC development is drastically compressed over accelerated approval (Fig. 2), it is becoming increasingly important to prioritize and develop solid analytical tools early on in development. This allows building stronger knowledge from the start and insights on the product characteristics likely to influence the clinical outcome. This strategy also provides a stronger baseline to support accelerated development as it reinforces process and product characterization, understanding of stability, and allows supporting the process changes and comparability inevitable through product development towards commercial production.
Figure 2.
Accelerated CMC development.
6.Conclusion
Insights within innovative products approved recently reveals the importance of defining a CMC strategy early on as part of clinical development. This approach helps identify key challenges, anticipate manufacturing and release requirements, the potential use of platform technology, the specific constraints as well as cost considerations in order to avoid blocking questions upon submissions and increase the chances of successful approval and launch.
Innovative product development inevitably requires adapted CMC development and priorities, tailored to each product type and characteristics. Interactions with agencies at key milestones of development are essential to reduce risk. Sharing the incremental product and process knowledge on which the overall control strategy is designed provides opportunities to reach an agreement on the relevant CMC and analytical package supporting safety and quality while providing early access to patients.
Conflict of interest
None to report.
References
[1] | FDA – Coronavirus Treatment Acceleration Program. |
[2] | EMA – Initiatives for acceleration of development support and evaluation procedures for COVID-19 treatments and vaccine. |
[3] | EudraLex – Volume 4 Good Manufacturing Practice Guidelines on Good Manufacturing Practice specific to Advanced Therapy Medicinal Products. |
[4] | Q&A on the principles of GMP for the manufacturing of starting materials of biological origin used to transfer genetic material for the manufacturing of ATMPs. |
[5] | Draft toolbox guidance on scientific elements and regulatory tools to support quality data packages for PRIME marketing authorisation applications. |
[6] | EMA/707383/2020 Corr.1*1 Assessment report Comirnaty. |
[7] | EMA/15689/2021 Assessment report COVID-19 Vaccine Moderna (Spikevax). |




