Grouper bone nano-calcium and substitution long-chain triglyceride (LCT) into medium-chain triglyceride (MCT) enhanced calcium bioavailability and rat’s bone density
Abstract
BACKGROUND:
The most effective strategy to prevent osteopenia or osteoporosis in the old life is to consume an adequate amount of calcium from childhood through adulthood.
OBJECTIVE:
This study compared the bioavailability of CaCO3 which is the standard non-dairy calcium supplementation with grouper bone nano-calcium (GBN) combined with various percentages of Long-Chain Triglyceride (LCT) into Medium-Chain Triglyceride (MCT) on calcium bioavailability and rat’s bones density.
METHODS:
This study was carried out during the rat’s growth period, from the weaning until the rats reached 16 weeks. Thirty-five weaned rats were separated into seven groups and fed varied feeds for 12 weeks. The seven groups of feed were (1) CCa: standard feed AIN-93G, (2) C0: standard calcium deficient feed (without calcium), (3) G0: GBN + MCT:LCT 0 : 100%, (4) G25: GBN + MCT:LCT 25 : 75%, (5) G50: GBN + MCT:LCT 50 : 50%, (6) G75: GBN + MCT:LCT 75 : 25%, and (7) G100: GBN + MCT:LCT 100 : 0%. Parameters observed were serum levels of calcium, phosphorus, and alkaline phosphatase, femur bone characteristics, bone microarchitecture by histomorphometry, micro computed-tomography (μCT), and mechanical strength.
RESULTS:
The CCa and G100 groups had the best dietary results based on all parameters. The G100 group was superior to CCa in calcium and phosphorus bioavailability, rat’s bone strength and density.
CONCLUSIONS:
G100: GBN + MCT:LCT 100 : 0% group feed beneficially affected the bioavailability of calcium, was letting he rat’s bones to develop properly, had high density, and been strong throughout the growth phase.
Abbreviations
ALP | Alkaline Phosphatase |
BMD | Bone Mineral Density |
BV | Bone Volume |
GBN | Grouper Bone Nano-calcium |
GI | Grayscale Index |
LCT | Long-Chain Triglyceride |
MCT | Medium-Chain Triglyceride |
Tb.Th | Trabecular Thickness |
Tb.N | Trabecular Number |
Tb.Sp | Trabecular Separation |
TV | Tissue Volume |
VOI | Volume of Interest |
1Introduction
Osteoporosis is a degenerative illness that primari-ly impacts the elderly. In 2050, Indonesia’s elderly population will account for one-third of the country’s total population, making the osteoporosis risk rather alarming [1]. Osteoporosis is a micro-architecture of bone loss caused by a lack of calcium intake throughout growth period, resulting in a lack of bone mass density in adulthood. [2] reports that in 1994, WHO classified normal bone as having a T-score more than –1, osteopenia bone as having a T-score between –1 and –2.5, and osteoporotic bone as having a T-score less than –2.5. Inadequate calcium intake during the growth period leads to a continual process of bone calcium remodelling in the years leading up to old age, which causes the bones to become porous and brittle in old life [3]. According to [4], 95% of skeletal development happens between infancy and maturity. Therefore, the best strategy to avoid osteoporosis is to consume enough calcium throughout infancy and adolescence.
Not all calcium consumed can be absorbed by the body optimally because the bioavailability of calcium from each calcium source is different. The finest sources of calcium in the diet are dairy foods, including milk and dairy products.
Synthetic calcium carbonate (CaCO3) is a source of calcium in the mineral mixes in international standard feed AIN-93 [5]. Calcium carbonate is commonly manufactured in significant volumes from the exoskeletons of crustaceans and mollusk for use as a dietary additive, such as in animal feed and human consumption as a calcium supplement. [6, 7] demonstrated in vitro and in vivo calcium solubility and bioavailability, respectively, that calcium derived from natural sources, specifically fish bones, was assimilated more efficiently than synthetic calcium carbonate. According to [8. 9], calcium must be made nano-sized so that it is more readi-ly absorbed by intestinal epithelial cells in order to increase its bioavailability. Several nutrient components, including medium-chain triglyceride (MCT), calcium-binding peptides, and lactose, have been shown to enhance calcium absorption [10–12].
The fat source in international standard rat feed (AIN-93) is soybean oil [5] whose main content is long-chain triglyceride (LCT), triglycerides whose glycerol molecules have ester bonds with long-chain fatty acids, min. 12 C chains or more. There is medium-chain triglyceride (MCT) oil, where each glycerol molecule has an ester bond with a medium chain fatty acid, 6–10 C chains [13]. MCT is made by hydrolyzing coconut oil or palm kernel oil, purifying and esterifying it. Compared to long-chain triglyceride (LCT), the size of MCT is smaller so it is easily absorbed through a passive diffusion system along the digestive tract and then enters the blood circulation, attaching to albumin [14].
[15] were able to successfully extract grouper bone nano-calcium (GBN) from grouper bone debris with 47.45 nm size, which still includes diverse natural fatty acids and amino acids and had a calcium solubility of 26.14% based on an in vitro gastrointestinal simulation solubility test. This study aims to determine the effect of administering grouper bone nano-calcium (GBN) at various percentages substitute of long-chain triglyceride (LCT) into medium-chain triglyceride (MCT) on calcium bioavailability and rat’s bone density and strength.
2Materials and methods
2.1Materials
This study utilized a calcium-deficient AIN-93G-MX mineral mix (Dyets, Pennsylvania, USA) with two types of calcium: (1) food grade synthetic calcium carbonate (CaCO3) (Shuren Kechuang Food Additive Co., Lianyungang, Jiangsu, China) purchased from an online marketplace for the CCa feed group and (2) GBN for the G0-G100 feed groups. Table 1 provides a comparison of the nutrients from the two calcium sources. MCT (MCT-Max, CV. Segala, Bandung, Indonesia), LCT (Mazola Soya Bean Oil, PT. Sukadjaya Djaya, Bekasi, Indonesia), and other necessary materials for the rat feed manufacturer were acquired from the local market. Calcium, phosphorus, and alkaline phosphatase levels in rat serum were analyzed using Diasys Diagnostic System kit (Holzheim, Germany).
Table 1
The comparison of the nutritional value of two calcium sources
| Nutrients (%) | Grouper Bone Nano-calcium (GBN) (For G0-G100 groups) | Synthetic Calcium Carbonate (CaCO3) (For CCaGroup)_ |
| Moisture (%) | 1.73±0.53 | 0.4±0.2 |
| Ash (%) | 87.73±0.04 | 99.6±0.2 |
| Protein (%) | 0.63±0.04 | nd |
| Fat (%) | 0.63±0.04 | nd |
| Calcium (%) | 30.73±0.32 | 45.19±0.57 |
| Phosphorous (%) | 18.37±0.32 | 0.0195±0.00 |
| Ca/P mole rasio | 1.29 | 1790.9 |
2.2Grouper bone nano-calcium production
The grouper bone nano-calcium production method followed [15]. The dried grouper (Epinephelus sp.) fish bone was autoclaved with 3×3 hour cycle then dried in a drying cabinet (50°) for 24 hours. The fish bone was crushed in a mortar, grounded, and sifted with a 80 mesh sieve. The coarse grouper bone was soaked in 1 N 1 : 5 HCl (Mallinckrodt, 7647-01-0) (w/v) and agitated with a magnetic stirrer for 1 hour and incubated in room temperature for 24 hours.
HCl was separated and removed via centrifugation at 3000 rpm, the particle was transferred into a glass beaker and diluted at a 1 : 5 (w/v) ratio with a 1 N NaOH solution (Merck, 1310-73-2). The sediment was subsequently hydrolyzed three times for 60 minutes at 100°C using a hotplate stirrer. After each cycle of hydrolysis, the supernatant was removed from the residue via centrifugation.
After mixing the hydrolysed sediment into the distilled water, the solution was neutralized using HCl 1 N (Mallinckrodt, 7647-01-0) until a pH range of 6.9–7.1 was attained. Following that, it was recentrifuged. The sediment was then transferred into a ceramic tray and desiccated in a cabinet dryer at 50°C for 15 to 18 hours. Following a one-minute refining period in a laboratory disc mill (Kawasaki T-100, Kobe, Japan), the desiccated sediment was sifted through a 203-mesh sieve (Haver & Boecker 59302 OELDE, Germany) and the resulting particle size was approximately 47.47 nm.
