Tart cherry in amelioration of pain in the elderly
Abstract
Background:
Tart cherry, rich in bioactive polyphenols, has received attention in the past decade for reported health benefits due to its high polyphenolic content.
Objective:
To determine whether there is a potential role for tart cherry or its isolated components in amelioration of pain relief in chronic diseases that may affect the elderly.
Methods:
In vitro and in vivo human and animal studies that utilized tart cherry or extracts of tart cherry compounds to determine an effect on oxidative stress, inflammation, muscle damage, and pain were reviewed and summarized (Table 1).
Results:
Tart cherry and its isolated compounds have demonstrated antioxidant and anti-inflammatory effects both in vitro and in vivo which may improve self-reported pain. In humans, these include modest improvements in gout flare incidents, and self-reported pain in fibromyalgia, osteoarthritis (OA), and conditions of induced oxidative stress and muscle damage. Beneficial biochemical changes were also reported for inflammatory and oxidative biomarkers such as serum urate, C-reactive protein (CRP), and interleukin-6 (IL-6). However, most studies reported to date have insufficient sample size, treatment duration, and statistical power to draw any firm conclusions.
Conclusions:
Consumption of tart cherries and their bioactive constituents may be a potential novel therapy for reducing the pain associated with chronic diseases particularly common to an aged population. Larger, more rigorous trials are needed to reach any firm conclusions.
1Introduction
Inflammation and oxidative stress have been suggested to play a key role in the causation and progression of cardiovascular disease (CVD), stroke, diabetes, cancer, Alzheimer’s, and other age-related chronic diseases [1–3]. Many epidemiological studies show a strong association between fruit and vegetable consumption and a lower risk of chronic disease. Since fruits and vegetables are rich sources of polyphenols and their derivatives, beneficial effects of their intake may be related to their polyphenol content and composition [1, 4]. Flavonoids are polyphenolic plant compounds, of which the pigments anthocyanins are a subclass. These pigments have been shown to have antioxidant and anti-inflammatory activity among other beneficial properties [2, 5, 6].
During the last decade, tart cherry has received special attention with respect to its health benefits due to its high polyphenolic content [3, 5, 7]. The literature on the health benefits of tart cherry has generally focused on their role in the prevention, treatment, and recovery of soft tissue injuries and pain remediation [8, 9]. Figure 1 demonstrates the proposed protective effects of cherry polyphenols and their implication in chronic disease management. Pain is one of the features of many chronic diseases in the aged. The purpose of this review is to summarize and critically examine in vitro and in vivo human and animal studies (Table 1) on the efficacy of tart cherry products for pain relief in chronic diseases. Since the majority of the published human studies [10–23] are insufficiently powered, it is not possible to conduct a meta-analysis to ascertain the role of tart cherry in pain relief.
2Tart cherry phenolics and anthocyanins
Phenolics are compounds that possess in common an aromatic ring bearing one or more hydroxyl groups. There are thousands of naturally occurring plant phenolics and about half of these are flavonoids [24]. Both flavonoids and phenolics exhibit a wide spectrum of biological activities such as antioxidant, antimutagenic, and anticarcinogenic activity as well as the ability to modify gene expression [25]. The flavonoids have two benzene rings separated by a propane unit. The flavones and flavonols are the most widely distributed of all the phenolics. Flavonoids occur in different plant parts both in a free state and as glycosides [24]. Cyanidin 3-glucoside has stronger antioxidant activity compared to other anthocyanins present in tart cherries, and is used as a standard for quantification of tart cherry anthocyanin content. The antioxidant activity of cyanidin glycosides increases as attached sugar units decrease [3, 26–28]. Table 2 shows that total polyphenolic content is higher in tart cherries compared to sweet cherries (228.9 mg vs. 109.8 mg). Table 3 demonstrates that total phenolic and anthocyanin concentration also tends to be higher in certain varieties and forms of tart cherry [3, 6].
The tart cherry cultivars Montmorency and Balaton are grown commercially in the United States, with Montmorency accounting for 75% of tart cherry production [6, 29]. Balaton fruit is reported as having six times the total anthocyanin content as Montmorency, but Montmorency has higher levels of total phenolics [6, 30]. Kirakosyan et al. (2009) found total anthocyanin content ranging from 173–1741 g/g dry weight of CGE across 10 different types tart cherry commercial products. Products made from frozen tart cherries have the highest levels of total anthocyanins and phenolics, but the concentration of both is lowered when the products are processed with sugar [6]. Ou et al. (2012) confirmed that frozen tart cherry products had higher levels of total anthocyanins and pro-anthocyanins, but found total phenolics to be highest in tart cherry juice concentrate. The authors determined that processed tart cherry products do have antioxidant and anti-inflammatory activity, but per serving a juice concentrate has superior antioxidant and anti-inflammatory activity compared to frozen, canned, or dried cherries [29].
3Tart cherry, antioxidant activity, and inflammatory cytokines
3.1 In vitro studies
Early in vitro studies that used isolated tart cherry anthocyanins suggested a role for tart cherry in the inflammatory and oxidative stress pathways linked to chronic disease [28, 31–34]. Wang and colleagues have conducted several in vitro studies of the antioxidant activities of tart cherry extracts [31–33]. In the first study, Balaton and Montmorency cherry extracts inhibited lipid peroxidation in ranges of 9.9% to 28.3% . When further refined, two fractions of Balaton showed much higher inhibitory activity at 75.7% and 67.3% [32]. A second study showed that the inhibition of lipid peroxidation was comparable to the commercial antioxidants tert-butylhydroquinone (TBHQ) and butylated hydroxytoluene (BHT). Three novel antioxidant compounds were identified in the extract, with inhibitory activity ranging from 50% to 80% . Structurally, two of these compounds were analogues of caffeic acid [31]. Three anthocyanins and their more effective aglycone form, cyanidin, also exhibited lipid peroxidation comparable to TBHQ and BHT and superior to that of vitamin E [33].
Seeram et al. (2001) also used tart cherry extracts to examine the role of tart cherry antioxidants in two in vitro studies. In the first study mixed tart cherry anthocyanins exhibited antioxidant activity comparable to commercial antioxidants TBHQ, butylated hydroxyanisole (BHA) and BHT, and superior to that of vitamin E [28]. The second study examined the stability and degradation products of isolated tart cherry cyanidin glycosides to better understand their fate in processed foods. The degradative byproduct protocatechuic acid had comparable antioxidant activity to BHA, BHT, TBHQ, and superior to vitamin E [35].
In the second study from Seeram et al. (2001), tart cherry anthocyanins were deemed stable at room temperature and at pH 3, the conditions found in cherry fruit. High processing temperatures will hydrolyze the anthocyanins to cyanidin, increasing the anti-inflammatory and antioxidant properties in the short term, but prolonged exposure will lead to little to no anti-inflammatory action. For maximum potency in cherry products used for pain relief, the authors recommended storage at low pH and avoidance of prolonged high temperatures [35]. A more recent study from Mulabagal and colleagues also confirmed that Balaton and Montmorency cherry extracts inhibited lipid peroxidation in ranges of 38% to 58% [36].
Inflammatory cytokines such as interleukin (IL)-6 are linked to chronic disease, possibly through the activation of the inflammatory mediator C-reactive protein (CRP). Zhou et al. (2012) compared the activity of several tart cherry extracts and their isolated compounds to Lipitor (an HMG Co-A reductase inhibitor) on IL-6 secretion by adipose stem cells. Both Lipitor and the cherry extract components exhibited synergistic effects on IL-6 secretion by adipose stem cells. Therefore, it is conceivable that tart cherries may be an effective adjunct to statins in lowering circulating cholesterol [37]. The results of these in vitro studies provide impetus for conducting animal and human studies.
3.2 In vivo studies
Anthocyanin intake in animals may contribute to improvements in certain chronic diseases associated with aging, such as hyperlipidemia, obesity, atherosclerosis, and Alzheimer’s [10–12]. Matchynski et al. found that a tart cherry extract combined with a fish and emu oil supplement lessened induced cognitive deficits in an Alzheimer’s mouse model. Seventeen days after mu-p75 saporin (SAP) induction of Alzheimer’s, the mice receiving the combination treatment were protected against neuron loss and the SAP-induced inflammation compared to placebo [10]. Anthocyanins are also thought to influence the activity of tissue peroxisome proliferator-activated receptors (PPARs), important transcription factors involved in lipid metabolism and inflammation. Seymour et al. (2008) showed that whole tart cherries enhanced mRNA expression of PPAR-α and its target acyl-coenzyme A oxidase in lean rats fed a low-fat diet. These Dahl Salt-Sensitive rats were insulin resistant and hyperlipidemic, but had increased plasma antioxidant capacity and reductions in fasting blood glucose, hyperlipidemia, hyperinsulinemia, and fatty liver after cherry treatment [11].
