Effect of Momordica cochinchinensis extract on locomotor function and brain antioxidant enzyme activity in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated zebrafish Parkinson’s disease model
Abstract
BACKGROUND:
Gac fruit (Momordica cochinchinensis) belongs to the Cucurbitaceae family. The red aril of Gac fruit contains high concentrations of carotenoids, including lycopene and beta-carotene.
OBJECTIVE:
This study aimed to investigate the effect of Gac fruit aril extract on locomotor activities in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced zebrafish model and measure antioxidant enzyme activities in the zebrafish brain.
METHODS:
This study used adult male zebrafish (Danio rerio) as an animal model. MPTP was used as a toxin to induce movement dysfunction in zebrafish, while the standard drug selegiline acted as a monoamine oxidase-B inhibitor. Locomotion was recorded on day 7 after MPTP induction using a digital video tracking system, and parameters related to zebrafish swimming, including total distance, velocity, and immobility, were observed. The brain tissue of the zebrafish was collected for antioxidant enzyme activity analysis.
RESULTS:
The results showed that Gac fruit extract at doses of 200 and 400 mg/kg improved locomotor functions in MPTP-induced Parkinsonism in zebrafish. However, antioxidant enzyme activities, such as catalase and superoxide dismutase activities, in the zebrafish brain showed no significant differences among all groups.
CONCLUSIONS:
These findings provide insights into the further research of Gac fruit extract as a nutraceutical for preventing Parkinson’s disease.
1Introduction
Parkinson’s disease, the second most common neurodegenerative disease, is an age-related motor disorder. The pathology of Parkinson’s presents as a decrease in dopamine levels in the synaptic cleft, possibly due to the degeneration of dopaminergic neurons. The mechanism of action of anti-Parkinson’s drugs, such as dopamine agonists, dopamine precursors, and dopamine degradation enzyme inhibitors, emphasizes increasing the level of dopamine or ameliorating dopaminergic neuron damage and neurotoxicity.
Gac fruit (Momordica cochinchinensis) belongs to the Cucurbitaceae family and is a perennial melon grown throughout Southeast Asian countries and northeastern Australia [1]. Gac fruit has long been used as food and in traditional medicine. Gac seeds have traditionally been used in China to treat inflammation, swelling, and diarrhea. In Bangladesh, all fractions of Gac fruit (i.e., fruits, seeds, leaves, and roots) are used to treat various diseases, such as cancer, diabetes, liver diseases, itches, rheumatoid arthritis, colic, and purify the blood [2]. Gac fruit contains several phytochemical components, such as beta-carotene, lycopene, and essential fatty acids. The red aril of Gac fruit contains high concentrations of carotenoids, including lycopene and beta-carotene. The beta-carotene and lycopene content in the Gac aril is five- and eight-fold greater than the levels measured in carrots and tomatoes, respectively [3, 4]. Moreover, bioactive compounds, such as alpha-tocopherol (vitamin E), phenolic compounds, and flavonoids, are also found in the Gac fruit extract. However, no studies have evaluated the effects of these extracts on Parkinson’s disease in zebrafish. Therefore, we aimed to investigate the effect of Gac fruit aril extract on locomotor activities in a 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine (MPTP)-induced zebrafish model and measure antioxidant enzyme activities in the zebrafish brain.
2Materials and methods
2.1Animals and drug administration
Thirty male adult zebrafish (Danio rerio) measuring 3–5 cm and weighing 500 mg were cultured in an acrylic tank with reverse osmosis water and oxygen by keeping the water in the tank for 24 h before use. The density was maintained at 5 fish per 7.5 liters [5].
Water temperature and pH were maintained at 28±2°C and 6.5–7.5, respectively, and the fish were reared in a 14/10-h light/dark cycle. Fish were fed with artemia twice daily (morning-evening). Before beginning the experiment, the fish were kept in holding tanks for seven days. Zebrafish were divided into six groups (n = 5).
Group I: Control (fed with water)
Group II: MPTP (induced by 100 mg/kg of MPTP and fed with water)
Group III: MPTP + Selegiline (induced by MPTP and fed with 1.5 mg/kg of selegiline)
Group IV: MPTP + GAC 200 (induced by MPTP and fed with 200 mg/kg of GAC)
Group V: MPTP + GAC 400 (induced by MPTP and fed with 400 mg/kg of GAC)
Group VI: MPTP + GAC 800 (induced by MPTP and fed with 800 mg/kg of GAC)
Zebrafish were received substances by oral gavage, place the fish on a wet towel and fed using a plastic catheter attached to a micropipette. The fish were induced motor dysfunction with MPTP (100 mg/kg body weight). This study used selegiline (1.5 mg/kg), a monoamine oxidase-B inhibitor, as a positive control. The animals received selegiline or Gac fruit extract once daily until the end of the behavioral test.
Animal experiments were performed according to the guidelines of the Institute of Animals for Scientific Purposes Development Chemicals (IAD). Animal ethics no. UP-AE64-01-04-021 was approved by the Laboratory Animal Research Center at the University of Phayao, Thailand.