2.3Experimental animals and feed preparation
The Ethical Clearance Commission of the Integrated Research and Testing Institute, Universitas Gadjah Mada, has approved all animal experimentation procedures in this research (Certificate No. 00059/04/LPPT/II/2022). The experiment used 35 healthy female Sprague Dawley rats weighing between 40 and 65 g at the weaning age (3 weeks). In this study, female rats were utilized in consideration of the findings of [1], which indicate that the incidence of osteoporosis in elderly women is four times greater than that in elderly men.
All rats were housed in separate cages in a room temperature of 27°C with a 12 hours light-dark cycle. The feed standard corresponds to the composition of the AIN-93G feed [5] for rats before they reach maturity, and all feeds were composed of iso calcium, refers to the calcium content of each calcium source and iso fat. The feeds were (1) CCa group was an AIN-93G standard feed with CaCO3 as the calcium source. (2) C0 group was an AIN-93G standard feed with calcium-deficient mineral mix; (3) G0 group used GBN as the calcium source with MCT:LCT 0 : 100% substitution, (4) G25 group used GBN as the calcium source with MCT:LCT 25 : 75% substitution, (5) G50 group used GBN as the calcium source with MCT:LCT 50 : 50% substitution, (6) G75 group used GBN as the calcium source with MCT:LCT 75 : 25% substitution, (7) G100 group used GBN as the calcium source with MCT:LCT 100 : 0% substitution.
The result from previous research in Table 1 shows that GBN has 30.73% calcium, whereas CaCO3 contains 45.19% calcium. The AIN-93G feed formulation, which is an international standard, should contain 40.04% calcium in the form of CaCO3. This calcium content is specifically present in 35 g of mineral mix. To provide similar calcium levels in both forms of calcium, an iso-calcium method was applied. The weight conversion was determined such that it equated to 35 g of mineral mixture. The CCa feed required approximately 11.025 g of CaCO3 and 23.975 g of mineral mix lacking of calcium. Conversely, G0-G100 required 15.365 g of GBN and 19.639 g of calcium-free mineral mix. The content of each feed may be observed in Table 2.
Table 2
Composition of rat feed in various diets (in grams)
| Composition (g) | CCa | C0 | G0 MCT:LCT 0% :100% | G25 MCT:LCT 25% :75% | G50 MCT:LCT 50% :50% | G75 MCT:LCT 75% :25% | G100 MCT:LCT 100% :0% |
| Corn Starch | 397.486 | 397.486 | 397.486 | 397.486 | 397.486 | 397.486 | 397.486 |
| PotasiumCaseinat (≥85% protein) | 200 | 200 | 200 | 200 | 200 | 200 | 200 |
| Dextrinized corn starch (90–94% tetrasaccharides) | 132 | 132 | 132 | 132 | 132 | 132 | 132 |
| Sucrose | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Soy Bean Oil | 70 | 70 | 70 | 52.5 | 35 | 17.5 | – |
| MCT | – | – | – | 17.5 | 35 | 52.5 | 70 |
| Fiber | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| CaCO3 | 11.025 | – | – | – | – | – | – |
| GBN | – | – | 15.365 | 15.365 | 15.365 | 15.365 | 15.365 |
| Ca-deficient Mineral Mix | 23.975 | 35 | 19.639 | 19.639 | 19.639 | 19.639 | 19.639 |
| Vitamin mix | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| L-Sistein | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Choline Bitartrate | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Total | 999.986 | 999.986 | 999.986 | 999.986 | 999.986 | 999.986 | 999.986 |
Various Feeds:CCa: standard diet AIN-93G; C0: standard calcium deficient feed (without calcium); G0: GBN + MCT 0%; G25: GBN + MCT 25%; G50: GBN + MCT 50%; G75: GBN + MCT 75%; G100: GBN + MCT 100%.
In order to simplify the process of preparing the rat’s feed, the feed was manufactured in quantities of 999,986 grammes, which was then rounded up to 1 kilogramme and its multiples, based on the rat’s feed needs. Each morning, the rats were given an amount of food that was equal to 10% of their body weight (The calcium requirement for rats is 0.110 g CaCO3 for CCa and 0.153 g GBN for G0-G100 in 10 g of feed according to the AIN-93G standard). The feeds were stored in a refrigerator at 4°C before use. Distilled water was provided ad libitum.
2.4Acclimatization phase and maintenance phase in In vivo experiment
The acclimatization period was carried out for 7 days. Every rat was given AIN-93G feed. After the acclimatization phase, 35 rats were randomly separated into seven feed groups and housed in labelled individual cages.
On the eighth day, the customized feed for each feed group was administered. Every four weeks, the rat was anesthetized using ketamine-xylazine (60 mg/kg BW) to obtained blood sample from the orbital sinus using microhematocrit. The rat’s blood was centrifuged for 15 minutes at 10,000 rpm to get rat serum. Rat serum was used to measure the calcium (Calcium AS FS), phosphorus (Phosphat FS), and alkaline phosphatase (ALP IFOC FS) using kits from Diasys Diagnostic System (Holzheim, Germany). The results of the serum and kit reactions will be calibrated using aspectrophotometer.
After 12 weeks, all rats were weighed, blood samples were collected, and they were sacrificed with ketamine-xylaizine (100 mg/kg BW). Rat femur and tibiae bones were collected and then cleansed of any remaining flesh. The tibiae bones were submerged for 24 hours in 10% PBS formalin to make histological preparations for histomorphometric tests followed [16] and [17] methods. The rat femur bone was cleaned and then dried for two hours in a cabinet dryer (50°C). The femurs of rats were weighed, measured for length and diameter, and then divided into two groups. The left femur group was used to test the bone’s mechanical strength using a universal testing machine (UTM) (Zwick Z0.5, Ulm, Germany) with three-bending test followed [6] and [18] methods. The right femurs were used for bone density testing used a Micro-Computed Tomography (μCT) (Phoenix V|tome|x s240, Waygate Technologies, Wunstorf, Germany) followed [19] methods. Figure 1 depicts the flowchart for this in vivo research.
Fig. 1
In vivo experimental flowchart.
