Study on the mechanism of TMRK electroacupuncture in repairing synaptic plasticity in amygdala and hippocampus to relieve fear memory in PTSD rats
Abstract
BACKGROUND AND OBJECTIVE:
Post-traumatic stress disorder (PTSD) is a chronic mental disorder caused by mental or psychological trauma after sudden events of a catastrophic or threatening nature. Synaptic plasticity is the core mechanism of PTSD and the main point of treatment of this disease.
METHODS:
Male Sprague Dawley rats were randomly divided into blank control (Ctrl), SPS (single-prolonged stress) model, SPS&S model (SPS and foot electric shock), SPS+EA (SPS plus electroacupuncture), and SPS&S+EA groups. Tranquilize Mind and Regulate Kidney (TMRK) electroacupuncture method was performed in each rat in the SPS+EA and SPS&S+EA groups, the treatment lasted for 20 minutes per day, simultaneously for 3 consecutive weeks. Behavioral evaluations, molecular tests, electron microscopy, electrophysiological testing were conducted following the treatment.
RESULTS:
First, electro-acupuncture can significantly improve the PTSD-like symptoms. Second, electro-acupuncture can up-regulate the long-term potentiation (LTP) in hippocampus, repair the synaptic morphology and improve BDNF levels in amygdala and hippocampus. Third, electroacupuncture can significantly up-regulate SYN, GAP43, and PSD95 protein levels and mRNA expression in amygdala and hippocampus.
CONCLUSIONS:
The effect of TMRK electro-acupuncture method on the regression of fear memory of PTSD rats may be through its repair of synaptic plasticity in amygdala and hippocampus.
1.Introduction
Post-traumatic stress disorder (PTSD) refers to a long-term persistent mental disorder caused by sudden and severe disasters or extraordinary threats. The core symptoms can be described as intrusive re-experience, increased fear and loss of confidence in the future, etc. As a state of mental and psychological imbalance [1]. The pathological core of PTSD is the formation and enhancement of fear memory. Early fear is a predictive indicator of long-term changes in PTSD avoidance behavior [2].
Clinically, exposure therapy is often used in patients with PTSD. This method uses dissipative training as its basic principle to intervene in the acquired fear memory. Animal experiments use regression training to suppress the animal’s fear response to conditional stimuli, but this suppressed conditional fear response can be re-induced in a variety of situations, such as renewal, rapid acquisition, spontaneous recovery and reinstatement [3]. Some drugs, for example, selective serotonin reuptake inhibitors, have been used for the first-line treatments for PTSD [4, 5]. Nevertheless, there is still a subgroup of PTSD patients who have some persistent symptoms or maintain conditioned fear responses to threatens,and finally the disease becomes chronic [6].
In recent years, acupuncture treatment for PTSD has become a research hotspot. As a common means of PTSD, acupuncture has high efficiency, long duration of action, and low side effects, it can effectively reduce anxiety-like behaviors and improve the impaired learning-memory ability in PTSD rats [7]. Hollifield et al. first reported positive effects of acupuncture in patients with PTSD [8]. Acupuncture can improve clinical symptoms such as anxiety, panic and low learning and memory in patients with PTSD, and its efficacy is even better than that of paroxetine [9]. Electroacupuncture and drug therapy can improve the symptoms of patients with PTSD, but electroacupuncture is superior to drug therapy [10]. In the previous issue of “Diagnostic RCT study of PTSD after different acupuncture treatments for the May 12 earthquake”, we found that electroacupuncture improved the symptoms of fear in patients, and the efficacy was better than that of paroxetine [11].
Synaptic plasticity is the carrier structure and molecular mechanism of learning and memory, and is the basis of learning and memory. Animal models and human neuro-imaging findings suggested that synaptic plasticity may be one of the underlying mechanisms of PTSD. In the study of animal behavioral models using simulated PTSD, such as training animals to learn about fear conditions, and testing the regression of the animal’s fear memory, synaptic plasticity is a very important indicator [12].
Amygdala plays an important role in the formation, expression and regression of fear [12], its synapses are closely related to the function of learning and memory [13]. At the same time, synaptic plasticity of amygdala is also the cellular basis of fear memory [14]. In brain science research, LTP is considered to be a manifestation of synaptic plasticity, a functional basis for learning and memory, and an experimental model of synaptic plasticity. Research reported that LTP of amygdala was inhibited in acute phase of PTSD rats, and was enhanced in the late stage, revealing that amygdala dysfunction is part of the pathogenesis of PTSD [15]. Hippocampus is recognized as a core brain region involved in the regulation of learning and memory, its synaptic plasticity is closely related to learning and memory [16]. Memory-related signal capture and environmental memory extraction in hippocampus-dependent fear memory rely on the synaptic plasticity in the hippocampus which transmits environmental signals to the amygdala to complete the process of fear memory [17]. Bliss and Collingridge [18] first discovered that after intense stimulation of the hippocampus, hippocampal neurons produce LTP, which is characterized by an increase in the intensity of postsynaptic response, which is stimulated by the incoming end of a simple-single synaptic-repetitive stimulating excitability. In addition, there are studies confirmed that LTP in the hippocampal amygdala (especially the basolateral amygdala) may be the neural basis of stress-induced anxiety and the neural basis of conditional fear, it works together with other brain regions to collectively regulate the situation-specific fear regression [19].
In the past few years, researchers have observed the effects of acupuncture on synaptic structure and synaptic transmission efficiency through morphological and electrophysiological methods. A large number of studies have shown that acupuncture can alleviate central synaptic loss and synaptic ultrastructural damage, enhance synaptic transmission efficiency, and promote the function of synaptic plasticity of neurons to a certain extent [20]. Electron microscopy results suggested that electroacupuncture can improve the microstructural changes of the synaptic interface, such as narrowing the synaptic gap width, increasing the PSD thickness, and reducing the curvature of the synaptic interface [21]. These structural changes suggested an increase in synaptic transmission efficiency, and also indicated that acupuncture not only promotes the formation of new synapses, but also promotes the modification of synaptic structures. In addition, electrophysiological recordings in body and brain slices have also observed that acupuncture can facilitate the induction of hippocampal LTP and enhance basal synaptic transmission and LTP intensity [22, 23, 24, 25].
In view of the above discussion, our study targets PTSD, with modeling by SPS and SPS&S, and puts forward the hypothesis that “the effect of acupuncture on PTSD may be related to the repair of damaged synaptic plasticity in amygdala and hippocampus”, thus to confirm the hypothesis.
2.Methods and materials
2.1Subjects
All of the experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC, USA), The study protocol was approved by the Biomedical Ethics Committee of Chengdu University of TCM for animal use and protection. Adult male Sprague Dawley rats (2 month old, 180–220 g,
2.2Model
2.2.1SPS model
This study adopted the internationally recognized SPS model, which was recommended for PTSD modeling by the ‘Advances in Basic and Clinical Research’ International conference held by the Japanese Ministry of Education in 2005. SPS modeling induces psychological stress and physiological stress to simulate PTSD-like symptoms in rats. In the current experimental protocol, each rat was first restrained via placement inside a plastic tube for 2 hours, immediately following restraint, each rat underwent a 20-min forced swim, after the forced swim and placement in a cage for 15 minutes, each rat was anesthetized with ether in a cylinder until losing consciousness. The SPS procedure was based on previous studies [6]. After modeling, the rat was returned to its cage, and was conventionally housed/fed until later experiments were conducted.
2.2.2SPS&S model
The SPS&S and SPS&S+EA group were subjected to the footshock after SPS modeling: they were placed in a square electric shock box made of plexiglass (470 mm
2.3Electroacupuncture treatment
The SPS+EA and the SPS&S+EA group underwent electroacupuncture treatment on the fifteenth day after modeling. Rats need to be fixed before treatment, place the rat on a 6
Electroacupuncture was performed in each rat at the acupoints of Baihui (GV20), Shenting (GV24), and Shenshu (BL23, bilateral). Several documents and methods were utilized to determine acupoints within the test rats. Specifically, the Acupoint Standard for Experimental Animals, the Map of Acupoints for Experimental Animals, and the map of animal acupoints in Experimental Acupuncture, combined with comparative anatomical methods, were used. Table 1 shows the exact locations and operations of the 3 acupoints. Instruments used in the electroacupuncture included 32 G Hwato disposable acupuncture needles (0.25 mm diameter
Table 1
Locations and operations of electroacupuncture in the 3 acupoints
| Acupoint location | Operation | |
|---|---|---|
| Shenting (GV24) | On the anterior midline and on the fronto-parietal seam of the rat | Insert needle obliquely in an upward direction to a depth of 2 mm |
| Baihui (GV20) | Center of the parietal bone | Insert needle obliquely in an upward direction to a depth of 2 mm |
| Shenshu (BL23) | 7 mm away from the lateral spinous processes of second lumbar vertebrae on left and right sides | Insert needle vertically to a depth of 8 mm |
During electroacupuncture, the GV24 and left BL23 acupoints were stimulated in one setting by connecting the GV24 acupoint to the anode and the left BL23 acupoint to the cathode. Similarly, the GV20 and right BL23 acupoints were stimulated separately by connecting the GV20 acupoint to the anode and the right BL23 acupoint to the cathode. Electroacupuncture of all regions was performed 20 minutes a day simultaneously for 3 consecutive weeks, resulting in a total of 21 applications per target area. Parameters of electroacupuncture included a 2/100 Hz dilatational frequency wave with an automatic shift between 2 Hz and 15 Hz of stimulation (3-second duration of each shift), and a current intensity of 1 mA.
2.4Behavioral analysis
All groups underwent behavioral testing on the next day after the completion of electroacupuncture in the following sequence: Locomoto Activity, Radial Six-Arm Water Maze Test, Elevated Plus-Maze, and Fear Conditioning Test. The researchers performing the behavioral tests were blinded to the experimental grouping of the animals.
