Bacopa monnieri protects neuronal cell line and Caenorhabditis elegans models of Alzheimer’s disease through sigma-1 receptor antagonist sensitive and antioxidant pathways
Abstract
BACKGROUND:
Due to better health care and improved nutritional status of the world’s population, many people live into old age. This has resulted in more diseases related to aging, such as neurodegenerative diseases. Bacopa monnieri (BM) is a medicinal herb found in Southeast Asia and is a popular memory-enhancing supplement.
OBJECTIVE:
This study aimed to investigate how BM may provide protection in neurodegenerative disease, and whether the sigma-1 receptor is involved.
METHODS:
PC-12 cells were differentiated with the addition of nerve growth factor. The potentiation by BM of PC-12 neurite growth was measured by counting the number of differentiated cells and by measuring their length. Differentiated PC-12 cells were also subjected to amyloid-β (Aβ) toxicity in the presence and absence of BM. The cell survival (MTT and cell counting) and neurite lengths were then measured as indicators of cellular health. Total protein was extracted from control and treated cells and expression of various signalling pathway molecules was assessed via western blotting. We also assessed the effects of BM on the lifespans of various mutant strains plus wild-type C. elegans.
RESULTS:
We show that BM can protect against Aβ toxicity in PC-12 cells. Furthermore, BM can potentiate neurite outgrowth in PC-12, in a sigma-1 receptor antagonist sensitive fashion, and Neuro2A cell lines. BM induced a reduction in pAKT expression and upregulated BDNF expression in PC-12 cells. BM was also able to increase the lifespan and health-span of Aβ expressing C. elegans mutants via the DAF-16 mediated pathway. BM reduced oxidative stress in wild-type C. elegans exposed to UV-A with pre-exposure and post-exposure treatments.
CONCLUSIONS:
This all further identifies BM as a potential agent to treat neurodegenerative diseases, by modulating different pathways.
1Introduction
Neurodegenerative diseases are becoming a significant health problem, especially as people live longer than in the past. There are approximately 44 million Alzheimer’s disease (AD) patients worldwide, and with no cure and no drug to slow the progression of the disease, it is expected to rise to 135 million by 2050 [1]. Furthermore, there is an increased risk of neurodegenerative and neurological disorders because of the SARS-COV-2 pandemic [2–4]. Neurodegenerative diseases such as AD are characterized by brain atrophy, neuronal loss, and a buildup of extracellular amyloid-β (Aβ), leading to plaque formation. There is also the hyperphosphorylation of tau protein, which results in neurofibril tangles and loss of efficiency in neurotransmission due to damage to the neuron’s energy-producing mechanisms, which leads to oxidative stress and disruption in calcium homeostasis, which all lead to cell death.
Increasing age [5], along with long term lack of quality sleep [6, 7], poor diet [8] leading to high cholesterol [9, 10], hypertension, and type-2 diabetes are some of the acquired risk factors in the development of AD, as well other neurogenerative diseases. It is estimated that 70% of the risk of developing AD is derived from genetics [11]. Polymorphisms in genes such as amyloid precursor protein (APP), presenilin 1 (PSEN1), and presenilin 2 (PSEN2) are responsible for early-onset AD. In contrast, the apolipoprotein E gene (APOE) is associated with late-onset AD [12]. There are currently no drugs that slow the progression of AD, however, acetylcholine esterase drugs (such as donepezil and rivastigmine) have shown some benefit in managing the symptoms of AD and tend to be the go-to drugs after AD diagnosis [13]. However, the side effects of these drugs can be intolerable and in some cases lead to death [14]. Several novel AD drugs (such as ANAVEX2-73 and T-817 MA) have been shown to bind to the sigma-1 receptor and modulate its activities while producing beneficial effects in AD [15, 16].
The sigma-1 receptor is a chaperone protein that resides at the ER and mitochondria inside the cell. It is highly expressed in neuronal tissues. The sigma-1 receptor can interact with various other proteins and complexes such as ion channels [17, 18], G-proteins [19, 20], and receptors to influence cell survival in stress conditions [21–23]. There appears to be an upregulation of the sigma-1 receptor in normal aging, possibly to compensate for the loss of other types of receptors during the aging process [16]. However, sigma-1 receptors appear to be under-expressed in AD brains. Furthermore, studies have identified some sigma-1 receptor polymorphisms that appear to be associated with sigma-1 receptor expression and increased risk of AD [24, 25]. There is also evidence that polymorphisms in the APOE and sigma-1 genes may interact and affect the severity of the disease [26].
Bacopa monnieri (BM) is a medicinal herb known for its traditional uses as a memory enhancer. It has become a popular over-the-counter herbal supplement, with an Australian survey of 60 to 64-year-olds ranking it as the third-placed memory supplement behind G. biloba and vitamin E [27]. However, the evidence for its memory-enhancing properties in healthy people has yet to be conclusively shown. Several studies have measured the memory-enhancing properties of BM in healthy volunteers [28–32]. However, none of these studies appear to replicate each other’s results, with each carrying out a range of different neuropsychological tests and finding some significant improvements except for one study, which found no significant improvements in any memory tests [31]. Furthermore, meta-analyses of clinical studies have not revealed any significant cognitive improvement in healthy people [33, 34]. Clinical studies in diseased states have shown mixed results, with BM not improving the behavior of adolescent boys with attention deficit and hyperactivity disorder (ADHD) but there was a reported improvement in cognitive ability, mood, and sleep [35]. BM has been shown to reduce anxiety and depression in the elderly [36] (two common symptoms that come along with AD) [37, 38]. There have been very few studies (no placebo-controlled study involving BM per se) on people with AD. However, those studies that have investigated BM clinically in patients with AD have shown results in line with, or better than, the standard drug donepezil and improved other metrics such as depression scores [39–41].
Caenorhabditis elegans is a popular model for studying aging, stress resistance, and neurological disorders [42–44] due to its short life cycle and ease of handling. It is also relatively simple to generate single gene mutants making it an ideal model organism for studying aging and age-related diseases [45]. It has been observed that many plants or plant products including, green tea [46], Streblus asper [47, 48], Paullinia cupanna [49], Hibiscus sabdariffa [50], Cleistocalyx nervosum [51, 52], Kaempferia parviflora and mulberry [53] can extend the lifespan and health-span and protect against stress in C. elegans model. Previously we have shown that the hexane extract of BM can extend the ‘health-span’ and lifespan in wild-type C. elegans [54].
This study sets out to measure BM’s neuroprotective effects against AD using in vitro cell culture models, in vivo, C. elegans models, and in silico, docking analysis. We show that BM can induce neurite outgrowth in Neuro2A cells, protect nerve growth factor (NGF) differentiated PC-12 cells against Aβ toxicity, and potentiate the NGF-induced growth of neurites in a sigma-1 receptor antagonist sensitive manner. Furthermore, BM extends the median and maximum lifespan of transgenic C. elegans expressing Aβ. The lifespan extension that we described previously [54] could be mediated in a daf-16 dependent mechanism. Finally, we investigated the potential targets of compounds found in BM using in silico docking analysis at the human and C. elegans sigma-1 receptor and DAF-16 and SKN-1 in C. elegans.
2Materials and methods
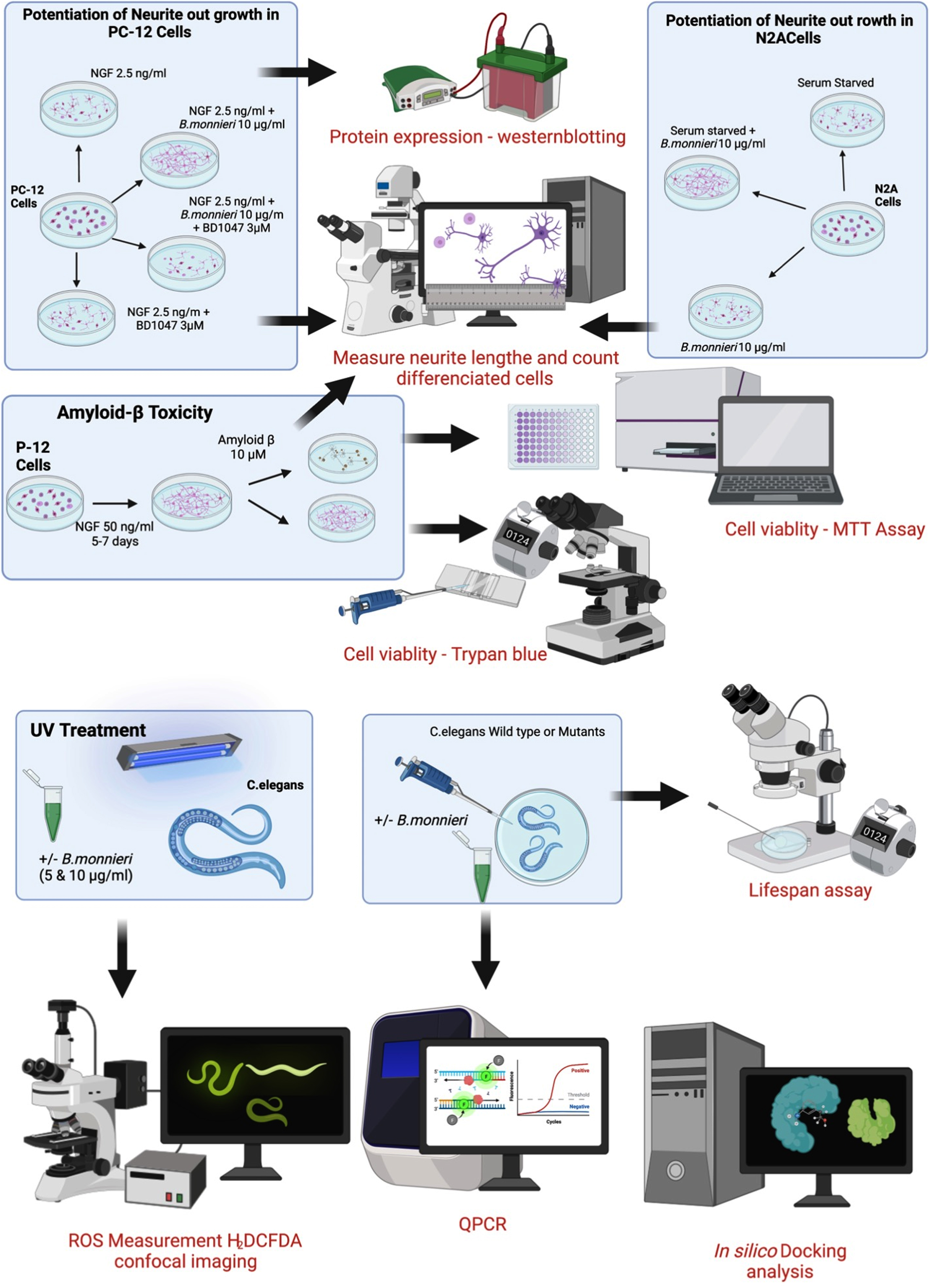
A summary of the experimental procedures involving cultured PC-12 and Neuro2A cells and the treatment and analysis of wild-type and mutant C. elegans is shown in Fig. 1.
Fig. 1
Summary of experimental cell culture and C. elegans procedures used in this study.
2.1BM extraction and identification
BM leaves were collected from Princess Maha Chakri Sirindhorn Herbal Garden (Rayong Province, Thailand) and identified by Nirun Vipunngeun, College of Pharmacy, Rangsit University, Thailand, voucher number 016299 (BUC). The leaves were shade dried before extraction in hexane by Soxhlet extraction. The resulting extract was collected, and solvents were removed before being suspended in DMSO at a concentration of 20 mg/ml. The extract was passed through a 0.2-μm filter and stored at –20°C before use. BM identity was confirmed using HPLC and described previously [58]. Briefly, The BM extract was analyzed using reverse-phase HPLC. The HPLC system consists of C18 column (Inertsil ODS-3:4.6x250 mm, 5 mL I.D.) and diode array at 205 nm. The mobile phase HPLC conditions namely mobile phase composition in A line containing 0.05% Phosphoric acid in water and Acetonitrile in B line. Flow rate was set at 20μL/min and temperature at 35 °C. The BM extract was compared to a standard consisting of mixture of bacoside A3, bacopaside II, bacopaside X and bacopasaponin C (Supplementary Figure 1).
2.2Metabolite prediction
From the selected compounds that are confirmed to be present in the BM extract we used the biotransformer 3.0 online resource to predict the possible metabolites produced by human and gut enzymes [55]. If there were more than five predicted metabolites the top five were presented (Supplementary Table 1).
2.3Cell culture
PC-12 cells were obtained from the Japanese Collection of Research Bioresources (Tokyo, Japan) and maintained in Roswell Park Memorial Institute Medium (RPMI) (GibThai, Bangkok) supplemented with 5% Fetal Bovine Serum (FBS) (GibThai, Bangkok), 10% Horse Serum (HS) (GibThai, Bangkok), penicillin (50 I.U./ml) and streptomycin (50μg/ml) (pen/strep) (GibThai, Bangkok). Neuro2A cells were obtained from the Health Science Research Resources Bank (Osaka, Japan) and cultured in Dulbecco’s Modified Eagle Medium (DMEM) (GibThai, Bangkok) supplemented with 10% FBS and pen/strep. All cells were maintained in a humidified, 5% CO2 atmosphere at 37°C, and were passed weekly with media changed every 2-3 days.
2.4Neurite growth analysis
PC-12 cells were centrifuged at 500 g and resuspended in RPMI medium containing 5% FBS and 10% HS. The cells were passed through a 22 G needle 20 times to disperse cell clumps. Viable cells were counted using trypan blue staining. The cells were diluted and plated onto Poly-d-lysine (PDL) coated 12-well plates at a density of 3000 cells/cm2. The following day the media was replaced with RPMI containing 0.5% HS, and the cells were treated with or without drugs and NGF.
Neurite outgrowth in Neuro2A cells was carried out as described previously [23]. Briefly, Neuro2A cells were trypsinized (0.05% trypsin EDTA (GibThai, Bangkok)), centrifuged at 500 g, then resuspended in DMEM 10% FBS, and plated at a density of 2000 cells/cm2. The following day the media was replaced with DMEM 0.5% FBS and added drugs. The cells were monitored for neurite outgrowth (Neuro2A over 3–5 days, PC-12 over 5–7 days), and micrographs were taken using Zeiss Axio Observer A1 Inverted Fluorescence Phase Contrast Microscope. Neurites were defied as extensions double the cell width; Image J was used to measure the neurites’ length (Neuron J plugin [56]) and count the percentage of neurite expressing cells.
2.5Aβ toxicity in NGF differentiated PC-12 cells
PC-12 cells were plated at a density of 30,000 cells/cm2 in PDL coated 96 well plates and allowed to adhere overnight at 37°C in a humidified, 5% CO2 atmosphere. For differentiated cells, NGF (50 ng/ml) in 2% HS supplemented RPMI was added, and the cells were incubated for 3–5 days. Once the cells were differentiated, the media was replaced with fresh media containing NGF (50 ng/ml), Aβ(25-35) (10μM), and BM (10–0.1μg/ml). After a further 24 hours at 37°C in a humidified, 5% CO2 atmosphere, cellular viability and damage were measured using the MTT and trypan blue exclusion assays. Damage to cells was quantified by measuring the lengths of the remaining neurites as described above.
2.6Western blotting analysis
PC-12 cells were plated at a density of 10,000 cells per cm2 and allowed to adhere overnight. The cells were treated with BM (1–100μg/ml) for 24 hours, in the presence and absence of 50 ng/ml NGF. Protein was then extracted following a wash in ice-cold PBS, using NP40 lysis buffer supplemented with protease inhibitor cocktail. The cells were incubated in the lysis buffer on ice for 30 minutes, then centrifuged at 12,000 g for 10 minutes, and the supernatant was collected and frozen until further use. Protein concentrations were calculated using the Bradford method as previously described [23, 54] and compared to a BSA standard curve.
