The role of zinc in the pathogenesis and treatment of COVID-19: A review
Abstract
Destructive outcomes of coronavirus pandemic call for medical research which can report all of the influential agents not only for the treatment of the disease but also preventing its severe impacts on the societal health in the most efficient manner. Zinc plays an integral role in the function of cellular enzymes and transcription factors. Owing to its anti-inflammatory and cellular immunity regulation activity, zinc is regarded to be effective on strengthening the immune system. Its crucial antiviral effects have long been established as well. Studies suggest that low serum zinc level predisposes the patient to severe COVID-19 infection, which makes patient’s zinc profile a potential determinant of prognosis and severity of this disease. Furthermore, zinc supplementation has indicated promising outcomes of coronavirus infection management. Zinc modulates cell-mediated immunity and participates in the killing of microorganisms in cytotoxic immune cells. Zn2+ has anti-inflammatory effects by inhibiting IL-6 production. Although there is still not enough evidence, it seems that zinc could be a promising supplementary treatment for COVID-19 especially in zinc-deficient patients. The aim of this review is to clarify the role of zinc in pathogenesis and therapy of COVID-19 in detail.
1Introduction
Since March 2020, a pandemic caused by coronavirus (SARS-COV 2) was declared by the World Health Organization. For the first time, COVID-19 was diagnosed in Wuhan, central China, in December 2019 [1, 2]. Up to September 15, 2021, more than 4 millions of deaths has been reported worldwide [3]. The disease is less frequent in children and the symptoms are less severe within this age group [2]. Male gender, age older than 60 years, obesity, pre-existing chronic metabolic diseases including hypertension, diabetes mellitus, chronic obstructive pulmonary disease (COPD), coronary artery disease, cancer, and immunodeficiency are related to the severity of infection [1].
An effective treatment protocol is crucial in tackling with this pandemic [4]. Although there is no known specific treatment yet, some drugs and supplement therapies such as zinc, selenium, vitamin C and vitamin D are highly recommended [5, 6].
Considering the role of zinc in boosting the immune system, its protective role against inflammation and reactive oxygen species (ROS) together with evidences of higher severity of the COVID-19 infections in zinc-deficient pateints, zinc supplementation has been assessed in several clinical trials [7–11]. Due to the synergistic effects of zinc on anti-viral drugs and its inhibitory role on SARS-CoV-2 replication it can be a remarkable candidate for COVID-19 treatment [10].
The aim of this review is to clarify the role of zinc in pathogenesis and therapy of COVID-19 in detail. Given the proven role of zinc in cellular function and immune system [10], hereby, we have prepared an overview of the latest clinical data on the role of zinc in the pathogenesis and treatment of COVID-19.
2Methods
This integrative review was performed by searching PUBMED data base using the following keywords: [“COVID-19” OR “SARS-CoV-2” OR “2019-nCoV”] AND [Zinc] up to October,1st 2021. The search was limited to clinical trials published in English and those conducted in humans and those mentioning zinc role in COVID-19 pathogenesis and treatment. At first, 428 articles met the inclusion criteria. After excluding articles that did not match our target scope based on their full-text content, 91articles entered the study.
Data were categorized according to zinc‘s metabolism, its role on health and immunity and the pathogenesis of zinc in SARS-COV-2 infections or treatment.
3Zinc metabolism
Zinc, the second most aboundant cation of the body, is a necessary trace element that acts as a cofactor in more than 300 enzymes [12]. Zinc can be found in different foods such as fish, meat, egg, nuts and legumes [12]. Absorption of this concentration dependent trace element is determined by the type of diet, the amount of phytic acids [13], iron [14] and proteins [15] of the foods and the zinc status of the body [16, 17]. Therefore it seems that it would be absorbed more efficiently in diets with poor sources of zinc by upregulating its retention [16].
In cell nucleus or other organelles, zinc is distributed by vesicles called zincosomes and in the cytoplasm it binds to metallothioneins. Zinc transporters are membrane transport proteins that regulate the concentration of zinc through influx or efflux of zinc [18]. Internal zinc hemostasis is mostly controlled by two zinc transporter families which are essential for survival [18, 19].
