Effect of ventricular myocardium characteristics on the defibrillation threshold
Abstract
Myocardium characteristics differ markedly among individuals and play an important role in defibrillation threshold. The accuracy of simulation models used in most published studies are still have room to be improved and most of them only discussed the effect of myocardial anisotropy on defibrillation threshold. In our manuscript, a rabbit ventricular finite-element (FE) volume conductor model with high precision was constructed. Ventricular myocardium characteristics include cardiomyocyte coupling and the degree of myocardial anisotropy, which are represented as the value and the ratio of anisotropic conductivity, respectively. Quantitative analysis was performed simultaneously in terms of cardiomyocyte coupling and the degree of myocardial anisotropy. Based on this, the combined effects of these two factors were further discussed. The electric field distributions of shocks and the defibrillation thresholds under different myocardial characteristics were simulated on this model. The simulation results revealed that as the degree of myocardial anisotropy increases, defibrillation threshold increases, and cardiomyocyte decoupling (decrease in electrical conductivity) can considerably increase the defibrillation threshold.
1.Background and objective
In China, more than 500,000 people die each year from sudden cardiac death (SCD) [1]; worldwide, this figure has reached an alarming 7 million [2]. One of the important causes of SCD is ventricular fibrillation, and the only effective clinical way to terminate it is by shock defibrillation. Myocardial characteristics play an important role in shock defibrillation effect, which differs markedly under different pathological or physiological conditions of different individuals. For instance, cardiomyocyte coupling will decrease with age; myocardial ischemia can lead to cardiomyocyte decoupling at the ischemic area; the degree of myocardial anisotropy increases for the patients with cardiac hypertrophy. Therefore, it is instructive to understand the effect of ventricular myocardium characteristics on the defibrillation threshold (DFT). A wrong assessment of DFT will result in a failed shock defibrillation [3]. Therefore, studying the effect of myocardium characteristics on defibrillation has not only important theoretical significance, but also important clinical value.
The distribution of electric field in the heart is closely related to the defibrillation results [4]. It is almost impossible to measure the distribution of electric field in the myocardium in vivo, so finite-element (FE) modeling has been widely used in such studies. During the previous three decades, various three-dimensional cardiac models have been established. Among them, volume conductor models can best reflect the electric field distribution in ventricles. Some examples include the human thoracic model constructed by Camacho et al. [5], the dog chest model developed by Karlon et al. [6], and the human thoracic model built by Kinst et al. [7]. Besides electric field, these models used current density as an indicator of defibrillation effects, which aimed to finding the relationship between current density and DFT. In 1975, the embryonic form of the critical mass hypothesis was proposed [8] and in the mid-nineties, this method for calculating the DFT has been generally accepted [9]. Using this hypothesis, many models were established to study the effect of defibrillation field on DFT, such as the porcine thoracic model constructed by Wang et al. [10] and the dog heart model built by Yang et al. [11]. With the understanding of the influence of myocardial anisotropy on the distribution of defibrillation electric field [12, 13], several cardiac volume conductor models have reported factors influencing the myocardial fibers. Such as the human torso models constructed by Eason et al. [13], Aguel et al. [14], Wang et al. [15] and Modre et al. [16], and the swine chest models constructed by Panescu et al. [17].
The aforementioned studies were committed to optimizing the electrode configuration [5, 6, 7, 10, 11] or researching the effects of myocardial anisotropy on DFT based on one group of anisotropic conductivities, which did not further discuss why fiber anisotropy could have this kind of effect on DFT [13, 14, 15, 16, 17].
To investigate the effect of myocardial characteristics on DFT, this manuscript used a rabbit ventricle as the anatomical model, which has higher resolution than aforementioned models [5, 6, 7, 10, 11, 14, 15, 16, 17, 18]. Rabbit heart has much smaller size compared to human heart, so using this model can evidently reduce the computational complexity, significantly shortening the simulation time. In addition, the structure and electrophysiological characteristics of the human and rabbit heart have a high degree of similarity, making this model reliable [19].
In this manuscript, the DFTs in different ventricular myocardium characteristics were given in the RESULTS section. The effects of cardiomyocyte coupling, the degree of myocardial anisotropy and the combined effects of these two factors on DFT were discussed in DISCUSSION section. Model development, model parameter selection and simulation methods were discussed in METHODS section. This study provided a basis for the modeling and parameter selection of myocardial conductivity in different conditions, and thus provides some guidance for defibrillation treatment.
