A 6-items Questionnaire (6-QMD) captures a Mediterranean like dietary pattern and is associated with memory performance and hippocampal volume in elderly and persons at risk for Alzheimer’s disease
Abstract
BACKGROUND:
There is evidence that adherence to Mediterranean-like diet reduces cognitive decline and brain atrophy in Alzheimer's disease (AD). However, lengthy dietary assessments, such as food frequency questionnaires (FFQs), discourage more frequent use.
OBJECTIVE:
Here we aimed to validate a 6-items short questionnaire for a Mediterranean-like diet (6-QMD) and explore its associations with memory performance and hippocampal atrophy in healthy elders and individuals at risk for AD.
METHODS:
We analyzed 938 participants (N = 234 healthy controls and N = 704 participants with an increased AD risk) from the DZNE-Longitudinal Cognitive Impairment and Dementia Study (DELCODE). The 6-QMD was validated against the Mediterranean Diet (MeDi) score and the Mediterranean-DASH Diet Intervention for Neurodegenerative Delay (MIND) score, both derived from a detailed FFQ. Furthermore, associations between the 6-QMD and memory function as well as hippocampal atrophy were evaluated using linear regressions.
RESULTS:
The 6-QMD was moderately associated with the FFQ-derived MeDi adherence score (ρ = 0.25, p < 0.001) and the MIND score (ρ = 0.37, p= < 0.001). Higher fish and olive oil consumption and lower meat and sausage consumption showed significant associations in a linear regression, adjusted for diagnosis, age, sex and education, with memory function (β = 0.1, p = 0.008) and bilateral hippocampal volumes (left: β = 0.15, p < 0.001); (right: β = 0.18, p < 0.001)).
CONCLUSIONS:
The 6-QMD is a useful and valid brief tool to assess the adherence to MeDi and MIND diets, capturing associations with memory function and brain atrophy in healthy elders and individuals at increased AD dementia risk, making it a valid alternative in settings with time constraints.
1Introduction
Several modifiable lifestyle factors influence the risk of cognitive decline and Alzheimer’s disease (AD) dementia [1], including smoking, depression and dietary habits. Adherence to a Mediterranean diet (MeDi)-like pattern appears to reduce the risk of dementia and AD [2– 4], however most evidence comes from epidemiological studies, unsuitable for establishing cause-and-effect relationships [5]. At the same time, a recent randomized controlled trial suggested a moderate beneficial effect of MeDi-like diet on cognitive composite scores in older adults [6], but the findings across the few trials published so far are still inconsistent [7].
In most studies assessing eating habits, lengthy and time-consuming food frequency questionnaires (FFQs), comprising 80 to 120 items, are used [8]. Time constraints in routine clinical and self-administered settings call for shorter assessments of dietary patterns to increase usability. Several short questionnaires have been developed and demonstrate good performance in predicting important health outcomes, such as the impact of a MeDi-like diet on reducing the risk of a first myocardial infarction [9– 11]. In the multicenter Prevención con Dieta Mediterránea (PREDIMED) study a comprehensive but comparatively short 14-point MeDi Adherence Screener (MEDAS) was developed to analyze the effects of a traditional MeDi in primary prevention programs [12].
In line with the positive impact of healthy dietary habits on clinical outcomes, evidence is emerging on their neuroprotective effects. Studies indicate that healthy older individuals with closer adherence to MeDi-like diet have larger MRI brain [13] and hippocampal volumes [14] and cortical thickness [15], slower change in brain atrophy over time [16], higher structural connectivity and white matter integrity [17, 18] and less amyloid-β (Aβ) pathology as measured in cerebrospinal fluid (CSF) [14]. Middle-aged healthy individuals following a MeDi-like diet display slower glucose metabolic decline on positron-emission-tomography (PET) [19– 21], with some, but not all [19], studies also showing lower Aβ accumulation on PET [20, 21].
In the present study we assessed how accurately a new 6-items Questionnaire for MeDi (6-QMD) reflects adherence to a MeDi-like diet compared with comprehensive FFQ-derived scores for adherence to MeDi and Mediterranean-DASH Diet Intervention for Neurodegenerative Delay (MIND). We used data from the prospective multicenter German Center for Neurodegenerative Disorders (Deutsches Zentrum für Neurodegenerative Erkrankungen, DZNE)-Longitudinal Cognitive Impairment and Dementia Study (DELCODE) and analyzed how the 6-QMD score was associated with cognitive performance and hippocampal atrophy in the entire subsample, and in sub-groups of healthy participants and individuals with elevated AD dementia risk. We compared this with results from our previous, smaller study from DELCODE where we had found FFQ-derived MeDi to be associated with the same outcomes [14].
2Methods
2.1Study population
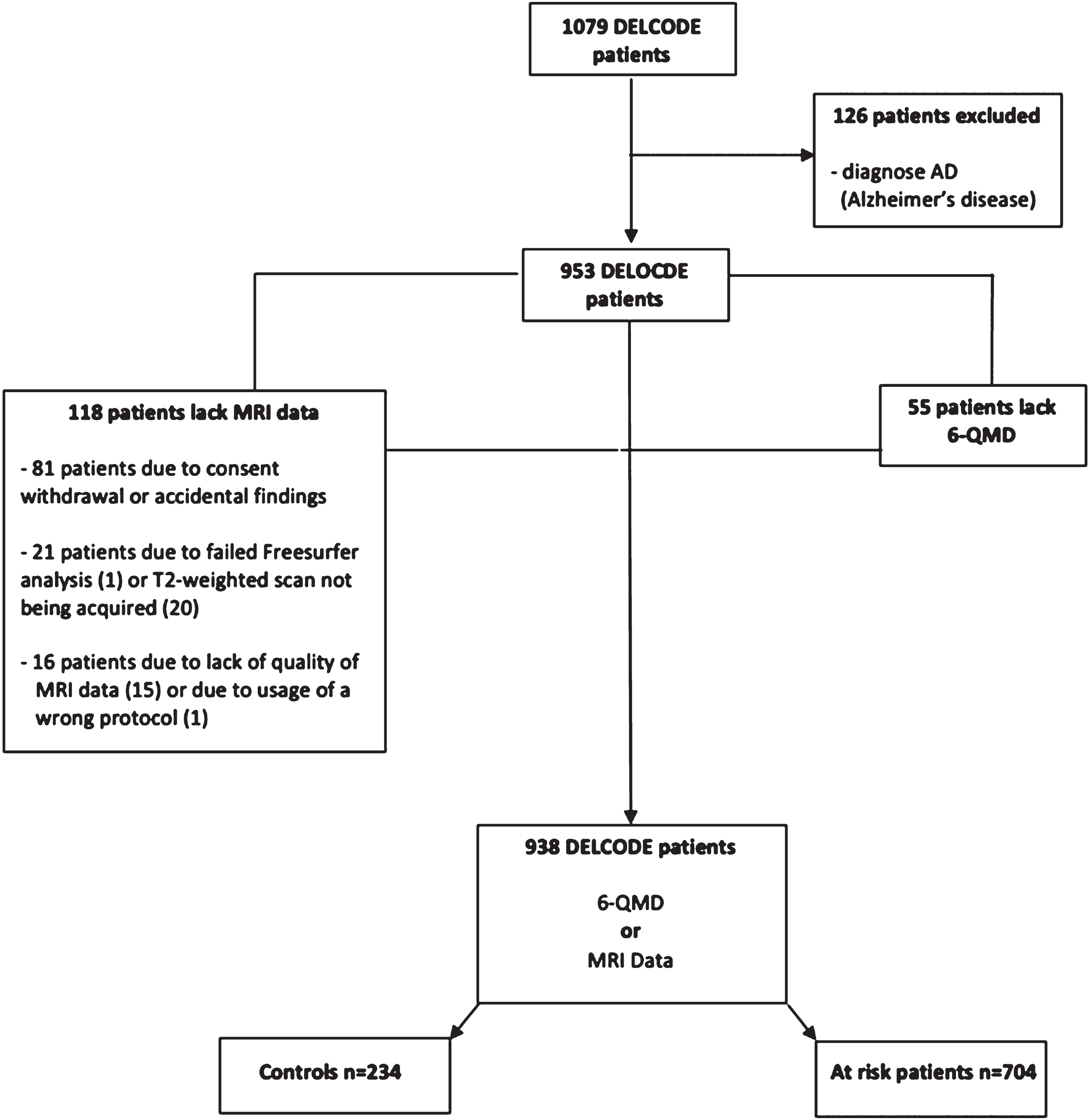
