Significance of vascular endothelium growth factor testing in exhaled breath condensate of patients with acute respiratory distress syndrome
Abstract
OBJECTIVE:
We aimed to observe and investigate the clinical significance of vascular endothelium growth factor (VEGF) levels in exhaled breath condensate (EBC) from patients with acute respiratory distress syndrome (ARDS).
METHODS:
An improved EcoScreen condenser was used to collect EBC from 31 ARDS patients on mechanical ventilation and from 22 healthy subjects. Serum and EBC VEGF levels were analyzed with ELISA. VEGF levels in the EBC of patients with different grades of lung injuries were analyzed. The correlation between VEGF levels and clinical indicators was analyzed.
RESULTS:
Serum and EBC VEGF levels were linearly and positively correlated with a correlation coefficient of 0.694 (
CONCLUSION:
The changes in VEGF levels in the EBC of ARDS patients can Respiratory Medicine, reflect the severity of lung injury. Therefore, VEGF level in EBC can be used as an auxiliary index for judging the severity and prognosis of ARDS patients.
1.Introduction
Acute respiratory distress syndrome (ARDS) is a diffused non-cardiogenic pulmonary edema that presents with impaired alveolar epithelial cells, increased pulmonary microvascular permeability and increased pulmonary interstitial and alveolar exudate proteins [1]. ARDS clinically manifests as acute respiratory failure that is characterised by reduced pulmonary compliance and refractory hypoxia. The mortality rate of ARDS in clinical practice is very high [2].
Systemic inflammatory response syndrome is a common route of infection, trauma and other factors that lead to ARDS. Inflammatory response is an important mechanism of ARDS pathogenesis. Neutrophils, alveolar macrophages and endothelial cells, which are inflammatory factors, mediate the occurrence and development of ARDS [3]. The accumulation and activation of neutrophils in the lung release injurious substances, such as proteolytic enzymes, superoxide and cytokines. Alveolar macrophages promote neutrophil infiltration and aggregation via the release of tumor necrosis factors (TNFs), interleukins (ILs) and leukotrienes. Pulmonary capillary endothelial cells release oxygen-free radicals and induce inflammatory cell injury in the vascular endothelium. These two processes can cause pulmonary edema. Alveolar epithelial cells and pulmonary vascular endothelial cells synthesize and release a variety of factors that mediate inflammation. These factors include oxygen free radicals, nitric oxide, 8-iso-prostaglandin, endothelin-1 and vascular endothelial growth factor (VEGF) [4].
VEGF is a multifunctional cytokine that promotes vascular permeability and induces angiogenesis. There are six subtypes of VEGF: A, B, C, D, E and placental growth factor (PLGF) [5]. Under normal physiological conditions, VEGF is moderately expressed in alveolar epithelial cells, bronchial epithelial cells and bronchial glandular cells.
Inflammatory reactions can be monitored as a simple, noninvasive, reliable and reproducible laboratory objective index for ARDS [6]. Therefore, this study aimed to determine VEGF levels in the exhaled breath condensate (EBC) of ARDS patients, to explore the relationship between VEGF levels and ARDS severity and to evaluate the clinical value of VEGF detection in EBC.
