Effect of polyphenols on inflammation related to cognitive function: a systematic review and meta-analysis of human randomized controlled trials
Abstract
BACKGROUND:
The decline of cognitive function could in part be caused by an increase in inflammation. Polyphenols have been widely investigated due to their anti-inflammatory property which may promote therapeutic effects on the brain and cognitive performance.
OBJECTIVE:
To evaluate the impact of polyphenols interventions on inflammation related to cognitive function in humans.
METHODS:
Three electronic databases: PubMed/Medline, Web of Science and PsycINFO were systematically searched until 30th May 2024 to find the study that have investigated the effect of polyphenols on both inflammatory response and cognitive function in human randomized controlled trials. The outcomes were pooled and calculated using inverse variance as mean difference (MD) with 95% confidence intervals (95% CI) for the inflammatory markers and standardized mean difference (SMD) with 95% CI for cognitive domains.
RESULTS:
Ten studies (451 participants, aged 20–81 years) assessed inflammatory markers and cognitive standardized tests responding to polyphenols interventions were included in this review and meta-analysis. Supplementation with polyphenols demonstrated a significant improvement of verbal memory (SMD: 0.33, 95% CI: 0.12 to 0.54, P = 0.002), executive function (SMD: 0.38, 95% CI: 0.03 to 0.72, P = 0.03) and attenuation in blood interleukin-6 (MD: – 1.23 pg/ml, 95% CI: –2.34, to –0.12, P = 0.03). No significant differences were observed in working memory (SMD: 0.13, 95% CI: –0.18 to 0.44, P = 0.42), attention (SMD: –0.19, 95% CI: –0.84 to 0.46, P = 0.57), and psychomotor skill (SMD: 0.09, 95% CI: –0.32 to 0.50, P = 0.66) as well as in c-reactive protein (MD: –0.10 mg/l, 95% CI: –0.28 to 0.09, P = 0.30), and tumor necrosis factor-α (MD: 0.11 pg/ml, 95% CI: –1.25 to 1.47, P = 0.87).
CONCLUSION:
Polyphenols supplementation decreases blood IL-6 as well as enhances verbal memory and executive function. Regular polyphenols consumption might prevent inflammation related to cognitive decline.
1Introduction
Inflammation, whether in the peripheral or in the brain, has been considered as an underlying mechanism of cognitive decline and neurodegenerative disorders. Previous studies demonstrated that elevated pro-inflammatory mediators have been related to poorer cognitive performance in various conditions, including older adults, dementia, Alzheimer’s disease, mild cognitive impairment (MCI), post-operative cognitive dysfunction, diabetes, and obesity [1–5]. Even though inflammation is a protective response of the body to external and internal stimuli, the uncontrolled process or prolonged response could deteriorate the bodily functions, including the brain [6]. The up-regulated pro-inflammatory molecules in the blood, such as interleukin-6 (IL-6), IL-1β, and tumor necrosis factor-α (TNF-α), could penetrate through the blood-brain barrier and stimulate the brain’s immune cells, resulting in neuroinflammation, neuronal damage, and brain pathologies which might have a negative impact on cognitive abilities [7, 8]. Several studies demonstrated that elevated blood inflammatory markers in either acute or chronic conditions could accelerate cognitive impairments in humans, particularly in tasks involving memory, attention, processing speed, and executive function [5, 9–13]. Due to the involvement of inflammation and cognitive function, studies focusing on anti-inflammatory interventions have gained increasing interest in delaying the onset of cognitive dysfunction and the progression of neurodegenerative diseases. Non-steroidal anti-inflammatory drugs (NSAIDs) have been examined for prevention or delaying the onset of inflammatory-related neurodegenerative diseases, especially AD and MCI [14, 15]. The outcomes have been satisfied to prevent an incidence of AD and cognitive impairment in observational studies [16]. Thus, attenuating the inflammatory response might be an approach to prevent or delay the progression of cognitive decline.
Dietary and non-dietary bioactive compounds have been widely studied due to their anti-inflammatory properties, which may promote therapeutic effects on the brain and cognitive performance [17, 18]. Polyphenols, non-nutrient bioactive compounds, commonly found in fruits, vegetables, and herbs, have been demonstrated to have several health benefits [18–20]. Polyphenols can be categorized into different groups based on their fundamental structural elements and functions, including phenolic acids, coumarins, flavonoids, stilbenes, and lignans. Their chemical structures are composed of molecules sharing an analogous elementary polyphenolic structure as well as molecules with a single phenol ring such as phenolic acids [21]. A number of studies have reported that polyphenols have the potential to attenuate neuroinflammation and peripheral inflammatory mediators as well as improve cognitive performance in animals and humans [17, 18, 22]. However, the role of polyphenols in the association between inflammation and cognitive function in humans has not been systematically elucidated. Therefore, this systematic review aims to evaluate the effect of polyphenols intervention on inflammation related to cognitive function in human randomized controlled trials to answer the question: do the polyphenols protect against cognitive decline through their potential to inhibit inflammatory processes in humans?
2Methodology
The systematic review was performed according to The Preferred Reporting items for Systematic Reviews and Meta-Analyses guideline (PRISMA) [23]. The outcome of this systematic review is to explore the effect of polyphenols intervention on cognitive function and inflammation in human randomized controlled trials.
2.1Search strategy
Searches were conducted using the following three electronic databases: PubMed/Medline, Web of Science and PsycINFO until 30th May 2024. The outcomes were carried out using the search terms: (neuroinflammat* OR inflammat* OR “inflammatory mediator*” OR cytokine* OR chemokine* OR “c-reactive protein” OR hsCRP) AND (cognit* OR cognition OR “cognitive function” OR “cognitive impairment” OR “cognitive decline” OR memory OR “executive function” OR “neuropsychological test*”) AND (“randomized controlled trial” OR “controlled trial” OR “cross-over stud*”).
2.2Inclusion and exclusion criteria
The inclusion criteria were as follows:
(i) original study with full-text papers published in English language in peer reviewed journals,
(ii) a randomized controlled trial with a parallel or cross-over group design,
(iii) conducted in humans aged≥18 years,
(iv) received polyphenols or polyphenols-rich food interventions,
(v) measured both a blood/cerebrospinal fluid inflammatory marker and a standardized cognitive test at baseline and post-intervention,
(vi) outcomes were presented as mean±SD/SEM or median and interquartile ranges (IQR).
Exclusion criteria were as follows:
(i) multicomponent supplementation or combined interventions,
(ii) measured the level of inflammatory mediators by cell culture assay,
(iii) assessed cognitive function by neuroimaging technique,
(iv) inflammatory status or cognitive outcomes were reported separately in another publication.
The selected studies were read in full to determine eligibility in accordance with the PICOs parameters as shown in Table 1.
Table 1
PICOS (participant, intervention, comparison, outcome, and study design) criteria for included studies
| PICOS parameters | Inclusion criteria |
| Population | Human participants aged more than 18 years |
| Intervention | Polyphenols or polyphenols-rich food interventions |
| Comparison | Placebo or appropriate comparable intervention |
| Outcome | Both inflammation (i.e., CRP, IL-6, TNF-β) and cognitive function (standardized cognitive tests) |
| Study design | Randomized controlled trial with a parallel or cross-over group design |
2.3Study selection and data extraction
The endnote programme was used to allocate all the results from the search strategy. The selected records were independently assessed for eligibility by two review authors (CM and DJL). A discussion with a third reviewer will be used to resolve any potential disagreements over the eligibility of a particular study. The data were extracted using a standardized form. The extracted information included the following: first author name and year of publication; study design; participant (condition, mean age, number of subjects); intervention (treatment, dose, and length); inflammatory marker; cognitive assessment; and main findings.
2.4Assessment of methodological quality
Studies selected for systematic review were assessed for overall quality of methodology and the potential risk of bias by two reviewers using Cochrane Collaboration’s risk of bias tool (RoB 2) [24]. For parallel randomized controlled trials, the assessed domains consisted of bias arising from randomized process, bias due to deviations from intended interventions, bias due to missing data outcome, bias in measurement outcome, bias in the selection of the reported result, and any adjustment to the overall study. The bias arising from period and carryover effects was additionally assessed in the cross-over design study. The risk of bias in each domain was rated as “low”, “some concern” and “high”. Any disagreements between the reviewers were resolved by discussion and a third researcher, where necessary.
2.5Meta-analysis
Due to various types of inflammatory mediators and cognitive tests, the outcomes of inflammation were clustered into CRP, IL-6, and TNF-α. The results of cognitive function were classified based on the cognitive domains assessed in the particular studies: working memory, verbal memory, executive function, attention, and psychomotor skill. For the study measuring outcomes in different periods, the longest time point was used for analysis. Quantitative extracted data were imported into RavMan 5.4 for meta-analysis. At least three studies reported similar post-intervention outcomes as mean±SD both in response to polyphenols and to placebo, were pooled and calculated using inverse variance as mean difference (MD) with 95% CI for inflammatory markers because the included studies used the same measurement scales. The standardized mean difference (SMD) with 95% CI was used with cognitive tests because of the different scales of measurements [24]. In the case of outcomes reported as median, the mean and SD were calculated following the study by Hozo et al., 2005 [25]. For the studies which reported the outcomes as mean and standard error of mean, the SD value was calculated from this equation: SEM = SD/sqrt (N). The heterogeneity among the included studies will be assessed by the Cochrane Q and I2 statistics [24]. Cochrane Q statistic with a p-value of less than 0.05 indicates statistical significance, while I2 statistics of more than 50% indicate substantial heterogeneity among the included studies. Effect size (ES) was interpreted as small (d = 0.2), medium (d = 0.5), and large (d > 0.8) according to Cohen’s criteria [26].