2.4.1Locomoto Activity
The test was used to investigate for anxiety-like behaviors as reported previously [26]. The rats were placed in a spontaneous activity test box (40
2.4.2Radial six-arm water maze test
Assessment of spatial learning and memory functions using the test as reported previously [27, 28]. Briefly, a black circular pool (200 cm in diameter, 60 cm in height) was filled with water at room temperature. A hidden black platform was submerged about 2 cm below the water level, and the camera is located 200 cm above the center of the pool. The testing was performed in a room with different pictures posted on the room walls to serve as cues for rats. Before the experiment, the rats were allowed to swim for 90 s without a platform in the pool, and then released into the pool with platform from different orientations and allowed to swim to find the escape platform. The rat was given 90 s to locate the platform in both the learning phase and the memory test phase. The rat was guided to the goal arm if it failed to locate the platform within 90 s. Then the rat was allowed to stay on the platform for about 30 s to observe the room before it was placed into the pool for the next trial.
2.4.3Elevated plus-maze
Anxiety-like behavior was evaluated using the test as described previously [29]. Briefly, our maze is made of a black fiberboard with a four-armed plus-shaped platform (two opposing arms, 50 cm long and 10 cm wide) raised about 50 cm from the ground. Each rat was placed in the central neutral area (10
2.4.4Fear conditioning test
The testing was performed as reported previously [30, 31]. The testing was performed in 2 chambers of similar size and material (A and B) starting on the day after the Elevated Plus-Maze was conducted. Contextual fear conditioning was only performed in chamber A, and cued fear conditioning was performed in both chambers A and B. Chambers A and B provided different context stimuli. Chamber A provided auditory stimulus and mild electric footshock, and chamber B provided only auditory stimulus. Proportional freezing duration of each rat induced by either auditory stimulus (cue) or context (chamber environment) was used as an index of conditional fear. In addition to acquisition of fear conditioning, animals were also tested in extinction and recall paradigms. Extinction was measured by placing rats in chamber A without foot shocks. One training session involved 10 placements (blocks). Four consecutive sessions were performed for contextual fear memory and 3 consecutive sessions for cued fear memory. After the extinction paradigm was completed, a recall paradigm was performed, in which rats were exposed again to foot shocks, followed by extinction-like training sessions.
Footshock sensitivity test was performed on the second day after the completion of the fear conditioning testing. The rats were placed in the A box for 3 minutes, and then footshock (0.05 mA) was administered, which was escalation by 0.05 mA. Recording minimum current intensity when the rats began to notice (staring at the electric shock rod does), flinch (the paws are quickly lifted from the shock rod) and vocalize.
2.5Electrophysiological testing in hippocampus
2.5.1Rat fixation and localization
After the behavioral analyses, the rats in each group were anesthetized with 20% urethane (i.p), then the incisors of rat were fixed on tooth holder of the three-dimensional brain stereotactic device (DW-5). Put the rat’s head in the middle of the two slides and placed the ear rod in the left and right ears external auditory canal. Then fix the screws on the two ear rods after observing the same scales. After the rat is fixed, the head surgery was performed. Separated the subcutaneous tissue, peeled off the fascia and muscle, and clean the skull surface with hydrogen peroxide, then the periosteum was pushed to expose the bregma, lambdoid suture and sagittal suture. Put the positioning needle on the bregma, then the stimulating electrode S (3.8 mm on the right side of the center line, 4.2 mm behind the Bregma) and the recording electrode R (2.5 mm on the right side of the center line and 3.4 mm behind the Bregma) were placed, the cortex of the two electrode insertion sites was exposed and the dura was peeled off.
2.5.2PS recording and LTP induction
Adjusted the depth of the recording and stimulation electrodes. Positive voltage stimulation (2.5
2.6Electron microscopic analysis
2.6.1Brain tissue preparation for the electron microscopic analysis
After an overdose of sodium pentobarbital (200 mg/ml dissolved in 10% ethanol) animals were transcardially perfused with ice cold 0.9% physiological saline followed by 4% paraformaldehyde containing 0.2% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4). The brains were removed and post fixed overnight in the same solution at 4
2.6.2Quantitative analysis of the synapses
Ten photos were randomly selected from each group for measurement, and analyzed by MoticImages Advanced 3.0 (MicroOptic Industrial Group Co. Ltd) image analysis system. The synaptic interface curvature, PSD thickness and active zone length, and synaptic gap width were measured.
2.7Western blot (WB) analysis
Total protein was extracted from brain tissue or cells with RIPA lysis buffer (Beyotime, Shanghai, China). Equal amount of protein were separated by SDS-PAGE and transferred to a PVDF (Millipore, Schwalbach, Germany). After blocked with 5% nonfat milk for 2 h at room temperature, the membrane was incubated with primary antibodies overnight at 4
2.8ELISA
The brain tissue was extracted for ELISA assays. Rat BDNF ELISA Kit (BOSTER Biological Technology, Wuhan, China) was used to assess the levels of BNDF proteins in brain tissue according to manufacturer’s protocol.
2.9Immunohistochemical (IHC)
All rats were executed, the brains were rapidly removed and washed with PBS, then immersion fixed in 4% PBS-buffered paraformaldehyde for 12h and then embedded in paraffin. The paraffin sections immunohistochemical staining was performed using UltraSensitive
Figure 1.
Effect of electroacupuncture on latency (A), exploration distance (B), number of stay in target quadrant (C) and exploration distance (D), Representative searching swimming paths by rats in different groups(E) during RAWM experiment in rats. The number of stay in target quadrant were performed with a Kruskal-Walls test followed by the Mann-Whitney U-test for multiple comparisons. Apart from number of stay in target quadrant, other data were presented as mean
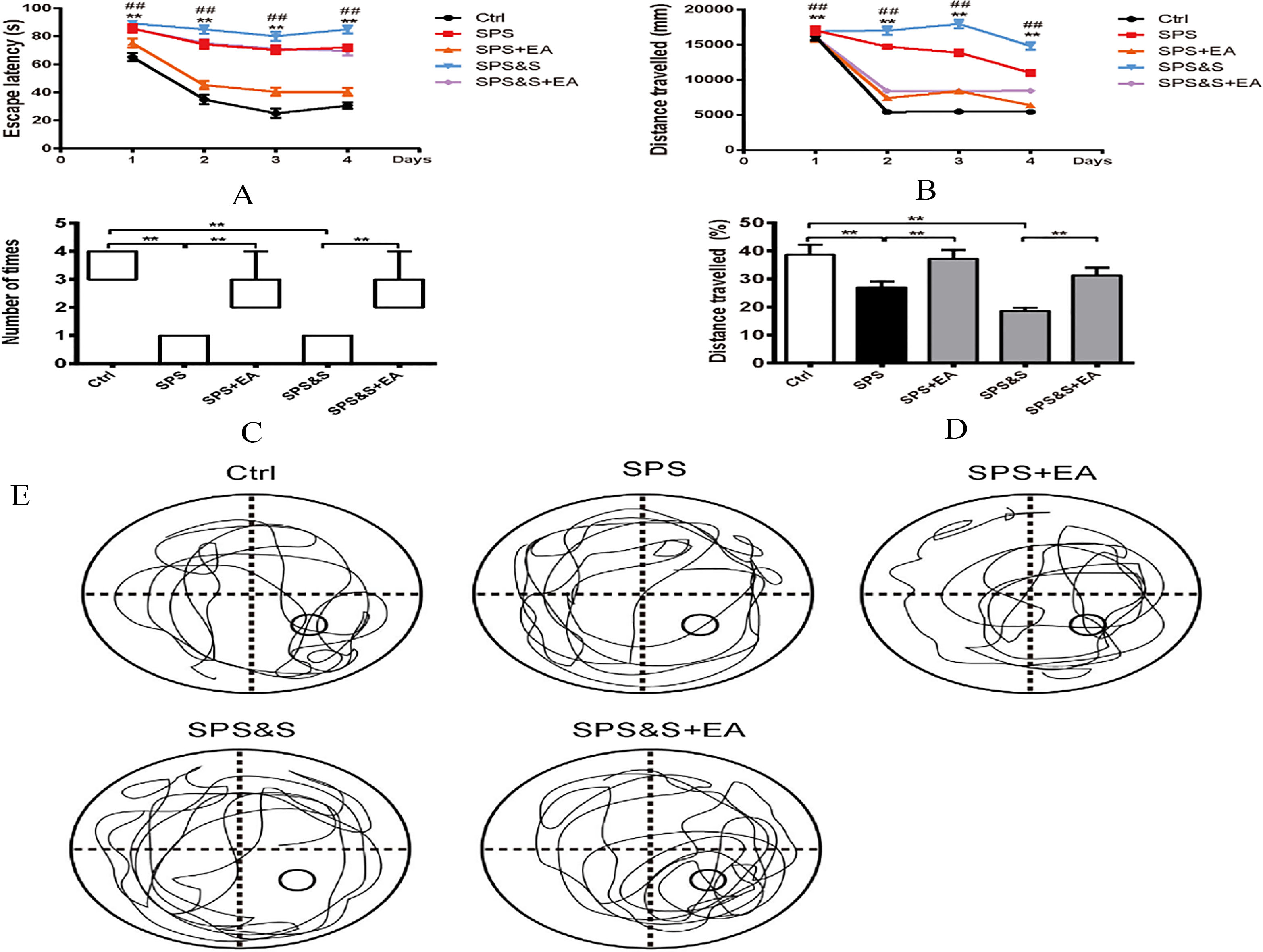
Figure 2.