50μg of protein was separated on a 12% SDS-PAGE gel and transferred to PVDF membranes. These were blocked in 5% BioRad blocking buffer (BioRad, Hercules, CA, USA) for two hours before overnight incubation with primary antibody (Cell Signalling Danvers, MA USA) at 4°C. The blots were then washed with pH 7.5 Tris-buffered saline with 0.05% Tween (TBS-T) three times for ten minutes each before incubation with the horseradish peroxidase-conjugated secondary antibody for one hour at room temperature. The blots were then rewashed in TBS-T three times for ten minutes each. Blots were visualized using a chemiluminescence detection system (ECL select western blotting detection agent: GE Healthcare Piscataway, NJ, USA) and exposure to X-ray film. Blots were analyzed using Image J software, and beta-actin was used as a loading control.
2.7C. elegans strains and propagation
The wild-type strain N2 (Bristol), Aβ transgenic strain (CL2006), daf-2 mutant (CB1370) and daf-16 mutant (CF1038) were acquired via the Caenorhabditis Genetics Center (University of Minnesota, USA). They were maintained in Nematode growth medium (NGM) at 15°C. Experiments were carried out using age synchronized young adult worms, with each experiment carried out in three independent trials, unless otherwise specified.
2.8C. elegans pharyngeal pumping
The pharyngeal pumping assay was carried out, as explained previously [51]. The known number of young adult stage nematodes (≈10) were transferred to NGM plates swabbed with different concentrations of BM. Pharyngeal pumping was observed once in every 24 hours using a stereomicroscope (Motic SMZ-171) for 30 consecutive seconds. Pharyngeal pumping of worms in NGM plates swabbed with E. coli OP50 was considered as the control group.
2.9C. elegans longevity (lifespan/survival) assay
The C. elegans lifespan assay has been described previously [51, 54]. Briefly, approximately ten age synchronized mutant worms were placed in M9 buffer along with E. coli OP50 and 5-Fluoro-2′-deoxyuridine (FUDR). The worms were treated with BM (7, 8, 9 and 10μg/ml), which was added to the liquid medium as such that it would be ingested via pharyngeal pumping or directly absorbed and the total number of live worms were counted every 24 hours. Dead worms did not respond to a gentle touch from the platinum loop or tapping on the side of the plate. Each experiment was carried out in five independent populations, and the data presented are average from these experiments.
2.10ROS imaging in C. elegans
C. elegans (N2) were exposed to UV light using a UV-A transilluminator lamp, SANKYO DENKI (F20T10BL), to simulate oxidated stress [42, 51]. ROS staining with 5μg/ml H2DCFDA was carried out as described previously [51]. Briefly, worms were either treated with BM (5 or 10μg/ml) before or after UV-A exposure, and in both sets’ BM treatment continued after UV exposure for five days. The worms were washed in M9 buffer and stained with H2DCFDA for 20 minutes and then rewashed and transferred to a drop of sodium azide on a glass slide. Fluorescent imaging was carried out on a minimum of ten randomly selected regions of the slide containing nematodes using a ZEISS LSM 700 confocal microscope. The images were analyzed using Image J software, and the relative fluorescence was represented as arbitrary units normalized to the untreated control (AU). The data were obtained from three independent repeats of the above protocol.
2.11Quantitative PCR
Total RNA from wild-type nematodes treated with different concentrations of BM extracts (5 and 10μg/ml) was extracted using the TRIzol kit (Invitrogen, Carlsbad, CA, USA). 1000 ng of total RNA was converted to cDNA using AccuPower RT Premix (Bioneer, Korea) using oligo dT primers per the manufacturer’s instructions. Real-time PCR was carried out using SYBR Green, Green Star PCR Master Mix (Bioneer, Korea), in the Exicycler Real-Time Quantitative Thermal Block (Bioneer, Daedeok- gu, Korea) using gene-specific primers (Table 1). Expression data were normalized to actin and then represented as fold change from the untreated control.
Table 1
Primers used in qPCR
| Gene name | Forward primer | Reverse primer |
| act-2 | ATCGTCCTCGACTCTGGAGATG | TCACGTCCAGCCAAGTCAAG |
| daf-2 | TCGAGCTCTTCCTACGGTGT | CATCTTGTCCACCACGTGTC |
| daf-16 | TGGTGGAATTCAATCGTGAA | ATGAATATGCTGCCCTCCAG |
| age-1 | ATAGAGCTCCACGGCACTTT | ATAGAGCTCCACGGCACTTT |
| utx-1 | GCAGAACACCAGCTCATCAG | ATCAACGCCATTCTTCTCGC |
| col-19 | CACACAAATGCTCCACCAAC | CTGGATTTCCCTTCTGTCCA |
| egl-8 | CGTATCGTTGCGCTTCTCA | AGTAGTGACACAGCGGTTG |
| dgk-1 | GTTGGGGAAGTGGTGCAAAT | GCGAGCTTGGATTGGATGAG |
| goa-1 | TGTTCGATGTGGGAGGTCAA | TCGTGCATTCGGTTTGTTGT |
| lin-45 | ACGTCATCGACCTCATTTCC | ATACATTCGGGGATTGTCCA |
| mpk-1 | GCCAGTTTGTGAGGAACCAT | CAGGATTCTGCCCTCCATTA |
| mek-2 | AATCTCGGTTGCAGTTGTCC | GTGGGATCCTGTGAGTCGTT |
| ace-1 | GGAGATCCGAACAAAAACGA | TGACGATTCAACGGTCATGT |
| ace-2 | AGCTCACCGGATATGAATGC | GTGGCCCAATATCTCCAGAA |
| lys-7 | TTGCAGTACTCTGCCATTCG | GCACAATAACCCGCTTGTTT |
Table 2
Confirmed and predicted components of BM extract. (*ND=not determined)
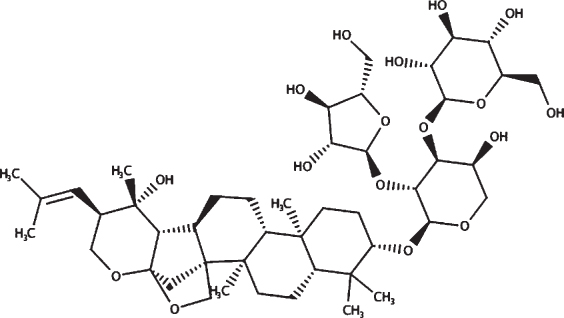
| HPLC confirmed compounds | Structure | HPLC retention time (mins) | Mwt |
| Bacopasaponin C |
 | 24.47 | 899.07 |
| Bacopaside II |
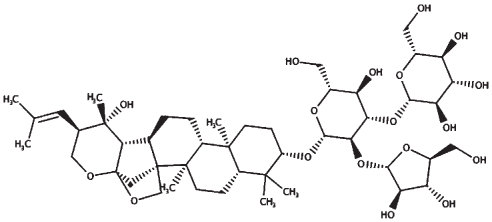 | 23.46 | 929.10 |
| Bacoside A3 |
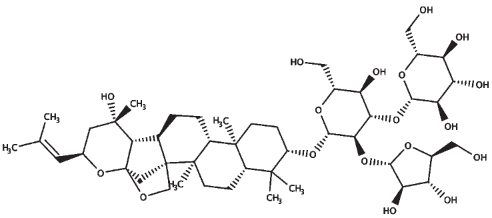 | 21.0 | 929.10 |
| Bacopaside X |
 | 21.6 | 899.07 |
| Other predicted compounds | |||
| Cucurbitacin E |
 | *ND | 556.69 |
| Cucurbitacin I |
 | *ND | 514.65 |
| Monnieriside A |
 | *ND | 379.31 |
2.12Molecular docking
The sequences of the proteins (σ non-opioid intracellular receptor 1 (human), σ 1-type opioid receptor (C. elegans), SKN-1, and DAF-16) were retrieved from UniProt (UniProt ID –Q99720, O02109, P34707, and O16850). The 3D structures were modeled using the online platform server trROSETTA, which builds a protein 3D model via Ab Initio modeling technique [60]. The stereochemical quality of the protein structures was estimated using PDBSum [61]. The ligands (selected from the known compounds in the BM extract plus other commonly found compounds from other studies) (Table 3) to be docked were extracted from the PubChem database, and molecular docking analysis was performed using a grid-based docking approach using DockThor [57] using epigallocatechin gallate (EGCG) as the reference compound for SKN-1 and DAF-16 and (+) Pentazocine for the sigma-1 receptor.
Table 3
Structures of compounds used in molecular docking analysis
| Compound name | Structure |
| Bacoside A |
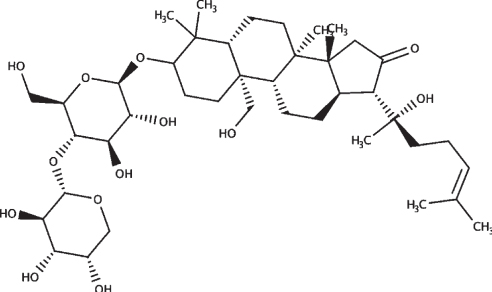 |
| Bacopaside II |
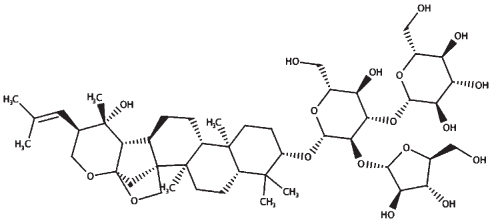 |
| Bacoside A3 |
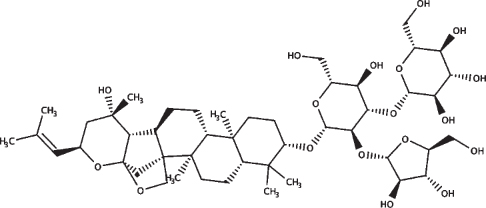 |
| Bacopaside X |
 |
| Cucurbitacin E |
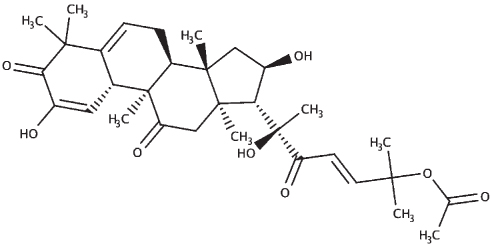 |
| Cucurbitacin I |
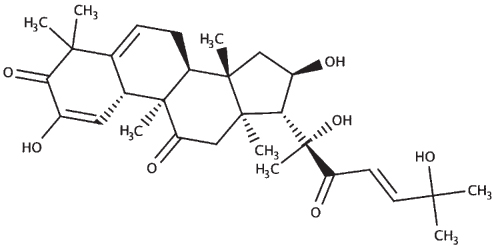 |
| Bacopasaponin C |
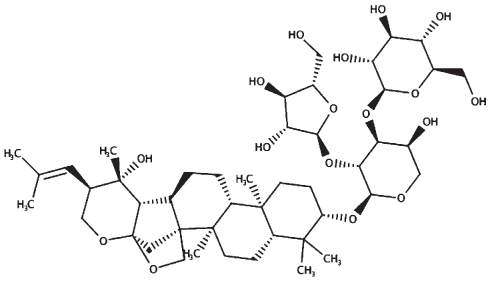 |
| Monnieriside A |
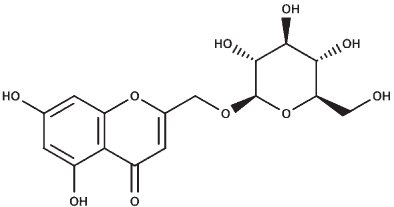 |
| Epigallocatechin gallate |
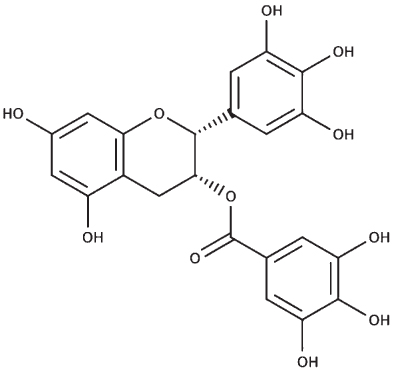 |
| (+) Pentazocine |
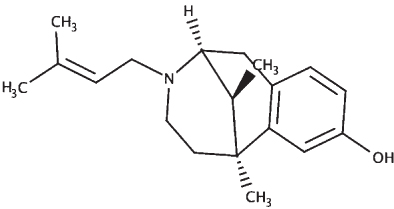 |
2.13Statistical analysis
Statistical analysis was carried out using GraphPad Prism version 9 for Macintosh. Statistical significance was measured using ANOVA followed by Dunnett’s or Tukey’s post hoc test, and p values below 0.05 were considered significant.
3Results
3.1Effect of BM on neurite outgrowth
Growing and replacing damaged neurons is an integral part of neuroprotection. We used two cell lines commonly used in the study of neurite outgrowth. Neuro2A cells are mouse neuroblastoma cells that will differentiate when serum-starved. PC-12 cells are the rat pheochromocytoma cells that differentiate in response to NGF. While cell culture studies are limited in that they make use of a monoculture of cells, which is in many respects unlike normal physiological conditions, cell culture study does allow for the study of how cells may respond to various compounds.
3.1.1Potentiation of neurite outgrowth in serum starved Neuro2A cells
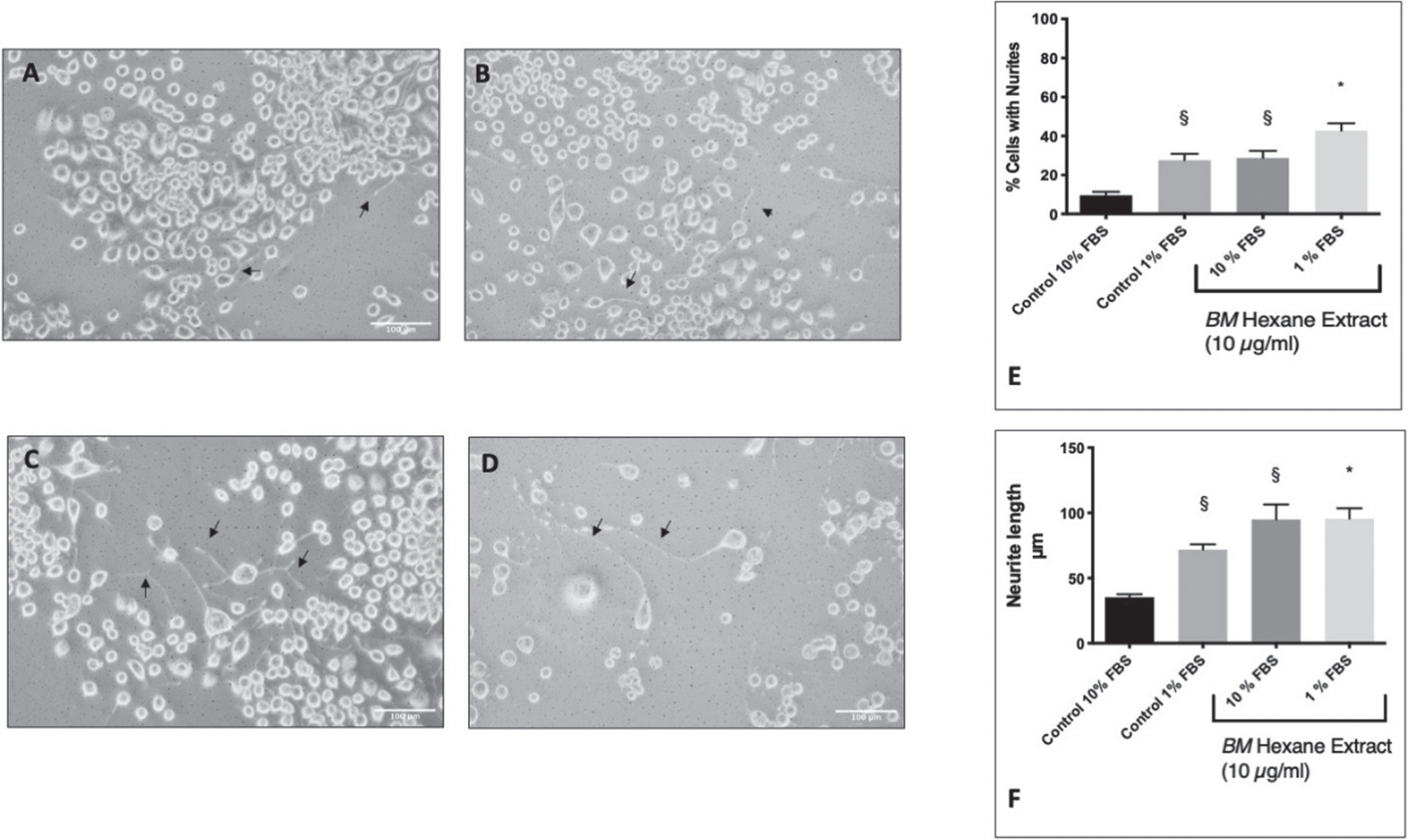
Differentiation of Neuro2A cells was induced by serum starvation in 1% FBS (Fig. 2A–D). This resulted in the formation of neurites in 27.6±3.3% of the cells compared to 9.5±1.8% in the control cells (10% FBS). In previous studies, we have identified 10μg/ml of BM (hexane extract) to be the most effective neuroprotective concentration in glutamate treated HT-22 cells [54, 58]. Therefore, we treated Neuro2A cells with 10μg/ml and measured the number of neurites. BM was able to potentiate neurite outgrowth in the serum-starved cells and induce neurite outgrowth in cells cultured in 10% FBS (Fig. 2E). We also measured the lengths of the neurites (Fig. 2F) using Neuron J [56, 59]. Neuro2A cells in 1% FBS had significantly longer neurites than those in 10% FBS (ANOVA followed by Tukey’s post hoc post hoc test) p = 0.0267). Furthermore, 10μg/ml BM was able to potentiate neurite growth in 1% FBS (ANOVA followed by Tukey’s post hoc test p = 0.04) and induce neurite growth in 10% FBS (Tukey’s post hoc test following ANOVA (p = 0.0007).