2.Methods
In this manuscript, a rabbit ventricular FE model with a resolution of approximately 250
To simplify the problem, all simulations in this manuscript used the implantable cardioverter defibrillator (ICD) electrode configuration: the anode (a thin cylindrical electrode with a height of 16 mm and a radius of 0.5 mm) was inserted into the right ventricular cavity and the cathode (a circular patch electrode with 4 mm radius) was placed on the upper part of the left ventricular epicardium (Fig. 1). A defibrillation threshold voltage was applied between the cylindrical electrode (anode) and the circular patch electrode (cathode) such that the critical mass hypothesis was met. In this study, the DFTs for isotropic, anisotropic, and different anisotropic conductivities were simulated.
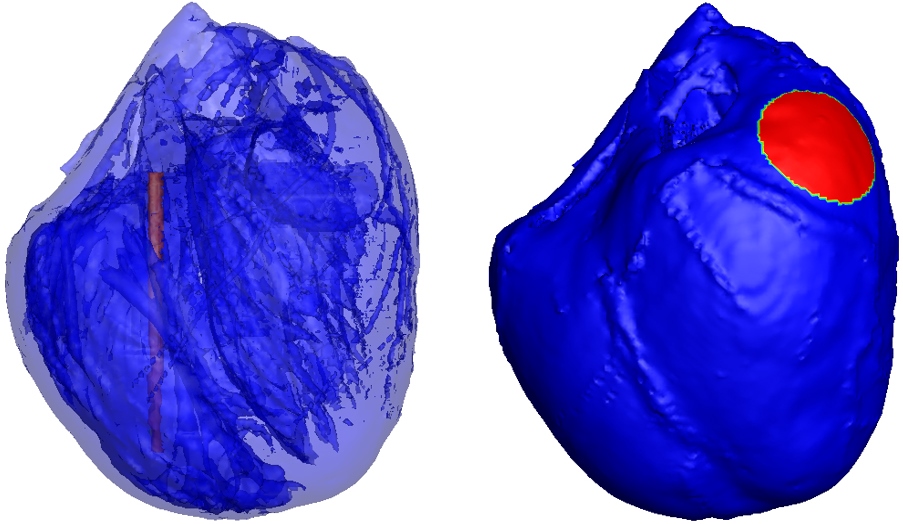
Figure 1.
ICD electrode configuration was used in the simulation. The red part of in the right ventricle as shown in left picture is the columnar electrode, the red part of on left ventricular epicardium as shown in right picture is the circular patch electrodes.
The electric shock defibrillation problem is equivalent to the constant electric field problem in the volume conductor model. The potential in the volume conductor satisfies the Laplace equation:
(1)
where
(2)
(3)
Equation (2) represents the Dirichlet boundary condition, which defines the myocardial surface (
The fiber conductivity tensor
(4)
where the coefficient
The FE simulation software, Comsol Multiphysics 5.2 (COMSOL Inc.), was used. For an isotropic cardiac volume model, the electrical conductivity of myocardial fibers was set to
Table 1
Electrical conductivity of myocardial fibers given by different literatures
| Cited | The model used | Anisotropic | Myocardial anisotropic |
| Blood conductivity | DFT | POD |
|---|---|---|---|---|---|---|---|
| paper | ratio | conductivity (S/m) | (S/m) | (V) | (%) | ||
| [5] | Chest model of human | 1:1 | 0.25 | 0.8 | 113 | 0 | |
| [15] | Chest model of swine | 2:1 | 0.2526 | 0.649 | 122 | 7.96 | |
| [16] | Ventricular model of human | 4:1 | 0.375 | 0.6 | 116 | 2.65 | |
| [14] | Torso model of human | 3.74:1 | 0.396 | 0.775 | 113 | 0 | |
| [13] | Torso model of human | 2.648:1 | 0.4305 | 0.775 | 106 | ||
| [17] | Chest model of human | 2:1 | 0.375 | 0.667 | 107 |
Figure 2.
Simulation result of the distribution of ventricular electric field. The red part represents the high electric field area and the blue part represents the low electric field area. The unit of electrical field (EF) is V/cm.
