Hylocereus undatus extends lifespan and exerts neuroprotection in Caenorhabditis elegans via DAF-16 mediated pathway
Abstract
BACKGROUND:
Hylocereus undatus is a traditional medicinal plant known for its medicinal, nutritional and commercial uses.
OBJECTIVE:
To address the anti-aging and neuroprotective efficacies of fruit peel extracts of H. undatus using Caenorhabditis elegans model.
METHODS:
C. elegans (wild-type (N2), transgenic and mutant strains) were treated with H. undatus and monitored for lifespan and neuroprotection through physiological assays, fluorescence microscopy and qPCR analysis. LC-MS/MS analysis was performed to identify the phytochemicals present in the extract. Molecular docking studies were employed to identify the interaction mode of selected phytochemicals with Aβ, DAF-16 and SKN-1.
RESULTS:
The extract was able to extend the lifespan of C. elegans (N2), extend the lifespan and reduce paralysis of Aβ transgenic strains CL2006 and CL4176, suggesting its anti-aging and neuroprotective potential. The LC-MS/MS analysis revealed the presence of phytochemicals including homostachydrine, betaine, syringic acid, typhaneoside, rutin, and behenic acid. The extract could activate antioxidant mechanism, through SKN-1, which was evident in qPCR and transgenic strain LG333. These effects were mediated through DAF-16 pathway as the extract was able to upregulate the expression of daf-16 in N2, increase the nuclear localization of daf-16 in transgenic strain TJ356, and not able to significantly alter the lifespan of both DAF-2 and DAF-16 mutants, CB1370 and CF1038 respectively. Finally, in molecular docking approach, typhaneoside and rutin showed better binding affinity with SKN-1 and DAF-16 when compared to resveratrol and similar binding affinity with Aβ when compared to donepezil.
CONCLUSION:
Taken together, this study indicates that H. undatus activates anti-aging and neuroprotection via DAF-16 mediated pathway.
1Introduction
Aging is a consequence of molecular and cellular damage leading to the functional loss of tissues and organs over time [1]. Advancements in the field of medicine has increased the average life expectancy in developed and developing nations, and age-associated disorders including neurodegenerative diseases, caused by the imbalance among free radicals, reactive oxygen species (ROS), reactive nitrogen species (RNS), and endogenous antioxidants. According to World Health Organization (WHO), neurodegenerative diseases will bypass cancer to become the second leading cause of death globally by 2040 [2]. Exogenous antioxidants could be supplemented to maintain the balance between the formation and elimination of free radicals, thus protecting the cell from their toxicity [1]. Polyphenols from plants and other herbal sources could be used, as they have abundant source of antioxidants naturally, with negligible or minimal side effects.
Hylocereus undatus, which is commonly known as dragon fruit or pitaya, has high value as an edible fruit and ornamental plant, and the fruit can be consumed raw or transformed into wine, juice, jelly, yogurt, jam, preserves, and other desserts [3–6]. It is majorly present in Mexico and Central America, whereas countries like, Israel, Malaysia, and Thailand use advanced technologies for growing them resulting in high yields [7]. Mayan civilization used H. undatus fruits as hypoglycemic, diuretic, cardioprotectant, wound disinfectant, anti-tumorigenic (stem sap), dysentery cure and other digestive system disorders [8–10].
The fruit is a rich source for vitamin C, calcium, and phosphorous, apart from organic acids (ascorbic, citric, isocitric, and malic acids), carbohydrates, amino acids (proline), minerals (potassium and magnesium), and lipids. Rutin, quercetin, kaempferol and isorhamnetin were also identified in the pulp of H. undatus [11]. Each 100 g of H. undatus pulp contains 1.4±1.4μg beta-carotene, 3.4±1.4μg lycopene and 0.26±0.06 mg vitamin E and up to 24 mg vitamin C [12]. Peel, which consists of dietary fibres and Vitamin C [13], can be used as a natural coloring agent as well. Seeds are mainly utilized to extract the oil containing about 50% essential fatty acids and can be used to make syrup, ice cream, sherbet, candy, yogurt, and pastries [14, 15].
The leaves, flowers and fruits of H. undatus exhibits wound healing properties [16]. The fruit peel has potential as an antibacterial [17], and antioxidant agent [18]. It could reduce hypertension, and diabetes, apart from mediating carbohydrate metabolism, heart tissue formation, fortification of teeth and bones, improving the function of kidneys, sharpness of eyes, strengthening the brain function, and prevents colon and prostate cancer [19]. Taken together, the extracts of H. undatus can impart antioxidant, anti-inflammatory, anti-obesity, anti-diabetic, anti-cancer, and anti-proliferative activities [11]. Apart from the nutritional benefits of mature fruit, the young stem, and fresh flower buds are also edible and can be used as a vegetable [6]. The dehydrated dragon fruit flowers were used for making antioxidant-rich tea [15]. Oligosaccharides from H. undatus promotes lactobacillus and bifidobacterias as well as shows complete and partial resistance to acidic conditions in the stomach, and human α-amylase respectively, indicating pre-biotic characteristics [20].
Caenorhabditis elegans is a multicellular transparent nematode that has been extensively used for research in the area of genetics, development, and neurobiology. The conserved mechanisms present in C. elegans, including insulin and TGF-β signaling, nutrient sensing, and autophagy have provided significant clues about universal mechanisms of longevity [21]. The smallest eukaryotic in vivo model has been employed to understand the anti-aging and neuroprotective properties of different plants including Cleistocalyx nervosum [22, 23], Bacopa monnieri [24], Streblus asper [25], Hibiscus sabdariffa [26], and Kaempferia parviflora [27, 28].
Recently, Tamagno et al. had reported that microencapsulated pulp extract of H. undatus was effective to prevent and repair the damages caused by juglone induced oxidative stress and copper-induced metal toxicity in C. elegans [29, 30]. The pulp of H. undatus contains antioxidant compounds and can serve as a potential nutraceutical product. In this regard, the present manuscript tries to understand the anti-aging and neuroprotective properties of fruit peels of H. undatus and the mechanism involved in the nematode and the metabolites mediating the activity through in vivo and in silico approach.
2Materials and methods
2.1Chemicals, reagents, and equipments used
The chemicals and reagents used in the study were obtained from Sigma-Aldrich (St. Louis, MO, USA) and HiMedia Laboratories (Mumbai, India), unless otherwise specified.
2.2Plant collection and extraction
The H. undatus fruits were collected from the local market in Nonthaburi Province, Thailand. The plant was authenticated and deposited at the herbarium of Kasin Suvatabhandhu (Department of Botany, Faculty of Science, Chulalongkorn University, Thailand) with voucher specimen number 016446(BCU). The fruit was peeled and the peels were washed, shade-dried and powdered using a blender, 6further subjected to extraction with absolute ethanol with the sample to solvent ratio of 1 : 10 by using the Soxhlet extractor for 24 h. The extract was dissolved in dimethyl sulfoxide (DMSO) to a final concentration of 100 mg/mL as the stock solution and stored at –20 °C until further use.
2.3C. elegans strains used and culture conditions
Wild type strain N2 (Bristol), daf-16 mutant CF1038, daf-16::GFP transgenic strain TJ356, daf-2 mutant CB1370, Aβ transgenic strains CL2006 and CL4176, skn-1::GFP transgenic strain LG333 and the bacterial food source E. coli OP50 were procured from Caenorhabditis Genetics Center, (University of Minnesota, USA). All strains were grown, maintained and propagated in Nematode growth medium (NGM) at 15 °C as per the standard protocol [31]. Age synchronized young adult worms were used for conducting all the experiments.
2.4Lifespan assay
Analysis of lifespan was performed in liquid media. Briefly, 10 age synchronized young adult nematodes (wild type or mutants) were transferred into a 24 well microtiter plate with M9 buffer along with E. coli OP50 and 5-Fluoro-2′-deoxyuridine (FUDR). Different concentrations of H. undatus extract (1–100μg/ml for wild type and 8–10μg/ml for mutants) dissolved in DMSO were added to each well. The worms alive on the well were counted every 24 h. Nematodes were considered to be dead when they did not respond to gentle touch or prodding using the platinum loop. Worms with no extract treatment but only with E. coli OP50 served as the control and DMSO as the vehicle control. The experiments were carried out in five independent trials [22].
2.5Fluorescence imaging
Accumulation of lipofuscin in the wild type nematodes and the skn-1::GFP tagged strain LG333 and daf-16::GFP transgenic strain TJ356, at young adult stage were treated with varying concentrations of H. undatus (8–10μg/ml) consecutively for 5 days, unless otherwise specified, with E. coli OP50 fed worms as control. After incubation, the worms were thoroughly washed using M9 buffer several times and then placed on a glass slide into a drop of sodium azide. Fluorescent imaging was performed using ZEISS LSM 700 Confocal microscope under 10X magnification. The images were further analyzed using Image J software, and the relative fluorescence was represented as arbitrary units (AU). The experiment was carried out in three independent trials [23].
2.6Paralysis assay
Synchronized eggs of CL4176 were maintained at 15 °C, on the NGM plates for 36 h. Then the worms were treated with different concentrations of H. undatus (8–10μg/ml) and were incubated at 25 °C to induce expression of Aβ. After 24 h of temperature shift, paralyzed nematodes were monitored every hour. The worms that only moved their head or did not show a full-body wave when gently touched with a platinum loop were noted as paralyzed [28, 32].
2.7Total RNA isolation and real-time PCR analysis
The nematodes were treated with varying concentrations of H. undatus (8–10μg/ml) and after treatment period total RNA was isolated using Trizol kit (Invitrogen, Carlsbad, CA, USA). From this, 1000 ng was reverse transcribed to cDNA using Accupower RT Premix (Bioneer, Korea) and oligo dT primers through following the manufacturer’s protocol. Gene specific primers were designed using Primer 3 software (Table 1) to carry out Real-time PCR using SYBR Green. The Green Star PCR Master Mix (Bioneer, Korea) was used in the Exicycler Real-Time Quantitative Thermal Block (Bioneer, Daedeok-gu, Korea). The expression data were normalized to the internal control actin and then represented as upregulated or downregulated by normalizing with untreated control [22].
Table 1
List of Primers used
| Gene Name | Forward Primer | Reverse Primer |
| daf-16 | TGGTGGAATTCAATCGTGAA | ATGAATATGCTGCCCTCCAG |
| age-1 | ATAGAGCTCCACGGCACTTT | ATAGAGCTCCACGGCACTTT |
| skn-1 | ATCCATTCGGTAGAGGACCA | GGCGCTACTGTCGATTTCTC |
| sir-2.1 | CGGGGAAGTGCAAGAAATAA | GAGTGGCACCATCATCAAGA |
| act-2 | ATCGTCCTCGACTCTGGAGATG | TCACGTCCAGCCAAGTCAAG |
2.8LC-MS/MS analysis
The liquid chromatography-mass spectrometry (LC-MS/MS) analysis of phytochemical components in H. undatus extract was carried out at the Institute of Systems Biology (Universiti Kebangsaan Malaysia, Malaysia). The LC-MS system consists of a DionexTM UltiMate 3000 UHPLC system (Thermo Fisher Scientific, Rockford, IL, USA) coupled with a high-resolution micrOTOF-Q III (Bruker Daltonics GmbH, Bremen, Germany). The injection volume of sample was 3μL. The chromatographic separation was performed on an AcclaimTM Polar Advantage II C18 (3 mm x 150 mm, 3μm) column (Thermo Fisher Scientific) using a gradient mobile phase consisting of 0.1% (v/v) aqueous formic acid (A) and 100% acetonitrile (B) at a flow rate of 0.4 ml/min with 22 min total run time. The gradient elution program was set as follows: 0–3 min, 5% B; 3–10 min, 80% B; 10–15 min, 80% B; 15–22 min, 5% B. The MS analysis was operated in the positive electrospray ionization (ESI) mode. The operating conditions were as follows: drying gas flow at 8 L/min, drying gas temperature at 250 °C, nebulizer pressure at 2.0 bar, capillary voltage at 4500 V, and m/z scan range of 50 to 1000. The identification of putative compounds detected was carried out by comparing their observed (experimental) m/z values with the METLIN and the KNApSAcK databases and with the calculated (theoretical) mass values of previously reported compounds in published literature, with an accepted mass error less than 30 ppm.