2.Objects and methods
2.1Research object
We selected 31 patients with ARDS who underwent mechanical ventilation at the Intensive Care Unit (ICU) of the Second Affiliated Hospital of Nantong University from September 2015 to June 2018. None of the patients had a primary pulmonary disease prior to mechanical ventilation. The ARDS group comprised of 18 males and 13 females with ages of 21–84 years, with an average of 60.1
Table 1
General clinical situation of ARDS patients
| Name of underlying diseases | Cases | Operation cases | Mild ARDS mortality (%) | Moderate-severe ARDS mortality (%) | ||
|---|---|---|---|---|---|---|
| Multiple injuries | 15 | 8 | 33.3 | (2/6) | 44.4 | (4/9) |
| Acute perforation of digestive tract | 4 | 4 | 0.0 | (0/2) | 50.0 | (1/2) |
| Acute upper gastrointestinal bleeding | 3 | 1 | 100.0 | (1/1) | 50.0 | (1/2) |
| Acute intestinal obstruction | 3 | 2 | 0.0 | (0/2) | 0.0 | (0/1) |
| Pelvic fractures | 2 | 0 | 0.0 | (0/1) | 0.0 | (0/1) |
| Femoral fractures | 2 | 1 | 0.0 | (0/1) | 0.0 | (0/1) |
| Postoperative ovarian cancer | 1 | 1 | 0.0 | (0/1) | 0.0 | (0/0) |
| Placenta previa | 1 | 0 | 0.0 | (0/0) | 100.0 | (1/1) |
| Total | 31 | 17 | 21.4 | (3/14) | 41.2 | (7/17) |
2.2Methods
2.2.1Sample collection
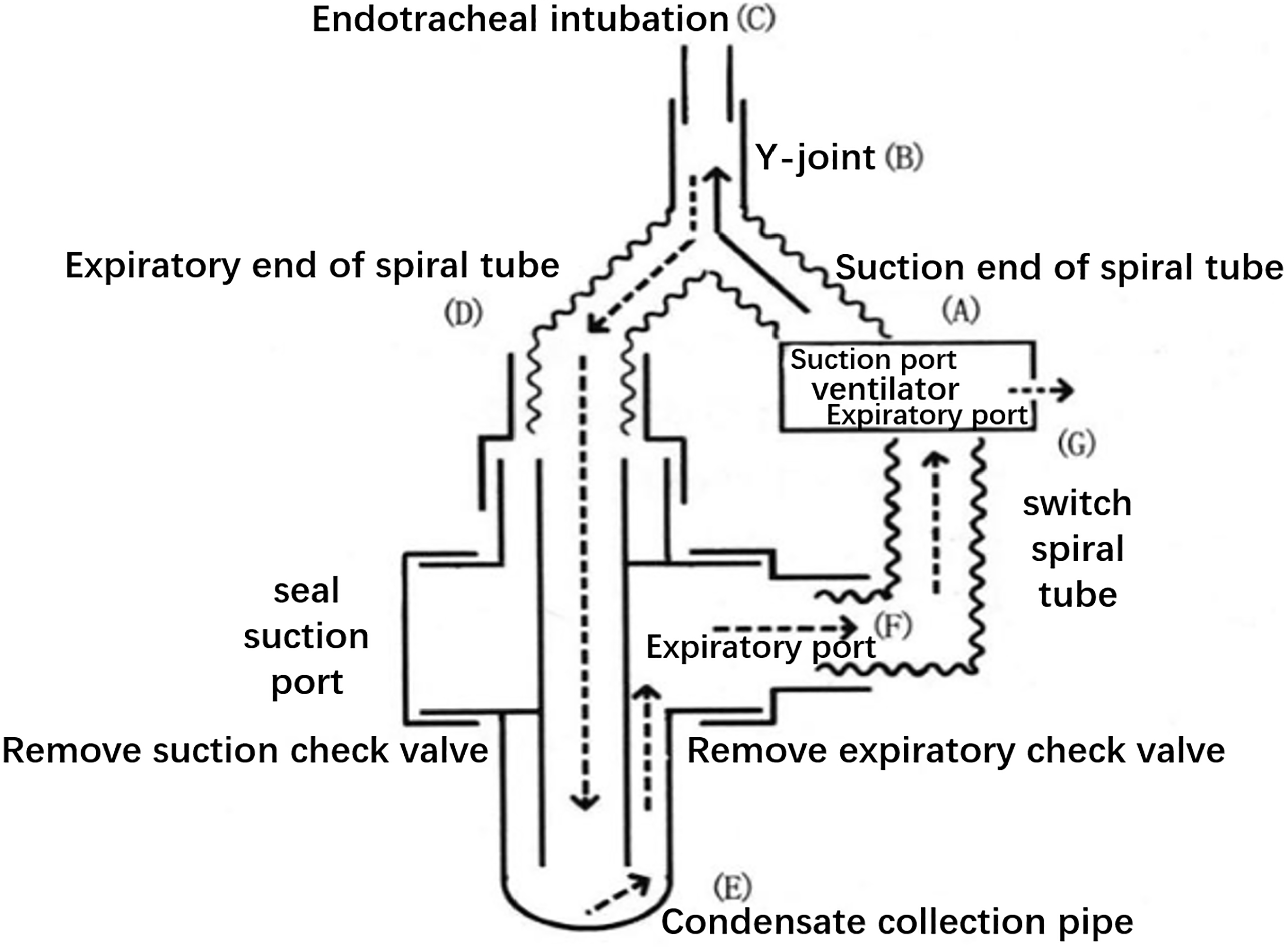
EBC specimens were collected within 24 h after definitive ARDS diagnosis. Prior to collection, the ventilator pipe was replaced with a dry threaded pipe and disconnected from the humidifier. The modified EcoScreen condenser was connected in series to the end of the ventilator pipe (Fig. 1). The condenser collected 2 ml of EBC for 20 min. EBC was collected from the control group by mouth breathing. A total of 5 ml of venous blood was simultaneously collected per subject. To extract serum, blood samples were centrifuged at 4000 rpm for 5 min. The samples were preserved in a
Figure 1.
Schematic diagram of the connection between the modified condenser and the ventilator.
2.2.2Arterial blood gas analysis
Prior to EBC collection, arterial blood (radial artery or femoral artery or dorsal artery) was collected with a Roche OMNIC blood gas analyser.