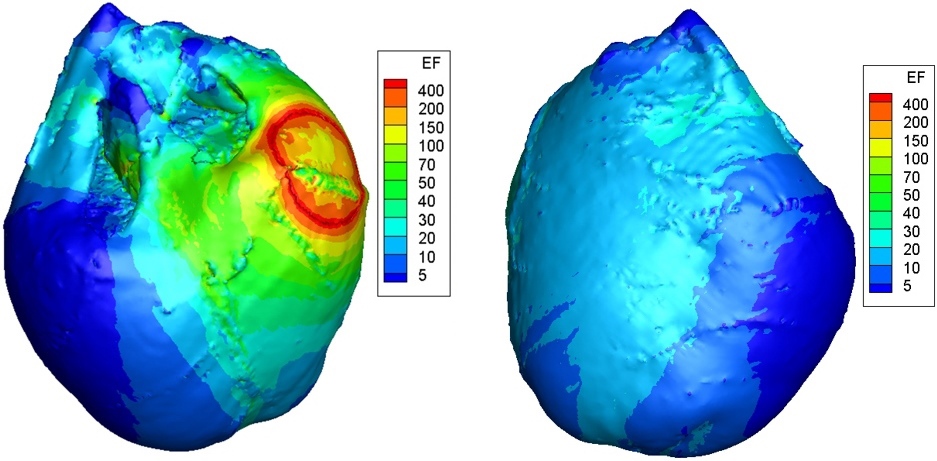
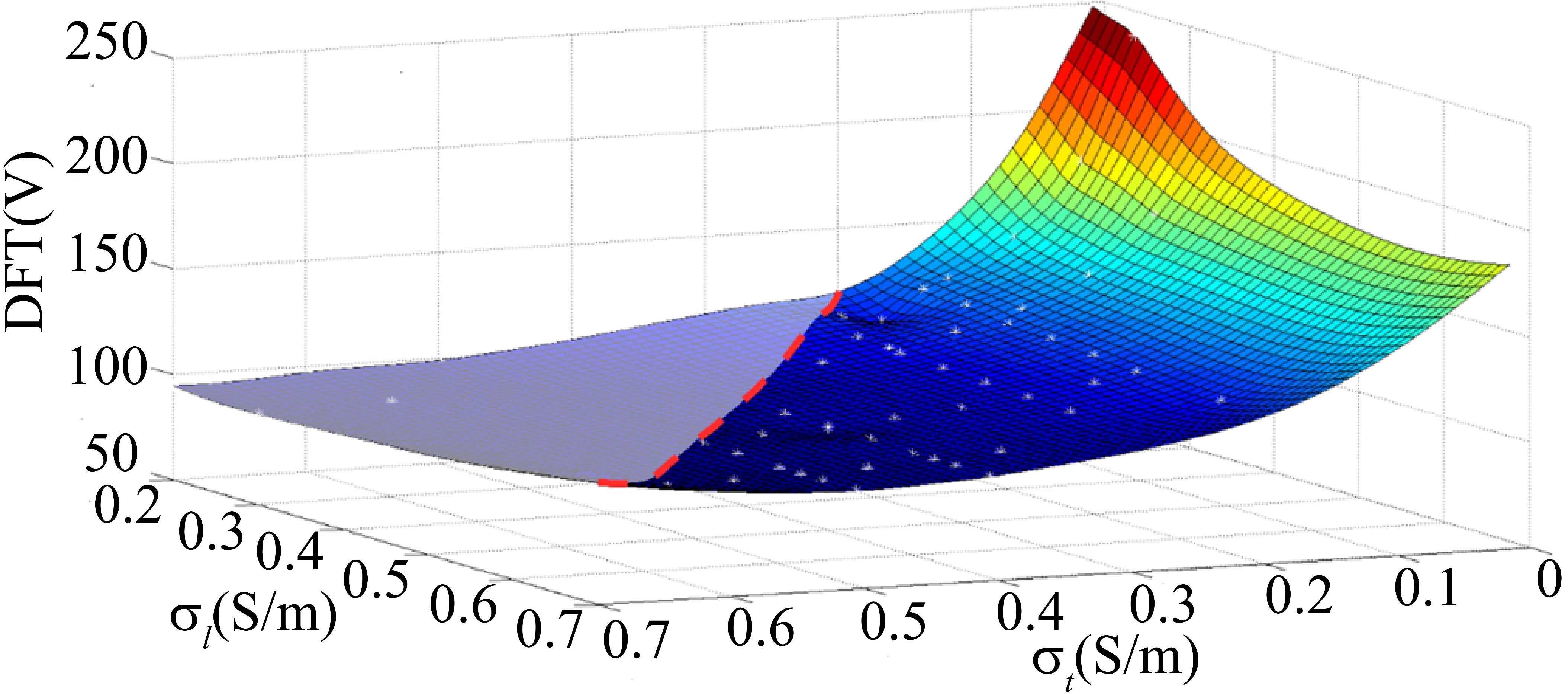
Figure 3.
