Hippocampal involvement in glucose facilitation of recognition memory: Event-related potential components in a dual-task paradigm
Abstract
BACKGROUND: Glucose administration may facilitate hippocampus-mediated recognition memory (‘remember’ rather than familiarity ‘know’ responses).
OBJECTIVE: This study aimed to investigate the electrophysiological correlates of this phenomenon in a cohort of older individuals.
METHODS: In this double-blind placebo-controlled cross-over study, 12 older participants (mean age = 69.33 ± 1.69 years) completed the remember-know paradigm both with and without a concurrent tracking task while recording event-related potentials (ERPs).
RESULTS: Counter to predictions, glucose reduced overall accuracy. No treatment effects were found for proportion of Remember, Know and Guess responses, although there was a trend towards greater accuracy for ‘Remember’ responses following glucose. There was weak evidence for dissociation of drink effects on tracking with glucose being associated with preferential allocation of resources to ‘Remember’ over ‘Know’ responses. At P3 and F3 electrode sites, a significantly greater left parietal (LP) recollection effect and greater FN400 effect respectively were found for glucose.
CONCLUSIONS: These findings do not support task effort modulation of the memory-enhancing effects of glucose. There was evidence of a greater glucose facilitatory effect for hippocampus-mediated LP recollection.
1Introduction
Glucose is a primary source of energy in the brain, with the aerobic metabolism of glucose requiring a steady supply of oxygen and glucose from the blood [1]. For this reason, acute changes in glucose supply have been found to have a significant facilitatory effect on cognitive function [2, 3], particularly under conditions of increased task demand [4, 5]. A recent meta-analysis Hoyland et al. [6] provided evidence to suggest that glucose may affect memory performance to a greater extent than other cognitive domains. While the exact mechanism by which glucose improves memory performance is yet to be elucidated, increased acetylcholine and insulin production and resultant effects on hippocampal function have emerged as important factors [3, 7–11].
The partitioning of recognition memory into ‘recollection’ and ‘familiarity’ processes [12] provides a framework by which the involvement of the hippocampus may be tested. The neurocognitive process of recollection, which involves the retrieval of spatiotemporal contextual details associated with the original stimuli, has been found to be associated with the hippocampus [13, 14]. In contrast the process of familiarity, which provides only a sense that the stimuli was previously encountered, has been found to be associated with the perirhinal cortex [15]. Elsewhere, fMRI has also provided strong evidence for non-overlapping regions subserving recollection and familiarity [16]. Indeed, in that study the hippocampus as well as associated anterior-medial pre-frontal and lateral parietal networks was linked to recollection. On the other hand, familiarity based recognition revealed activation in the lateral pre-frontal, superior lateral parietal cortex and the precuneus.
The Remember/Know paradigm [17, 18] provides a behavioural means of testing for recollection versus familiarity. During ageing the proportion of ‘know’ to ‘remember’ responses increases [19–21], an effect which is consistent with the documented age-related decline in hippocampal volume and function [22–24]. ‘Remember’ and ‘know’ responses can also be differentiated pharmacologically by, for example, lorazepam [25] and alcohol [26]. In relation to studies of the glucose facilitatory effect, Sunram-Lea et al. [27] reported that glucose preferentially increased the proportion of responses based on recollection, but had no effect on the proportion of responses based on familiarity.
Whilst the research of Sünram-Lea et al. (2008) provides support for the view that the cognitive enhancing effects of glucose are ‘domain’ (hippocampal) specific, a recent study from our laboratory [28] provided contrary evidence. In this study glucose was found to have no effect on the proportion of responses categorised as recollected or familiar. However, greater overall recognition accuracy following glucose consumption was found when a secondary hand movement task was included during word encoding [28]. These findings raise the possibility that the degree of mental effort required to complete a task, rather than hippocampal involvement, is the more important determinant of task sensitivity to glucose enhancement [28]. A corollary of this theory is that glucose may facilitate memory function by targeting brain regions more globally, with effects not strictly isolated to the hippocampus [10].
The use of event-related potentials (ERPs; derived from the EEG) is another means by which hippocampal involvement in glucose facilitation of memory may be tested (Riby et al. 2009). The Left Parietal old/new ‘recognition’ effect (LP) has been identified in previous research utilising old/new recognition paradigms, and represents more positive going amplitude for correct responses to old items in comparison to correct rejections for new items. The LP effect is typically maximal over left parietal sites in the 400–800 ms latency range post-stimulus [29, 30]. Another related ERP component is the FN400 ‘familiarity’ component which also represents more positive going amplitude for old versus new stimuli when utilising an old/new recognition task. In contrast to the LP effect, the FN400 peaks earlier in the 300–500 ms latency range suggesting faster retrieval mechanisms and is maximal over mid-frontal sites [30, 31]. In a recent study using healthy adolescents, the hippocampal theory of glucose facilitation was brought into question when Smith et al. [32] reported that glucose ingestion was associated with enhancement of both the LP effect as well as the frontal FN400 effect.
Recollection is relatively more impaired in older individuals [33] and glucose impacts more in deficit populations with known hippocampal dysfunction [10, 11]. One reason that glucose may be more effective in enhancing recollection in older adults is because glucose regulation becomes increasingly compromised with advancing age [2, 34]. The presence of a high concentration of insulin receptors in the hippocampus, together with the associated GLUT-4 insulin-sensitive glucose transporter, suggests that the hippocampus may be particularly vulnerable to shortages in glucose supply [2, 8]. The aim of the present study was therefore to further investigate the involvement of the hippocampus in the glucose facilitatory effect of episodic memory using the Remember/Know paradigm in conjunction with the measurement of familiarity and recollection ERP components in an elderly sample. Moreover, in order to better delineate the effects of task effort from hippocampal involvement in relation to glucose facilitation, an additional dual task condition was also included whereby participants were required to complete a tracking task during memory encoding.
If glucose differentially enhances hippocampal function in comparison to other brain regions then one would hypothesise that it should differentially increase ‘remember’ responses, particularly in elderly. Conversely, if the amount of mental effort involved in task performance is a more important determinant then it would be hypothesised that ‘remember’ and ‘know’ responses should be equally enhanced by a glucose load and that this effect would be more marked during conditions of higher mental effort. Similarly, hippocampus-mediated glucose enhancement would also be expected to result in greater enhancement of the left parietal (LP) ERP component in comparison to the FN400 component. Conversely, if task effort is a more important determinant in glucose facilitation then it would be expected that LP and FN400 components would not be differentially affected by a glucose load. Further, it would also be expected that glucose would enhance LP and FN400 components equally in the more difficult dual-task condition in comparison to the single-task condition.
2Method
2.1Participants
A total of 12 right-handed healthy elderly participants (7 males and 5 females) volunteered to take part in the study (M = 69.33 years, SD = 1.69 years). The study was approved by the Human Research Ethics Committee of Swinburne University of Technology. All participants reported that they were aged over 65 years, healthy, and not taking any medication, herbal extracts, or vitamin supplements expected to interfere with blood glucose levels.