2.9In silico studies
2.9.1Protein and ligand preparation
The protein 3D structures of DAF-16 and SKN-1 were modelled via homology modelling approach. Initially, the Fasta sequence of each protein was retrieved from Uniprot database (UniProtKB –P34707 and O16850) and were submitted to online webserver trROSETTA [33], which models the 3D structure via Ab initio modelling technique. Subsequently, the structures were checked for their stereochemical quality by using PDBSum [34]. It predicts the confirmation of the polypeptide backbone of the protein by analyzing the phi/psi torsion angles. If the structure modelled is reliable then this will presume that most of the amino acid residues will lie in the favored region of the Ramachandran Plot. In addition, the 3D structure for Beta-amyloid (PDB ID: 1AAP) was extracted from PDB databank (https://www.rcsb.org/). The chemical structure of ligands was downloaded from PUBCHEM database (https://pubchem.ncbi.nlm.nih.gov/) in SDF format and were converted into MOL2 and PDB format using Open Babel [35] tool for docking analysis.
2.9.2Docking simulation study
Molecular docking was done to understand the behavior of ligand molecule in the protein binding pocket and the biochemical processes involved [36]. Docking study was done for 6 phytocompounds (Behenic Acid, Betaine, Homostachydrine, Syringic Acid, Rutin and Typhaneoside) obtained from LC-MS/MS analysis of H. undatus along with the reference drug candidates Resveratrol (DAF-16, SKN-1) and Donepezil (Aβ). In the present study we have employed two different docking tools Swiss Dock and CB-Dock, which enables blind docking and calculates the energies based on docking poses [37–39].
2.10Statistical analysis
One-way ANOVA (SPSS 17) was used to compare the mean values of each treatment in every experiment unless otherwise specified. The data were represented as average of the independent experiments. Significant differences between the means of parameters were determined by using Duncan’s test (P < 0.05) comparing between the groups control vs treated.
3Results
3.1H. undatus extended the lifespan of C. elegans
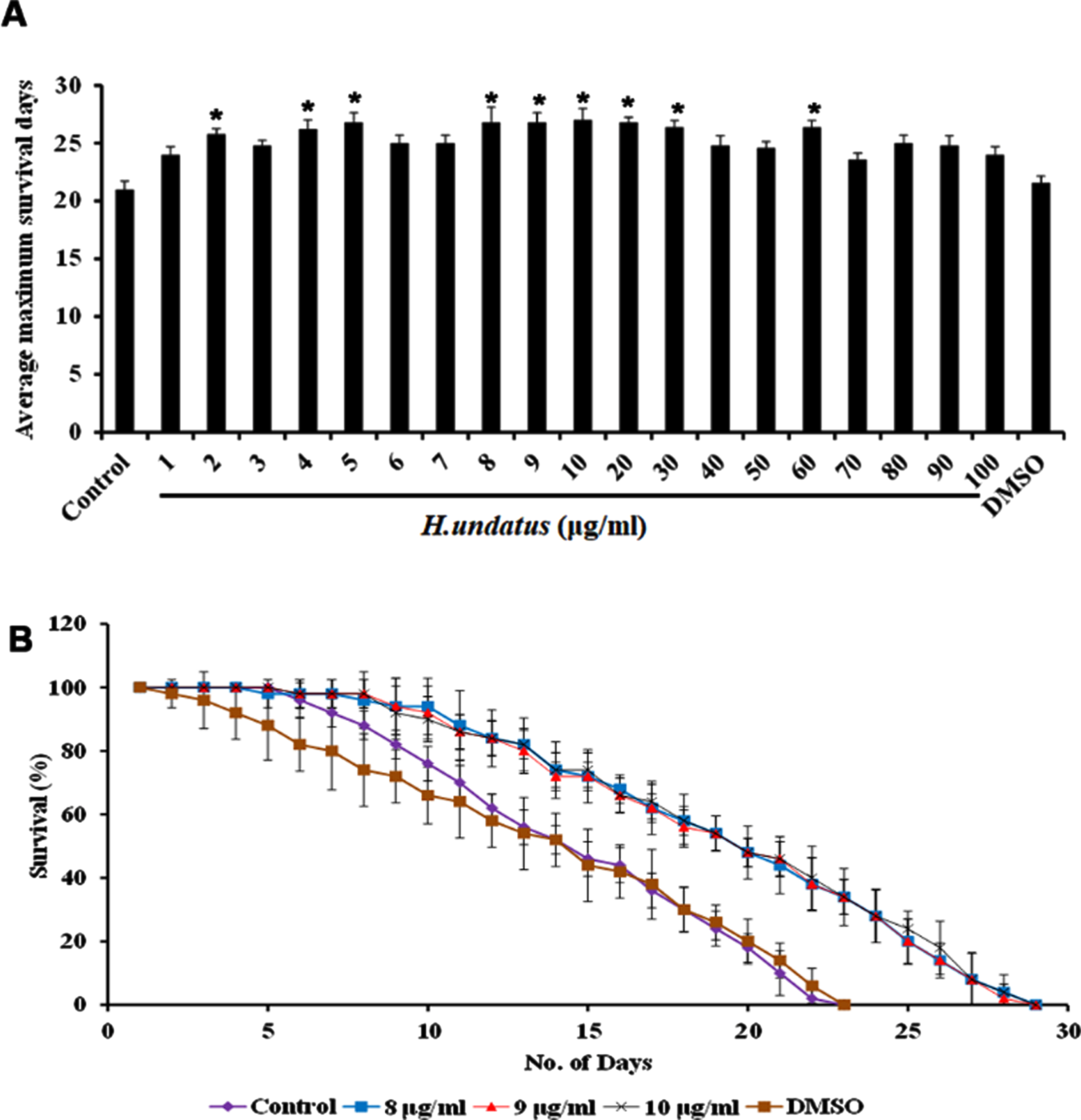
H. undatus was monitored for its efficacy in extending the lifespan of C. elegans by treating varying doses of extract ranging from 1–100μg/ml to the wild type nematodes. It was observed that all the doses could extend the lifespan of the nematodes when compared to untreated control indicating that the extract was not toxic to the nematodes. Interestingly, the doses ranging from 8–10μg/ml exhibited significant (p < 0.05) and maximum extension of lifespan up to 28 days when compared to untreated control, which survived up to 22 days. DMSO was used as vehicle control which also exhibited maximum lifespan of 22 days (Fig. 1).
Fig. 1
H. undatus extract could extend the maximum lifespan in C. elegans. (A) Average maximum survival rate of the extract (1 –100μg/ml) in N2 worms (B) H. undatus extract (8 –10μg/ml) significantly (p < 0.05) prolonged the lifespan of nematodes to 28, 28 and 28 days respectively.
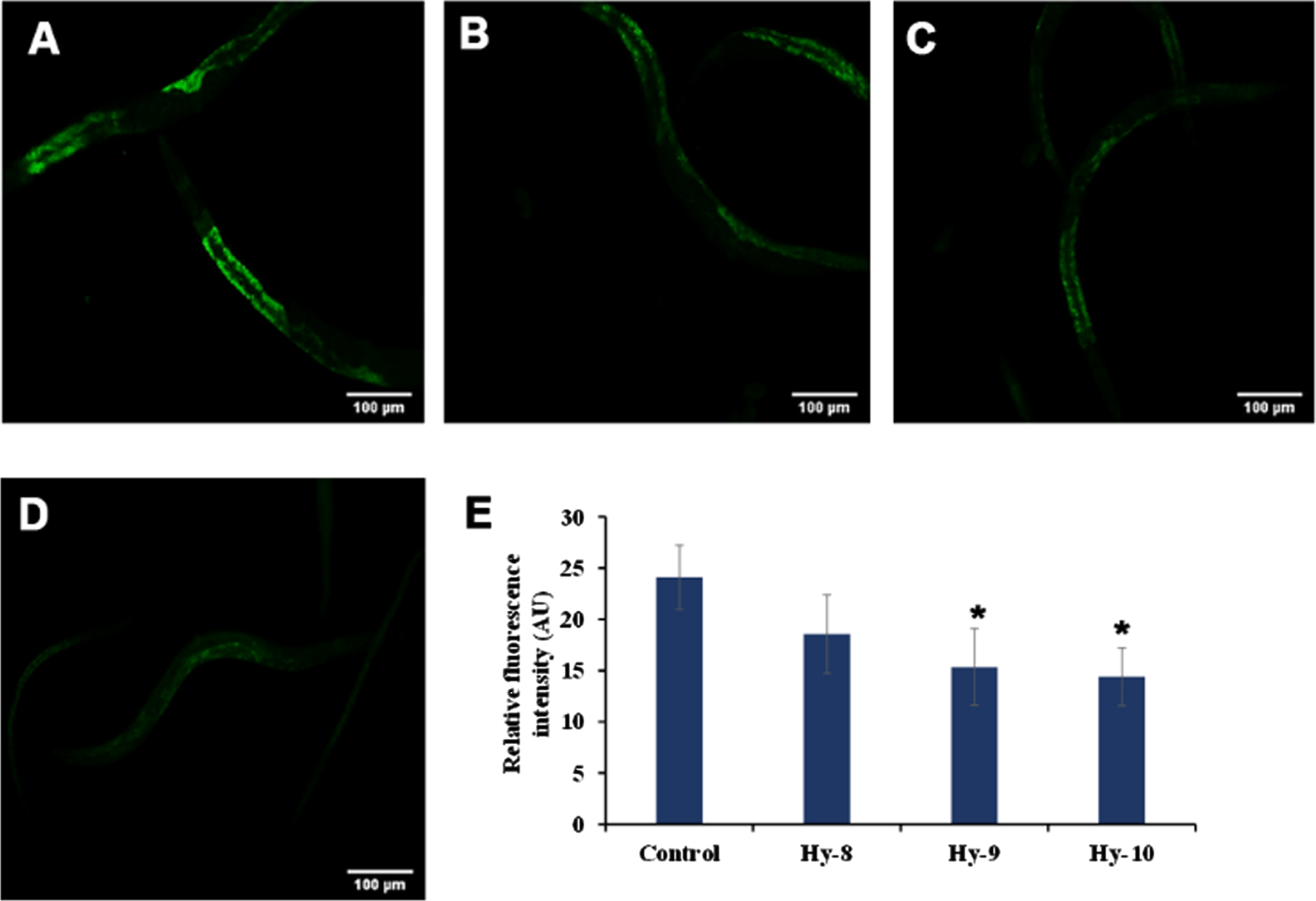
The level of autofluorescent protein lipofuscin, which is considered as a marker of aging, as it increases inside the nematode which is directly proportional to aging, was monitored after treating the nematodes with H. undatus extract at 8–10μg/ml. It was observed that the extract could significantly (p < 0.05) reduce the accumulation of lipofuscin indicating the anti-aging potential of H. undatus (Fig. 2).