3Results
3.1Study identification
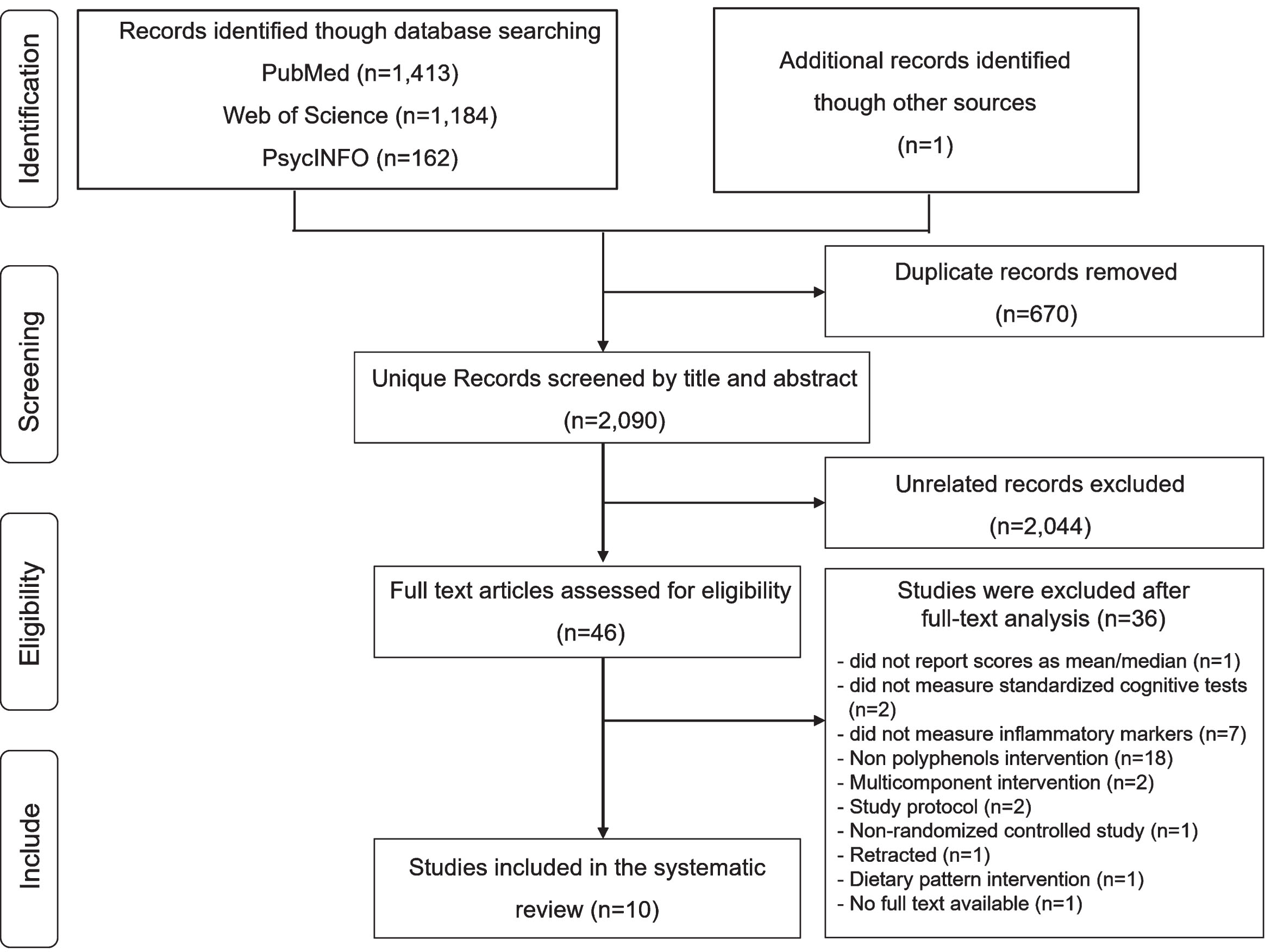
A total of 2,760 records were obtained after the initial search of the PubMed/Medline, Web of Science, and PsycINFO databases. 670 duplicated studies were removed, and then the titles and abstracts of 2,090 unduplicated studies were preliminary screened to identify eligibility. After that, the 46 potential eligible articles were read in full text according to the previously mentioned criteria, and 10 articles were retrieved in the systematic review and meta-analysis, respectively (Fig. 1).
Fig. 1
Flow chart illustrating the identification of studies for the literature.
3.2The study characteristics
Ten studies met the inclusion criteria were included in a systematic review to determine the effect of polyphenols on both cognitive function and inflammation in human randomized controlled trials. Eight studies were parallel randomized controlled trials [27–29, 32–36], and two studies were cross-over designs [30, 31]. The included studies were published between 2012 and 2024, with sample sizes ranging from 26 to 60 subjects. A total of 451 participants were enrolled, with a mean age range of 20 to 81 years. The 94 participants were grouped as young adults (age 18–40 years), and the remaining 357 participants were older adults (age≥60 years). The subjects were general healthy status for five studies [27, 30, 31, 33, 34] overweight or obese for two studies [32, 35], mild cognitive impairment for two studies [28, 29], and adults with self-reported depressive symptoms for one study [36]. The polyphenols intervention was categorized into anthocyanin-rich foods (anthocyanin contents between 10 and 387 mg/day), resveratrol supplements (200 mg/day), and polyphenol-rich extracts: Brazilian green propolis extract (11 mg/day of artepillin C) and polyphenol-rich seaweed extract (polyphenols 560 mg/day). All included studies investigated the chronic effects (5 weeks to 24 months) of polyphenols supplementation on cognitive function and blood inflammatory status. The summarized characteristics and interventions of the included studies are in Table 2.
Table 2
The impact of polyphenols intervention on inflammation and cognitive function
| Author | Design | Population | Intervention | Inflammatory | Cognitive | Outcomes | Main findings | |||||
| Conditions | Mean age | Sample size | Treatment | Dose | Length | measurement | tests | Inflammation | Cognition | |||
| Bowtell et al. (2017) [27] | Double-blind randomized controlled trial | Healthy older adults | I: 68C: 69 | 26I: 12C:14 | Blueberry concentrate | 387 mg.day–1anthocyanins | 12 wks. | hs-CRP | The cogstate: a detection task, the Groton maze timed chase test, the Groton maze learning test with a delayed recall, international shopping list task with delayed recall, 1-2 back memory tasks | ↔ | ↑ 2-back test | Blueberry concentrate consumption did not significantly change hsCRP levels but can improve brain activities related to working memory. |
| Kent et al. (2017) [28] | Double-blind randomized controlled trial | Mild-moderate dementia | I: 79C: 81 | 49I: 24C:25 | Anthocyanin-rich cherry juice | 138 mg.day-1 anthocyanins | 12 wks | CRP, IL-6 | RAVLT, the self-ordered pointing task (SOPT), the Boston naming test, the trail making test (TMT), digit span backwards task and category and letter verbal fluency | ↔ | ↑ Verbal fluency, short-term long-term memory | Anthocyanin-rich juice intake improved verbal fluency and memory, as well as decreased BP. However, CRP and IL-6 levels did not alter compared to the control. |
| Bohn et al. (2021) [29] | Double-blind randomized controlled trial | Aged men with subjective memory impairment | I: 72C: 71 | 60I: 30C:30 | Bilberry and red grape-juice | 660 ml.day–1 | 9 wks. | IL8, IP10, MCP, MIP-1β, IL-4, IL-6, IL-7, IL-9, IL-10, IL-12, IL-13, IL-17, TNF-α, IFN-g | The CANTAB; Delayed matching to sample, Paired associates learning, Pattern recognition memory, Spatial recognition memory, Grooved Pegboard Test | ↓ L-6, TNF-α, IL-9, IL-10, MIP-1β | ↔ | Bilberry juice significantly reduced IL-6, TNF-α, IL-9, IL-10, and MIP-1β but did not change cognitive performance. |
| Igwe et al. (2020) [30] | Randomized, crossover clinical trial | Healthy elderly adults | 70 | 31 | Queen garnet plum nectar | 10 mg.day–1 anthocyanins | 8 wks. | CRP | The Rey Auditory Verbal Learning Test (RAVLT), digit-span backwards task, stroop task, and counting span | ↔ | ↔ | Low anthocyanin queen garnet plum nectar consumption did not alter both serum CRP and cognitive function. |
| Nilsson et al. (2017) [31] | Randomized cross-over study | Healthy non-smoker adults | 63 | 40 | Berry beverage | 795 mg/l of polyphenols, 248 mg/l of anthocyanins | 5 wks. | IL-6, IL-18 | Verbal working memory (WM) test, selective attention test | ↔ | ↑ Working memory | Berry drinks improved working memory and reduced LDL cholesterol, but they did not change serum cytokine levels. |
| Witte et al. (2014) [32] | Double blind placebo-controlled study | Healthy overweight older adults | I: 65C: 64 | 46I: 23C:23 | Resveratrol | 200 mg.day–1 | 26 wks. | IL-6, TNF-α, hsCRP | The auditory verbal learning task; retention, delayed recall, recognition, learning ability, fifth learning trial | ↓ TNF-α | ↑ Retention of word | Resveratrol intake significantly reduced TNF-α levels and increased retention of words as well as functional activity in the hippocampus. |
| Huhn et al. (2018) [33] | Double-blind randomized controlled trial | Healthy elderly participants | I: 69C: 68 | 53I: 27C:26 | Resveratrol | 200 mg.day–1 | 26 wks. | IL-6, TNF-α, hsCRP | The German version of the California Verbal Learning Task (CVLT), Trail making test A&B | ↑ IL-6, TNF-α, hsCRP | ↔ | Resveratrol supplement slightly increased serum IL-6, TNF-α, and hsCRP, but no change in cognition was found in both groups. However, the resveratrol trend preserved pattern recognition memory. |
| Zhu et al. (2018) [34] | Randomized, placebo-controlleddouble-blind | Elderly people living at high altitude | I: 72C:73 | 46I: 30C: 30 | Brazilian green propolis extract | 11 mg.day–1 Artepillin C | 6,12,24 months | IL-1β, IL-6, TNF-α, IL-10, TGF-β1 | MMSE | ↓ IL-1β, IL-6 | ↑ MMSE | Propolis supplements decreased plasma IL-1β and IL-6 and improve global cognition in a time-dependent manner. |
| Murray et al. (2021) [35] | Double-blind, placebo-controlled, randomized parallel trial | Overweight adults | I: 34C: 45 | 34I: 17C:17 | Polyphenol-rich seaweed (F. vesiculosus) extract | 560 mg.day–1 of total polyphenols | 12 wks. | IL-2, IL-6, IL-8, IL-10, TNF-α | The immediate word recall task, 2-back, serial three and serial seven subtraction tasks, corsi blocks, digit vigilance task, delayed word recall task, and delayed word recognition task | ↔ | ↔ | No changes were found in inflammatory markers or cognitive function between treatment and placebo after receiving seaweed extract, but an increase HDL was observed in the intervention group. |
| Velichkov et al. (2024) [36] | Double-blind randomized controlled trial | Adults with self-reported depressive symptoms | I: 20C: 20 | 60I: 30C: 30 | Blueberry drink | 121 mg.day–1anthocyanins, 55 mg.day–1 chlorogenic acid | 2 h, 6 wks. | hs-CRP, IL-6 | The task-switching paradigm | ↔ | ↑ Task-switching at acute phase | Acute blueberry consumption enhanced executive function and mood, but long-term supplementation did not affect serum indicators or cognition. |
↓: significantly reduce, ↑ significantly increase, ↔ non-significantly change, I = intervention, C: control.