2.2Materials
2.2.1Glucose treatment
At each visit the participant consumed a drink containing 20 mL sugar-free orange cordial diluted with 200 mL tap water. In the glucose condition 25 g glucose was dissolved into the drink, while in the placebo condition 30 mg saccharine as dissolved into the drink. Scholey et al. have previously shown that the two drinks are indistinguishable in terms of taste [1, 35].
2.2.2Word recognition and tracking tasks
At each testing session an ‘encoding’ block of 22 computer-generated spoken words was played through speakers placed within 1-metre of the participant at bench level. A fixation cross appeared for 5000 ms, followed by auditory presentation for each word lasting approximately 1000 milliseconds. The inter-stimulus interval for each presentation, including the time taken to display the fixation cross, was 4500 milliseconds. Immediately following the auditory presentation of the words, participants’ memory for the words was tested using another ‘retrieval’ block of words that were presented visually.
The retrieval block consisted of 41 words, which included the 20 words from the preceding encoding block as well as 21 new words, not previously presented. The first and final words presented at encoding were not included at retrieval due to primacy and recency effects. The first word presented at retrieval was also excluded due to potential novelty effects. Timing for each trial during retrieval was as follows: a fixation cross was presented variably for 500–1000 ms, then visual presentation of a word for 3000 ms, before the user was prompted for a response with the slide ‘(1) old or (2) new?’ If the participant indicated that the word was ‘new’ then there was an inter stimulus interval of 500 ms before the next trial began. If the participant indicated that the word was ‘old’ then a subsequent slide was presented ‘(1) Remember (2) Know (3) Guess?’ Once they had provided a response then there was an inter stimulus interval of 500 ms before the next trial began. Pressing the key for ‘Remember’ indicated conscious recollection of the item’s appearance in the original list, while ‘Know’ indicated that the item seemed familiar but they could not recall its actual occurrence, and ‘Guess’ indicated that they were uncertain whether an item had appeared in the list. After the completion of the retrieval block a second encoding block using a different set of words was presented to the participant, together with a subsequent retrieval block.
In the dual task (tracking) version of the task, participants were required to track a moving target during the auditory presentation of the words at encoding. A circle of 10 pixels in radius appeared at a random location on the screen then proceeded to move in a random direction for 4000 milliseconds in duration. If the circle reached the boundary of the screen it would automatically ‘bounce’ back and begin moving in the opposite direction. Participants were instructed to attempt to keep the cursor within the moving circle as best as they could. The tracking task has previously been evidenced to be sensitive to age-related change and impair memory consolidation for words presented simultaneously (Scholey, unpublished data). The experiment was generated using E-Prime Software 2.0.8.90 (Psychology Software Tools). Stimuli were presented in black, bold and capitalised 18-point courier new font on a white background at the vertical and horizontal centre of the screen. The circle used in the tracking task was also presented in black on a whitebackground.
Each participant completed four tasks in total, one at each testing session. Participants were randomized to receive either glucose or placebo and single or dual tracking tasks according to a Latin square. The four treatment/task combinations were as follows: placebo/single task, placebo/dual task, glucose/single task and glucose/dual task.
2.2.3Procedure
Each participant was required to attend the laboratory on five separate occasions. The first visit was a training session to familiarise participants with the study procedures and tests. Demographic information and written informed consent was collected at this time. The following four testing sessions were scheduled at least 48 hours apart and participants were randomised as to order in which they received the two treatment drinks (glucose/placebo) and the (single/dual) taskversions.
Participants were requested to abstain from food and drink from 10 pm the night before the training session and at each testing session. Testing commenced between 8 a.m and 9 a.m. For each session, participants’ blood glucose was measured upon arrival at the laboratory. The experimenter then proceeded to fit the EEG electrode cap. Following the EEG cap being fitted, participants were given their (glucose or placebo) drink. A second blood glucose measurement was taken twenty minutes after drink administration. This was followed by the word recognition task with or without the tracking task. The following instructions were presented to participants on the computer monitor prior to commencing the encoding segment of the task: “In this test you will be read a series of words. Please listen carefully and try to remember each word. Your memory of these words will be tested shortly after so it is important to try to remember as many words as possible.” For the dual task (tracking) version of the task, additional instructions were also displayed: “At the same time you will be required to complete a tracking task on the computer using the mouse. Move the ’+’ with the mouse and try to keep the ’+’ in the circle. Try to minimise eye movements and keep you head as still as possible. It is important that while you complete the tracking task you continue to pay attention to the words as they are presented to you.”
Prior to commencing the retrieval segment of the task additional instructions were also presented: “In this test there is a list of words; some of these words you heard read to you a moment ago, others were not. As you see each word presented on the screen, please indicate whether or not you confidently recognize the word from the original study set, bearing in mind the following: When we remember something, we consciously recollect and become aware of aspects of its occurrence. For example, you might remember a recent movie and be able to recall specific details about it, like where and with whom you saw it. At other times, we simply know that something has occurred before, but without being able to consciously recollect what was experienced at the time of its occurrence. For example, you might recognize a person and be certain that you know him or her, but be unable to recall any specific details about the person, such as the person’s name. For this test, remembering a word would signify that the word evokes specific memories of what was experienced during its presentation, such as how it sounded, the way in which it was presented, or even what you were thinking or doing at the time you heard it. There will also be times when you do not remember the word, nor do you know it, but you might want to guess that it was one of the words you heard read to you. A series of words will be presented on the screen. When you see each word appear DO NOT MAKE A RESPONSE. You will then be prompted to indicate whether you think the word is ‘old’ or ‘new’. You press (1) to indicate that you have already seen it before (old). You press (2) to indicate that it is new. If you pressed (1) for old then a second screen will also appear which prompts you to press (1) to indicate that you consciously recollect the occurrence of the word, (2) if you simply know that the word was in the original study set, or (3) if you’re guessing that the word was studied. Knowing a word would signify that you are confident the word was presented, but you cannot recollect any aspects of its presentation.”
Upon completion of the word recognition task, blood glucose was measured once more to conclude the testing session (approximately 40 min post-drink).
2.2.4Blood glucose measurement
At each testing session blood glucose levels were measured using an Optium Xceed Blood Glucose Sensor and disposable Optium Blood Glucose Test Strips (Abbott Diabetes Care Ltd., Witney, UK).