Fig. 2
The effect of BM on serum starvation induction of neurite outgrowth in Neuro2A cells. Black arrows indicate examples of neurites. A Control Neuro2A cells in DMEM 10% FBS. B Control Neuro2A cells in DMEM 1% FBS. C BM (10 μg/ml) treated Neuro2A cells in DMEM 10% FBS. D BM (10 μg/ml) treated Neuro2A cells in DMEM 1% FBS. E The percentage of cells showing neurite growth. §Statistical significance compared to control 10% (ANOVA followed by Tukey’s post hoc test) p = 0.018 (control 1% FBS) p = 0.013 (10% FBS+BM 10 μg/ml). * Statistical significance compared to control 1% FBS (ANOVA followed by Tukey’s post hoc test) p = 0.04. F Neurite length. §Statistical significance compared to control 10% (ANOVA followed by Tukey’s post hoc post hoc test) p = 0.0267 (control 1% FBS) p = 0.0007 (10% FBS+BM 10 μg/ml). * Statistical significance (ANOVA followed by Tukey’s post hoc multiple comparison test) compared to control 1% FBS p = 0.047.
3.1.2Potentiation of neurite outgrowth in NGF treated PC-12 cells
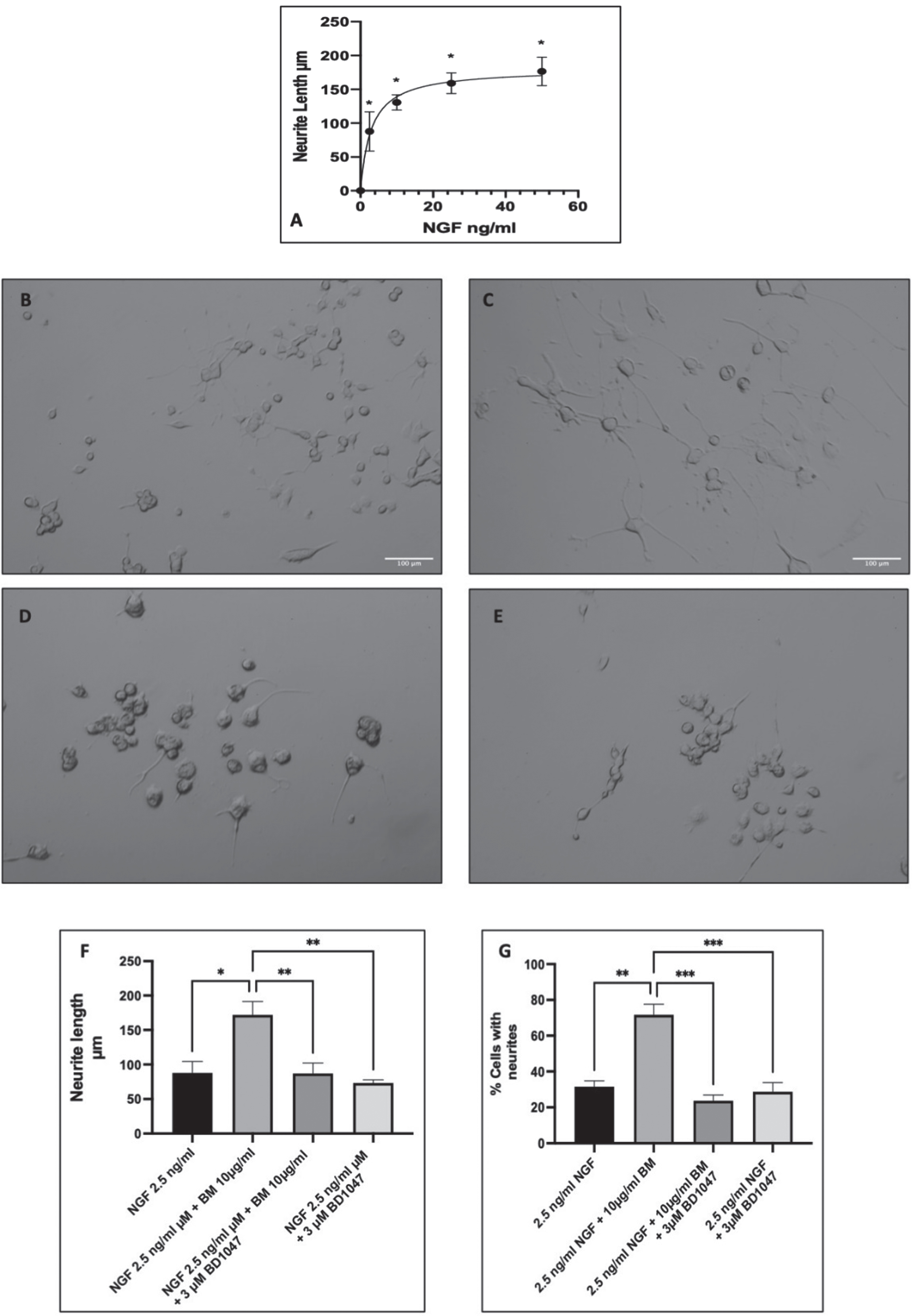
Differentiation of PC-12 cells was induced by NGF, with many studies using varying concentrations from 2.5 ng/ml to 100 ng/ml [58–60]. Therefore, we carried out a dose-response to NGF in PC-12 cells and measured the lengths of the neurites at day seven (Fig. 3A) and then selected a submaximal dose of NGF for the potentiation of neurite outgrowth experiments just below the EC50 of 2.9 ng/ml (selected dose 2.5 ng/ml). Treatment with 2.5 ng/ml NGF significantly increased neurite outgrowth compared to control (untreated cells) (ANOVA followed by Dunnett’s post hoc multiple comparisons test (p < 0.001)). Subsequently, co-treatment with BM (10μg/ml) and NGF (2.5 ng/ml) resulted in potentiation of neurite outgrowth with more cells with neurites and longer neurites (Fig. 3B–E). Previous studies have indicated that the potentiation of neurite outgrowth in PC-12 is regulated by the sigma-1 receptor [52]. Therefore, to evaluate the role of the sigma-1 receptor in the potentiation of NGF-induced neurite outgrowth, we also treated the cells with 10μg/ml BM in the presence and absence of the sigma-1 receptor antagonist BD1047 (3μM) (Fig. 3F & G). The length of the neurites and the percentage of cells expressing neurites were quantified with Image J. BM significantly increased the neurite length from 97.1±4.9μm to 155.3±0.9μm (ANOVA followed by Dunnett’s post hoc test p < 0.05). However, NGF 2.5 ng/ml and BM treatment in the presence of the sigma-1 receptor antagonist BD1047 (3μM) resulted in neurites that were no different in length to the control. Furthermore, BD1047 did not affect the growth of neurites in response to 2.5 ng/ml NGF alone.
Fig. 3
BM potentiation of neurite outgrowth in PC-12 cells. Representative images of PC-12 cells at day seven of treatment with A dose-response to NGF in PC-12 cells. Measuring neurite length after 7 days of incubation with varying concentrations of NGF. EC50 2.9 ng/ml (95% CI 1.600 to 5.2). * Statistically significant difference from control cells (0 ng/ml NGF) using ANOVA followed by Dunnett’s post hoc multiple comparison test p < 0.001. B 2.5 ng/ml NGF; C 2.5 ng/ml NGF and 10 μg/ml BM; D 2.5 ng/ml NGF, 10 μg/ml BM and 3 μM BD1047; E 2.5 ng/ml NGF and 3 μM BD1047; F analysis of neurite length using Image J. Significant difference in neurite length ANOVA followed by Dunnett’s post hoc test (*p < 0.05, **p < 0.01) n = 3; G number of cells with neurites in response to 2.5 ng/ml NGF±BM (100 μg/ml) and/or 3 μM BD1047. The significant difference in % cells with neurites ANOVA followed by Dunnett’s post hoc test (*p < 0.05, **p < 0.01).
3.2Aβ toxicity in NGF differentiated PC-12 cells
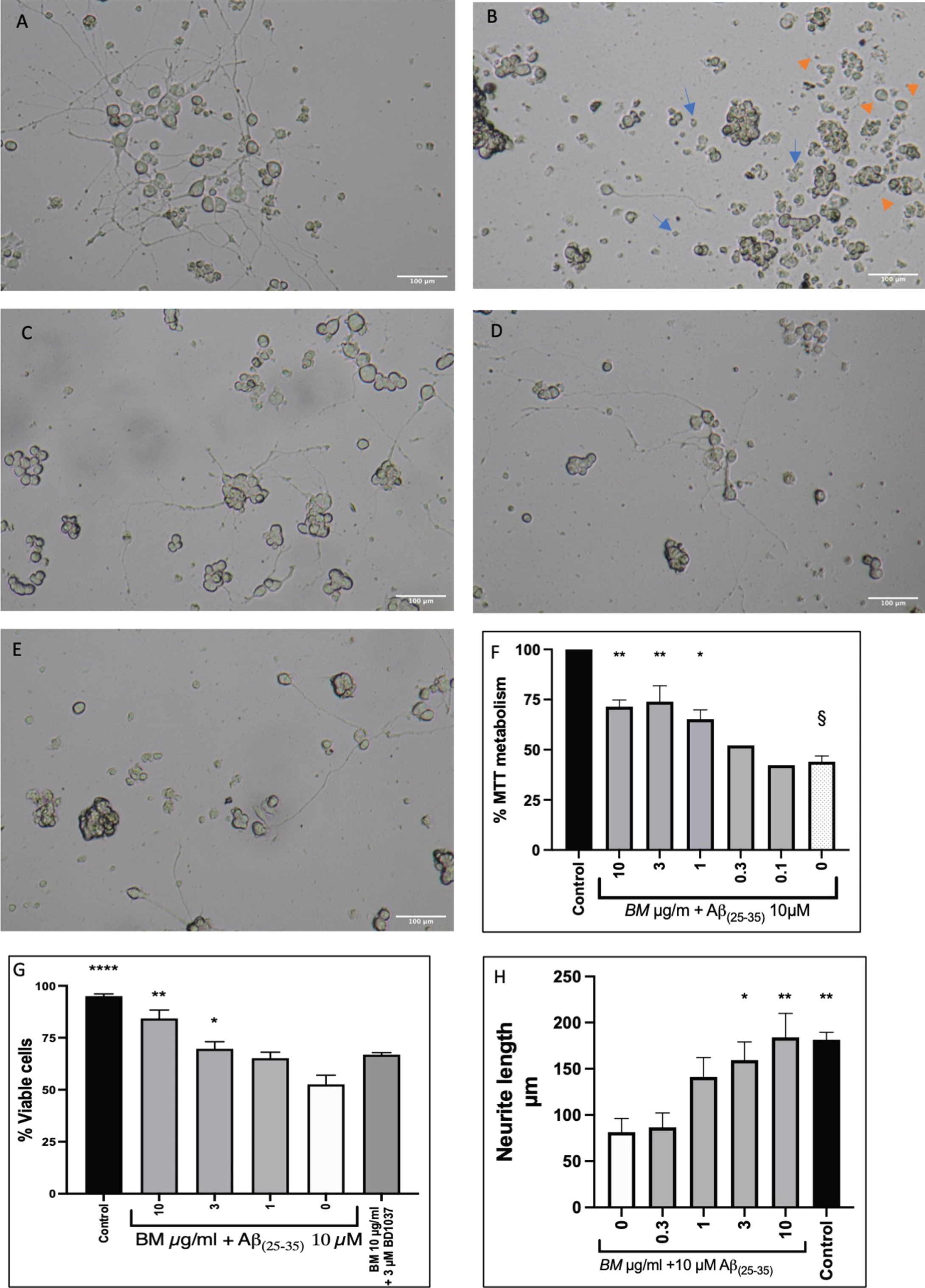
PC-12 cells that had been differentiated with NGF (50 ng/ml) for seven days were treated with 10μM Aβ(25-35) alone or with varying concentrations of BM (10–0.1μg/ml). Representative micrographs are shown in Fig. 4A-E, showing the difference in morphology between control cells and cells treated with 10μM Aβ(25-35) . Treatment with BM appears to prevent damage to the neurites and reduce the number of shrunken cells. We quantified cell survival using the MTT assay (Fig. 4F). BM dose-dependently improved cell survival from 44.00±2.91% to 71.45±3.32% (BM 10μg/ml), 73.96±7.90 (BM 3μg/ml) and 65.17±4.73 (BM 1μg/ml). The damage to the neurites caused by Aβ(25-35) treatment was quantified by measuring the length of the neurites (Fig. 4H). In the untreated control, the neurites were 181.4±8.1μm long, whereas treatment with Aβ(25-35) resulted in neurites 81.5±14.8μm long. BM treatment dose dependently improved neurite length in the Aβ(25-35) treated cells, with 10 and 3μg/ml BM resulting in a statistically significant increase (184±26.0μm and 159.3±19.7μm respectively) compared to Aβ(25-35) treatment alone.
Fig. 4
Protective effect of BM in Aβ(25-35) treated PC-12 cells. A Control cells (NGF 50 ng/ml difficilitated PC-12 cells). B Differentiated PC-12 cells treated with Aβ(25-35)10 μM. The image shows shrunken cells (Blue arrows) and damaged neurites (orange arrows). C Differentiated PC-12 cells treated with Aβ(25-35)10 μM and BM 3 μg/ml. D Differentiated PC-12 cells treated with Aβ(25-35) 10 μM and BM 1 μg/ml. E Differentiated PC-12 cells treated with Aβ(25-35) 10 μM and BM 0.3 μg/ml. F BM dose-dependently improved cell survival in PC-12 cells treated with 10 μM Aβ(25-35). G Cell viability quantified by Trypan blue exclusion assay. H Quantification of neurite damage by Aβ(25-35). Means±SEM from 3 independent experiments; * statistical significance compared to 10 μM Aβ(25-35) treated cells ANOVA followed by Dunnett’s post hoc test p < 0.05, **p < 0.01, ****p < 0.0011; §statistical significance compared to control cells ANOVA, followed by Dunnett’s post hoc test p < 0.0001.
