The effects of dexamethasone on 17β-HSD1 levels at the rat optic nerve
Abstract
Dexamethasone (DEX) is associated with many inflammation and metabolic diseases. We analyzed the effects of DEX on the expression of estrogen metabolism enzyme 17
1.Introduction
Dexamethasone is a kind of glucocorticoid, it has anti-inflammatory, and anti-endotoxin effects, suppress the immune response and the resistance to shock and enhance the stress response among other pharmacological effects, so it is widely used for the treatment of many diseases [1, 2]. DEX may cause hypokalemia syndrome, peptic ulcers, pancreatitis, children’s growth is restrained, and worsen glaucoma, cataract and diabetes [3]. As a steroid hormone, it is degraded by many enzymes such as 3
The relationship between hormones and eye diseases has recently gained increased attention. The corneal thickness changes along the female menstrual cycle. It is thicker at the time of ovulation to be gradually thinner up to the menstrual period [12, 13]. The incidence of cataracts is higher in woman than in men, especially in postmenopausal women, because of the lack of estrogens that is a well-known risk factor [14]. Hormones also play important roles in neural regulation and protection, avoiding neural degeneration [15, 16]. It has also been found that estradiol can enhance the viability of primary nerve cell cultures. The activation of estrogen receptors result in nerve protective effects, improve the interaction between antioxidants and intracellular signal transduction pathways, inhibit apoptosis, enhance synaptic sprouting and axonal regeneration, and improve the function of cholinergic nerves [17, 18].
In the early 90s, the steroid hormonal, pregnenolone, dehydroepiandrosterone and progesterone were found in the human retina [19]. In 2008, hormonal receptors were located at the rat retina by immunohistochemistry [20]. Zhang designed a study to explore the ovarectomy and sham-ovarectomy for studying the effect of estrogen on ocular diseases and its role in retinal neovascularization. He found decrease of estrogen level in blood contributes to retinal neovascularization, but too much damage to the optic nerve and cause SD rats visual deterioration [21]. Garg et al. measured the serous steroid and 24 h urinary steroid content in 30 (central serous chorioretinopathy, CSC) patients and the control group, and found that the steroid content in the patients group was significantly higher than control group, and the female patients were significantly higher than the male patients. Steroid hormones are involved in regulating choroidal microcirculation, causing retinal pigment epithelium damage and local ion flow changes in high dosage. The local metabolism of hormones plays an important role in gene regulation, nerve regulation and immune regulation [22]. Several studies demonstrated that hormones and the related enzymes are closely involved with eye diseases [23]. We have studied the expression of 17
2.Materials and methods
2.1Materials
Sprague Dawley rats (SD rats) were purchased from the laboratory animal center of Jilin University (Changchun, China), Dexamethasone sodium phosphate 5 mg/ml (Zhuo Feng Pharmaceutical Factory of Zhengzhou, China), urethane (Heng Yuan biotech company of Shanghai, China) 15%. Paraformaldehyde solution (Sen Bei Jia Pharmaceutical Factory of Nanjing, China) 4%. Phosphate Buffer solution (PBS, 0.01 mol/L, HP 7.4), Tris-TBS concentration is 0.05 mol/L, Western Blot Buffer (Glycine 2.9 g. Tris 5.8 g, SDS 0.37 g, Methanol, 200 ml, H2O, 1000 ml), TBS Buffer (1 mol/L Tris
The rat holder, retort stand about 1 meter height, A pool, Y maze (the arms is 450 mm length, 145 mm breadth, 125 mm height), SYU Gel Imaging System (BIO-RAD,USA), FV1000 Laser Scanning Confocal Microscope (Olympus, Japan), CM 850 freezing microtome (Leica, Germany), OCT. Compound (Leica, Germany), UV spectrophotometer (Pu Yuan Metrologic Instruments of Shanghai, China) beaker, glass rod, test tube, graduated pipette, measuring cylinder, Constant temperature and humidity incubator, glass slide.
2.2Dexamethasone treatment assay
The experimental animals were 6–8 weeks of age, 400–600 g weight, they are healthy, lively, and curious about everything. Rats fed for three days at the laboratory to adapt the animals to the environment. Then, DEX was intraperitoneally injected daily for 7 days. There were 5 groups (2 rats per group). The DEX was given at 5 different concentrations (2 mg/kg, 1.5 mg/kg, 1 mg/kg, 0.5 mg/kg, 0.25 mg/kg).
After 7 days, the health status of the rats was observed. Y maze, tail suspension test and forced swimming among DEX and control groups were recorded.