2.3EEG recording and data reduction
EEG was recorded from a 60 channel electrode cap which contained all International 10–20 positions [36]. The montage included eight midline sites (FPZ; FZ; FCZ; CZ; CPZ; PZ; POZ; OZ), 26 sites over the left hemisphere (FP1; AF3; F1; F3; F5; F7; FC1; FC3; FC5; FT7; C1; C3; C5; T7; CP1; CP3; CP5; TP7; P1; P3; P5; P7; PO3; PO5; PO7; O1) and 26 sites over the right hemisphere (FP2; AF4; F1; F4; F6; F8; FC2; FC4; FC6; FT8; C2; C4; C6; T8; CP2; CP4; CP6; TP8; P2; P4; P6; P8; PO4; PO6; PO8; O2). The EEG signal was referenced to linked electrodes placed on the mastoids, and impedance was kept below 10kΩ for all electrode sites. A high-pass filter of 0.05 Hz and a low-pass filter of 200 Hz were used for data acquisition, with the signal digitised at 1000 Hz. Horizontal-electrooculograms were recorded from electrodes placed at the outer canthi of each eye. To assess eye blink motion, separate electrodes were placed above and below the left eye to record the vertical-electrooculogram. An EEG calibration task was recorded at the beginning of each testing session, which was subsequently used to correct for eye movement and blink artefact off line. This calibration task required the participant to track a small red square from the left to the right edge of the screen as well as from the top to the bottom of the screen. The final phase of the task required the participant to focus on a square in the middle of the screen which alternated from blue to red, blinking each time the square changed colour. The task ran for approximately 5 minutes in duration. Following the calibration task, recording for the main memory task was conducted which took approximately 20 minutes. EEG data was not able to be recorded for four individual sessions (out of a total 48 sessions) due to equipment malfunction.
After recording all continuous EEG recordings for both the calibration and memory tasks were visually inspected for bad electrode channels. In cases where bad channels were detected they were replaced with the average of the nearest surrounding electrodes (3-4 electrodes). Next, ocular artefact was removed from all continuous EEG recordings using a Spatial Singular Value Decomposition (SVD) technique [37, 38]. In this technique the EOG calibration recording was first epoched into Horizontal, Vertical and Eye blink events, then a Spatial Singular Value Decomposition (SVD) transform was applied to the epochs in order to derive two independent components accounting for vertical/horizontal eye movements and blinks. Ocular artefact was then removed from all subsequent EEG recordings by spatial filtering for these components. All files were manually scanned to ensure that ocular artefact rejection had worked effectively.
All event files were merged with behavioural accuracies and response times obtained from E-Prime. Responses at retrieval were categorized according to ‘remember’, ‘know’, ‘guess’ or correctly identified new words. Presentation of stimulus words at the time of encoding were also categorized according to their subsequent ‘remember’ ‘know’ or ‘guess’ status at the time of retrieval. EEG epochs recorded from 200 ms pre-stimulus onset to 1400 ms post-stimulus onset were extracted for averaging. All epochs were baseline corrected using the pre-stimulus interval and low-pass filtered at 30 Hz. Automatic artefact rejection was used for all trials where ERPs extended beyond the range of −100 to 100 μV. ERP averaging was conducted using Edit 4.5 software (Neuroscan). ERP averages were only used for analysis if they comprised a minimum of 16 artefact-free trials.
3Results
3.1Blood glucose
A 2 (drink: glucose, placebo) × 2 (tracking: + tracking, − tracking) × 3 (time: baseline, 20 mins, post-testing) repeated measures analysis of variance (ANOVA) was conducted, whereby all three variables were within subject variables. While homogeneity of covariance was shown for time, this assumption was violated for the interactions between drink and time (Mauchly’s Test of Sphericity <0.05), and tracking and time (Mauchly’s Test of Sphericity <0.05), thus Huynh-Feldt corrections were used for the two interactions. As expected, the ANOVA revealed a significant main effect of time (F (2, 20) = 144.36, p < 0.001, η 2 = 0.31), drink (F (1, 10) = 188.81, p < 0.001, η2 = 0.25), as well as a significant interaction between drink and time (F (1.30, 13.02) = 98.95, p < 0.001, η2 = 0.29). Post-hoc analysis revealed that for glucose administration compared to placebo, blood glucose levels were significantly higher after testing compared to baseline (F (1, 10) = 210.95, p < 0.001), and 20 minutes after administration (F (1, 10) = 56.31, p < 0.001). There were no main effects of tracking, nor did tracking interact with the other factors significantly. Mean blood glucose levels are presented graphically in Fig. 1.
3.2Behavioural results
Due to a strong positive correlation between correct responses and false alarms (r = 0.56, p < 0.001) a sensitivity index was calculated according to the following formula, where h = probability of a correct hit and f = probability of false alarm [39]:
Mean Number of Correct Responses, False Alarms and Sensitivity Index calculations are displayed in Table 1 according to condition and task. Repeated measures ANOVA on SI, with both treatment (glucose, placebo) and task (single, dual/tracking) as within-subject factors revealed that the main effect for treatment was significant (F(1,9) = 11.607, p = 0.008), indicating that SI values were higher for placebo than glucose. The main effect for task was also significant (F(1,9) = 10.994, p = 0.009), indicating that SI values were higher for the single task in comparison to the dual task. The treatment by task interaction was non-significant (F(1,9) = 0.078, p > 0.05). SI means and SEs according to treatment and task are displayed in Fig. 2.
The proportions of remember, know and guess responses were found to be highly skewed and for this reason the non-parametric Wilcoxon signed-rank test was used to test for significant differences between placebo and glucose treatments according to task type. In the single task condition, no significant differences between glucose and placebo groups were found for proportion remember (Z =−0.089, p = 0.929), know (Z =−0.051, p = 0.959) or guess responses (Z =−0.357, p = 0.721). Similarly for the dual task condition, no significant differences between glucose and placebo groups were found for proportion remember (Z =−1.173, p = 0.241), know (Z =−1.071, p = 0.284) or guess responses (Z =−1.275, p = 0.202). Median proportions of remember, know and guess responses according to treatment and task are displayed in Table 2 and Fig. 3.
3.3Tracking accuracy
Tracking accuracy over the first second of encoding for correctly recalled words (remember, know) are displayed in Fig. 4 according to treatment (glucose, placebo).
Repeated-measures ANOVA on tracking accuracy for correctly-recalled ‘remember’ responses, fitting an auto-regressive covariance, AHR(1), structure to time within each treatment and a subject-specific random intercept, revealed that the main effect for treatment was approaching significance (F(1,179) = 3.51, p = 0.06) while the treatment by time interaction was non-significant (F(1,179) = 0.76, p = 0.65). Differences of Least Square means revealed that tracking accuracy was significantly better for glucose in comparison to placebo at 600 ms (p = 0.02) and 700 ms (p = 0.04). Repeated-measures ANOVA on tracking accuracy for correctly-recalled ‘know’ responses, also fitting an auto-regressive covariance structure to time and a subject-specific random intercept, revealed that the main effect for treatment was significant (F(1,123) = 4.28, p = 0.04) but the treatment by time interaction was non-significant (F(1,123) = 1.50, p = 0.15). Differences of Least Square means revealed that tracking accuracy was significantly better for placebo in comparison to glucose at 300 ms (p = 0.02), 400 ms (p = 0.006) and 500 ms (p = 0.04).