2.2Preparation of extracts
The whole Gac fruit was scooped out, and the red aril surrounding the seeds was completely separated. Red aril (0.5 kg) was blended in distilled water (2.5 L) at a ratio of 1:5 in a laboratory blender. The resulting juice was filtered twice and freeze-dried. The operational conditions were a condenser temperature of –20°C and a pressure of 250 Pa for 48 h. Powder samples of freeze-dried aril were packed separately in aluminum foil and kept at –20°C until use.
2.3The polyphenolic compound determination in Gac fruit extract
The polyphenolic compound of Gac fruit extract has been analyzed using HPLC coupled to diode-array detection and tandem mass spectrometry with electrospray ionization followed the Gavrilova et al., 2011 [6] method. This analysis performed by Central Laboratory (Thailand) Co., Ltd.
2.4Behavioral tests
Locomotor activity tests were performed using a digital video tracking system to observe zebrafish swimming. The zebrafish were allowed to swim for 1 min before testing, and their behavior was observed for 10 min [7]. The total distance, velocity, and immobility time were recorded on day 7 after MPTP induction; the experimental plan of the behavioral tests is shown in Fig. 1.
Fig. 1
Experimental plan of the behavioral tests in zebrafish.
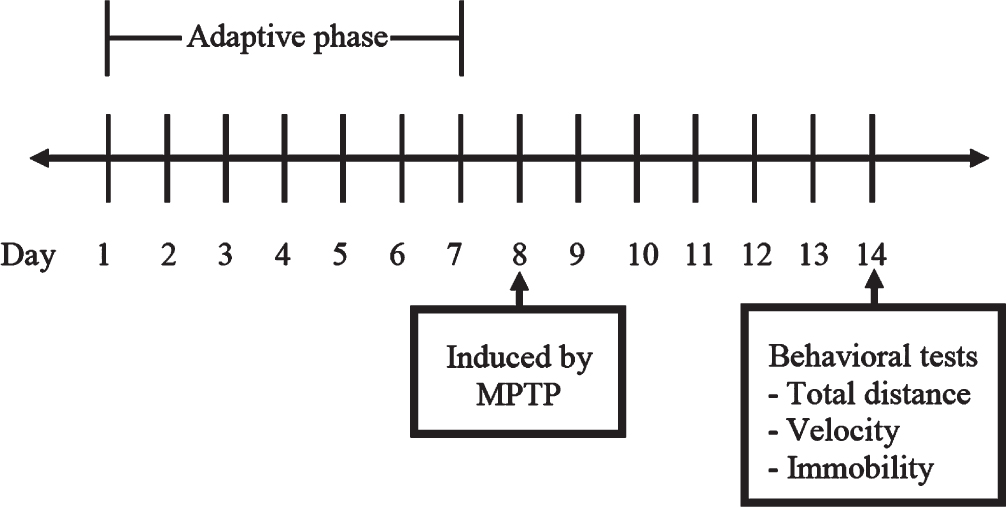
In this experiment, the total distance (cm) and velocity (cm/s) of zebrafish movement within 10 min were analyzed using the open-source Fiji ImageJ software. Zebrafish were considered immobile when velocity was less than or equal to 3 mm/sec [8].
After finishing the behavioral test, zebrafish brains were collected and stored at –80°C. Frozen brain tissues were homogenized using a hand homogenizer in 0.1 M potassium phosphate buffer (pH 8.0). The homogenate was centrifuged at 3000×g for 10 min at 4°C to obtain the supernatant for enzyme activity analysis.
2.5Catalase activity in the zebrafish brain
Catalase activity was assayed following the method described by Grilo et al. [9], with modifications. Briefly, the reaction mixture contained 20μL of zebrafish brain supernatant, 180μL of 50 mM phosphate buffer (pH 7.8), and 100μL of 90 mM H2O2 solution in a 96-well plate. Next, 100μL of H2O2 was added, and absorbance was measured at 340 nm using a multimode microplate reader (Biotek SynergyTM H1). Enzyme activity was expressed as units/mL/mg of tissue.
2.6Superoxide dismutase (SOD) activity in the zebrafish brain
SOD activity was measured following the method of Gazsi et al. [10], with modifications. In brief, the 10 mL reaction mixture contained 2.5 mL of 216 mM phosphate buffer (pH 8.0), 0.1 mL of 10.7 mM EDTA, 0.1 mL of 1.1 mM cytochrome C, 5.0 mL of 0.108 mM xanthine, and 2.3 mL of distilled water. Standards for SOD were prepared at concentrations of 0, 0.005, 0.01, 0.02, 0.03, 0.04, and 0.05 U/mL.
The assay was performed with 200μL of the reaction mix, 10μL of sample or SOD standard, and 20μL of diluted xanthine oxidase enzyme at 0.05 U/ml (on ice). Absorbance was measured at 450 nm using a multimode microplate reader (Biotek SynergyTM H1), and the standard curve of the SOD enzyme was plotted. SOD activity was calculated from the linear equation of the standard curve and is expressed as units/mL/mg of tissue.