Effect of electroacupuncture on Locomotor activity (A), Foot shock sensitivity (B), and the percentage of open arm retention time (C) and open arm entries (D) during EPM test in rats. The results were presented as mean
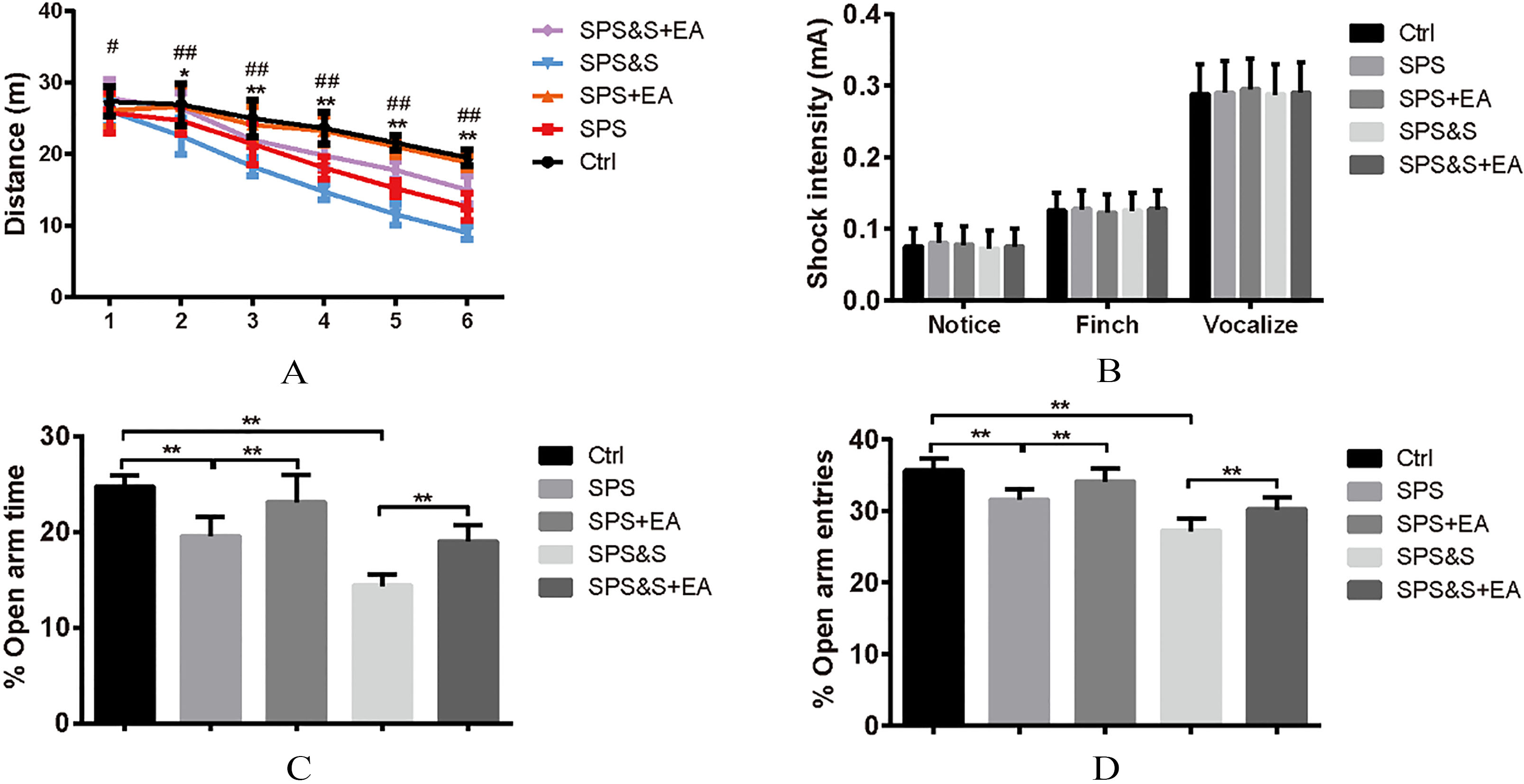
Figure 3.
Effect of electroacupuncture on percentage of freezing of fear conditioning acquisition (A), Fatigue (B–E) and Reconstruction Detection (F) during Scene fear conditioning training in rats. The results were presented as mean
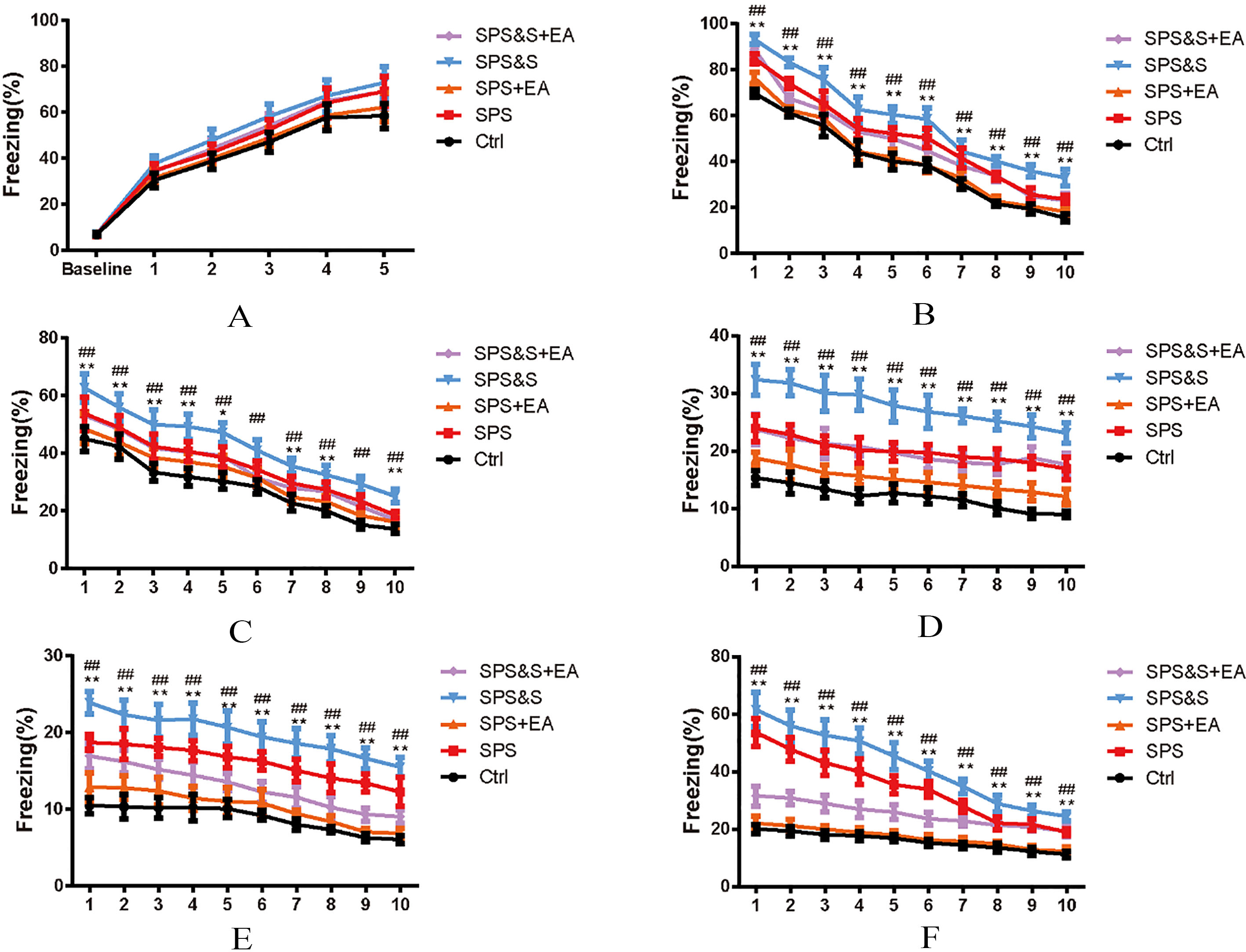
Figure 4.
Effect of electroacupuncture on percentage of freezing of fear conditioning acquisition (A), Fatigue (B–D) and Reconstruction Detection (E) during Clue fear conditioning training in rats. The results were presented as mean
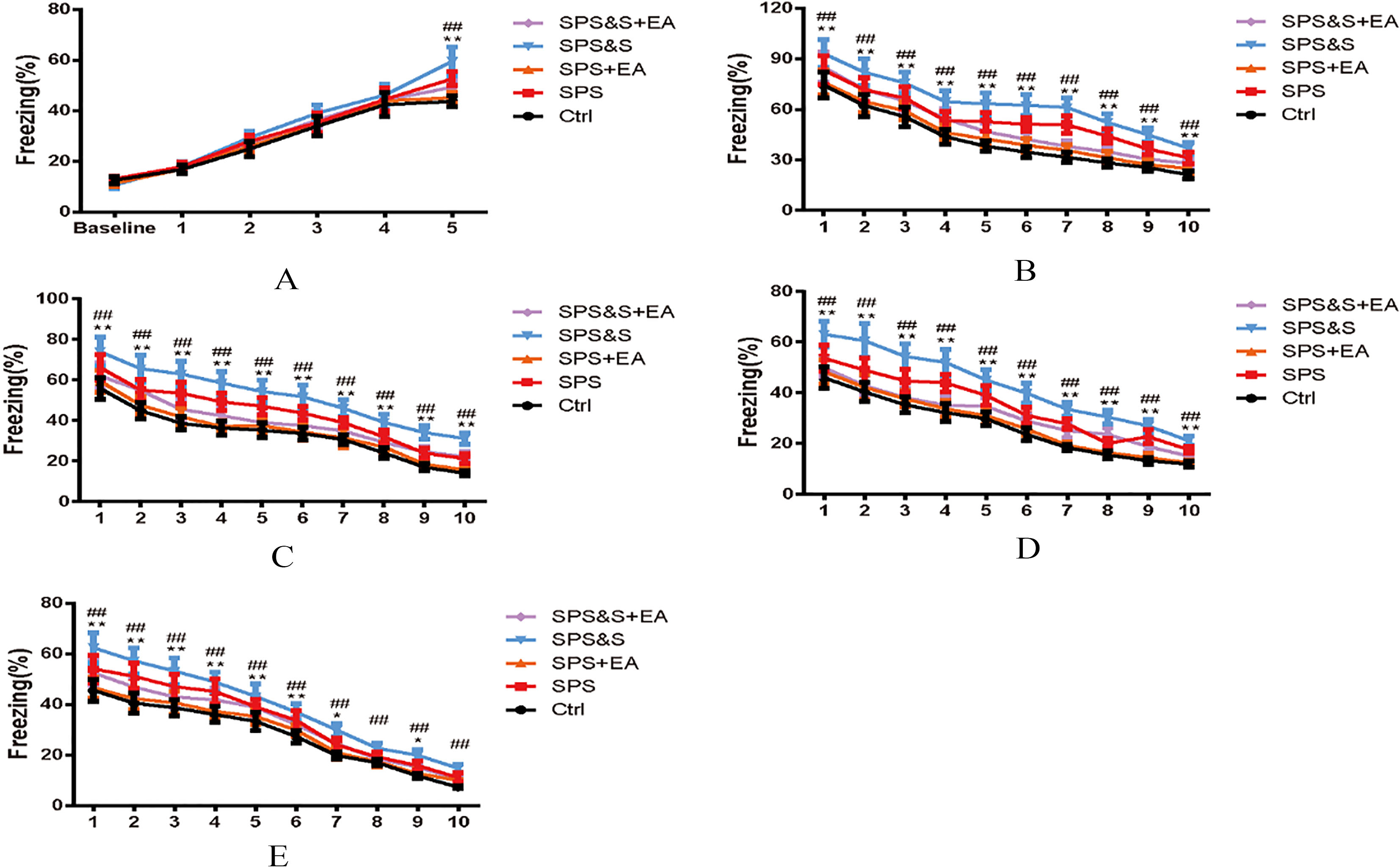
Figure 5.