3.4Electrophysiological results
In order to capture the ERP components of interest a targeted analysis was performed on the scalp sites known to reveal the LP and FN400 effects. This approach has been used elsewhere when examining the impact of glucose on cognition (Smith et al. 2009). The Frontal Negativity effect (FN400) was taken as the average difference in amplitude between old [correct] and new [correct] words in the 300–600 ms latency range of frontal sites F3, Fz and F4. The Left Parietal effect (LP) was taken as the average difference in amplitude between old [correct] and new [correct] words in the 600 –900 ms latency range over parietal sites P3, Pz and P4. The grand average ERP for old [correct] versus new [correct] waveforms is displayed in Fig. 5 for frontal and parietal sites.
Left Parietal (LP) effect. For electrode P3, repeated measures ANOVA revealed a significant main effect for treatment (F(1,11) = 6.24, p = 0.03), with average old-new amplitude difference greater in the glucose treatment group in comparison to placebo. However, the main effect for task (F(1,11) = 0.0, p = 0.98) and the treatment x task interaction (F(1,11) = 0.02, p = 0.88) were found to be non-significant. For electrode PZ the main effect for treatment was non-significant (F(1,11) = 1.92, p = 0.19), as well as the main effect for task (F(1,11) = 0.84, p = 0.38) and the treatment x task interaction (F(1,11) = 0.13, p = 0.72). For electrode P4 the main effect for treatment was also non-significant (F(1,11) = 0.41, p = 0.53), as well as the main effect for task (F(1,11) = 0.19, p = 0.67) and the task x treatment interaction (F(1,11) = 1.61, p = 0.23).
FN400 Familiarity effect. For electrode F3, repeated measures ANOVA revealed that the main effect for treatment was non-significant (F(1,11) = 2.65, p = 0.13), although there was a trend towards the average old-new amplitude difference being greater in the glucose treatment group in comparison to placebo. The main effect for task (F(1,11) = 1.30, p = 0.28) and the treatment x task interaction (F(1,11) = 0.01, p = 0.91) were also found to be non-significant. For electrode FZ the main effect for treatment was non-significant (F(1,11) = 0.21, p = 0.65), as well as the main effect for task (F(1,11) = 1.37, p = 0.27) and the treatment x task interaction (F(1,11) = 0.50, p = 0.49). For electrode F4 the main effect for treatment was also non-significant (F(1,11) = 0.25, p = 0.62), as well as the main effect for task (F(1,11) = 0.91, p = 0.36) and the task x treatment interaction (F(1,11) = 0.56, p = 0.47).
4Discussion
The finding of a lowered Sensitivity Index (SI) with glucose in comparison to placebo is in contrast to previous research by both Sünram-Lea et al. [27] and Scholey et al. [28] who reported a treatment effect in favour of glucose, and is also in contrast to the finding of no effect by Smith et al. [32]. This finding is difficult to reconcile with current theories regarding the glucose-facilitatory effect. However, the finding of a decrease in the SI for the dual-task condition in comparison to the single task is in accordance with previous research by Scholey et al. [28] where a decrease in accuracy was also reported when an additional hand-moving task was employed. In contrast to the work of Scholey et al. [28] however, no interaction between task and treatment was found, failing to corroborate the theory that task effort is an important determinant of glucose facilitation. Similarly, the proportion of remember, know and guess responses were also found to be unaffected by task and condition as well as their interaction. This also contrasts with previous researchshowing a significantly greater proportion of remember and guess responses were reported for the dual task condition, and a trend towards a greater proportion of remember responses was reported for the placebo condition [28]. It is difficult to compare the current findings to those of Sünram-Lea et al. [27], as absolute rather than proportionate measures of remember, know and guess responses werereported.
In regards to tracking accuracy during word presentation, the finding of poorer tracking accuracy in the first 500 ms for subsequent know responses in the glucose condition in comparison to placebo is intriguing. A possible interpretation of this finding is that in the glucose condition poorer attention to the task at hand resulted concurrently in words that were only encoded for familiarity, rather than recollection, and impaired accuracy in the dual tracking task. In contrast, for the placebo condition a trend towards poorer tracking accuracy was observed for words subsequently categorized as ‘Remember’ responses. This may be interpreted as evidence to suggest that poorer attention to the task at hand resulted in words encoded for recollection rather than familiarity. While this interpretation is speculative, it is in line with the notion that glucose may preferentially enhance encoding of words for recollection rather than familiarity [3, 7–9]. Additionally we cannot rule out the possibility the effects previously seen in younger participants are not observed in this cohort of older individuals.
Somewhat more informative is the pattern of ERPs presented here. We employed a remember/know paradigm to elicit two components related to successful memory retrieval; namely the LP effect associated with recollection and the FN400 associated with familiarity. The finding of a significantly greater Left Parietal effect for the glucose condition in comparison to placebo at electrode site P3 is in line with previous findings by Smith et al. [32]. However, the lack of an interaction between task and condition again brings in to question the notion that the LP effect is modulated by task effort [28]. The lack of a significant treatment effect for the FN400 familiarity ERP component at frontal sites is in contrast to previous research by Smith et al. [32]. Our data are more consistent with the suggestion that glucose impacts on individuals who are not performing at their cognitive optimum [40, 41]. Indeed, our sample comprised older adults with known impairment of recollection (hippocampal deficit) compared to relative stability in familiarity processes. It is hardly surprising that selective enhancement was only observed in those processes known to decline in ageing. One possible explanation for the findings of Smith et al. [32] is that the adolescent population had not fully developed the familiarity based processing and therefore enhancement was also observed. This notion is consistent with ERP studies failing to elicit frontal old/new effect [42]. However, it is important to note that at electrode site F3 there was also a trend towards an FN400 effect for glucose. Taken together the current electrophysiological findings suggest that there was a stronger facilitatory effect of glucose on the hippocampal-mediated LP effect in comparison to the FN400 familiarity effect, yet it cannot be ruled out that with a larger sample size a significant FN400 effect may have alsoemerged.
The fact that electrophysiological differences between glucose and placebo were found in the absence of behavioural differences is an interesting issue. It may be argued that the measurement of ERPs is a more reliable and robust measure of the recollection effect than the subjective reporting of ‘remember’ and ‘know’ responses. The dichotomous classification of words during cued recall is a highly subjective and difficult task, perhaps even more so for elderly participants. It is highly likely that many participants had a favoured mode of response, which appeared to be ‘remember’ for the majority of participants. Unfortunately, due to the fact that there were far fewer ‘know’ responses in comparison to ‘remember’ responses, there was insufficient statistical power to compare the ERP profiles of these two different responses.
Whilst the current research did not provide evidence of task effort being a moderating factor in glucose facilitation, it is important to acknowledge that a possible reason for the lack of effect was that the task was not hard enough. The tracking task employed in the current study used a target position that was reset each time that a new word was presented. In future research it may be advantageous to use a tracking task that does not reset on stimulus presentation but is continuous throughout the encoding interval. In this way, with a more constant level of dual task involvement, it is foreseeable that task demands may be increased. Similarly, the use of a graded dual task with more than one level of difficulty would provide valuable information regarding whether there the difficulty level must reach a required threshold before modulation of glucose facilitation is observed.