3.3Quality assessment of included studies
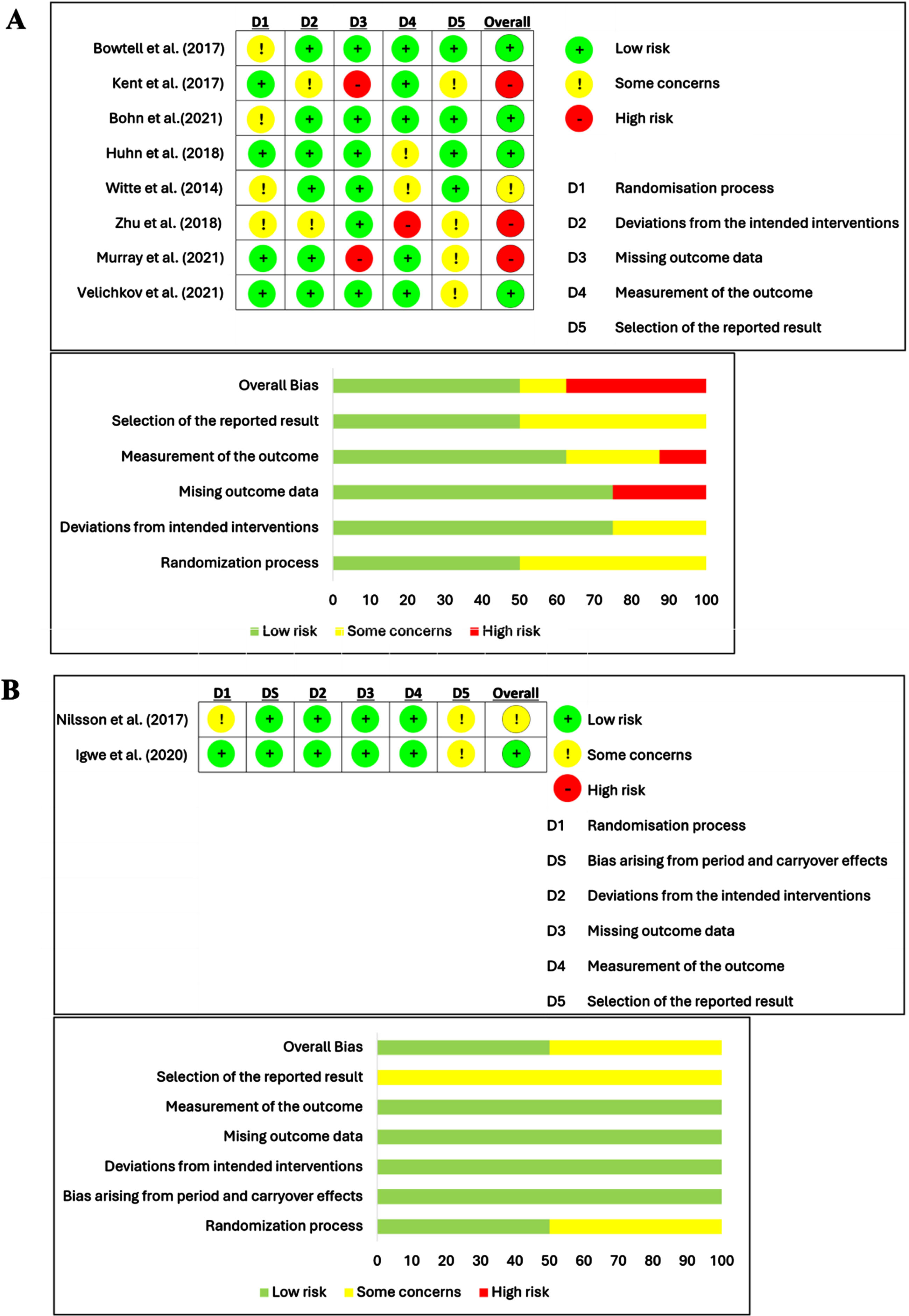
ROB2 was used to assess the quality of the included studies. The ROB summary and graph were demonstrated in Fig. 2A, parallel randomized controlled design, and Fig. 2B, cross-over design. The overall ROB was graded “low” for five studies [27, 29, 30, 33, 36], “some concern” for two studies [31, 32], and “high” for three studies [28, 34, 35]. The randomization process was rated “some concern” for five studies because they did not specify the process for randomization and allocation [27, 29, 31, 32, 34]. The risk of deviations from the intended intervention was assessed as “some concern” in two studies due to the fact that participants and carers might be aware of their assigned intervention [28, 34]. The “high” risk of missing data outcome was assessed in two studies because the reports did not mention the extent of missing data outcomes [28, 35]. For the risk of measurement data outcome was rated as “high” risk for one study [34] and “some concern” for two studies [32, 33] due to the assessors may not be blinded by the intervention status. The selection of the reported data was rated “some concern” for six studies because the results were likely to have been selected to be reported in the papers [28, 30, 31, 34, 35, 36]. Lastly, the risk of carryover effects was rated “low” in both cross-over design studies [30, 31].
Fig. 2
Risk of bias and graph for parallel design study (A) and cross-over design study (B).
3.4Narrative synthesis
3.4.1Polyphenols intervention on inflammation and cognitive benefits
This systematic review includes ten studies that assessed blood inflammatory markers as well as standardized cognitive assessments in human RCTs. Eight studies were conducted with older participants, whereas the others were in a younger age group with obesity and self-reported depression symptoms. [35, 36]. Out of ten studies, three (30%) demonstrated a significant reduction of inflammatory markers, including L-6, TNF-α, IL-9, IL-10, MIP-1β, and IL-1β, by polyphenols interventions [29, 32, 34]. One study reported an increase in some inflammatory markers such as IL-6, TNF-α, and hs-CRP, but in both the interventional and control groups [33]. The others demonstrated no significant reduction in blood inflammatory indicators after polyphenols treatment compared to the placebo [27, 28, 30, 31, 35, 36]. Regarding cognitive outcomes, six studies (60%) demonstrated that polyphenols supplementation significantly improved cognitive performance when compared to controls [27, 28, 31, 32, 34, 36]; however, one of these only showed a substantial improvement during the acute period (2 h) [36]. The domains of memory, especially verbal memory, and working memory were predominantly improved. In addition, one study indicated improvements in executive function and psychomotor skill [28]. However, four studies found that the polyphenols supplementation did not change cognitive function in comparison to the control [29, 30, 33, 35]. The association between inflammation and cognitive performance was summarized based on studies indicating anti-inflammatory benefits of polyphenols treatment. Three studies indicated that polyphenols significantly reduced the levels of TNF-α, IL-6 and IL-1β mediators [29, 32, 34], and two of them identified an association with improved verbal memory and global cognition [32, 34]. Therefore, polyphenols supplementation might enhance cognitive performance underlining the anti-inflammatory processes. Four studies found that polyphenols, primarily anthocyanins, improved cognitive performance while having no effect on inflammatory responses [27, 28, 31, 36]. The improvement of cognitive functions, however, could be underlined by various mechanisms. Other putative mechanisms were highlighted in the included research, such as improving cerebral blood flow [27], activating brain regions relevant to cognitive function [27, 32], lowering blood pressure [28], and changing lipid metabolic risk indicators [31–35].