A subsequent study from Seymour et al. showed whole tart cherry powder reduced circulating plasma levels of tumor necrosis factor alpha (TNF-α) and IL-6 after 90 days of treatment in obesity-prone Zucker rats fed a high fat diet [12]. The authors observed plasma reductions of 44% in IL-6 and 40% in TNF-α after 90 days. Furthermore, whole tart cherry treatment also reduced expression of mRNA for TNF-α and IL-I in retroperitoneal fat depots by 2.9 to 4.5 fold. However, PPAR-α mRNA was increased 2.7-fold. It is interesting that the cherry treatment had no effect on body weight gain in the earlier study using lean rats, but in the study with the rat model prone to obesity and inflammation there was a significant reduction in body weight and abdominal fat mass [11, 12]. The results suggest that tart cherry intake resulted in reduced inflammation in retroperitoneal fat, though specificity of the action to cell type within the adipose tissue was not determined [11, 12].
Human studies have also given some evidence of antioxidant and anti-inflammatory response at doses equal to about 100 tart cherries daily. Lynn et al. (2014) found that consumption of a tart cherry juice concentrate evoked a minor increase (p = 0.012) in plasma ferric reducing ability in a group of 25 healthy adults. However, the effect was mostly due to a decreased antioxidant status in the control group. The study did not show an effect on arterial stiffness or improved CVD markers, but there were several design flaws that may have accounted for the failure to find a significant effect. The authors conceded the ferric reducing ability of plasma (FRAP) assay used has limited sensitivity to detect changes in antioxidant status after cherry juice consumption. While the cherry treatment had no effect on CRP, the authors speculated this was due to using a single daily dose. It is possible that CVD markers examined were simply not responsive to tart cherry in normal weight, healthy adults. Finger prick capillary blood samples did not render complete data for all analyses [13].
Traustadottir et al. (2009) examined the effect of tart cherry juice intake in a small group of 11 older adults, a population with impaired resistance to oxidative stress. In response to a forearm ischemia-reperfusion oxidative stress challenge, the F2-isoprostane response (a sensitive marker of acute oxidative damage) was reduced in the cherry group compared to placebo. There was no effect on F2-isoprostane response under resting conditions. Urinary markers of sustained DNA and RNA damage (8-hydroxy-2’-deoxyguanosine and 8-hydroxyguanosine) were also significantly lower in the cherry group compared to placebo, leading the authors to speculate that anthocyanins may form cyanidin-DNA complexes resistant to oxidative damage. However, plasma or urinary anthocyanin levels were not examined [14].
These in vivo studies offer promising, but limited, evidence that tart cherry may influence antioxidant and anti-inflammatory pathways. This is particularly so in adipose tissue; animals prone to obesity, insulin resistance and hyperlipidemia; and older adults with compromised resistance to oxidative stress. However, the sample sizes studied are very small and the authors did not provide evidence of sufficient statistical power to detect a true difference between placebo and treatment groups [10–14].
4Tart cherry inhibits cyclooxygenase enzymes
The prostaglandin-endoperoxide synthase (cyclo-oxygenase or COX) enzyme mediates the synthesis of prostaglandin from arachidonic acid as part of the inflammatory response, which generates pain and inflammation. There are two isoforms of the COX enzyme, COX-1 being a constitutive enzyme and COX-2 inducible in inflammatory conditions [38]. Therefore, COX-2 inhibition is a target of therapies that aim to reduce inflammation and pain in certain disease states such as osteoarthritis. However, there can be adverse cardiovascular and gastrointestinal effects of such pharmacotherapy [39, 40].
Ou et al. (2012) were the first to report in vitro inhibitory effects on COX enzyme activity by processed whole tart cherry products. However, inhibitory activity was toward COX-1 and not COX-2 [29]. In contrast, an anti-inflammatory assay by Wang et al. (1999) found that three tart cherry anthocyanins failed to inhibit COX at a 300 mM concentration, and at higher concentrations two of the anthocyanins actually increased enzyme activity. However, cyanidin, a type of anthocyanin, had inhibitory activity against COX-1 and COX-2. Inhibition of COX-1 by cyanidin was superior to that of aspirin, though less than that of naproxen and ibuprofen [33]. A later study subsequently found that kaempferol, quercetin, and luteolin extracted from Balaton tart cherries had COX-1 inhibition activity that was also superior to aspirin but less than naproxen and ibuprofen [34].
Seeram et al. (2001) also found that a cyanidin aglycone derived from tart cherry anthocyanin powder inhibited COX-1 and COX-2 at 38.7 and 46.8% , respectively. This was comparable to inhibition of COX-1 and COX-2 by ibuprofen (47.5 % inhibition of COX-1, 39.8% inhibition of COX-2), and naproxen (54.3% inhibition of COX-1, 41.3% inhibition of COX-2). Mixed anthocyanins inhibited both COX-1 and COX-2, though to a lesser degree (26.6 and 24.9 % inhibition of COX-1 and 38.3 and 36.6% inhibition of COX-2 from Balaton and Montmorency cherries, respectively). However, a follow up study that tested the degradative products of tart cherry anthocyanins failed to find significant inhibition of COX-1 or COX-2 [28].
A more recent study confirmed that water extracts of Balaton and Montmorency tart cherries inhibited both COX-1 and COX-2 enzymes (84% and 91% Balaton; 77% and 87% Montmorency) [36]. While these in vitro studies have reported some mixed results, inhibition of COX may be a mechanism for the pain-reducing effects of tart cherry products in chronic disease. In particular, COX-2 inhibition by cyanidin could be an important advance in anti-inflammatory therapy, because adverse effects of non-steroidal anti-inflammatory drug (NSAID) treatments are due to COX-1 inhibition while the positive effects are a result of COX-2 inhibition [33].
5Tart cherry and arthritis
Arthritic conditions are characterized by inflamed joints that generate inflammatory mediators, such as TNF-α and prostaglandin E2, which produce reactive oxygen species that perpetuate the inflammatory response. He et al. (2006) studied the effect of tart cherry anthocyanins in a rheumatoid arthritis rat model, confirming a dose-dependent antioxidant and anti-inflammatory response. Antioxidant capacity and levels of the potent antioxidant scavenger superoxide dismutase were significantly higher in rats given cherries compared to untreated rats. The results suggest a role for tart cherry products in modulating inflammatory cytokines and improving antioxidant status in arthritic conditions [15].
A recent randomized, placebo-controlled trial in 20 middle aged and elderly females with osteoarthritis gave limited evidence of a reduction in inflammation with tart cherry juice consumption [41]. These authors found the adults with moderate OA pain in the tart cherry juice group had a significant decrease in CRP from baseline, but only after an apparent outlying high value participant was removed from calculation. This was one of the few studies in this review that provided a statistical power analysis. The authors estimated that 20 subjects were required to detect a 14% difference in CRP between treatments for a statistical power of 80% . No significant changes were found in IL-6, IL-10, or TNF-α. Surprisingly, though the study purported to measure pain via Western Ontario McMaster Osteoarthritis Index (WOMAC) and a visual analog scale (VAS), the results were not reported [41].
In contrast, a tart cherry juice blend was found to be partially effective against mild to moderate knee osteoarthritis pain in a crossover human trial by Schumacher et al. (2013). WOMAC scores were measured in 58 middle-aged adults with subscales assessed in pain, stiffness, and function. WOMAC pain scores decreased 15% in the treatment groups. Pain and function improved during the cherry juice treatment, but stiffness only improved in those that started out in the cherry juice group and followed with placebo. High sensitivity CRP (hsCRP) also declined by 23% during cherry treatment compared to placebo where it rose 51% . This effect was associated with the improved WOMAC scores in those whose hsCRP decreased by 10% or more. Power to detect a treatment effect in the change in WOMAC scores was lower than estimated (change score of 4.4 vs. 5.5 points calculated to provide 80% power). While the participants were primarily obese, African American males, WOMAC score treatment effects were not significantly affected by race, BMI, or gender. However, this study did not clearly establish the efficacy of tart cherry for osteoarthritis pain, as improvement was seen during cherry treatment but not when compared to placebo (15% vs. 6%) [42].