2.7Statistical analysis
All results are presented as the mean±SEM. Statistical comparisons between control and treatment groups were performed using one-way analysis of variance (ANOVA), followed by post-hoc Tukey’s test. All statistical analysis were performed using SigmaPlot 12.0 software. The results were considered statistically significant at p < 0.05.
3Results
3.1The polyphenolic compound in Gac fruit extract
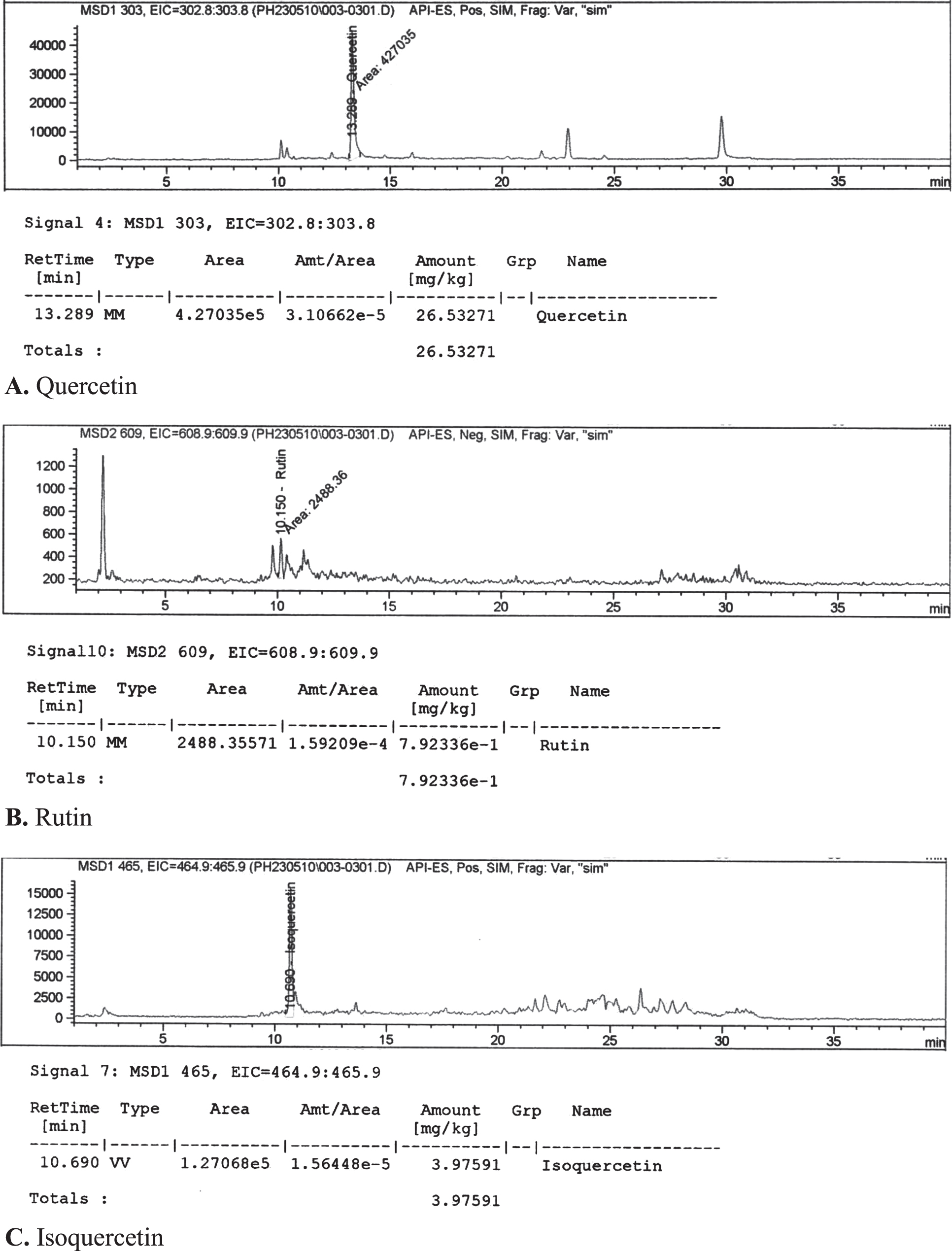
The Characteristic profiles of Gac fruit extract measured by HPLC-DAD method. The results found that the Gac fruit extract contain the polyphenolic compoud including quercetin, rutin, and isoquercetin in 26.53, 7.92 and 3.98 mg/kg, respectively. (Fig. 2A-2C).
Fig. 2
HPLC chromatogram of polyphenolic compound including quercetin (2A), rutin (2B), and isoquercetin (2C) in Gac fruit extract. A. Quercetin. B. Rutin. C. Isoquercetin.