Fig. 2
Effect of KP and DMF on the aging marker lipofuscin in N2 nematodes. (A) Control (B) H. undatus extract 8μg/ml (C) H. undatus extract 9μg/ml (D) H. undatus extract 10μg/ml.
3.2Phytochemical profiling of H. undatus extract
In the present study, phytochemical profiling of H. undatus extract was carried out using LC-MS/MS analysis. A total of 11 compounds were tentatively identified in the positive mode [M + H]+ or [M + Na]+ by comparing their m/z values of precursor ions with those in databases and the literature. Among this, six compounds were previously reported in H. undatus including homostachydrine [40], betaine, syringic acid [41], typhaneoside [41], rutin [40, 41], and behenic acid [42]. All identified compounds were annotated by number and detailed in Table 2.
Table 2
Putative phytochemical constituents in H. undatus extract
| No. | Proposed compound | Molecular formula | RT (min) | Precursor ion (m/z) | Mass error (ppm) | Database |
| 1 | Homostachydrine | C8H15NO2 | 1.9 | 158.1176 [M + H]+ | 0 | METLIN |
| 2 | Lepidine alkaloid (B, D, E, F) | C20H18N4O2 | 3.4 | 347.1552 [M + H]+ | 14 | METLIN |
| 3 | Betaine | C5H11NO2 | 6.2 | 118.0867 [M + H]+ | 3 | METLIN, KNApSAcK |
| 4 | Malvidin 3-(6”-p-coumaryl glucoside)-5-dimalonylglucoside | C44H45O25 | 7.3 | 995.1839 [M + Na]+ | 22 | METLIN |
| 5 | Syringic acid | C9H10O5 | 7.5 | 199.0577 [M + H]+ | 12 | METLIN |
| 6 | Dihydrochalcone | C15H14O | 8.0 | 211.1081 [M + H]+ | 17 | METLIN |
| 7 | Apovincamine | C21H24N2O2 | 8.2 | 359.1713 [M + Na]+ | 4 | METLIN |
| 8 | Tricycloekasantal | C12H18O | 8.5 | 201.1228 [M + Na]+ | 10 | METLIN |
| 9 | Typhaneoside | C34H42O20 | 8.7 | 771.2313 [M + H]+ | 3 | METLIN |
| 10 | Rutin | C27H30O16 | 8.9 | 611.1607 [M + H]+ | 0 | METLIN |
| 11 | Behenic acid | C22H44O2 | 10.3 | 366.3356 [M + Na]+ | 17 | METLIN |
3.3H. undatus induced neuroprotection in C. elegans
The neuroprotective potential of H. undatus was monitored by treating the extract at 8–10μg/ml to the transgenic strain of C. elegans CL2006, that express Aβ1 - 42 constitutively and the level of survival was monitored. It was observed that the extract was able to significantly (p < 0.05) extend the lifespan of the transgenic strain by 19, 20 and 20 days wherein the untreated control survived up to 17 days (Fig. 3A-B).
Fig. 3
H. undatus extract can impart neuroprotection in Aβ transgenic strain CL2006 (A) Maximum lifespan upon treatment with 8 –10μg/ml (B) H. undatus extract (8 –10μg/ml) prolonged the lifespan in CL2006 nematodes to offer neuroprotection against Aβ expression (C) Treatment with H. undatus extract significantly (p < 0.05) diminished Aβ-induced paralysis when compared to control.
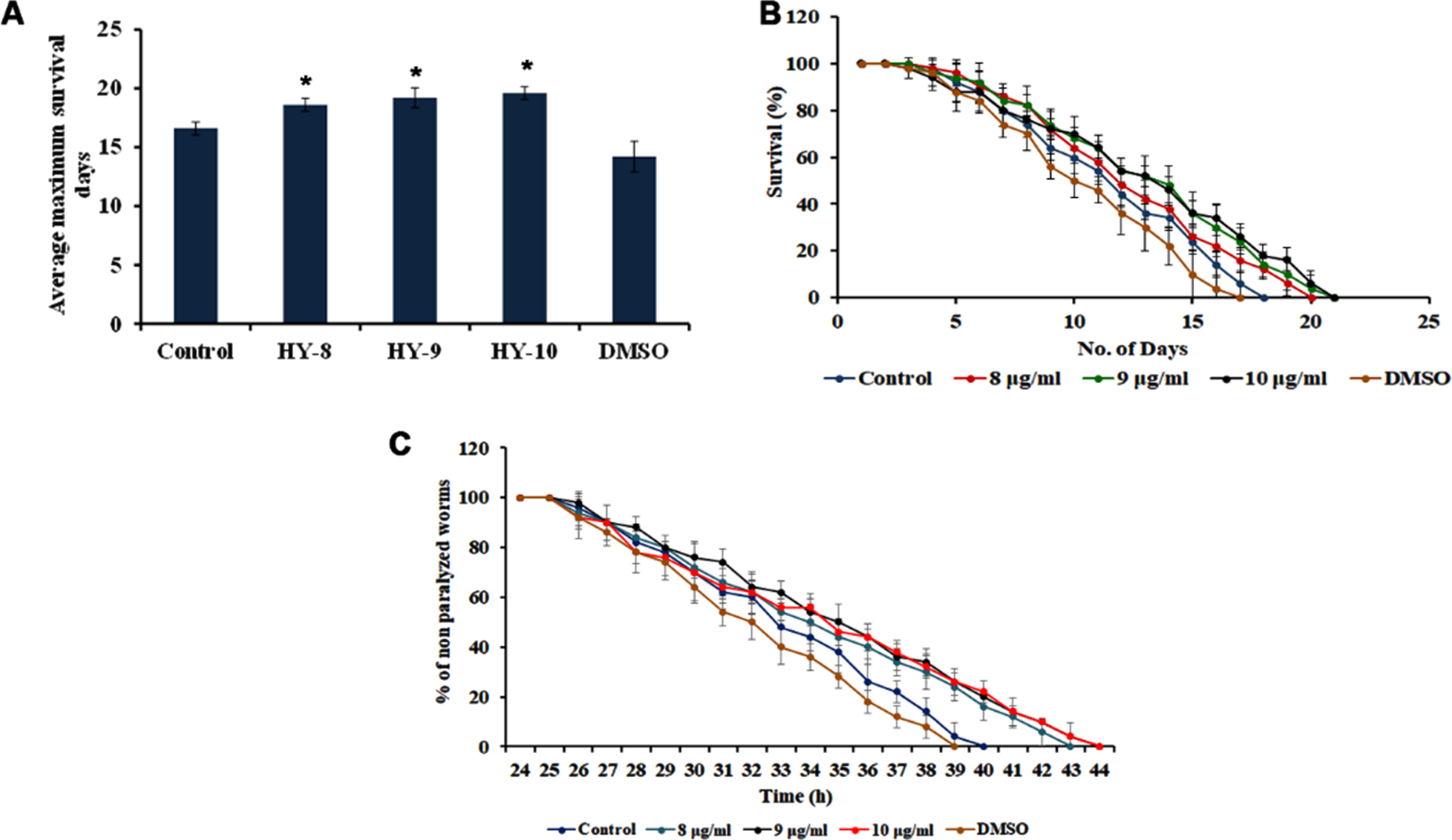
The transgenic strain CL4176, which expresses Aβ1 - 42 in the body wall muscles while temperature upshift, will lead to the paralysis of the nematodes. The H. undatus extract was able to significantly (p < 0.05) delay the paralysis of the CL4176 worms, as the worms remained active until 43, 44, and 44 h after treatment with H. undatus extract when compared to control, which remained active until 40 h (Fig. 3C) suggesting the neuroprotective potential of the extract.
3.4H. undatus potentiates antioxidant effect by activating SKN-1