6Tart cherry, gout, and changes in uric acid
An early anecdotal clinical report linked consumption of cherries to pain relief in gout, an inflammatory condition associated with high levels of uric acid in the blood [43]. Deposition of uric acid crystals in the joints and other parts of the body then triggers an inflammatory arthritis [18, 43]. Using a hyperuricemic rat model, Haidari et al. (2009) confirmed a hypouricemic effect of tart cherry juice consumption. Allopurinol is a xanthine oxidoreductase (XOR) inhibitor that has been used for decades to lower uric acid in gout, though it can have severe adverse side effects. Tart cherry inhibited one form of XOR (xanthine dehydrogenase or XDH) activity by up to 22.36% in normal rats and 29.58% in hyperuricemic rats. The effect was less against xanthine oxidase (XO), the predominant form in pathological conditions (13.20% and 20.08% in normal and hyperuricemic animals, respectively). Tart cherry juice resulted in increased serum total antioxidant capacity in normal rats vs. control groups (340 vs. 275 mmol/L) and in hyperuricemic rats (260 vs. 212 mmol/L). In hyperuricemic rats, both allopurinol and tart cherry juice decreased uric acid after 1 and 14 days of consumption, respectively. However, allopurinol did so to levels below normal, which might be counterproductive as uric acid also possesses antioxidant activity [16].
In the earliest human case study suggesting a role for cherry consumption in gout relief, twelve patients recorded consumption of approximately one-half pound of fresh or canned cherries (sweet and tart) daily. These patients experienced greater joint movement and symptom relief accompanied by decreases in blood uric acid [43]. More recent human studies have confirmed a potential role for tart cherry in the prevention and treatment of gout (17, 44). Schlesinger and Schlesinger (2012) recently published the results of three small pilot studies on the use of a cherry juice concentrate as a prophylactic treatment for gout flares that typically follow urate-lowering therapy (ULT). In the first randomized controlled study, the group of nine adults that received daily cherry juice concentrate equivalent to 90 to 120 cherries had a significant reduction of gout flare incidents from 4.99 to 1.56 over four months. Five of the nine cherry group members were free of gout flares within four months of cherry intake. In contrast, this was the case for only one of five patients receiving the pomegranate juice concentrate control. In those receiving ULT there was a synergistic effect in reducing gout flares at 4–6 months (36% flare-free without ULT vs. 62% flare-free on ULT). No patients in the pomegranate group discontinued NSAID use, but five of the nine in the cherry group discontinued NSAIDs within 60 days of starting treatment [Schlesigner 12].
The second retrospective pilot from Schlesinger and Schlesigner (2012) was slightly larger, and found that in 24 gout patients there was a significant reduction in gout flares from 6.85 to 1.34 flares over at least four months of cherry juice concentrate intake, without a significant change in serum urate. Their third in vitro pilot study showed cherry juice concentrate treatment in human monocytes that were stimulated by urate crystals led to inhibition of IL-1β by 60% and TNF-α by up to 45% . Pomegranate juice by comparison only had a weak effect or even some stimulation of IL-1β, and a weak or no effect on TNF-α. Though these were small pilot studies in a mostly Caucasian population, they do suggest tart cherry to be effective against prevention of gout pain over the course of several months via an anti-inflammatory action [Schlesigner 12]. Unlike the hyperuricemic rat model [16], the changes in this study were not accompanied by changes in serum urate [17].
In contrast, a compelling new randomized crossover study from Bell et al. (2014) found that larger daily doses equal to 180 or more cherries were sufficient to decrease serum urate as early as 2 hours after supplementation with a Montmorency cherry extract. The decrease was accompanied by an increase in urinary urate excretion. Furthermore, serum hsCRP decreased by up to 29% at the 5 hour post-supplement time point, providing evidence of an anti-inflammatory effect following tart cherry consumption. It should be noted this was a small study of twelve healthy, mostly male (n = 11), adults that lasted a total of four days and did not employ a control group. It is unknown whether a similar outcome would be seen in different populations or time frames. The authors did provide an estimated statistical power of 80% to detect a 17% decrease in uric acid with 12 participants. Participants were instructed to follow a low-polyphenolic diet throughout the supplementation period for 48-hours prior to participating in each arm of the trial. There was no benefit found in doubling the treatment dose for the second arm of the study, though plasma uptake of the major anthocyanin, cyanidin-3-O-glucosiderutinoside, was increased at 1 hour post-supplementation with the 60 mL dose compared to the 30 mL dose [44].
Zhang et al. (2012) conducted a large prospective study in 633 middle-aged adults to identify triggers associated with chronic gout. While the authors did not draw a distinction between tart or sweet cherries, two days of cherry consumption prior to a gout attack was linked with a 35% reduction in risk of recurrent gout when compared to no cherry intake. Risk reduction peaked at three half-cup servings daily over the two days (52% reduction). Cherry extract intake was associated with a 45% reduction. Their appeared to be a synergistic effect with allopurinol resulting in a 75% reduction in risk of gout attack. Interestingly, the authors noted that the effect on risk was stronger when it coincided with increased dietary purine, and avoidance of alcohol, diuretics, or NSAIDs. This observational study would not detect a causal link between cherry consumption and decreased gout flares, but it does give further evidence that cherry may be effective as a short-term prophylactic or adjunctive treatment for gout. The study was internet-based, allowing for a larger (but self-selected) population to participate. The average age of the participants was 54 and they were mostly Caucasian, college educated males. Yet the association did remain across subgroups of both sex and BMI [18].
7Tart cherry, pain, and muscle damage
There have been few studies investigating the effect of tart cherry on pain reduction in normal populations, but the results of two studies where pain was triggered suggest tart cherry anthocyanins may reduce acute inflammatory pain [19, 45]. Whether that would translate to pain reduction in long-term chronic diseases is unclear. Tall and colleagues demonstrated that tart cherry anthocyanins reduced inflammation-induced thermal hyperalgesia, mechanical hyperalgesia, and paw edema in a dose-dependent manner in rats [45]. The effect on thermal hyperalgesia was present at the early stages (1.5 hours), and at high doses was effective at 4.5 hours. A 400 mg/kg anthocyanin dose was comparable to the highly effective NSAID indomethacin at 5 mg/kg. Motor function was not affected at doses up to 400 mg/kg, giving evidence that the observed effects were not due to impairments in motor function. However, the tart cherry effects were shorter acting and less effective against mechanically-induced pain than thermal-induced pain [45].
Fibromyalgia is a chronic pain disorder that may be responsive to tart cherry intake. Elliot et al. (2010) conducted a small study of tart cherry juice intake equivalent to about 100 cherries daily in 14 women with fibromyalgia. Results showed a marginal benefit in about 1/3 of the women. After an eccentric exercise challenge, which can exacerbate symptoms, there was a non-significant trend toward greater muscle strength in the tart cherry group when baseline and follow-up day one values were combined (26.5 vs. 22.4 lbs.). This was noteworthy as strength loss was predicted to be highest at this time point. Five participants in the cherry group did have significant reduction in overall pain as measured by VAS pain scores. The authors note that in prior studies a VAS difference of 20 mm between VAS scores was considered clinically significant, which this subset clearly achieved with a 25 mm difference. The pain tolerance and threshold in this group of women was below that usually seen in women without fibromyalgia, which may explain in part why tart cherry had limited effectiveness in this group [19].
It is interesting to note that a 2009 study by Ducharme et al. suggested that tart cherry treatment in horses may decrease the magnitude of changes in the skeletal muscle enzyme aspartate amino transferase (AST) seen during an exercise protocol. However, inferences cannot be made about human muscle response to tart cherry based on this study [20]. A small number of human studies have been conducted in athletes to investigate whether tart cherry treatment is linked to pain associated with muscle damage [21, 22, 46]. Pain that occurs following an acute tissue injury may be due in part to oxidative damage, which triggers an inflammatory response [21, 22]. Both mechanical and oxidative tissue damage are seen in chronic diseases of aging, as well as in competitive athletes. In either case muscle pain is often treated with NSAIDs, yet tart cherry may provide a potential safe treatment or adjunct with fewer side effects [21].