3.3Western blotting analysis
Protein expression of total AKT and pAKT were measured in PC-12 cells treated with BM (in the presence (Fig. 5A) and absence of 50 ng/ml NFG (Fig.5 B)). In the absence of NGF, BM treatment appeared to do reduce total AKT expression and pAKT expression, with no significant change in pAKT/AKT ratio. However, in the presence of NGF the expression of total AKT remains unchanged. In contrast, the expression of pAKT significantly reduced in a dose-dependent manner when the bands’ densities were analyzed with Image J (Fig. 5C-D).
Fig. 5
Representative images of western blot analysis of PC-12 cells protein expression in response to varying concentrations of BM A in the absence of NGF; B in the presence of 50 ng/ml NGF. C Densities analyzed with Image J from three independent repeats in the presence of BM and absence of NGF. D Densities analyzed with Image J from three independent repeats in the presence of BM and NGF. The significant difference in protein expression measured by ANOVA followed by Dunnett’s post hoc test. (*p < 0.05, **p < 0.01, ****p < 0.0001).
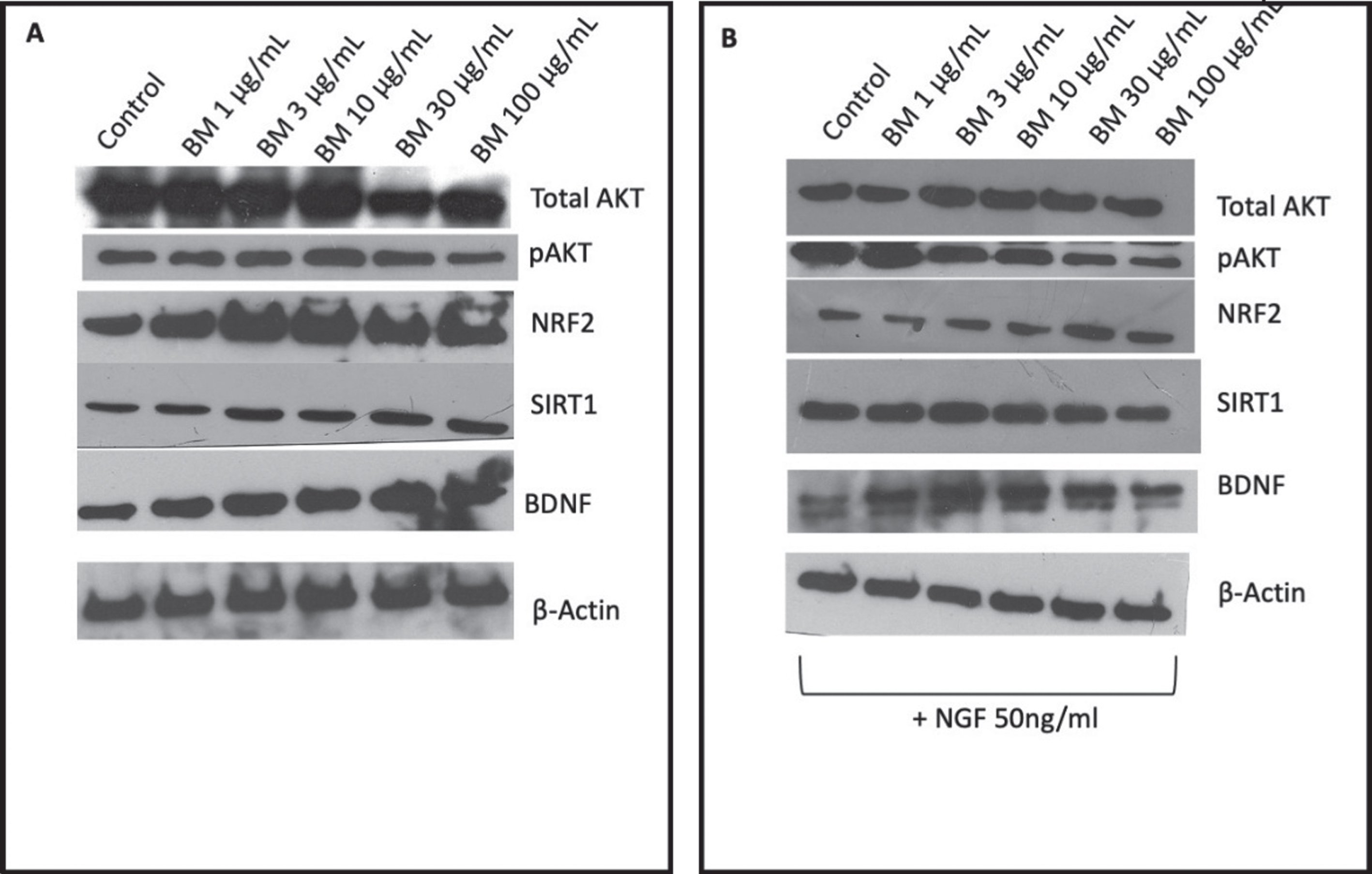
NRF2 expression shows a bell-shaped dose-response to BM with 3μg/ml being the peak increase in expression when normalized to β-actin and statistically significant when the band densities were analyzed with Image J (Fig. 5A-D). In the absence of NGF, the effect of BM treatment appears reduced, with the increase in NGF expression no longer statistically significant.
The expression of SIRT1 remains unchanged when treated with BM in the absence of NGF (Fig. 5A), however, when PC-12 cells are cotreated with BM and NGF there is a bell-shaped response (Fig. 5B), with 3μg/ml showing a statistically significant increase in expression (when the densities of the bands were analyzed with Image J and normalized to β-actin expression Fig. 5C-D).
The expression of BDNF in PC-12 cells treated with BM (in the absence of NGF) dose-dependently increased (Fig. 5A), whereas in the presence of NGF the response to BM was bell-shaped with the peak expression being seen at 3μg/ml (Fig. 5B).
3.4C. elegans pharyngeal pumping assay
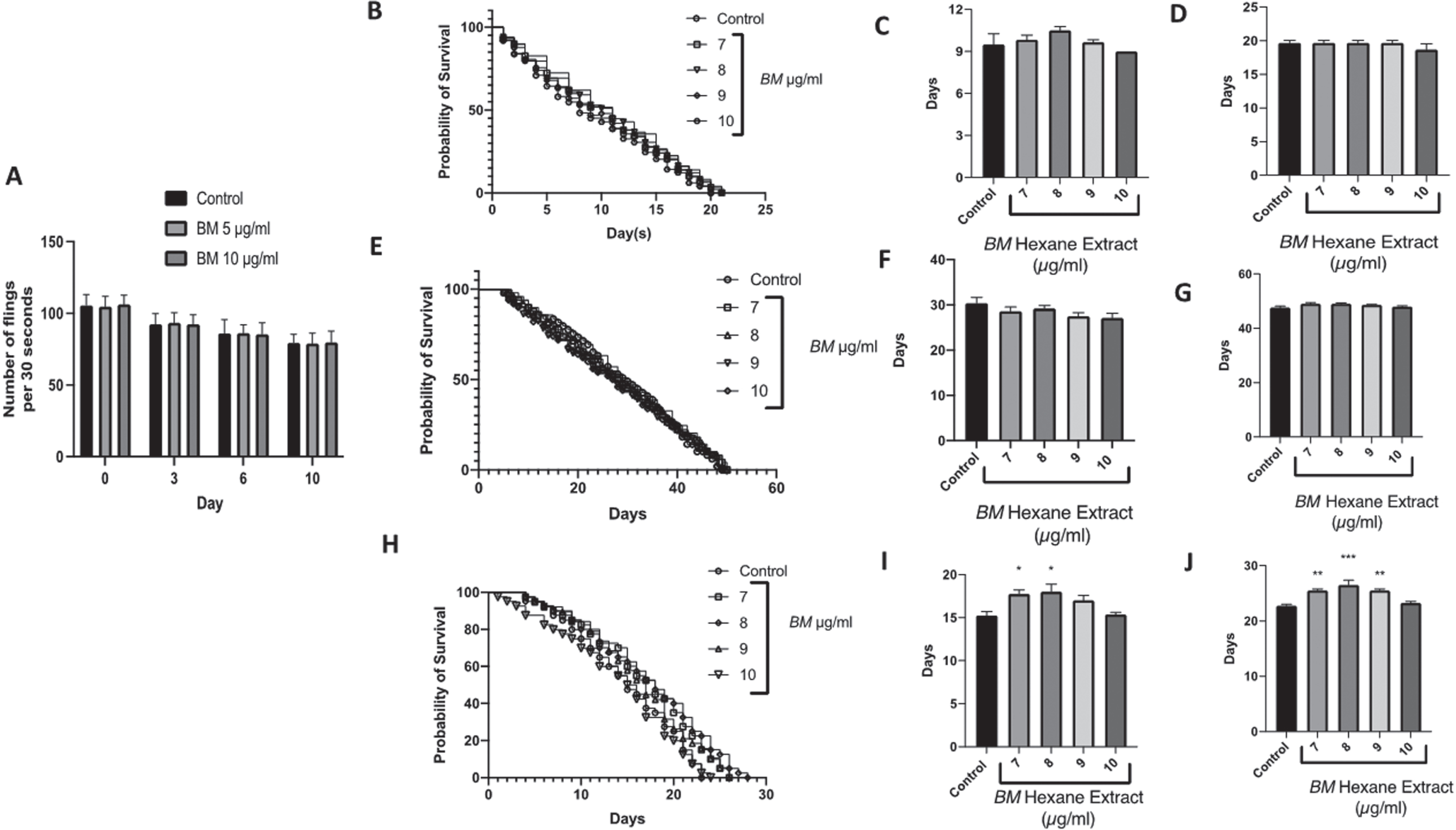
It was observed that both doses of BM did not alter the pharyngeal pumping of the nematodes and showed a similar pumping rate when compared to the control worms fed with laboratory food source E. coli OP50, indicating that the worms consumed BM normally, without any hindrance (Fig. 6A).
Fig. 6
The effect of the hexane extract of Bacopa monnieri on the lifespan of C. elegans mutants: A Pharyngeal pumping assay showing no difference in number of flings at 5 and 10μg/ml compared to control over 10 days of treatment. B Survival curve of daf-16 mutants treated with Bacopa monnieri (7–10μg/ml). C Median lifespan of daf-16 mutant worms treated with Bacopa monnieri (7–10μg/ml). (mean±SEM, n = 5) D Maximum lifespan of daf-16 mutant worms treated with Bacopa monnieri (7–10μg/ml). (mean±SEM, n = 5) E Survival curve of daf-2 mutants treated with Bacopa monnieri (7–10μg/ml). F Median lifespan of daf-2 mutant worms treated with Bacopa monnieri (7–10μg/ml). (mean±SEM, n = 5) G Maximum lifespan of daf-2 mutant worms treated with Bacopa monnieri (7–10μg/ml). (mean±SEM, n = 5) H Survival curve of Aβ expressing transgenic strains treated with Bacopa monnieri (7–10μg/ml). I Median lifespan of Aβ expressing transgenic strains treated with Bacopa monnieri (7–10μg/ml). (mean±SEM, n = 5) J Maximum lifespan of Aβ expressing transgenic strains treated with Bacopa monnieri (7–10μg/ml). (mean±SEM, n = 5). Significant changes from control (ANOVA followed by Dunnett’s post hoc multiple comparison test) *p < 0.05 **p < 0.01.
3.5C. elegans longevity (lifespan/survival) assay
We have previously shown that BM extends the lifespan of wild-type C. elegans, with 7–10μg/ml being the most effective dose. BM was able to extend the median lifespan from approximately 14 days (control) to approximately 18 days, and the maximum lifespan from approximately 20 days (control) to approximately 25 days [54]. Therefore, we selected these doses for measuring the lifespans of the daf-16, daf-2 negative mutants, and Aβ expressing transgenic strains. BM had no significant effect on the lifespans of either the daf-16, daf-2 mutants (Fig. 6B–G). However, BM did significantly improve the median and maximum lifespans of the Aβ mutants, with 7 and 8μg/ml significantly improving the median lifespan from 15.3±0.5 days to 17.8±0.5 and 18.0±0.9 days respectively (Fig. 6H-F) (statistically significant difference ANOVA followed by Dunnett’s post hoc multiple comparisons test p < 0.05). BM (7–9μg/ml) also improved the maximum lifespan from 22.8±0.3 to 25.5±0.3, 26.5±0.9, and 25.5±0.3 days respectively (statistically significant difference ANOVA followed by Dunnett’s post hoc multiple comparisons test p < 0.01).
3.6The antioxidant effect of BM in C. elegans exposed to UV light
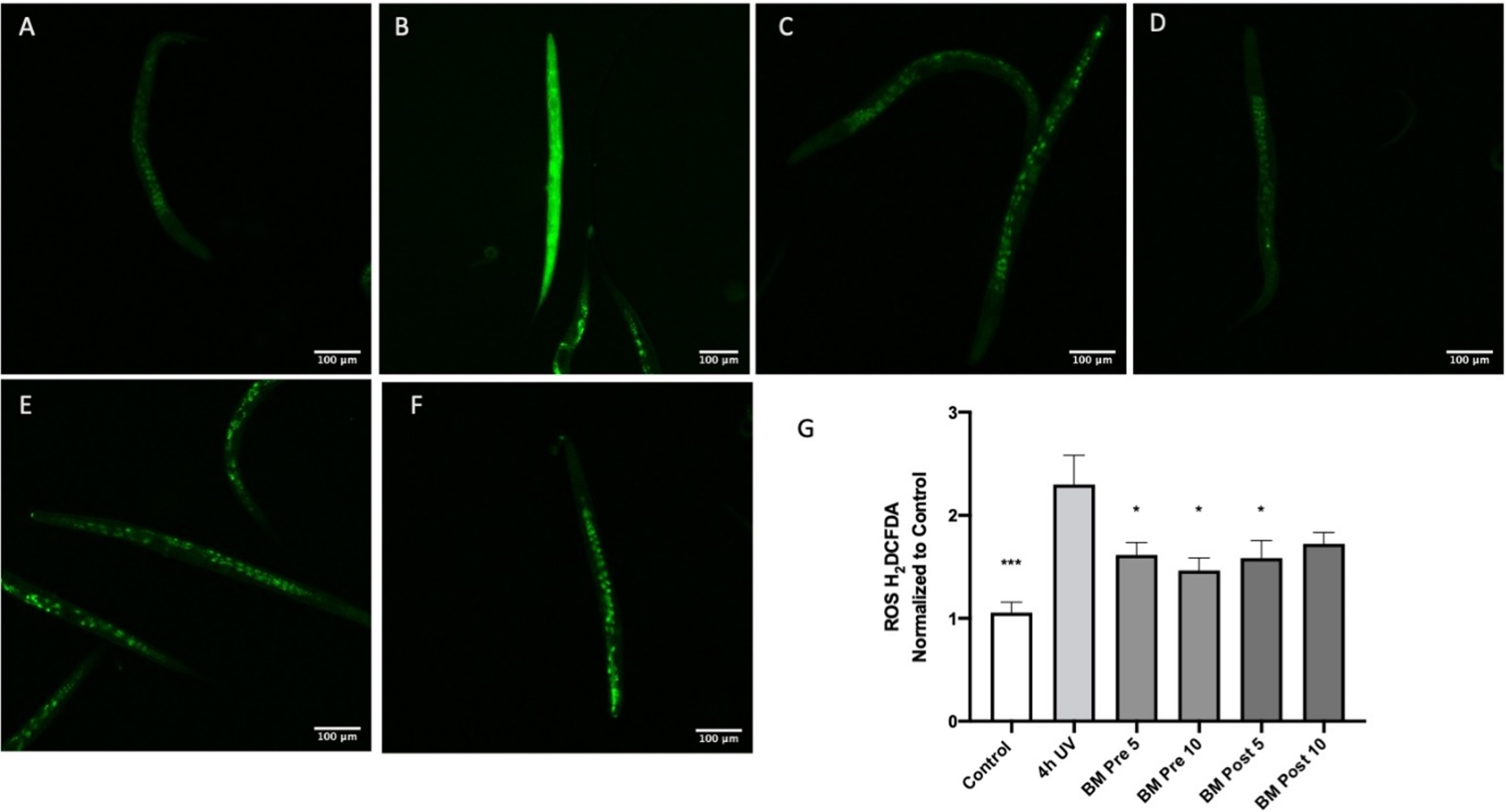
Oxidative stress is a key component of several neurodegenerative diseases including AD [61]. Therefore, to analyze the antioxidant potential of BM, oxidative stress was induced in C. elegans by exposure to UV-A [51]. BM was analyzed for its protective and repair effects by treating the extracts before and after induction of oxidative stress, respectively. There was a significant (p < 0:05) reduction of the oxidative stress level observed, which was directly proportional to the reduction in fluorescence when treated with BM (Fig. 7). Treatment of the worms with UV-A for four hours significantly increased H2DCFDA fluorescence compared to the control (Fig. 7A-B), indicating an increase in oxidative stress (p < 0.001 using ANOVA followed by Dunnett’s post hoc multiple comparisons test). Pretreatment with BM (5 & 10μg/ml) significantly reduced H2DCFDA fluorescence compared to four hours of UV-A alone (Fig. 7 B-D), suggesting that BM can prevent oxidative stress (p < 0.05 using ANOVA followed by Dunnett’s post hoc multiple comparisons test). Treatment with BM (5μg/ml) after four hours of UV exposure significantly reduced H2DCFDA fluorescence compared to four hours of UV alone (Fig. 7B, E), suggesting that BM can reverse oxidative stress in the UV-exposed worms (p < 0.05 using ANOVA followed by Dunnett’s post hoc multiple comparisons test).