One thousand seventy-nine participants were recruited for the DELCODE baseline dataset between May 2014 and August 2018. After excluding N = 141 participants (reasons see Fig. 1), 938 participants were eligible for this study, including N = 234 healthy individuals (mean age 69, range 61– 88, 132 female), N = 441 subjects with subjective cognitive decline (SCD, mean age 71 years, range 59– 87 years, 205 female), N = 183 with mild cognitive impairment (MCI, mean age 73 years, range 61– 86 years, 80 female) and N = 80 older first-degree relatives of patients with AD dementia (mean age 66 years, range 60– 78 years, 48 female) (Table 2). Participants with SCD, MCI and first-degree relatives were subsumed as participants with increased AD dementia risk (N = 704) (Fig. 1). The detailed study design, including the inclusion and exclusion criteria and definitions of the diagnostic groups in DELCODE are reported elsewhere [22]. Informed signed consent has been obtained from all subjects in advance. Per DELCODE protocols, all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee. All local institutional review boards and ethical committees approved the study protocol. The protocol approval number is 171/13.
Fig. 1
Flow charts of inclusion/exclusions. Abbreviations: AD, Alzheimer’s disease; DELCODE, DZNE-Longitudinal Cognitive Impairment and Dementia Study; MRI, magnetic resonance imaging; 6-QMD, 6-items Questionnaire for a Mediterranean-like Diet.
2.2Assessment of dietary parameters
A validated German version of the European Prospective Investigation into Cancer and Nutrition (EPIC) [23] semi-quantitative FFQ (EPIC-FFQ) was completed by the DELCODE participants, assessing their nutrition intake over the past year. The 148 food items and the associated nutrients were selected based on recommendations of the German Institute for Food Research [24]. Based on the EPIC-FFQ, the MeDi and MIND scores were calculated using published algorithms [25, 26].
The MeDi score is a tool to assess the adherence to Mediterranean dietary patterns and comprises 9 categories, each of which can be scored 0 or 1. A sex-specific median is used as a cut-off for each category, except for the category covering the consumption of alcohol, for which the sex-specific consumption quantity of alcohol was used as a cut-off. The MeDi score ranges from 0– 9, with higher scores indicating a higher adherence to a MeDi-like diet [27]. The MIND score is based on 15 food groups, including 10 beneficial food groups and 5 unfavorable food groups, with either 0, 0.5 or 1 points given in each category, according to predefined cut-offs. Therefore, the MIND score ranges from 0 to 15, with higher scores indicating a higher adherence to the MIND diet [27].
2.3Generation of the 6-QMD
In addition to the EPIC-FFQ, a 14-item short form questionnaire (FoodScreener), specifically created for a quick assessment of diet in the German Study on Aging, Cognition and Dementia in Primary Care Patients (AgeCoDe) [28], was used to assess the participants’ nutrition covering the past 12 months. This questionnaire includes 14 food item categories (olive oil; fish; red wine; white wine; other alcoholic beverages; green tea; black tea; coffee; fresh fruit; raw vegetables; cooked vegetables (without potatoes); potatoes, dairy- cheese, yogurt, milk- and meat/ sausage). Patients were asked to report the frequency of consumption for each of the 14 categories. There were five consumption frequencies to select from: each day, several times per week, once a week, less than once a week and never. To analyze questions relevant for a MeDi-like dietary pattern, we decided to include only 6 food item categories in our analysis, based on the accepted main characteristics of a MeDi-like diet [25]: olive oil, fish, fresh fruit, raw and cooked vegetables (as one category), cheese, yogurt and milk as dairy products and meat and sausages (as one category). Although low-to-moderate alcohol consumption in some studies was found to protect against dementia [29], we decided not to include alcohol consumption as a category in the 6-QMD, due to the fact that the information generated by the FoodScreener was very unspecific regarding the quantity of alcohol consumption. As only the consumption frequency per week, not the consumed quantity (in milliliters or consumed glasses) was obtained in the FoodScreener, it is not possible to assess whether the alcohol consumption was low, moderate or high in total.
As the traditional MeDi is marked by a high intake of olive oil, fruit, vegetables and fish, as well as a low intake of meat and dairy products [25], we distinguished two groups, one including beneficial and the other one including non-beneficial food categories, for the generation of the 6-QMD. In the four categories covering healthy food items (olive oil, fish, fresh fruit and vegetables) 1 point was given for daily consumption or multiple times per week and 0 points for consumption once per week, less than once per week or never. In the categories covering the consumption of meat and sausages and the consumption of dairy products, 1 point was given if the food items were consumed once per week, less than once per week or never and 0 points were given if the food items were consumed multiple times per week or daily. Therefore, the resulting 6-QMD score ranges from 0– 6 points, with higher scores indicating a more MeDi-like dietary pattern. The information regarding the 6-QMD is summed up in the info box in Table 1.