2.3Extraction and measure of total protein
Eyes from ethyl urethane anesthetized rats were obtained and the neural tissues were carefully dissected. Tissues were homogenized in 250
The protein concentration of the samples was determined following the coomassie brilliant blue method in J. Steroid. Biochem. Mol. Biol [24], The OD is determined at 595 wavelength absorption values.
2.4Western Blot of 17β
First the optic nerves just extracted from control rats, the retina detachment lysis buffer in homogenizer. centrifugation, collected supernatant liquor, supernatant was used for Western Blot. The sample protein concentration is 1.94 mg/ml. The Western Blot was identified by the method of E. Maser in J. Steroid. Biochem. Mol. Biol [24]. Added rabbit anti-HSD17B1 (200
2.5Immunohistochemistry of 17β
Retina tissues were got from DEX rats and controls. There are four samples from each group, right eye or left eye. The optic nerves put into the paraformaldehyde solution 30 min, 4
2.6Elisa of 17β
ELISA was used to measure the rat optic nerve of DEX-treated and control group. The control group was given sterile water, while the experiment group was given 17
3.Results
3.1Dexamethasone treatment results
The SD rats injected intraperioneally with EDX in 2 mg/kg, 1.5 mg/kg, 1 mg/kg are death, 0.5 mg/kg and 0.25 mg/kg groups are alive so the limiting concentration chose 0.75 mg/kg for the experimental group. Control group was also measured by giving the same amount of physiological saline.
Table 1
The correct times rats found food in Y maze assay
| A1 | A2 | A3 | A4 | A5 | C1 | C2 | C3 | C4 | C5 | |
| 1 day | 1 | 2 | 1 | 1 | 0 | 2 | 2 | 2 | 2 | 3 |