Fig. 7
The antioxidant effect of BM in worms exposed to UV-A: A Control; B Four hours of UV-A exposure; C Pretreatment with BM 5 μg/ml and four hours of UV-A exposure; D Pretreatment with BM 10 μg/ml and four hours of UV-A exposure; E Four hours of UV-A exposure, followed by treatment with BM 5 μg/ml; F Four hours of UV-A exposure, followed by treatment with BM 10 μg/ml; G Image J analysis of H2CDFDA fluorescence normalized to control worms. *p < 0.05, ***p < 0.001 using ANOVA followed by Dunnett’s post hoc multiple comparisons test.
3.7mRNA expression in wild-type C. elegans treated with BM
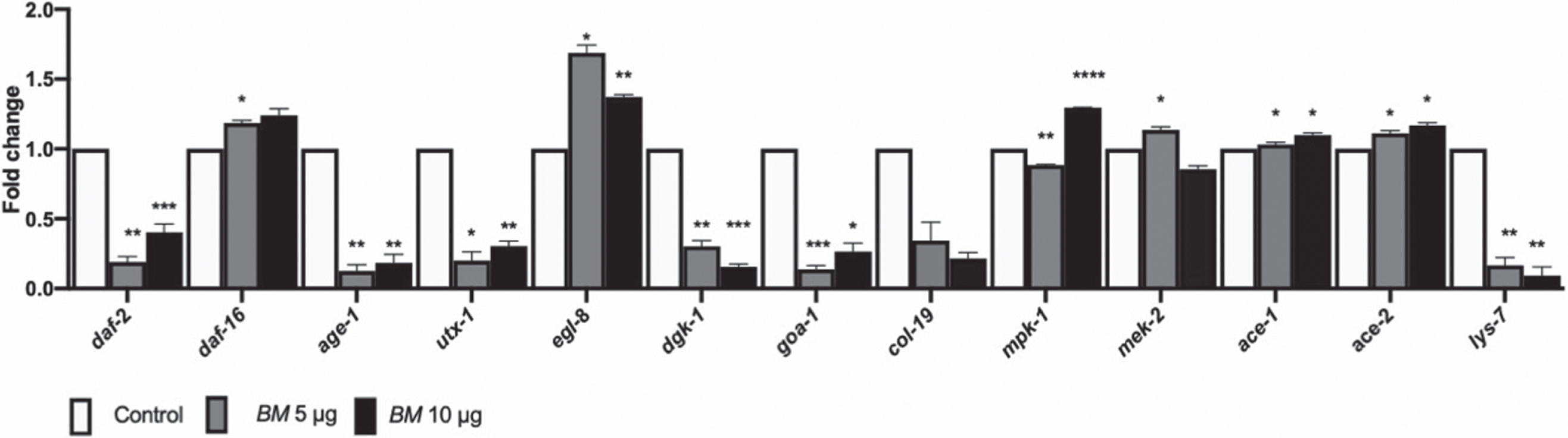
Finally, we assessed the expression of genes which are involved in mediating lifespan, like the DAF-16 mediated pathway (daf-2, daf-16, age-1, utx-1), and ERK MAPK pathway (lin-45, mpk-1, mek-2) and health-span (egl-8, dgk-1, goa-1, col-19, ace-1, ace-2, lys-7) in wild-type worms, treated with BM (5 & 10μg/ml) (Fig. 8). The genes related to reduced lifespans, daf-2, age-1 and utx-1 were downregulated; whereas, daf-16, mpk-1 and mek-2, related to increased lifespan was upregulated. Of the genes related to heath-span egl-8, ace-1 and ace-2 were upregulated, whereas dgk-1, goa-1 and col-19 were downregulated by BM treatment (Fig. 8).
Fig. 8
qPCR analysis. mRNA expression of daf-2, daf-16, age-1, utx-1, egl-8, dgk-1, goa-1, col-19, lin-45, mpk-1, mek-2, ace-1 ace-2 and lys-7 in wild-type nematodes treated with BM (5 & 10μg/ml). BM upregulated daf-16, egl-8, mpk-1, mek-2, ace-1 and ace-2, and downregulated daf-2, age-1, utx-1, dgk-1, goa-1, col-19, lin-45 and lys-7. Significant changes from control were assessed using two-way ANOVA followed by Tukey’s post hoc multiple comparisons test, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3.8Docking analysis
The 3D structure of proteins modelled with trROSETTA were validated using Ramachandran plots. The Ramachandran plots showing SKN-1, DAF-16, C. elegans sigma-1-type opioid receptor, and human sigma non-opioid intracellular receptor 1 (Fig. 9A) revealed that the amino acid residues were found in the favored region indicating a reliable model. The interaction between the protein targets and the compounds in BM were visualized using in silico docking approaches (Fig. 9 B and 10 A-B). The binding scores for each protein were represented in Table 4 and compared with the reference compound EGCG (SKN-1 & DAF-16) or (+) Pentazocine (the human and C.elegans sigma-1 receptor). Against DAF-16, the compounds Bacoside A3 (–9.2 kcal/mol), Bacopaside II (–9.1 kcal/mol), Bacoside A (–8.7 kcal/mol) showed highest binding score compared to that of EGCG (–7.4 kcal/mol). Bacoside A3 exhibited similar hydrogen bonding as that of EGCG with the residues Ala134, Glu244, Arg393 along with other residues Gln146, Ala190, Pro192. In the case of SKN-1, Bacopaside II, Bacoside A, and Bacopaside X showed the highest interaction with binding energies of –9.1, –9.1, and –8.9 kcal/mol respectively when compared to EGCG (–6.6 kcal/mol). EGCG showed hydrogen bonding with Asn238, Glu242, Asp243, which is also observed with Bacoside A and Bacopaside X alongside other interactions including Alkyl, Pi-Alkyl, and carbon-hydrogen bonding (Fig. 9B). The results indicate that the compounds of BM can positively bind with DAF-16 and SKN-1.
Fig. 9
A. Ramachandran plots showing SKN-1, DAF-16, and human sigma non-opioid intracellular receptor 1; B. Interactions of Bacopaside II, Bacoside A, Bacoside A3 and EGCG with amino acids in Daf-16 and SKN-1.
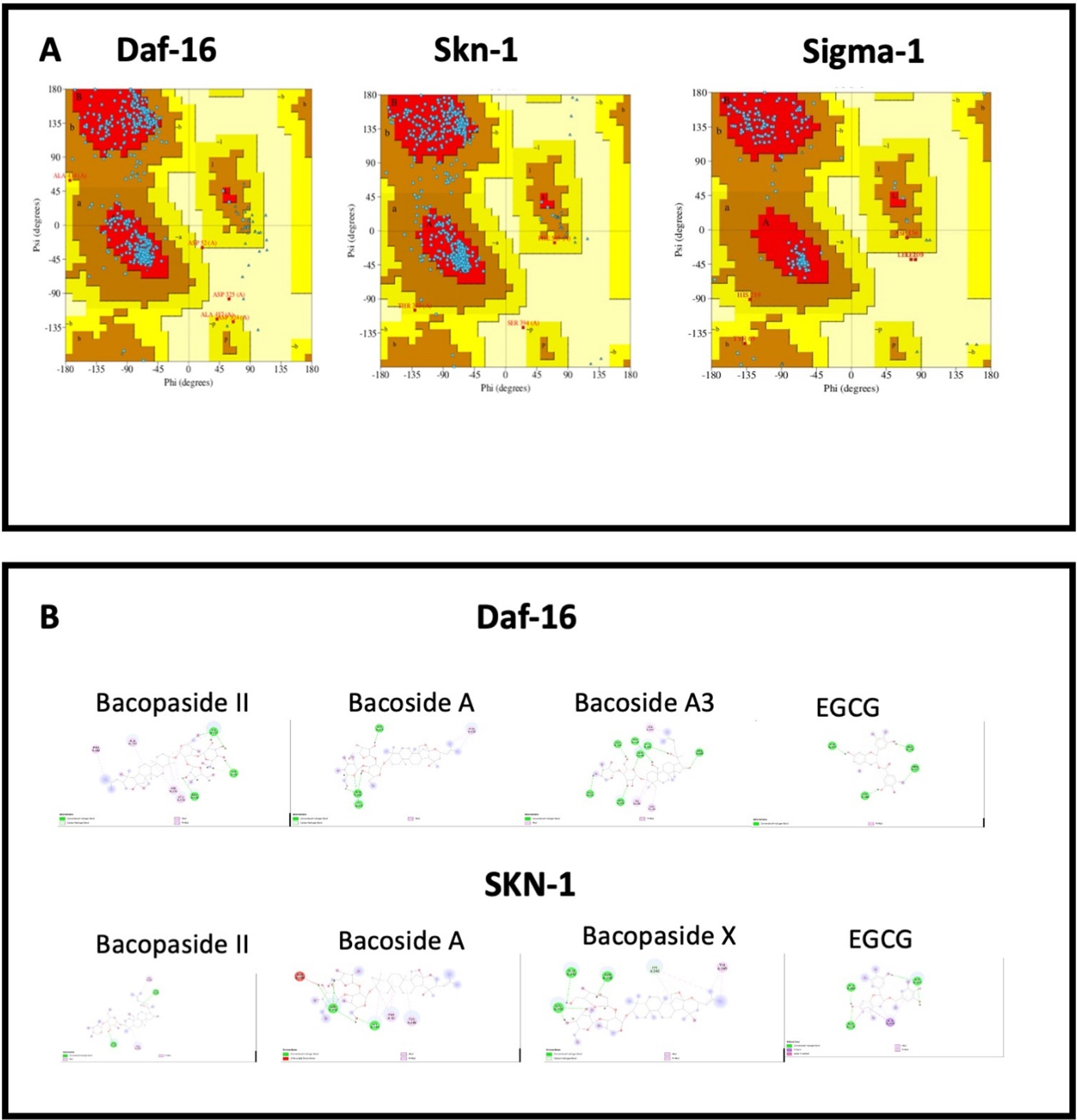
Fig. 10
Interactions of bacopa extract compounds with amino acids in A. C. elegans sigma-1 receptor and B. human sigma-1 receptor.