Kuehl et al. (2010) conducted a randomized, placebo-controlled trial in a group of 54 long-distance runners. While both treatment and placebo groups showed increases in post-race muscle pain, tart cherry juice intake for one week prior to the race resulted in a smaller increase on the VAS pain scale compared to a placebo (12 mm increase vs. 37 mm increase). The runners that took cherry juice were also more satisfied with their treatment and associated pain reduction with the treatment. Possible mechanisms of action are anti-inflammatory or antioxidant effects that blunt a secondary inflammatory response and myofibrillar disruption, thus prevention of oxidative tissue damage [21].
Connolly et al. (2006) evaluated elbow pain and range of motion after an eccentric exercise challenge in 14 adult males given tart cherry juice daily for eight days. While range of motion parameters and tenderness did not significantly differ, the cherry juice group did have less strength loss and subjective pain. The average strength loss across four days was 4% in the cherry group and 22% in the placebo group. At 96 hours after the exercise challenge muscle strength was actually 6% above baseline in the cherry group. Pain peaked at 24 hours with cherry juice and 48 hours with placebo. Data were reanalyzed in five subjects who started with cherry to ensure the effect was not due to crossover protective effect. Treatment by time interaction was not significant for pain in this group, but the pattern was similar to the entire group with a decline in pain after 24 hours in cherry and peak of 48 hours in placebo. The authors estimated a statistical power of 80% with a sample size of 16 in order to detect a 14% difference in strength loss between placebo andcherry [46].
A small study of 10 male athletes demonstrated that consumption of a tart cherry juice concentrate resulted in greater muscle force recovery with decreased serum protein carbonyls associated with reduced oxidative damage [22]. Knee extension maximum voluntary contraction recovery was 90.9% of baseline at 24 hours and 92.9% of baseline at 48 hours in in the cherry group, compared to 84.9% and 88.5% in the placebo group, respectively. There was no significant reduction in muscle soreness because the cherry group had no change in muscle pressure pain threshold (reduction a maximum of 27%) [22]. Protein carbonyls were statistically elevated at baseline, which could have been a confounder indicating increased oxidative damage in the cherry group. However, the authors noted there is no known mechanism for this to affect response to the exercise protocol [22]. Protein carbonyls, commonly known as advanced glycation end products (AGEs), are known to increase in chronic diseases of aging, such as CVD, chronic kidney disease (CKD), and type II diabetes (47).
Two human studies have included indices of muscle damage and strength recovery rather than pain or soreness in response to tart cherry. These include creatine kinase, lactate dehydrogenase, muscle soreness, and isometric strength [23, 48]. Howatson et al. (2010) observed that tart cherry juice consumption in the five days leading up to and for 48 hours following a marathon run led to faster muscle strength recovery in 10 adults. This effect was a possible indication that the secondary inflammatory response from muscle damage was curbed. Total antioxidant status (TAS) was approximately 10% higher in the cherry juice group than placebo group at all time points, and was significantly higher than the placebo group at 24 hours (114% of baseline vs. 103% of baseline). Elevation in post-race serum IL-6 was 49% smaller in the cherry group (41.8 vs. 82.1 pg/mL), though IL-6 returned to baseline at 24 hours post-race. There were also smaller elevations in uric acid up to 24 hours post-race and CRP up to 48 hours post-race, with CRP being 34% lower in the cherry group 24 hours post-race. The authors calculated statistical power to be 80% with 10 subjects per treatment group, and reported all dependent variables having a power of equal to or greater than74% [48].
A very recent study by Bell and colleagues (2014) used dosages up to 180 tart cherries daily as a juice concentrate in a small group of males on a low-polyphenolic diet. The results showed a reduced inflammatory response and oxidative stress in response to three days of simulated high-intensity cycling. Sixteen cyclists consumed tart cherry juice or placebo for seven days, with a high intensity, stochastic road cycling trial of 109 minutes on each of the last three days. Lipid hydroperoxide increases (a measure of oxidative stress) were lower in the cherry group, by up to 29.8% in the third day of cycling. On average, pre-to-post trial lipid hydroperoxide increases were 5% in the cherry group and 11% in the placebo group. The inflammatory markers IL-6 and hsCRP both decreased with tart cherry juice intake. IL-6 was lower in the cherry group after days 2 and 3 of cycling, and hsCRP was lower across all three days of cycling [23].
These studies of pain, muscle damage, and muscle recovery were all short term, and most showed a modest effect at best on self-reported pain and short-term muscle recovery in very small groups [19, 21–23, 46, 48]. Some authors did not measure oxidative stress or inflammatory markers, which might have given a clearer picture of the response [19, 21, 46]. In the case of Bowtell et al. (2011) and Howatson et al. (2010) the functional changes were accompanied by some reduced serum markers of oxidative damage and inflammation [22, 48]. These results suggest tart cherry should be further studied as an agent for amelioration in conditions of both mechanical and metabolic stress.
8Conclusions
The in vitro antioxidant and anti-inflammatory activity of tart cherry flavonoids such as anthocyanin are well established [28, 29, 31–35, 37]. However, in vivo effects are influenced by consumption, absorption, metabolism, and resulting tissue concentration of flavonoids. There is a lack of data showing that dosage calculations of tart cherry or anthocyanins are based on body weight. Anthocyanins are unstable at neutral pH, so their antioxidant activity in vivo has not been clearly established [2]. Ou et al. (2012) noted that oxygen radical absorbance capacity (ORAC) values were significantly correlated to total phenolics and not total anthocyanins. Therefore, high anthocyanin content alone may not be a marker of higher antioxidant activity [29]. Anthocyanin and polyphenol content in tart cherry can also vary based on variety of cherry, growing season, climate, and other factors.
Most of the in vivo studies reviewed were too small in sample size and of limited duration to draw meaningful conclusions. Several authors did not appear to exclude the use of pain medication or anti-inflammatories, while some at least required a stable medication dosage [13, 19, 22, 41, 44]. Some of these studies were conducted on patients who were receiving standard routine care for a chronic disease condition, which may have included NSAIDs, COX-2 inhibitors, or other agents that affect inflammation [17, 19, 40, 41]. Dosages in human studies were generally equal to about 100 cherries daily, but anthocyanin and phenolic content varied (80–274 mg and 1100–1200 mg daily, respectively) [13, 14, 17, 19, 21, 41, 42, 46, 48]. In the case of the Tall study (2004), rats were given doses of up to 400 mg anthocyanins [45]. No serious adverse effects were reported in animal or human studies, and compliance was good. There were a few instances of attrition due to gastrointestinal discomfort, and in at least two cases withdrawal due to elevated blood sugar resulting from treatment [8, 13, 17, 21, 42, 44]. This could be a concern with the use of cherry juice products in an aging population where type 2 diabetes is more common. Commercially available tart cherry products could contain amounts of sugar sufficient to affect blood glucose, depending upon their form, concentration, and serving sizes. While it is likely that phytochemical interactions in whole food sources provide synergistic effects greater than that of individual bioactive components [6], development of suitable tart cherry extracts lower in sugar could be helpful in this group.
During the aging process, mechanical and metabolic oxidative stress can trigger an inflammatory response that promotes further stress and damage. The results of this review suggest that anthocyanins and other tart cherry bioactives do reach cellular concentrations that are sufficient to positively alter metabolic processes [10–23, 41, 42, 44–46, 48]. It is conceivable that such alterations might affect clinical outcomes in an aging population. Other compounds that may contribute significantly to tart cherry antioxidant and anti-inflammatory properties are numerous and include quercetin, kaempferol, catechin, epicatechin, and melatonin among others [6, 29, 34, 48]. Consumption of tart cherries and their bioactive constituents may be a potential novel therapy for reducing the pain associated with chronic diseases particularly common to an aged population. Future clinical trials should examine pain and other functional changes coupled with oxidative and inflammatory biomarkers, particularly those representative of COX-1 and COX-2 activity. Compliance to treatment could be measured by plasma or urinary anthocyanins, and special attention should be paid to diet records, polyphenolic content in the diet, and use of anti-inflammatory medications.
Acknowledgments
The authors declare no conflicts of interest, financial or material support for this review.