Table 1
Info box about the 6-QMD
| The 6-items short questionnaire for a MeDi-like diet (6-QMD) is a brief screener comprising 6 food categories (olive oil, fish, fruit, vegetables, dairy products and meat, sausages) typical for a MeDi. In each category either 0 or 1 point is given, depending on the consumption frequency of the respective food category. In total the 6-QMD ranges from 0-6 points, with higher scores indicating a more MeDi. |
Table 2
Sociodemographic and clinical characteristics of the study cohort
| Variable | Controls (n = 234) | At risk patients (n = 704) | SCD (n = 441) | MCI (n = 183) | 1st degree Relatives of AD patients (n = 80) | p-value |
| Sex | 132/234 (56%) f; 102/234 (44%) m | 333/704 (47%) f 371/704 (53%) m | 205/441 (47%) f; 236/441 (53%) m | 80/183 (44%) f; 103/183 (56%) m | 48/80 (60%)f; 32/80 (40%) m | <0.01 |
| Age (years) | 69.4 (5,4) | 71.2 (6,1) | 71.4 (6,0) | 73.0 (5,6) | 66,2 (4.5) | <0.01 |
| MEM score *1 | 0.61 (0.45) | 0.10 (0.77) | 0.38 (0.55) | – 0.76 (0.67) | 0.54 (0.54) | <0.01 |
| Left hippocampal volume (mm3)*2 | 3060 (315) | 2945 (400) | 3023 (365) | 2704 (424) | 3057 (298) | <0.01 |
| Right hippocampal volume (mm3)*2 | 3163 (332) | 3061 (401) | 3128 (364) | 2864 (448) | 3140 (338) | <0.01 |
| 6-QMD*3 | 2.98 (0.99) | 2.96 (1.00) | 2.99 (1.05) | 2.82 (0.92) | 3.05 (0.91) | 0.271 |
| MIND score*4 | 6.40 (1.35) | 6.14 (1.40) | 6.16 (1.36) | 5.90 (1.47) | 6.56 (1.40) | <0.01 |
| MeDi score*5 | 4.65 (1.66) | 4.45 (1.65) | 4.52 (1.62) | 4.13 (1.81) | 4.70 (1.40) | 0.066 |
At risk patients: SCD patients, MCI patients and 1st degree relatives of AD patients. *1: n (Controls) = 234; n(At risk) = 702; n (SCD) = 440; n(MCI) = 182; n(Relatives of AD patients) = 80. *2 : n(Controls) = 223; n(At risk) = 612; n(SCD) = 378; n(MCI) = 158; n(Relatives of AD patients) = 76. *3: n(Controls) = 230; n(At risk) = 668; n(SCD) = 423; n(MCI) = 167; n(Relatives of AD patients) = 78. *4: n(Controls) = 177; n(At risk) = 369; n(SCD) = 222; n(MCI) = 93; n(Relatives of AD patients) = 54. *5: n(Controls) = 178; n(At risk) = 380; n(SCD) = 231; n(MCI) = 95; n(Relatives of AD patients) = 54. Abbreviations: AD, Alzheimer’s Disease; f, female; m, male; MCI, mild cognitive impairment; MeDi score, Mediterranean-like diet score; MEM score, composite score of memory function; MIND score, Mediterranean-DASH Diet Intervention for Neurodegenerative Delay score; 6-QMD, 6-items Questionnaire for a Mediterranean-like Diet; SCD, subjective cognitive decline.
2.4Assessment of cognitive function
Cognitive functioning was assessed using a comprehensive battery of several detailed neuropsychological assessments, including multiple tests for each individual cognitive domain. Due to its clinical relevance, we report a composite memory factor score (MEM score) as an evidence-based and specific measure of memory function in DELCODE. A detailed method description of the development of the MEM score is found elsewhere [30].
2.5Magnetic resonance imaging acquisition and analysis
Structural brain MR images were acquired using a synchronized 3D T1-weighted 1 mm3 isovoxel Magnetization Prepared-RApid Gradient Echo (MPRAGE) sequences (repetition time: 2500 ms; echo time: 4.37 ms; flip angle: 7°; field of view: 256×256×192 mm) on SIEMENS MAGNETOM TrioTim, Verio or Skyra 3 tesla scanners (Siemens Healthcare, Erlangen, Germany) using identical acquisition parameters and harmonized instructions. To ensure high image quality throughout the acquisition phase, all scans had to pass a semi-automated quality check during the study conduction, such that protocol deviations could be reported to the study sites, and the acquisition at the respective site could be adjusted.
Hippocampal volumes were computed using FreeSurfer 6.0 [31] and a previously described module for improved segmentation of the hippocampus, based on ultra-high resolution (∼0.1 mm isotropic) ex vivo MRI data to create a statistical atlas which is used to generate a segmentation of the hippocampal subfields [32]. The algorithm has been validated across different sites [33]. To enhance the accuracy of the hippocampal segmentation, two MR images, a T1-weighted and a high resolution T2-weighted image, were used [34]. In our analysis we used the estimated whole hippocampal volumes for the left and the right hippocampus. Following a visual inspection for completeness, cuts, subject motion, and other artifacts (such as blurring, echoes, and ghosting), only images classified as acceptable were included. The structural MRI data of one MCI participant was excluded, as it was classified as unusable due to strong motion artifacts.
2.6Statistical analysis
All statistical analyses were performed using SPSS, version 26.0 (IBM Corp., Armonk, NY). All tests were two-sided, with p < 0.05 considered significant. Kruskal-Wallis tests and Chi-square tests were used to compare the baseline sociodemographic, clinical, and genetic variables between the study groups as appropriate. Partial Spearman correlation was used to assess the association between the MeDi and MIND scores and the 6-QMD as well as to assess the correlation between the single 6-QMD items and the corresponding items in the FFQ. Linear regression was used to analyze the associations between the 6-QMD and cognitive decline and hippocampal atrophy. All analyses were adjusted for age, sex, diagnosis and education (only whole group). Corrections for multiple testing were performed using false discovery rate (FDR) in subgroup analyses.
3Results
Sociodemographic and clinical characteristics of the studied cohort are presented in Table 2. Subjects with MCI were older, had lower MEM scores and smaller bilateral hippocampal volumes compared to healthy individuals, subjects with SCD and relatives of AD dementia patients. Sex distribution was approximately equal across all groups. The healthy and AD dementia risk groups were comparable regarding their age. The at-risk participants exhibited lower MEM scores and bilateral hippocampal volumes.
3.1Validation of the 6-QMD with the FFQ-derived MeDi and MIND scores
In the subsample with both the FFQ and the 6-QMD available, we assessed the associations between all 6-QMD items with MeDi and MIND scores and corresponding FFQ items. After adjusting for age, sex, diagnosis and education, a positive association between the 6-QMD and the MIND score (ρ= 0.37, p < 0.001; N = 535) and MeDi score (ρ= 0.25, p < 0,001; N = 546) was found for the entire subsample (Figs. 2 and 3 respectively), the AD dementia risk group (MIND (ρ= 0.37, p < 0.001, N = 360), MEDI (ρ= 0.27, p < 0.001, N = 370)), and the control group (MIND (ρ= 0.43, p < 0.001, N = 175), MeDi (ρ= 0.22, p < 0.003, N = 176)). Reported intake of olive oil, fish, fruit and vegetables showed significant associations with the MIND and the MeDi scores. Furthermore, five single items from the 6-QMD (fish, fruit, vegetables, meat and sausages and dairy products) showed significant correlations with the corresponding food components (measured in consumption in grams per day) from the FFQ (Table 3). Olive oil consumption cannot be deduced from the FFQ, as the FFQ does not differentiate between olive oil and other vegetable oils.
Fig. 2
Correlation between 6-QMD and MIND score. Abbreviations: MIND score, Mediterranean-DASH Diet Intervention for Neurodegenerative Delay Score; 6-QMD, 6-items Questionnaire for a Mediterranean-like Diet.