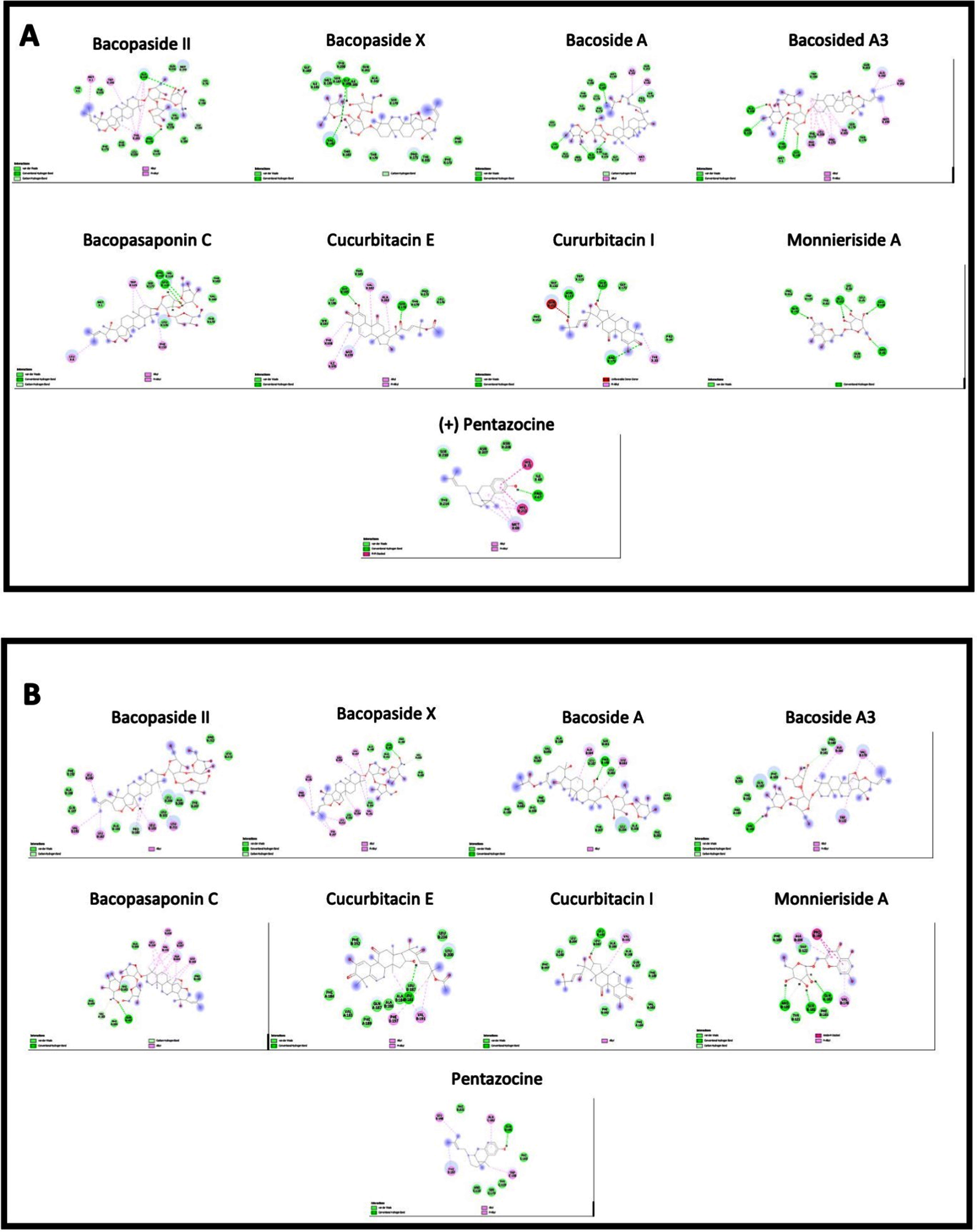
Table 4
Molecular docking analysis of compounds found in BM against DAF-16 SKN-1 and the C. elegans and human sigma-1 receptor. Results presented as kcal/mol
| Compound | Daf-16 (kcal/mol) | SKN-1 (kcal/mol) | C. elegans σ 1-type opioid receptor (kcal/mol) | Human σ non-opioid intracellular receptor 1 (kcal/mol) |
| Bacopasaponin C | –8.303 | –7.921 | –8.951 | –8.879 |
| Bacopaside II | –9.158 | –9.077 | –10.064 | –9.39 |
| Bacopaside X | –8.369 | –8.954 | –9.138 | –8.898 |
| Bacoside A | –8.716 | –9.06 | –8.951 | –9.15 |
| Bacoside A3 | –9.234 | –7.027 | –9.661 | –8.648 |
| Cucurbitacin E | –8.309 | –6.989 | –8.231 | –8.648 |
| Cucurbitacin I | –8.136 | –6.997 | –8.231 | –8.437 |
| Monnieriside A | –7.891 | –6.444 | –8.231 | –8.437 |
| (+) Pentazocine | – | – | –8.461 | –8.437 |
| EGCG | –7.473 | –6.632 | – | – |
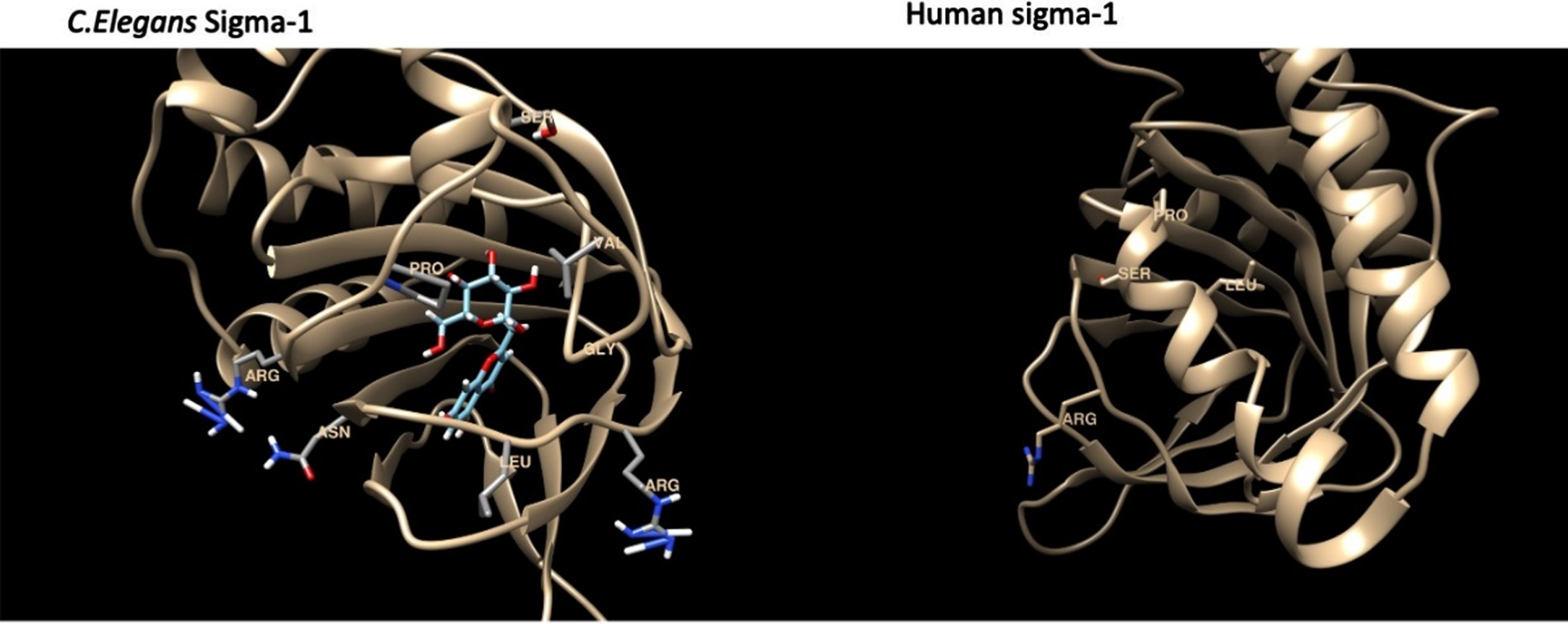
The binding energies for the C. elegans sigma-1 type opioid receptor were highest for Bacopaside II (–10.1 kcal/mol), Bacoside A3 (–9.2 kcal/mol) and Bacopaside X (–9.1 kcal/mol), when compared to the standard reference compound, (+) Pentazocine (–8.5 kcal/mol). The docking analysis for the C.elegans sigma-1 type opioid receptor indicated that there was hydrogen (H)-bonding interactions with amino acids in the binding pocket of the sigma-1 receptor, with Bacopaside II H-bonding with Pro175, Bacopaside X showed predicted H-bonding interactions with Val182 and Gly185 and Bacoside A and cucurbitacin E both having predicted H-bonding interactions with Gly 185. There were also interesting H-bonding predicted interactions for Bacopasaponin C and Curcurbitacin I which interreacted with amino acids on the outside of the binding pocket (Fig. 11). Bacopasaponin C was predicted to interact with Arg110 and Leu113, whereas Cucurbitacin I was predicted to interact with Asn117 and Arg171 (Fig. 11).
Fig. 11
Model of the sigma-1 receptor from both C. elegnans and humans showing the binding pocket and interacting amino acids.
4Discussion
Many plant extracts have been previously investigated for their beneficial effects against neurodegenerative diseases, including C. asiatica which shows protection against Aβ toxicity in PC-12 and IMR32 cells [62], Pomegranate extract prevents oxidative damage in the brains of AD model transgenic Swedish mutant mice [63], and T. divaricata root extract protects mice from Aβ25–35 neurotoxicity [64]. We have previously shown BM to have antioxidant activities in HT-22 cells subjected to glutamate toxicity [54]. However, in this study, we aimed to investigate whether BM is more than just an antioxidant and whether it had any further beneficial effects that could explain its potential for treating AD.
Herein, we have shown that BM can not only potentiate NGF-induced neurite outgrowth in PC-12 cells, but it can also induce neurite outgrowth in Neuro2A cells. Moreover, BM was able to induce neurite outgrowth in the presence of FBS for Neuro2A cells. Furthermore, BM protected differentiated PC-12 cells from damage caused by Aβ and extended the lifespan of C. elegans that expressed Aβ. As far as we are aware, this is the first study to show that BM’s hexane extract has these effects in PC-12, Neuro2A and C. elegans models.
Targeting multiple receptors and pathways, using traditional pharmacological compounds or those extracted from medicinal plants may be beneficial for many neurological diseases [16, 65, 66]. One receptor that is common to several pathways as well as many neurological diseases, including AD, Parkinson’s disease (PD), Huntington’s disease (HD), and depression (MD) is the sigma-1 receptor [16, 67, 68]. Multiple studies have shown that neurite outgrowth is dependent on the sigma-1 receptor in both PC-12 [69–73] and Neuro2A cells [23]. Furthermore, several pre-clinical studies have indicated that BM treatment in vivo, can induce neurogenesis and increase CREB, AKT, ERK and BDNF expression [74–76]. The expression of BDNF, AKT, ERK, and CREB are potentially under the control of sigma-1 receptor activity [77–83]. We have shown that the sigma-1 receptor antagonist BD1047 is able to prevent the BM-induced potentiation of neurite outgrowth in PC-12 cells. We also show that BDNF expression is increased by BM treatment. BDNF expression plays a central role in cellular proliferation, migration, differentiation, and maintenance in the developing brain [84]. Moreover, the potentiation of neurite outgrowth in PC-12 cells by BM mimics that of several antidepressants such as fluvoxamine and fluoxetine that also have affinities for the sigma-1 receptor and upregulate the expression of BDNF in cultured PC-12 and cortical neurons in vitro.
The PI3K/AKT/mTOR pathway is essential in the control of the cell cycle [85]. It is, therefore, essential to the longevity and survival of the cell. AKT activation has been shown to inhibit neuronal differentiation and drive cell proliferation [86]. Here we have shown that BM is able to downregulate total AKT and pAKT expression in the absence of NGF and in the presence of NGF downregulate pAKT. This suggests that BM is modulating the PI3K/AKT/mTOR pathway to induce differentiation into neuron-like cells. We also see an upregulation of NRF-2 in the absence of NGF, which could possibly be linked to sigma-1 receptor activation [87, 88]. The upstream regulators of Nrf2 (PKC, MAPK and PI3K) [89] are also potentially regulated by sigma-1 receptor activity. However, it is possible that BM acts directly on NRF-2 considering its active compounds’ potential binding affinities to the C. elegans ortholog of NRF-2, SKN-1.
The molecular docking analysis in the sigma-1 receptor (human and predicted C. elegans) suggests that several of BM’s compounds may have high affinity toward the sigma-1 receptor. The docking analysis indicated that Bacopaside II, Bacopaside X, Bacoside A, and Cucurbitacin E all showed predicted H-bonding interactions with amino acids within the predicted binding pocket of the sigma-1 receptor, which further adds to the evidence for sigma-1 receptor modulation by BM extracts as a potential mechanism for BMs positive neurological effects. Furthermore, Bacopasaponin C and Curcurbitacin I interreacted with amino acids on the outside of the binding pocket and could possibly act allosterically to induce their effects. Allosteric interactions with the sigma-1 receptor are regularly being reported with regard to neurological disease [90–92]. Several studies have identified BM to have anti-seizure effects in various models [93–95]. Allosteric binding to the sigma-1 receptor has also been shown to alleviate seizure in similar models [96, 97], suggesting the potential mechanism for these BM extracts.
One major drawback of this study is that it revolves around the use of cultured (non-human) cells which brings the question of physiological relevance when extrapolating to the use of BM as a nutritional supplement in humans. The possible metabolism of BM’s compounds to produce further active/inactive compounds and the activities of these compounds in a more complex system (rather than a single cell type culture) requires further investigation. With this in mind, we used in silico analysis to predict possible metabolites produced by human and gut bacterial enzymes, that could be used in future study of BM’s effects in neuroprotection.
To understand BMs activities in a more complex system, we have employed the model organism C. elegans. In a previous study, we have shown that BM is able to extend the lifespan of C. elegans and reduce the deposition of lipofuscin [54], (a pigment widely regarded as a sign of aging [42, 51]). In the present study, we tried to understand the mechanism of lifespan extension. The insulin-like-signaling pathway, otherwise known as the DAF-16 mediated pathway is one of the major pathways that mediate aging in C. elegans wherein daf-2 is a negative regulator of lifespan and daf-16 is a positive regulator [98, 99]. Mutants of both daf-2 and daf-16 did not exhibit any significant changes in lifespan irrespective of BM treatment, indicating the importance of the pathway in extending lifespan. In C. elegans, a dietary restriction or calorie restriction process can get activated, which can also extend lifespan, which is interconnected to many other pathways [100–102]. To confirm that the lifespan extension observed in the present study was mediated by BM and was not by dietary restriction, the pharyngeal pumping assay was carried out in C. elegans, which showed that there was no difference in the pharyngeal pumping rate in worms fed with BM when compared to the control. This suggests that the extension of lifespan observed was mediated by BM, and that the worms had no trouble ingesting BM through their gut system.
qPCR analysis of candidate genes, namely daf-2, daf-16, age-1 and utx-1 [98, 99] were monitored in wild-type worms wherein daf-16 was upregulated, and all other genes were downregulated, indicating the activation of DAF-16 mediated pathway. Interestingly, age-1, which is orthologous to mammalian PI-3-kinase, was also downregulated [103, 104]. The western blot also validated the downregulation of the PI-3-kinase pathway (via AKT-phosphorylation) indicating the involvement of the pathway during BM treatment. The lysozyme gene, lys-7, is a target downstream of DAF-16 [105]. It is known to provide immune protection against pathogens [106] and the downregulation of the same in the present study suggests that BM was not considered as a toxic or harmful substance by the worms. Additionally, the role of DAF-16 was further confirmed through docking analysis. We observed that the compounds of BM were able to efficiently bind with BM than the reference compound EGCG, which is known to activate DAF-16 [107].
The ERK/MAPK (extracellular signal-regulated kinase/mitogen-activated protein kinase) pathway regulates major biological processes including metabolism. It can also aid in the activation of DAF-16 mediated lifespan extension [108, 109]. Candidate genes of the pathway, mpk-1 and mek-2 were analyzed for their role in BM mediated lifespan extension. Both mpk-1 and mek-2 were upregulated indicating the activation of DAF-16 mediated lifespan extension [109].
To confirm health-span-related gene activation, qPCR analysis of health-span candidate genes was measured. Col-19 is thought to be an adult-specific marker [110, 111] since its expression begins at the late larval stages and increases into adulthood [112]. In the present study, the expression of col-19 was downregulated compared to control (Fig. 3B) indicating the anti-aging potential of BM.
The Diacylglycerol (DAG) pathway includes the orthologs of the Go ligands (egl-8, goa-1, and dgk-1). These are essential for health-span [42, 51] including C. elegans functions such as pharyngeal pumping, movement/locomotion and egg-laying where egl-8 is regulated positively [113] and dgk-1 and goa-1 are regulated negatively [114, 115]. In this study, qPCR analysis showed, the expression of egl-8 was upregulated, whereas the expression of dgk-1 and goa-1 were downregulated. The acetylcholinesterase genes, ace-1 and ace-2 were also upregulated which could help in improving egg laying [116], which is also another marker for health span.
The transgenic Aβ expressing worms have a reduced lifespan compared to the wild-type worms. Treating these worms with BM significantly increased their lifespan suggesting that BM has some positive effect on the toxicity of Aβ in the worm. Additionally, BM was able to reduce the level of oxidative stress in C. elegans, which was induced by exposure to UV-A [117]. Moreover, the docking analysis of SKN-1 (which reduces oxidative stress [118, 119]), indicates that the compounds of BM (except for Monnieriside A) had better binding potential towards SKN-1 than the reference compound EGCG, pointing to the activation of SKN-1. This aligns with the data we present in the differentiated PC-12 cells treated with Aβ and could be the result of the antioxidant effects seen in C. elegans treated with BM, since excess Aβ has been shown to induce oxidative stress in both cell lines and C. elegans in a large number of studies [120–123].
5Conclusion
In conclusion, we have further shown that BM is neuroprotective in cell culture models of AD, as well as being able to induce neurite growth. These neurodifferentiation and neuroprotective effects are possibly a result of sigma-1 receptor activation (with several BM’s compounds showing possible high sigma-1 receptor affinity in the molecular docking analysis) as well as antioxidant effects which we have shown in the C. elegans models. Since BM failed to extend the lifespans of the daf-16 and daf-2 mutants, we can conclude that this pathway is likely involved in the lifespan-extending mechanism activated by BM. Furthermore, BM also regulated genes involving the improvement of health span. This study suggests that BM could potentially be of future use in the treatment of neurodegenerative diseases such as AD, by reducing oxidative stress and controlling neuron survival and growth. However, further research into the physiological relevance of these findings are required plus further preclinical and clinical study of BM.
Acknowledgments
JMB and MIP were supported by the Rachadapisek Sompote Fund for Postdoctoral Fellowship, Chulalongkorn University, Thailand. DSM was supported by the Second Century Fund (C2F) for postdoctoral fellows at Chulalongkorn University, Thailand. We also thank BioRender.com for help creating some of the figures.
Supplementary material
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/NHA-220161.