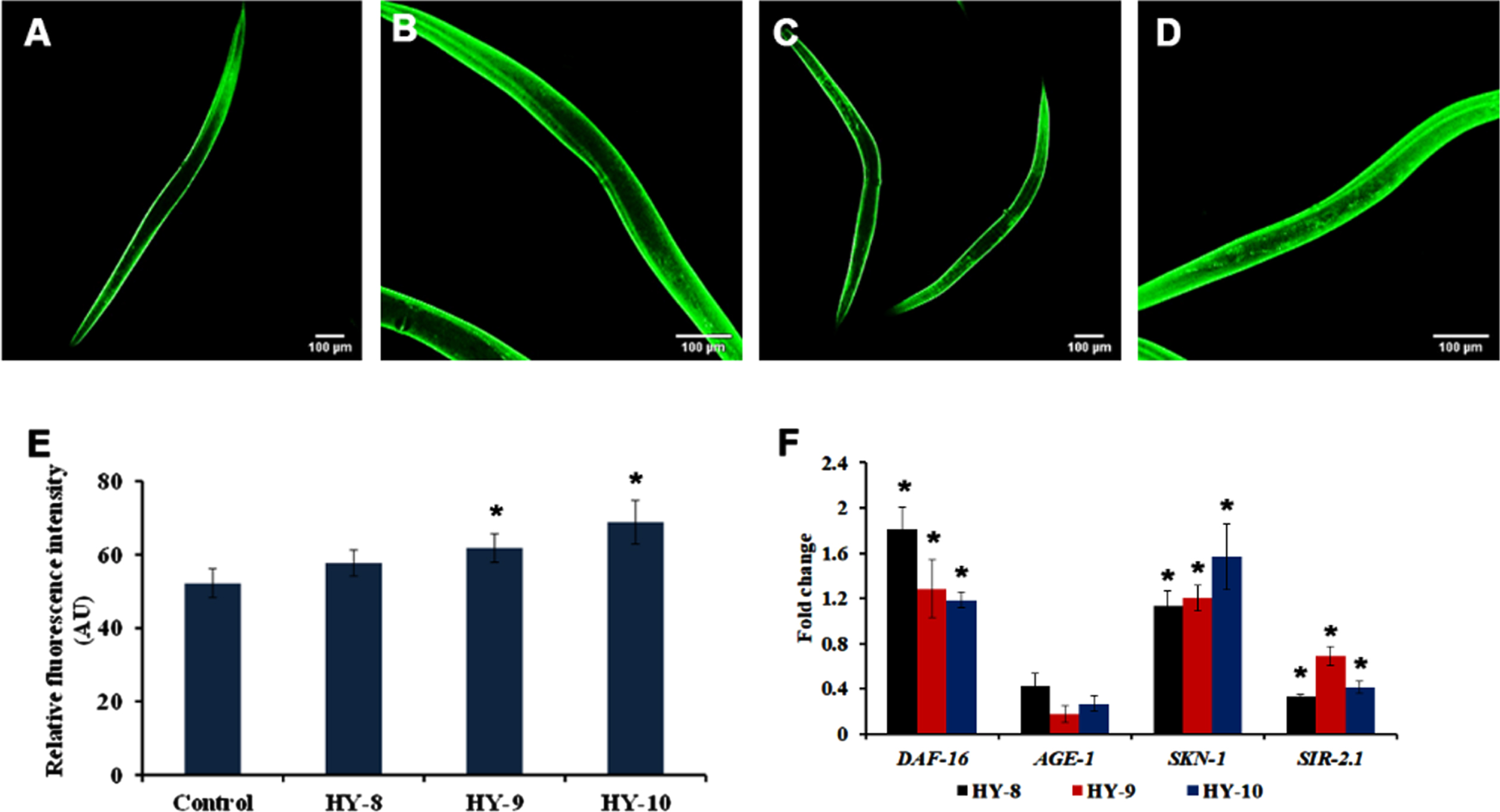
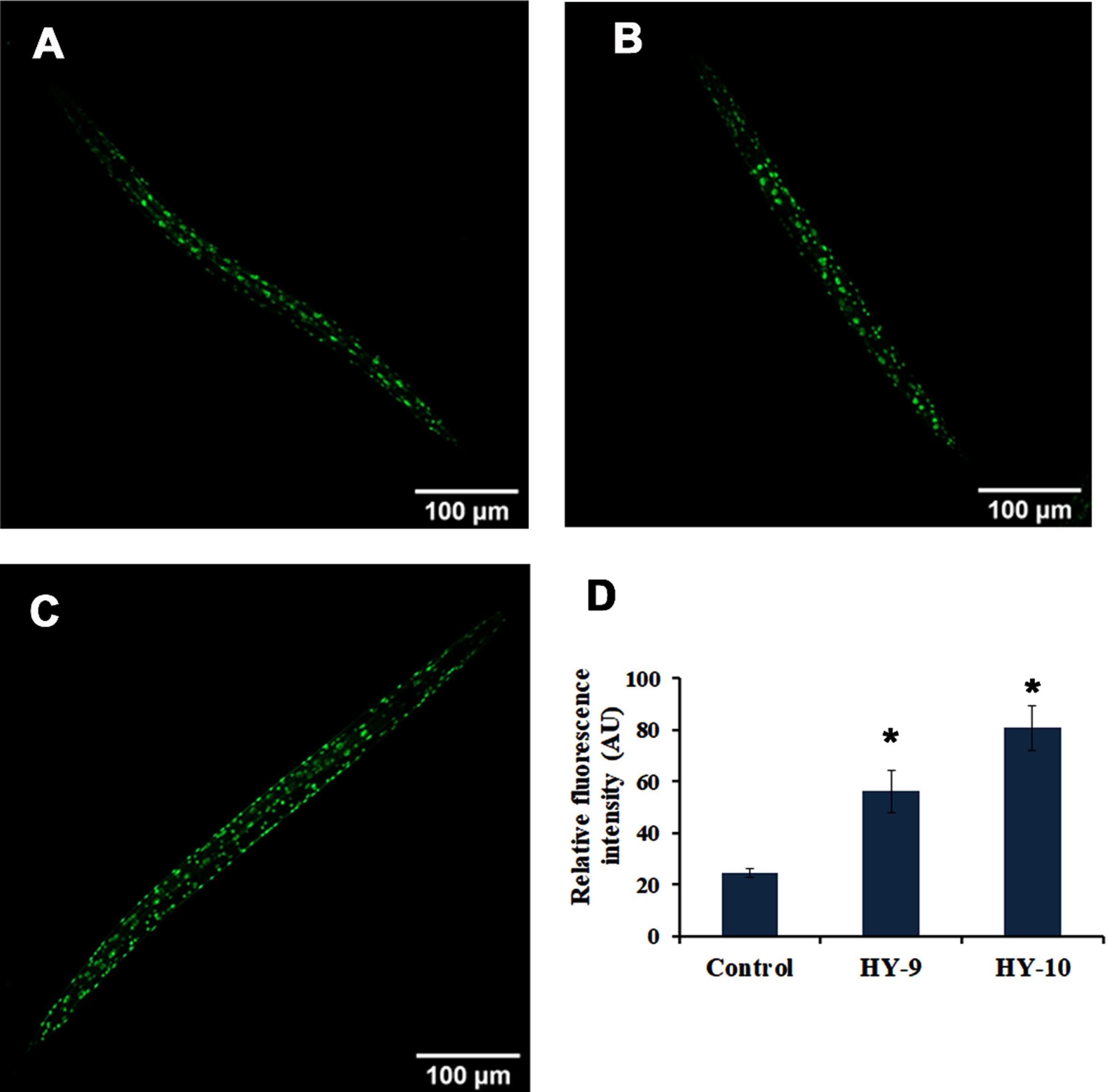
SKN-1 is one of the predominant mediators of antioxidant activity in C. elegans. The effect of H. undatus in activating SKN-1 was monitored using a skn-1::GFP transgenic strain, LG333. It was observed that the extract (8–10μg/ml) treated worms were able to significantly activate SKN-1 when compared to untreated controls (Fig. 4A–E). Additionally, qPCR expression of candidate antioxidant genes, skn-1 and sir-2.1 were analyzed in wild type nematodes treated with H. undatus extract. Both the genes expressed significant upregulation indicating the activation of antioxidant potential (Fig. 4F).
Fig. 4
H. undatus extract can activate antioxidant potential by activating SKN-1 in C. elegans. (A) LG333 Control (B-D) LG333 treated with 8 –10μg/ml of H. undatus extract. (E) Quantification of fluorescence indicates significant increase in expression of SKN-1 at concentrations 9 and 10μg/ml of H. undatus extract (F) Real Time PCR analysis of daf-16, age-1, skn-1 and sir-2.1 was done in nematodes treated with 8 –10μg/ml of H. undatus extract.
3.5H. undatus mediated effects are dependent on DAF-16 pathway
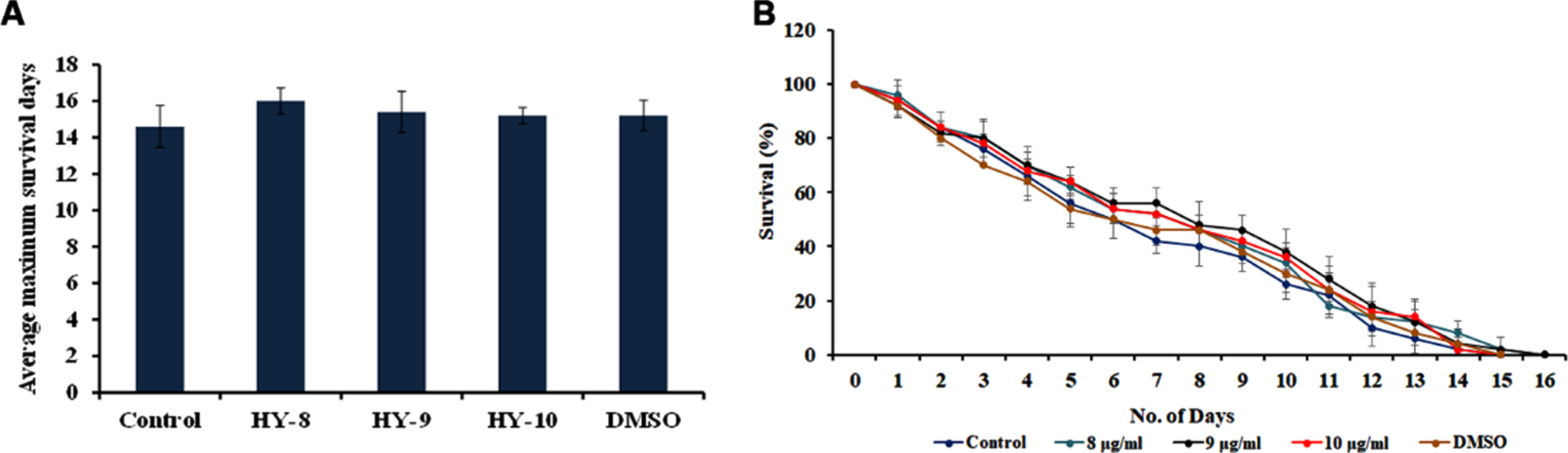
The DAF-16 mediated pathway is one of the predominant pathways that regulate anti-aging. The mutants of DAF-2 and DAF-16, two predominant regulators of the pathway were monitored for their lifespan after treatment with H. undatus extract at 8–10μg/ml. It was observed that the DAF-2 mutant worms survived up to 50, 50 and 50 days when compared to untreated controls, which survived up to 49 days (Fig. 5). In the case of DAF-16 mutant worms, the extract treated worms survived up to 16, 16 and 15 days whereas, the untreated control survived up to 15 days (Fig. 6). The results suggest that the lifespan extension mediated by H. undatus extract could be mediated by the DAF-16 pathway. To further support this, qPCR expression of daf-16 and age-1 was analyzed in wild type nematodes treated with H. undatus extract, which showed the upregulation of daf-16 indicating the activation of the pathway (Fig. 4F) thereby exhibiting anti-aging property.
Fig. 5
H. undatus mediated lifespan extension is dependent of DAF-16 pathway (A) Maximum lifespan of daf-2 mutants showed no significant change when treated with 8 –10μg/ml of H. undatus extract (B) Graph representing the average of maximum lifespan extension of daf-2 mutants when treated with 8 –10μg/ml of H. undatus extract.
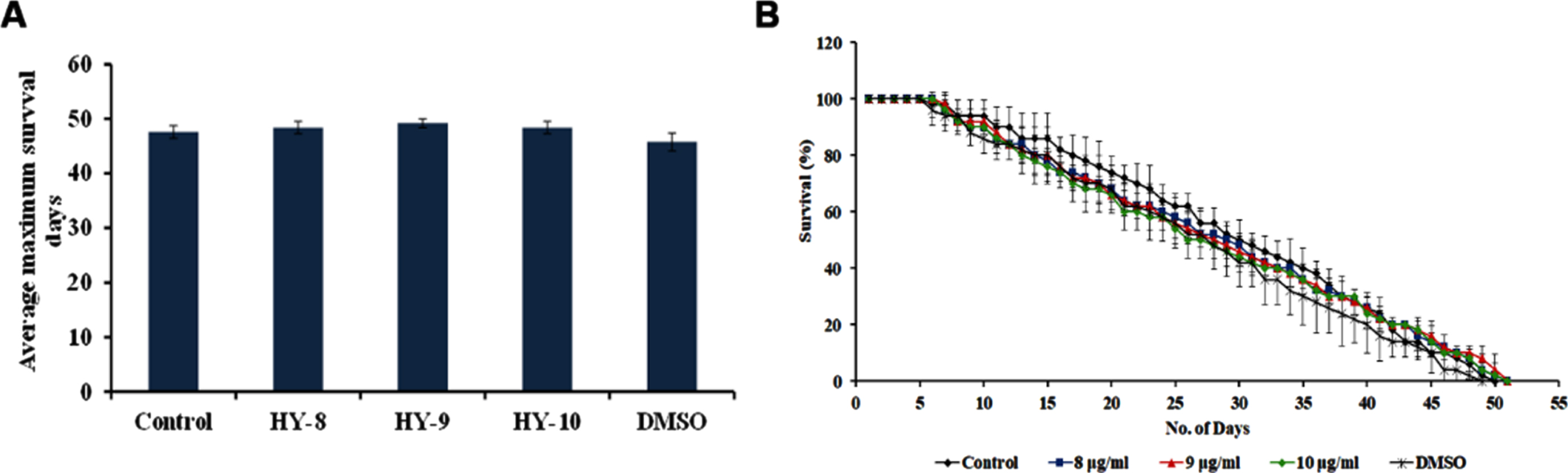
Fig. 6
H. undatus mediated lifespan extension is dependent of DAF-16 pathway (A) Maximum lifespan of daf-16 mutants showed no significant change when treated with 8 –10μg/ml of H. undatus extract (B) Graph representing the average of maximum lifespan extension of daf-16 mutants when treated with 8 –10μg/ml of H. undatus extract.
Additionally, the effect of H. undatus in activating the nuclear translocation of DAF-16 was monitored using a daf-16::GFP transgenic strain, TJ356. It was observed that the extract (9–10μg/ml) treated worms were able to significantly (p < 0.05) improve the nuclear translocation of DAF-16 when compared to untreated control (Fig. 7).