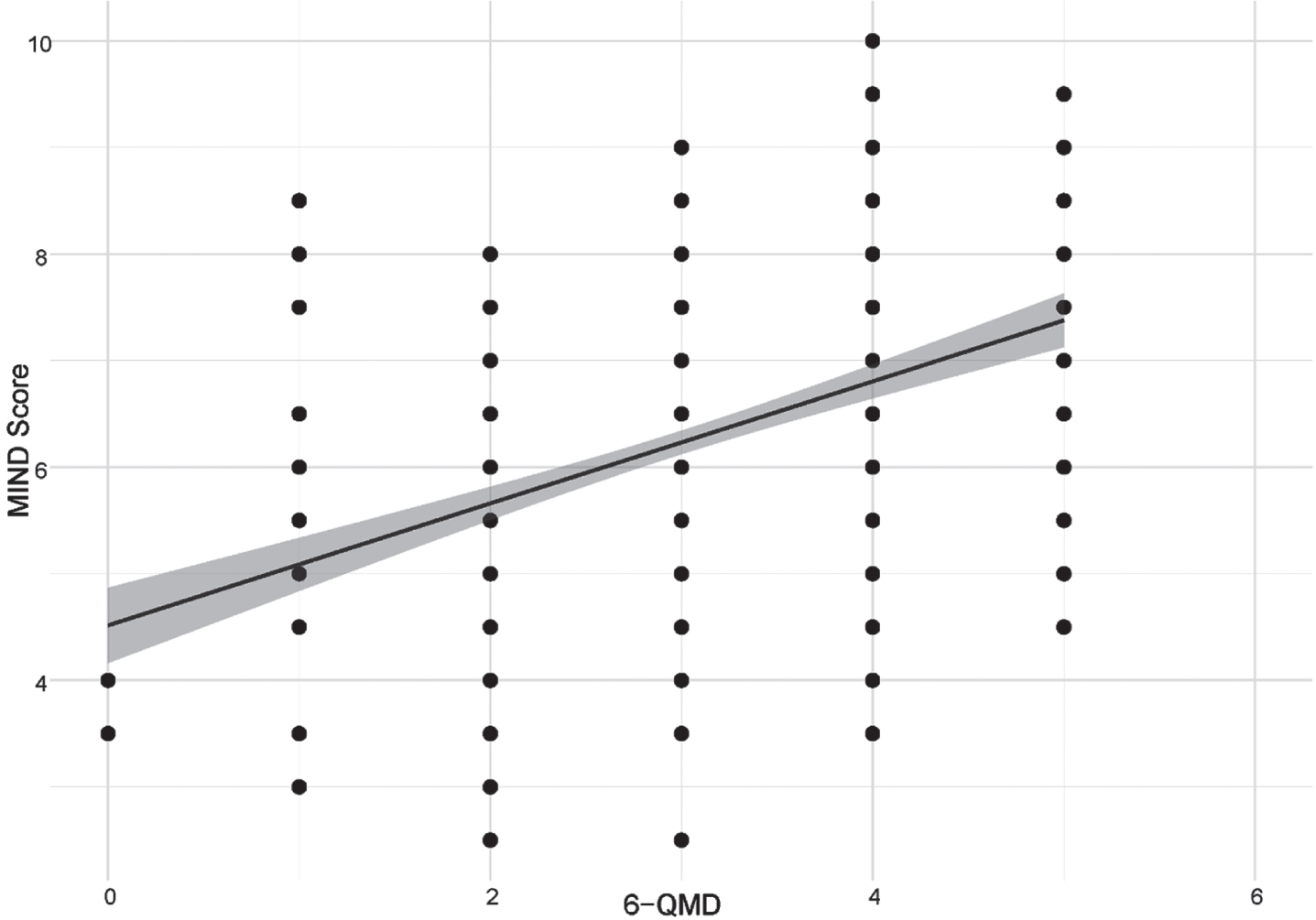
Fig. 3
Correlation between 6-QMD and MeDi score. Abbreviations: MeDi score, Mediterranean-like diet score; 6-QMD, 6-items Questionnaire for a Mediterranean-like Diet.
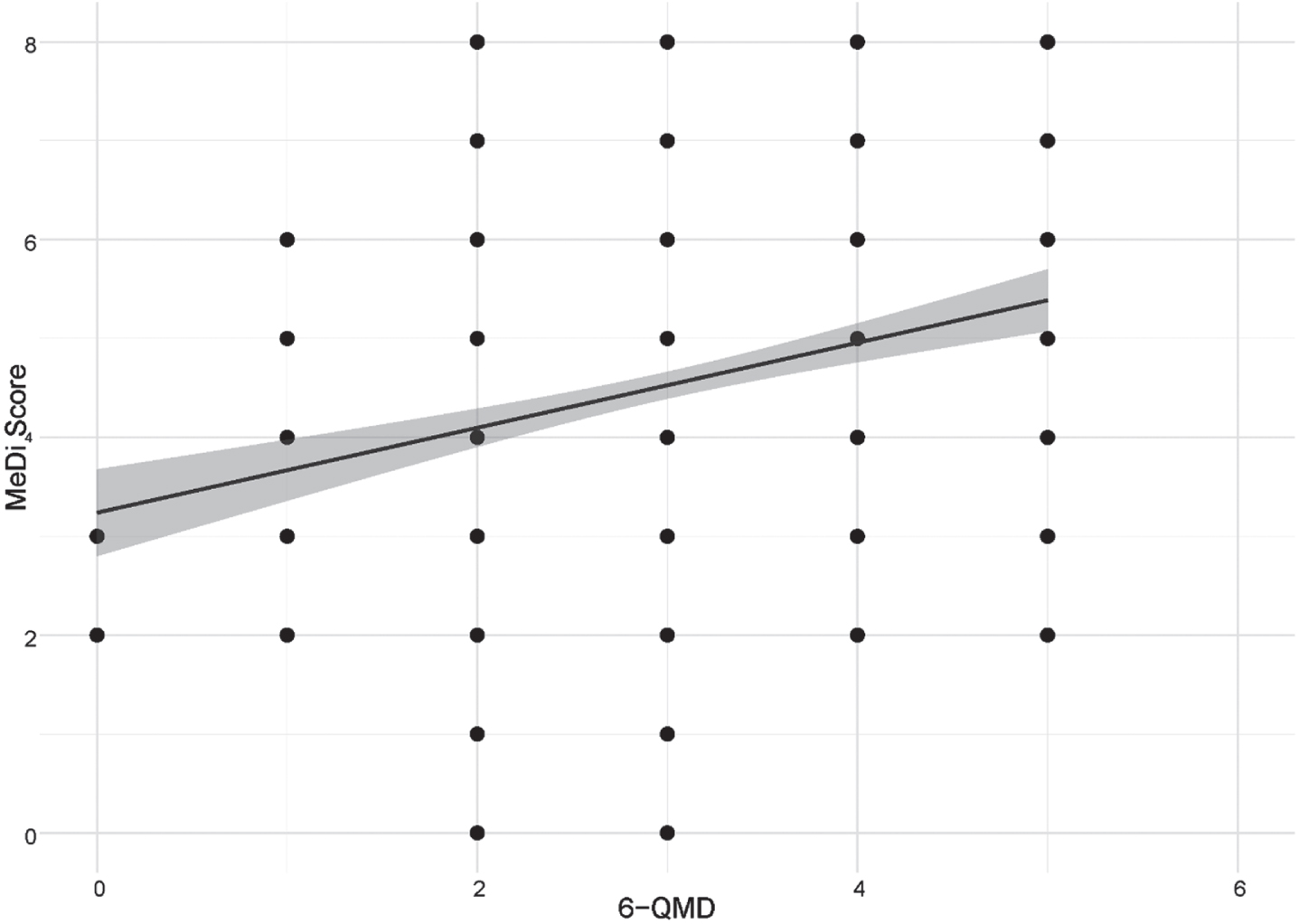
Table 3
Associations between the individual items of the 6-QMD with the MIND score, the MeDi score, the 6-QMD and the corresponding items from the FFQ in the entire cohort
| Variables | MIND score ρ | MeDi score ρ | 6-QMD ρ | FFQ items ρ |
| Olive oil | 0.25** | 0.15** | 0.64** | No corresponding items in the FFQ |
| Fish | 0.23** | 0.14* | 0.52** | 0.49** |
| Fruit | 0.95* | 0.15** | 0.35** | 0.24** |
| Vegetables | 0.11* | 0.13* | 0.39** | 0.14* |
| Meat &sausages | 0.22** | 0.04 | 0.49** | – 0.37** |
| Dairy products (Cheese, yogurt, milk) | – 0.07 | 0.03 | 0.15** | – 0.24** |
Abbreviations: *p < 0.05; **p < 0.001; FFQ, food frequency questionnaire; MeDi score, Mediterranean-like diet score; MIND score, Mediterranean-DASH Diet Intervention for Neurodegenerative Delay score; 6-QMD, 6-items Questionnaire for a Mediterranean-like Diet.