REFERENCES
[1] | Prince MJ , Prina M , Guerchet M World Alzheimer Report 2013: journey of caring: an analysis of long-term care for dementia, in, Alzheimer’s Disease International, London, 2013, pp. 1-8. |
[2] | Brimson JM , Prasanth MI , Malar DS , Brimson S , Thitilertdecha P , Tencomnao T , Drugs that offer the potential to reduce hospitalization and mortality from SARS-CoV-2 infection: The possible role of the Sigma-1 receptor and autophagy, Expert Opinion on Therapeutic Targets (2021) ;25: :401–14. |
[3] | Hascup ER , Hascup KN , Does SARS-CoV-2 infection cause chronic neurological complications? GeroScience (2020) ;42: :1083–7. |
[4] | Ellul MA , Benjamin L , Singh B , Lant S , Michael BD , Easton A , Kneen R , Defres S , Sejvar J , Solomon T , Neurological associations of COVID-19, The Lancet Neurology (2020) ;19: :767–83. |
[5] | Jorm AF , Alzheimer’s disease: Risk and protection, Medical Journal of Australia (1997) ;167: :443–6. |
[6] | Troussière A-C , Charley CM , Salleron J , Richard F , Delbeuck X , Derambure P , Pasquier F , Bombois S , Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer’s disease, Journal of Neurology, Neurosurgery & Psychiatry (2014) ;85: :1405–8. |
[7] | Benedict C , Byberg L , Cedernaes J , Hogenkamp PS , Giedratis V , Kilander L , Lind L , Lannfelt L , Schiöth HB , Self-reported sleep disturbance is associated with Alzheimer’s disease risk in men, Alzheimer’s & Dementia (2015) ;11: :1090–7. |
[8] | Solfrizzi V , Panza F , Frisardi V , Seripa D , Logroscino G , Imbimbo BP , Pilotto A , Diet and Alzheimer’s disease risk factors or prevention: The current evidence, Expert Review of Neurotherapeutics (2011) ;11: :677–708. |
[9] | Iwagami M , Qizilbash N , Gregson J , Douglas I , Johnson M , Pearce N , Evans S , Pocock S , Blood cholesterol and risk of dementia in more than 1.8 million people over two decades: A retrospective cohort study. The Lancet Healthy Longevity. 2021. |
[10] | Ishii M , Midlife dyslipidaemia as a modifiable risk factor for later-life dementia. The Lancet Healthy Longevity. 2021. |
[11] | Gauthier S , Cummings J , Ballard C , Brodaty H , Grossberg G , Robert P , Lyketsos C , Management of behavioral problems in Alzheimer’s disease, International Psychogeriatrics (2010) ;22: :346. |
[12] | Giri M , Zhang M , Lü Y , Genes associated with Alzheimer’s disease: An overview and current status, Clinical Interventions in Aging (2016) ;11: :665. |
[13] | Colovic MB , Krstic DZ , Lazarevic-Pasti TD , Bondzic AM , Vasic VM , Acetylcholinesterase inhibitors: Pharmacology and toxicology, Current Neuropharmacology (2013) ;11: :315–35. |
[14] | Ali TB , Schleret TR , Reilly BM , Chen WY , Abagyan R , Adverse effects of cholinesterase inhibitors in dementia, according to the pharmacovigilance databases of the United-States and Canada, PLoS ONE (2015) ;10: :e0144337. |
[15] | Prasanth MI , Malar D , Tencomnao T , Brimson J , The emerging role of the sigma-1 receptor in autophagy: Hand-in-hand targets for the treatment of Alzheimer’s Disease, Expert Opinion on Therapeutic Targets (2021) ;25: :435–49. |
[16] | Brimson JM , Brimson S , Chomchoei C , Tencomnao T , Using Sigma-ligands as part of a multi-receptor approach to target diseases of the brain, Expert Opinion on Therapeutic Targets (2020) ;24: :1009–28. |
[17] | Aydar E , Palmer CP , Klyachko VA , Jackson MB , The sigma receptor as a ligand-regulated auxiliary potassium channel subunit, Neuron (2002) ;34: :399–410. |
[18] | Aydar E , Palmer CP , Djamgoz MBA , Sigma receptors and cancer: Possible involvement of ion channels, Cancer Research (2004) ;64: :5029–35. |
[19] | Natsvlishvili N , Goguadze N , Zhuravliova E , Mikeladze D , Sigma-1 receptor directly interacts with Rac1-GTPase in the brain mitochondria, BMC Biochemistry (2015) ;16: :1–7. |
[20] | Brimson JM , Brown CA , Safrany ST , Antagonists show GTP-sensitive high-affinity binding to the sigma-1 receptor, British Journal of Pharmacology (2011) ;164: :772–80. |
[21] | Marrazzo A , Caraci F , Salinaro ET , Su T-P , Copani A , Ronsisvalle G , Neuroprotective effects of sigma-1 receptor agonists against beta-amyloid-induced toxicity, Neuroreport (2005) ;16: :1223–6. |
[22] | Nguyen L , Lucke-Wold BP , Mookerjee S , Kaushal N , Matsumoto RR , Sigma-1 receptors and neurodegenerative diseases: Towards a hypothesis of sigma-1 receptors as amplifiers of neurodegeneration and neuroprotection. Sigma Receptors: Their Role in Disease and as Therapeutic Targets. 2017;133-52. |
[23] | Brimson JM , Safrany ST , Qassam H , Tencomnao T , Dipentylammonium binds to the sigma-1 receptor and protects against glutamate toxicity, attenuates dopamine toxicity and potentiates neurite outgrowth in various cultured cell lines, Neurotoxicity Research (2018) ;34: :263–72. |
[24] | Fehér Á , Juhász A , László A , Kálmán J Jr , Pákáski M , Kálmán J , Janka Z , Association between a variant of the sigma-1 receptor gene and Alzheimer’s disease, Neuroscience Letters (2012) ;517: :136–9. |
[25] | Maruszak A , Safranow K , Gacia M , Gabryelewicz T , Słowik A , Styczyńska M , Pepłońska B , Golan MP , Zekanowski C, Barcikowska M, Sigma receptor type 1 gene variation in a group of polish patients with Alzheimer’s disease and mild cognitive impairment. Dementia and Geriatric Cognitive Disorders (2007) ;23: :432–8. |
[26] | Huang Y , Zheng L , Halliday G , Dobson-Stone C , Wang Y , Tang H-D , Cao L , Deng Y-L , Wang G , Zhang Y-M , Genetic polymorphisms in sigma-1 receptor and apolipoprotein E interact to influence the severity of Alzheimer’s disease, Current Alzheimer Research (2011) ;8: :765–70. |
[27] | Jorm AF , Rodgers B , Christensen H , Use of medications to enhance memory in a large community sample of 60–64 year olds, International Psychogeriatrics (2004) ;16: :209–17. |
[28] | Stough C , Lloyd J , Clarke J , Downey L , Hutchison C , Rodgers T , Nathan PJ , The chronic effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy human subjects, Psychopharmacology (2001) ;156: :481–4. |
[29] | Peth-Nui T , Wattanathorn J , Muchimapura S , Tong-Un T , Piyavhatkul N , Rangseekajee P , Ingkaninan K , Vittaya-areekul S , Effects of 12-week bacopa monnieri Consumption on attention, cognitive processing, working memory, and functions of both cholinergic and monoaminergic systems in healthy elderly volunteers, Evidence-Based Complementary and Alternative Medicine (2012) ;2012: :1–10. |
[30] | Morgan A , Stevens J , Does Bacopa monnieri improve memoryperformance in older persons? Results of a randomized,placebo-controlled, double-blind trial, The Journal of Alternativeand Complementary Medicine (2010) ;16: :753–9. |
[31] | Sathyanarayanan V , Thomas T , Einöther SJL , Dobriyal R , Joshi MK , Krishnamachari S , Brahmi for the better? New findings challenging cognition and anti-anxiety effects of Brahmi (Bacopa monniera) in healthy adults, Psychopharmacology (2013) ;227: :299–306. |
[32] | Mandal AK , Hedge S , Patki PS , A clinical study to evaluate the efficacy and safety of Bacopa caplets in memory and learning ability: A double blind placebo controlled study, Australian Journal of Medical Herbalism (2011) ;23: :122–5. |
[33] | Norman Scholfield C , Dilokthornsakul P , Limpeanchob N , Thanarangsarit P , Kongkeaw C , Meta-analysis of randomized controlled trials on cognitive effects of Bacopa monnieri extract, Journal of Ethnopharmacology (2013) ;151: :528–35. |
[34] | Brimson JM , Brimson S , Prasanth MI , Thitilertdecha P , Malar DS , Tencomnao T , The effectiveness of Bacopa monnieri (Linn, ) Wettst. as a nootropic, neuroprotective, or antidepressant supplement: Analysis of the available clinical data. Scientific Reports (2021) ;11: :1–11. |
[35] | Kean JD , Downey LA , Sarris J , Kaufman J , Zangara A , Stough C , Effects of Bacopa monnieri (CDRI 08®) in a population of males exhibiting inattention and hyperactivity aged 6 to 14 years: A randomized, double-blind, placebocontrolled trial. Phytotherapy Research. |
[36] | Calabrese C , Gregory WL , Leo M , Kraemer D , Bone K , Oken B , Effectsof a standardized Bacopa monnieri Extract on cognitiveperformance, anxiety, and depression in the elderly: A randomized,double-blind, placebo-controlled trial, The Journal of Alternativeand Complementary Medicine (2008) ;14: :707–13. |
[37] | Lyketsos CG , Olin J , Depression in Alzheimer’s disease: Overview and treatment, Biological Psychiatry (2002) ;52: :243–52. |
[38] | Ferretti L , McCurry SM , Logsdon R , Gibbons L , Teri L , Anxiety and Alzheimer’s disease, Journal of Geriatric Psychiatry and Neurology (2001) ;14: :52–8. |
[39] | Goswami S , Saoji A , Kumar N , Thawani V , Tiwari M , Thawani M , Effect of Bacopa monnieri on cognitive functions in Alzheimer’s disease patients, International Journal of Collaborative Research on Internal Medicine and Public Health (2011) ;3: :285–93. |
[40] | Sadhu A , Upadhyay P , Agrawal A , Ilango K , Karmakar D , Singh GPI , Dubey GP , Management of cognitive determinants in senile dementia of Alzheimer’s type: Therapeutic potential of a novel polyherbal drug product, Clinical Drug Investigation (2014) ;34: :857–69. |
[41] | Cicero AF , Bove M , Colletti A , Rizzo M , Fogacci F , Giovannini M , Borghi C , Short-term impact of a combined nutraceutical on cognitive function, perceived stress and depression in young elderly with cognitive impairment: A pilot, double-blind, randomized clinical trial, The Journal of Prevention of Alzheimer’s Disease (2017) ;4: :12–5. |
[42] | Prasanth MI , Santoshram GS , Bhaskar JP , Balamurugan K , Ultraviolet-A triggers photoaging in model nematode Caenorhabditis elegans in a DAF-16 dependent pathway, Age (2016) ;38: :1–13. |
[43] | Sharika R , Subbaiah P , Balamurugan K , Studies on reproductive stress caused by candidate Gram positive and Gram negative bacteria using model organism, Caenorhabditis elegans, Gene (2018) ;649: :113–26. |
[44] | Yang J , Huang X-B , Wan Q-L , Ding A-J , Yang Z-L , Qiu M-H , Sun H-Y , Qi S-H , Luo H-R , Otophylloside B protects against Aβ toxicity in Caenorhabditis elegans models of Alzheimer’s disease, Natural Products and Bioprospecting (2017) ;7: :207–14. |
[45] | Qian H , Xu X , Niklason LE , PCH-2 regulates Caenorhabditis elegans lifespan, Aging (Albany NY) (2015) ;7: :1. |
[46] | Deusing DJ , Winter S , Kler A , Kriesl E , Bonnländer B , Wenzel U , Fitzenberger E , A catechin-enriched green tea extract prevents glucose-induced survival reduction in Caenorhabditis elegans through sir-2, 1 and uba-1 dependent hormesis. Fitoterapia (2015) ;102: :163–70. |
[47] | Prasansuklab A , Meemon K , Sobhon P , Tencomnao T , Ethanolic extract of Streblus asper leaves protects against glutamate-induced toxicity in HT22 hippocampal neuronal cells and extends lifespan of Caenorhabditis elegans, BMC Complementary and Alternative Medicine (2017) ;17: :1–14. |
[48] | Prasanth MI , Brimson JM , Sheeja Malar D , Prasansuklab A , Tencomnao T , Streblus asper Lour, exerts MAPK and SKN-1 mediated anti-aging, anti-photoaging activities and imparts neuroprotection by ameliorating Aβ in Caenorhabditis elegans. Nutrition and Healthy Aging (2021) ;6: :211–27. |
[49] | Boasquívis PF , Silva GMM , Paiva FA , Cavalcanti RM , Nunez CV , dePaula Oliveira R , (Paullinia cupana) extract protects Caenorhabditis elegans models for Alzheimer disease and Huntington disease through activation of antioxidant and protein degradation pathways. Oxidative Medicine and Cellular Longevity. 2018;2018.. |
[50] | Malar DS , Prasanth MI , Brimson JM , Verma K , Prasansuklab A , Tencomnao T , Hibiscus sabdariffa extract protects HT-22 cells from glutamate-induced neurodegeneration by upregulating glutamate transporters and exerts lifespan extension in C, elegans via DAF-16 mediated pathway. Nutrition and Healthy Aging (2021) ;6: :229–47. |
[51] | Prasanth MI , Brimson JM , Chuchawankul S , Sukprasansap M , Tencomnao T , Antiaging, stress resistance, and neuroprotective efficacies of cleistocalyx nervosum var. paniala fruit extracts using caenorhabditis elegans model. Oxidative Medicine and Cellular Longevity. 2019;2019. |
[52] | Brimson JM , Prasanth MI , Isidoro C , Sukprasansap M , Tencomnao T , Cleistocalyx nervosum var, paniala seed extracts exhibit sigma-1 antagonist sensitive neuroprotective effects in PC12 cells and protect C. elegans from stress via the SKN-1/NRF-2 pathway. Nutrition and Healthy Aging (2021) ;6: :131–46. |
[53] | Yan F , Chen Y , Azat R , Zheng X , Mulberry anthocyanin extract ameliorates oxidative damage in HepG2 cells and prolongs the lifespan of Caenorhabditis elegans through MAPK and Nrf2 pathways. Oxidative Medicine and Cellular Longevity. 2017;2017.. |
[54] | Brimson JM , Prasanth MI , Plaingam W , Tencomnao T , Bacopa monnieri (L, ) wettst. Extract protects against glutamate toxicity and increases the longevity of Caenorhabditis elegans. Journal of Traditional and Complementary Medicine (2020) ;10: :460–70. |
[55] | Djoumbou-Feunang Y , Fiamoncini J , Gil-de-la-Fuente A , Greiner R , Manach C , Wishart DS , BioTransformer: A comprehensive computational tool for small molecule metabolism prediction and metabolite identification, Journal of Cheminformatics (2019) ;11: :1–25. |
[56] | Meijering E , Jacob M , Sarria JC , Steiner P , Hirling H , Unser M , Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images, Cytometry Part A: The Journal of the International Society for Analytical Cytology (2004) ;58: :167–76. |
[57] | Santos KB , Guedes IA , Karl AL , Dardenne LE , Highly flexible ligand docking: Benchmarking of the DockThor program on the LEADS-PEP protein–peptide data set, Journal of Chemical Information and Modeling (2020) ;60: :667–83. |
[58] | Terada K , Migita K , Matsushima Y , Sugimoto Y , Kamei C , Matsumoto T , Mori M , Matsunaga K , Takata J , Karube Y , Cholinesterase inhibitor rivastigmine enhances nerve growth factor-induced neurite outgrowth in PC12 cells via sigma-1 and sigma-2 receptors, PLoS ONE (2018) ;13: :1–16. |
[59] | Rossi D , Pedrali A , Urbano M , Gaggeri R , Serra M , Fernández L , Fernández M , Caballero J , Ronsisvalle S , Prezzavento O , Schepmann D , Wuensch B , Peviani M , Curti D , Azzolina O , Collina S , Identification of a potent and selective σ1 receptor agonist potentiating NGF-induced neurite outgrowth in PC12 cells, Bioorganic & Medicinal Chemistry (2011) ;19: :6210–24. |