PSD thickness (A), Synaptic gap width (nm) (B), Curvature of synaptic interface (C) and BDNF levels (D) in amygdala from rats in the indicated groups. Electron microscopy of Amygdala (E). scale
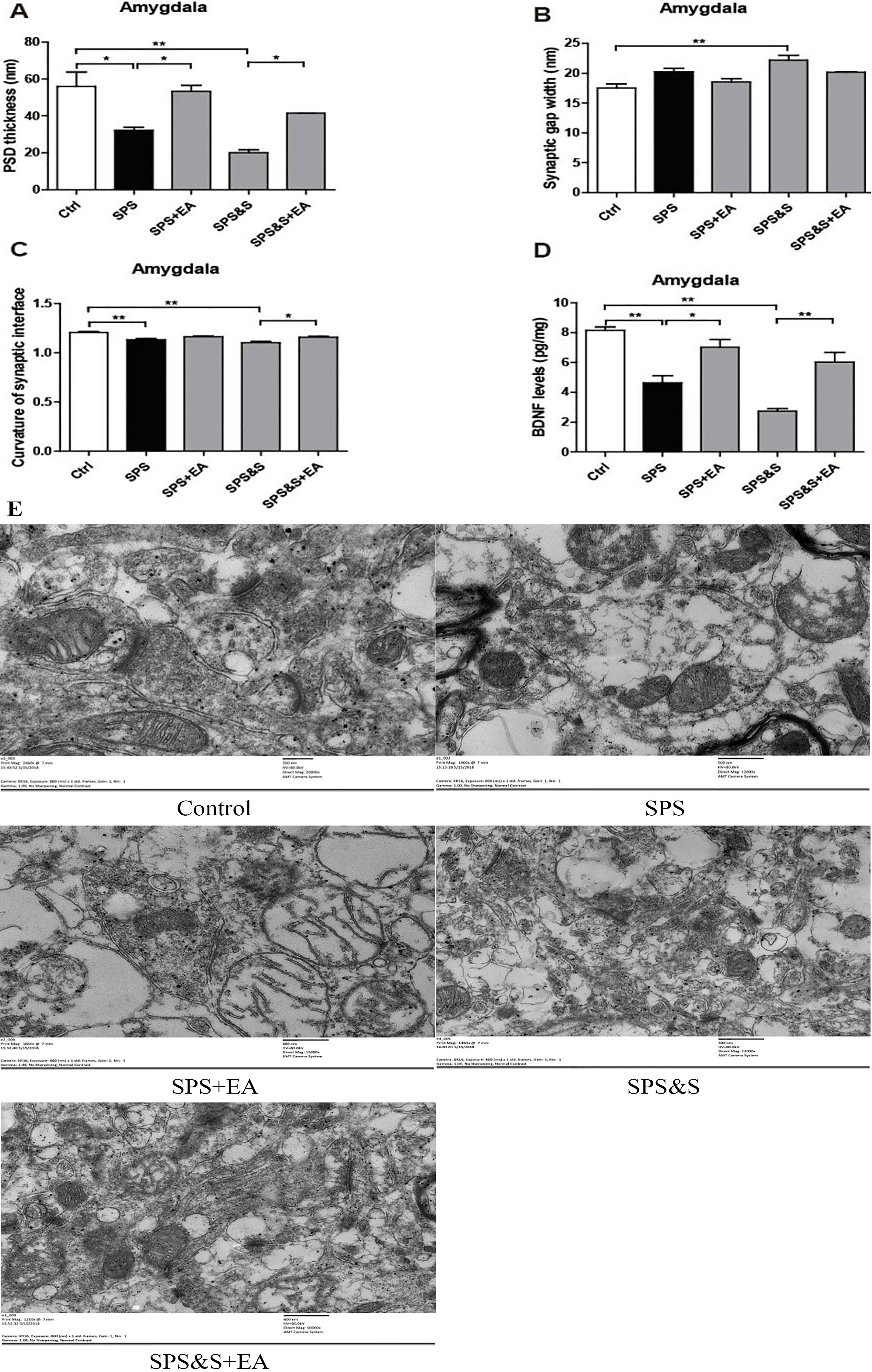
Figure 6.
PSD thickness (A), Synaptic gap width (nm) (B), Curvature of synaptic interface (C) and BDNF levels (D) in hippocampus from rats in the indicated groups. Electron microscopy of Hippocampus (E). scale
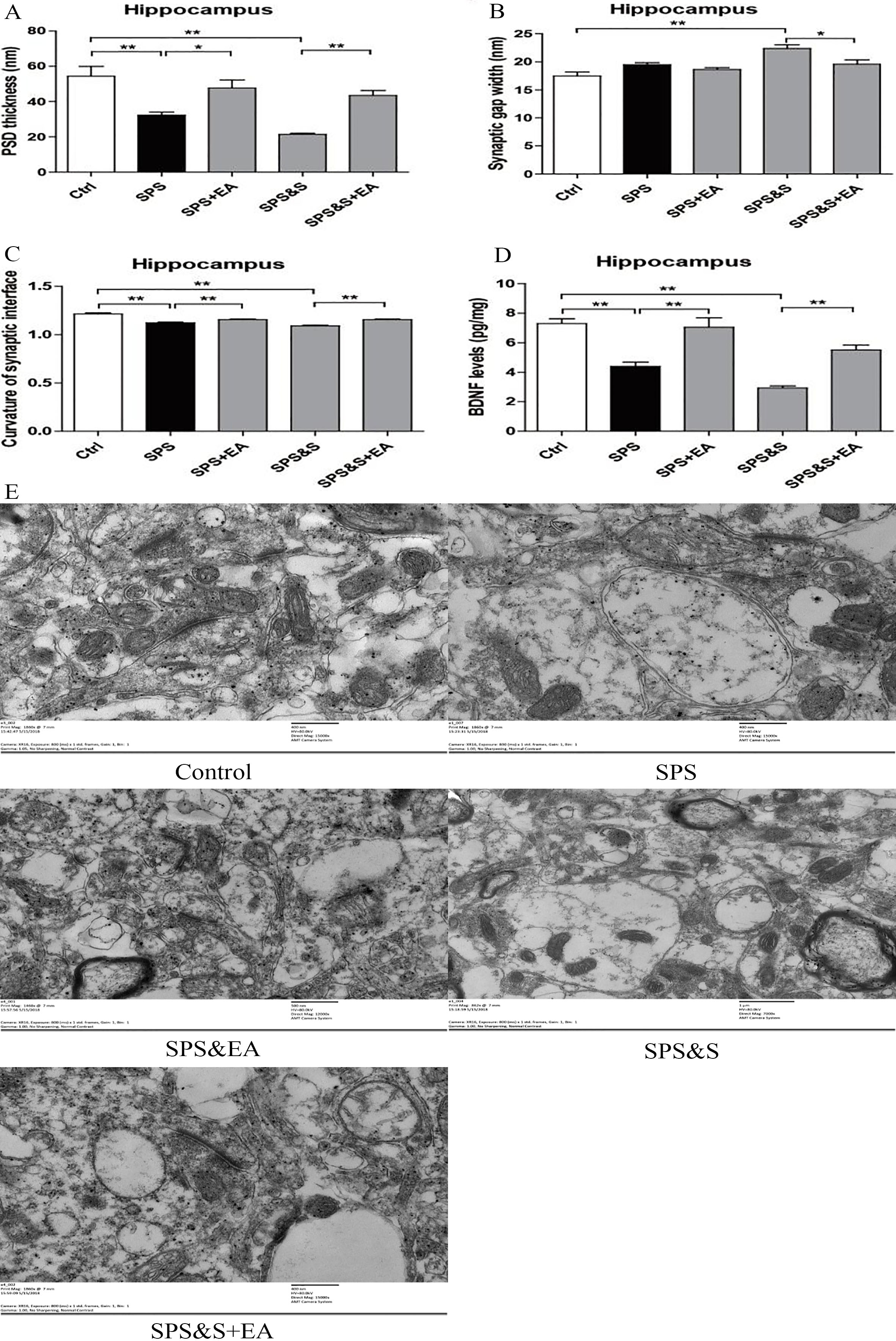
Figure 7.
The average fEPSP amplitude of hippocampal brain regions in each group of PTSD models.