References
[1] | Liu RH . Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. (2003) ;78: (suppl)517S–520S. |
[2] | Prior RL . Fruits and vegetables in the prevention of cellular oxidative damage. Am J Clin Nutr. (2003) ;78: (suppl)570S–578S. |
[3] | Ferretti G , Bacchetti T , Belleggia A , Neri D . Cherry antioxidants: From farm to table. Molecules. (2010) ;15: :6993–7005. |
[4] | Burkhardt S , Tan DX , Manchester LC , Hardeland R , Reiter RJ . Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunus cerasus). J Agric Food Chem. (2001) ;49: :4898–4902. |
[5] | Tall JM , Raja SN . Dietary constituents as novel therapies for pain. Clin J Pain. (2004) ;20: (1):19–26. |
[6] | Kirakosyan A , Seymour EM , Urcuyo Llanes DE , Kaufman PB , Bolling SF . Chemical profile and antioxidant capacities of tart cherry products. Food Chem. (2009) ;115: :20–25. |
[7] | Kim DO , Heo HJ , Kim YJ , Yang HS , Lee CY . Sweet and sour cherry phenolics and their protective effects on neuronal cells. J Agric Food Chem. (2005) ;53: (26):9921–9927. |
[8] | Kuehl KS . Cherry juice targets antioxidant potential and pain relief. Med Sport Sci. (2012) ;59: :86–93. |
[9] | Volpe SL . Tart cherry juice and inflammatory response. ACMS’s Health & Fit J. (2014) ;18: (1):32–33. |
[10] | Matchynski JJ , Lowrance SA , Pappas C , Rossignol J , Puckett N , Sandstrom M , et al. Combinatorial treatment of tart cherry extract and essential fatty acids reduces cognitive impairments and inflammation in the mu-p75 saporin-induced mouse model of Alzheimer’s disease. J Med Food. (2013) ;16: (4):288–295. |
[11] | Seymour EM , Singer AAM , Kirakosyan A , Urcuyo-Llanes DE , Kaufman PB , Bolling SF . Altered hyperlipidemia, hepatic steatosis, and hepatic peroxisome proliferator-activated receptors in rats with intake of tart cherry. J Med Food. (2008) ;11: (2):252–259. |
[12] | Seymour EM , Lewis SK , Urcuyo-Llanes DE , Tanone I , Kirakosyan A , Kaufman PB , et al. Regular tart cherry intake alters abdominal adiposity, adipose gene transcription, and inflammation in obesity-prone rats fed a high fat diet. J Med Food. (2009) ;12: (5):935–942. |
[13] | Lynn A , Mathew S , Moore CT , Russell J , Robinson E , Soumpasi V , et al. Effect of a tart cherry juice supplement on arterial stiffness and inflammation in healthy adults: A randomised controlled trial. Plant Foods Hum Nutr. (2014) ;69: (2):122–127. |
[14] | Traustadottir T , Davies SS , Stock AA , Su Y , Heward CB , Roberts LJ II , et al. Tart cherry juice decreases oxidative stress in healthy older men and women. J Nutr. (2009) ;139: :1896–1900. |
[15] | He Y , Zhou J , Wang Y , Xiao C , Tong Y , Tang J , et al. Anti-inflammatory and anti-oxidative effects of cherries on Freund’s adjuvant-induced arthritis in rats. Scand J Rheumatol. (2006) ;35: :356–358. |
[16] | Haidari F , Mohammad SM , Keshavarz SA , Rashidi MR . Inhibitory effects of tart cherry (Prunus cerasus) juice on xanthine oxidoreductase activity and its hypouricemic and antioxidant effects on rats. Mal J Nutr. (2009) ;15: (1):53–64. |
[17] | Schlesinger N , Schlesinger M . Pilot studies of cherry juice concentrate for gout flare prophylaxis. J Arthritis. (2012) ;1: :101. |
[18] | Zhang Y , Neogi T , Chen C , Chaisson C , Hunter D , Choi HK . Cherry consumption and the risk of recurrent gout attacks. Arthritis Rheum. (2012) ;64: (12):4004–4011. |
[19] | Elliot DL , Kuehl KS , Dupree Jones K , Dulacki K . Using an eccentric exercise-testing protocol to assess the beneficial effects of tart cherry juice in fibromyalgia patients. Integ Med. (2010) ;9: (6):25–29. |
[20] | Ducharme NG , Fortier LA , Kraus MS , Hobo S , Mohammad HO , McHugh MP , et al. Effect of a tart cherry juice blend on exercise-induced muscle damage in horses. Am J Vet Res. (2009) ;70: :758–763. |
[21] | Kuehl KS , Perrier ET , Elliot DL , Chesnutt JC . Efficacy of tart cherry juice in reducing muscle pain during running: A randomized controlled trial. J Int Soc Sports Nutr. (2010) ;7: :17. |
[22] | Bowtell JL , Sumners DP , Dyer A , Fox P , Mileva KN . Montmorency cherry juice reduces muscle damage caused by intensive strength exercise. Med Sci Sports Exerc. (2011) ;43: (8):1544–1551. |
[23] | Bell PG , Walshe IH , Davison GW , Stevenson E , Howatson G . Montmorency cherries reduce the oxidative stress and inflammatory responses to repeated days high-intensity stochastic cycling. Nutrients. (2014) ;6: :829–843. |
[24] | Harborne JB , Herbert H . Phytochemical dictionary: A handbook of bioactive compounds from plants. London: Taylor and Francis; (1993) . |
[25] | Liu RH . Health-promoting components of fruits and vegetables in the diet. Adv Nutr. (2013) ;4: (3)384S–392S. doi: 10.3945/an.112.003517 |
[26] | Blando F , Gerardi C , Nicoletti I . Sour cherry (Prunus cerasus L) anthocyanins as ingredients for functional foods. J Biomed Biotech. (2004) ;5: :253–258. |
[27] | Chandra A , Rana J , Yingqin LF . Separation, identification, quantification, and method validation of anthocyanins in botanical supplement raw materials by HPLC and HPLC-MS. J Agric Food Chem. (2001) ;49: :3515–3521. |
[28] | Seeram NP , Momin RA , Nair MG , Bourquin LD . Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomed. (2001) ;8: (5):362–369. |
[29] | Ou B , Bosak KN , Brickner PR , Iezzoni DG , Seymour EM . Processed tart cherry products - Comparative phytochemical content, in vitro antioxidant capacity and in vitro anti-inflammatory activity. J Food Sci. (2012) ;77: (5):H105–H112. |
[30] | Chandra A , Nair MG , Iezzoni A . Evaluation and characterization of the anthocyanin pigments in tart cherries (Prunus cerasus L. ). J Agric Food Chem. (1992) ;40: :967–969. |
[31] | Wang H , Nair MG , Strasburg GM , Booren AM , Gray JI . Novel antioxidant compounds from tart cherries (Prunus cerasus). J Nat Prod. (1999) ;62: :86–88. |
[32] | Wang H , Nair MG , Strasburg GM , Booren AM , Gray JI . Antioxidant polyphenols from tart cherries (Prunus cerasus). J Agric Food Chem. (1999) ;47: :840–844. |
[33] | Wang H , Nair MG , Strasburg GM , Chang Y , Booren AM . Antioxidant and anti-inflammatory activities of anthocyanins and their aglycone, cyanidin, from tart cherries. J Nat Prod. (1999) ;62: :294–296. |
[34] | Wang H , Nair MG , Strasburg GM , Booren AM , Gray I , Dewitt DL . Cyclooxygenase active bioflavonoids from Balaton tart cherry and their structure activity relationships. Phytomed. (2000) ;7: (1):15–19. |
[35] | Seeram NP , Bourquin LD , Nair MG . Degradation products of cyanidin glycosides from tart cherries and their bioactives. J Agric Food Chem. (2001) ;49: :4924–4929. |
[36] | Mulabagal V , Lang GA , DeWitt DL , Dalavoy SS , Nair MG . Anthocyanin content, lipid peroxidation and cyclooxygenase enzyme inhibitory activities of sweet and sour cherries. J Agric Food Chem. (2009) ;57: :1239–1246. |
[37] | Zhou Z , Nair MG , Claycombe KJ . Synergistic inhibition of interleukin-6 production in adipose stem cells by tart cherry anthocyanins and atorvastatin. Phytomed. (2012) ;19: :878–881. |
[38] | Subbaramaiah K , Dannenberg AJ . Resveratrol inhibits the expression of cyclooxygenase-2 in mammary epithelial cells. Advances in Experimental Medicine and Biology. (2001) ;492: :147–157. |
[39] | Laine L , White WB , Rostom A , Hochberg M . COX-2 selective inhibitors in the treatment of osteoarthritis. Seminars in Arthritis & Rheumatism. (2008) ;38: (3):165–187. |
[40] | Zhang Y , Jordan JM . Epidemiology of osteoarthritis. Rheumatic Diseases Clinics of North America. (2008) ;34: (3)515–529. doi: 10.1016/j.rdc.2008.05.007 |
[41] | Kuehl KS , Elliot DL , Sleigh AE , Smith JI . Efficacy of tart cherry juice to reduce inflammation biomarkers among women with osteoarthritis (OA). J Food Stud. (2012) ;1: (1):14–25. |
[42] | Schumacher HR , Pullman-Mooar S , Gupta SR , Dinnella JE , Kim R . Randomized double-blind crossover study of the efficacy of a tart cherry juice blend in treatment of osteoarthritis (OA) of the knee. Osteoarthr Cartilage. (2013) ;21: :1035–1041. |
[43] | Blau LW . Cherry diet control for gout and arthritis. Tx Reports Biol Med. (1950) :309–311. |
[44] | Bell PG , Gaze DC , Davison GW , George TW , Scotter MJ , Howatson G . Montmorency tart cherry (Prunus cerasus L. ) concentrate lowers uric acid, independent of plasma cyanidin-3-O-glucosiderutinoside. J Funct Foods. (2014) ;11: :82–90. |
[45] | Tall JM , Seeram NP , Zhao C , Nair MG , Meyer RA , Raja SN . Tart cherry anthocyanins suppress inflammation-induced pain behavior in rat. Beh Brain Res. (2004) ;153: :181–188. |
[46] | Connolly DAJ , McHugh MP , Padilla-Zakour OI . Efficacy of a tart cherry juice blend in preventing the symptoms of muscle damage. Br J Sports Med. (2006) ;40: :679–683. |
[47] | Prasad C , Imrhan V , Juma S , Vijayagopal P , Marotta F . Lifestyle and advanced glycation end products (AGEs) burden: Its relevance to healthy aging. Aging & Dis. (2014) ;5: :212–217. |
[48] | Howatson G , McHugh MP , Hill JA , Brouner J , Jewell AP , van Someren KA , et al. Influence of tart cherry juice on indices of recovery following marathon running. J Med Sci Sports. (2010) ;20: :843–852. |
Figures and Tables
Fig.1
Protective Effects of Cherry Polyphenols in Chronic Disease Management. Adapted from Ferretti et al., Molecules 2010, 15, 6993-7005.