3.2Locomotor activity
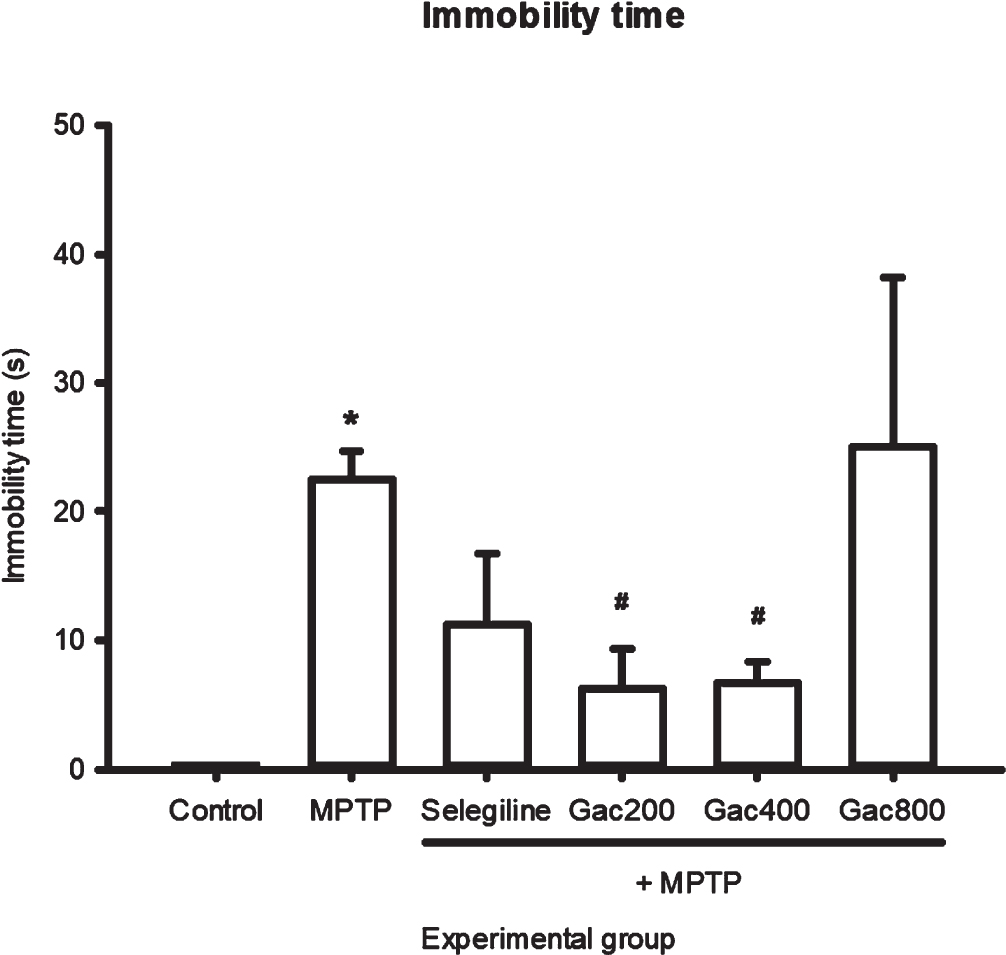
The effects of MPTP, selegiline, and Gac extract on total distance, velocity, and immobility are expressed as mean±SEM (Fig. 3–5). We found that a significantly greater total distance was traveled by zebrafish that received selegiline (3735.60±87.22 cm) and Gac extract at doses of 200 and 400 mg/kg (3950.57±252.39 and 4143.41±195.72 cm) when compared to the MPTP group (2592.82±23.38 cm). In terms of velocity, zebrafish in the MPTP group (4.9401±0.34 cm/s) had significantly decreased velocity when compared to the control group (6.2958±0.15 cm/s). In addition, zebrafish that received selegiline (6.2783±0.15 cm/s) and Gac extract at doses of 200 and 400 mg/kg (6.6396±0.42 and 7.2408±0.23 cm/s) had significantly increased velocity when compared to the MPTP group. The results showed that zebrafish in the MPTP group had significantly increased immobility time when compared to the control group. In addition, zebrafish that received Gac extract at doses of 200 and 400 mg/kg (6.25±3.15 and 6.67±1.67 s) had significantly decreased immobility time compared to the MPTP group (22.50±2.16 s). It should be noted that immobility was considered when the velocity was≤3 mm/s.
Fig. 3
Effect of MPTP, selegiline, and Gac extract on total distance (cm) expressed as mean±SEM. Zebrafish that received selegiline and Gac extract at doses of 200 and 400 mg/kg had significantly increased total distance when compared to the MPTP group. *p value < 0.05 when compared to the control group, #p value < 0.05 when compared to the MPTP group.