Fig. 7
H. undatus extract can improve nuclear translocation of DAF-16 in C. elegans. (A) TJ356 Control (B-C) TJ356 treated with 9–10μg/ml of H. undatus extract. (E) Quantification of fluorescence indicates significant increase in expression of DAF-16 at concentrations 9 and 10μg/ml of H. undatus extract.
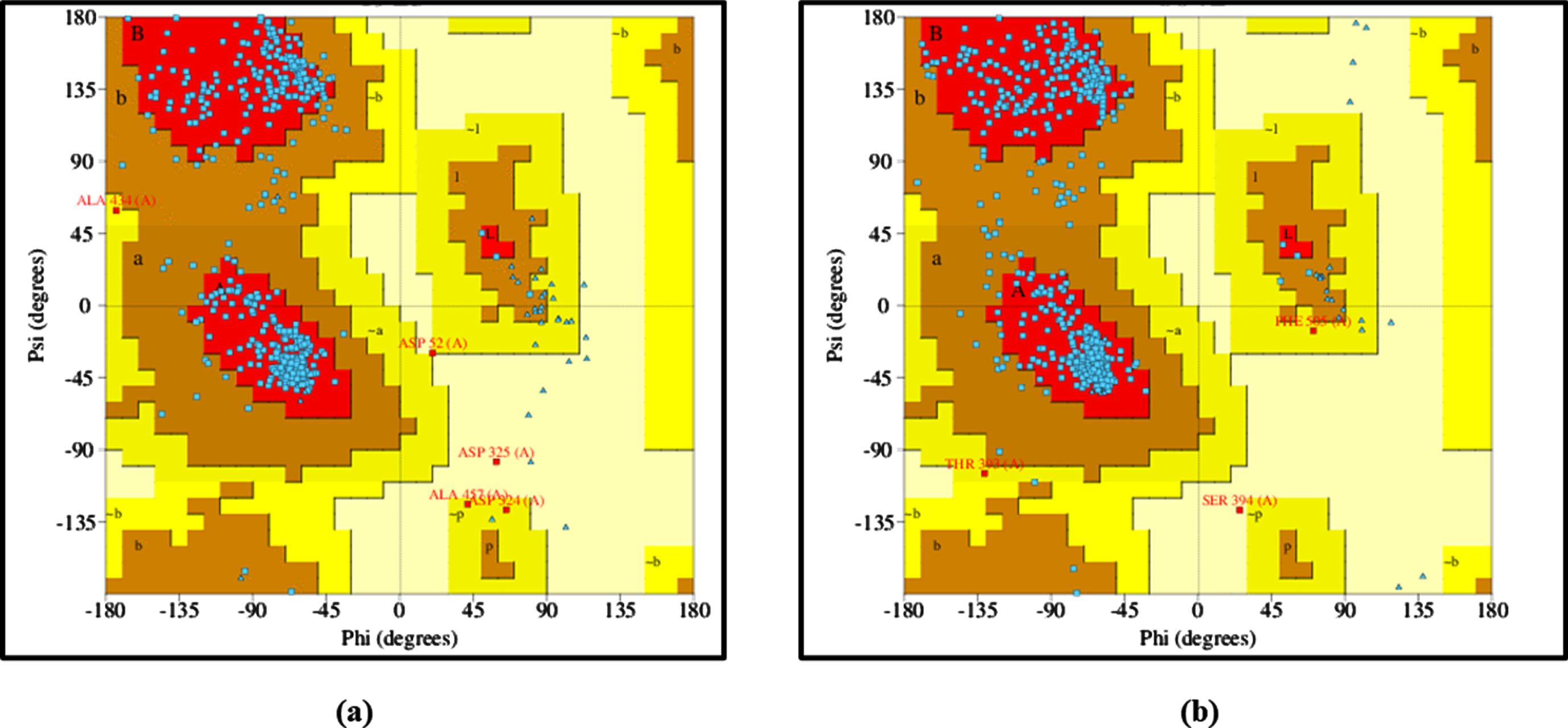
3.6In silico analysis confirms the effects of H. undatus
The 3D structures of DAF-16 and SKN-1 protein models were generated using trROSETTA and was estimated for its stereochemical quality check using PDBSUM. The Ramachandran plot clearly depicts that the angles psi and phi are mainly presented in favored regions of the plot and mostly lie in the allowed regions, indicating that protein model is reliable (Fig. 8).
Fig. 8
Ramachandran Plot analysis of (a) DAF-16 and (b) SKN-1.
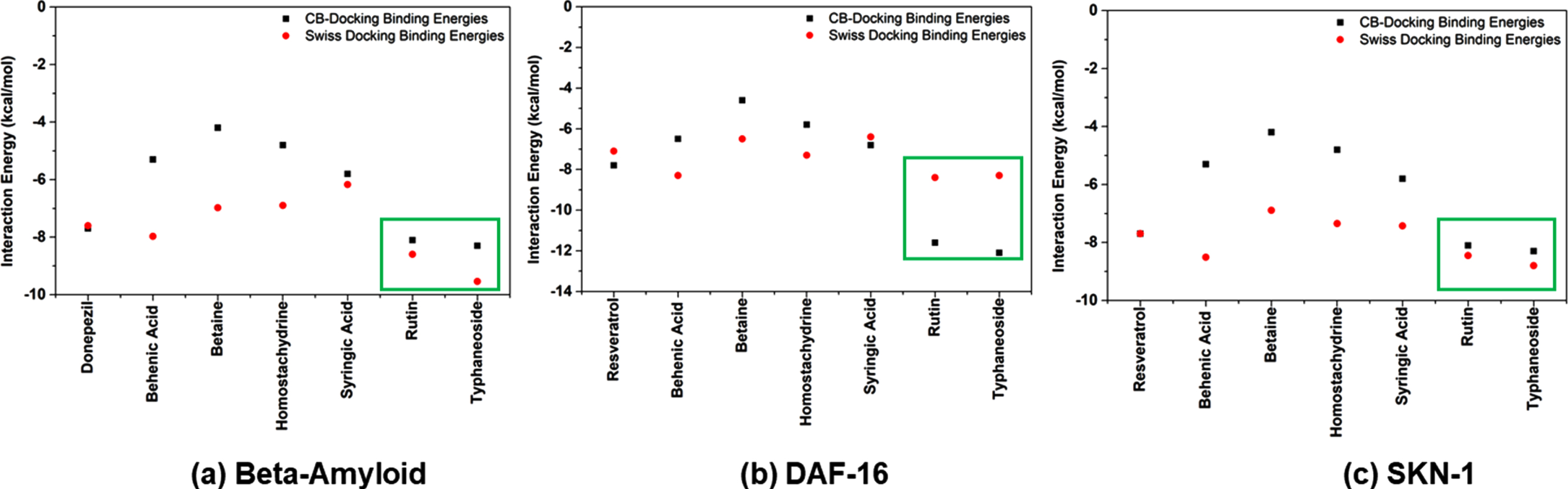
The interaction pattern of phytocompounds identified through LC-MS/MS analysis (homostachydrine, typhaneoside, behenic acid, betaine, syringic acid, and rutin) with target proteins Aβ, DAF-16 and SKN-1 were analyzed through molecular docking approach. The reference drugs, Donepezil (Aβ) and Resveratrol (DAF-16 and SKN-1) showed binding affinity of –7.66, –7.10 and –7.71 kcal/mol in Swiss Dock analysis and –7.7, –7.8 and –7.7 kcal/mol in CB-Dock analysis respectively. Among the 6 phytocompounds, rutin and typhaneoside were able to show better binding affinities towards the target proteins. In the case of Aβ, rutin and typhaneoside showed binding affinity of –8.6, and –8.1 kcal/mol in Swiss Dock and –9.5, –8.3 kcal/mol in CB-Dock respectively. The binding affinity of these compounds against DAF-16 was identified as –8.4, and –8.5 kcal/mol in Swiss Dock and –11.6, and –12.1 kcal/mol in CB-Dock respectively. Likewise, against SKN-1, the binding efficacy of the compounds were –8.4, and –8.8 kcal/mol in Swiss Dock and –9.1, and –9.3 kcal/mol in CB-Dock respectively (Fig. 9).
Fig. 9
Docking Simulation analysis of phytocompounds with (a) Aβ (b) DAF-16 and (c) SKN-1 using Swiss Dock and CB-Dock programs.
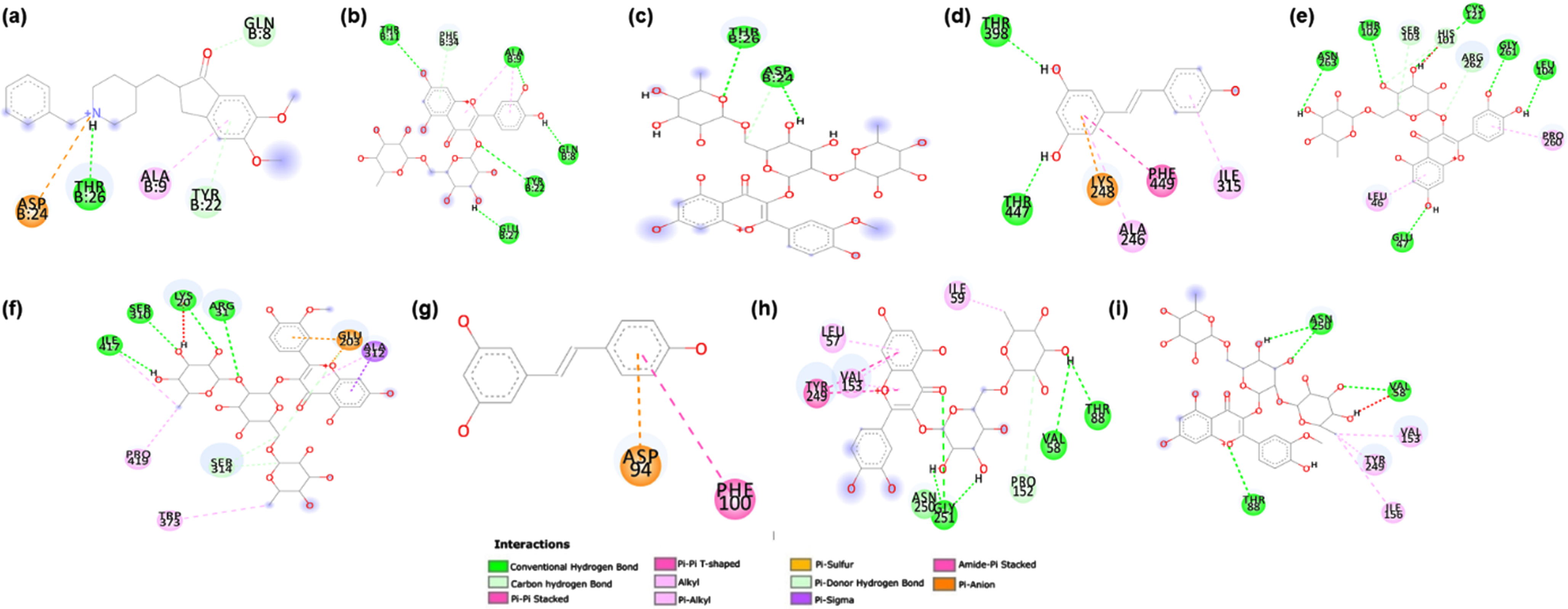
Further, the ligand interacting residues for rutin and typhaneoside were analyzed against each target protein along with the reference drug using Discovery Studio Visualizer 2019 (Biovia DS. Discovery studio visualizer) (Fig. 10). It was observed that the phytocompounds and reference drugs were able to maintain different types of interactions with different amino acids of the target proteins, wherein common residue interactions were present. In the case of Aβ interaction with rutin and typhaneoside, the amino acids Thr11, Gln8, Glu27, Thr26, ASP24, Tyr22 and Ala9 were observed to form Hydrogen bond interactions, which is main interaction force for the binding. Similarly, in DAF-16, the amino acid residues Thr398, Thr447, Thr102, Asn263, Gly261, Leu104, Glu47, Cys121, Ile417, Ser310, Lys20 and Arg31 contributed to the hydrogen bond interaction with the ligands. Interestingly, there were no hydrogen bonds interaction between SKN-1 and Resveratrol, and only Pi-sulfur and Pi-Pi interactions were maintained by residues Asp and Phe respectively, whereas, rutin and typhaneoside were able to maintain Hydrogen bond interactions with the target protein SKN-1 via residues Asn250, Gly251, Val58 and Thr88. Overall, from the results of interaction study we conclude that Hydrogen bond interactions are the major forces for strong and efficient binding of rutin and typhaneoside towards target protein Aβ, DAF-16 and SKN-1.