Effect of cell coupling degree on defibrillation electric field distribution. As shown, the two axes of the horizontal plane respectively represent electrical conductivity along the myocardial fiber
3.Results
Electrical conductivity of myocardial fibers varies in different literatures. For all myocardial conductivities shown in Table 1, the corresponding distributions of electric field in the ventricle were gotten by our computer simulation (Fig. 2). The DFT (minimum defibrillation voltage that meets the critical mass hypothesis) is showed in seventh column of Table 1. Using Eq. (4), we calculated all the percentages of difference (POD, eighth column, Table 1) between the DFT of isotropic conductivity (
(5)
The values of anisotropic conductivites were changed depending on the possible cell coupling conditions: 1. because the cells are not prone to over-coupling, the
In the next part, three aspects of the problem will be discussed: 1. The effect of intercellular coupling on the DFT, which was represented as the value of the conductivity in volume conductor model (Section 3.1); 2. The effect of the degree of the myocardial anisotropy on the DFT, which was represented as the ratio of anisotropic conductivity in volume conductor model (Section 3.2); 3. The combined effect of the above two aspects on the DFT (Section 3.3).
3.1Effects of intercellular coupling of cardiomyocytes on the defibrillation thresholds
Studies have shown that cardiomyocyte coupling directly affects the degree of dispersion in myocardial repolarization and provides a major basis for reentrant arrhythmias [24]. This section investigated the effects of the degree of cardiomyocyte coupling on the DFT. By comparing the DFTs when
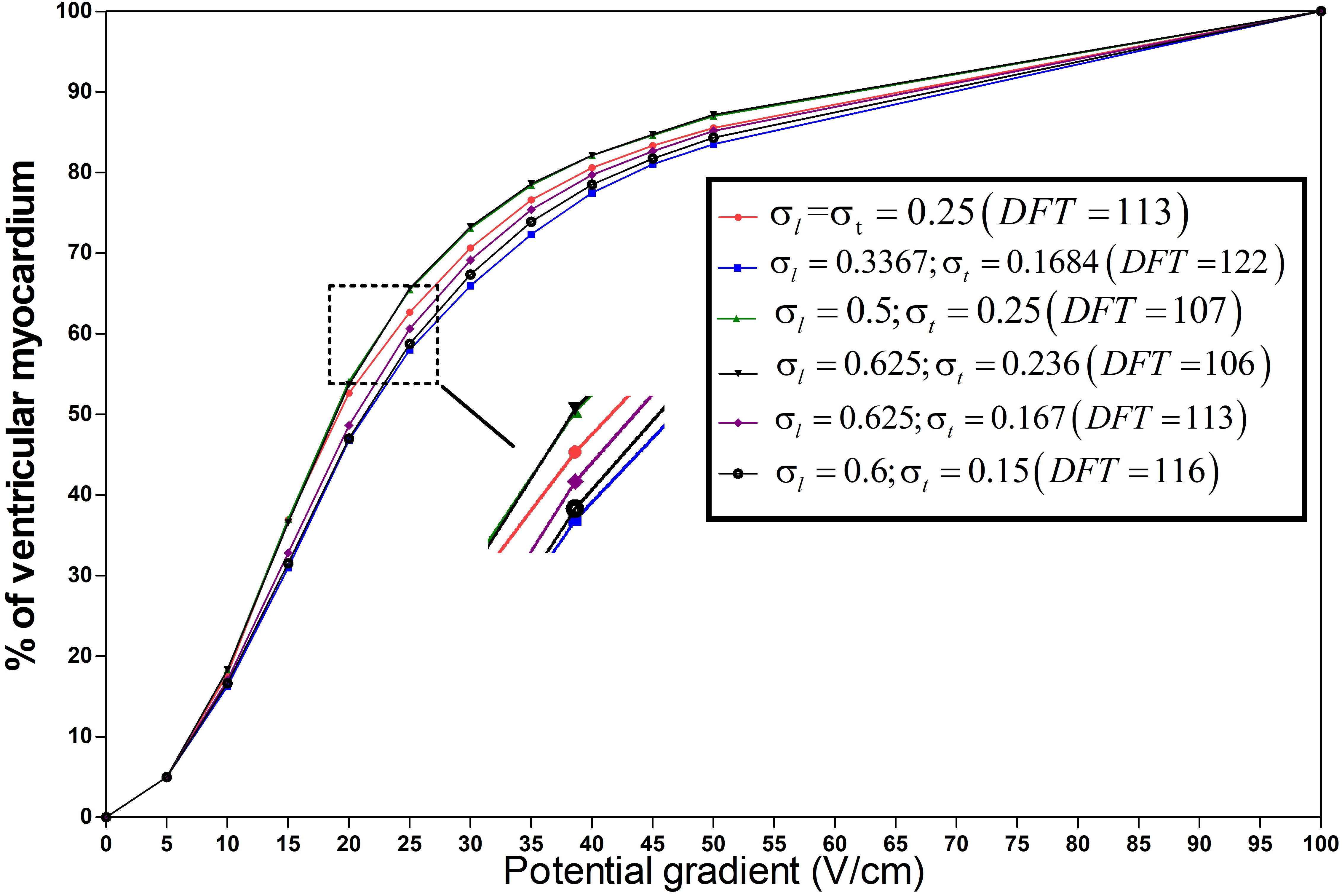
Figure 4.