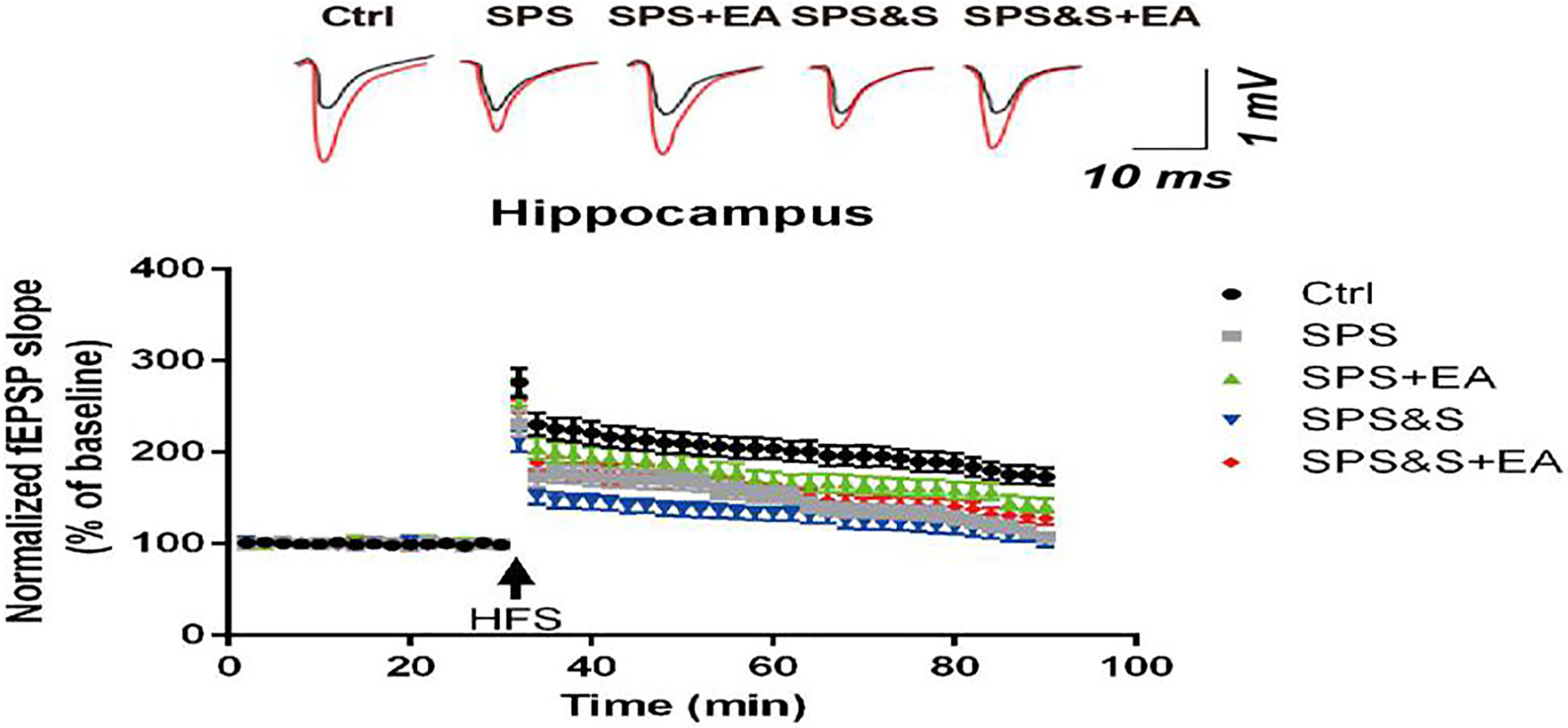
Figure 8.
Effect of electroacupuncture on the expression of SYN, GAP43 and PSD95in amygdala from rats in the indicated groups. (A–D) Expression of SYN, GAP43 and PSD95 were analyzed by immunofluorescence cytochemistry (scale bar, 50
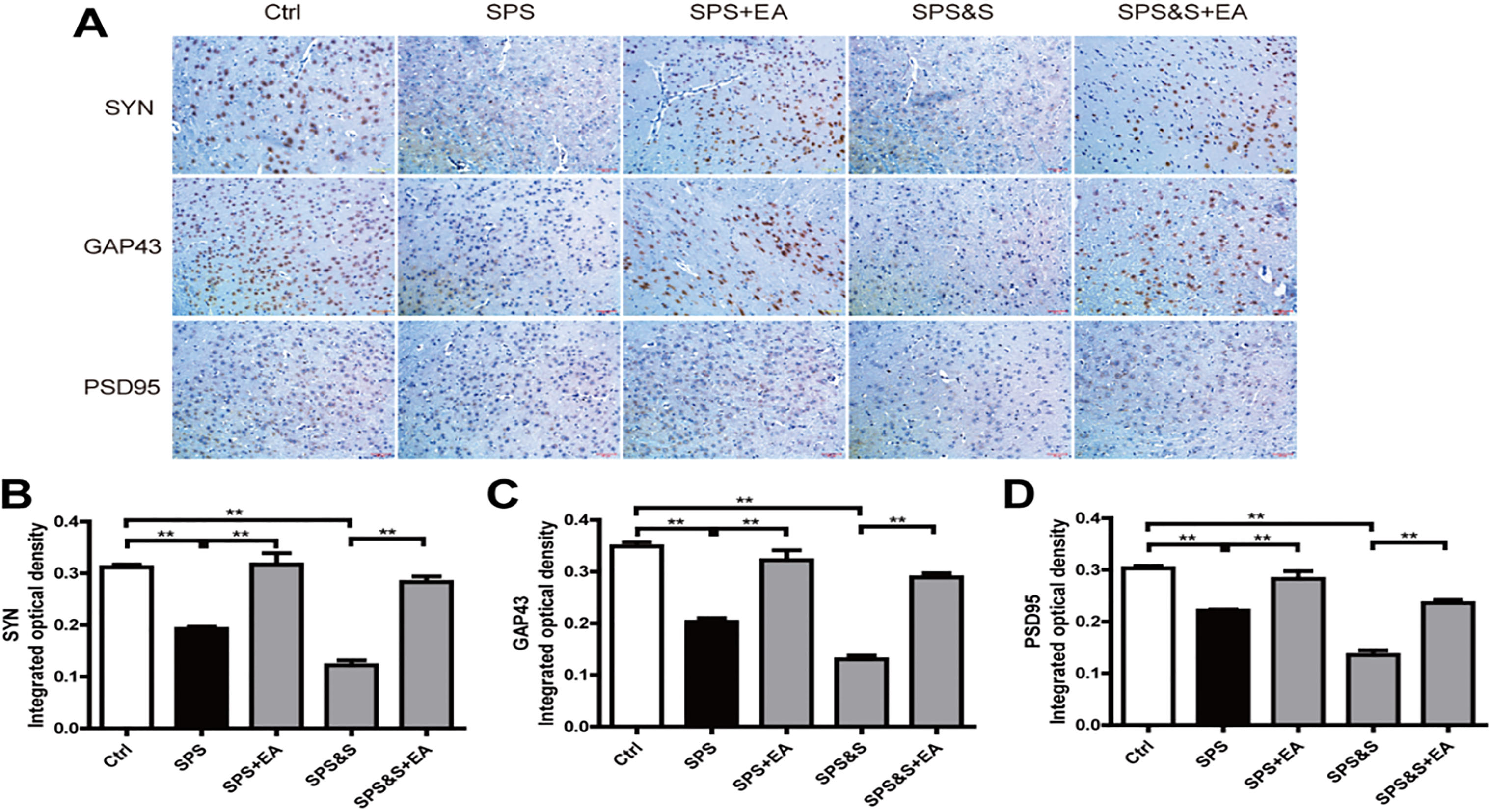
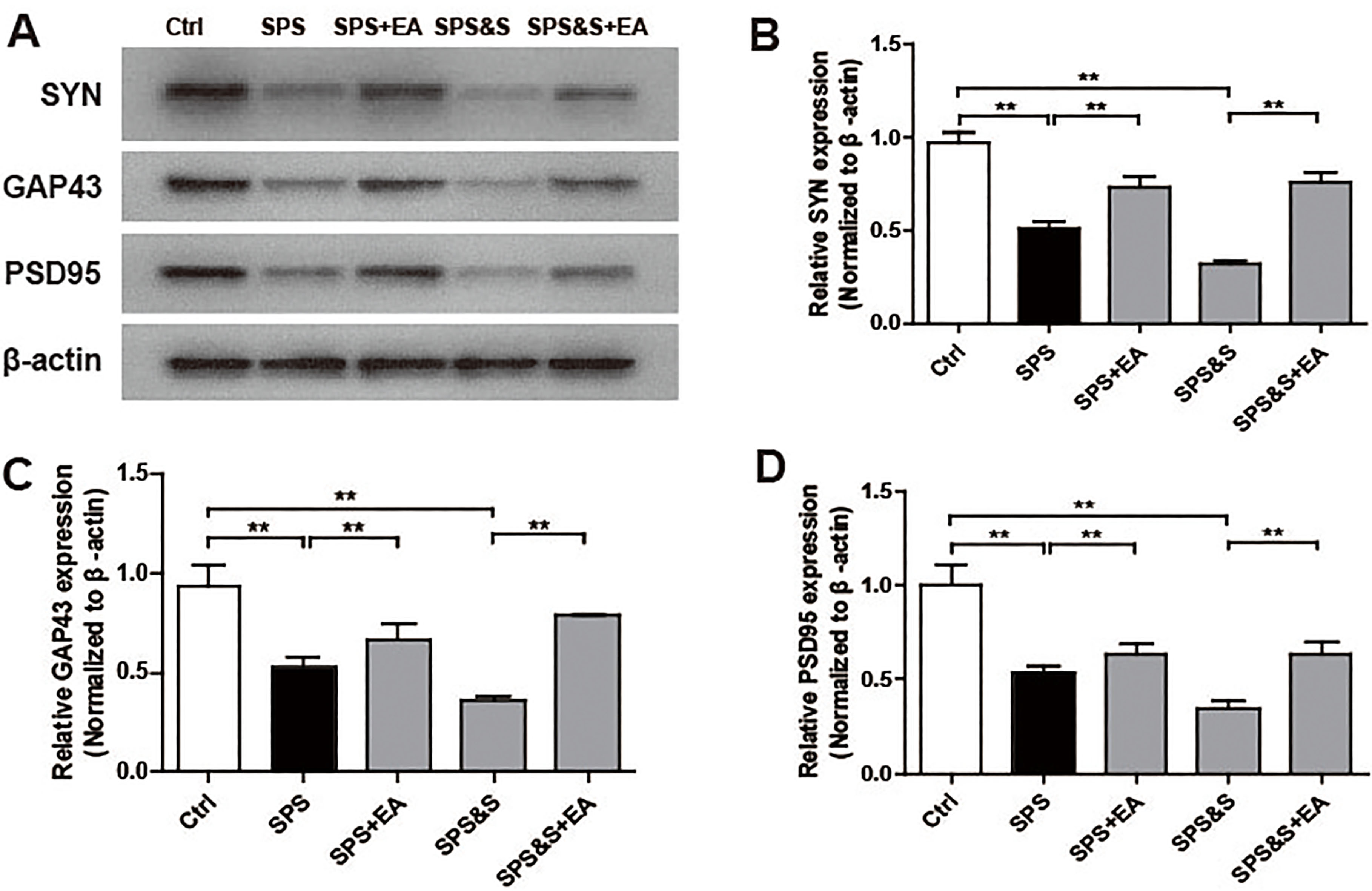
Figure 9.