Finally it is possible that predicted behavioural effects were not found because of the relatively low N (12 individuals crossed over). While other studies in this field have used similar cell sizes (typically 11 to 20 in crossover studies [3]), it is possible that increased variability in an older population may require a higher N. On the other hand it is clear that the N was adequate to detect drink-related changes to tracking accuracy and ERP in this population. Nevertheless, we recommend that future similar studies should consider using a higher number of participants.
In summary, the current study did not find that the addition of a secondary tracking increased susceptibility to the glucose-facilitation of recognition memory, although there was evidence of better performance in the secondary tracking task for subsequently recalled ‘remember’ responses when compared to placebo. Whilst the current study did not provide conclusive evidence to suggest that the glucose facilitatory effect is specific to hippocampus-mediated recollection (as opposed to familiarity), a stronger effect of glucose facilitation was observed for the LP component in comparison to the FN400 component of ERPs. Future research employing a larger sample size and a more refined tracking task will help to further elucidate the brain mechanisms by which glucose facilitates episodic memory.
Acknowledgements
Funded by grant number DP1093834 from the Australian Research Council.
References
[1] | Scholey AB, Harper S, Kennedy DO(2001) Cognitive demand and blood glucosePhysiology and Behavior73: 4585592 |
[2] | Messier C(2004) Glucose improvement of memory: A reviewEuropean Journal of Pharmacology490: 1-33357 |
[3] | Riby LM(2004) The impact of age and task domain on cognitive performance: A meta-analytic review of the glucose facilitation effectBrain Impairment5: 2145165 |
[4] | Fairclough SH, Houston K(2004) A metabolic measure of mental effortBiological Psychology66: 2177190 |
[5] | Scholey AB, Laing S, Kennedy DO(2006) Blood glucose changes and memory: Effects of manipulating emotionality and mental effortBiological Psychology71: 11219 |
[6] | Hoyland A, Lawton CL, Dye L(2008) Acute effects of macronutrient manipulations on cognitive test performance in healthy young adults: A systematic research reviewNeuroscience and Biobehavioral Reviews32: 17285 |
[7] | Wenk GL(1989) An hypothesis on the role of glucose in the mechanism of action of cognitive enhancersPsychopharmacology99: 4431438 |
[8] | Park CR(2001) Cognitive effects of insulin in the central nervous systemNeuroscience and Biobehavioral Reviews25: 4311323 |
[9] | Hoyer S, Henneberg N, Knapp S, Lannert H, Martin E(1996) Brain glucose metabolism is controlled by amplification and desensitization of the neuronal insulin receptor374379 |
[10] | Smith MA, Riby LM, Eekelen JAMV, Foster JK(2011) Glucose enhancement of human memory: A comprehensive research review of the glucose memory facilitation effectNeuroscience and Biobehavioral Reviews35: 3770783 |
[11] | Riby LM, Riby DM, Ballesteros S(2006) Glucose, ageing and cognition: The hippocampus hypothesisAge, Cognition and Neuroscience/Envejecimiento |
[12] | Mandler G(2008) Familiarity breeds attempts: A critical review of dual-process theories of recognitionPerspectives on Psychological Science3: 5390399 |
[13] | Aggleton JP, Brown MW(2006) Interleaving brain systems for episodic and recognition memoryTrends in Cognitive Sciences10: 10455463 |
[14] | Tulving E(1985) Memory and consciousnessCanadian Psychologist26: 112 |
[15] | Brown MW, Aggleton JP(2001) Recognition memory: What are the roles of the perirhinal cortex and hippocampus?Nature Reviews Neuroscience2: 15161 |
[16] | Yonelinas AP, Otten LJ, Shaw RN, Rugg MD(2005) Separating the brain regions involved in recollection and familiarity in recognition memoryJournal of Neuroscience25: 1130023008 |
[17] | Gardiner JM, Java RI(1990) Recollective experience in word and nonword recognitionMemory and Cognition18: 12330 |
[18] | Gardiner JM, Richardson-Klavehn A(2000) Remembering and knowingThe Oxford Handbook of Memory229244 |
[19] | Perfect TJ, Dasgupta ZRR(1997) What underlies the deficit in reported recollective experience in old age? Memory and Cognition25: 6849858 |