| 2 day | 0 | 1 | 3 | 2 | 1 | 1 | 3 | 1 | 2 | 3 |
| 3 day | 0 | 3 | 4 | 1 | 0 | 3 | 4 | 1 | 3 | 2 |
| 4 day | 1 | 3 | 5 | 3 | 2 | 0 | 3 | 3 | 4 | 3 |
| 5 day | 0 | 4 | 4 | 2 | 0 | 1 | 4 | 2 | 3 | 4 |
| 6 day | 2 | 3 | 2 | 3 | 1 | 2 | 5 | 2 | 3 | 4 |
| 7 day | 2 | 4 | 3 | 2 | 1 | 2 | 6 | 2 | 4 | 5 |
| Average | 0.86 | 2.57 | 2.85 | 2.0 | 0.71 | 1.57 | 3.86 | 1.86 | 3.0 | 3.38 |
The table show correct times rats found food in Y maze assay. A1, A2, A3, A4, A5 group rats which treated with DEX, the C1, C2, C3, C4, C5 was the control groups. It is obviously in 7 days training healthy rats give more times in founding food average 2.8, but the experimental group just 2 in one minute.
Figure 1.
The picture was drawn by the average data in 7 days, each data measured 3 times, A1, A2, A3, A4, A5 group rats which treated with DEX, the C1, C2, C3, C4, C5 was the control groups. Mean is 171.6
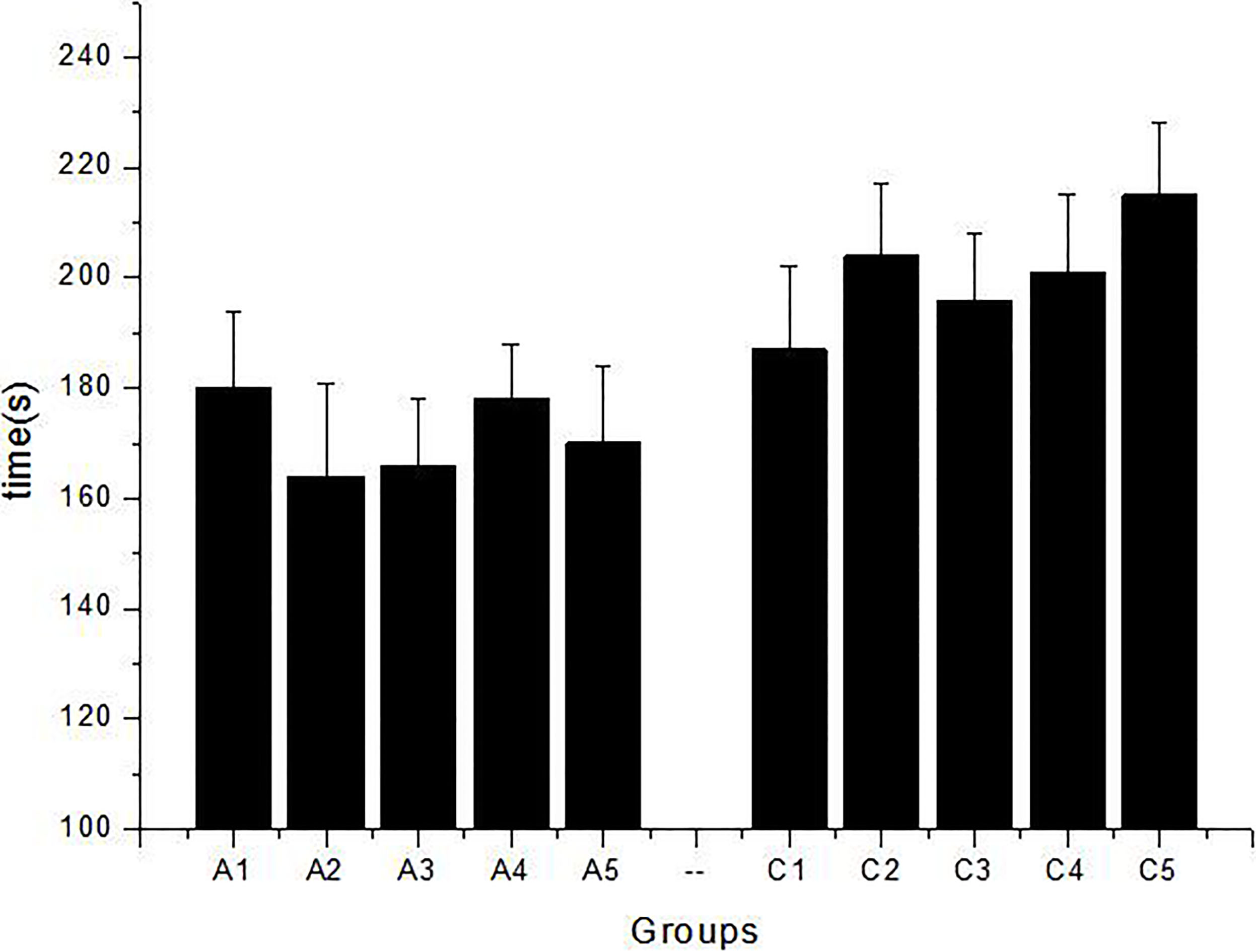
Seven day’s data were taken, the average value were calculated and given in Table 1, A1, A2, A3, A4, A5 are the rats injected with DEX, indicating the food average is 2 s/min, but C1, C2, C3, C4, C5 of the control group indicate the food average is 2.8 s/min in Y maze. Tail suspension test shows the average time to give up struggling is 172 s in, but the blank is 200.6 s in average, which is decreased by 14.3%. So, the DEX has negative influence for rats in emotion or exercise capacity (Fig. 1). Forced swimming test observation records the time of rats got in desperate state in the water. Struggling time reduces to 19.25% for DEX group, but it is increased to 33.68% for control group (Fig. 2).