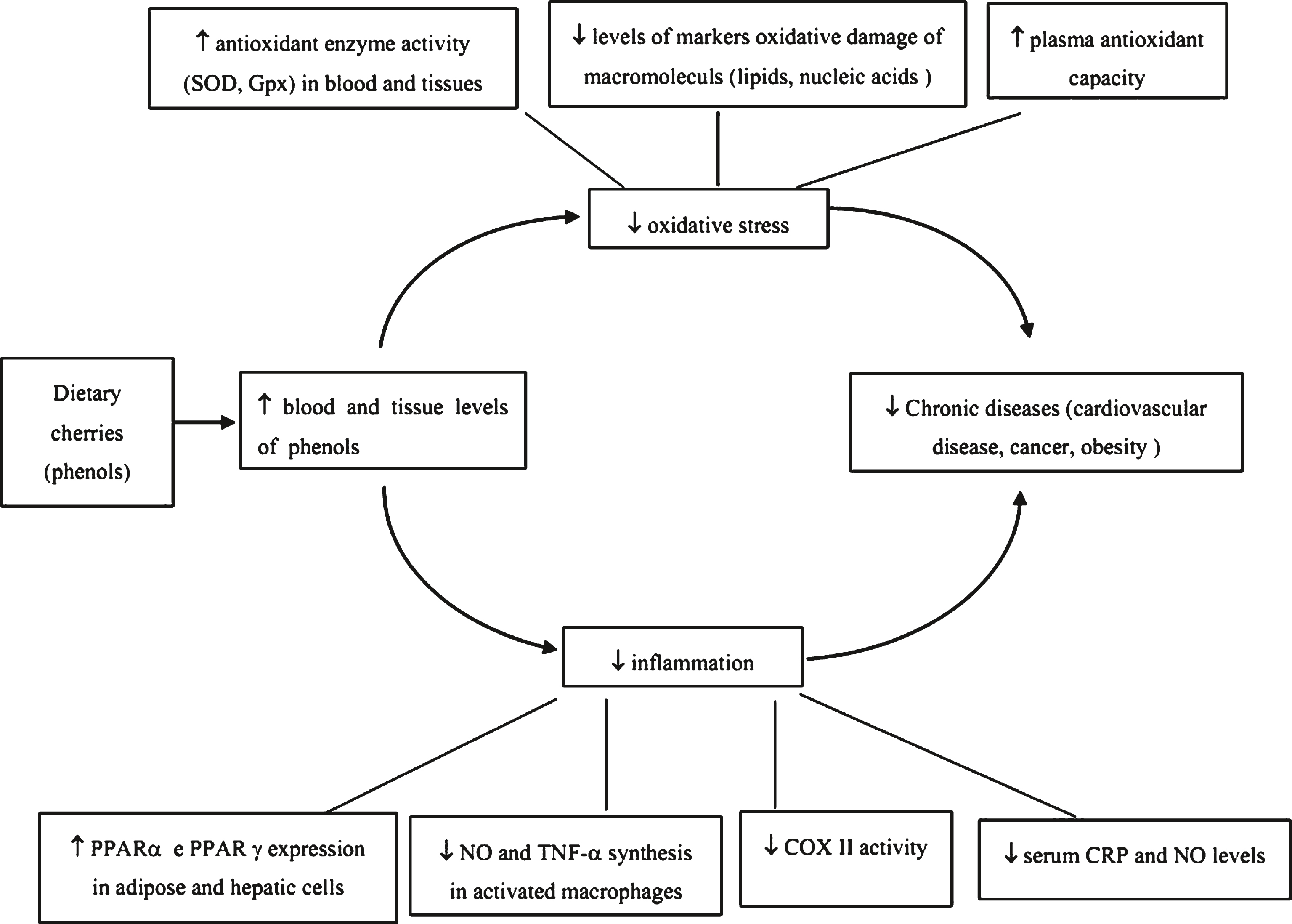
Table 1
Antioxidant and Anti-inflammatory Response of Tart Cherry
| Author/Date | Study Design | Results |
| IN VITRO STUDIES | ||
| Wang et al. 1999 [31] | Antioxidant response of a purified extract from Balaton tart cherries to lipid peroxidation | •Antioxidant activity of compounds 3 and 4 comparable to BHT, TBHQ, and caffeic acid at 20μM |
| •Antioxidant activity of compound 1 comparable to chlorogenic acid at 20μM | ||
| •No antioxidant activity of compound 2 even at concentrations up to 100μM | ||
| Wang et al. 1999 [33] | Antioxidant and anti-inflammatory response of anthocyanin and cyanidin extracted from tart cherries to lipid peroxidation and COX-1 and COX-2 activity | •Antioxidant activity of all compounds comparable to BHA and BHT and superior to vitamin E at 2 mM •No inhibition of COX-1 or COX-2 at 300 mM with pure anthocyanins 1–3 •Increased activity of COX-1 and COX-2 with >300 mM concentrations of anthocyanins 1-2 •Cyanidin inhibited COX-1 and COX-2 at 15 mM (IC50 of 90 and 60 mM, respectively) •Cyanidin inhibition of COX-1 superior to that of aspirin, though less than that of naproxen and ibuprofen |
| Wang et al. 1999 [32] | Antioxidant response of extracts from Balaton and Montmorency tart cherries to lipid peroxidation | At 25 ppm: •Methanolic extractions inhibited lipid peroxidation |
| •Fractions I and II of the Balaton extract showed considerably higher inhibition of lipid peroxidation | ||
| •A mix of compounds 1–8 in fraction I inhibited lipid peroxidation, with compounds 3 and 8 being the highest contributors to peroxidation | ||
| Wang et al. 2000 [34] | Anti-inflammatory response of bioflavonoid extracts from Balaton tart cherries to COX-1 activity | •Genistein had the highest COX-1 inhibitory activity among the isoflavonoids |
| •Kaempferol had the highest COX-1 inhibitory activity among the flavonoids and was superior to genistein | ||
| •Kaempferol, quercetin, and luteolin inhibition of COX-1 was superior to that of aspirin, though less than that of naproxen and ibuprofen | ||
| •Isoflavonoid hydroxyl groups at carbon 4, 5 and 7 were essential for COX-1 inhibition | ||
| •A double bond between carbons 2 and 3 in flavonoids is important for COX-1 inhibition, but a hydroxyl group at this position decreases COX-1 inhibition | ||
| Seeram et al. 2001 [28] | Antioxidant and anti-inflammatory response of anthocyanin extracts from Balaton and Montmorency tart cherries to lipid peroxidation and COX-1 and COX-2 activity | •Antioxidant activity of anthocyanins comparable to TBHQ, BHA and BHT and superior to vitamin E at 125μg/mL •Cyanidin fraction 4 inhibited COX-1 and COX-2 comparable to ibuprofen and naproxen at 5μM •Anthocyanin mixtures inhibited COX-1 and COX-2 at 10μM, but isolated anthocyanin compounds 1 and 2 had little activity against COX |
| Seeram et al. 2001 [35] | Antioxidant and anti-inflammatory response of anthocyanin extracts from Balaton tart cherries to lipid peroxidation and COX-1 and COX-2 activity | •Antioxidant activity of degradative compound 5 comparable to BHA and BHT at 50μM and superior to vitamin E at 10μM |
| •No anti-inflammatory activity from degradation products of anthocyanins at 50μM concentrations compared to NSAIDs | ||
| Mulabagal et al. 2009 [36] | Antioxidant and anti-inflammatory response of extracts from Balaton and Montmorency tart cherries to lipid peroxidation and COX-1 and COX-2 activity | •Inhibition of lipid peroxidation by 59% (Balaton) and 49% (Montmorency) at 250μg/mL with water extracts •All extracts of Montmorency and Balaton had inferior inhibition of lipid peroxidation compared to the BHA, BHT, and TBHQ used as positive controls at 1μg/mL •Inhibition of COX-1 and COX-2 by 84 and 91% (Balaton) and 77 and 87% (Montmorency) at 250μg/mL, respectively, with water extracts •Results were similar for methanolic and ethyl acetate extracts (values not reported) •Ethyl acetate extracts of both cultivars were superior to Celebrex (26 nM), and Vioxx (32 nM) for COX-1 inhibition, and superior to aspirin (60μM) for COX-2 inhibition |
| Zhou et al. 2012 [37] | In vitro anti-inflammatory effect of tart cherry extracts on LPS-induced IL-6 secretion in adipose stem cells after pretreatment of 4 hours and treatment of 18 hours | •Reduction in IL-6 secretion with tart cherry extract at 50μL/L •Synergistic effect with Lipitor added at 50 and 100μM/L •Dose-dependent reduction in IL-6 secretion with mixed anthocyanins at 250 and 500μg/mL, synergistic effect with Lipitor added at 50μM/L •Dose-dependent reduction IL-6 secretion with Cyanidin 3-glucoside at 100, 250 and 500μg/mL |
| Ou et al. 2012 [29] | In vitro anti-inflammatory response of whole Montmorency tart cherry products to COX-1 and COX-2 activity | •COX-1 was inhibited in juice concentrate >frozen cherries >canned cherries •No effect on COX-2•Total anthocyanin content highest in frozen cherries >juice concentrate >dried cherries >canned cherries |
| ANIMAL STUDIES | ||
| Tall et al. 2004 [45] | Two-part experiment with tart cherry anthocyanin extract from Balaton cherries in Sprague-Dawley rats | Part I: ∘ suppression of hyperalgesia and greater paw withdrawal latency at 1.5, 2.5 and 4.5 hrs. with indomethacin and anthocyanins but not saline |
| •Part I: pre-treated for 3 days with 400 mg/kg anthocyanins, indomethacin or saline and then treated with anthocyanins on day 4 | ∘ no change in paw withdrawal latency after anthocyanin treatment compared to baseline or saline ∘ subsequent group with no pre-treatment had suppression of carrageenan-induced paw thickness and higher paw withdrawal threshold at 0.5 and 1.5 hrs. with anthocyanin and indomethacin but not saline | |