2.2.3Chest X-ray examination
Prior to EBC collection, patients were examined with a bedside X-ray.
2.2.4Detection method
VEGF levels in EBC and serum were measured by enzyme-linked immunosorbent assay (ELISA) kits in accordance with the manufacturer’s protocols [11]. The kits were purchased from Bender Med Systems GmbH (Austria).
2.2.5Observation index
The lung injury score (LIS) included PaO
Table 2
Lung injury score (LIS)
| Score | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| X-ray score | 0 | 0 | 1/4 | 2/4 | 3/4 |
| PaO | 225 | 175 | 100 | ||
| PEEP (cmH | 6 | 9 | 12 | ||
| Static compliance (ml/cmH | 60 | 40 | 20 |
2.3Statistical analysis
Statistical analysis was performed with SPSS13.0 statistical software. Quantitative data were subjected to the normal distribution test and
3.Results
3.1Comparison of EBC and serum VEGF levels between ARDS group and healthy group
The VEGF level in the EBC of the ARDS group (49.22
Table 3
Comparison of EBC and serum VEGF levels between ARDS and the healthy group (
| N | EBC | Serum | |
| VEGF (ng/L) | VEGF (ng/L) | ||
| ARDS group | 31 | 49.22 | 1248.12 |
| Healthy group | 22 | 56.62 | 1373.11 |
|
| 3.566 | 1.312 | |
|
| 0.195 |
3.2Correlation between EBC and serum VEGF levels
Serum and EBC VEGF levels were positively correlated with a correlation coefficient of 0.694 (
3.3Comparison of EBC and serum VEGF levels in mild ARDS group and moderate-severe ARDS group
The VEGF level in the EBC of the mild ARDS group (55.44
Table 4
Comparison of EBC and serum VEGF levels in mild ARDS group and moderate-severe ARDS group (
| N | EBC | Serum | |
| VEGF (ng/L) | VEGF (ng/L) | ||
| Mild ARDS | 14 | 55.44 | 1150.62 |
| Moderate-severe ARDS | 17 | 44.09 | 1328.41 |
|
| 6.031 | 1.603 | |
|
| 0.120 |
3.4Comparison of EBC and serum VEGF levels in survival and mortality groups
The VEGF level in the EBC of the survival group (52.25
Table 5
Comparison of EBC and serum VEGF levels in survival and mortality groups (
| N | EBC | Serum | |
| VEGF (ng/L) | VEGF (ng/L) | ||
| Survival group | 21 | 52.25 | 1282.84 |
| Mortality group | 10 | 42.84 | 1175.21 |
|
| 3.843 | 0.885 | |
|
| 0.383 |
Figure 2.