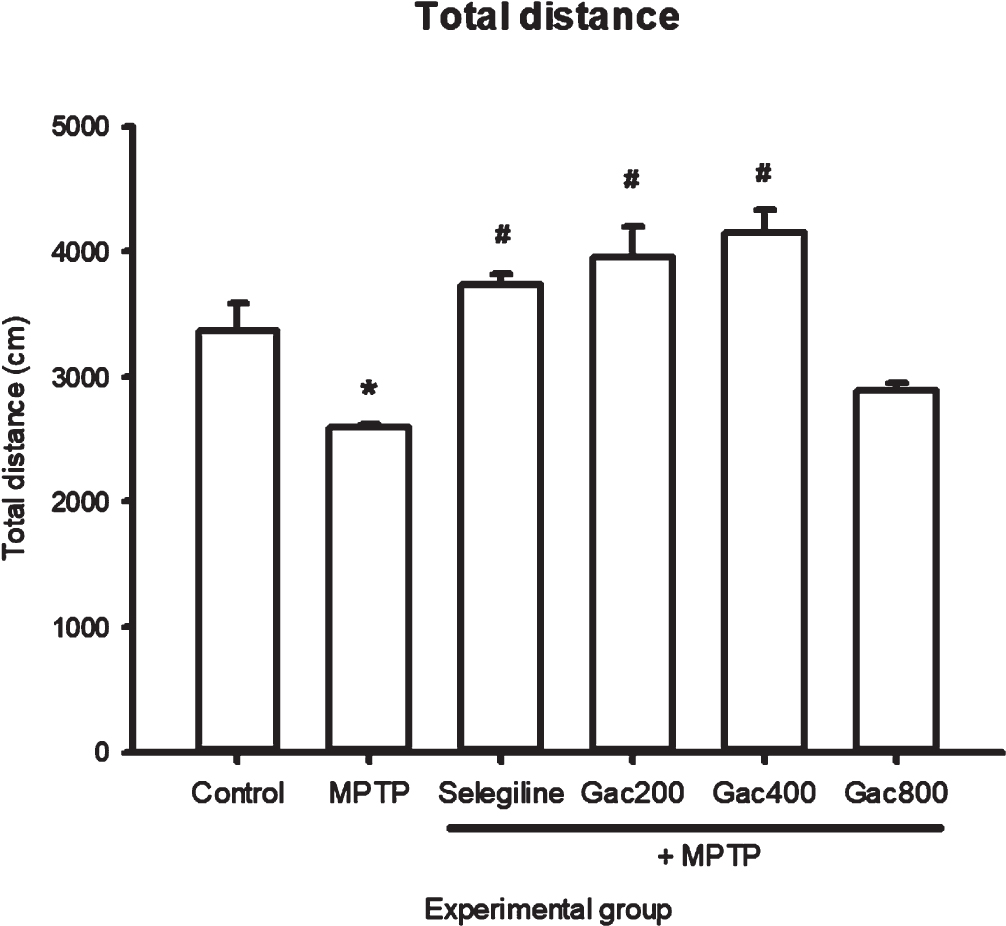
Fig. 4
Effect of MPTP, selegiline, and Gac extract on velocity (cm/s) expressed as mean±SEM. Zebrafish in the MPTP group had significantly decreased velocity when compared to the control group. In addition, zebrafish that received selegiline and Gac extract at doses of 200 and 400 mg/kg had significantly increased velocity when compared to the MPTP group. *p value < 0.05 when compared to the control group, #p value < 0.05 when compared to the MPTP group. **p value < 0.05 when compared to the control, selegiline, Gac200, Gac400, and Gac800 groups.
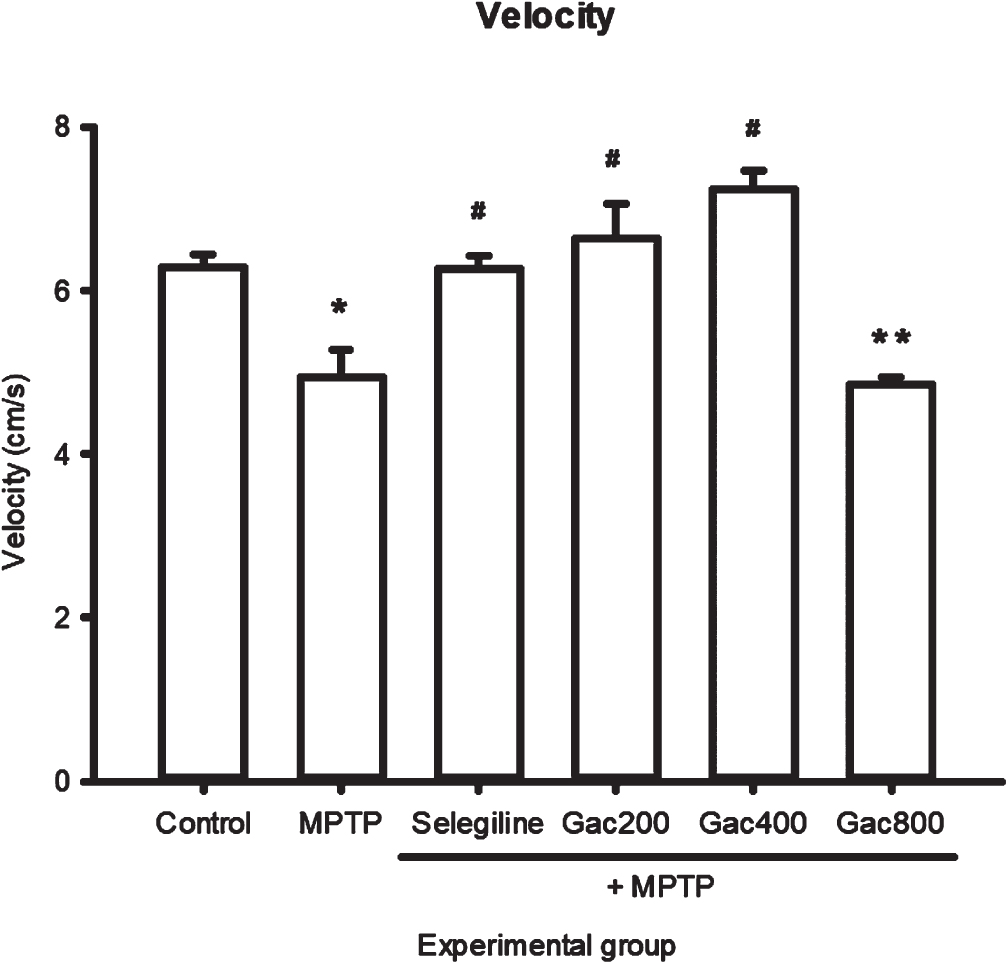
Fig. 5
Effect of MPTP, selegiline, and Gac extract on immobility time (s) expressed as mean±SEM. Zebrafish in the MPTP group had significantly increased immobility time when compared to the control group. In addition, zebrafish that received Gac extract at doses of 200 and 400 mg/kg had significantly decreased immobility time when compared to the MPTP group. *p value < 0.05 when compared to the control group, #p value < 0.05 when compared to the MPTP group,
3.3Antioxidant enzyme activity in the brain
The CAT activity in zebrafish brain, expressed as mean±SEM and reported in U/mL/mg tissue, is shown in Table 1. The zebrafish that received GAC 400 mg/kg appeared to have the highest CAT activity; however, there was no significant difference between groups. Table 2 shows the SOD activity in the zebrafish brain, expressed as mean SEM and reported in U/mL/mg tissue. Similar to CAT activity, the GAC 400 mg/kg-treated zebrafish appeared to have the highest SOD activity, but there was not a significant difference between the groups.
Table 1
CAT activity in zebrafish brains
| Experimental group | CAT activity (U/mL/mg tissue) |
| Control | 128.41±11.69 |
| MPTP | 162.03±13.52 |
| MPTP + Selegiline | 180.53±42.98 |
| MPTP + GAC200 | 168.76±22.22 |
| MPTP + GAC400 | 202.37±42.00 |
| MPTP + GAC800 | 170.69±18.20 |