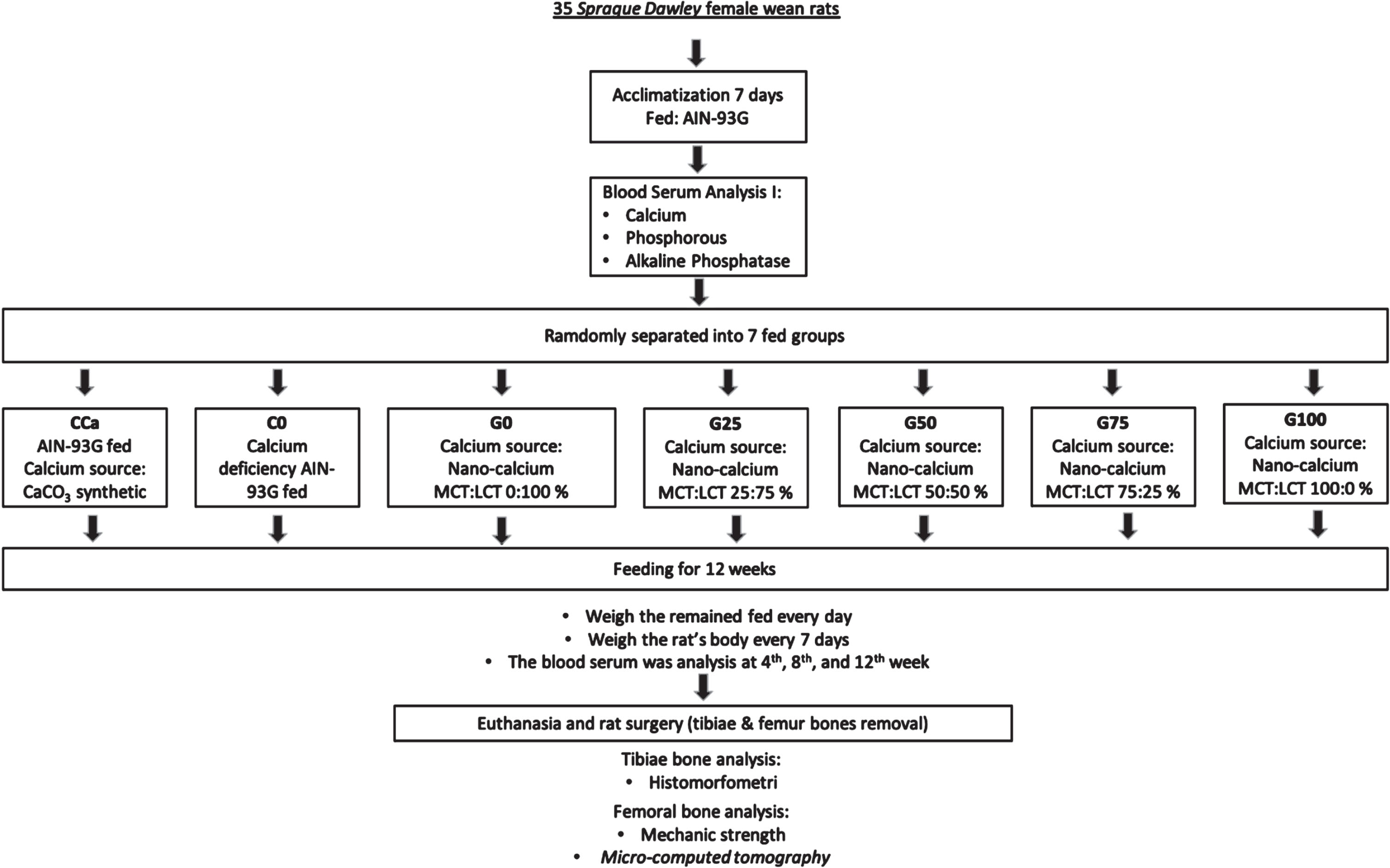
2.5Statistical analysis
All data (excuding μCT data) were analyzed using a one-factor Completely Randomized Design (CRD), which consisted of 7 groups in feed composition, each with 5 replicates. The data were evaluated for diversity (ANOVA), and if the findings indicated a significant difference, the Duncan Multiple Range Test was performed (DMRT). If the two best values were not substantially different from one another, the two data were re-tested using the Independent T-Test differentiating test. The data were processed using the SPSS application (IBM SPSS 26 version, IL, USA) according to [20].
3Results
3.1In vivo nutrient bioavailability
3.1.1Calcium level of rat’s serum
The aim of the calcium test in rat serum was to determine how much GBN was effectively absorbed during the digestion of feed. Calcium concentrations in rat serum in response to different feed are shown in Fig. 2. According to Fig. 2, serum calcium levels at the beginning of maintenance for all feed groups were almost similar, ranging between 10.2–11 mg/dL. At 4th week, blood calcium levels decreased in all feeds, particularly the C0 therapy. At week 8, serum calcium levels were reasonably steady between 9.3 and 10 mg/dL, while the C0 therapy had shown 25% reduction. At week 8, the serum calcium concentrations in the G100 and CCa groups were almost identical. In the 12th week, the G0–G75 therapy group repeatedly reduced blood calcium levels by 5–10%, but the C0 feed group demonstrated a serum calcium reduction of almost 30%. The C0 feed group which provided calcium-deficient feed for 12 weeks, the blood calcium level continued to decline, indicating the remodelling of calcium in the bones, which might cause the bones shrink and brittle. It will be evaluated in the rat femur bone’sstrength test.
Fig. 2
Calcium levels in rat serum every 4 weeks for 12 weeks of maintenance. Various feed formulas: CCa: standard diet AIN-93G; C0: standard calcium deficient feed (without calcium); G0: GBN + MCT:LCT 0:100%; G25: GBN + MCT:LCT 25:75%; G50: GBN + MCT 50:50%; G75: GBN + MCT:LCT 75:25%; G100: GBN + MCT:LCT 100:0%.
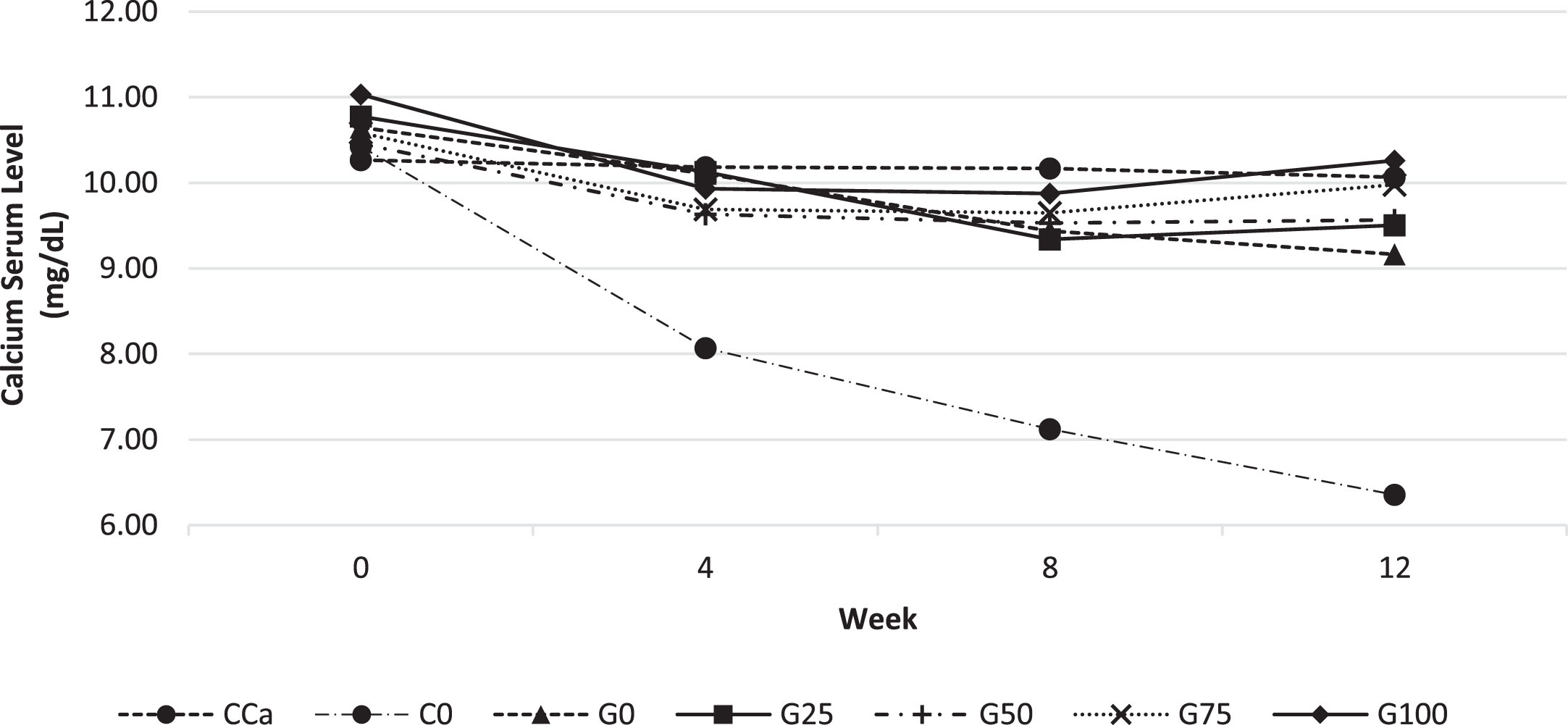
The CCa feed group was able to maintain constant blood calcium levels of around 10 mg/dL for a period of 12 weeks. The G100 feed group had the best GBN feed compared to the other GBN groups, which, despite a fall in blood calcium levels at week 8, continued to rise until 12th week.