Correlation scatter diagram of EBC and serum level of VEGF (ng/L).
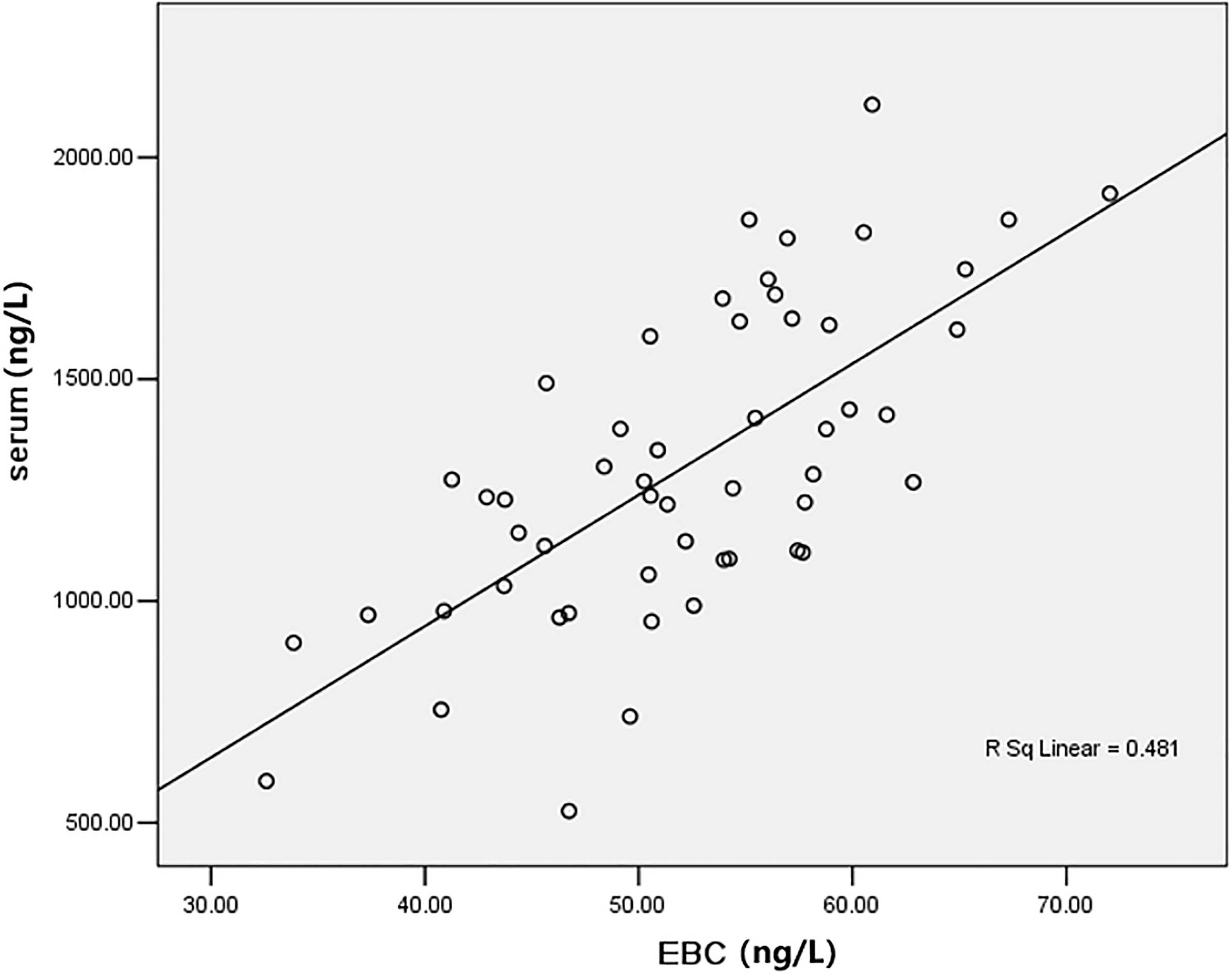
Table 6
Correlation analysis between VEGF and clinical indexes in patients with ARDS
| Clinical index | EBC VEGF | Serum VEGF | ||
|---|---|---|---|---|
|
|
|
|
| |
| LIS | ||||
| Static compliance | 0.132 | 0.069 | ||
| PaO | 0.579 | 0.256 | ||
| A-aDO | 0.078 | |||
| PaO | 0.601 | 0.271 | ||
3.5Correlation analysis between VEGF and clinical indexes in patients with ARDS
Table 6 shows that the VEGF level in EBC was positively correlated with PaO
4.Discussion
ARDS is caused by excessive and uncontrolled inflammatory reactions that are triggered by neutrophil activation and oxygen-free radical injuries. Inflammation changes lung microvascular permeability and the morphology and function of pulmonary microvascular endothelial cells [12]. Moreover, inflammation causes the vascular leakage of albumin to tissue, which results in hypoxemia. In this process, VEGF increases endothelial cell vesicles to act as vascular permeability regulator. VEGF is 50,000 times more potent than histamine [13]. The effects of VEGF cannot be blocked by antihistamines and platelet activating factor inhibitors. VEGF has a protective effect on ARDS and an important role in the survival of epithelial cells that are cultured in vitro. Alveolar epithelial cells proliferate in the presence of exogenous VEGF. Moreover, VEGF can repair damaged alveolar capillary membrane barriers, improve pulmonary edema and promote ARDS recovery [14].
The diagnosis and staging of ARDS mainly depends on comprehensive judgment that uses patient history, chest X-rays and arterial blood gas results. Clinical workers have focused on identifying the severity of ARDS by detecting the level of inflammatory factors and changes in the patient’s body. The detection of EBC components has provided a new way to further study the mechanism of oxidative stress response in critically ill patients.