Fig. 10
Ligand interacting residues for rutin and typhaneoside against Aβ, DAF-16 and SKN-1 along with the reference drug using Discovery Studio Visualizer 2019.
4Discussion
Aging is a conserved and unique degenerative process characterized by loss of cellular contents and functions leading to many age-related diseases including neurodegenerative diseases and eventually leading to mortality. Phytochemicals from plant sources, majorly polyphenols, terpenoids, alkaloids, saponins, phytosterols, and organosulfur compounds, are being identified for their medicinal and nutraceutical properties [43]. The anti-aging effects of these phytochemicals are mostly related to their anti-oxidant properties and their ability to scavenge free radicals, thereby preventing and treating many age-related disorders [44]. The C. elegans model, with 60–80% genome similarity with humans, can be used as a model for aging research using plant species [43].
H. undatus is one such plant with fruits having many traditional, medicinal and commercial benefits. It has already reported to have antioxidant, anti-inflammatory, anti-obesity, antidiabetic, anti-cancer and anti-proliferative activities [11]. In the present study, H. undatus extract exhibited anti-aging activity by significantly enhancing the lifespan of C. elegans (Fig. 1). Additionally, the extract was also able to reduce the accumulation of lipofuscin (Fig. 2) suggesting the possible role of the plant as an anti-aging agent. Previous reports of plant extracts including Cleistocalyx nervosum [22, 23], Bacopa monnieri [24], Streblus asper [25], Hibiscus sabdariffa [26], and Kaempferia parviflora [27, 28] in C. elegans showed extension of lifespan and reduction of lipofuscin accumulation indicating its anti-aging potential.
Even though crude extracts may exert therapeutic effects, the components present inside it are responsible for the specific effects. It is therefore important to elucidate and characterize the specific components, as this will eventually pave the way for new strategic pharmacological designs [43]. In this regard, the extract was subjected to LC-MS/MS analysis and the major components present inside the extract were identified. Homostachydrine, typhaneoside, behenic acid, betaine, syringic acid, and rutin were the major components identified in the present study, which were already been reported for their presence in the fruit apart from lepidine alkaloid (B, D, E, F), malvidin 3-(6”-p-coumaryl glucoside)-5-dimalonylglucoside, dihydrochalcone, apovincamine, and tricycloekasantal (Table 2).
Homostachydrine is a compound found majorly in citrus and coffee plants [45, 46]. It was reported to potentially deteriorate pentylenetetrazole induced seizures in mice models, thereby control brain neurons [47]. Syringic acid, a natural phenolic compound, has been found in medicinal herbs and dietary plants, and is reported to have anti-oxidative, chemoprotective, anti-angiogenic, anti-glycation, anti-proliferative, anti-hyperglycemic, anti-endotoxic, anti-microbial, anti-inflammatory, anti-diabetic, anti-depressant, and neuroprotective properties, along with extending anti-aging and cognitive functions [48, 49].
Behenic acid belongs to the class of very long chain fatty acids, which can modulate metabolic syndromes, cardiovascular disorders, diabetes and other age-associated diseases [50–52] and thereby promote healthy aging [53]. Betaine, a small compound present in Lycium barbarum can exert anti-aging and neuroprotective properties [54]. The compound can induce autophagy and inhibit the accumulation of Aβ [55]. Additionally, it can protect liver health, preserve myocardial function, and prevent pancreatic steatosis, along with regulating oxidative, endoplasmic reticulum stress, inflammation, and cancer development [56].
Typhaneoside, a compound identified to reach the brain of mice after oral intake [57], was identified in Shaofu Zhuyu decoction showing analgesic potential [58], and the pollen of Typha angustata inhibiting rat aortic vascular smooth muscle cell proliferation [59]. Typha angustifolia has been reported to exhibit anti-nociceptive properties wherein, typhaneoside was the major constituent behind it [60]. The compound was observed to improve cardiac morphological structure and myocardial remodeling by modulating interleukins and matrix metalloproteases in rats thereby enhancing heart function. Additionally, it can modulate autophagy through the PI3K/Akt/mTOR pathway [61]. It can also suppress the release of glutamate by inhibiting the voltage-dependent calcium entry in rat cerebrocortical nerve terminals via the Mitogen Activated Protein Kinase (MAPK) pathway [62].
Rutin, a glycoside of the flavonoid quercetin, was found in many plants and fruits, including buckwheat, apricots, cherries, grapes, grapefruit, plums, and oranges. The compound could reduce the proinflammatory cytokines, improve antioxidant enzyme activities, activate mitogen-activated protein kinase cascade, downregulate mRNA expression of proapoptotic genes, upregulate the ion transport and antiapoptotic genes, and restore the activities of mitochondrial complex enzymes [63]. The compound was also identified in mung beans, which aided in improving the neuroprotective potential [64]. The presence of rutin in crude extracts enables the extract to have antioxidant properties thereby reducing ROS production or activating antioxidant mechanisms, and reduce metabolic disorders including cardiac disorders [65, 66].
H. undatus extract was able to extend the lifespan of transgenic strain expressing Aβ (Fig. 3), indicating its neuroprotective potential [67]. Another Aβ transgenic strain, CL4176, expresses Aβ in the body wall muscle, which is accompanied by paralysis upon temperature upshift from 15 °C to 25 °C [32]. The H. undatus extract was able to significantly delay the paralysis phenotype suggesting that it could induce neuroprotection in C. elegans (Fig. 3). Further, the molecular docking analysis was done to identify the effect of phytochemicals identified from H. undatus against Aβ using Donepezil, one of the most widely used, FDA approved drug which inhibit cholinesterase activity [68], as the reference.
Molecular docking is an important approach for the process of drug discovery. There are many programs for docking which are based on different algorithms. This approach can be utilized to model the ligand and protein interactions at the atomic level, which also allows to depict the behavior of ligand molecule in the protein binding pocket. Moreover, it helps to explain the fundamental biochemical processes of the ligand when in complex with protein [36]. The docking tools used in the present study were Swiss Dock and CB-Dock, which can be used for blind docking and to calculate the energies involved. Swiss Dock is based on EADock DSS engine which is joined through setup scripts, which helps in regulating common problems and in preparing the input files of both protein and the ligand molecules. Also, it searches for the thermodynamically favorable sites for ligand binding on the protein and calculates the energies based on CHARMM force field on the cluster for the docked poses [37, 39]. CB-Dock web server is based on cavity detection-guided blind docking of protein and ligand. This server predicts the ligand binding site of the target protein and evaluates the various centers and sizes with a novel curvature-based cavity detection method. To be noted, the CB-Cock executes docking with a popular docking approach, AutoDock Vina [38]. The phytochemicals in this study, rutin and typhaneoside, exhibited similar binding affinity to that of donepezil indicating that they can have significant activity against Aβ (Fig. 8). A recent study reported that H. undatus extract was able to induce neuroprotection by activating antioxidant mechanism in zebrafish model [69].
H. undatus extract was reported to have antioxidant potential, along with anti-aging and neuroprotection [29, 30, 69]. The betacyanins purified from the fruit peels were able to reduce obesity in high fat fed mice, indicating its antioxidant potential [70]. In this regard, the present study analyzed the expression levels of skn-1 and sir-2.1, which are the major regulators of antioxidant mechanism in C. elegans [71] and observed that the expression of skn-1 was significantly higher indicating the activation of the gene (Fig. 4). Further, the transgenic strain of skn-1 pointed towards the activation of skn-1 after treatment with the extract (Fig. 4) [72]. This was validated by the molecular docking approach, which showed that rutin and typhaneoside were able to bind efficiently with SKN-1, when compared to the reference drug, resveratrol (Fig. 4), validating its antioxidant potential. It has to be noted that, the fruit peels were already being used in food industry as a dietary antioxidant without affecting the quality and acceptability of products, rather improving it [73].
Further, the mechanism of action initiated by H. undatus extract was investigated. It has been reported that forkhead box O (FoxO) transcription factors, which corresponds to DAF-16 mediated pathway in C. elegans, had been implicated in the regulation of aging and longevity, thereby extending neuroprotection [74, 75]. In C. elegans, molecular cloning and characterization studies revealed that DAF-2 encodes a receptor tyrosine kinase homolog, AGE-1, a phosphatidyl inositol-3-kinase (PI3K) homolog, and DAF-16, a FoxO transcription factor, which together are termed as insulin/insulin-like growth factor 1 (IGF-1) signaling (IIS) pathway [76]. The H. undatus extract was not able to bring in any significant changes in the lifespan of DAF-2 and DAF-16 mutants, indicating that the lifespan extension and neuroprotection exhibited by the extract could be dependent on the pathway (Fig. 5, 6).
The qPCR analysis of candidate genes of the pathway, daf-16 and age-1 were quantified in wild type nematodes treated with H. undatus extract. It was observed that both the genes were upregulated wherein daf-16 expressed significant upregulation indicating its activation (Fig. 4) which aided the anti-aging potential [77]. Also, the daf-16 transgenic strains showed significant upregulation of nuclear accumulation of DAF-16 while treated with H. undatus extract (Fig. 7). The nuclear localization of DAF-16 is very crucial in the activation of anti-aging, stress resistance and neuroprotection [78–80]. Multiple pathways including mitogen activated protein kinase pathway and mammalian target of rapamycin aids in the nuclear localization of DAF-16, thereby activating DAF-16 and induce longevity [81]. Finally, molecular docking analysis were done with the phytochemicals identified from H. undatus extract. It was observed that both rutin and typhaneoside were able to bind efficiently with DAF-16, when compared to the reference drug, resveratrol (Fig. 9). Our study has thereby validated the role of DAF-16/FOXO and SKN-1 in exerting anti-aging and neuroprotective effects.
5Conclusion
Aging and age-associated neurodegeneration is on the rise and the use of natural therapies is on demand to reduce the severity. Hylocereus undatus is a fruit with medicinal, economic and commercial values. The present study shows that the fruit peels can exert anti-aging, neuroprotective and antioxidant mechanism via DAF-16 mediated pathway through in vivo and in silico approach. Further, clinical trials have to be done to validate the impact of this fruit, peels and related compounds in humans.
Funding
This study was supported by a Grant from the Ratchadaphiseksomphot Endowment Fund (T.T).
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
M.I.P. and J.M.B. wish to thank the Ratchadaphiseksomphot Endowment Fund for Postdoctoral Fellowship and Chulalongkorn University, Thailand, for the support. D.S.M wishes to thank the Second Century Fund (C2F), Chulalongkorn University, Thailand, for the support. The authors would like to thank Natural Products for Neuroprotection and Anti-ageing Research Unit and Ratchadaphiseksomphot Endowment Fund for the support.