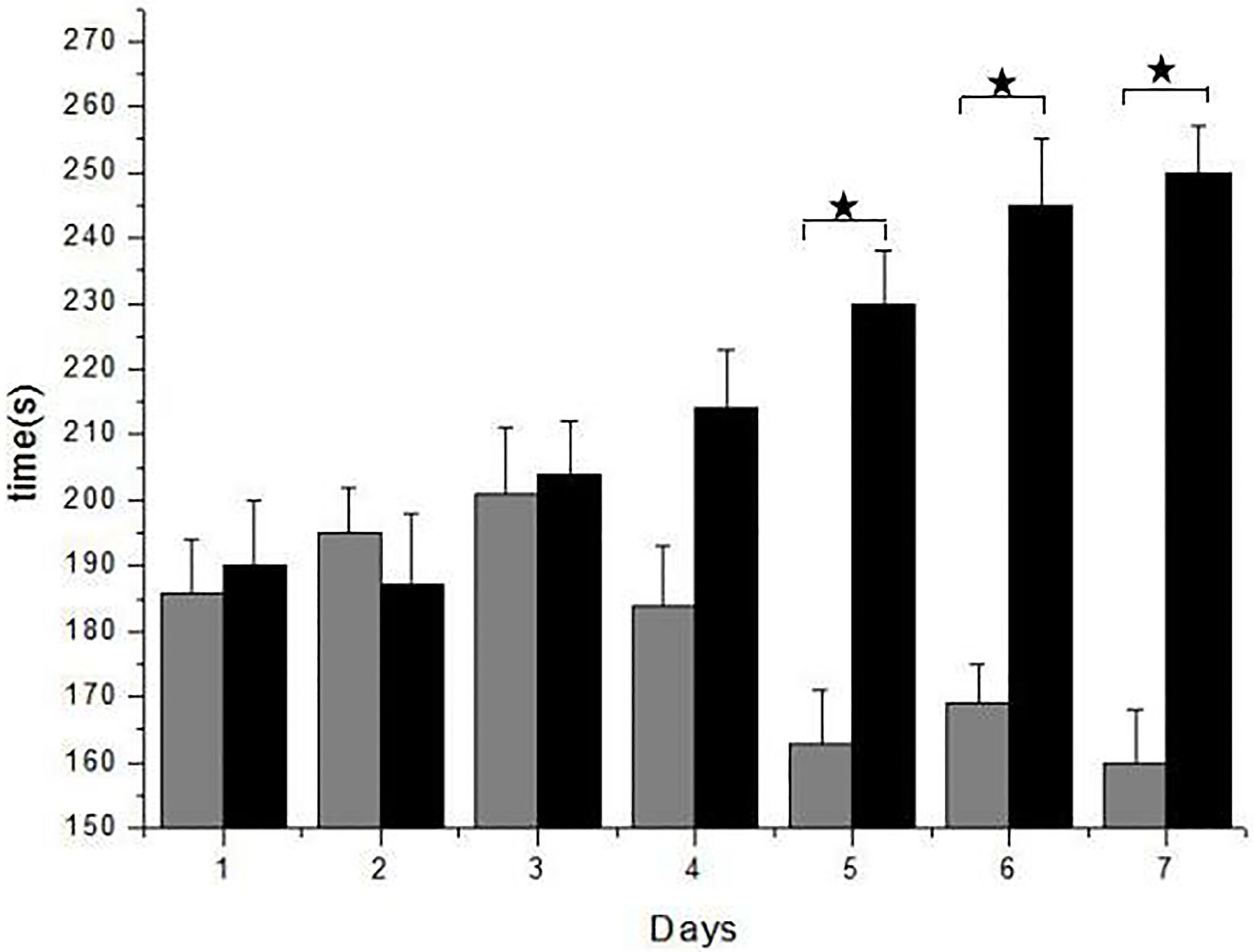
Figure 2.
Forced Swimming Test (FST) shows rats with the DEX exercise capacity and the faculty of memory. It is obvious the test results are similar in 3 days, but after the fourth day, the score improved. In the control group rats adapting the swimming, the physical strength and memory became much worse than control rats. Especially the fifth, sixth, seventh day, experimental group took more time, it is 67
3.2Extract and measure total protein
Standard curve of protein was drawn by coomassie brilliant blue method, based on the gradient dilution technique of BSA solution. The linear regression equation was
The sample was measured by three times, the absorption values were got at a wavelength of 595 nm, and then calculated according to the standard curve. The average concentration of total protein was 1.94 mg/ml.