| •Part II: Treatment with 15, 85 and 400 mg/kg anthocyanins, indomethacin or saline | Part II: ∘ dose-dependent increase in paw withdrawal latency at 1.5 and 4.5 hrs. with indomethacin and anthocyanins but not saline ∘ paw withdrawal latency at highest anthocyanin dose comparable to indomethacin ∘ dose-dependent increase in paw withdrawal thresholds at 1 hr. with 85 mg or 400 mg anthocyanins and indomethacin but not saline or low dose anthocyanins ∘ smaller CFA-induced paw thickness at 2 hrs. with anthocyanins and indomethacin but not saline ∘ paw thickness reduction continuing into 5 hrs. with 85 mg or 400 mg anthocyanins and indomethacin, and 24 hrs. with 400 mg anthocyanins and indomethacin | |
| He et al. 2006 [15] | Treatment with tart cherry anthocyanins at 10, 20, and 40 mg/kg for 2 weeks and 4 weeks in Sprague Dawley rats | •Dose-dependent increase in total anti-oxidant capacity and SOD activity at all dosesAt 40 mg/kg: •decreased serum TNF-α and malondialdehyde •decreased paw PGE2 •stronger total anti-oxidative capacity |
| Seymour et al. 2008 [11] | 1% of diet by weight of freeze-dried Montmorency tart cherries or placebo for 90 days in Dahl Salt-Sensitive rats with insulin resistance and hyperlipidemia | •Increased plasma antioxidant capacity, hepatic PPAR-α expression, expression and activity of the PPAR-α target ACO •Reduced hepatic lipid accumulation, hyperlipidemia, fasting glucose and hyperinsulinemia, BUN and amylase •No effect on body weight gain or liver function |
| Seymour et al. 2009 [12] | 1% of diet by weight of freeze-dried whole tart cherries or placebo for 90 days in Zucker fatty rat model of obesity and metabolic syndrome | •Reduced hyperlipidemia, fasting glucose, insulin, percentage fat mass, retroperitoneal abdominal fat, plasma IL-6 and TNF-α, and expression of retroperitoneal IL-6, TNF-α and NFKB •Increased retroperitoneal fat PPAR-α mRNA |
| Haidari et al. 2009 [16] | Treatment with tart cherry juice at 5 ml/kg compared to allopurinol at 5 mg/kg and saline control for 2 weeks in normal and hyperuricemic Wistar rats | •Time-dependent reduction in serum uric acid in hyperuricemic rats •Reduction in malondialdehyde in hyperuricemic rats •Inhibited activity of hepatic xanthine oxidase/dehydrogenase in both normal and hyperuricemic rats •Increased serum total antioxidant capacity in both normal and hyperuricemic rats •No reduction in serum uric acid with cherry in normal rats |
| Ducharme et al. 2009 [20] | Crossover study with 1.42 L tart cherry juice blend daily for 2 weeks in female horses | •Decreased serum aspartate aminotransferase (AST) activity |
| •No effect on malondialdehyde or serum amyloid A | ||
| Matchynski et al. 2013 [10] | Combination Montmorency tart cherry extract (426.7μg/mg anthocyanins) with omega-3 (65%), omega-6 (26%) and omega-9 (9%) fatty acids, and zinc and magnesium stearate (18.5μg.mg) daily for 2 weeks prior and 17 days after induction in mu-p75 saporin (SAP)-induced mouse model of Alzheimer’s | •Treatment at 30, 60, and 90 mg/kg decreased SAP-induced cognitive deficits in object-recognition, place-recognition, and Morris water maze tasks •Histology showed treatment at 60 and 90 mg/kg protected against SAP-induced inflammatory response and cholinergic neuron loss around the medial septum •No significant differences seen in rotarod or open field distance traveled, or percent weight change |
| HUMAN STUDIES | ||
| Connolly et al. 2006 [46] | RCT crossover in young males given 12 oz. tart cherry juice blend from Montmorency cherries (CherryPharm) or placebo twice daily for 8 days | •Reduced strength loss and pain compared to placebo •No difference in range of motion loss or muscle tenderness between treatments |
| Traustadottir et al. 2009 [14] | RCT crossover in older adults given 240 mL tart cherry juice blend from Montmorency cherries (CherryPharm) or placebo twice daily for 14 days | •Increased capacity to resist oxidative damage as measured by reduced plasma F2-isoprostane response •Reduced oxidative damage to nucleic acids as measured by basal urinary excretion of oxidized nucleic acids •No change in urinary excretion of isoprostanes |
| Elliot et al. 2010 [19] | RCT crossover in females with fibromyalgia given 10.5 oz. tart cherry juice from Montmorency cherries or placebo twice daily for 10 days | •A subset had improvements in pain VAS >25 mm after eccentric exercise •No change in overall pain scores, muscle pain threshold or muscle pain tolerance |
| Kuehl et al. 2010 [21] | RCT in adult marathon runners given 335 mL tart cherry juice blend from Montmorency cherries or placebo twice daily for 7 days prior to event and at race start and end | •Smaller increase in post-race subjectively reported muscle pain •Higher satisfaction with treatment and pain reduction attributed to treatment |
| Howatson et al. 2010 [48] | Pseudo-randomized controlled trial in adult marathon runners given 8 oz. tart cherry juice blend from Montmorency cherries (CherryPharm) or placebo twice daily for 5 days prior to event, the day of and 2 days after event (8 days total) | •Faster recovery of isometric strength in knee extension •No changes in other indices of muscle damage •Greater total antioxidant status post-race •Reduced IL-6, uric acid, and CRP •TBARS lower in cherry group at 48 hours •No difference in protein carbonyls |
| Bowtell et al. 2011 [32] | RCT crossover in adult male athletes given 30 mL tart cherry juice concentrate from Montmorency cherries (CherryActive) or placebo for 7 days prior to intensive knee extensions, the day of and 2 days after extensions | •Improved recovery of knee extensor maximum isometric muscle strength •Reduced serum protein carbonyls at 24 and 48 hours post-extensions •No significant reduction in muscle soreness, creatine kinase, total antioxidant capacity, or hsCRP |