EBC, a mixture of mucus and volatile substances in respiratory tract liquid, can reflect various physiological and pathological changes in the body [15]. The collection and detection of EBC are advantageous in detecting early pathological changes in the lower respiratory tract and lung parenchyma. Given that EBC is directly derived from the airway, the physiological and biochemical function of the lungs can be detected without damaging bronchial mucosa [16]. In addition, EBC can produce reliable and reproducible test results and can be collected using simple methods that are suitable for patients of any age and any illness [17]. EBC collection is especially advantageous for diagnosing patients on mechanical ventilation. The repeated collection of EBC can dynamically detect airway inflammation and oxidative stress in critically ill patients [18, 19].
This research revealed that VEGF levels in the EBC of ARDS patients on mechanical ventilation were lower than those in the control group. And our results demonstrated that VEGF levels in the EBC of the mild ARDS group were significantly higher than those in the moderate-severe ARDS group and significantly higher in the survival than that in the mortality group. These results were consistent with decreased VEGF levels in the lung tissue and bronchoalveolar lavage fluid (BALF) of ARDS patients. VEGF levels decreased because: 1) The pulmonary vascular permeability of ARDS patients increased and more liquid accumulated in the alveolar dilution [20]. 2) Pulmonary edema directly damaged alveolar epithelial cells. The expression of IL-6, IL-8, TNF and other cytokines can indirectly damage alveolar epithelial cells. 3) Proteolytic enzymes that were released from neutrophils and other inflammatory cells accelerated the degradation of VEGF [21]. 4) Damaged alveolar capillary barriers released VEGF from the lungs to the blood. 5) VEGF was released as a self-protective mechanism. Given that the level of VEGF in EBC reflects the severity of lung injury and contributes to disease prognosis, EBC is a good objective index for evaluating the condition of ARDS [22].