3.1.2Phosphorous level of rat’s serum
The pattern of phosphorus levels in rat serum in Fig. 3 was almost identical to that of calcium level in Fig. 2; all feed groups began with serum phosphorus levels of around 7 mg/dL. The phosphorus level serum decreased in all calcium CaCO3 and GBN feed groups, notably in the C0 group at 4 and 8 weeks. Even though the adjusted feed in group C0 had a calcium deficiency and did not diminish the demand for phosphorus in the feed, it nonetheless affected the phosphorus level serum of rats, which declined with time. This suggests that calcium deficiency impacts phosphorus bioavailability. The blood phosphorus levels of rats were similarly impacted by the calcium-deficient feed, as shown by [21] study.
Fig. 3
Phosphorous levels in rat serum every 4 weeks for 12 weeks of maintenance. Various feed formulas: CCa: standard diet AIN-93G; C0: standard calcium deficient feed (without calcium); G0: GBN + MCT:LCT 0:100%; G25: GBN + MCT:LCT 25:75%; G50: GBN + MCT 50:50%; G75: GBN + MCT:LCT 75:25%; G100: GBN + MCT:LCT 100:0%.
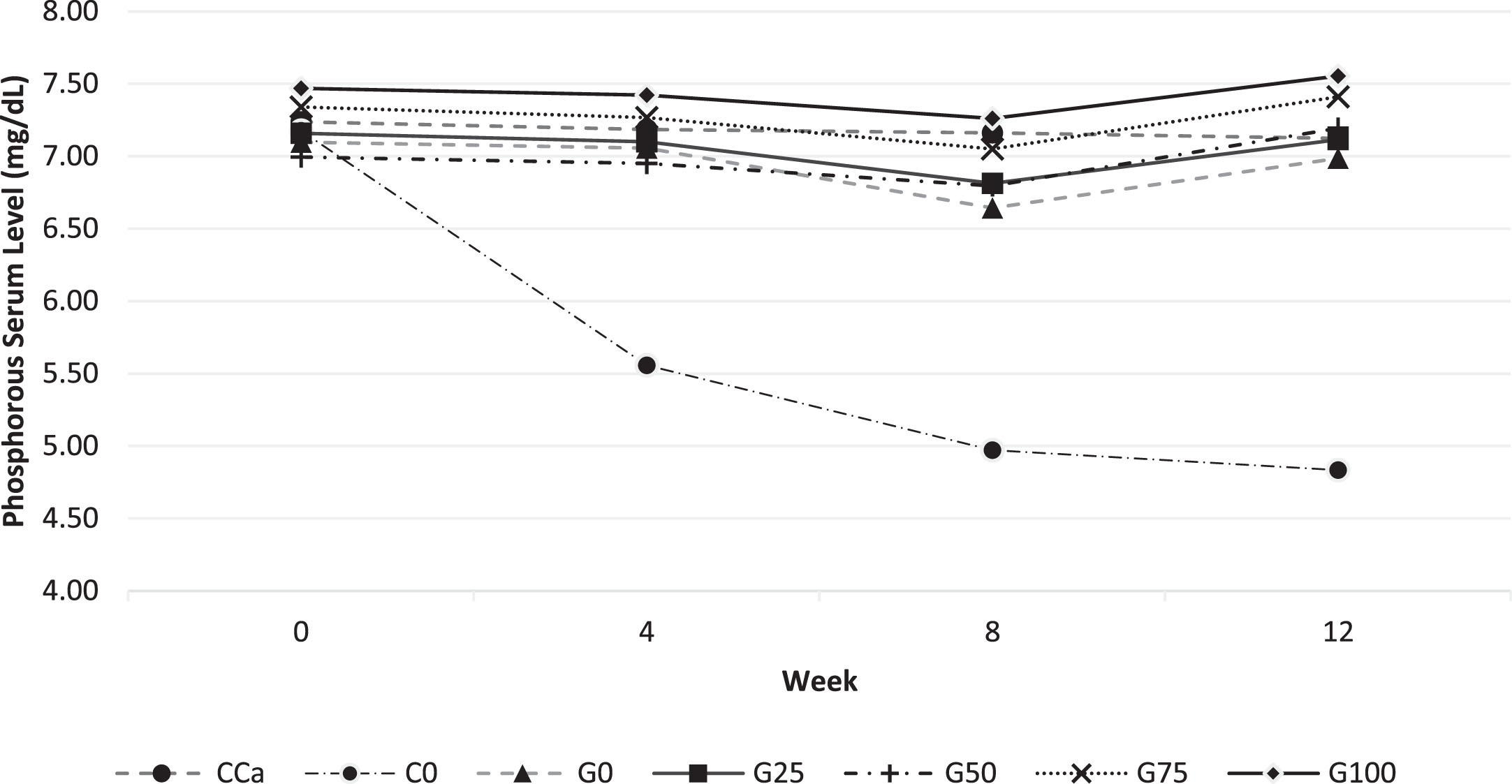
At the end of the maintenance period (after 12 weeks), all phosphorus levels in the calcium source of CaCO3 and GBN feed were comparable to their baseline values. This did not happen in the C0 feed group, where phosphorus levels kept descending until they were 32.6% lower at the end of the maintenance period than they were at the beginning. In addition to phosphorus from the mineral mix, feed G0–G100 also includes natural phosphorus from GBN so that phosphorus level serum can be kept consistently high for the 12 weeks of maintenance.
According to [22], sufficient calcium consumption without phosphorus intake will lead to phosphorus insufficiency inside the body. G0–G100 feed groups also get phosphorus through mineral mix, and GBN contains natural phosphorus, allowing normal blood phosphorus levels to be maintained for 12 weeks.
3.1.3Alkaline phosphatase level of rat’s serum
Alkaline Phosphatase/ALP is an enzyme that is extensively expressed in cells of mineralized tissue and plays a crucial role in the development of hard tissues. Intestinal, placenta, liver, and bone are the four primary sites of ALP in the human body [23]. ALP serum level is a particular indicator of bone activity. A rise in ALP suggests that the parathyroid gland is responsible for maintaining calcium levels in the blood when calcium levels is below normal which trigger the remodelling of bone calcium. Bone ALP is physiologically bound to the membrane of osteoblast cells, and only a little quantity is discharged into the serum. Increasing ALP serum level indicating an increase in bone resorption [24–26].
ALP level in rat serum in response to different feed are shown in Fig. 4. The C0 feed group had considerably greater blood ALP levels than those other feeds. C0 ALP serum level was the highest and substantially distinct from those of other feed groups. It suggests that only the C0 feed group, which began in the 4th week, suffered bone remodelling. Bone remodelling is the release of calcium from bones in response to low blood calcium levels; hence, calcium stores in the bones must be replenished to fulfil the body’s calcium requirements in order to maintain homeostasis [27]. This condition will lead to osteopenia or even osteoporosis if it is not promptly treated with an adequate calcium intake.
Fig. 4
Alkaline Phosphatase levels in rat serum every 4 weeks for 12 weeks. Various feed formulas: CCa: standard diet AIN-93G; C0: standard calcium deficient feed (without calcium); G0: GBN + MCT:LCT 0:100%; G25: GBN + MCT:LCT 25:75%; G50: GBN + MCT 50:50%; G75: GBN + MCT:LCT 75:25%; G100: GBN + MCT:LCT 100:0%.