References
[1] | Rivas F , Poblete-Aro C , Pando ME , Allel MJ , Fernandez V , Soto A , Nova P , Garcia-Diaz D . Effects of Polyphenols in Aging and Neurodegeneration Associated with Oxidative Stress. Curr Med Chem. (2022) ;29: (6):1045–60. |
[2] | Peterson CT . Dysfunction of the Microbiota-Gut-Brain Axis in Neurodegenerative Disease: The Promise of Therapeutic Modulation With Prebiotics, Medicinal Herbs, Probiotics, and Synbiotics. J Evid Based Integr Med. (2020) ;25: :2515690X20957225. |
[3] | Ortiz-Hernández YD , Carrillo-Salazar JA . Pitahaya (Hylocereus spp.): A short review. Comunicata Scientiae. (2012) ;3: (4):220–37. |
[4] | Shetty AA , Rana MK , Preetham SP . Cactus: a medicinal food. J Food Sci Technol. (2012) ;49: (5):530–6. |
[5] | Oguntibeju OO . Medicinal plants and their effects on diabetic wound healing. Vet World. (2019) ;12: (5):653–63. |
[6] | Jalgaonkar K , Mahawar MK , Bibwe B , Kannaujia P . Postharvest Profile, Processing and Waste Utilization of Dragon Fruit (Hylocereus Spp.): A Review. Food Rev Int. (2022) ;38: (4):733–59. |
[7] | Mizrahi Y , Nerd A . Climbing and columnar cacti- new arid lands fruit crops. In: Janick J. ed. Perspective in new crops and new crops uses. ASHS Press, Alexandria, USA. (1999) ; 358–66. |
[8] | Ankli A , Sticher O , Rich MH . Yucatec maya medicinal plants versus non medicinal plants: indigenous characterization and selection. Hum Ecol. (1999) ;27: (4):557–80. |
[9] | Hopkins AL , Stepp JR . Distribution of herbal remedy knowledge in Tabi, Yucatan, Mexico. Econ Bot. (2012) ;66: (3):249–54. |
[10] | Das G , Lim KJ , Tantengco OAG , Carag HM , Gonçalves S , Romano A , Das SK , Barrera EC , Shin HS , Grijalva EPG , Heredia JB , Patra JK . Cactus: Chemical, nutraceutical composition and potential bio-pharmacological properties. Phytother Res. (2021) ;35: (3):1248–83. |
[11] | de Araújo FF , de Paulo Farias D , Neri-Numa IA , Pastore GM . Underutilized plants of the Cactaceae family: Nutritional aspects and technological applications. Food Chem. (2021) ;362: :130196. |
[12] | Charoensiri R , Kongkachuichai R , Suknicom S , Sungpuag P . Beta-carotene, lycopene, and alpha-tocopherol contents of selectedThai fruits. Food Chem. (2009) ;113: :202–7. |
[13] | Attar ŞH , Gündesli MA , Urün I , Kafkas S , Kafkas NE , Ercisli S , Ge C , Mlcek J , Adamkova A . Nutritional Analysis of Red-Purple and White-Fleshed Pitaya (Hylocereus) Species. Molecules. (2022) ;27: (3):808. |
[14] | Ariffin AA , Bakar J , Tan CP , Rahman RA , Karim R , Loi CC . Essential Fatty Acids of Pitaya (Dragon Fruit) Seed Oil. Food Chem. (2009) ;114: (2):561–4. |
[15] | Gunasena HPM , Pushpakumara DKNG , Kariyawasam M . Dragon Fruit Hylocerus undatus Haw. Britton and Rose. In Underutilized Fruit Trees in Sri Lanka; Pushpakumara D. K.N.G., Gunasena H.P.M., Singh V.P., Eds.; World Agroforestry Centre, South Asia Office: New Delhi, 2007; pp. 110-141. |
[16] | Perez RM , Varga R , Ortiz YD . Wound healing properties of Hylocereus undatus on diabetic rats. Phytother Res. (2005) ;19: (8):665–8. |
[17] | Nurliyana R , Syed Zahir I , Mustapha Suleiman K , Aisyah MR , Kamarul Rahim K . Antioxidant Study of Pulps and Peels of Dragon Fruits: A Comparative Study. Int Food Res J. (2010) ;17: :367–75. |
[18] | Lourith N , Kanlayavattanakul M . Antioxidant and Stability of Dragon Fruit Peel Colour. Agro FOOD Ind. Hi Tech. (2013) ;24: (3):56–8. |
[19] | Kumar SB , Issac R , Prabha ML . Functional and health-promoting bioactivities of dragon fruit. Drug Invent Today. (2018) ;10: (Special Issue 3):3307–10. |
[20] | Wichienchot S , Jatupornpipat M , Rastall RA . Oligosaccharides of pitaya (dragon fruit) flesh and their prebiotic properties. Food Chem. (2010) ;120: (3):850–7. |
[21] | Dixit A , Bhattacharya B . Sensory perception of environmental cues as a modulator of aging and neurodegeneration: Insights from Caenorhabditis elegans . J Neurosci Res. (2021) ;99: (10):2416–26. |
[22] | Prasanth MI , Brimson JM , Chuchawankul S , Sukprasansap M , Tencomnao T . Anti-aging, stress resistance and neuroprotective efficacies of Cleistocalyx nervosum var. paniala fruit extracts using Caenorhabditis elegans model. Oxid Med Cell Longev. (2019) ;2019: :7024785. |
[23] | Brimson JM , Prasanth MI , Isidoro C , Sukprasansap M , Tencomnao T . Cleistocalyx nervosum var. paniala seed extracts exhibit sigma-1 antagonist sensitive neuroprotective effects in PC12 cells and protect C. elegans from stress via the SKN-1/NRF-2 pathway. Nutrition and Healthy Aging. 2021. DOI: 10.3233/NHA-200108. |
[24] | Brimson JM , Prasanth MI , Plaingam W , Tencomnao T . Bacopa monnieri (L.) wettst. Extract protects against glutamate toxicity and increases the longevity of Caenorhabditis elegans. J Tradit Complement Med. (2019) ;10: (5):460–70. |
[25] | Prasanth MI , Brimson JM , Malar DS , Prasansuklab A , Tencomnao T . Streblus asper Lour. exerts MAPK and SKN-1 mediated anti-aging, anti-photoaging activities and imparts neuroprotection by ameliorating Aβ in Caenorhabditis elegans. Nutrition and Healthy Aging. 2021. DOI: 10.3233/NHA-210121. |
[26] | Malar DS , Prasanth MI , Brimson JM , Verma K , Prasansuklab A , Tencomnao T . Hibiscus sabdariffa extract protects HT-22 cells from glutamate-induced neurodegeneration by upregulating glutamate transporters and exerts lifespan extension in C. elegans via DAF-16 mediated pathway. Nutrition and Healthy Aging. 2021. DOI: 10.3233/NHA-210131. |
[27] | Tonsomboon A , Prasanth MI , Plaingam W , Tencomnao T . Kaempferia parviflora Rhizome Extract Inhibits Glutamate-Induced Toxicity in HT-22 Mouse Hippocampal Neuronal Cells and Extends Longevity in Caenorhabditis elegans . Biology. (2021) ;10: (4):264. |
[28] | Prasanth MI , Malar DS , Brimson JM , Verma K , Tonsomboon A , Plaingam W , Tencomnao T . DAF-16 and SKN-1 mediate Anti-aging and Neuroprotective efficacies of “thai ginseng” Kaempferia parviflora Rhizome extract in Caenorhabditis elegans. Nutrition and Healthy Aging. 2022. DOI: 10.3233/NHA-210148. |
[29] | Tamagno WA , Vanin AP , Sutorillo NT , Bilibio D , Dada RA , Colla LM , Zamberlan DC , Kaizer RR , Barcellos LJG . Fruit extract of red pitaya (Hylocereus undatus) prevents and reverses stress-induced impairments in the cholinergic and antioxidant systems of Caenorhabditis elegans. J Food Biochem. 2021;e13981. |
[30] | Tamagno WA , Santini W , Dos v A , Alves C , Bilibio D , Sutorillo NT , Zamberlan DC , Kaizer RR , Barcellos LJG . Pitaya fruit extract ameliorates the healthspan on copper-induced toxicity of Caenorhabditis elegans. J Food Biochem. (2022) ;46: (3):e14050. |
[31] | Brenner S . The genetics of Caenorhabditis elegans . Genetics. (1974) ;77: (1):71–94. |
[32] | DanQing L , YuJie G , ChengPeng Z , HongZhi D , Yi H , BiSheng H , Yan C . N-butanol extract of Hedyotis diffusa protects transgenic Caenorhabditis elegans from Aβ-induced toxicity. Phytother Res. (2021) ;35: (2):1048–61. |
[33] | Yang J , Anishchenko I , Park H , Peng Z , Ovchinnikov S , Baker D . Improved protein structure prediction using predicted interresidue orientations. Proc Natl Acad Sci U S A. (2020) ;117: (3):1496–503. |
[34] | Laskowski RA , Hutchinson EG , Michie AD , Wallace AC , Jones ML , Thornton JM . PDBsum: a Web-based database of summaries and analyses of all PDB structures. Trends Biochem Sci. (1997) ;22: (12):488–90. |
[35] | O’Boyle NM , Banck M , James CA , Morley C , Vandermeersch T , Hutchison GR . Open Babel: An open chemical toolbox. J Cheminform. (2011) ;3: :33. |
[36] | McConkey BJ , Sobolev V , Edelman M . The performance of current methods in ligand–protein docking. Curr Sci. (2002) ;83: (7):845–56. |
[37] | Grosdidier A , Zoete V , Michielin O . SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. (2011) ;39: (suppl_2):W270–7. |
[38] | Liu Y , Grimm M , Dai WT , Hou MC , Xiao ZX , Cao Y . CB-Dock: a web server for cavity detection-guided protein–ligand blind docking. Acta Pharmacol Sin. (2020) ;41: (1):138–44. |
[39] | Lohning AE , Levonis SM , Williams-Noonan B , Schweiker SS . A practical guide to molecular docking and homology modelling for medicinal chemists. Curr Top Med Chem. (2017) ;17: (18):2023–40. |
[40] | Angonese M , Motta GM , de Farias NS , Molognoni L , Daguer H , Brugnerotto P , de Oliveira Costa AC , Müller CMO . Organic dragon fruits (Hylocereus undatus and Hylocereus polyrhizus) grown at the same edaphoclimatic conditions: Comparison of phenolic and organic acids profiles and antioxidant activities. LWT –Food Sci Technol. (2021) ;149: :111924. |
[41] | Tang W , Li W , Yang Y , Lin X , Wang L , Li C , Yang R . Phenolic Compounds Profile and Antioxidant Capacity of Pitahaya Fruit Peel from Two Red-Skinned Species (Hylocereus polyrhizus and Hylocereus undatus). Foods. (2021) ;10: (6):1183. |
[42] | Ibrahim SRM , Mohamed GA , Khedr AIM , Zayed MF , El-Kholy AAES . Genus Hylocereus: Beneficial phytochemicals, nutritional importance, and biological relevance—A review. J Food Biochem. (2018) ;42: :e12491. |
[43] | Okoro NO , Odiba AS , Osadebe PO , Omeje EO , Liao G , Fang W , Jin C , Wang B . Bioactive Phytochemicals with Anti-Aging and Lifespan Extending Potentials in Caenorhabditis elegans . Molecules. (2021) ;26: (23):7323. |