3.3Western Blot of 17β
To explore the 17
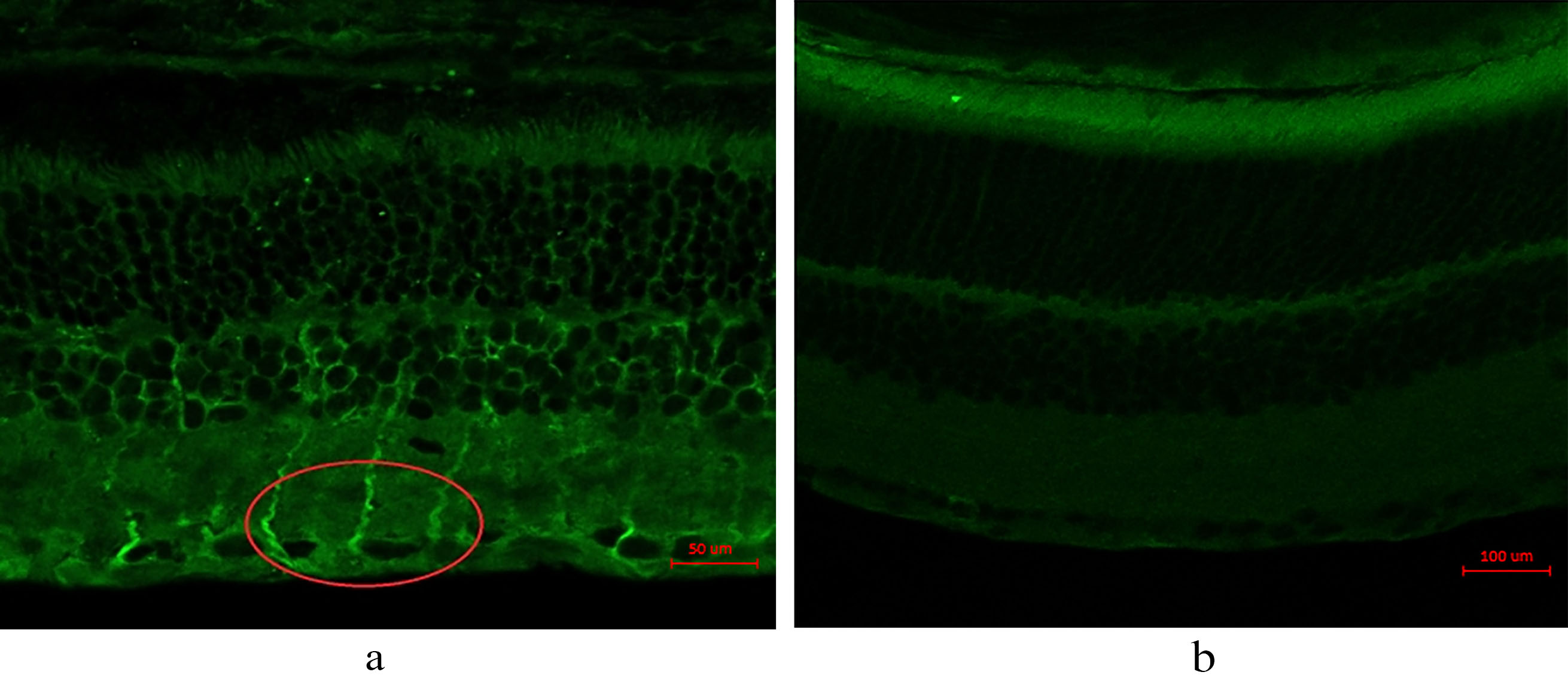
3.4Immunohistochemistry of 17β
The result of Immunohistochemistry (IHC) test 17
Table 2
Elisa assay OD595 of 17
| Sample | 17 | 1:50 | 1:100 | 1:200 | 1:400 | 1:600 | 1:800 | 1:1000 | 1:1200 | Control |
|---|---|---|---|---|---|---|---|---|---|---|
| DEX | 1.74 | 1.51 | 1.37 | 1.11 | 0.51 | 0.2 | 0.12 | 0.09 | 0.08 | 0.18 |
| 0.07 | 0.08 | 0.09 | 0.07 | 0.12 | 0.08 | 0.05 | 0.02 | 0.03 | 0.03 | |
| Control | 0.73 | 0.57 | 0.44 | 0.31 | 0.23 | 0.17 | 0.12 | 0.1 | 0.09 | 0.02 |
| 0.08 | 0.07 | 0.1 | 0.06 | 0.07 | 0.04 | 0.05 | 0.03 | 0.04 | 0.02 |
Figure 3.
Western Blot of 17

Figure 4.