Effect of MPTP, selegiline, and Gac extract on CAT activity in zebrafish brains, expressed as mean±SEM. No significant difference was observed between groups.
Table 2
SOD activity in zebrafish brains
| Experimental group | SOD activity (U/mL/mg tissue) |
| Control | 0.0134±0.0019 |
| MPTP | 0.0272±0.0117 |
| MPTP + Selegiline | 0.0339±0.0112 |
| MPTP + GAC200 | 0.0230±0.0054 |
| MPTP + GAC400 | 0.0346±0.0099 |
| MPTP + GAC800 | 0.0299±0.0052 |
Effect of MPTP, selegiline, and Gac extract on SOD activity in zebrafish brains, expressed as mean±SEM. No significant difference was observed between groups.
4Discussion
MPTP-induced animal models are widely used to investigate the pathophysiology of Parkinson’s disease [11]. MPTP induces a state of oxidative stress in the brain, which has been reported to significantly decrease SOD and CAT activities in the brains of C57BL/6 mice [12, 13]. However, the present results show no significant differences in the antioxidant enzyme activity of the zebrafish brain, including the activities of CAT and SOD. This is consistent with the report of Mu et al. [14], in which the results showed that exposure to beta-CYP could lead to varying degrees of increases in hepatic SOD, CAT, GR, and GPx activities 7 and 15 days after exposure, but the activities of SOD and CAT in the zebrafish brain showed no detectable response to beta-CYP throughout the treatment period [14].
Previous studies have shown that quercetin, rutin, and isoquercetin, which the Gac fruit contains [15], had significant neuroprotective effect against 6-OHDA toxicity in PC-12 cells [16]. Quercetin has shown neuroprotective potential through several mechanisms, such as Tau phosphorylation, attenuate oxidative stress, and ameliorate neuroinflammation [17]. These polyphenolic compounds exhibit anti-neurodegenerative effects. Therefore, natural products containing these phytochemicals may have benefits for neurodegenerative disorders, including Parkinson’s disease.