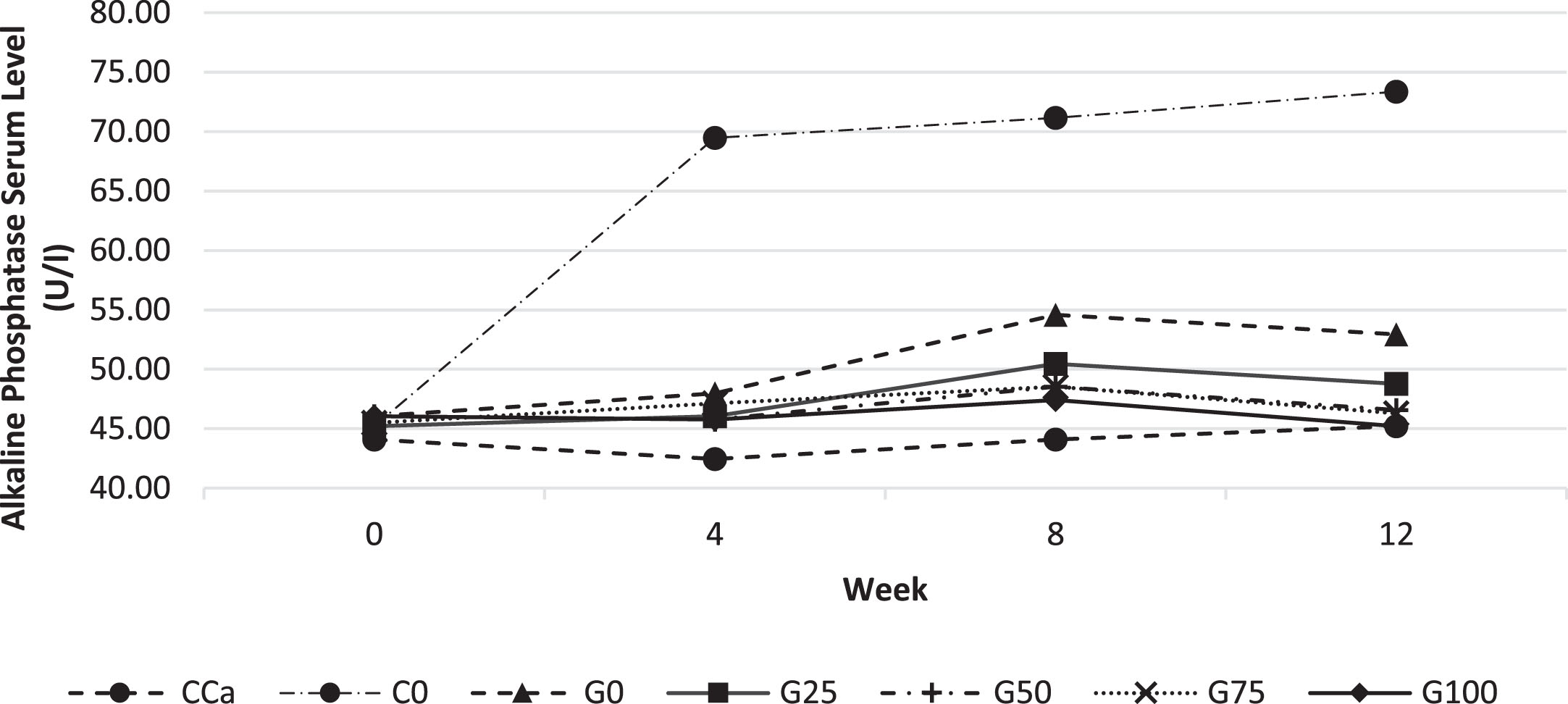
3.2Physical parameter and mechanical strength of rat’s femur bone
3.2.1Femur length
Based on Table 3, the G0–G100 feed group did not show significantly different results. Femur length indicates the rate of bone growth which is influenced by adequate calcium intake in the rat’s feed.
Table 3
Physical parameter and mechanical strength of rat’s femur bone
| Diet Group | Femur Physical Parameters | Mechanical Strength/Ultimate Strength (N) | ||
| Length (mm) | Thickness (mm) | Weight (mg) | ||
| CCa | 29.68±2.36c | 2.618±0.17 | 257.82±71.51c | 73.50±1.14de |
| C0 | 24.25±1.97a | 2.442±0.14 | 143.48±24.64a | 43.54±4.67a |
| G0 | 26.59±2.49ab | 2.474±0.14 | 162.1±21.65ab | 46.73±8.84ab |
| G25 | 27.26±1.16bc | 2.468±0.17 | 170.86±17.75ab | 52.38±6.54bc |
| G50 | 27.71±1.87bc | 2.468±0.13 | 171.62±19.59ab | 62.36±16.02bcd |
| G75 | 27.74±1.45bc | 2.438±0.06 | 212.22±37.31bc | 66.15±16.03cde |
| G100 | 29.17±1.89bc | 2.634±0.2 | 264.86±62.7c | 82.39±4.59e |
The mean ± standard deviation (n = 5). Using one-way ANOVA and Duncan’s test, superscript letters in the same column denoted differences of significance (p < 0.05). Various feed formulas:CCa: standard diet AIN-93G; C0: standard calcium deficient feed (without calcium); G0: GBN + MCT:LCT 0 : 100%; G25: GBN + MCT:LCT 25 : 75%; G50: GBN + MCT 50 : 50%; G75: GBN + MCT:LCT 75 : 25%; G100: GBN + MCT:LCT 100 : 0%.
Adequate intake of calcium and other balanced nutrients will ensure optimal bone growth as evidenced by an increase in optimal bone length. The femur size of the G25–G100 rat group was not statistically significantly different from the femur size of the CCa group. It showed that MCT played role in the bioavailability of GBN.
3.2.2Femur thickness
The thickness of the femur was measured at the center of the bone using a digital calliper. The measurement results table shows that the thickness of the seven femurs in the feed group was not significantly different. A thicker bone diameter will make the bone stronger and less easily broken, compared to thinner bones. [21] was using different calcium to grow up 16 weeks old rats, the rat femur had a diameter of 2.31–2.78 mm, a wider range compared to this study, 2.43–2.63 mm.
3.2.3Femur weight
Based on the Duncan test on femur bone weight, it was found that the G100 feed group was not significantly different from the G75 and CCa feed groups. The C0, G0, G25, and G50 feed groups appeared to have lighter femurs and were not significantly different compared to all groups, this indicates that calcium deficiency feed or the lower percentage of MCT substitution for LCT (0–50%) reduces bone formation ability. The GBN calcium feed, namely G75–G100, showed an increase in the percentage of MCT substitution for LCT along with an increase in rat’s bone weight. Elevated levels of MCT have been suggested to result in enhanced calcium bioavailability within the body, thereby positively influencing the bone matrix mass of rat femurs.
3.2.4Mechanical strength of rat’s femur bone
Bone mechanical tests were carried out to determine the level of femur bone strength of rats from the feed treatment group carried out in this study. Of the seven feed groups, the CCa and G100 feed groups did not show any significant differences. The results of femur bone mechanical strength testing were in line with the calcium levels in Fig. 2 which shows that CCa, G100 and G75 contain higher calcium than the other feed groups. Better calcium bioavailability would support better bone calcification, thicker bone layers, and increased bone mechanical strengths.
[28] using tuna bone meal as a source of calcium in In vivo tests on rats obtained higher mechanical strength of rat femurs (84.8–89.7 N) compared to this study. This is due to the age of the mice euthanized in the study by [28] was 18 weeks, in contrast to this study which used rats aged 16 weeks. The type of feed and age of the mice greatly influence the level of strength of the mice’s femur bones.
3.3Rat’s tibiae histomorphometry
Bone volume fraction in the 2D field is defined as the area of bone (Bone Volume) relative to the overall area of the ROI (Tissue Volume) [17], which is displayed as a percentage (%) in Table 4. The greater the bone volume percentage, the thicker or broader the microstructure of the cross-section of the bone.
Table 4
Histomorphometry result of rat’s tibia bone
| Diet Group | Histomorphometry Parameters | |
| Bone Volume Fraction (BV/TV)% | Trabecular Thickness (mm) | |
| CCa | 27.85±6.48c | 0.1083±0.034b |
| C0 | 21.14±1.71a | 0.0939±0.041ab |
| G0 | 22.53±9.97ab | 0.0828±0.024a |
| G25 | 23.43±2.96ab | 0.0954±0.024ab |
| G50 | 25.72±5.33bc | 0.0925±0.06ab |
| G75 | 27.97±5.22c | 0.0967±0.041ab |
| G100 | 28.27±5.22c | 0.1087±0.035b |
The mean ± standard deviation (n = 5). Using one-way ANOVA and Duncan’s test, superscript letters in the same column denoted differences of significance (p < 0.05). Various feed formulas:CCa: standard diet AIN-93G; C0: standard calcium deficient feed (without calcium); G0: GBN + MCT:LCT 0 : 100%; G25: GBN + MCT:LCT 25 : 75%; G50: GBN + MCT 50 : 50%; G75: GBN + MCT:LCT 75 : 25%; G100: GBN + MCT:LCT 100 : 0%.