IHC shows 17
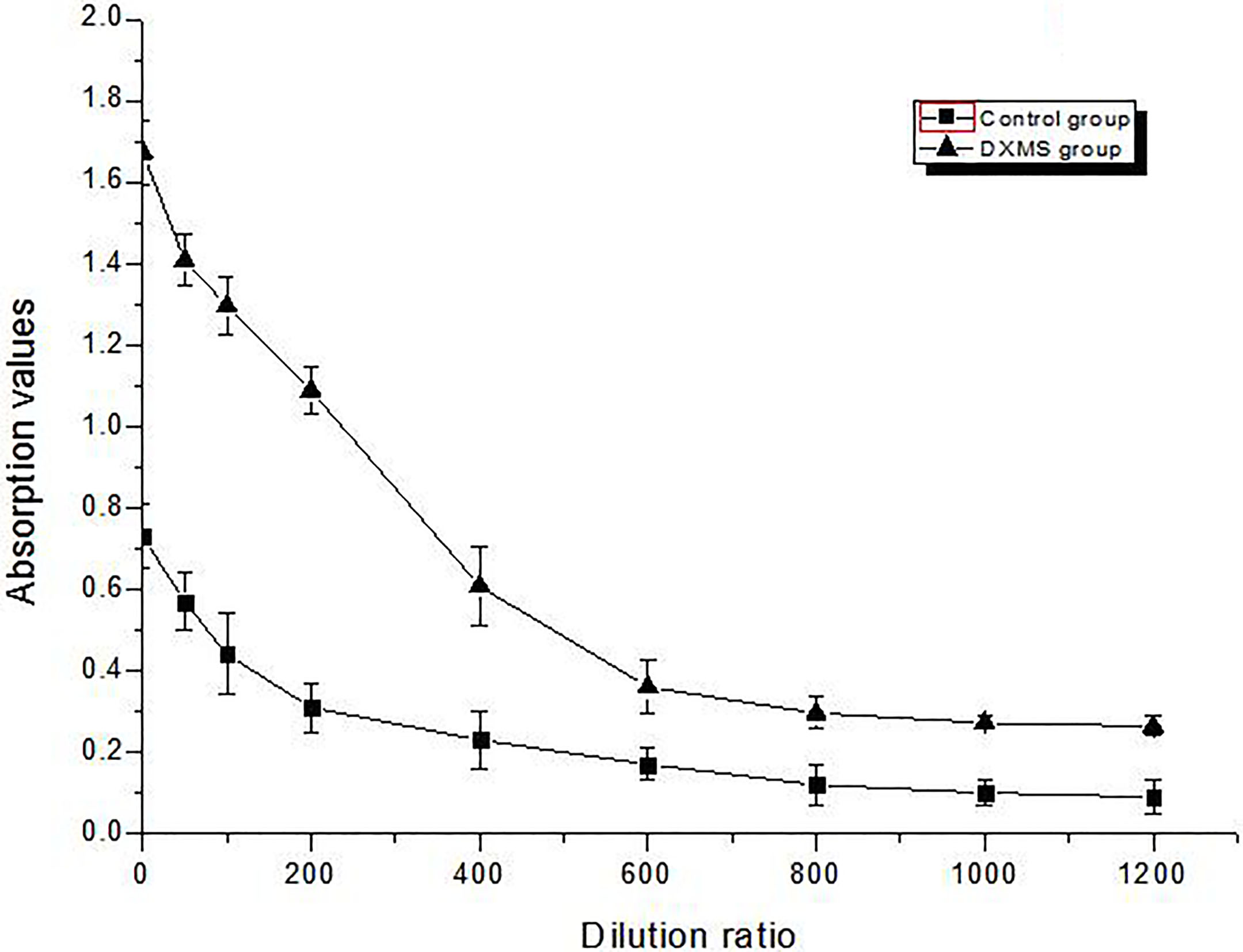
3.5Elisa of 17β
The results show the 17
Figure 5.
Elisa assays the content of 17
4.Discussion
Bisphenol A, pesticide and DEX are synthetic estrogen; they can combine with hormone receptor or mimic hormones signaling pathways. They act like endogenous estrogen, because their structure are highly similar to endogenous hormones [26], leading to excessive accumulation of endogenous hormone, and making the body endocrine disorder, may even suffer from some diseases [27]. The endogenous hormone also plays an important role in our eyes, and involve in many biochemical reactions, so the abnormal level of hormone often causes eye diseases [28]. DEX is belong to steroid hormone and widely used in our life such as anti-inflammatory drugs, rheumatic drugs, allergies drugs, asthma drugs, chronic obstructive pulmonary drugs. So it has important significance to study the effects of excess DEX on our retina.
This paper has studied about the relationship between DEX and retina. DEX has negative effect on exercise, metabolism, memory of rat (Table 1, Figs 1 and 2), and this damage could not recover. Western Blot results show that 17
In short, we have studied the effects of hormone on optic nerve, the regulation relationship of enzymes involved in hormone, enzyme activity and optic nerve physiology, which is worth exploring.
Acknowledgments
This scientific research project was supported by the Education Development Plan of Jilin Province of the People’s Republic of China (JJKH20190603KJ).
Conflict of interest
None to report.