Considering the type of interventions, six studies investigated anthocyanin-rich foods [27–31, 36]; two studies demonstrated resveratrol supplementation [32, 33]; and two studies assessed polyphenol-rich extracts [34, 35]. Following anthocyanins treatment, three out of six studies reported that chronic anthocyanin consumption at concentrations of 138–387 mg/day for 5–12 weeks demonstrated a substantial improvement in cognitive performance in older adults, particularly in the domain of working memory and executive function; however, the interventions did not impact any blood inflammatory biomarkers (IL-6, CRP, and IL-18) [27, 28, 31]. Bowtell et al. (2017) reported that the consumption of blueberry concentrates (387 mg/day of anthocyanins) for 12 weeks significantly improved working memory (2-back memory test, p = 0.05) with no change in serum CRP levels in healthy elderly compared to controls. However, they found an increase in blood perfusion to the grey matter and the activation of some brain-related cognitive areas, including the Brodmann, anterior cingulate, precuneus, and insula/thalamus, which may result in the enhancement of working memory [27]. Nilsson et al. (2017) found similarly that working memory significantly enhanced (p < 0.05) after 5 weeks of berry beverage consumption (248 mg/day of anthocyanins) in non-smoker healthy elderly subjects, while the serum IL-6 and IL-18 cytokines were not altered. Meanwhile, a reduction of LDL cholesterol was reported after supplementation [31]. Kent et al. (2017) reported that 12-week anthocyanin-rich juice intake (138 mg/day of anthocyanins) in elderly people with MCI significantly improved executive function (P = 0.014), short-term memory (P = 0.014), and long-term memory (p≤0.001), as well as reduced systolic blood pressure (P = 0.038). However, the serum CRP and IL-6 levels were not significantly different compared to the control [28]. On the contrary, the study by Bohn et al. (2021) demonstrated that bilberry juice consumption (660 ml/day) for 9 weeks significantly reduced plasma IL-6 (P = 0.04), TNF-α (P = 0.04), IL-9 (P = 0.05), IL-10 (P = 0.05), and MIP-1β (P = 0.02) levels as well as plasma tissue damage biomarkers in aged men with subjective memory impairment without affecting working memory or episodic memory [29]. Another anthocyanin study by Igwe et al. (2020) found that neither serum CRP levels nor cognitive performance significantly differed after low anthocyanin queen garnet plum nectar (10 mg/day of anthocyanins) supplementation in healthy elderly compared to controls [30]. Additionally, Velichkov et al. (2024) demonstrated that six weeks of blueberry drink intake (121 mg/day of anthocyanins) had no effect on serum inflammatory markers or cognitive performance, but that acute consumption (2 h) enhanced executive function and mood in subjects with self-reported symptoms of depressive disorders [36]. To summarize, the improvement of cognitive outcomes by anthocyanin-rich food consumption, especially working memory, reported consistency in three studies with doses of 138–387 mg/day in healthy elderly participants, with non-significant changes in the makers of inflammation. The enhancement of cognitive performance by anthocyanins was unlikely to be dependent on a specific anti-inflammatory mechanism. These investigations revealed several probable underlying processes, such as increased cerebral blood flow, activation of brain regions associated with cognitive ability, and a decrease in oxidative stress indicators. In order to establish the cognitive benefits of anthocyanins supplementation, a minimum dose of at least 138 mg/day should be recommended over a long-term period (≥5 weeks).
The impacts of resveratrol supplementation on cognitive function and inflammatory status were evaluated in healthy older adults and older persons with overweight status [32, 33]. Witte et al. (2014) found that taking 200 mg/day of resveratrol for 26 weeks significantly reduced blood TNF-α levels and improved verbal memory (word retention test, P = 0.038) compared to a placebo in the elderly with overweight status. In addition, the enhancement of functional activity in the hippocampus, a main brain area of memory, has been reported [32]. Another study by Huhn et al. (2018) demonstrated that serum IL-6, TNF-α, and hs-CRP levels significantly increased after consuming 200 mg of resveratrol for 6 months, but in both intervention and control groups. The psychomotor skills (Trail Making Tasks A and B) were not altered in healthy elderly volunteers; however, the recognition trend to be preserved (P = 0.07) following supplementation compared to control [33]. These studies demonstrated that prolonged resveratrol supplementation (≥6 months) at a dosage of 200 mg/day might maintain memory, psychomotor skills, and recognition in elderly subjects whilst additionally decreasing inflammatory mediators, particularly in overweight individuals.
Another two studies investigated the effects of polyphenol-rich extracts on inflammation and cognitive function in healthy elderly and overweight adults. Zhu et al. (2018) investigated the long-term effects of artepillin C (11 mg/day)-rich propolis extract on cognition and inflammatory mediators in elderly people living at high altitude. The chronic propolis supplementations for 6, 12, and 24 months significantly reduced serum IL-6 and IL-1β concentrations and improved the MMSE scores in a time-dependent manner compared to the controls. Interestingly, the prolonged duration of supplementation could extend the effects of anti-inflammatory and cognitive benefits [34]. On the other hand, Murray et al. (2021) reported the negative effect of 12-weeks polyphenol-rich seaweed supplementation (560 mg/day of total polyphenols) on both cognitive function and inflammatory mediators in overweight adults. However, they reported a significant increase in HDL cholesterol after supplementation [35].
3.5Meta-analysis results
3.5.1The effect of polyphenols intervention on blood inflammatory markers
3.5.1.1. CRP. The effect of polyphenols intervention on serum CRP concentration (mg/l) was evaluated in seven RCTs with 356 participants. The pooled estimate demonstrated that dietary polyphenols did not significantly change the level of CRP (MD: –0.10 mg/l, 95% CI: –0.28 to 0.09, P = 0.30). Considering I2 index (0.0%) and Cochrane Q test (P = 0.79), very low inter-trial heterogeneity was detected (Fig. 3A).
Fig. 3
Forest plot of studies demonstration of polyphenols interventions on CRP (A), IL-6 (B) and TNF-α (C).
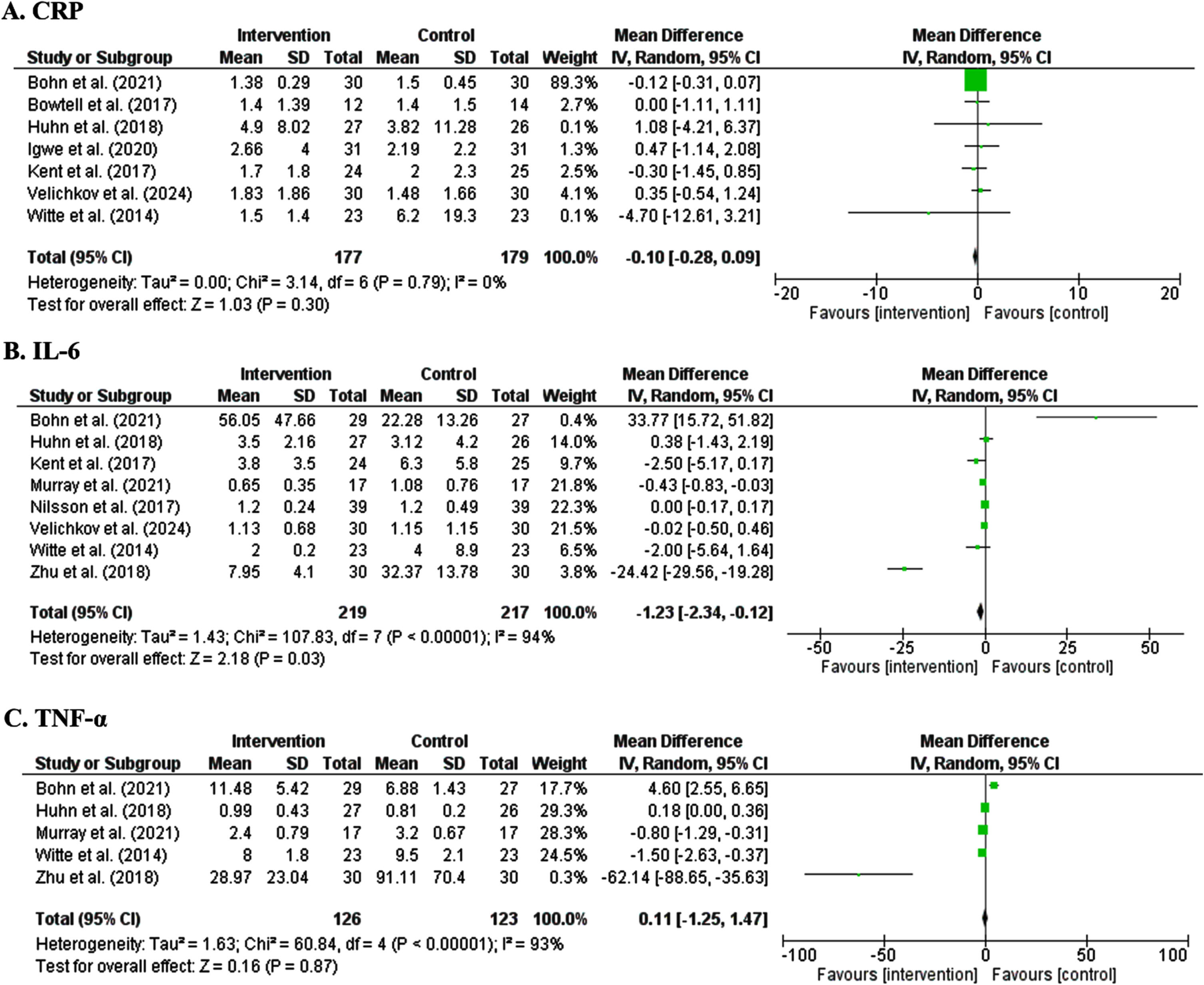
3.5.1.2. IL-6. Data from eight RCTs, with 436 participants, in which the effect of polyphenols interventions on serum IL-6 concentration (pg/ml) was evaluated. The pooled estimate demonstrated that dietary polyphenols significantly reduced the level of IL-6 (MD: –1.23 pg/ml, 95% CI: –2.34 to 0.12, P = 0.03). Considering I2 index (94.0%) and Cochrane Q test (P < 0.00001), substantial inter-trial heterogeneity was detected (Fig. 3B).