A balance between zinc absorption and intestinal excretion maintains zinc hemostasis [20]. Although absorbed zinc is secreted through biliary tract and intestines, a great amount is reabsorbed. Gastrointstinal tract is responsible for more than half of the eliminated zinc [16]. The excreted zinc is in balance with the dietary intake, the amount of absorption and the physiologic needs [17]. Daily requirement of zinc increases during infancy, childhood, adolescence, pregnancy and lactation [16]. Moreover, elderly people have low level of zinc in their diet [21]. Figure 1 shows the summary of zinc metabolism.
Fig. 1
Zinc metabolism.
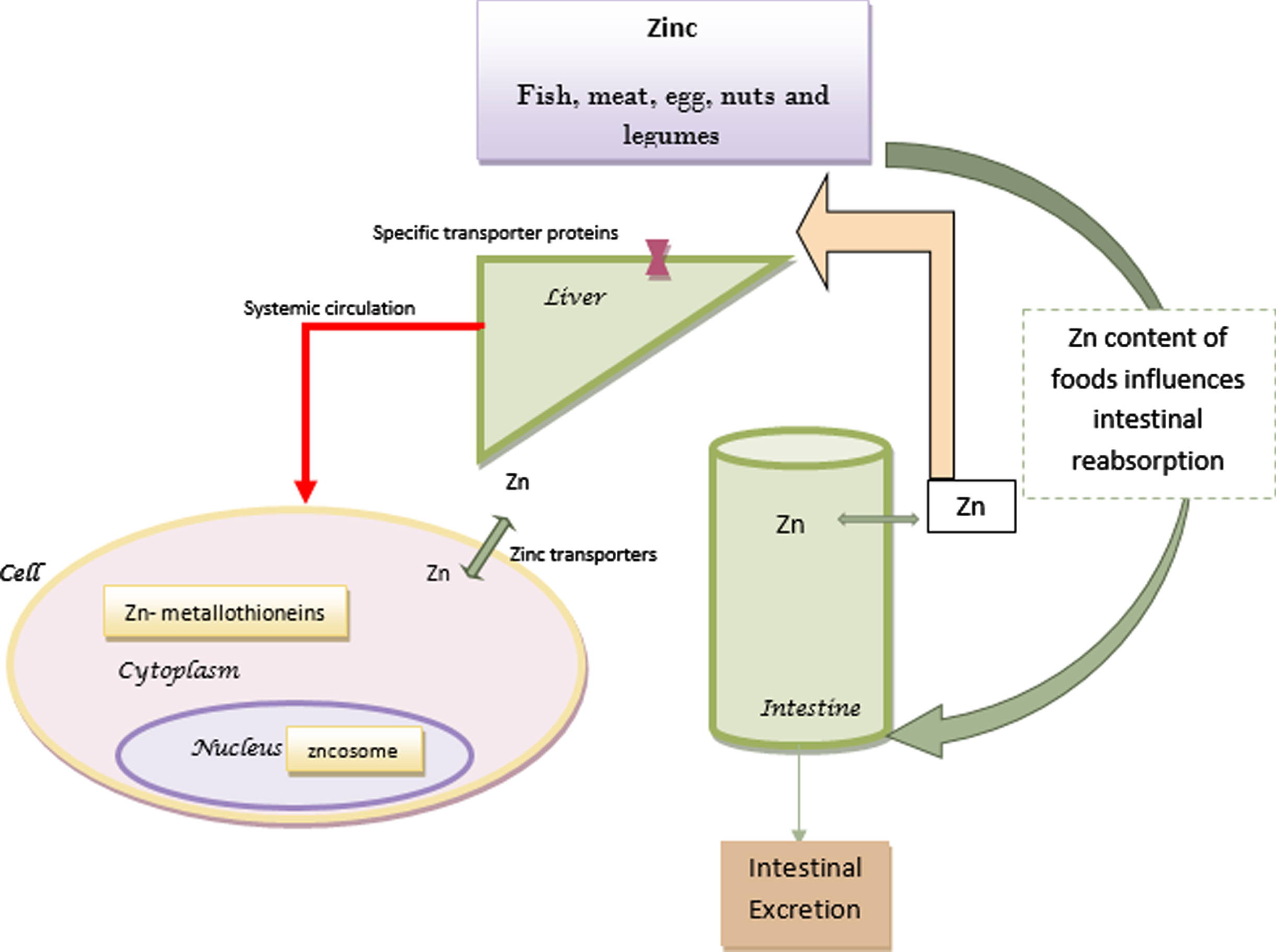
The majority of zinc in the plasma is bound to albumin, alpha-2-macroglobulin (A2M), and transferrin, however, there is a very small amount of free zinc in the plasma that is still very important for its homeostasis [18, 22]. Insufficient zinc in the diet, increased requirments, impaired absorption and abnormal losses may lead to zinc deficiency [16].