[20] | Parkin AJ, Walter BM(1992) Recollective experience, normal aging, and frontal dysfunctionPsychology and Aging7: 2290298 |
[21] | Mantyla T(1993) Knowing but not remembering: Adult age differences in recollective experienceMemory and Cognition21: 3379388 |
[22] | Miller DB, O’Callaghan JP(2005) Aging, stress and the hippocampusAgeing Research Reviews4: 2123140 |
[23] | Du AT, Schuff N, Chao LL, Kornak J, Jagust WJ, Kramer JH(2006) Age effects on atrophy rates of entorhinal cortex and hippocampusNeurobiology of Aging27: 5733740 |
[24] | Driscoll I, Sutherland RJ(2005) The aging hippocampus: Navigating between rat and human experimentsReviews in the Neurosciences16: 287121 |
[25] | Curran HV, Gardiner JM, Java RI, Allen D(1993) Effects of lorazepam upon recollective experience in recognition memoryPsychopharmacology110: 3374378 |
[26] | Curran HV, Hildebrandt M(1999) Dissociative effects of alcohol on recollective experienceConsciousness and Cognition8: 4497509 |
[27] | Sünram-Lea SI, Dewhurst SA, Foster JK(2008) The effect of glucose administration on the recollection and familiarity components of recognition memoryBiological Psychology77: 16975 |
[28] | Scholey A, MacPherson H, Sünram-Lea S, Elliott J, Stough C, Kennedy D(2013) Glucose enhancement of recognition memory: Differential effects on effortful processing but not aspects of ‘remember-know’ responsesNeuropharmacology64: 544549 |
[29] | Allan K, Wilding EL, Rugg MD(1998) Electrophysiological evidence for dissociable processes contributing to recollectionActa Psychologica98: 2-3231252 |
[30] | Rugg MD, Curran T(2007) Event-related potentials and recognition memoryTrends in Cognitive Sciences11: 6251257 |
[31] | Curran T(1999) The electrophysiology of incidental and intentionalretrieval: ERP old/new effects in lexical decision andrecognition memoryNeuropsychologia37: 7771785 |
[32] | Smith MA, Riby LM, Sünram-Lea SI, Van Eekelen JAM, Foster JK(2009) Glucose modulates event-related potential components of recollection and familiarity in healthy adolescentsPsychopharmacology205: 11120 |
[33] | Yonelinas AP(2002) The nature of recollection and familiarity: A review of 30 years of researchJournal of Memory and Language46: 3441517 |
[34] | Riby LM, Meikle A, Glover C(2004) The effects of age, glucose ingestion and gluco-regulatory control on episodic memoryAge and Ageing33: 5483487 |
[35] | Scholey AB, Fowles KA(2002) Retrograde enhancement of kinesthetic memory by alcohol and by glucoseNeurobiology of Learning and Memory78: 2477483 |
[36] | Jasper HH(1958) The ten-twenty electrode system of the international federationElectroencephalogr Clin Neurophysiol10: 371375 |
[37] | Lagerlund TD, Sharbrough FW, Busacker NE(1997) Spatial filtering of multichannel electroencephalographic recordings through principal component analysis by singular value decompositionJournal of Clinical Neurophysiology14: 17382 |
[38] | Ille N, Berg P, Scherg M(2002) Artifact correction of the ongoing EEG using spatial filters based on artifact and brain signal topographiesJournal of Clinical Neurophysiology19: 2113124 |
[39] | Frey PW, Colliver JA(1973) Sensitivity and responsivity measures for discrimination learningLearning and Motivation4: 3327342 |
[40] | Foster JK, Lidder PG, Sünram SI(1998) Glucose and memory: Fractionation of enhancement effects? Psychopharmacology137: 3259270 |
[41] | Riby LM, Marriott A, Bullock R, Hancock J, Smallwood J, McLaughlin J(2009) The effects of glucose ingestion and glucose regulation on memory performance in older adults with mild cognitive impairmentEuropean Journal of Clinical Nutrition63: 4566571 |
[42] | Czernochowski D, Mecklinger A, Johansson M, Brinkmann M(2005) Age-related differences in familiarity and recollection: ERP evidence from a recognition memory study in children and young adultsCognitive, Affective and Behavioral Neuroscience5: 4417433 |
Figures and Tables
Fig.1
Mean Blood Glucose Levels over the Duration of Testing. 20 mins = 20 minutes after drink administration; Final = after conclusion of testing. Significant main effects of drink at 20 mins and Final (approximately 40 min post-drink), are indicated (***p < 0.001).
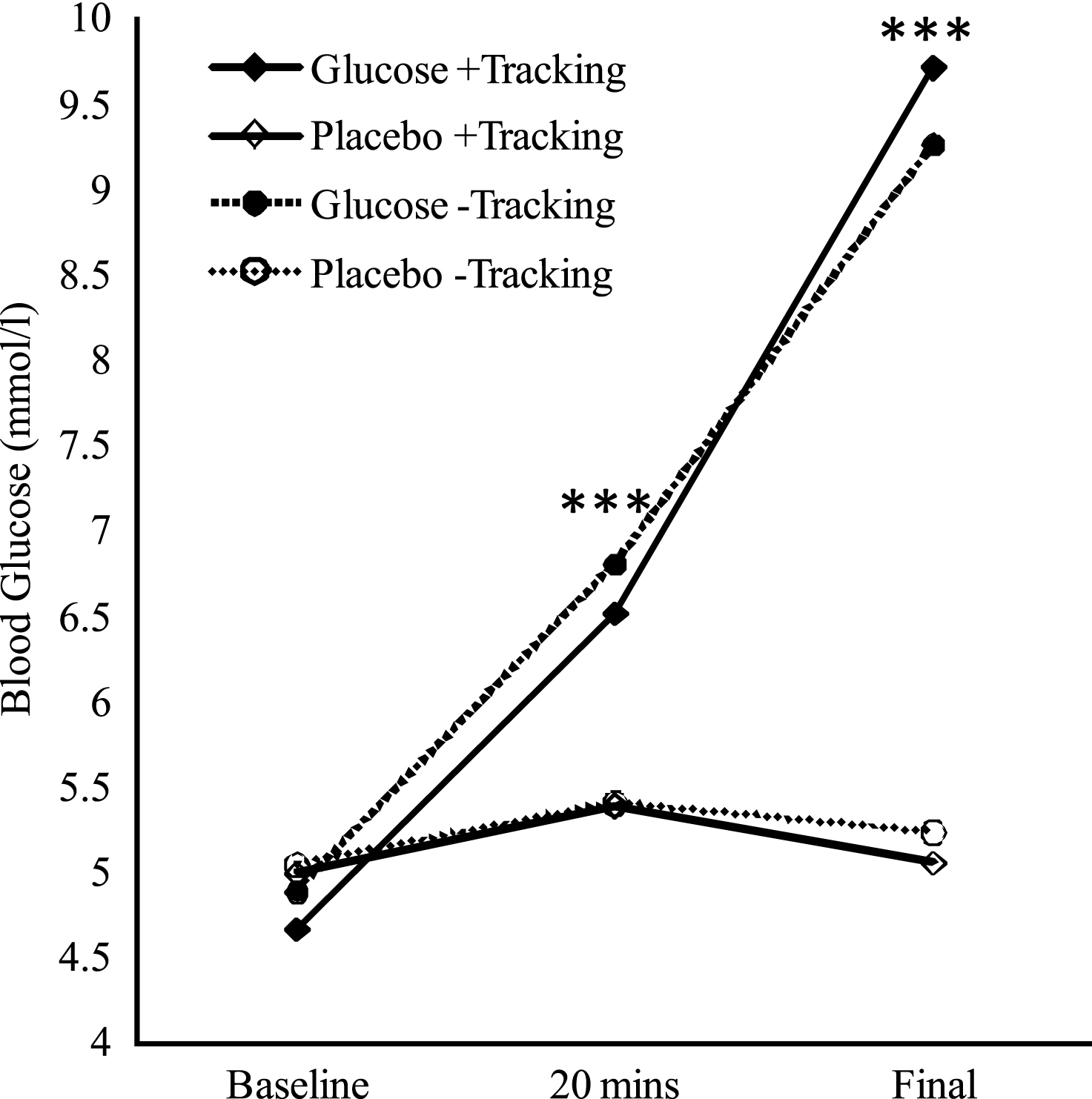
Fig.2
Mean Sensitivity Index (±SE) for Glucose and Placebo treatments according to Task (single/dual).