3.5.1.3. TNF-α. Data from five RCTs, with 249 participants, in which the effect of polyphenols interventions on serum TNF-α level (pg/ml) was assessed. The pooled estimate found that dietary polyphenols did not significantly change the level of TNF-α (MD: 0.11 pg/ml, 95% CI: –1.25 to 1.47, P = 0.87). Considering I2 index (93.0%) and Cochrane Q test (P < 0.00001), substantial inter-trial heterogeneity was detected (Fig. 3C).
3.5.2The effect of polyphenols on cognitive performance
3.5.2.1. Verbal memory. The results from each study measuring cognitive ability in the domain of verbal memory were considered an independent study. There were eleven studies, comprising 542 participants, in which the effect of polyphenols on working memory was pooled in our meta-analysis. The pooled estimate revealed that dietary polyphenols significantly improve verbal memory. The summarized effect of the findings showed a small effect. (SMD: 0.33, 95% CI: 0.12 to 0.54, P = 0.002). Considering I2 index (34.0%) and Cochrane Q test (P = 0.13), substantial inter-trial heterogeneity was detected (Fig. 4A).
Fig. 4
Forest plot of studies demonstration of polyphenols interventions on verbal memory (A) and working memory (B).
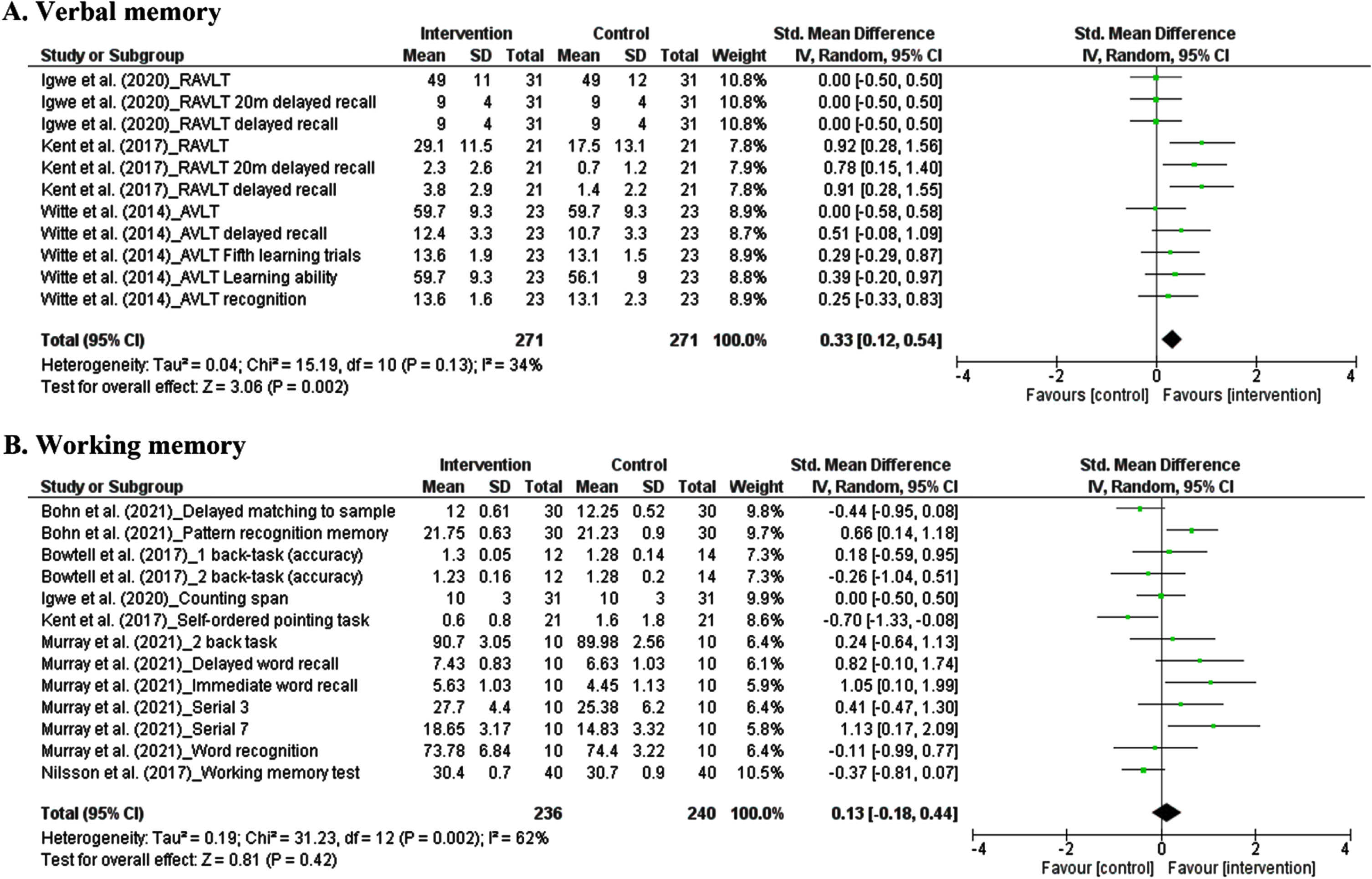
3.5.2.2. Working memory. The results from each study measuring cognitive performance in domain of working memory were considered as an independent study. There were thirteen studies, comprising 476 participants, in which the effect of polyphenols on working memory was pooled in our meta-analysis. The pooled estimate revealed that dietary polyphenols did not significantly improve working memory. The summarized effect of the findings showed a small effect (SMD: 0.13, 95% CI: –0.18 to 0.44, P = 0.42). Considering I2 index (62.0%) and Cochrane Q test (P = 0.002), substantial inter-trial heterogeneity was detected (Fig. 4B).
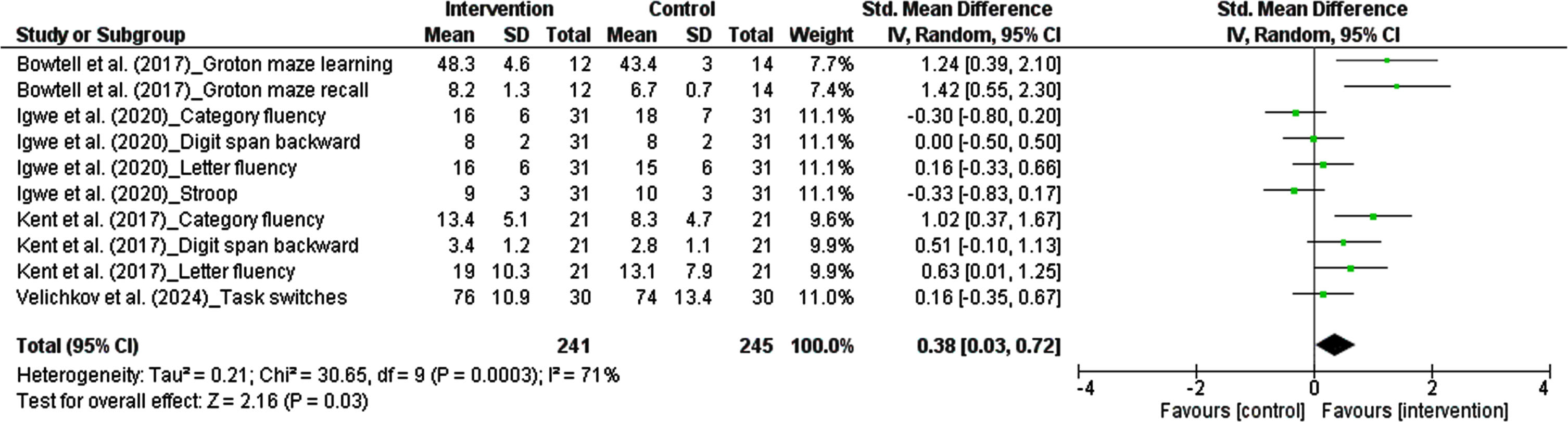
3.5.2.3. Executive function. The results from each study measuring cognitive ability in domain of executive function were considered as an independent study. There were ten studies, comprising 486 participants, in which the effect of polyphenols on working memory was pooled in our meta-analysis. The pooled estimate revealed that dietary polyphenols significantly enhance executive function. The summarized effect of the findings showed a small effect (SMD: 0.38, 95% CI: 0.03 to 0.72, P = 0.03). Considering I2 index (71.0%) and Cochrane Q test (P = 0.0003), substantial inter-trial heterogeneity was detected (Fig. 5).