3.2Associations of memory performance and hippocampal atrophy with the 6-QMD
In the entire subsample, positive associations between the 6-QMD were found using a linear regression, adjusted for diagnosis, age, sex and education, and the MEM score (β= 0.1, p = 0.008) and left (β= 0.15 p < 0.001) and right hippocampal volume (β= 0.18, p < 0.001); in the AD dementia risk group, the 6-QMD was associated with MEM (β= 0.18, p = 0.003) and left (β= 0.19, p < 0.001) and right hippocampal volume (β= 0.22, p < 0.001); no associations were found in the healthy control group and for right hippocampal volume. In a subsequent analysis we explored the associations between individual 6-QMD items with hippocampal volume and memory performance in the AD dementia risk group. A higher consumption of olive oil and fish and a lower consumption of meat was associated with higher bilateral hippocampal volumes. Lower meat consumption and increased olive oil consumption were further associated with increased memory performance (Table 4).
Table 4
Association between the 6-QMD, the individual items of the 6-QMD and the left and right hippocampal volume and the MEM score in a linear regression model l in the AD risk group
| Left hippocampal volume | Right hippocampal volume | MEM score | ||||
| Variables | β (std) | p | β (std) | p | β (std) | p |
| 6-QMD | 0.19 | <0.001 | 0.22 | <0.001 | 0.18 | 0.003 |
| Olive oil consumption | 0.09 | 0.03 | 0.09 | 0.009 | 0.08 | 0.02 |
| Fish consumption | 0.09 | <0.001 | 0.09 | 0.01 | 0.05 | 0.17 |
| Fruit consumption | 0.04 | 0.26 | 0.05 | 0.13 | 0.02 | 0.56 |
| Vegetable consumption | 0.03 | 0.34 | 0.06 | 0.11 | 0.01 | 0.76 |
| Meat consumption | 0.08 | 0.03 | 0.09 | 0.02 | 0.09 | 0.02 |
| Dairy product consumption | 0.02 | 0.54 | 0.04 | 0.27 | – 0.4 | 0.31 |
Abbreviations: 6-QMD, 6-items Questionnaire for a Mediterranean-like Diet, β (std), standardized beta.
4Discussion
Extensive food questionnaires such as the EPIC-FFQ provide reliable estimates of nutrient intake in diverse settings [35], and have been validated in diverse populations [36]. However, the administration of FFQs is a time-consuming process and the computation of dietary patterns is relatively complex. Therefore, FFQs are not suitable for clinical settings, requiring a rapid, but still reasonably reliable, assessment of individual dietary habits, for example, to explore lifestyle-based risk of AD and other brain disorders. FFQs require a high level of motivation and a relatively large burden is passed onto the respondents, which can be reduced by short questionnaires, increasing the willingness of individuals to participate in the lifestyle assessments. The main findings of our study are: The 6-QMD is (i) correlated with the established FFQ-derived MeDi and MIND scores; (ii) subjects at risk for AD dementia, but not healthy controls, with low adherence to a MeDi-like dietary pattern show memory impairment and hippocampal atrophy; (iii) high daily intake of fish and olive oil as well as low intake of meat and sausages are associated with higher hippocampal volume in subjects at risk for AD dementia, but not in healthy controls and (iv) high intake of olive oil and low intake of meat and sausages are associated with less memory impairment in subjects at risk of AD. In general, these findings for the 6-QMD align well with our study on the FFQ-derived MeDi in a DELCODE subsample [14].
The significant association between MeDi and MIND scores and the 6-QMD, and the association with memory and hippocampal atrophy, suggest that this short screening tool is a valid method to analyze the adherence to beneficial, MeDi-like dietary patterns at least in German samples. In accordance with previous studies, we revealed an association between non-adherence to a MeDi-like diet and hippocampal atrophy using the 6-QMD [13, 14, 37]. In addition to a possible impact on brain structure integrity, a possible explanation for these findings is that fish and olive oil lower the risk of cardiovascular disease [38, 39], known to be associated with neurodegeneration and AD [40– 42]. An increase of HDL cholesterol, a decrease of LDL and total serum cholesterol as well as a decrease of triglycerides in the blood have been associated with the consumption of monounsaturated fatty acids (MUFAs), n-6 polyunsaturated fatty acids (PUFAs) and n-3 PUFAs, which are all contained in olive oil [43]. As it is suggested that an increased risk for any type of dementia emerges from high mid-life total cholesterol [44] and a part of the constituents of olive oil go along with a reduction of the total serum cholesterol, this might be one of the main pathways of how olive oil reduces the risk of AD. In addition to that, research suggests that high levels of n-3 PUFAs in fish and MUFAs as well as n-6 PUFAs in olive oil exhibit protective effects against neurodegeneration [43, 45, 46]. Furthermore, n-3 PUFAs are known to protect against neuroinflammation, playing a key role in the development of AD [47, 48]. The polyphenols, antioxidants and anti-inflammatory agents contained in olive oil [49] may also improve Aβ clearance from the brain and reduce neuroinflammation [50]. In particular, a reduced risk for AD has been found in connection with the consumption of oleocanthal, a phenolic component in extra virgin olive oil [50]. But not only do polyphenols exhibit antioxidant and anti-inflammatory qualities, it was also suggested that they inhibit the self-assembly of Aβ and tau [51]. Therefore, intake of polyphenols could be another reasonable explanation for the reduced AD-related neurological pathologies in association with the consumption of olive oil. Moreover, an increased intake of extra virgin olive oil has been associated with enhanced memory, increased hippocampal synaptic activity along with a reduction of tau neuropathology in a mouse model [52], in line with our findings.