We found that VEGF levels in EBC were significantly correlated with the index of oxygenation, positively correlated with PaO
Although the differences in EBC VEGF levels among groups were statistically significant, there was no significant difference in serum VEGF levels. These results implied that changes in VEGF levels in EBC reflect the severity of lung injury, as previously reported by Pickkers [24].
In conclusion, the EBC assay can be used to analyze the changes of VEGF content in the epithelial lining of the respiratory tract to directly understand the degree of inflammation in the lung [25]. This diagnostic method is suitable for ARDS patients on mechanical ventilation. Hence, EBC levels of VEGF can be used as an index for judging the severity of illness and evaluating therapeutic effect.
Acknowledgments
This research was supported by the Natural Science Foundation of Jiangsu Province (no. BK20191207) and the Science and Technology Project of Nantong (no. HS2018002).
Conflict of interest
None to report.
References
[1] | Zinter MS, Spicer A, Orwoll BO, et al. Plasma angiopoietin-2 outperforms other markers of endothelial injury in prognosticating pediatric ARDS mortality. Am J Physiol Lung Cell Mol Physiol. (2016) ; 310: (3): L224-231. doi: 10.1152/ajplung.00336.2015. |
[2] | Cochi SE, Kempker JA, Annangi S, et al. Mortality trends of acute respiratory distress syndrome in the united states from 1999 to 2013. Ann Am Thorac Soc. (2016) ; 13: (10): 1742-1751. doi: 10.1513/annalsats.201512-841oc. |
[3] | Rong LQ, Di Franco A, Gaudino M, et al. Acute respiratory distress syndrome after cardiac surgery. J Thorac Dis. (2016) ; 8: (10): E1177-E1186. doi: 10.21037/jtd.2016.10.74. |
[4] | Maretta M, Toth S, Jonecova Z, et al. Immunohistochemical expression of MPO, CD163 and VEGF in inflammatory cells in acute respiratory distress syndrome: A case report. Int J Clin Exp Pathol. (2014) ; 7: (7): 4539-4544. |
[5] | Barratt S, Medford AR, Millar AB. Vascular endothelial growth factor in acute lung injury and acute respiratory distress syndrome. Respiration. (2014) ; 87: (4): 329-342. doi: 10.1159/000356034. |
[6] | Umbrello M, Formenti P, Bolgiaghi L, et al. Current concepts of ARDS: A narrative review. International Journal of Molecular Sciences. (2016) ; 18: (1): 64. doi: 10.3390/ijms18010064. |
[7] | ARDS Definition Task Force. Acute respiratory distress syndrome: The berlin definition. JAMA. (2012) ; 307: (23): 2526-2533. doi: 10.1001/jama.2012.5669. |
[8] | Sine CR, Belenkiy SM, Buel AR, et al. Acute respiratory distress syndrome in burn patients: A comparison of the berlin and american-european definitions. J Burn Care Res. (2016) ; 37: (5): e461-469. doi: 10.1097/bcr.0000000000000348. |
[9] | Chen JL, Chen JR, Huang FF, et al. Analysis of p16 gene mutations and their expression using exhaled breath condensate in non-small-cell lung cancer. Oncol Lett. (2015) ; 10: (3): 1477-1480. doi: 10.3892/ol.2015.3426. |
[10] | Kuban P, Frantisek F. Exhaled breath condensate: Determination of non-volatile compounds and their potential for clinical diagnosis and monitoring. A review. Analytica Chimica Acta. (2013) ; 805: : 1-18. doi: 10.1016/j.aca.2013.07.049. |