References
[1] | Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. (2010) ; 115: : 168–186. DOI: 10.1182/blood-2009-06-225565. |
[2] | Mohan K, Kecova H, Hernandez-Merino E, Kardon RH, Harper MM. Retinal ganglion cell damage in an experimental rodent model of blast-mediated traumatic brain injury. Investigative Opthalmology & Visual Science. (2013) ; 54: (5): 3440–3450. DOI: 10.1167/iovs.12-11522. |
[3] | Gracitelli CP, Duque-Chica GL, Moura AL, Nagy BV, Paranhos A Jr, et al. A positive association between intrinsically photosensitive retinal ganglion cells and retinal nerve fiber layer thinning in glaucoma. Investigative Ophthalmology & Visual Science. (2014) ; 55: (12): 7997–8005. DOI: 10.1167/iovs.14-15146. |
[4] | Cascio C, Deidda I, Russo D, Guarneri P. The estrogenic retina: The potential contribution to healthy aging and age-related neurodegenerative diseases of the retina. Steroids. (2015) ; 103: : 31–41. DOI: 10.1016/j.steroids.2015.08.002. |
[5] | Prokai-Tatrai K, et al. 17β-estradiol eye drops protect the retinal ganglion cell layer and preserve visual function in an in vivo model of glaucoma. Molecular Pharmaceutics. (2013) ; 10: (8): 3253–3261. DOI: 10.1021/mp400313u. |
[6] | Kador KE, Montero RB, Venugopalan P, Hertz J, Zindell AN, Valenzuela DA, Uddin MS, Lavik EB, et al. Tissue engineering the retinal ganglion cell nerve fiber layer. Biomaterials. (2013) ; 17: : 4242–4250. DOI: 10.1016/j.biomaterials.2013.02.027. |
[7] | Cassetta A, Krastanova I, Kristan K, et al. Insights into subtle conformational differences in the substrate-binding loop of fungal 17β-hydroxysteroid dehydrogenase: a combined structural and kinetic approach. Biochemical Journal. (2012) ; 441: (1): 151–160. DOI: 10.1042/BJ20110567. |
[8] | Marchais-Oberwinkler S, Henn C, Möller G, Klein T, Negri M, Oster A, Spadaro A, Werth R, et al. 17β-hydroxysteroid dehydrogenases (17β-HSDs) as therapeutic targets: protein structures, functions, and recent progress in inhibitor development. Steroid. Biochem. Mol. Biol. (2011) ; 125: : 66–82. DOI: 10.1016/j.jsbmb.2010.12.013. |
[9] | Svegelj MB, Stojan J, Rizner TL. The role of Ala231 and Trp227 in the substrate specificities of fungal 17β-hydroxysteroid dehydrogenase and trihydroxynaphthalene reductase: steroids versus smaller substrates. Journal of Steroid Biochemistry & Molecular Biology. (2012) ; 129: (1-2): 92–98. DOI: 10.1016/j.jsbmb.2011.03.019. |
[10] | Lajic S, Nordenström A, Hirvikoski T. Long-term outcome of prenatal dexamethasone treatment of 21-hydroxylase deficiency. Endocrine Development. (2011) ; 20: : 96–105. DOI: 10.1159/000321228. |
[11] | Kawa L, Barde S, Arborelius UP, Theodorsson E, Agoston D, Risling M, Hökfelt T. Expression of galanin and its receptors are perturbed in a rodent model of mild, blast-induced traumatic brain injury. Experimental Neurology. (2016) ; 279: : 159–167. DOI: 10.1016/j.expneurol.2016.02.019. |
[12] | Adamski J, Jakob FJ. A guide to 17β-hydroxysteroid dehydrogenases. Mol. Cell. Endocrinol. (2001) ; 171: (1-2): 1–4. DOI: 10.1016/S0303-7207(00)00383-X. |
[13] | Li M, Xiong G, Maser E. A novel transcriptional repressor PhaR for the steroid-inducible expression of the 3, 17β-hydroxysteroid dehydrogenase gene in Comamonas testosteroni ATCC11996. Chem-biol. Interact. (2013) ; 202: (1-3): 116–125. DOI: 10.1016/j.cbi.2012.12.014. |
[14] | Morrison JC, Moore CG, Deppmeier LMH, et al. A rat model of chronic pressure-induced optic nerve damage. Experimental Eye Research. (1997) ; 64: (1): 85–96. DOI: 10.1006/exer.1996.0184. |
[15] | Ma W, Cojocaru R, Gotoh N, et al. Gene expression changes in aging retinal microglia: relationship to microglial support functions and regulation of activation. Neurobiology of Aging. (2013) ; 34: (10): 2310–2321. DOI: 10.1016/j.neurobiolaging.2013.03.022. |