We show for the 6-QMD that food items related to MeDi-like diets are associated with both, changes on the neurobiological level (i.e. hippocampal atrophy) and on the cognitive level, suggesting slower cognitive decline in participants at risk for AD dementia with closer adherence to more beneficial dietary patterns. An association between a MeDi-like dietary pattern and decreased cognitive decline over-time has been reported previously in several studies [6, 30, 53], making our findings even more relevant with regard to the role of a MeDi-like dietary pattern in the prevention of AD and in delaying the preclinical and clinical disease stages.
Our findings support and extend previous studies reporting that high consumption of fish and olive oil and low consumption of meat and sausages are associated with higher hippocampal volume. High fish intake and low meat consumption have previously shown to exhibit neuroprotective effects against brain atrophy [13, 54– 56], in AD typically affecting the medial temporal brain regions, including the hippocampus, early in the disease course, indicating that these findings are related to prodromal AD as well as healthy aging.
There are potential limitations of our study. Although the analyses were performed using a relatively large prospective cohort, no longitudinal data was available at the time of the analyses. This should be addressed in future studies. Furthermore, reverse causation may have affected our results because AD progression is related to changes in eating habits and nutrient uptake. We attempted to mitigate those effects by including only participants at risk for AD dementia, in whom dietary changes are still relatively minor, if present at all. Furthermore, it has been shown that MeDi-like dietary patterns remain stable in older adults [57]. Hippocampal atrophy is a reasonably specific finding in individuals with AD-type cognitive impairment, supporting our conclusions on the neurobiological substrates of MeDi in early AD.
To conclude, our data support the application of short assessment tools for MeDi in clinical and research settings where time constraints and limited resources do not allow a more extensive evaluation. Furthermore, our study indicates that a MeDi-like dietary pattern, particularly high olive oil and fish and low meat consumption, may protect against cognitive decline and brain atrophy in individuals at risk for AD dementia. Further research is warranted to replicate our findings in independent datasets, including longitudinal measurements and biomarker-diagnosed subjects.
Acknowledgments
The authors have no acknowledgements.
Consent for publication
Not applicable.
Availability of data and material
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.
Funding
Study funded by the German Center for Neurodegenerative Diseases (Deutsches Zentrum für Neurodegenerative Erkrankungen, DZNE) reference number BN01. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authors’ contributions
All authors had access to the data.
| Name | Location | Role | Contribution |
| Boris-Stephan Rauchmann | Department of Psychiatry and Psychotherapy, University Hospital, LMU Munich, Germany | Author | Conceptualization and design of the study; Statistical Analysis; Interpretation of data; Drafting and/or revision of manuscript for important intellectual content |
| Patrizia Gross | Department of Psychiatry and Psychotherapy, University Hospital, LMU Munich, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Ersin Ersoezlue | Department of Psychiatry and Psychotherapy, University Hospital, LMU Munich, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Michael Wagner | DZNE Bonn Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Tommaso Ballarini | DZNE Bonn Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Carolin Kurz | Department of Psychiatry and Psychotherapy, University Hospital, LMU Munich, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Maia Tato | Department of Psychiatry and Psychotherapy, University Hospital, LMU Munich, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Julia Utecht | Department of Psychiatry and Psychotherapy, University Hospital, LMU Munich, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Boris Papazov | Department of Radiology, University Hospital, LMU Munich, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Selim Gürsel | Department of Psychiatry and Psychotherapy, University Hospital, LMU Munich, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Maria Totzke | Department of Psychiatry and Psychotherapy, University Hospital, LMU Munich, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Lena Trappmann | Department of Psychiatry and Psychotherapy, University Hospital, LMU Munich, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Lena Burow | Department of Psychiatry and Psychotherapy, University Hospital, LMU Munich, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Gabrielle Koller | Department of Psychiatry and Psychotherapy, University Hospital, LMU Munich, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Sophia Stöcklein | Department of Radiology, University Hospital, LMU Munich, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Daniel Keeser | Department of Psychiatry and Psychotherapy, University Hospital, LMU Munich, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Slawek Altenstein | German Center for Neurodegenerative Diseases (DZNE), Berlin, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Claudia Bartels | Department of Psychiatry and Psychotherapy, University Medical Center Goettingen, University of Goettingen, Goettingen, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Katharina Buerger | DZNE Munich Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Peter Dechent | Georg-August-University Göttingen, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Laura Dobisch | DZNE Magdeburg, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Michael Ewers | German Center for Neurodegenerative Diseases (DZNE, Munich), Munich, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Klaus Fliessbach | DZNE Bonn Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Silka Dawn Freiesleben | German Center for Neurodegenerative Diseases (DZNE), Berlin, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Wenzel Glanz | German Center for Neurodegenerative Diseases (DZNE), Magdeburg, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Doreen Goeerss | Department of Psychosomatic Medicine, Rostock University Medical Center, Rostock, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Daria Gref | German Center for Neurodegenerative Diseases (DZNE), Berlin, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| John Dylan Haynes | Bernstein Center for Computational Neuroscience, Charité — Berlin, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Daniel Janowitz | Ludwig-Maximilians- University, Munich, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Ingo Kilimann | DZNE Rostock, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Okka Kimmich | German Center for Neurodegenerative Diseases (DZNE) Bonn, Bonn, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Luca Kleineidam | German Center for Neurodegenerative Diseases (DZNE) Bonn, Bonn, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Christoph Laske | DZNE Tübingen, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Andrea Lohse | Department of Psychiatry and Psychotherapy, Charité, Berlin, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Franziska Maier | Department of Psychiatry, University of Cologne, Medical Faculty, Cologne, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Coraline D. Metzger | DZNE Magdeburg, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Matthias H. Munk | DZNE Tübingen, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Oliver Peters | DZNE Berlin Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Lukas Preis | Charité – Universitätsmedizin Berlin, Department of Psychiatry and Psychotherapy, Campus Benjamin Franklin, Berlin, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Roeske, Sandra | German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Nina Roy | DZNE Bonn Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Carolin Sanzenbacher | German Center for Neurodegenerative Diseases (DZNE), Tübingen, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Klaus Scheffler | University of Tübingen, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Stefan Teipel | DZNE Rostock, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Debora Melo van Lent | Glenn Biggs Institute for Alzheimer’s and Neurodegenerative Diseases, UT Health San Antonio, Texas, USA | Author | Drafting and/or revision of manuscript for important intellectual content |
| Jens Wiltfang | DZNE Goettingen, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Steffen Wolfsgruber | Department of Psychiatry, Charité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health (BIH) | Author | Drafting and/or revision of manuscript for important intellectual content |
| Renat Yakupov | German Center for Neurodegenerative Diseases (DZNE), Magdeburg, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Emrah Düzel | DZNE Magdeburg, Germany | Author | Drafting and/or revision of manuscript for important intellectual content |
| Frank Jessen | DZNE Bonn Germany | Author | Conceptualization and design of the study; Drafting and/or revision of manuscript for important intellectual content |
| Robert Perneczky | DZNE Munich Germany | Author | Conceptualization and design of the study; Interpretation of data; Drafting and/or revision of manuscript for important intellectual content |
Conflict of interest
Robert Perneczky received fees for consultation from Janssen, Roche, Biogen, Eli Lilly, Novo Nordisk and Grifols. All remaining authors declare that they have no competing interests.