[11] | Vernes JM, Meng YG. Detection and quantification of VEGF isoforms by ELISA. Methods Mol Biol. (2015) ; 1332: : 25-37. doi: 10.1007/978-1-4939-2917-7_2. |
[12] | Oliveira GP, Silva JD, Marques PS, et al. The effects of dasatinib in experimental acute respiratory distress syndrome depend on dose and etiology. Cell Physiol Biochem. (2015) ; 36: (4): 1644-58. doi: 10.1159/000430325. |
[13] | Villar J, Cabrera-Benítez NE, Ramos-Nuez A, et al. Early activation of pro-fibrotic WNT5A in sepsis-induced acute lung injury. Crit Care. (2014) ; 18: (5): 568. doi: 10.1186/s13054-014-0568-z. |
[14] | Azamfirei L, Gurzu S, Solomon R, et al. Vascular endothelial growth factor: A possible mediator of endothelial activation in acute respiratory distress syndrome. Minerva Anestesiol. (2010) ; 76: (8): 609-616. |
[15] | Chen JL, Lv XD, Ma H, et al. Detection of cancer embryo antigen and endothelin-1 in exhaled breath condensate: A novel approach to investigate non-small cell lung cancer. Molecular and Clinical Oncology. (2016) ; 5: (1): 124-128. doi: 10.3892/mco.2016.902. |
[16] | Xiao P, Chen JR, Zhou F, et al. Methylation of P16 in exhaled breath condensate for diagnosis of non-small cell lung cancer. Lung Cancer. (2014) ; 83: : 56-60. doi: 10.1016/j.lungcan.2013.09.008. |
[17] | Chen JL, Chen JR, Han HN, et al. Clinical significance of miRNA21 in exhaled breath condensate of non-small-cell lung cancer. Int J Clin Exp Med. (2016) ; 9: (9): 17232-17238. |
[18] | Vaschetto R, Corradi M, Goldoni M, et al. Sampling and analyzing alveolar exhaled breath condensate in mechanically ventilated patients: A feasibility study. J Breath Res. (2015) ; 9: (4): 047106. doi: 10.1088/1752-7155/9/4/047106. |
[19] | Walsh BK, Davis MD, Hunt JF, et al. The effects of lung recruitment maneuvers on exhaled breath condensate pH. J Breath Res. (2015) ; 9: (3): 036009. doi: 10.1088/1752-7155/9/3/036009. |
[20] | Ortolan LS, Sercundes MK, Barboza R, et al. Predictive criteria to study the pathogenesis of malaria-associated ALI/ARDS in mice. Mediators Inflamm. (2014) ; 2014: : 872464. doi: 10.1155/2014/872464. |
[21] | Yang S, Cao S, Li J, et al. Association between vascular endothelial growth factor + 936 genotype and acute respiratory distress syndrome in a Chinese population. Genet Test Mol Biomarkers. (2011) ; 15: (10): 737-740. doi: 10.1089/gtmb.2011.0054. |
[22] | Yang Y, Hu S, Xu X, et al. The vascular endothelial growth factors-expressing character of mesenchymal stem cells plays a positive role in treatment of acute lung injury in vivo. Mediators Inflamm. (2016) ; 2016: : 2347938. doi: 10.1155/2016/2347938. |
[23] | Mittal N, Sanyal SN. Exogenous surfactant protects against endotoxin induced acute respiratory distress syndrome in rodents via vascular endothelial growth factor. Pathol Res Pract. (2011) ; 207: (5): 279-284. doi: 10.1016/j.prp.2011.01.010. |
[24] | Pickkers P, Sprong T, EjjK LV, et al. Vascular endothelial growth factors increased during the first 48 hours of human septic shock and correlates with vascular permeability. Shock. (2005) ; 24: (6): 508-512. |
[25] | Chen JL, Lv XD, Ma H, et al. Could detection of VEGF in exhaled breath condensate be more helpful for non-small cell lung cancer? Int J Clin Exp Pathol. (2016) ; 9: (5): 5582-5587. |