Our results showed that Gac fruit extract at doses of 200 and 400 mg/kg improved locomotor function in MPTP-induced Parkinson’s-like symptoms in zebrafish. Gac fruit has a very high nutritional content and contains high levels of carotenoids such as lycopene and β-carotene, which possess several health benefits [15, 18]. Lycopene is a natural pigment in plants belonging to lipophilic isoprenes and possesses diverse bioactivity against cardiovascular diseases, Type II diabetes mellitus, neurodegenerative disease, and cancer [19, 20]. In addition, lycopene has antioxidant and anti-inflammatory activities related to neurodegenerative diseases [21, 22]. Moreover, lycopene has been reported to stimulate the activation of antioxidant enzymes and translocation of the transcription factor Nrf2 [23]. Nrf2 activation is known to prevent neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease [24]. MPTP-induced damage by increasing cyto-c release and decreasing the expression of bcl-2 with increasing expressions of bax, caspases-3, 8 and 9 [25]. Lycopene reversed MPTP induced neuronal damage by its antioxidant activity and controlling apoptosis at the gene level. The orally pre-treatment lycopene could enhance striatal dopamine levels and its metabolites, ameliorated oxidative stress by attenuated the levels of TBARS and activities of SOD and catalase with increased GSH and activities of GPx in the MPTP-treated mice, dose dependently. Moreover, it significantly enhanced the expressions of bcl-2 and cyto-c, whereas decreased in expressions of bax, caspases-3, 8 and 9 as compared with MPTP alone treated animals [26]. Similar results had been observed by Fujita et al. [27] which reported that lycopene could attenuate ischemia-related neuronal damage in gerbil hippocampal tissue by enhancing bcl-2, diminishing caspase- 3 levels, and increasing SOD activities. These reports provide evidence for the effect of Gac fruit on behavioral tests in a Parkinson’s model. These findings may aid further research on zebrafish brain analysis to develop Gac fruit extract as a treatment option for Parkinson’s disease.
Acknowledgments
We are very grateful to the Laboratory Animal Research Center (UP-LARC) and School of Pharmaceutical Sciences, University of Phayao, for supporting the facilities of this research.
Funding
This research project was supported by the Thailand Science Research and Innovation Fund and the University of Phayao (grant no. FF64-RIM022 and FF66-UoE024).
Author Contributions
Kanathip: Principal investigator, conception of the study experimental design, analysis of behavioral studies, SOD enzymatic test, and writing-original draft; Nopphakarn: Implement a system and maintain zebrafish management in research; Supawadee: Data analysis of locomotor test and behavioral studies; Pimchanok: Catalase enzymatic test. All the authors provided conceptual advice and approved the final version of the manuscript for submission.
Conflict of interest
The authors have no conflict of interest to report.
References
[1] | Kubola J , Siriamornpun S . Phytochemicals and antioxidant activity of different fruit fractions (peel, pulp, aril and seed) of Thai gac (Momordica cochinchinensis Spreng). Food Chem. (2011) ;127: (3):1138–45. |
[2] | Abdulqader A , Ali F , Ismail A , Esa NM . Antioxidant compounds and capacities of Gac (Momordica cochinchinensis Spreng) fruits. Asian Pac. J. TroBiomed. (2019) ;9: (4):158–67. |
[3] | Chuyen HV , Nguyen MH , Roach PD , Golding JB , Parks SE . Gac fruit (Momordica cochinchinensis Spreng. ): a rich source of bioactive compounds and its potential health benefits. Int. J. Food Sci. (2015) ;50: (3):567–77. |
[4] | Müller-Maatsch J , Sprenger J , Hempel J , Kreiser F , Carle R , Schweiggert RM . Carotenoids from gac fruit aril (Momordica cochinchinensis Spreng. ) are more bioaccessible than those from carrot root and tomato fruit. Food Res. Int (Ottawa, Ont.). (2017) ;99: (Pt 2):928–35. |
[5] | Singsai K , Ladpala N , Dangja N , Boonchuen T , Jaikhamfu N , Fakthong P . Effect of Streblus asper Leaf Extract on Scopolamine-Induced Memory Deficits in Zebrafish: The Model of Alzheimer’s Disease. Adv. Pharmacol. Pharm. Sci. (2021) 6666726. |
[6] | Gavrilova V , Kajdzanoska M , Gjamovski V , Stefova M . Separation, Characterization and Quantification of Phenolic Com-pounds in Blueberries and Red and Black Currants by HPLC–DAD–ESI-MSn. J. Agric. Food Chem. (2011) ;59: (8):4009–18. |
[7] | Anichtchik OV , Kaslin J , Peitsaro N , Scheinin M , Panula P . Neurochemical and behavioural changes in zebrafish Danio rerio after systemic administration of 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J. Neurochem. (2004) ;88: (2):443–53. |