4Zinc and health
Zinc maintains cell membrane integrity; is important in cellular proliferation, DNA and RNA replication, gene expression and regulates the function of T, B and natural killer cells [23].
Skin, gastrointestinal tract, central nervous system, immunologic system, bones and genitourinary tract may be affected by zinc deficiency [16]. The role of zinc in human health have been clarified since the detection of a patient with anemia, hypogonadism and dwarfism in Iran in 1961 [24]. The relationship of zinc deficiency and febrile seizure is well considered in different studies [25, 26]. Acrodermatitis enteropathica is a well-known disease caused by zinc deficiency [16]. Many studies pointed out the effect of zinc deficit on impaired growth and development [27]. Moreover zinc may have a protective effect on inflammation and atherosclerosis [28].
In the elderly it is suggested that reduced immunocompetence, wound healing impairments (non-healing skin ulcers) and neurological problems are partly due to zinc deficiency. Psychological changes such as impairment of memory, mentality, cognitive functions and behavioral problems may be caused by zinc deficiency [16]. Furthermore, growth retardation and alopecia can be seen in zinc-deficient children. Given that malnutrition and infections are among the most common causes of mortality and morbidity in childhood, zinc supplementation may have preventive effects on childhood death [29]. Adminstration of this trace element can protect children against childhood obesity and metabolic syndrome [30, 31].
The consequenses of zinc deficiency in infections has been extensively studied. It is reasonable to control serum zinc level during infections, due to transient hypozincemia during infection and inflammation [13, 17]. It is hypothesized that in patients with prior hypozincemia, decreased transient zinc level during infections may cause hyperinflammation [18]. Due to poor hepatic reserve of functional zinc, during increased zinc demands like infections, the required amount could not be delivered to plasma [12]. The relation between diarrhea and zinc deficiency can be explained by the alteration of zinc transport across intestinal mucosal cell and the negative impact of zinc deficiency on immune system [16]. Zinc supplementation in children has also significantly decreased the frequency of pneumonia in developing countries [16] and malaria episodes in endemic areas [32].
5Zinc and immunity
The function and folding of cellular enzymes and transcription factors depend highly on zinc cations. Zinc is also a co-factor of viral proteins. It is also noteworthy that zinc affects viral proteases and interferes with viral polyprotein folding [33, 34].
In the human body, the first defensive mechanisms against infectious agents are ciliary mucous membrane and anti-microbial peptides [35]. By controling tight junction proteins, zinc supports mucous membrane coherence [12] and protects cells with blockage of virus entry [33]. Moreover, it increases ciliary beat frequency [36] and improves ciliary length of bronchial epithelium [37].
In viral infections, zinc can interfere with membrane fusion, protein translation and stabilization of viral envelope, the function of viral polymerase, liberation of viral particles and uncoating [35, 38].The inhibitory action of zinc on viral replication has been illustrated on rhinoviruses, HCV, and influenza virus. In Nidoviruses, of which SARS COV- 2 is a member, zinc can decrease RNA synthesis [33]
Zinc has favourable impacts on adaptive and innate immune cells and enhances their growth, development, and function [39, 40] such as: development of Th17, Th9, and regulatory T cells, production of IFN γ, complement system activity, cytokine production and release, cytotoxic function of NK cells, proliferation and differentiation of T cells [41, 42], and IgG production [42, 43].