[60] | Niitsu T , Iyo M , Hashimoto K , Sigma-1 receptor agonists as therapeutic drugs for cognitive impairment in neuropsychiatric diseases, Current Pharmaceutical Design (2012) ;18: :875–83. |
[61] | Andersen JK , Oxidative stress in neurodegeneration: Cause or consequence? Nature Medicine, S (2004) ;10: :18–25. |
[62] | Chen C-L , Tsai W-H , Chen C-J , Pan T-M , Centella asiatica extract protects against amyloid β1–40-induced neurotoxicity in neuronal cells by activating the antioxidative defence system, Journal of Traditional and Complementary Medicine (2016) ;6: :362–9. |
[63] | Subash S , Essa MM , Al-Asmi A , Al-Adawi S , Vaishnav R , Braidy N , Manivasagam T , Guillemin GJ , Pomegranate from oman alleviates the brain oxidative damage in transgenic mouse model of Alzheimer’s disease, Journal of Traditional and Complementary Medicine (2014) ;4: :232–8. |
[64] | Khongsombat O , Nakdook W , Ingkaninan K , Inhibitory effects of Tabernaemontana divaricata root extract on oxidative stress and neuronal loss induced by amyloid β25–35 peptide in mice, Journal of Traditional and Complementary Medicine (2018) ;8: :184–9. |
[65] | Szczepańska K , Kuder KJ , Kieć-Kononowicz K , Dualtargeting approach on histamine H3 and sigma-1 receptor ligands as promising pharmacological tools in the treatment of CNS-linked disorders. Current Medicinal Chemistry. 2020. |
[66] | Khan A , Jahan S , Imtiyaz Z , Alshahrani S , Antar Makeen H , Mohammed Alshehri B , Kumar A , Arafah A , Rehman MU , Neuroprotection: Targeting multiple pathways by naturally occurring phytochemicals, Biomedicines (2020) ;8: :284. |
[67] | Dreser A , Vollrath JT , Sechi A , Johann S , Roos A , Yamoah A , Katona I , Bohlega S , Wiemuth D , Tian Y , The ALS-linked E102Q mutation in Sigma receptor-1 leads to ER stress-mediated defects in protein homeostasis and dysregulation of RNA-binding proteins, Cell Death & Differentiation (2017) ;24: :1655–71. |
[68] | Miki Y , Tanji K , Mori F , Wakabayashi K , Sigma-1 receptor is involved in degradation of intranuclear inclusions in a cellular model of Huntington’s disease, Neurobiology of Disease (2015) ;74: :25–31. |
[69] | Rossi D , Pedrali A , Urbano M , Gaggeri R , Serra M , Fernández L , Fernández M , Caballero J , Ronsisvalle S , Prezzavento O , Schepmann D , Wuensch B , Peviani M , Curti D , Azzolina O , Collina S , Identification of a potent and selective σ1 receptor agonist potentiating NGF-induced neurite outgrowth in PC12 cells, Bioorganic & Medicinal Chemistry (2011) ;19: :6210–24. |
[70] | Ishima T , Fujita Y , Hashimoto K , Interaction of new antidepressants with sigma-1 receptor chaperones and their potentiation of neurite outgrowth in PC12 cells, European Journal of Pharmacology (2014) ;727: :167–73. |
[71] | Takebayashi M , Hayashi T , Su T-P , Nerve growth factor-induced neurite sprouting in PC12 cells involves σ-1 receptors: Implications for antidepressants, Journal of Pharmacology and Experimental Therapeutics (2002) ;303: :1227–37. |
[72] | Ishima T , Nishimura T , Iyo M , Hashimoto K , Potentiation of nerve growth factor-induced neurite outgrowth in PC12 cells by donepezil: Role of sigma-1 receptors and IP3 receptors, Progress in Neuro-Psychopharmacology and Biological Psychiatry (2008) ;32: :1656–9. |
[73] | Hellewell SB , Bowen WD , A sigma-like binding site in rat pheochromocytoma (PC12) cells: Decreased affinity for (+)-benzomorphans and lower molecular weight suggest a different sigma receptor form from that of guinea pig brain, Brain Research (1990) ;527: :244–53. |
[74] | Banerjee R , Hazra S , Ghosh AK , Mondal AC , Chronic administration of bacopa monniera increases BDNF protein and mRNA expressions: A study in chronic unpredictable stress induced animal model of depression, Psychiatry Investigation (2014) ;11: :297. |
[75] | Hazra S , Banerjee R , Das BK , Ghosh AK , Banerjee TK , Hazra US , Biswas SK , Mondal AC , Evaluation of antidepressant activity of Bacopa monnieri in rat: A study in animal model of depression, Drug Discov (2012) ;2: :8–13. |
[76] | Hazra S , Kumar S , Saha GK , Mondal AC , Reversion of BDNF, Akt and CREB in hippocampus of chronic unpredictable stress induced rats: Effects of phytochemical, Bacopa Monnieri, Psychiatry Investigation (2017) ;14: :74–74. |
[77] | Zhang J-C , Yao W , Hashimoto K , Brain-derived neurotrophic factor (BDNF)-TrkB signaling in inflammation-related depression and potential therapeutic targets, Current Neuropharmacology (2016) ;14: :721–31. |
[78] | Yagasaki Y , Numakawa T , Kumamaru E , Hayashi T , Su T-P , Kunugi H , Chronic antidepressants potentiate via sigma-1 receptors the brain-derived neurotrophic factor-induced signaling for glutamate release, Journal of Biological Chemistry (2006) ;281: :12941–9. |
[79] | Fujimoto M , Hayashi T , Urfer R , Mita S , Su TP , Sigma-1 receptor chaperones regulate the secretion of brain-derived neurotrophic factor, Synapse (2012) ;66: :630–9. |
[80] | Ji LL , Peng JB , Fu CH , Tong L , Wang ZY , Sigma-1 receptor activation ameliorates anxiety-like behavior through NR2A-CREB-BDNF signaling pathway in a rat model submitted to single-prolonged stress, Molecular Medicine Reports (2017) ;16: :4987–93. |
[81] | Eraso-Pichot A , Larramona-Arcas R , Vicario-Orri E , Villalonga R , Pardo L , Galea E , Masgrau R , CREB decreases astrocytic excitability by modifying subcellular calcium fluxes via the sigma-1 receptor, Cellular and Molecular Life Sciences (2017) ;74: :937–50. |
[82] | Nakano M , Osada K , Misonoo A , Fujiwara K , Takahashi M , Ogawa Y , Haga T , Kanai S , Tanaka D , Sasuga Y , Fluvoxamine and sigma-1 receptor agonists dehydroepiandrosterone (DHEA)-sulfate induces the Ser473-phosphorylation of Akt-1 in PC12 cells, Life Sciences (2010) ;86: :309–14. |
[83] | Zhao J , Mysona BA , Wang J , Gonsalvez GB , Smith SB , Bollinger KE , Sigma 1 receptor regulates ERK activation and promotes survival of optic nerve head astrocytes, PLoS ONE (2017) ;12: :e0184421. |
[84] | Zhang E , Shen J , So KF , Chinese traditional medicine and adult neurogenesis in the hippocampus, Journal of Traditional and Complementary Medicine (2014) ;4: :77–81. |
[85] | Wymann MP , Zvelebil M , Laffargue M , Phosphoinositide 3-kinase signalling–which way to target? Trends in Pharmacological Sciences (2003) ;24: :366–76. |
[86] | Peltier J , O’Neill A , Schaffer DV , PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation, Developmental Neurobiology (2007) ;67: :1348–61. |
[87] | Prasanth MI , Tencomnao T , Brimson JM , Letter to the Editor, the Sigma-1 receptors role in neuroprotection: Comment on Nrf2 as a therapeutic target in ischemic stroke, Expert Opinion on Therapeutic Targets (2021) ;25: :613–4. |
[88] | Wang J , Shanmugam A , Markand S , Zorrilla E , Ganapathy V , Smith SB , Sigma 1 receptor regulates the oxidative stress response in primary retinal Müller glial cells via NRF2 signaling and system xc–, the Na+-independent glutamate–cystine exchanger, Free Radical Biology and Medicine (2015) ;86: :25–36. |
[89] | Mazur A , Fangman M , Ashouri R , Arcenas A , Doré S , Nrf2 as a therapeutic target in ischemic stroke, Expert Opinion on Therapeutic Targets (2021) ;25: :163–6. |
[90] | Chen J , Li G , Qin P , Chen J , Ye N , Waddington JL , Zhen X , Allosteric modulation of the sigma-1 receptor elicits antipsychotic-like effects. Schizophrenia Bulletin. 2021. |
[91] | Wu Z , Li L , Zheng LT , Xu Z , Guo L , Zhen X , Allosteric modulation of sigma-1 receptors by SKF 9 inhibits microglia-mediated inflammation, Journal of Neurochemistry (2015) ;134: :904–14. |
[92] | Zvejniece L , Vavers E , Svalbe B , Vilskersts R , Domracheva I , Vorona M , Veinberg G , Misane I , Stonans I , Kalvinsh I , The cognition-enhancing activity of E1R, a novel positive allosteric modulator of sigma-1 receptors, British Journal of Pharmacology (2014) ;171: :761–71. |
[93] | Mishra A , Mishra AK , Jha S , Effect of traditional medicine brahmi vati and bacoside A-rich fraction of Bacopa monnieri on acute pentylenetetrzole-induced seizures, amphetamine-induced model of schizophrenia, and scopolamine-induced memory loss in laboratory animals, Epilepsy & Behavior (2018) ;80: :144–51. |
[94] | Sudha S , Kumaresan S , Amit A , David J , Venkataraman B , Anti-convulsant activity of different extracts of Centella asiatica and Bacopa monnieri in animals, Journal of Natural Remedies (2002) ;2: :33–41. |
[95] | Pandey R , Gupta S , Tandon S , Wolkenhauer O , Vera J , Gupta SK , Baccoside A suppresses epileptic-like seizure/convulsion in Caenorhabditis elegans, Seizure (2010) ;19: :439–42. |
[96] | Guo L , Chen Y , Zhao R , Wang G , Friedman E , Zhang A , Zhen X , Allosteric modulation of sigma-1 receptors elicits anti-seizure activities, British Journal of Pharmacology (2015) ;172: :4052–65. |
[97] | Rodríguez-Muñoz M , Onetti Y , Cortés-Montero E , Garzón J , Sánchez-Blázquez P , Cannabidiol enhancesmorphine antinociception, diminishes NMDA-mediated seizures andreduces stroke damage via the sigma 1 receptor, Molecular Brain (2018) ;11: :1–12. |
[98] | Tissenbaum HA , DAF- FOXO in the context of C, elegans, in: Current topics in developmental biology. Elsevier (2018) ;16: :1–21. |
[99] | Kenyon C , Chang J , Gensch E , Rudner A , Tabtiang R , AC elegans mutant that lives twice as long as wild type, Nature (1993) ;366: :461–4. |
[100] | Tissenbaum HA , Genetics, life span, health span, and the aging process in Caenorhabditis elegans, Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences (2012) ;67: :503–10. |
[101] | Kapahi P , Kaeberlein M , Hansen M , Dietary restriction and lifespan: Lessons from invertebrate models, Ageing Research Reviews (2017) ;39: :3–14. |
[102] | Wu Z , Isik M , Moroz N , Steinbaugh MJ , Zhang P , Blackwell TK , Dietary restriction extends lifespan through metabolic regulation of innate immunity, Cell Metabolism (2019) ;29: :1192–205. e1198. |
[103] | Kenyon C , The first long-lived mutants: Discovery of the insulin/IGF-1 pathway for ageing, Philos Trans R Soc Lond B Biol Sci (2011) ;366: :9–16. |
[104] | Johnson TE , 25 years after age- Genes, interventions and the revolution in aging research, Exp Gerontol (2013) ;48: :640–3. |
[105] | Murphy CT , McCarroll SA , Bargmann CI , Fraser A , Kamath RS , Ahringer J , Li H , Kenyon C , Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans, Nature (2003) ;424: :277–83. |
[106] | Dierking K , Yang W , Schulenburg H , Antimicrobial effectors in the nematode Caenorhabditis elegans: An outgroup to the Arthropoda, Philosophical Transactions of the Royal Society B: Biological Sciences (2016) ;371: :20150299. |
[107] | Prasanth MI , Sivamaruthi BS , Chaiyasut C , Tencomnao T , A review of the role of green tea (Camellia sinensis) in antiphotoaging, stress resistance, neuroprotection, and autophagy, Nutrients (2019) ;11: :474. |
[108] | Qu M , Li D , Qiu Y , Wang D , Neuronal ERK MAPK signaling in response to low-dose nanopolystyrene exposure by suppressing insulin peptide expression in Caenorhabditis elegans. Science of The Total Environment. 2020;138378.. |
[109] | Okuyama T , Inoue H , Ookuma S , Satoh T , Kano K , Honjoh S , Hisamoto N , Matsumoto K , Nishida E , The ERK-MAPK pathway regulates longevity through SKN-1 and insulin-like signaling in Caenorhabditis elegans, Journal of Biological Chemistry (2010) ;285: :30274–81. |
[110] | Thein MC , McCormack G , Winter AD , Johnstone IL , Shoemaker CB , Page AP , Caenorhabditis elegans exoskeleton collagen COL- An adult-specific marker for collagen modification and assembly, and the analysis of organismal morphology, Developmental Dynamics: An Official Publication of the American Association of Anatomists (2003) ;226: :523–39. |
[111] | Li Y , Paik Y-K , A potential role for fatty acid biosynthesis genes during molting and cuticle formation in Caenorhabditis elegans, BMB Rep (2011) ;44: :285–90. |
[112] | Hada K , Asahina M , Hasegawa H , Kanaho Y , Slack FJ , Niwa R , The nuclear receptor gene nhr-25 plays multiple roles in the Caenorhabditis elegans heterochronic gene network to control the larva-to-adult transition, Developmental Biology (2010) ;344: :1100–9. |
[113] | Govorunova EG , Moussaif M , Kullyev A , Nguyen KC , McDonald TV , Hall DH , Sze JY , A homolog of FHM2 is involved in modulation of excitatory neurotransmission by serotonin in Celegans, PLoS ONE (2010) ;5: :e10368. |
[114] | Matsuki M , Kunitomo H , Iino Y , Goalpha regulates olfactory adaptation by antagonizing Gqalpha-DAG signaling in Caenorhabditis elegans, Proc Natl Acad Sci U S A (2006) ;103: :1112–7. |
[115] | Avery L , You YJ , C. elegans feeding.Worm Book. 2012;1-23. |
[116] | Bany IA , Dong M-Q , Koelle MR , Genetic and cellular basis for acetylcholine inhibition of Caenorhabditis elegans egg-laying behavior, Journal of Neuroscience (2003) ;23: :8060–9. |
[117] | Liu H , Wang Y , Zhang W , Sun W , Ji X , Zhang S , Qiao K , Lentinan extends lifespan and increases oxidative stress resistance through DAF-16 and SKN-1 pathways in Caenorhabditis elegans, Int J Biol Macromol (2022) ;202: :286–95. |
[118] | Prasanth MI , Venkatesh D , Murali D , Bhaskar JP , Krishnan V , Balamurugan K , Understanding the role of DAF-16 mediated pathway in Caenorhabditis elegans during UV-A mediated photoaging process, Arch Gerontol Geriatr (2019) ;82: :279–85. |
[119] | Prasanth MI , Gayathri S , Bhaskar JP , Krishnan V , Balamurugan K , Analyzing the synergistic effects of antioxidants in combating photoaging using model nematode, Caenorhabditis elegans, Photochemistry and Photobiology (2020) ;96: :139–47. |
[120] | Cheignon C , Tomas M , Bonnefont-Rousselot D , Faller P , Hureau C , Collin F , Oxidative stress and the amyloid beta peptide in Alzheimer’s disease, Redox Biology (2018) ;14: :450–64. |
[121] | Drake J , Link CD , Butterfield DA , Oxidative stress precedes fibrillar deposition of Alzheimer’s disease amyloid β-peptide (1–42) in a transgenic Caenorhabditis elegans model, Neurobiology of Aging (2003) ;24: :415–20. |
[122] | Melo JB , Agostinho P , Oliveira CR , Involvement of oxidative stress in the enhancement of acetylcholinesterase activity induced by amyloid beta-peptide, Neuroscience Research (2003) ;45: :117–27. |
[123] | Butterfield DA , Boyd-Kimball D , Amyloid β-peptide (1-42) contributes to the oxidative stress and neurodegeneration found in Alzheimer disease brain, Brain Pathology (2004) ;14: :426–32. |