References
[1] | Livingston G , Huntley J , Sommerlad A , Ames D , Ballard C , Banerjee S , et al Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. (2020) ;396: (1048):413–46. |
[2] | Pistollato F , Iglesias RC , Ruiz R , Aparicio S , Crespo J , Lopez LD , et al. Nutritional patterns associated with the maintenance of neurocognitive functions and the risk of dementia and Alzheimer’s disease: A focus on human studies. Pharmacol Res. (2018) 131: :32–43. |
[3] | Lourida I , Soni M , Thompson-Coon J , Purandare N , Lang IA , Ukoumunne OC , et al. Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology. (2013) 24: (4):479–89. |
[4] | van den Brink AC , Brouwer-Brolsma EM , Berendsen AAM , van de Rest O The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer’s Disease-A Review. Adv Nutr. (2019) 10: (6):1040–65. |
[5] | Petersson SD , Philippou E Mediterranean Diet, Cognitive Function, and Dementia: A Systematic Review of the Evidence. Adv Nutr. (2016) 7: (5):889–904. |
[6] | Valls-Pedret C , Sala-Vila A , Serra-Mir M , Corella D , de la Torre R , Martínez-González MÁ , et al. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern Med. (2015) 175: (7):1094–103. |
[7] | Radd-Vagenas S , Duffy SL , Naismith SL , Brew BJ , Flood VM , Fiatarone Singh MA Effect of the Mediterranean diet on cognition and brain morphology and function: a systematic review of randomized controlled trials. Am J Clin Nutr. (2018) 107: (3):389–404. |
[8] | Cantin J , Latour E , Ferland-Verry R , Morales Salgado S , Lambert J , Faraj M , et al. Validity and reproducibility of a food frequency questionnaire focused on the Mediterranean diet for the Quebec population. Nutr Metab Cardiovasc Dis. (2016) 26: (2):154–61. |
[9] | Martínez-González MA , Fernández-Jarne E , Serrano-Martínez M , Marti A , Martinez JA , Martín-Moreno JM Mediterranean diet and reduction in the risk of a first acute myocardial infarction: an operational healthy dietary score. Eur J Nutr. (2002) 41: (4):153–60. |
[10] | Martínez-González MA , Fernández-Jarne E , Serrano-Martínez M , Wright M , Gomez-Gracia E Development of a short dietary intake questionnaire for the quantitative estimation of adherence to a cardioprotective Mediterranean diet. Eur J Clin Nutr. (2004) 58: (11):1550–2. |
[11] | Schröder H , Fitó M , Estruch R , Martínez-González MA , Corella D , Salas-Salvadó J , et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J Nutr. (2011) 141: (6):1140–5. |
[12] | Martínez-González MA , García-Arellano A , Toledo E , Salas-Salvadó J , Buil-Cosiales P , Corella D , et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: the PREDIMED trial. PLoS One. (2012) 7: (8):e43134. |
[13] | Gu Y , Brickman AM , Stern Y , Habeck CG , Razlighi QR , Luchsinger JA , et al. Mediterranean diet and brain structure in a multiethnic elderly cohort. Neurology. (2015) 85: (20):1744–51. |
[14] | Ballarini T , Melo van Lent D , Brunner J , Schröder A , Wolfsgruber S , Altenstein S , et al. Mediterranean Diet, Alzheimer Disease Biomarkers and Brain Atrophy in Old Age. Neurology [Internet]. (2021) May 5; Available from: https://dx.org/10.1212/WNL.0000000000012067 |
[15] | Staubo SC , Aakre JA , Vemuri P , Syrjanen JA , Mielke MM , Geda YE , et al. Mediterranean diet, micronutrients and macronutrients, and MRI measures of cortical thickness. Alzheimers Dement. (2017) 13: (2):168–77. |
[16] | Luciano M , Corley J , Cox SR , Valdés Hernández MC , Craig LCA , Dickie DA , et al. Mediterranean-type diet and brain structural change from 73 to 76 years in a Scottish cohort. Neurology. (2017) 88: (5):449–55. |
[17] | Pelletier A , Barul C , Féart C , Helmer C , Bernard C , Periot O , et al. Mediterranean diet and preserved brain structural connectivity in older subjects. Alzheimers Dement. (2015) 11: (9):1023–31. |
[18] | Rodrigues B , Coelho A , Portugal-Nunes C , Magalhães R , Moreira PS , Castanho TC , et al. Higher Adherence to the Mediterranean Diet Is Associated With Preserved White Matter Integrity and Altered Structural Connectivity. Front Neurosci. (2020) 14: :786. |
[19] | Walters MJ , Sterling J , Quinn C , Ganzer C , Osorio RS , Andrews RD , et al. Associations of lifestyle and vascular risk factors with Alzheimer’s brain biomarker changes during middle age: a 3-year longitudinal study in the broader New York City area. BMJ Open. (2018) 8: (11):e023664. |
[20] | Matthews DC , Davies M , Murray J , Williams S , Tsui WH , Li Y , et al. Physical Activity, Mediterranean Diet and Biomarkers-Assessed Risk of Alzheimer’s: A Multi-Modality Brain Imaging Study. Adv J Mol Imaging. (2014) 4: (4):43–57. |
[21] | Berti V , Walters M , Sterling J , Quinn CG , Logue M , Andrews R , et al. Mediterranean diet and 3-year Alzheimer brain biomarker changes in middle-aged adults. Neurology. (2018) 90: (20):e1789–98. |
[22] | Jessen F , Spottke A , Boecker H , Brosseron F , Buerger K , Catak C , et al. Design and first baseline data of the DZNE multicenter observational study on predementia Alzheimer’s disease (DELCODE). Alzheimers Res Ther. (2018) 10: (1):1–10. |
[23] | Riboli E , Hunt KJ , Slimani N , Ferrari P , Norat T , Fahey M , et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. (2002) 5: (6B):1113–24. |
[24] | Nöthlings U , Hoffmann K , Bergmann MM , Boeing H Fitting portion sizes in a self-administered food frequency questionnaire. J Nutr. (2007) 137: (12):2781–6. |
[25] | Trichopoulou A , Costacou T , Bamia C , Trichopoulos D Adherence to a Mediterranean Diet and Survival in a Greek Population [Internet], Vol. 348: , New England Journal of Medicine. (2003) 2599–608. Available from: https://dx.doi.org/10.1056/nejmoa025039 |
[26] | Morris MC , Tangney CC , Wang Y , Sacks FM , Barnes LL , Bennett DA , et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. (2015) 11: (9):1015–22. |
[27] | Wesselman LMP , van Lent DM , Schröder A , van de Rest O , Peters O , Menne F , et al. Dietary patterns are related to cognitive functioning in elderly enriched with individuals at increased risk for Alzheimer’s disease. Eur J Nutr. (2021) 60: (2):849–60. |