The bone volume fraction revealed that the G100 feed group had the greatest bone volume fraction (28.27%), which did not vary from the CCa and G75 feed groups. The microstructure of the large bone reflects the bone’s strength, which is also proportional to bone density. [6] reported 12–16% for tibiae bone volume percentage which were lower than in this study. This was probably because the rats they used were younger than the ones used in this study. In addition, the rats used in their research were post-lactating rats, which bones experienced remodelling and a decrease in bone volume fraction during lactation.
Trabeculae are microstructures of cancellous/spongy bone shaped like spicules. The trabeculae serve as a structural support that provides substantial strength without substantially increasing the bone’s mass. If the periosteum covers the surface of the compact bone, the endosteum covers the trabecular surface of the cancellous/spongy bone [27]. Trabecular thickness is the average thickness of the trabeculae in 2D or 3D, measured in mm or μm [19]. The trabeculae thickness in this study as shown in Table 4.
The trabeculae thickness in the G100 group was not substantially different from the CCa and G75 feed group, similar to the bone volume percentage. The trabecular thickness in this study was higher than the tibial trabecular thickness in [6] research.
Histomorphometric trabecular thickness was calculated using tibial histology photo files which were opened using Image Rasher software. The trabecular thickness was determined by drawing 5 lines at random and measuring their length (in μm units) to determine the trabecular thickness. This calculation was subjective because the starting point of the trabecular line was selected at randomly, as was the positioning of the line drawing, making it hard to standardise. To assure its validity, the histomorphometric measurement of trabecular thickness should be confirmed by other techniques, such as the μCT method.
3.4Micro-computed tomography (μCT) analysis of rat femur bones
The samples tested using μCT were 3 rat femur samples from 3 selected feed groups: CCa, C0, and G100. The CCa sample represents the AIN-93G feed group which utilises a synthetic CaCO3 calcium source, the C0 sample represents the calcium deficiency feed group, and G100 was the best treatment and was significantly different from the other four GBN + MCT:LCT feed groups based on the Duncan Test (against the five G0–G100 feed groups), especially on calcium, phosphorus and ALP levels, as well as on measurements of femur weight and strength.
The aim of this test is to validate the microstructure of the bone tissue tested using the μCT instrument. The appearance of the femur bone sample using μCT can be observed in Fig. 5. The VOI or volume studied in this study was the area of the proximal epiphysis up to the epiphyseal plate as the boundary of the spongy bone, which is 8.5 mm high from the proximal epiphysis.
Fig. 5
3D structure of femur bone sample reconstructed image from µCT (a) CCa, (b) C0, dan (c) G100.
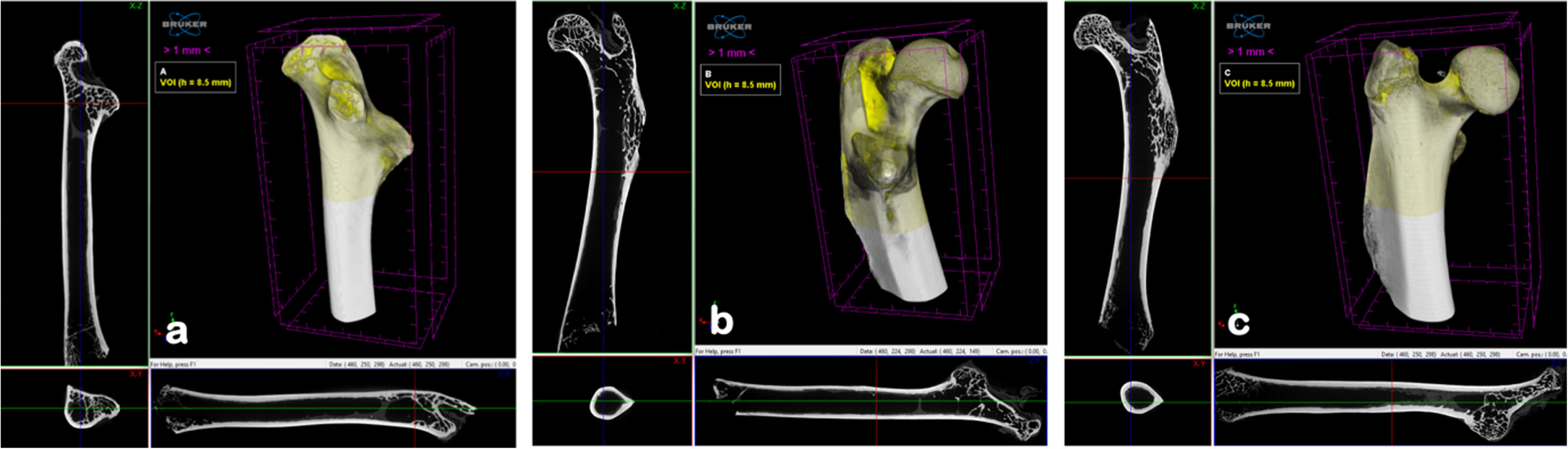
Trabeculae in the femur are most commonly found in the spongy bone, because this is where the most bone growth occurs during the growth period. In old age, indicators of bone loss are also easiest to detect in the spongy bone. The μCT data analysis process was carried out by trans-section scanning/cross-section of the bone using the SkyScanTM CT-analyzer software program. The pixel size resolution was 14.25μm, resulting in 597 layers with a height of 8.5 mm.
3.4.1Percent object volume
According to [29], percent object volume can be equated with percent bone volume or bone volume fraction (BV/TV)%. This parameter measures the area of the bone and the microstructure within it detected via μCT compared to a previously defined volume of interest (VOI) area.
Based on Table 5, the G100 sample has the largest percent object volume even though it is almost the same as the CCa sample. Similar results were also confirmed in the bone volume fraction in Table 4, that the G100 group also had results that were not significantly different from the CCa group. The higher the percent object volume indicates the denser the bone microstructure. A dense microstructure indicates high bone density and good bone strength. This was proven in Table 4 which showed that the strength of G100 bone was not significantly different from that of CCa bone.
Table 5
Micro-computed tomography result of rat’s femur bone
| Parameters | Diet Groups | |||
| No. | CCa | C0 | G100 | |
| 1 | Percent Object Volume (%) | 52.044 | 39.531 | 52.739 |
| 2 | Structure Thickness (mm) | 0.28 | 0.18 | 0.24 |
| 3 | Structure Linear Density (1/mm) | 1.87 | 2.16 | 2.19 |
| 4 | Structure Separation (mm) | 0.66 | 0.59 | 0.53 |
| 5 | VOI Density (in Grayscale Index) | 140.97 | 137.72 | 135.86 |
| 6 | Whole Femur Density (in Grayscale Index) | 145.76 | 144.03 | 159.77 |
Various Diets: CCa: standard diet AIN-93G; C0: standard calcium deficient feed (without calcium); G100: GBN + MCT:LCT 100 : 0%.
3.4.2Structure thickness
According to [29], structure thickness can be equated with trabecular thickness (Tb.Th). While histomorphometry measures a single average bone Tb.Th value from a 2D cross-section of trabecular bone, in μCT, the 3D trabecular bone volume is calculated from the distribution of trabecular thickness.
Based on Table 5, it showed that the CCa femur has the thickest structural thickness compared to the other 2 femurs. This indicated that the CCa femur had more robust microstructure (trabeculae) than the other two femur. While the results of trabecular thickness measurements in Table 4 showed that CCa thickness was not significantly different from G100, the results are different from these structural thickness measurements.
The three femurs have structural thickness values that are thicker compared to the results of trabecular thickness measurements in 2D tibial histomorphometry measurements in Table 4. This proved that 3D structural thickness measurements could be very different from 2D trabecular thickness measurements. Analysis based on the histomorphometric method is often reconfirmed using the μCT method [17].