Effect of electroacupuncture on the expression of SYN, GAP43, PSD95 in amygdala from rats in the indicated groups. (A–D) Expression of SYN, GAP43, PSD95 were analyzed by Western Blot. The results were presented as mean
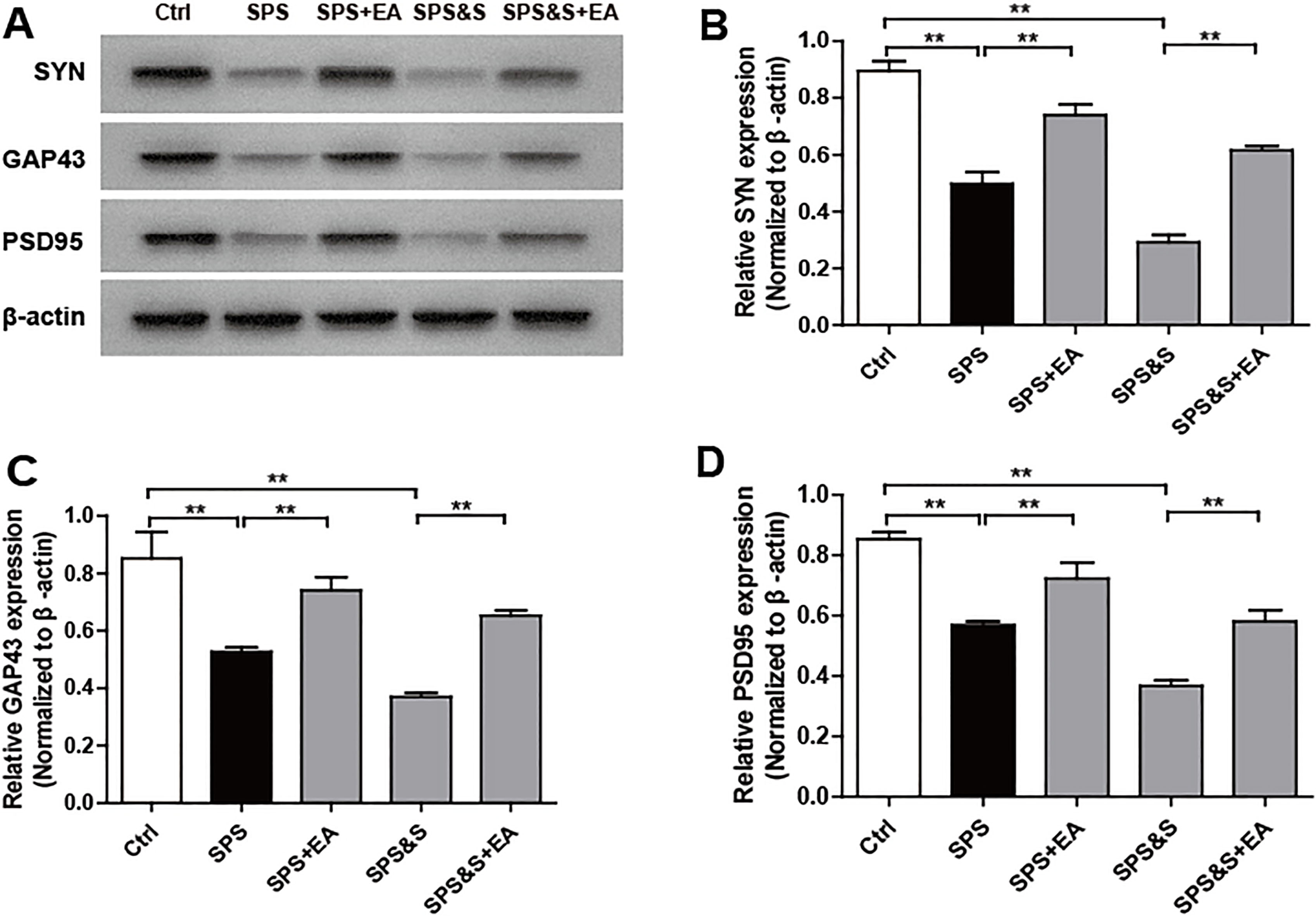
Figure 10.
Effect of electroacupuncture on the mRNA expression of SYN, GAP43, PSD95 in amygdala from rats in the indicated groups. (A–C) mRNA expression of SYN, GAP43, PSD95 were analyzed by Real-time PCR. The results were presented as mean
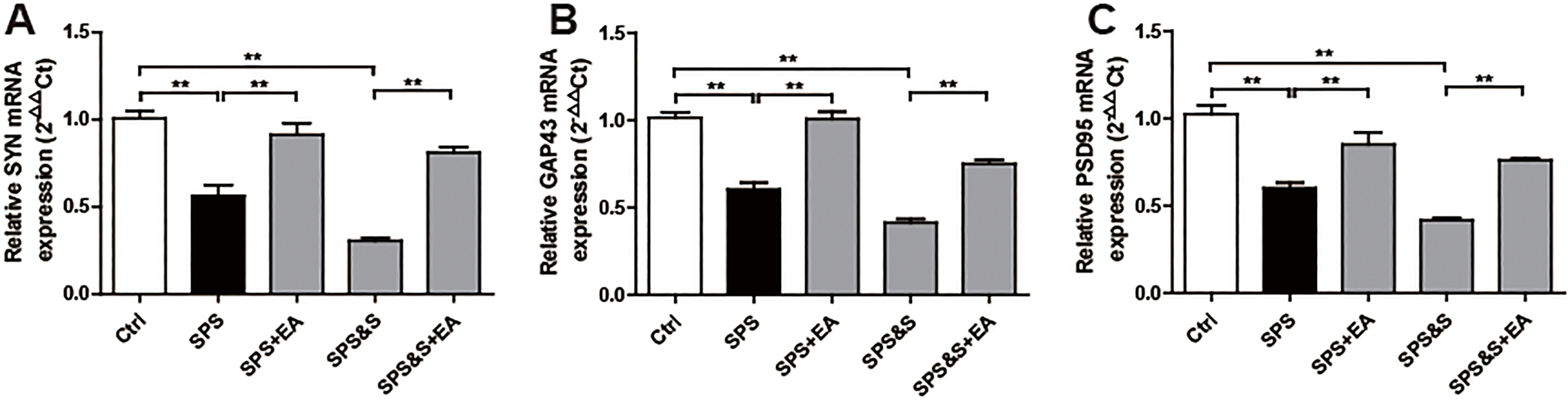
Figure 11.
Effect of electroacupuncture on the expression of SYN, GAP43 and PSD95 in hippocampus from rats in the indicated groups. (A-D) Expression of SYN, GAP43 and PSD95 were analyzed by immunofluorescence cytochemistry (scale bar, 50
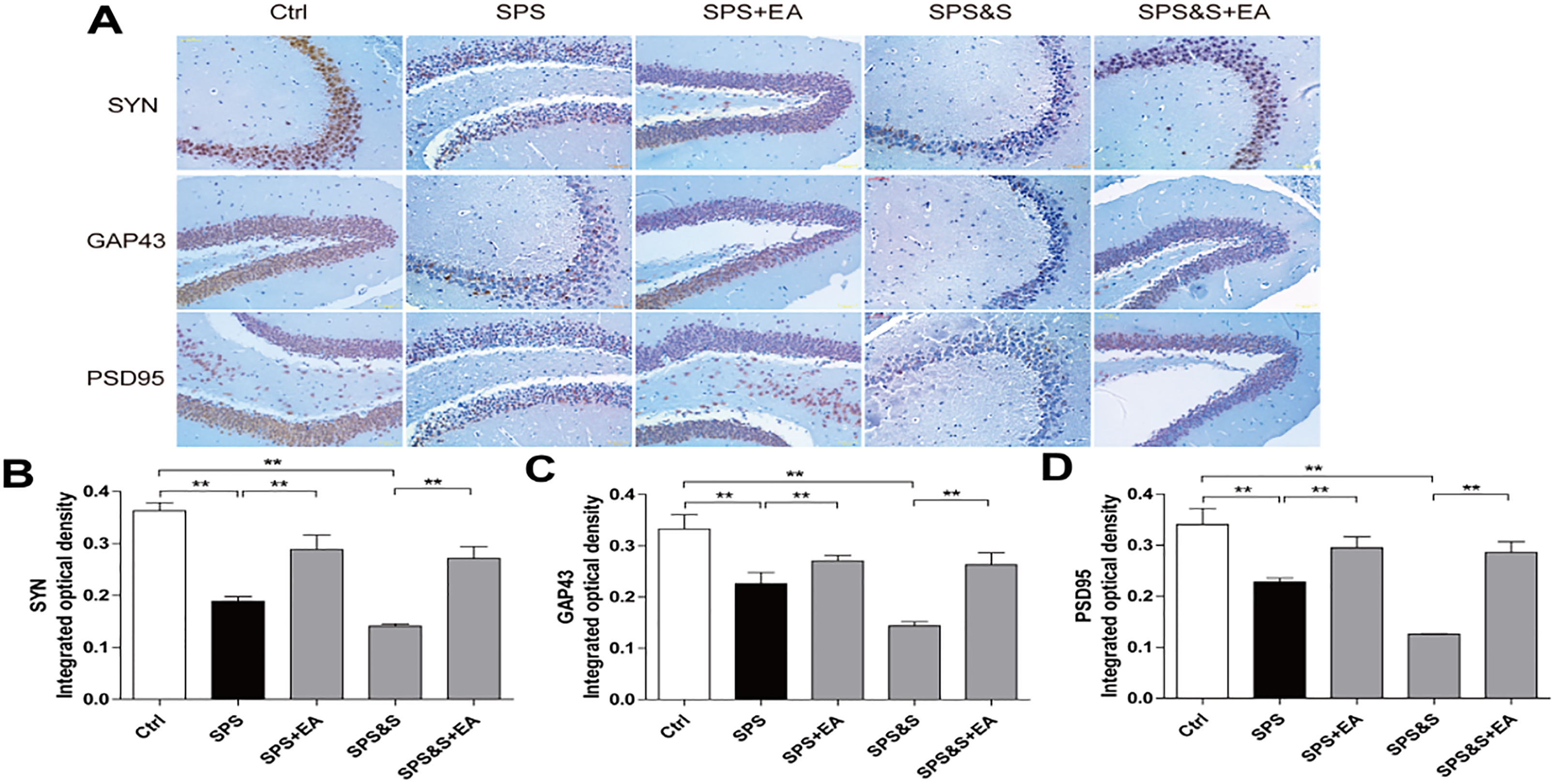
Figure 12.