Fig. 5
Forest plot of studies demonstration of polyphenols interventions on executive function.
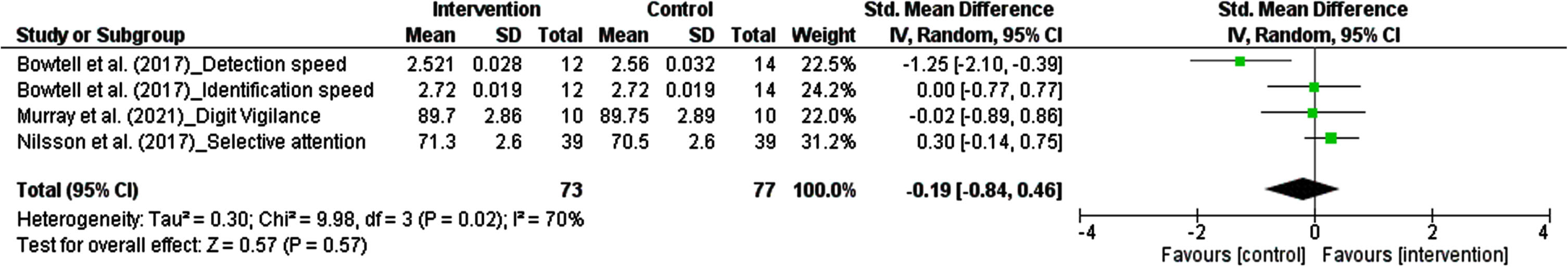
3.5.2.4. Attention. The results from each study measuring cognitive ability in domain of attention were considered as an independent study. There were four studies, comprising 150 participants, in which the effect of polyphenols on attention was pooled in our meta-analysis. The pooled estimate revealed that dietary polyphenols did not significantly enhance attentive skills. The summarized effect of the findings showed a small effect (SMD: –0.19, 95% CI: –0.84 to 0.46, P = 0.57). Considering I2 index (70.0%) and Cochrane Q test (P = 0.02), substantial inter-trial heterogeneity was detected (Fig. 6).
Fig. 6
Forest plot of studies demonstration of polyphenols interventions on attention.
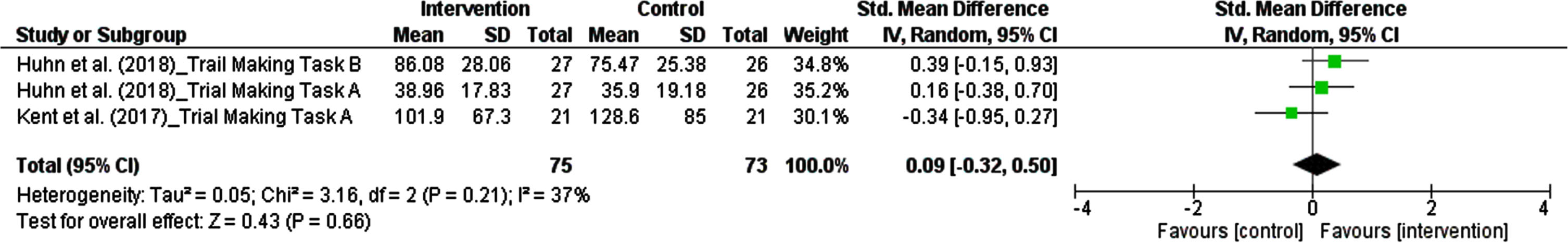
3.5.3Psychomotor skill
The results from each study measuring cognitive ability in domain of psychomotor skill were considered as an independent study. There were three studies, comprising 148 participants, in which the effect of polyphenols on psychomotor skill was pooled in our meta-analysis. The pooled estimate revealed that dietary polyphenols did not significantly increase psychomotor skill. The summarized effect of the findings showed a small effect (SMD: 0.09, 95% CI: –0.32 to 0.50, P = 0.66). Considering I2 index (37.0%) and Cochrane Q test (P = 0.21), a low inter-trial heterogeneity was detected (Fig. 7).
Fig. 7
Forest plot of studies demonstration of polyphenols interventions on psychomotor skill.
4Discussions
This comprehensive review with meta-analysis first establishes the associations between cognition and inflammation in response to polyphenols intervention in human RCTs. The included ten studies were primarily conducted in older age groups and supplemented anthocyanins with a daily dosage of 10–387 mg, followed by resveratrol at 200 mg, polyphenol-rich extract at 560 mg, and propolis extract containing 11 mg of artepillin C. All studies examined chronic supplementation for 5 weeks to 24 months, with one examining both acute and chronic phrases. Significant improvements were observed in verbal memory and executive function as well as an attenuation in blood IL-6 concentrations following the chronic supplementation of polyphenols. The serum TNF-α and CRP concentrations, as well as the domains of working memory, attention, and psychomotor skill, were not statistically changed (summary in Table 3).
Table 3
Summary of the effect of polyphenols intervention on inflammation and cognitive function
| Outcomes | No. of | No. of | Heterogeneity | Pooled effect | ||||
| study | participant | χ2 | df (p) | I2 | d | 95% CI | P | |
| Inflammatory markers | ||||||||
| CRP (mg/L) | 7 | 356 | 3.14 | 6 (0.79) | 0% | –0.10 | [–0.28, 0.09] | 0.30 |
| IL-6 (pg/ml) | 8 | 436 | 107.83 | 7 (<0.000001) | 94% | –1.23 | [–2.34, –0.12] | 0.03* |
| TNF-α (pg/ml) | 5 | 249 | 60.84 | 4 (<0.000001) | 93% | 0.11 | [–1.25, 1.47] | 0.87 |
| Cognitive domains | ||||||||
| Verbal Memory | 11 | 542 | 15.19 | 10 (0.13) | 34% | 0.33 | [0.12, 0.54] | 0.002* |
| Working memory | 13 | 476 | 31.23 | 12 (0.002) | 62% | 0.13 | [–0.18, 0.44] | 0.42 |
| Executive function | 10 | 486 | 30.65 | 9 (0.0003) | 71% | 0.38 | [0.03, 0.72] | 0.03* |
| Attention | 4 | 150 | 9.98 | 3 (0.02) | 70% | –0.19 | [–0.84, 0.46] | 0.57 |
| Psychomotor skill | 3 | 148 | 3.16 | 2 (0.21) | 37% | 0.09 | [–0.32, 0.05] | 0.66 |
*Level of significance at P≤0.05.
Cognitive function refers to the mental processes involved in receiving knowledge, manipulating information, and being able to understand and think rationally, and it can be measured in a variety of domains such as attention, memory, learning, perception, decision making, processing speed, psychomotor skill, language, and executive function [37]. In general, cognitive performance, particularly memory, deteriorates with age due to various mechanisms such as loss of brain volume and neuron cells, a decrease in synaptic plasticity, a decrease in vascular function and cerebral blood flow, as well as an increase in systemic inflammation [38]. These factors may have an impact on brain function, including cognitive performance, personality, and behavior. This meta-analysis found that polyphenols supplementation predominantly improved verbal memory and executive function, particularly in older adults. Regarding individual research, six studies reported a significant increase in cognitive performance after polyphenols supplementation compared to controls [27, 28, 31, 32, 34, 36]. The beneficial effects on domains of memory, executive function, and psychomotor skill were primarily observed in anthocyanin-rich food interventions [27, 28, 31, 36]. The supplementation with resveratrol and propolis extract demonstrated the improvement of memory and global cognitive function, respectively [32, 34]. Consistent with a recent meta-analysis by Cheng et al. (2022), they demonstrated the positive effects of dietary flavonoids on long-term memory, processing speed, and mood. Chronic dietary flavonoids supplementation with a low to moderate dosage had a stronger favorable impact in middle-aged to older adults [39]. In addition, a previous meta-analysis study suggested that either acute or chronic anthocyanin supplementation could maintain cognitive processing speed in older adults [40]. The systematic review by Ahles et al. (2021) comprehensively reported the improvement of cognitive outcomes, including memory, attention, psychomotor speed, and executive function after anthocyanins supplementation. The researchers suggested that the beneficial effect of anthocyanins on cognitive function might differ depending on other factors such as the domain of cognition, the forms of products (powder, juice, or extract), and the targeted populations [41]. Furthermore, polyphenol-rich treatments have been reported to enhance cognitive function in young and middle-aged adults [42]. Another meta-analysis demonstrated that taking more than 200 mg of resveratrol per day enhanced delayed recognition and mood in adults [43]. In accordance with previous systematic reviews and meta-analyses, our findings confirm that chronic supplementation with polyphenols may improve cognitive abilities in adults and the elderly.