3.5Structure linear density
Struture linear density or also known as trabecular number (Tb.N) is the number of trabeculars per unit length [19]. The number of trabeculae is the inverse of the average distance from the central axis of the structure [30]. The higher the number of linear density structures implies the greater the number of trabeculae in the VOI, thus the spongy bone microstructure will be stronger. Based on Table 5, it was known that the linear structure density of the G100 femur sample is the highest compared to other samples.
CCa femur had fewer spongy/trabecular structures but were thicker than the other 2 samples. Meanwhile, the G100 femur had greater number of spongi/trabecular but the structure was thinner. Meanwhile, the C0 femur, although it has a thinner spongy/trabecula structure thickness than the CCa femur, apparently has a greater number of spongi/trabecula structures than the CCa femur.
3.6Structure separation
Structural separation can be defined as trabecular separation (Tb.Sp), which is the area of the cavity between the trabeculae measured in 3D [19]. The Tb.Sp value is calculated using the same method as Tb.Th but the cavity part is considered as a non-bone part, so it can be said that Tp.Sp is the thickness of the cavity in the bone [30].
Based on Table 5, it was known that the structure separation was the smallest in the G100 femur and the highest in the CCa sample. Based on calculations of structure separation, structure linear density, and structure thickness, it can be seen that CCa has the thickest trabecula thickness compared to the other two femurs, but it turned out that the number of trabeculae was the smallest, resulting in the largest cavity compared to the other two femurs. Even the G100 femur had slimmer trabeculae than CCa, but it had greater number of trabeculae and resulting in the lowest structure separation to the other two femurs.
3.6.1Bone density (in grayscale index)
Bone Mineral Density (BMD) measurements require a calibrator in the form of pure hydroxyapatite at various concentrations. If calibrator is not available, density measurements can be represented using the grayscale index (GI) method, where 0 is black and 255 is light gray. [31] have successfully validated the use of the grayscale index to measure bone mineralization density distribution in rat femur bones.
The measurement of the grayscale index on each sample was carried out twice, (1) on the volume of interest (8.5 mm) from the proximal and (2) on the entire femur sample. The VOI density was highest in the CCa sample compared to other samples. However, the highest whole femur density was in the G100 sample.
Based on the bone density calculation on the VOI, the proximal epiphysis area up to the epiphyseal plate as the spongy bone boundary at 8.5 mm. Based on Table 5, it was known that the density of the CCa femur is the highest compared to the other two femurs. However, after measuring the density of whole femur, it was discovered that the G100 femur had the highest density. Thus, it was suspected that the highest density of the G100 femur is in the diaphysis/shaft of the femur.
3.7Discussions
The objective of the four-weekly calcium serum examination in rats is to evaluate the bioavailability of calcium derived from different feed groups. The AIN-93G feed was able to maintain stable calcium levels for 12 weeks, whereas the G0-G100 nano-calcium grouper demonstrated that the greater the percentage of MCT, the greater the bioavailability of calcium in rat serum. Similarly, serum phosphorus levels in rats exhibited a similar trend to serum calcium levels. In contrast, alkaline phosphatase (ALP) levels exhibited an inverse relationship with calcium and phosphorus. ALP is extensively secreted by osteoblast cells during mineralization and calcification of bone tissue [27, 32].
The results of in vivo research showed that among 5 GBN feeds with various percentages of MCT substitution for LCT. The independent T-Test statistical test was then carried out on CCa and G100 to test the differences between the two, whether they were purely not significantly different or whether they were actually significantly different between the two. The results of the T-Test test showed that G100 feed was significantly different from CCa feed in testing bioavailability of calcium, bone strength/ultimate strength and bone volume fraction (BV/TV) % by histomorphometry. Thus, calcium sourced from GBN plus MCT:LCT 100 : 0% was superior to synthetic calcium carbonate (CaCO3) in calcium and phosphorus bioavailability, bone strength/ultimate strength and bone volume fraction (BV/TV) % by histomorphometry.
The VOI section examination of bone density did not reveal the true state of a bone density. According to [18] if the length of the bone is considered 100%, the transect position of the femur in the distal part is at positions 15, 17, and 20% of the length of the femur, midway at 40, 42.5, and 45%, and proximal at 64, 66.5, and 69% of the length of the femur.
[6] research revealed that calcium carbonate (CaCO3) and tuna bonepowder, as calcium supplements, increased the bone mineral density (BMD) of the mother rat. On the contrary, bone histomorphometry demonstrated that the maternal bone microstructure was beneficially impacted by tuna bone. This was evidenced by enhanced bone formation, reduced bone resorption, and increased bone volume.
The advantage of the GBN calcium source, apart from its nano size, GBN also contains various amino acids [15] which support the absorption of fish bone nano-calcium to pass through the transcellular pathway for active absorption.
The findings of [33] study indicated that Protein Bonito Hydrolysate (PBH-Ca) enhanced rat growth, increased the apparent calcium absorption and quantity of bone trabeculae, stabilised serum calcium and phosphorus levels, also inhibited ALP activity, reduced bone trabeculae separation, accelerated rat bone growth, and elevated BV/TV and femoral BMD. PBH-Ca has considerable potential as an ingredient in a calcium-rich feed.
The mechanism of MCT’s role in calcium absorption is still debated. [34] assume that MCT basically does not directly increase calcium absorption, but MCT intake improves fat absorption in the intestinal tract. This is thought to increase Vitamin D absorption, where increasing Vitamin D absorption will increase calcium absorption.
According to [27], the bone mineralization process begins with osteoblast cells secreting type I collagen, several glycoproteins and proteoglycans. Some of these factors, especially osteocalcin and certain glycoproteins, bind Ca2 + with high affinity, increasing local concentrations of calcium ions. Osteoblasts also release very small membrane-enclosed matrix vesicles containing alkaline phosphatase and other enzymes. These enzymes hydrolyze PO4 - ions from various matrix macromolecules, creating high concentrations of these ions locally. High concentrations of Ca2 + and PO4 - ions cause calcified nanocrystals to form in and around the matrix vesicles. The crystals grow and mineralize further with the formation of small masses of calcium hydroxyapatite [Ca10(PO4)6(OH)2], which surround the collagen fiber and all other macromolecules. Eventually the hydroxyapatite masses coalesce as a solid, fused bone matrix when matrix calcificationis complete.
The presence of natural protein in calcium supplements is very important to help osteoblast cells convert calcium into hydroxyapatite crystals. The mineralization process aims to thicken the trabeculae or cortical bone or to replace calcium lost due to osteoclast cell activity if calcium breakdown has previously occurred.
3.8Conclusion
Administration of grouper bone nano-calcium with 100% MCT substitution for LCT has been shown to increase calcium bioavailability and bone density in rats. It beneficially affected the bioavailability of calcium, was letting the rat’s bones to develop properly, had high density, and been strong throughout the growth phase.
Acknowledgments
The authors would like to thank Sugiyono, DVM, M.Sc., from the Department of Pathology, Faculty of Veterinary Medicine, UGM, and Dr. Fourier Dzar Eljabbar Latief, from the Physics Department, Science Faculty, ITB, for their willingness to discuss and assist with the discussion of this work.
Author’s contributions
All authors have contributed to the study. PK collected and analyzed the data as well as authored the article. PT, SA, and YP; prepared the core idea, control, and analyzed the article’s core ideas. YP as corresponding author. Every author discussed the results and contributed to the final manuscript.
Confict of interest
The authors state that there were no commercial or financial links that could be interpreted as a potential conflict of interest throughout the study’s conduct.
Funding Information
This work was supported by a Doctoral Dissertation Research grant from the Indonesian Government’s National Competitive Program-DIKTI in 2022 (grant number: 1939/UN1/DITLIT//PT.01.03/2022).