Effect of electroacupuncture on the expression of SYN, GAP43, PSD95 in hippocampus from rats in the indicated groups. (A-D) Expression of SYN, GAP43, PSD95 were analyzed by Western Blot. The results were presented as mean
2.10Quantitative real-time PCR (RT-PCR)
Total RNA was extracted using TRIzol reagent (Invitrogen, CA, USA). RNA was reverse transcribed to cDNA with the PrimeScript
2.11Statistical analysis
The latency data, spontaneous activity data and fear conditional data in the RAWM experiment were statistically analyzed by two-way ANOVA. The Post hoc test was performed using the Tukey HSD method; The number of stay in target quadrant were performed with a Kruskal-Walls test followed by the Mann-Whitney U-test for multiple comparisons, and the remaining data were statistically analyzed using the Student’s t-test. Apart from number of stay in target quadrant, other data were presented as mean
3.Results
3.1Efficacy evaluation of electroacupuncture to improve PTSD-like behaviors
In the Radial Six-Arm Water Maze Test experiment, analysis of variance showed that there was a significant efficacy on the latency of SPS+EA and SPS groups as well as SPS&S+EA and SPS&S groups (
Figure 13.
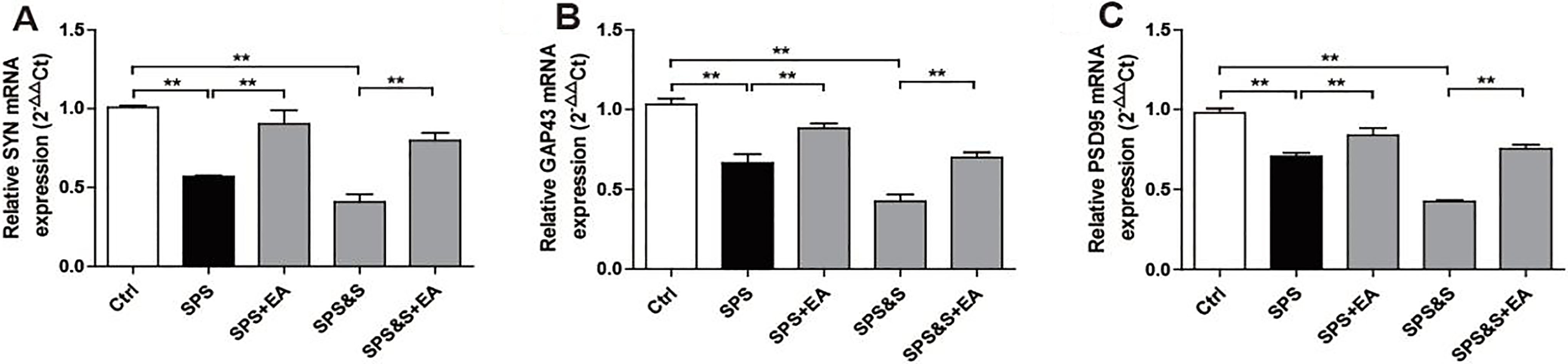
Effect of electroacupuncture on the mRNA expression of SYN, GAP43, PSD95 in hippocampus from rats in the indicated groups. (A-C) mRNA expression of SYN, GAP43, PSD95 were analyzed by Real-time PCR. The results were presented as mean
Locomoto Activity was assessed in an open field arena for all groups. Results indicated no significant difference among groups during the beginning of testing block. In blocks 2–6 of free exploration, the SPS and SPS&S rats moved less and shorter distances than the SPS+EA, SPS&S+EA and Ctrl groups (block 2,
Comparison of responses toward different electric footshocks with different current intensity showed no significant differences regarding current levels that elicited notice reactions, withdrawal reactions (flinch), and/or vocalization (vocalize) among all groups (
Elevated Plus-Maze: Percentage of time spent in the open arms and the number of entry to the open arms were significantly lower in the SPS and SPS&S groups when compared to the SPS+EA, SPS&S+EA and Ctrl groups (
The above findings suggested that electroacupuncture significantly increased locomotor activity and reduced levels of anxiety-like behavior in PTSD rats.
Assessment of conditional fear response: No significant difference in the proportional time of freezing was found among the groups 3 minutes before the acquisition of contextual fear conditioning and training (baseline,
3.2Efficacy evaluation of electroacupuncture for repairing synaptic plasticity in amygdala and hippocampus of PTSD rats
3.2.1Efficacy of electroacupuncture on repairing amygdala, hippocampal synaptic morphology and up-regulating BDNF expression
Compared with the control group, the PSD thickness of the SPS and SPS&S groups was significantly reduced (
3.2.2Efficacy of electroacupuncture on improving LTP in Hippocampus
As shown in Figure, the average value of the basic fEPSP before HFS was taken as the reference value (100%). After the HFS stimulation, the average amplitude of the normal group (Ctrl) fEPSP was maintained (276.35
The results above suggested that electroacupuncture treatment can improve the learning and memory ability of PTSD rats, which may be related to its up-regulation of LTP in hippocampus, maintenance of synaptic morphology (mainly up-regulation of PSD thickness, improvement of synaptic interface curvature) and up-regulation of BDNF levels in hippocampus and amygdala.
3.2.3Molecular effects of electroacupuncture on synaptic plasticity in amygdala and hippocampus of PTSD rats
IHC, WB, RT-PCR were used to observe the effects of electroacupuncture on the transcriptional levels and protein expression levels of synaptophysin (SYN), growth-associated protein 43 (GAP43) and postsynaptic density 95 (PSD95) in amygdala and hippocampus of PTSD rats.
Compared with the Ctrl group, SPS and SPS&S groups down-regulated SYN, GAP43, and PSD95 protein levels and mRNA expression, which were significantly up-regulated by electroacupuncture treatment (SPS+EA and SPS&S+EA groups), and all comparisons were significantly different (
4.Discussion
According to the relevant data of the behavioral test, it can be known that the two modeling methods can obviously induce anxiety, depression and fear-like behaviors and can significantly reduce spatial learning and memory ability in rats. According to the fear-related behavioral data, the percentage of stiffness in the SPS&S group was higher than that in the SPS group at different time points, suggesting that the fear behaviors were more obvious in SPS&S modeled rats. Through this experiment and reviewing previous studies, it is not difficult to find that all model animals could show symptoms similar to PTSD, but each kind of modeling method cannot cover all the symptoms and biological changes, because of the variable and individualized differences in PTSD. However, various animal models can be used not only to study similar symptoms and biological changes of PTSD, but also to combine the results of various models to obtain a more comprehensive research progress on the disease. In the experimental research, different modeling methods should be selected according to the different emphasis of the PTSD symptoms for the experimental design. Therefore, the main problem of current research is how to effectively combine individual findings in animal models and serve patients with PTSD.
According to TCM theories, PTSD is the result of internal and external factors. The external causes are mainly catastrophic stimuli from the outside world, the internal factors are mainly the lack of physical endowment and the disorder of visceral function caused by strong panic. “The brain is the seat of the mentality”, charging consciousness, the central part of the emotional activity is the brain. And the kidney is the foundation of the congenital constitution, which has a regulating effect on the human body’s emotional activities. Essence in the kidney is the material basis for the formation, development and function of the brain and for maintaining the mental activity and behavior of the entire human body. Traditional Chinese Medicine Classics Chinese Medicine Summary
Su Wen
In summary, in response to the decline of fear memory, we should start from tonifying the kidney and waking up the brain. In the clinical research on PTSD, we have confirmed that electroacupuncturing Baihui, Shenting to refresh the mind and nerves, Shenshu to regulate the kidney, significantly promoted the decline of fear memory [11, 34]. As the most commonly used traditional treatment for neurological diseases, electroacupuncture is to give a suitable pulse current while acupoints treatment,it can improve the spatial learning and memory ability of patients with PTSD, and may have protective effects on hippocampal neurons [35]. Our previous study suggested under electroacupuncture treatment, the local consistency of the BOLD signal in the affected brain area was increased [36], the glucose metabolism changed accordingly [37], the clinical symptoms improved significantly as well. Based on previous clinical confirmation, we still choose the above treatment program in this study. In addtion, the treatment program has been proved to be a superior solution, so we did not set up a control group of western medicine or other therapies in this study, as this study focused on the mechanism of acupuncture effectiveness. Certainly, we again confirmed the advantages of electroacupuncture in improving PTSD like symptoms through a series of behavioral tests,and these results are more objective than clinical trials.