Regarding inflammation, IL-6, an important inflammatory cytokine, could act as both a pro-inflammatory and anti-inflammatory substance depending on conditions. IL-6 is often used as a marker for the acute phase response after tissue damage or infection [44]. The higher levels of blood IL-6 have been associated with poorer cognitive performance in either acute or chronic inflammatory responses [45–47]. Additionally, the higher blood IL-6 concentrations have been associated with poorer global cognitive performance in non-dementia older people [48–50]. Our findings demonstrated a significant difference in the reduction of IL-6 after polyphenols supplementation compared to controls. Consistent with the previous meta-analysis, dietary anthocyanins significantly decreased the levels of blood inflammatory markers such as CRP, TNF-α, IL-6, intercellular adhesion molecule-1, and vascular adhesion molecule-1 [51]. On the other hand, another meta-analysis reported that polyphenol-rich interventions did not alter the level of CRP, TNF-α, and IL-6 inflammatory markers in older adults [52]. The contradictory findings might be explained by differences in the included criteria, including the types of polyphenols and the target population. Considering our findings from individual studies, three of ten RCTs (30%) reported a significant reduction of inflammatory markers, including L-6, TNF-α, IL-9, IL-10, MIP-1β, and IL-1β, following polyphenols treatment compared to controls [29, 32, 34]. Furthermore, the levels of inflammation appeared to be significantly reduced in specific population conditions, such as the elderly with overweight or obese subjects and MCI patients who might have low-grade or chronic inflammatory status.
The improvement of cognitive abilities by polyphenols could be underlined by various possible mechanistic pathways such as inhibition of inflammation, an increase in cerebral blood flow, enhancement of endothelial function, stimulation of neural synaptic plasticity, activation of brain areas related to cognitive abilities, and modulation of the gut-brain axis [53–56]. To elucidate, whether or if polyphenols protect against cognitive impairment in humans by inhibiting inflammatory processes. The quantitative findings found that the polyphenols could ameliorate verbal memory, and executive function as well as reduce blood IL-6, mainly in older participants. As a result, polyphenols’ anti-inflammatory properties may contribute to enhanced cognitive function in humans, particularly in those with low-grade inflammation. Consistent with two individuals’ studies, lowering blood inflammatory mediators (TNF-α, IL-1β, and IL-6) may contribute to improved global cognitive function and verbal memory after 26 weeks and 6 months following treatments with resveratrol and propolis extract [32, 34]. Remarkably, supplementing with propolis extract for extended periods of time (12 and 24 months) may prolong the benefits of anti-inflammation and overall cognitive enhancement compared to 6 months [34]. In addition, the potential of polyphenols on inflammation related to cognition seems to be noticeable in elderly participants with obesity and MCI. This suggests that the effect of polyphenols intervention on inflammation linked to cognitive function may be more prominent over a longer period and in older people with chronic inflammatory conditions rather than in healthy individuals. However, the effects of polyphenols on the interaction between inflammation and cognitive function are complicated and vary depending on other factors such as population conditions, inflammatory status, severity of cognitive impairment, domain of cognitive function, and type and duration of polyphenols supplementation. In this review, the number of available studies reporting the properties of polyphenols on inflammation and cognitive outcomes was limited, and most were conducted in older individuals. A number of human trials in specific subjects with ongoing chronic inflammatory status (e.g., dementia, Alzheimer’s disease, MCI, stroke, diabetes, obesity, cancer, and metabolic syndrome) are required in order to clarify the effect of polyphenols intervention on the interaction between cognitive function and inflammation. Furthermore, additional variables might influence the relationship between cognition and inflammation through polyphenols intervention, such as the modulation of the gut microbiota and the blood-brain barrier, which should be further explored.
A limitation of this review is that the number of available studies which assessed both inflammatory markers and cognitive function, as well as the number of sample sizes, was rather small. In addition, the eligible studies used various types and dosages of polyphenols supplementation as well as different population conditions. So, the retrieved data from available studies was insufficient to perform the subgroup analysis, in particular the age group, subject’s condition, type of polyphenols intervention, concentration, and duration. Therefore, substantial inter-trial heterogeneity was significantly detected in some parameters, including IL-6, TNF-α, working memory, executive function, and attention. These might confound the efficacy of the polyphenol’s interventions on the actual outcomes.
5Conclusion
This review provides comprehensive findings from the available evidence on the effects of polyphenol treatments on the outcomes of inflammation related to cognitive function in human randomized controlled trials. According to the findings, the reduction of IL-6 may be attributed to enhanced cognitive performance, particularly in verbal memory and executive function, following polyphenols interventions. Even though the other domains of cognition and inflammatory markers did not reach statistical significance, the findings revealed that polyphenols interventions tend to attenuate inflammatory markers as well as improve cognitive abilities. These findings shed light on the benefits of polyphenols in the link between inflammation and cognitive function, which could be promoted for the prevention of inflammatory-related cognitive dysfunction or neurodegenerativediseases.
Funding
The authors report no funding.
Acknowledgments
The authors are grateful to The Royal Thai Government for financing the Ph.D. scholarship of author Chusana Mekhora
Conflicts of interest
The authors declare no conflicts of interest.
References
[1] | Bradburn S , Sarginson J , Murgatroyd CA . Association of Peripheral Interleukin-6 with Global Cognitive Decline in Non-demented Adults: A Meta-Analysis of Prospective Studies. Frontiers in Aging Neuroscience. (2017) ;9: :438. |
[2] | Shen XN , Niu LD , Wang YJ , Cao XP , Liu Q , Tan L , et al. Inflammatory markers in Alzheimer’s disease and mild cognitive impairment: a meta-analysis and systematic review of 170 studies. The Journal of Neurology, Neurosurgery, and Psychiatry. (2019) ;90: (5):590–8. |
[3] | Anita NZ , Zebarth J , Chan B , Wu CY , Syed T , Shahrul D , et al. Inflammatory Markers in Type 2 Diabetes and Cognitive Impairment: A Systematic Review and Meta-Analysis. Biological Psychiatry. (2022) ;91: (9):S124–5. |
[4] | Liu X , Yu Y , Zhu S . Inflammatory markers in postoperative delirium (POD) and cognitive dysfunction (POCD): A meta-analysis of observational studies. PLoS One. (2018) ;13: (4):e0195659. |
[5] | Moreno-Navarrete JM , Blasco G , Puig J , Biarnés C , Rivero M , Gich J , et al. Neuroinflammation in obesity: circulating lipopolysaccharide-binding protein associates with brain structure and cognitive performance. International Journal of Obesity. (2017) ;41: (11):1627–35. |
[6] | Lyman M , Lloyd DG , Ji X , Vizcaychipi MP , Ma D . Neuroinflammation: The role and consequences. Neuroscience Research. (2014) ;79: :1–12. |
[7] | Huang LT , Zhang CP , Wang YB , Wang JH . Association of Peripheral Blood Cell Profile With Alzheimer’s Disease: A Meta-Analysis. Frontiers in Aging Neuroscience. (2022) ;14: :888946. |
[8] | Takata F , Nakagawa S , Matsumoto J , Dohgu S . Blood-Brain Barrier Dysfunction Amplifies the Development of Neuroinflammation: Understanding of Cellular Events in Brain Microvascular Endothelial Cells for Prevention and Treatment of BBB Dysfunction. Frontiers in Cellular Neuroscience. (2021) ;15: :661838. |
[9] | Dyer AH , McKenna L , Batten I , Jones K , Widdowson M , Dunne J , et al. Peripheral Inflammation and Cognitive Performance in Middle-Aged Adults With and Without Type 2 Diabetes: Results From the ENBIND Study. Frontiers in Aging Neuroscience. (2020) ;12: :605878. |
[10] | Sartori AC , Vance DE , Slater LZ , Crowe M . The impact of inflammation on cognitive function in older adults: implications for healthcare practice and research. Journal of Neuroscience Nursing. (2012) ;44: (4):206–17. |
[11] | Brydon L , Harrison NA , Walker C , Steptoe A , Critchley HD . Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biological Psychiatry. (2008) ;63: (11):1022–9. |
[12] | Reichenberg A , Yirmiya R , Schuld A , Kraus T , Haack M , Morag A , et al. Cytokine-associated emotional and cognitive disturbances in humans. Archives of General Psychiatry. (2001) ;58: (5):445–52. |
[13] | Grigoleit JS , Kullmann JS , Wolf OT , Hammes F , Wegner A , Jablonowski S , et al. Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS One. (2011) ;6: (12):e28330. |