[44] | Liu L , Guo P , Wang P , Zheng S , Qu Z , Liu N . The Review of Anti-aging Mechanism of Polyphenols on Caenorhabditis elegans . Front Bioeng Biotechnol. (2021) ;9: :635768. |
[45] | Servillo L , Giovane A , Balestrieri ML , Ferrari G , Cautela D , Castaldo D . Occurrence of pipecolic acid and pipecolic acid betaine (homostachydrine) in Citrus genus plants. J Agric Food Chem. (2012) ;60: (1):315–21. |
[46] | Servillo L , Giovane A , Casale R , Cautela D , D’Onofrio N , Balestrieri ML , Castaldo D . Homostachydrine (pipecolic acid betaine) as authentication marker of roasted blends of Coffea arabica and Coffea canephora (Robusta) beans. Food Chem. (2016) ;205: :52–7. |
[47] | Nishiyama M , Nakamichi N , Yoshimura T , Masuo Y , Komori T , Ishimoto T , Matsuo JI , Kato Y . Homostachydrine is a Xenobiotic Substrate of OCTN1/SLC22A4 and Potentially Sensitizes Pentylenetetrazole-Induced Seizures in Mice. Neurochem Res. (2020) ;45: (11):2664–78. |
[48] | Ogut E , Armagan K , Gül Z . The role of syringic acid as a neuroprotective agent for neurodegenerative disorders and future expectations. Metab Brain Dis. (2022) ;37: (4):859–80. |
[49] | Rutledge GA , Sandhu AK , Miller MG , Edirisinghe I , Burton-Freeman BB , Shukitt-Hale B . Blueberry phenolics are associated with cognitive enhancement in supplemented healthy older adults. Food Funct. (2021) ;12: (1):107–18. |
[50] | Yamazaki Y , Kondo K , Maeba R , Nishimukai M , Nezu T , Hara H . Proportion of nervonic acid in serum lipids is associated with serum plasmalogen levels and metabolic syndrome. J Oleo Sci. (2014) ;63: (5):527–37. |
[51] | Fretts AM , Mozaffarian D , Siscovick DS , Djousse L , Heckbert SR , King IB , McKnight B , Sitlani C , Sacks FM , Song X , Sotoodehnia N , Spiegelman D , Wallace ER , Lemaitre RN . Plasma phospholipid saturated fatty acids and incident atrial fibrillation: the Cardiovascular Health Study. J Am Heart Assoc. (2014) ;3: (3):e000889. |
[52] | Fretts AM , Imamura F , Marklund M , Micha R , Wu JHY , Murphy RA , Chien KL , McKnight B , Tintle N , Forouhi NG , Qureshi WT , Virtanen JK , Wong K , Wood AC , Lankinen M , Rajaobelina K , Harris TB , Djoussé L , Harris B , Wareham NJ , Steffen LM , Laakso M , Veenstra J , Samieri C , Brouwer IA , Yu CI , Koulman A , Steffen BT , Helmer C , Sotoodehnia N , Siscovick D , Gudnason V ; InterAct Consortium, Wagenknecht L , Voutilainen S , Tsai MY , Uusitupa M , Kalsbeek A , Berr C , Mozaffarian D , Lemaitre RN . Associations of circulating very-long-chain saturated fatty acids and incident type 2 diabetes: a pooled analysis of prospective cohort studies. Am J Clin Nutr. (2019) ;109: (4):1216–23. |
[53] | Bockus LB , Biggs ML , Lai HTM , de Olivera Otto MC , Fretts AM , McKnight B , Sotoodehnia N , King IB , Song X , Siscovick DS , Mozaffarian D , Lemaitre RN . Assessment of Plasma Phospholipid Very-Long-Chain Saturated Fatty Acid Levels and Healthy Aging. JAMA Netw Open. (2021) ;4: (8):e2120616. |
[54] | Chang RC , So KF . Use of anti-aging herbal medicine, Lycium barbarum, against aging-associated diseases. What do we know so far? Cell Mol Neurobiol. (2008) ;28: (5):643–52. |
[55] | Mueed Z , Mehta D , Rai PK , Kamal MA , Poddar NK . Cross-Interplay between Osmolytes and mTOR in Alzheimer’s Disease Pathogenesis. Curr Pharm Des. (2020) ;26: (37):4699–711. |
[56] | Arumugam MK , Paal MC , Donohue TM Jr, Ganesan M , Osna NA , Kharbanda KK . Beneficial Effects of Betaine: A Comprehensive Review. Biology (Basel). (2021) ;10: (6):456. |
[57] | Luo L , Wu S , Zhu X , Su W . The profiling and identification of the absorbed constituents and metabolites of Naoshuantong capsule in mice biofluids and brain by ultra- fast liquid chromatography coupled with quadrupole-time-of-flight tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. (2019) ;1129: :121791. |
[58] | Ma H , Su S , Duan J , Tang Y , Zhou J , Guo J , Zhan Z . Evaluation of the analgesic activities of the crude aqueous extract and fractions of Shao Fu Zhu Yu decoction. Pharm Biol. (2011) ;49: (2):137–45. |
[59] | Nhiem NX , Kiem PV , Minh CV , Lee JJ , Ku JH , Myung CS , Kim YH . A potential inhibitor of rat aortic vascular smooth muscle cell proliferation from the pollen of Typha angustata . Arch Pharm Res. (2010) ;33: (12):1937–42. |
[60] | Zeng G , Wu Z , Cao W , Wang Y , Deng X , Zhou Y . Identification of anti-nociceptive constituents from the pollen of Typha angustifolia L. using effect-directed fractionation. Nat Prod Res. (2020) ;34: (7):1041–5. |
[61] | Zhang X , Yang K , Zhang H , Dong W , Peng W , Zhao Y . Effect of typhaneoside on ventricular remodeling and regulation of PI3K/Akt/mTOR pathway. Herz. (2020) ;45: (Suppl 1):113–22. |
[62] | Chiu KM , Lin TY , Lee MY , Lu CW , Wang MJ , Wang SJ . Typhaneoside Suppresses Glutamate Release Through Inhibition of Voltage-Dependent Calcium Entry in Rat Cerebrocortical Nerve Terminals. Chem Res Toxicol. (2021) ;34: (5):1286–95. |
[63] | Enogieru AB , Haylett W , Hiss DC , Bardien S , Ekpo OE . Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxid Med Cell Longev. (2018) ;2018: :6241017. |
[64] | Xu H , Zhou Q , Liu B , Cheng KW , Chen F , Wang M . Neuroprotective Potential of Mung Bean (Vigna radiata L.) Polyphenols in Alzheimer’s Disease: A Review. J Agric Food Chem. (2021) ;69: (39):11554–71. |
[65] | Kong YR , Jong YX , Balakrishnan M , Bok ZK , Weng JKK , Tay KC , Goh BH , Ong YS , Chan KG , Lee LH , Khaw KY . Beneficial Role of Carica papaya Extracts and Phytochemicals on Oxidative Stress and Related Diseases: A Mini Review. Biology (Basel). (2021) ;10: (4):287. |
[66] | Ferenczyova K , Kalocayova B , Bartekova M . Potential Implications of Quercetin and its Derivatives in Cardioprotection. Int J Mol Sci. (2020) ;21: (5):1585. |
[67] | Malar DS , Prasanth MI , Jeyakumar M , Balamurugan K , Devi KP . Vitexin prevents Aβ proteotoxicity in transgenic Caenorhabditis elegans model of Alzheimer’s disease by modulating unfolded protein response. J Biochem Mol Toxicol. (2021) ;35: (1):e22632. |
[68] | Kareem RT , Abedinifar F , Mahmood EA , Ebadi AG , Rajabi F , Vessally E . The recent development of donepezil structure-based hybrids as potential multifunctional anti-Alzheimer’s agents: highlights from 2010 to 2020. RSC Adv. (2021) ;11: (49):30781–97. |
[69] | Tamagno WA , Santini W , Alves C , Vanin AP , Pompermaier A , Bilibio D , Sutorillo NT , Kaizer RR , Barcellos LJG . Neuroprotective and antioxidant effects of pitaya fruit on Cu-induced stress in adult zebrafish. J Food Biochem. 2022;e14147. |
[70] | Song H , Chu Q , Xu D , Xu Y , Zheng X . Purified Betacyanins from Hylocereus undatus Peel Ameliorate Obesity and Insulin Resistance in High-Fat-Diet-Fed Mice. J Agric Food Chem. (2016) ;64: (1):236–44. |
[71] | Dinda B , Dinda M , Kulsi G , Chakraborty A , Dinda S . Therapeutic potentials of plant iridoids in Alzheimer’s and Parkinson’s diseases: A review. Eur J Med Chem. (2019) ;169: :185–99. |
[72] | Prasanth MI , Gayathri S , Bhaskar JP , Krishnan V , Balamurugan K . Understanding the role of p38 and JNK mediated MAPK pathway in response to UV-A induced photoaging in Caenorhabditis elegans . J Photochem Photobiol B. (2020) ;205: :111844. |
[73] | Madane P , Das AK , Nanda PK , Bandyopadhyay S , Jagtap P , Shewalkar A , Maity B . Dragon fruit (Hylocereus undatus) peel asantioxidant dietary fibre on quality and lipid oxidation of chickennuggets. J Food Sci Technol. (2020) ;57: (4):1449–61. |
[74] | Oli V , Gupta R , Kumar P . FOXO and related transcription factors binding elements in the regulation of neurodegenerative disorders. J Chem Neuroanat. (2021) ;116: :102012. |
[75] | Graczyk N , Youngman M . Regulation of the Age-Dependent Activity of the FOXO Transcription Factor DAF-16 in Adult Caenorhabditis elegans Roundworms. FASEB J. (2022) ;36 Suppl 1: . doi: 10.1096/fasebj.2022.36.S1.L8126. |
[76] | Zecić A , Braeckman BP . DAF-16/FoxO in Caenorhabditis elegans and Its Role in Metabolic Remodeling. Cells. (2020) ;9: (1):109. |
[77] | Prasanth MI , Venkatesh D , Murali D , Bhaskar JP , Krishnan V , Balamurugan K . Understanding the role of DAF-16 mediated pathway in Caenorhabditis elegans during UV-A mediated photoaging process. Arch Gerontol Geriatr. (2019) ;82: :279–85. |
[78] | Balkrishna A , Gohel V , Pathak N , Singh R , Tomer M , Rawat M , Dev R , Varshney A . Anti-oxidant response of lipidom modulates lipid metabolism in Caenorhabditis elegans and in OxLDL-induced human macrophages by tuning inflammatory mediators. Biomed Pharmacother. (2023) ;160: :114309. |
[79] | Wang W , Feng X , Du Y , Liu C , Pang X , Jiang K , Wang X , Liu Y . Synthesis of Novel Pinocembrin Amino Acid Derivatives and Their Antiaging Effect on Caenorhabditis elegans via the Modulating DAF-16/FOXO. Drug Des Devel Ther. (2021) ;15: :4177–93. |
[80] | Song X , Sun Y , Wang Z , Su Y , Wang Y , Wang X . Exendin-4 alleviates β-Amyloid peptide toxicity via DAF-16 in a Caenorhabditis elegans model of Alzheimer’s disease. Front Aging Neurosci. (2022) ;14: :955113. |
[81] | Zecić A , Braeckman BP . DAF-16/FoxO in Caenorhabditis elegans and Its Role in Metabolic Remodeling. Cells. (2020) ;9: (1):109. |