The relevant indicators of synaptic plasticity in this experiment suggesed that compared with the ctrol group, the synaptic efficacy of the hippocampus and amygdala brain regions of the PTSD model rats was significantly impaired, and the TMRK electroacupuncture method significantly repaired the damaged synaptic morphology, activated the inhibited LTP and the expression of key proteins, so this experiment initially clarified a relevant neurobiochemical mechanism of fear memory regression by TMRK electroacupuncture, layed the foundation for future translational medical research on prevention of fear-related mental illness.
However, synaptic plasticity is a complex, multi-directional regulation system, including neurotrans-mitter-receptor, neurotrophic, energy metabolism, cell adhesion, protein pathway, etc. This experimental group will further deepen the research in future experiments. At present, this direction has been supported by the National Natural Science Foundation of China and is under study. In addition, the specificity between the amygdala and hippocampal synaptic plasticity and the PTSD fear memory regression needs to be further explored.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (81574086).
Conflict of interest
The authors have no conflict of interest to report.
References
[1] | Luke R, McGuire JJ, Lazarus R, Palmer A. Pavlovian fear memory circuits and phenotype models of PTSD. Neuropharmacology. (2012) ; 62: (2): 638-646. |
[2] | Chen X, Li Y, Li S, Kirouac GJ. Early fear as a predictor of avoidance in a rat model of post-traumatic stress disorder. Behav Brain Res. (2012) ; 226: (1): 112-117. |
[3] | Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. (2002) ; 4: (36): 567-584. |
[4] | Ipser J, Seedat S, Stein DJ. Pharmacotherapy for post-traumatic stress disorder – a systematic review and meta-analysis. S Afr Med J. (2006) ; 96: (10): 1088-1096. |
[5] | Corchs F, Nutt DJ, Hood S, Bernik M. Serotonin and sensitivity to trauma-related exposure in selective serotonin reuptake inhibitors-recovered posttraumatic stress disorder. Biol Psychiatry. (2009) ; 66: (1): 17-24. |
[6] | Fang Q, Li Z, Huang GD, Zhang HH, Chen YY, Zhang LB, Rose ME. Traumatic Stress Produces Distinct Activations of GABAergic and Glutamatergic Neurons in Amygdala. Front Neurosci. (2018) ; 12: : 387. |
[7] | Zhao YK, Han YD, Zhang YF, Zhu TT, Ma CB, Zhao ZT, Rose ME. Acupuncture Intervention Improves Behavior Reactions and Learning-memory Ability in Post-traumatic Stress Disorder Rats. Zhen Ci Yan Jiu. (2018) ; 43: (9): 562-566. |
[8] | Hollifield M, Sinclair-Lian N, Warner TD, Hammerschlag R. Acupuncture for posttraumatic stress disorder: a randomized controlled pilot trial. J Nerv Ment Dis. (2007) ; 195: (6): 504-513. |
[9] | Zhe D, Fang H, Yuxiu S. Expressions of hippocampal mineralocorticoid receptor (MR) and glucocorticoid receptor (GR) in the single-prolonged stress-rats. Acta Histochem Cytochem. (2008) ; 41: (4): 89-95. |
[10] | Wang Y, Hu YP, Wang WC, Pang RZ, Zhang AR. Clinical studies on treatment of earthquake-caused posttraumatic stress disorder using electroacupuncture. Evid Based Complement Alternat Med. (2012) ; 2012: : 1-7. |
[11] | Zhang H, Yuan CF, Ran LH, Yuan Q, Yuan XL, Hu Y. Randomized-controlled clinical trial of different acupuncture therapies in treating posttraumatic stress disorder after 512. Wenchuan Earthquake. China Journal of Traditional Chinese Medicine and Pharmacy. (2010) ; 25: (9): 1505-1510. |
[12] | Zhang LM, Zhang YZ, Li YF. The progress of neurobiological mechanisms on PTSD. Chinese Pharmacological Bulletin. (2010) ; 26: (6): 704-707. |
[13] | Sears RM, Schiff HC, LeDoux JE. Molecular mechanisms of threat learning in the lateral nucleus of the amygdala. Prog Mol Biol Transl Sci. (2014) ; 122: : 263-304. |
[14] | Toyoda H, Li XY, Wu LJ, Zhao MG, Descalzi G, Chen T, Rose ME. Interplay of amygdala and cingulate plasticity in emotional fear. Neural Plast. (2011) ; 2011: : 813749. |
[15] | Leanne M. Williams Andrew Al. Trauma modulates amygdale and medial prefrontal responses to consciously attended fear. Neuro Image. (2006) ; 2: (29): 347-357. |
[16] | Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. (2006) ; 313: (5790): 1093-1097. |
[17] | Honey RC, Good M. Selective hippocampal lesions abolish the contextual specificity of latent inhibition and conditioning. Behav Neurosci. (1993) ; 107: (1): 23-33. |
[18] | Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. (1993) ; 361: (6407): 31-39. |
[19] | Garcia R, Spennato G, Nilsson-Todd L, Moreau JL, Deschaux O. Hippocampal low-frequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiol Learn Mem. (2008) ; 89: (4): 560-566. |
[20] | Feng M, Hou TS, Yu ML, Wu QF, Lu SF, Yu SG. Advances in research on the influence of acupuncture on synaptic plasticity and its mechanism. Lishizhen Medicine and Material Medical Research. (2014) ; 25: (1): 172-174. |
[21] | Lu SF, Shao X, Tang Y, Yin HY, Chen J, Yu SG. Effects of electroacupuncture on neural cell adhesion of synaptic plasticity in the hippocampus of SAMP8. Chinese Journal of Rehabilitation Medicine. (2008) ; 23: (12): 1057-1060. |
[22] | Xu Y, Zhang ZX, Wang XY, Li Y. Effect of electroacupuncture on learning and memory and LTP in hippocampal CA1 in senile rats. Chinese Journal of Gerontology. (2008) ; 28: (16): 1568-1570. |
[23] | Jing XH, Shi H, Cai H, Chen SL, Jin ZG. Effect of Acupuncture on Long-term Potentiation of Hippocampal CA1 Area in Diabetic Rats with Concurrent Cerebral Ischemia. Acupuncture Research. (2008) ; 33: (6): 397-401. |
[24] | Yang ZX, Yu HB, Wang L, Pi M, Zhang JW. Effect of Electroacupuncture on Synaptic Transmission in Hippocampal Dentate Gyrus of Rats with Cerabral Ischemic Damage. Journal of Guangzhou University of Traditional Chinese Medicine. (2005) ; 22: (2): 118-122. |
[25] | Effects of electrical acupuncture on long-term potentiation of excit atory postsynaptic potential in rat hippocampus. Chinese Journal of Clinical Rehabilitation. (2004) ; 8: (13): 2587-2589. |
[26] | Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. (2007) ; 2: (2): 322-328. |
[27] | Solanki N, Alkadhi I, Atrooz F, Patki G, Salim S. Grape powder prevents cognitive, behavioral, and biochemical impairments in a rat model of posttraumatic stress disorder. Nutr Res. (2015) ; 35: (1): 65-75. |
[28] | El-Elimat T, Alzoubi KH, AbuAlSamen MM, Subeh ZY, Graf TN, Oberlies NH. Silymarin Prevents Memory Impairments, Anxiety, and Depressive-Like Symptoms in a Rat Model of Post-Traumatic Stress Disorder. Planta Med. (2018) . |
[29] | Komada M, Takao K, Miyakawa T. Elevated plus maze for mice. J Vis Exp. (2008) ; 22: (1088): e1088. |
[30] | Souza RR, Robertson NM, Pruitt DT, Noble L, Meyers EC, Gonzales PA, Rose ME. The M-Maze task: An automated method for studying fear memory in rats exposed to protracted aversive conditioning. J Neurosci Methods. (2018) ; 298: : 54-65. |
[31] | Anagnostaras SG, Wood SC, Shuman T, Cai DJ, Leduc AD, Zurn KR, Rose ME. Automated assessment of pavlovian conditioned freezing and shock reactivity in mice using the video freeze system. Front Behav Neurosci. (2010) ; 4. |
[32] | Csabai D, Wiborg O, Czeh B. Reduced Synapse and Axon Numbers in the Prefrontal Cortex of Rats Subjected to a Chronic Stress Model for Depression. Front Cell Neurosci. (2018) ; 12: : 24. |
[33] | Ye W, Wu L, Yan C. Effect of Kidney Essence in Tranquilizing the Mind: Based on Formation and Extinction of Fear Memory. J Tradit Chin Med. (2017) ; 58: (13): 1117-1120. |
[34] | Zheng CQ, Tan LX, Zhou TX, Zhang H. Effects of electroacupuncture on resting-state encephalic functional connectivity network in patients with PTSD. Chinese Acupunture & Moxibustion. (2015) ; 35: (5): 469-473. |
[35] | Hou LQ, Liu W, Xiong KR. Effects of Electroacupuncture on Expression of Neuronal Nitric Oxide Synthase in Hippocampus of Rats with Post-Traumatic Stress Disorder. Chinese Acupunture & Moxibustion. (2013) ; 33: (7): 632-636. |
[36] | Yang S, Li B, Yuan Q, Wang YZ, Zhang H. Effect of electroacupuncture on the local consistency of resting brain function in patients with post-traumatic stress disorder. Chinese Journal of TCM. (2013) ; 28: (4): 1102-1106. |
[37] | Zhang H, Chen WW, Song WZ, Zhu MJ, Feng Y. Effect of electroacupuncture on brain glucose metabolism in patients with post-traumatic stress disorder. Chinese Journal of TCM. (2010) ; 25: (11): 1882-1884. |