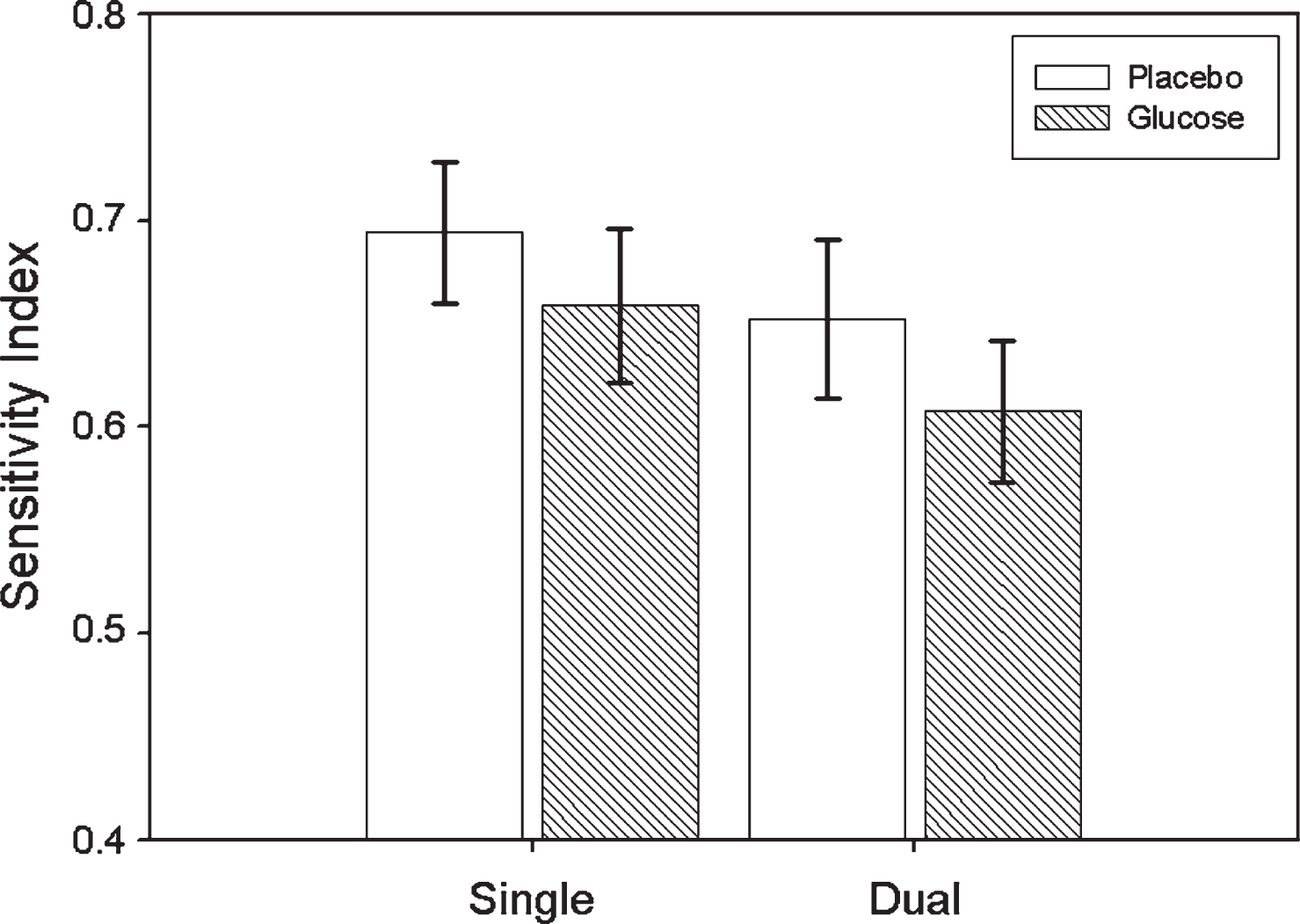
Fig.3
Boxplots for median proportions of a) Remember, b) Know and c) Guess responses according to treatment (Glucose, Placebo) and Task (Single, Dual).
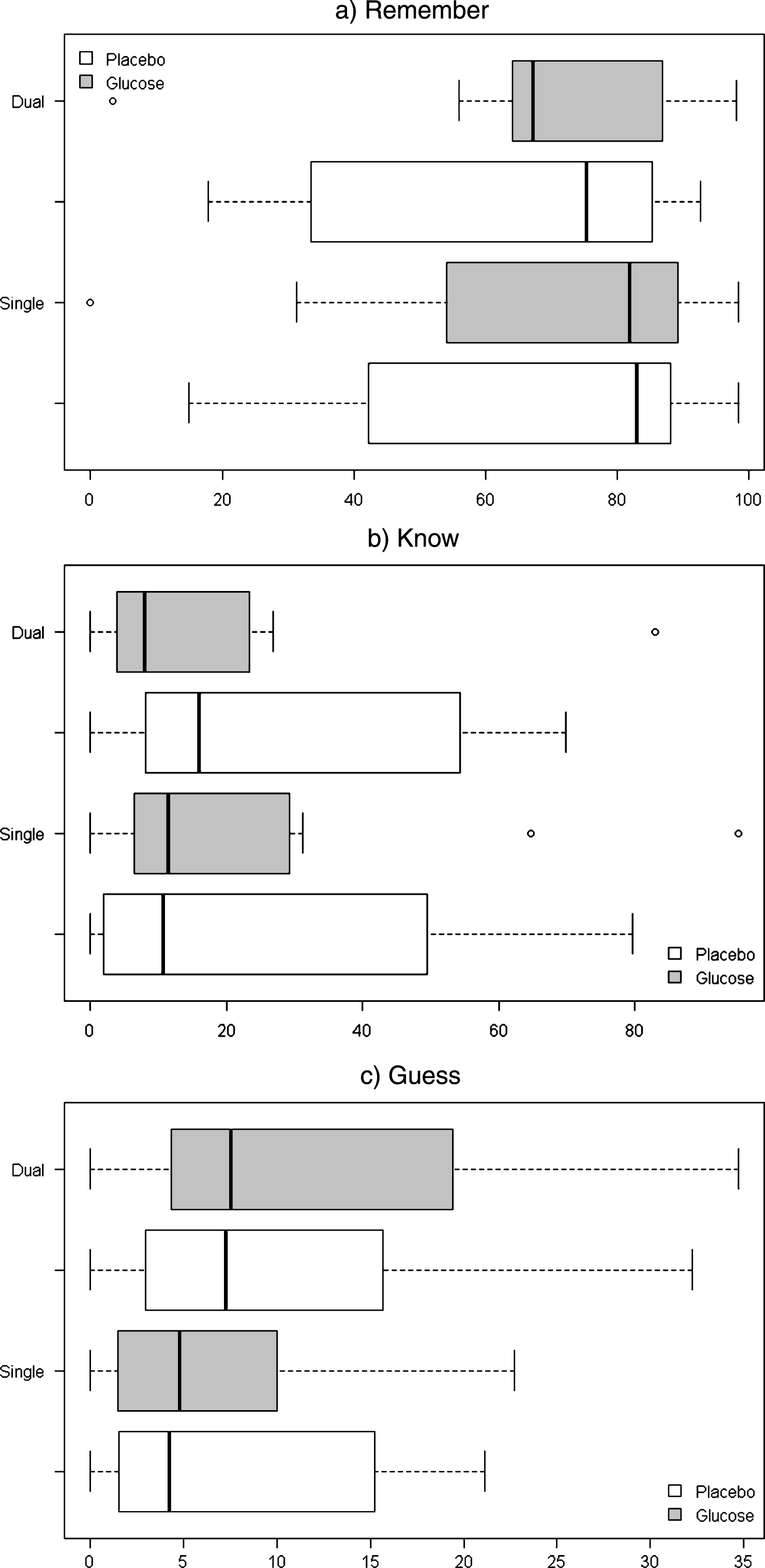
Fig.4
Tracking Accuracy for correctly recalled words according to condition (Glucose, Placebo) with higher values reflecting lower tracking accuracy. *p < 0.05, **p < 0.01.