The percentage of ventricular myocardial mass below discrete potential gradients in different anisotropic conductivity. The right diagram shows the case in Table 1 with different anisotropy ratios(
3.2Effects of myocardial anisotropic conductivity on the defibrillation thresholds
The shape of the myocardial cell is fusiform, which has long axis and short axis. The gap junctions between the long axis of the cells connection are more intensive, and thus the electrical conductivity is higher; gap junctions between the short axes of the cells are rare, and thus the electric conductivity is lower (
To investigate the effect of anisotropic conductivity on DFT, the DFTs of the isotropic conductivity (first row, Table 1) and anisotropic conductivity with 2:1 anisotropic ratio (second row, Table 1) were compared. The
Furthermore, both the conductivity of 4:1 anisotropic ratio (third row, Table 1) and the conductivity of 2:1 anisotropic ratio (sixth row, Table 1) have
3.3The effect of cardiomyocyte coupling and the degree of anisotropy on defibrillation thresholds
Previous studies in literature [8] have already provided that myocardial fibers have substantial electrical anisotropy, and myocardial anisotropy affects the distribution of voltage and defibrillation electric field. James Eason et al. compared the DFTs of the isotropic conductivity (
As shown in Fig. 4, with the increasing proportion of the low-intensity region, the curves shifted left, indicating a smaller or constant DFT. The DFTs of the red line (isotropic conductivity,
4.Conclusions
This study focused on the effects of ventricular myocardium characteristics on DFT. It is concluded that the DFTs and electrical conductivity of myocardial fibers are closely related. Higher electrical conductivities correlates with lower DFTs. In addition, the DFTs are affected by the degree of myocardial anisotropy. Higher degree of myocardial anisotropy correlates with higher DFTs.
Cardiac modeling and simulation are important techniques for evaluating defibrillation electric field distribution; the accuracy and complexity of the model directly affect the simulation results and efficiency. This study not only provides a basis for cardiac modeling and simulation of defibrillation electric field distribution, but also provides a new insight into the differences in the DFTs of clinical patients.
Acknowledgments
The authors would like to thank the Department of Computer Science, Oxford University for providing the data of rabbit ventricular model. This work received financial support from Science and Technology Commission of Shanghai (Grant No. 16441907900).
Conflict of interest
None to report.