References
[1] | Anonymous, IOF Region osteoporosis meeting in Hongkong. Individual Country Reports: Indonesia. Hongkong, 2013. |
[2] | Wilson DJ . Osteoporosis and sport. Eur J Radiol. (2019) ;110: (November 2018):169–74. doi: 10.1016/j.ejrad.2018.11.010. |
[3] | Qin D , Zhang H , Zhang H , Sun T , Zhao H , Lee WH . Anti-osteoporosis effects of osteoking via reducing reactive oxygen species. J Ethnopharmacol. (2019) ;244: (112045):1–11. doi: 10.1016/j.je2019.112045. |
[4] | Hodges JK , Cao S , Cladis DP , Weaver CM . Lactose intolerance and bone health: The challenge of ensuring adequate calcium intake. Nutrients. (2019) ;11: (4):1–17. doi: 10.3390/nu11040718. |
[5] | Reeves PG . Components of the AIN-93 diets as improvements in the AIN-76A diet. in Symposium: Animal diets for nutritional and toxicological research, (1997) , vol. 127: , pp. 838–841. |
[6] | Suntornsaratoon P , Charoenphandhu N , Krishnamra N Fortified tuna bone powder supplementation increases bone mineral density of lactating rats and their offspring. J Sci Food Agric. (2017) ;98: (5):2027–34. doi: 10.1002/jsfa.8688. |
[7] | Benjakul S , Mad-Ali S , Sookchoo P . Characteristics of biocalcium powders from pre-cooked tongol (Thunnus tonggol) and yellowfin (Thunnus albacores) tuna bones. Food Biophys. (2017) ;12: (4):412–21. doi: 10.1007/s11483-017-9497-0. |
[8] | Suptijah P , Jacoeb AM , Deviyanti, N . Karakterisasi dan bioavailabilitas nanokalsium cangkang udang Vannamei (Litopenaeus vannamei). J Akuatika. (2012) ;III: (1):63–73. |
[9] | Prinaldi WV , Suptijah P , Uju . Karakteristik sifat fisikokimia nano-kalsium ekstrak tulang ikan tuna sirip kuning (Thunnus albacares). J Pengolah Has Perikan Indones. (2018) ;21: (3):385–95. |
[10] | Wongdee K , Rodrat M , Teerapornpuntakit J , Krishnamra N , Charoenphandhu N . Factors inhibiting intestinal calcium absorption: hormones and luminal factors that prevent excessive calcium uptake. J Physiol Sci. (2019) ;69: (5):683–96. doi: 10.1007/s12576-019-00688-3. |
[11] | Peng Z , Hou H , Zhang K , Li B . Effect of calcium-binding peptide from Pacific cod (Gadus macrocephalus) bone on calcium bioavailability in rats. Food Chem. (2017) ;221: :373–8. doi: 10.1016/j.foodchem.2016.10.078. |
[12] | Li Y , Zhang H , Yang L , Zhang L , Wang T . Effect of medium-chain triglycerides on growth performance, nutrient digestibility, plasma metabolites and antioxidant capacity in weanling pigs. Anim Nutr. (2015) ;1: (1):12–8. doi: 10.1016/j.aninu.2015.02.001. |
[13] | Kinsella R , Maher T , Clegg ME . Coconut oil has less satiating properties than medium chain triglyceride oil. Physiol Behav. (2017) ;179: (February):422–26. doi: 10.1016/j.physbeh.2017.07.007. |
[14] | Shah ND , Limketkai BN . The use of medium-chain triglycerides in gastrointestinal disorders. Pract Gastroenterol. (2017) ;41: (2);20–8. |
[15] | Kusumawati P , Triwitono P , Anggrahini S , Pranoto Y . Autoclaving and alkaline hydrolysis effects on the particle size and solubility of grouper (Epinephelus sp.) nano-calcium powder in in vitro gastrointestinal tract simulation. J Ilm Perikan dan Kelaut. (2022) ;14: (2):176–202. doi: 10.20473/jipk.v14i2.36261. |
[16] | Egan KP , Brennan TA , Pignolo RJ . Bone histomorphometry using free and commonly available software. Histopathology. (2012) ;61: (6):1168–73. doi: 10.1111/j.1365-2559.2012.04333.x. |
[17] | Nenda MM , Lewicki M , Mandalunis PM . Histomorphometry of the tibia and mandible of healthy female wistar rats at different stages of growth. Exp Anim. (2016) ;65: (2):109–16. 10.1538/expanim.15-0069 |
[18] | Prodinger PM , et al. Whole bone testing in small animals: Systematic characterization of the mechanical properties of different rodent bones available for rat fracture models. Eur J Med Res. (2018) ;23: (1);231–11. doi: 10.1186/s40001-018-0307-z. |
[19] | Bouxsein ML , Boyd SK , Christiansen BA , Guldberg RE , Jepsen KJ , Müller R . Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. (2010) ;25: (7):1468–86. doi: 10.1002/jbmr.141. |
[20] | Harsojuwono BA , Arnata IW , Puspawati GAKD . Rancangan percobaan teori, aplikasi SPSS dan Excel, no. March. 2011. |
[21] | Ekantari N , Harmayani E , Pranoto Y , Marsono Y . Calcium of Spirulina platensis has higher bioavailability than those of calcium carbonate and high-calcium milk in sprague dawley rats fed with vitamin D-deficient diet. Pakistan J Nutr. (2017) ;16: (3):179–86. doi: 10.3923/pjn.2017.179.186. |
[22] | Heaney RP , Nordin BEC . Calcium effects on phosphorus absorption: Implications for the prevention and co-therapy of osteoporosis. J Am Coll Nutr. (2002) ;21: (3):239–44. doi: 10.1080/07315724.2002.10719216. |
[23] | Vimalraj, S . Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene. (2020) ;754: :1–8. doi: 10.1016/j.gene.2020.144855. |
[24] | Hlaing TT , Compston JE . Biochemical markers of bone turnover – uses and limitations. Ann Clin Biochem. (2014) ;51: (2):189–202. doi: 10.1177/0004563213515190. |
[25] | Prihastuti CC , Ratnasari W , Hernayanti . The effect of potato (Solanum tuberosum L.) skin extract on alkaline phosphatase level in periodontitis. in IOP Conference Series: Earth and Environmental Science, (2019) , vol. 255: , no. 1, pp. 1–8, doi: 10.1088/1755-1315/255/1/012027. |
[26] | Tariq S , Tariq S , Lone KP , Khaliq S . Alkaline phosphatase is a predictor of bone mineral density in postmenopausal females. Pakistan J Med Sci. (2019) ;35: (3):749–53. doi: 10.12669/pjms.35.3.188. |
[27] | Mescher AL . Junqueira’s basic histology, Fourteenth. Indiana USA: McGraw-Hill Education, 2016. |
[28] | Jung W-K , Lee B-J , Kim S-K . Fish-bone peptide increases calcium solubility and bioavailability in ovariectomised rats. Br J Nutr (2006) ;95: (1):124–8. doi: 10.1079/bjn20051615. |
[29] | Bruker-micro CT . Morphometric parameters measured by Skyscan™ CT – analyser software. Ref. Man., pp. 1–49, 2012. |
[30] | Kim HJ , Park KH , Kim DH , Chae HJ , Sung GH , Kim YO . In vitro assessments of bone microcomputed tomography in an aged male rat model supplemented with Panax ginseng . Saudi J Biol Sci. (2018) ;25: (6):1135–9. doi: 10.1016/j.sjbs.2018.04.006. |
[31] | Tu SJ , Wang SP , Cheng FC , Chen, YJ . Extraction of gray-scale intensity distributions from micro computed tomography imaging for femoral cortical bone differentiation between low-magnesium and normal diets in a laboratory mouse model. Sci Rep. (2019) ;9: (1):1–11. doi: 10.1038/s41598-019-44610-8. |
[32] | Golub EE , Boesze-Battaglia K . The role of alkaline phosphatase in osteogenesis. J Exp. Med. (2007) ;18: :444–8. doi: 10.1084/jem.93.5.415. |
[33] | Ji W , Chen M , Ji H . The calcium supplementation effect of calcium-binding oligopeptides from bonito (Auxis thazard) hydrolysate in rats. Food Sci Technol. (2022) ;42: (e101621):1–7. doi: 10.1590/fst.101621. |
[34] | Griessen M , et al. Comparison of the effect of medium-chain and long-chain triacylglycerols on calcium absorption in healthy subjects. Am J Clin Nutr. (1999) ;69: (6):1237–42. doi: 10.1093/ajcn/69.6.1237. |