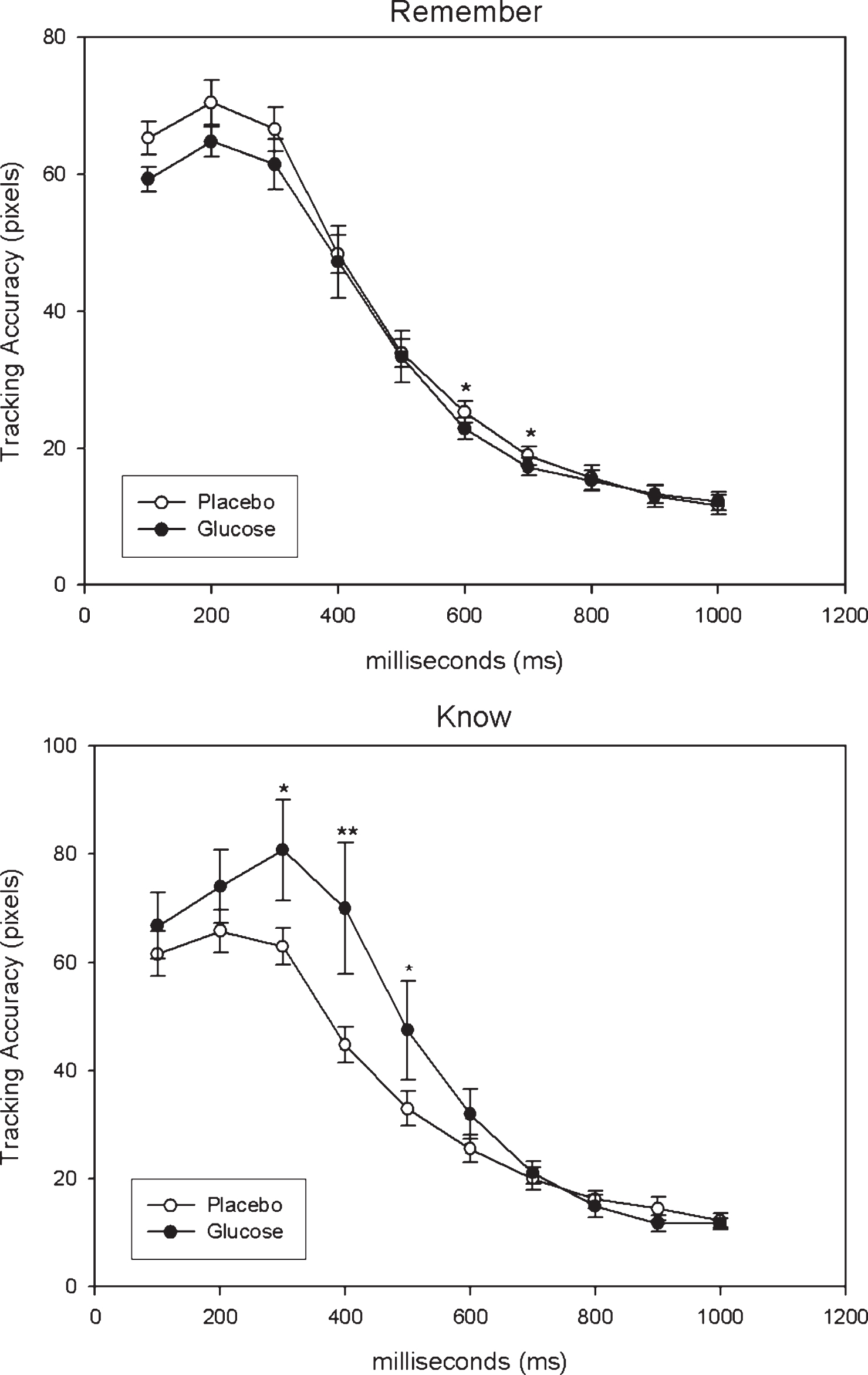
Fig.5
Grand average ERP old [correct] -new [correct] for frontal sites (F3, FZ, F4) and parietal sites (P3, PZ, P4), illustrating the FN400 and LP effects.
![Grand average ERP old [correct] -new [correct] for frontal sites (F3, FZ, F4) and parietal sites (P3, PZ, P4), illustrating the FN400 and LP effects.](https://content.iospress.com:443/media/nua/2015/3-1/nua-3-1-nua042/nua-3-1-nua042-g005.jpg)
Fig.6
Grand average ERP Glucose old [correct] and Placebo old [correct] for frontal and parietal sites.
![Grand average ERP Glucose old [correct] and Placebo old [correct] for frontal and parietal sites.](https://content.iospress.com:443/media/nua/2015/3-1/nua-3-1-nua042/nua-3-1-nua042-g006.jpg)
Table 1
Mean (with standard error) proportion of words correctly recognized (Hits) and non-studied words falsely recognized (FA = false alarms) according to drink (placebo, glucose) and task (single, dual)
| Placebo | Glucose | |||
| Single | Dual | Single | Dual | |
| Overall | 84.41 (3.26) | 79.09 (4.73) | 79.09 (4.11) | 71.94 (6.06) |
| False Alarms | 16.90 (4.57) | 19.70 (6.29) | 18.51 (6.81) | 18.95 (6.53) |
| Sensitivity Index | 0.694 (0.035) | 0.652 (0.085) | 0.659 (0.037) | 0.608 (0.034) |
Table 2
Proportion responses categorized as Remember, Know and Guess according to condition and task type. Numbers are presented as means with standard error (SE), and medians with interquartile range (IQR)
| Placebo | Glucose | ||||
| Single | Dual | Single | Dual | ||
| Remember | |||||
| Mean (±SE) | 67.97 (8.34) | 61.74 (8.95) | 68.41 (9.21) | 68.59 (7.70) | |
| Median (±IQR) | 82.95 (41.13) | 81.94 (35.10) | 75.36 (51.82) | 67.16 (67.16) | |
| Know | |||||
| Mean (±SE) | 24.34 (7.79) | 27.56 (7.94) | 24.01 (9.08) | 18.34 (7.12) | |
| Median (±IQR) | 10.61 (46.51) | 11.39 (22.51) | 15.94 (46.25) | 7.94 (19.50) | |
| Guess | |||||
| Mean (±SE) | 7.69 (2.29) | 10.70 (3.11) | 7.58 (2.44) | 13.06 (3.65) | |
| Median (±IQR) | 4.22 (13.10) | 4.76 (8.54) | 7.25 (12.71) | 7.50 (15.09) | |