[14] | Veronese N , Stubbs B , Maggi S , Thompson T , Schofield P , Mueller C , et al. Low-Dose Aspirin Use and Cognitive Function in Older Age: A Systematic Review and Meta-analysis. Journal of the American Geriatrics Society. (2017) ;65. |
[15] | Jaturapatporn D , Isaac M , McCleery J , Tabet N . Aspirin, steroidal and non-steroidal anti-inflammatory drugs for the treatment of Alzheimer’s disease. Cochrane database of systematic reviews (Online). (2012) ;2: :CD006378. |
[16] | Wang J , Tan L , Wang HF , Tan CC , Meng XF , Wang C , et al. Anti-inflammatory drugs and risk of Alzheimer’s disease: an updated systematic review and meta-analysis. Journal of Alzheimer’s Disease. (2015) ;44: (2):385–96. |
[17] | Spencer JP , Vafeiadou K , Williams RJ , Vauzour D . Neuroinflammation: modulation by flavonoids and mechanisms of action. Molecular Aspects of Medicine. (2012) ;33: (1):83–97. |
[18] | Joseph SV , Edirisinghe I , Burton-Freeman BM . Fruit Polyphenols: A Review of Anti-inflammatory Effects in Humans. Critical Reviews in Food Science and Nutrition. (2016) ;56: (3):419–44. |
[19] | Lamport DJ , Williams CM . Polyphenols and Cognition In Humans: An Overview of Current Evidence from Recent Systematic Reviews and Meta-Analyses. Brain Plasticity. (2021) ;6: (2):139–53. |
[20] | Macready AL , Kennedy OB , Ellis JA , Williams CM , Spencer JP , Butler LT . Flavonoids and cognitive function: a review of human randomized controlled trial studies and recommendations for future studies. Genes & Nutrition. (2009) ;4: (4):227–42. |
[21] | Pandey KB , Rizvi SI . Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. (2009) ;2: (5):270–8. |
[22] | Vauzour D , Rendeiro C , D’Amato A , Waffo-Téguo P , Richard T , Mérillon JM , et al. Anthocyanins Promote Learning through Modulation of Synaptic Plasticity Related Proteins in an Animal Model of Ageing. Antioxidants (Basel). (2021) ;10: (8). |
[23] | Matthew JP , Joanne EM , Patrick MB , Isabelle B , Tammy CH , Cynthia DM , et al. The PRISMA statement: an updated guideline for reporting systematic reviews. BMJ. (2021) ;372: :n71. |
[24] | Higgins JPT , Thomas J , Chandler J , Cumpston M , Li T , Page MJ , Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 Cochrane, (2023) . Available from: www.training.cochrane.org/handbook. |
[25] | Hozo SP , Djulbegovic B , Hozo I . Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology. (2005) ;5: (1):13. |
[26] | Cohen J . Statistical Power Analysis for the Behavioral Sciences. New York, NY: Routledge Academic. (1988) . |
[27] | Bowtell JL , Aboo-Bakkar Z , Conway ME , Adlam AR , Fulford J . Enhanced task-related brain activation and resting perfusion in healthy older adults after chronic blueberry supplementation. Applied Physiology, Nutrition, and Metabolism. (2017) ;42: (7):773–9. |
[28] | Kent K , Charlton K , Roodenrys S , Batterham M , Potter J , Traynor V , et al. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. European Journal of Nutrition. (2017) ;56: (1):333–41. |
[29] | Bøhn SK , Myhrstad MCW , Thoresen M , Erlund I , Vasstrand AK , Marciuch A , et al. Bilberry/red grape juice decreases plasma biomarkers of inflammation and tissue damage in aged men with subjective memory impairment – a randomized clinical trial. BMC Nutrition. (2021) ;7: (1):75. |
[30] | Igwe EO , Roodenrys S , Probst YC , do Rosario V , Netzel ME , Hong HT , et al. Low anthocyanin plum nectar does not impact cognition, blood pressure and gut microbiota in healthy older adults: A randomized crossover trial. Nutrion Ressearch. (2020) ;82: :74–87. |
[31] | Nilsson A , Salo I , Plaza M , Björck I . Effects of a mixed berry beverage on cognitive functions and cardiometabolic risk markers; A randomized cross-over study in healthy older adults. PLoS One. (2017) ;12: (11):e0188173. |
[32] | Witte AV , Kerti L , Margulies DS , Flöel A . Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. The Journal of Neuroscience. (2014) ;34: (23):7862–70. |
[33] | Huhn S , Beyer F , Zhang R , Lampe L , Grothe J , Kratzsch J , et al. Effects of resveratrol on memory performance, hippocampus connectivity and microstructure in older adults – A randomized controlled trial. NeuroImage. (2018) ;174: :177–90. |
[34] | Zhu A , Wu Z , Zhong X , Ni J , Li Y , Meng J , et al. Brazilian Green Propolis Prevents Cognitive Decline into Mild Cognitive Impairment in Elderly People Living at High Altitude. Journal of Alzheimer’s Disease. (2018) ;63: (2):551–60. |
[35] | Murray M , Dordevic AL , Cox K , Scholey A , Ryan L , Bonham MP . Twelve weeks’ treatment with a polyphenol-rich seaweed extract increased HDL cholesterol with no change in other biomarkers of chronic disease risk in overweight adults: A placebo-controlled randomized trial. The Journal of Nutritional Biochemistry. (2021) ;96: :108777. |
[36] | Velichkov M , Bezur Z , van Reekum CM , Williams CM . A biphasic response to blueberry supplementation on depressive symptoms in emerging adults: a double-blind randomized controlled trial. European Journal of Nutrition. (2024) ;63: :1071–88. |
[37] | Sachdev P , Blacker D , Blazer D , Ganguli M , Jeste D , Paulsen J , et al. Classifying neurocognitive disorders: The DSM-5 approach. Nature Reviews Neurology. (2014) ;10: :634–42. |
[38] | Murman DL . The Impact of Age on Cognition. Seminars in Hearing. (2015) ;36: (3):111–21. |
[39] | Cheng N , Bell L , Lamport DJ , Williams CM . Dietary Flavonoids and Human Cognition: A Meta-Analysis. Molecular Nutrition & Food Resesrch. (2022) ;66: (21):e2100976. |
[40] | Feng RC , Dong YH , Hong XL , Su Y , Wu XV . Effects of anthocyanin-rich supplementation on cognition of the cognitively healthy middle-aged and older adults: a systematic review and meta-analysis of randomized controlled trials. Nutrition Reviews. (2023) ;81: (3):287–303. |
[41] | Ahles S , Joris PJ , Plat J . Effects of Berry Anthocyanins on Cognitive Performance, Vascular Function and Cardiometabolic Risk Markers: A Systematic Review of Randomized Placebo-Controlled Intervention Studies in Humans. International Journal of Molecular Sciences. (2021) ;22: (12). |
[42] | Ammar A , Trabelsi K , Boukhris O , Bouaziz B , Müller P , J MG , et al. Effects of Polyphenol-Rich Interventions on Cognition and Brain Health in Healthy Young and Middle-Aged Adults: Systematic Review and Meta-Analysis. Journal of Clinical Medicine. (2020) ;9: (5). |
[43] | Marx W , Kelly JT , Marshall S , Cutajar J , Annois B , Pipingas A , et al. Effect of resveratrol supplementation on cognitive performance and mood in adults: a systematic literature review and meta-analysis of randomized controlled trials. Nutrition Reviews. (2018) ;76: (6):432–43. |
[44] | Tanaka T , Narazaki M , Kishimoto T . IL-6 in inflammation, immunity, and disease. Cold Spring Harbor Perspectives in Biology. (2014) ;6: (10):a016295. |
[45] | Wright CB , Sacco RL , Rundek T , Delman J , Rabbani L , Elkind M . Interleukin-6 is associated with cognitive function: the Northern Manhattan Study. Journal of Stroke & Cerebrovascular Diseases. (2006) ;15: (1):34–8. |
[46] | Harrison NA , Doeller CF , Voon V , Burgess N , Critchley HD . Peripheral inflammation acutely impairs human spatial memory via actions on medial temporal lobe glucose metabolism. Biological Psychiatry. (2014) ;76: (7):585–93. |
[47] | Reichenberg A , Yirmiya R , Schuld A , Kraus T , Haack M , Morag A , et al. Cytokine-associated emotional and cognitive disturbances in humans. Archives of General Psychiatry. (2001) ;58: (5):445–52. |
[48] | Yaffe K , Lindquist K , Penninx BW , Simonsick EM , Pahor M , Kritchevsky S , et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. (2003) ;61: (1):76–80. |
[49] | Bradburn S , Sarginson J , Murgatroyd CA . Association of Peripheral Interleukin-6 with Global Cognitive Decline in Non-demented Adults: A Meta-Analysis of Prospective Studies. Frontiers in Aging Neuroscience. (2017) ;9: :438. |
[50] | Capogna E , Watne LO , Sørensen Ø , Guichelaar CJ , Idland AV , Halaas NB , et al. Associations of neuroinflammatory IL-6 and IL-8 with brain atrophy, memory decline, and core AD biomarkers – in cognitively unimpaired older adults. Brain, Behavior, and Immunity. (2023) ;113: :56–65. |
[51] | Fallah AA , Sarmast E , Fatehi P , Jafari T . Impact of dietary anthocyanins on systemic and vascular inflammation: Systematic review and meta-analysis on randomised clinical trials. Food and Chemical Toxicology. (2020) ;135: :110922. |
[52] | Ammar A , Trabelsi K , Müller P , Bouaziz B , Boukhris O , Glenn JM , et al. The Effect of (Poly)phenol-Rich Interventions on Cognitive Functions and Neuroprotective Measures in Healthy Aging Adults: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. (2020) ;9: (3). |
[53] | Spencer JP , Vauzour D , Rendeiro C . Flavonoids and cognition: the molecular mechanisms underlying their behavioural effects. Archives of Biochemistry and Biophysics. (2009) ;492: (1-2):1–9. |
[54] | Lamport DJ , Williams CM . Polyphenols and Cognition In Humans: An Overview of Current Evidence from Recent Systematic Reviews and Meta-Analyses. Brain Plasticity. (2021) ;6: (2):139–53. |
[55] | Song Z , Zhang X , Hong M , Wu Z , Luo S , Cheng K . Oolong tea polyphenols affect the inflammatory response to improve cognitive function by regulating gut microbiota. Journal of Functional Foods. (2023) ;105: :105584. |
[56] | Láng L , McArthur S , Lazar AS , Pourtau L , Gaudout D , Pontifex MG , et al. Dietary (Poly)phenols and the Gut–Brain Axis in Ageing. Nutrients. (2024) ;16: (10). |