| Zhang et al. 2012 [18] | Prospective case-crossover study of association of risk factors 2 days prior to recurrent gout attacks compared to controls | •Reduced risk of gout attack with intake of cherries and cherry extract compared to no intake •Decreased risk was dose-dependent up to 3 servings of cherries daily, and stronger when combined with allopurinol use |
| Schlesinger and Schlesinger 2012 [17] | Three pilot studies on the use of a cherry juice concentrate for gout flare prophylaxis •Study I: RCT in gout patients given 1 tablespoon cherry juice concentrate or pomegranate juice concentrate twice daily for 4 months •Study II: retrospective study of flare prophylaxis and association of cherry juice concentrate intake over 4 months or longer •Study III: In vitro effect of cherry juice concentrate compared to pomegranate juice concentrate on IL secretion in human monocytes exposed to urate crystals | Study I: ∘ Reduction in number of gout flares from baseline ∘ No change in serum urate or serum creatinine from baseline Study II: ∘ Reduction in number of gout flares across at least four months ∘ No change in serum urate Study III: ∘ Inhibited IL-1β and TNF-α secretion at dilutions of 1:1600 or higher without toxic effects |
| Kuehl et al. 2012 [8] | RCT in middle aged and elderly females with inflammatory OA given 10.5 oz. tart cherry juice from Montmorency cherries or placebo twice daily for 21 days | •WOMAC scores not reported •Significant reduction in CRP compared to placebo •No significant changes in IL-6, IL-10 and TNF-α |
| Schumacher et al. 2013 [42] | RCT crossover in middle aged adults with knee OA given 8 oz. tart cherry juice blend from Montmorency cherries (CherryPharm) or placebo twice daily for 6 weeks | •Decrease in WOMAC scores from baseline •No difference in change in total WOMAC scores between treatments •Decreased hsCRP, the decline also associated with improved WOMAC scores •Improved WOMAC pain and function scores •Improved WOMAC stiffness scores in those starting with cherry treatment •No difference in walking performance, acetaminophen use, plasma urate or creatinine |
| Lynn et al. 2014 [13] | RCT parallel open-label intervention in healthy adults given 30 mL of tart cherry juice concentrate diluted to 250 mL (Cherry Active) from Montmorency cherries or control drink daily for 6 weeks | •Increased plasma antioxidant capacity measured as ferric reducing ability of plasma (FRAP) •No effect on CRP, arterial stiffness, blood pressure, total cholesterol or HDL cholesterol |
| Bell et al. 2014 [23] | RCT in healthy adult male cyclists given 30 mL tart cherry juice concentrate from Montmorency cherries in 100 mL water or placebo twice daily for 7 days | •Reduced lipid hydroperoxides, IL-6 and hsCRP compared to placebo •No changes in TNF-α, IL-8, IL-1β and creatine kinase |
| Bell et al. 2014 [44] | Single-blind, two-phase, randomized crossover trial in healthy young adults given 30 or 60 mL tart cherry juice concentrate from Montmorency cherries in 100 mL water for 48 hours | •Increased plasma cyanidin-3-O-glucosiderutinoside at 3 hours and 5 hours compared to baseline, with greater uptake at 1 hour in the 60 mL dose compared to the 30 mL dose. •Decreased serum urate at all time points after 2 hours, which peaked at 8 hours •Increased urinary urate excretion, peaking at 2 hours with a second smaller peak at 26 hours •Decreased serum hsCRP for up to 8 hours, with the largest decrease at 5 hours. Return to baseline at 24 hours, with a second decrease at 26 hours lasting until 48 hours. •No differences in lipid-soluble antioxidants α-tocopherol, χ-tocopherol, retinol or lycopene. |
Table 2
Phytonutrients in Sweet and Tart Cherries (in 100 g) (from Ferretti et al., Molecules 2010, 15, 6993-7005 [3])
| Nutrient | Sweet cherries | Tart cherries |
| Vitamin C | 7 mg | 10 mg |
| Niacin | 0.2 mg | 0.4 mg |
| Pantothenic Acid | 0.2 mg | 0.1 mg |
| Vitamin A | 64 IU | 1283 IU |
| Vitamin E | 0.1 mg | 0.1 mg |
| (Alpha Tocopherol) | ||
| Vitamin K | 2.1μg | 2.1μg |
| Beta Carotene | 38μg | 770μg |
| Lutein+Zeaxanthin | 85μg | 85μg |
| Total Phenols | 109.8 mg | 228.9 mg |
Table 3
Total Anthocyanin and Total Phenols in Different Cultivars of Sweet or Sour Cherries; GAE, gallic acid equivalent; CGE, cyanidin-3-glucoside equivalents. (from Ferretti et al., Molecules 2010, 15, 6993-7005 [3] and Kirakosyan et al., Food Chemistry 2009, 115, 20-25 [6])
| Cultivar | Total | Total |
| phenolics | anthocyanins | |
| Sweet Cherries | ||
| Brooks | 60±13a | 10±2b |
| Newstar | 75±14a | 20±5b |
| Black Gold | 92±12a | 30±9b |
| Hedelfingen | 96±20a | 40±7b |
| Regina | 104±6a | 41±2b |
| Hartland | 147±19a | 76±12b |
| Christalina | 155±20a | 79±5b |
| Sour Cherries | ||
| Danube | 162±1a | 65±3b |
| Schatternmorelle | 295±34a | 72±6b |
| Sumadinka | 312±8a | 109±6b |
| Balaton | 254.1±60a | 45±2.3b |
| Balaton (dried, | 6343±776c | 564±65d |
| no added sugar) | ||
| Balaton (dried with sugar) | 3522±512c | 273±33d |
| Balaton (frozen) | 6742±786c | 1741±287d |
| Balaton concentrate | 2541±371c | 722±87d |
| Balaton individually | 7752±932c | 1063±178d |
| quick frozen (IQF) powder | ||
| Montmorency (dried, | 7813±855c | 173±31d |
| no added sugar) | ||
| Montmorency | 5103±455c | 62±5.3d |
| (dried with sugar) | ||
| Montmorency (frozen) | 12665±1321c | 533±47d |
| Montmorency concentrate | 4013±578c | 213±41d |
| Montmorency IQF powder | 10323±1468c | 482±56d |
aexpressed as mg gallic acid equivalent (GAE)/100 g fresh cherries. bexpressed as cyanidin-3-glucoside equivalents (CGE)/100 g fresh cherries. cexpressed as 1 g/g dry weight of GAE. dexpressed as 1 g/g dry weight of CGE.