References
[1] | Hua W, Zhang L, Wu Y, et al. Incidence of sudden cardiac death in china analysis of 4 regional populations. Journal of the American College of Cardiology (2009) ; 54: (12): 1110-1118. |
[2] | Smith TW, Cain ME. Sudden cardiac death: Epidemiologic and financial worldwide perspective. Journal of Interventional Cardiac Electrophysiology (2007) ; 17: (3): 199-203. |
[3] | Li Y, Ristagno G, Yu T, et al. A comparison of defibrillation efficacy between different impedance compensation techniques in high impedance porcine model. Resuscitation (2009) ; 80: (11): 1312-1317. |
[4] | Jianfei W, Lian J, Xiaomei W, et al. Low-energy defibrillation research using a rabbit ventricular model: Optimizing the potential gradient distribution using multiple epicardial electrodes. Conference Proceedings Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference (2016) ; 2753-2756. |
[5] | Camacho MA, Lehr JL, Eisenberg SR. A 3-dimensional finite-element model of human transthoracic defibrillation-paddle placement and size. IEEE Transactions on Biomedical Engineering (1995) ; 42: (6): 572-578. |
[6] | Karlon WJ, Eisenberg SR, Lehr JL. Effects of paddle placement and size on defibrillation current distribution – a 3-dimensional finite-element model. IEEE Transactions on Biomedical Engineering (1993) ; 40: (3): 246-255. |
[7] | Kinst TF, Sweeney MO, Lehr JL, et al. Simulated internal defibrillation in humans using an anatomically realistic three-dimensional finite element model of the thorax. Journal of Cardiovascular Electrophysiology (1997) ; 8: (5): 537-547. |
[8] | Zipes DP, Fischer J, King RM, et al. Termination of ventricular-fibrillation in dogs by depolarizing a critical amount of myocardium. American Journal of Cardiology (1975) ; 36: (1): 37-44. |
[9] | Aguel F, Eason JC, Trayanova NA, et al. Impact of transvenous lead position on active-can ICD defibrillation: A computer simulation study. Pace-Pacing and Clinical Electrophysiology (1999) ; 22: (12): 158-164. |
[10] | Wang YQ, Schimpf PH, Haynor DR, et al. Analysis of defibrillation efficacy from myocardial voltage gradients with finite element modeling. IEEE Transactions on Biomedical Engineering (1999) ; 46: (9): 1025-1036. |
[11] | Yang F, Sha Q, Patterson RP. A novel electrode placement strategy for low-energy internal cardioversion of atrial fibrillation: A simulation study. International Journal of Cardiology (2012) ; 158: (1): 149-152. |
[12] | Plonsey R, Barr R. The 4-electrode resistivity technique as applied to cardiac-muscle. IEEE Transactions on Biomedical Engineering (1982) ; 29: (7): 541-546. |
[13] | Eason J, Schmidt J, Dabasinskas A, et al. Influence of anisotropy on local and global measures of potential gradient in computer models of defibrillation. Annals of Biomedical Engineering (1998) ; 26: (5): 840-849. |
[14] | Aguel F, Eason JC, Trayanova NA, et al. Impact of transvenous lead position on active-can ICD defibrillation: A computer simulation study. Pace-Pacing and Clinical Electrophysiology (1999) ; 22: (12): 158-164. |
[15] | Wang YQ, Haynor DR, Kim Y. An investigation of the importance of myocardial anisotropy in finite-element modeling of the heart: Methodology and application to the estimation of defibrillation efficacy. IEEE Transactions on Biomedical Engineering (2001) ; 48: (12): 1377-1389. |
[16] | Modre R, Seger M, Fischer G, et al. Cardiac anisotropy: Is it negligible regarding noninvasive activation time imaging? IEEE Transactions on Biomedical Engineering (2006) ; 53: (4): 569-580. |
[17] | Panescu D, Webster JG, Tompkins WJ, et al. Optimization of cardiac defibrillation by 3-dimensional finite-element modeling of the human thorax. IEEE Transactions on Biomedical Engineering (1995) ; 42: (2): 185-192. |
[18] | Eason J, Schmidt J, Dabasinskas A, et al. Influence of anisotropy on local and global measures of potential gradient in computer models of defibrillation. Annals of Biomedical Engineering (1998) ; 26: (5): 840-849. |
[19] | Arevalo HJ, Boyle PM, Trayanova NA. Computational rabbit models to investigate the initiation, perpetuation, and termination of ventricular arrhythmia. Progress in Biophysics and Molecular Biology (2016) ; 121: (2): 185-194. |
[20] | Bordas R, Gillow K, Lou Q, et al. Rabbit-specific ventricular model of cardiac electrophysiological function including specialized conduction system. Progress in Biophysics and Molecular Biology (2011) ; 107: (1): 90-100. |
[21] | Clayton RH, Panfilov AV. A guide to modelling cardiac electrical activity in anatomically detailed ventricles. Progress in Biophysics and Molecular Biology (2008) ; 96: (1-3): 19-43. |
[22] | Jolley M, Stinstra J, Pieper S, et al. A computer modeling tool for comparing novel ICD electrode orientations in children and adults. Heart Rhythm (2008) ; 5: (4): 565-572. |
[23] | Bishop MJ, Plank G, Burton RAB, et al. Development of an anatomically detailed MRI-derived rabbit ventricular model and assessment of its impact on simulations of electrophysiological function. AJP: Heart and Circulatory Physiology (2010) ; 298: (2): H699-H718. |
[24] | Spach MS. Changes in the topology of gap-junctions as an adaptive structural response of the myocardium. Circulation (1994) ; 90: (2): 1103-1106. |