[16] | Ma W, Wong WT. Aging changes in retinal microglia and their relevance to age-related retinal disease. Advances in Experimental Medicine and Biology. (2016) ; 854: : 73–78. DOI: 10.1007/978-3-319-17121-0_11. |
[17] | Xiong G, Maser E. Regulation of the steroid-inducible 3α-hydroxysteroid dehydrogenase/carbonyl reductase gene in comamonas testosteroni. J. Biol. Chem. (2001) ; 276: (13): 9961–9970. DOI: 10.1074/jbc.M010962200. |
[18] | Meier M, Möller G, Adamski J. Perspectives in understanding the role of human 17β-hydroxysteroid dehydrogenases in health and disease. Annals of the New York Academy of Sciences. (2009) ; 1155: (1): 15–24. DOI: 10.1111/j.1749-6632.2009.03702.x. |
[19] | Jaliffa CO, Howard S, Hoijman E, et al. Effect of neurosteroids on the retinal gabaergic system and electroretinographic activity in the golden hamster. Journal of Neurochemistry. (2005) ; 94: (6): 1666–1675. DOI: 10.1111/j.1471-4159.2005.03321.x. |
[20] | Kumar DM, Simpkins JW, Agarwal N. Estrogens and neuroprotection in retinal diseases. Molecular Vision. (2008) ; 14: (14): 1480–1486. |
[21] | Dhindsa S, Furlanetto R, Vora M, et al. Low estradiol concentrations in men with subnormal testosterone concentrations and type 2 diabetes. Diabetes Care. (2011) ; 34: (8): 1854–1859. DOI: 10.2337/dc11-0208. |
[22] | Jiang M, Luo X, Shen Y, et al. Neuroprotection of estrogen receptor GPR30 in retinal ganglion cell. Medical Journal of Wuhan University. (2016) ; 37: (2): 195–199. DOI: 10.14188/j.1671-8852.2016.02.006. |
[23] | Sposato V, Parisi V, Manni L, et al. Glaucoma alters the expression of NGF and NGF receptors in visual cortex and geniculate nucleus of rats: Effect of eye NGF application. Vision Research. (2009) ; 49: (1): 54–63. DOI: 10.1016/j.visres.2008.09.024. |
[24] | Gong W, Xiong G, Maser E. Cloning, expression and characterization of a novel short-chain dehydrogenase/reductase (SDRx) in Comamonas testosteroni. Journal of Steroid Biochemistry and Molecular Biology. (2012) ; 129: (1-2): 15–21. DOI: 10.1016/j.jsbmb.2010.11.008. |
[25] | Saiegh L, Keren D, Rainis T, Sheikh-Ahmad M, et al. Dexamethasone suppressed corticotropin-releasing hormone stimulation test in morbid obese adults. Obesity Research & Clinical Practice. (2016) ; 10: (3): 275–282. DOI: 10.1016/j.orcp.2015.07.004. |
[26] | Fudvoye J, Bourguignon JP, Parent AS. Chapter one-endocrine-disrupting chemicals and human growth and maturation: a focus on early critical windows of exposure. Vitamins & Hormones-advances in Research & Applications. (2014) ; 94: (1): 1–25. DOI: 10.1016/B978-0-12-800095-3.00001-8. |
[27] | Warris LT, van den Akker ELT, Aarsen FK, et al. Predicting the neurobehavioral side effects of dexamethasone in pediatric acute lymphoblastic leukemia. Psychoneuroendocrinology. (2016) ; 72: : 190–195. DOI: 10.1016/j.psyneuen.2016.07.006. |
[28] | Cacciaglia R, Nees F, Grimm O, et al. Trauma exposure relates to heightened stress, altered amygdala morphology and deficient extinction learning: Implications for psychopathology. Psychoneuroendocrinology. (2017) ; 76: : 19–28. DOI: 10.1016/j.psyneuen.2016.11.012. |
[29] | Opere CA, Heruye S, Njiembye YF, et al. Regulation of excitatory amino acid transmission in the retina: Studies on neuroprotection. J Ocul Pharmacol Ther. (2018) ; 34: (1-2): 245–256. DOI: 10.1093/ijnp/pyx096. |
[30] | Talalay P, Dobson MM. Purification and properties of a beta-hydroxysteroid dehydrogenase. Biological Chemistry. (1953) ; 205: (2): 823–837. DOI: 10.1016/S0074-7696(08)61042-6. |
[31] | Labrie F, Luu-The V, Lin SX, Labrie C, Simard J, Breton R, Bélanger A. The key role of 17 beta-hydroxysteroid dehydrogenases in sex steroid biology. Steroids. (1997) ; 62: (1): 148–158. |