Zinc may restrict infection by the production of IFN-γ. The production and antiviral effects of IFN-α (human interferon alpha) are also influenced by this trace element [18, 44]. Zinc downregulates IL-1b and TNF-alpha expression and inhibits Nuclear Factor kappa-light-chain-enhancer of activated B cells activity (NF-kB) and therefore decreases inflammatory cytokines that are important in severe lung inflammation [16]. IL-6 is a key factor in cytokine storm and higher IL-6 responses are seen in hypozincemia [18].The zinc deficiency in obese and elderly patients may increase IL-6 levels. In addition, gene expression of IL-1 alpha, IL-1 beta, and IL-6 are increased in obese patients with reduced zinc levels [18].
Zinc is responsible for the secretion of pro-inflammatory cytokines and it suppresses inflammation by inhibition of leukocyte function-associated antigen-1 binding to ICAM-1 [45].
This trace element can inhibit the transfer of proteins across capillaries and reduces edema and inflammation [46]. Moreover, zinc is essential in the IL-2-dependent proliferation of T cells [47] and increases cell’s resistance to apoptosis [48] which can result in the increase of T helper cells [46]. It also protects against the adverse effects of Reactive Oxygen Species (ROS), that has a main function in the progression of inflammation [10].
It was proposed that the excess level of intracellular zinc causes metal ion poisoning of pathogens in the innate immune cells. The excess zinc results in mismetallation of essential proteins and impairment of other metal trace elements of microorganisms. It seems that zinc can also influence nitric oxide production in the innate immune cells and therefore help the free radicals to attack intracellular pathogens. The resistance of these pathogens depends on their detoxification systems [49].
Alternatively, zinc deficit is accompanied by thymic atrophy, decreased number and impaired function of T cells and changes in the production of thymic hormones. It is supposed that thymulin which is important in the function of peripheral T cells and differentiation of immature T cells in the thymus is decreased in zinc deficiency. Therefore an increased risk of infection can be seen with zinc deficiency because of altered cellular- and antibody-mediated immune responses [33]. Furthermore, zinc deficiency is responsible for impaired phagocytosis and neutrophil function, lymphopenia, decreased antibody and IFNγ production which may increase the risk of infection [12].
Adequate immunity needs balanced zinc levels because elevated zinc levels cause inhibition of T and B cell function, impairment of a destructive function of macrophages, and overload of regulatory T–cells, that may harm the immune function as well [12].
5.1The pathogenesis of zinc in SARS-COV-2 infections
SARS-COV-2 the newly introduced member of Coronaviridae family, is approximately 50–200 nm in diameter. It has a single stranded nonsegmented positive-sense RNA covered with neucleocapsid, membrane proteins and spike proteins [50]. The first infected cells with this virus are nasal and bronchial epithelial cells and pneumocytes [51]. SARS-COV-2 attaches to angiotensin-converting enzyme 2 (ACE2) receptors via spike glycoprotein (S protein), and then enters these cells [10]. ACE2 is a zinc metalloenzyme [52] and its expression is influenced by this trace element. Therefore, any impairment of gene expression in pneumocytes influenced by zinc can modify viral entry [35]. After entry, the virus is transported by endosomes in the cytoplasm, subsequent to the low pH of the endosome, viral envelope fuses with endosomal membrane, releasing the positive-strand viral RNA (+RNA) [10].
SARS-COV-2 poly proteins (pp1a and pp1ab polyproteins) are translated by using the 5′-proximal open reading frame (ORF1a and ORF1b) which at last result in the formation of sixteen nonstructural proteins [10]. These nonstructural proteins bind to the replicase–transcriptase complex and undergo cloning and transcription and participate in the formation of viral membrane structures [10, 53]. RNA-dependent RNA polymerase (RdRp) domain which is in nonstructural protein 12, together with the two encoded cysteine proteases, main protease (Mpro) and papain-like protease (PLpro), are targets for SARS-COV-2 future therapy. For instance, Disulfiram can have an inhibitory effect on PLpro and Mpro. This is caused by releasing zinc from these enzymes and destabilizing them concurrent with an increase in cytoplasmic zinc levels [53]. Some studies showed that high levels of cytoplasmic zinc could inhibit RdRp action and replicase polyproteins [54]. RdRp is united with a viral enzyme complex leading to the formation of a negative RNA chain which will be a template for viral mRNA synthesis [10]. The similarity of more than 95%RdRp with SARS-COV-2 protease together with the inhibitory effect of zinc on RdRp, can lead to the idea that zinc can have antiviral effects on this virus [33].