[28] | Fischer K , Melo van Lent D , Wolfsgruber S , Weinhold L , Kleineidam L , Bickel H , et al. Prospective Associations between Single Foods, Alzheimer’s Dementia and Memory Decline in the Elderly. Nutrients [Internet]. (2018) 10: (7). https://dx.doi.org/10.3390/nu10070852 |
[29] | Visontay R , Rao RT , Mewton L Alcohol use and dementia: new research directions. Curr Opin Psychiatry. (2021) 34: (2):165–70. |
[30] | Wolfsgruber S , Kleineidam L , Guski J , Polcher A , Frommann I , Roeske S , et al. Minor neuropsychological deficits in patients with subjective cognitive decline. Neurology. (2020) 95: (9):e1134–43. |
[31] | Fischl B FreeSurfer. Neuroimage. (2012) 62: (2):774–81. |
[32] | Iglesias JE , Augustinack JC , Nguyen K , Player CM , Player A , Wright M , et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage. (2015) 115: :117–37. |
[33] | Quattrini G , Pievani M , Jovicich J , Aiello M , Bargalló N , Barkhof F , et al. Amygdalar nuclei and hippocampal subfields on MRI: Test-retest reliability of automated volumetry across different MRI sites and vendors. Neuroimage. (2020) 218: :116932. |
[34] | Dounavi ME , Mak E , Wells K , Ritchie K , Ritchie CW , Su L , et al. Volumetric alterations in the hippocampal subfields of subjects at increased risk of dementia. Neurobiol Aging. (2020) 91: :36–44. |
[35] | Bingham SA , Gill C , Welch A , Cassidy A , Runswick SA , Oakes S , et al. Validation of dietary assessment methods in the UK arm of EPIC using weighed records, and 24-hour urinary nitrogen and potassium and serum vitamin C and carotenoids as biomarkers. Int J Epidemiol. (1997) 26: (Suppl 1):S137–51. |
[36] | Haftenberger M , Heuer T , Heidemann C , Kube F , Krems C , Mensink GBM Relative validation of a food frequency questionnaire for national health and nutrition monitoring. Nutr J. (2010) 9: :36. |
[37] | Mosconi L , Murray J , Tsui WH , Li Y , Davies M , Williams S , et al. Mediterranean Diet and Magnetic Resonance Imaging-Assessed Brain Atrophy in Cognitively Normal Individuals at Risk for Alzheimer’s Disease. J Prev Alzheimers Dis. (2014) 1: (1):23–32. |
[38] | Widmer RJ , Flammer AJ , Lerman LO , Lerman A The Mediterranean diet, its components, and cardiovascular disease. Am J Med. (2015) 128: (3):229–38. |
[39] | Estruch R , Ros E , Salas-Salvadó J , Covas MI , Corella D , Arós F , et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med. (2018) 378: (25):e34. |
[40] | Wanleenuwat P , Iwanowski P , Kozubski W Alzheimer’s dementia: pathogenesis and impact of cardiovascular risk factors on cognitive decline. Postgrad Med. (2019) 131: (7):415–22. |
[41] | Baumgart M , Snyder HM , Carrillo MC , Fazio S , Kim H , Johns H Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement. (2015) 11: (6):718–26. |
[42] | Qiu C , Kivipelto M , von Strauss E Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. (2009) 11: (2):111–28. |
[43] | Román GC , Jackson RE , Reis J , Román AN , Toledo JB , Toledo E Extra-virgin olive oil for potential prevention of Alzheimer disease. Rev Neurol. (2019) 175: (10):705–23. |
[44] | Anstey KJ , Lipnicki DM , Low LF Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry. (2008) 16: (5):343–54. |
[45] | Román GC , Jackson RE , Gadhia R , Román AN , Reis J Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev Neurol. (2019) 175: (10):724–41. |
[46] | Ajith TA A Recent Update on the Effects of Omega-3 Fatty Acids in Alzheimer’s Disease. Curr Clin Pharmacol. (2018) 13: (4):252–60. |
[47] | Trépanier MO , Hopperton KE , Orr SK , Bazinet RP N-3 polyunsaturated fatty acids in animal models with neuroinflammation: An update [Internet]. Vol. 785: , European Journal of Pharmacology. (2016) . pp. 187–206. Available from: https://dx.doi./org/10.1016/j.ejphar.2015.05.045 |
[48] | Rauchmann BS , Schneider-Axmann T , Alexopoulos P , Perneczky R , Alzheimer’s Disease Neuroimaging Initiative CSF soluble TREM2 as a measure of immune response along the Alzheimer’s disease continuum. Neurobiol Aging. (2019) 74: :182–90. |
[49] | Bayram B , Esatbeyoglu T , Schulze N , Ozcelik B , Frank J , Rimbach G Comprehensive analysis of polyphenols in 55 extra virgin olive oils by HPLC-ECD and their correlation with antioxidant activities. Plant Foods Hum Nutr. (2012) 67: (4):326–36. |
[50] | Abuznait AH , Qosa H , Busnena BA , El Sayed KA , Kaddoumi A Olive-oil-derived oleocanthal enhances β-amyloid clearance as a potential neuroprotective mechanism against Alzheimer’s disease: in vitro and in vivo studies. ACS Chem Neurosci. (2013) 4: (6):973–82. |
[51] | Zheng Q , Kebede MT , Kemeh MM , Islam S , Lee B , Bleck SD , et al. Inhibition of the Self-Assembly of Aβ and of Tau by Polyphenols: Mechanistic Studies. Molecules [Internet]. (2019) ;24: (12). Available from: https://dx.doi.org/10.3390/molecules24122316 |
[52] | Lauretti E , Nenov M , Dincer O , Iuliano L , Praticò D Extra virgin olive oil improves synaptic activity, short-term plasticity, memory, and neuropathology in a tauopathy model. Aging Cell. (2020) 19: (1):e13076. |
[53] | Loughrey DG , Lavecchia S , Brennan S , Lawlor BA , Kelly ME The Impact of the Mediterranean Diet on the Cognitive Functioning of Healthy Older Adults: A Systematic Review and Meta-Analysis. Adv Nutr. (2017) 8: (4):571–86. |
[54] | Daiello LA , Gongvatana A , Dunsiger S , Cohen RA , Ott BR , Alzheimer’s Disease Neuroimaging Initiative Association of fish oil supplement use with preservation of brain volume and cognitive function. Alzheimers Dement. (2015) 11: (2):226–35. |
[55] | Berti V , Murray J , Davies M , Spector N , Tsui WH , Li Y , et al. Nutrient patterns and brain biomarkers of Alzheimer’s disease in cognitively normal individuals. J Nutr Health Aging. (2015) 19: (4):413–23. |
[56] | Samieri C , Maillard P , Crivello F , Proust-Lima C , Peuchant E , Helmer C , et al. Plasma long-chain omega-3 fatty acids and atrophy of the medial temporal lobe. Neurology. (2012) 79: (7):642–50. |
[57] | Scarmeas N , Stern Y , Tang MX , Mayeux R , Luchsinger JA Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. (2006) 59: (6):912–21. |