[8] | Sumbre G , de Polavieja GG . The world according to zebrafish: how neural circuits generate behavior. Front. Neural. Circuits. (2014) ;8: :91. |
[9] | Grilo LF , Martins JD , Cavallaro CH , Nathanielsz PW , Oliveira PJ , Pereira SP . Development of a 96-well based assay for kinetic determination of catalase enzymaticactivity in biological samples. Toxicol. In Vitro. (2020) ;69: :104996. |
[10] | Gazsi G , Czimmerer Z , Ivánovics B , Berta IR , Urbányi B , Csenki-Bakos Z , Ács A . Physiological,Developmental, and Biomarker Responses of Zebrafish Embryos to Sub-Lethal Exposure of Bendiocarb. Water. (2021) ;13: (2):204. |
[11] | Braidy N , Selvaraju S , Essa MM , Vaishnav R , Al-Adawi S , Al-Asmi A , Al-Senawi H , Abd Alrahman Alobaidy A , Lakhtakia R , Guillemin GJ . Neuroprotective effects of a variety of pomegranate juice extracts against MPTP-induced cytotoxicity and oxidative stress in human primary neurons. Oxid. Med. Cell. Longev. (2013) ;2013: :685909. |
[12] | Anandhan A , Tamilselvam K , Vijayraja D , Ashokkumar N , Rajasankar S , Manivasagam T . Resveratrol attenuates oxidative stress and improves behaviour in 1 -methyl-4-phenyl1,2,3,6-tetrahydropyridine (MPTP) challenged mice. Ann. Neurosci. (2010) ;17: (3):113–9. |
[13] | Ardah MT , Bharathan G , Kitada T , Haque ME . Ellagic Acid Prevents Dopamine Neuron Degeneration from Oxidative Stress and Neuroinflammation in MPTP Model of Parkinson’s Disease. Biomolecules. (2020) ;10: (11):1519. |
[14] | Mu X , Wang K , Chen X , Pang S , Zhu L , Yang Y , Zhang J , Li X , Wang C . Impact of environmental concentrations of beta-cypermethrin onthe antioxidant system in the brain and liver of zebrafish(Danio rerio). Chem. Ecol. (2014) ;30: (7):643–52. |
[15] | Do TVT , Fan L , Suhartini W , Girmatsion M . Gac (Momordica cochinchinensis Spreng) fruit: A functional food and medicinal resource. J. Funct. Foods. (2019) ;62: :103512. |
[16] | Magalingam KB , Radhakrishnan A , Haleagrahara N . Protective effects of quercetin glycosides, rutin, and isoquercetrin against 6-hydroxydopamine (6-OHDA)-induced neurotoxicity in rat pheochromocytoma (PC-12) cells. Int J Immunopathol Pharmacol. (2016) ;29: (1):30–9. |
[17] | Khan H , Ullah H , Aschner M , Cheang WS , Akkol EK . Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules. (2019) ;10: (1):59. |
[18] | Praengam K , Sukboon P , Tuntipopipat S . Gac Fruit Extract Suppresses Oxidative Stress in LPS-induced RAW264. 7 Cells. J. Nutr. Assoc. Thailand. (2021) ;56: (1):37–51. |
[19] | Khan UM , Sevindik M , Zarrabi A , Nami M , Ozdemir B , Kaplan DN , Selamoglu Z , Hasan M , Kumar M , Alshehri MM , Sharifi-Rad J . Lycopene: Food Sources, Biological Activities, and Human Health Benefits. Oxid. Med. Cell. Longev. (2021) ;2021: ;2713511. |
[20] | Leh HE , Lee LK . Lycopene: A Potent Antioxidant for the Amelioration of Type II Diabetes Mellitus. Molecules (Basel, Switzerland). (2022) ;27: (7):2335. |
[21] | Choi J , Lim JW , Kim H . Lycopene Inhibits Toll-Like Receptor 4-Mediated Expression of Inflammatory Cytokines in House Dust Mite-Stimulated Respiratory Epithelial Cells. Molecules (Basel, Switzerland). (2021) ;26: (11):3127. |
[22] | Lee J , Lim JW , Kim H . Lycopene Inhibits Oxidative Stress-Mediated Inflammatory Responses in Ethanol/Palmitoleic Acid-Stimulated Pancreatic Acinar AR42J Cells. Int. J. Mol. Sci. (2021) ;22: (4):2101. |
[23] | Wang S , Wu YY , Wang X , Shen P , Jia Q , Yu S , Wang Y , Li X , Chen W , Wang A , Lu Y . Lycopene prevents carcinogen-induced cutaneous tumor by enhancing activation of the Nrf2 pathway through p62-triggered autophagic Keap1 degradation. Aging. (2020) ;12: (9):8167–90. |
[24] | Sun Y , Yang T , Leak RK , Chen J , Zhang F . Preventive and Protective Roles of Dietary Nrf2 Activators Against Central Nervous System Diseases. CNS. Neurol. Disord. Drug. Targets. (2017) ;16: (3):326–38. |
[25] | Nataraj J , Manivasagam T , Thenmozhi AJ , Essa MM . Lutein protects dopaminergic neurons against MPTP-induced apoptotic death and motor dysfunction by ameliorating mitochondrial disruption and oxidative stress. Nutr. Neurosci. (2016) ;19: (6):237–46. |
[26] | Prema A , Janakiraman U , Manivasagam T , Thenmozhi AJ . Neuroprotective effect of lycopene against MPTP-induced experimental Parkinson’s disease in mice, Neurosci. Lett. (2015) ;599: :12–19. |
[27] | Fujita K , Yoshimoto N , Kato T , Imada H , Matsumoto G , Inakuma T et al. Lycopene inhibits ischemia/reperfusion-induced neuronal apoptosis in gerbil hippocampal tissue, Neurochem. Res. (2013) ;38: :461–9. |