In vitro studies have shown the effectiveness of zinc on rhinovirus, RSV, and SARS-COV-2. They have demonstrated that Zn2+ has inhibitory effect on SARS-COV replication in cell culture [54]. Zinc can inhibit papain-like protein 2 that is crucial for SARS-COV virulence [55].
During the first phase of COVID-19 disease, the release of cytokines and chemokines are delayed. However, at the later phase of the disease, the level of interleukins (IL) IL-1β, IL-6, tumor necrosis factor (TNF), chemokines, and interferon are increased and is called as “cytokine storm” [56–58]. The inhibition of NF-kB (nuclear factor kappa light chain enhancer of activated B cells) by zinc may have anti-inflammatory effects and limit the cytokine storm [59, 60].
Lung damage in COVID-19 is related to increased IL-6; IL-6 reduces zinc bioavailability by inducing expression of zinc bounded proteins like metallothioneins (MT) and alpha-2-macroglobulin (A2M). Zinc reduces IL-6-mediated activation of the Signal transducer and activator of transcription 3 (STAT-3) thus has anti-inflammatory action [18].
5.2The role of zinc in inhancing drugs’ effects
The synergistic action of zinc with some antiviral agents in treatment of viruses like hepatitis C, HIV, SARS-CoV-1 has been demonstrated [33].
Inhibitors of Mpro may act against many types of coronaviruses but drugs for PLpro or RdRp may act against SARS-CoV-2 and SARS-CoV [33, 61]. Ionophores are molecules that facilitate ion transport across cell membrane and their action depends on the pH changes. Some drugs act as zinc ionophores like Disulfiram and Chloroquine [53]. Chloroquine disrupts lysosomal acidification and therefore has cytotoxic effects. Chloroquine can also increase zinc absorption and when combined together, its efficacy and cytotoxic effects are increased [10]. Same mechanism is explained for quercetin and epigallocatechin-gallate but with lower cytotoxic effects [62]. Other drug targets in viral infections are zinc fingers. Zinc fingers are protein structural motif in a great number of viral proteins, which contain cysteine, histidine, and attached zinc [63]. The three-dimensional structure of these proteins and their foldings are maintained by the presence of zinc [53]. “Labile zinc fingers” can release Zn2+ which will make the protein unfolded and increase cellular zinc level. Agents that are capable of removing zinc from zinc fingers in viral metalloenzymes are under investigation. They can destabilize viral proteins and increase intracellular zinc [53]. If zinc is administered with an ionophore, the increase of intracellular zinc will be remarkable which can inhibit viral RdRp and therefore viral mRNA synthesis. In addition to the decrease of protein synthesis, an increase of intracellular zinc can lead to apoptosis [53].
5.3Zinc and COVID-19
Surprisingly, diseases that are considered to be the risk factors of COVID-19 such as chronic obstructive pulmonary disease (COPD), obesity, diabetes mellitus, malignancy, coronary artery disease, cirrhosis, and suppression of the immune system are also accompanied with a low serum Zn2+ level [35].
Moreover, due to the reduced sense of smell and taste in zinc deficiency, these symptoms which are seen in COVID-19 infection as well, can be attributed to transient zinc deficiency during infection [18].
Also, elderly are at increased risks of infections, COVID-19, cancer, and autoimmune diseases. Aging leads to immunosenescence (age-related changes of the immune system). These changes may be contributed to zinc deficiency that may be a result of low intake, decreased absorption, and/or drug interactions with zinc [64].
Findings showed that the efficacy of some drugs used in the treatment of COVID-19 such as ribavirin, remdesivir, lopinavir/ritonavir, azithromycin and doxycycline is improved with zinc supplementation [10].
Despite numerous scientific data about the effect of Zn2+ on infection in the literature, very few clinical trials have been performed about the relation of zinc and COVID-19. As of October 1st 2021, 67 studies were found after searching with the keywords “zinc” and “COVID-19” at the clinical trial website https://clinicaltrials.gov/ct2/home. Among 55 clinical trials, studies with no intervention of Zn2+ supplementation to the patient were excluded. From 24 remained study, 14 used a combination of zinc with different drugs, vitamins or trace elements. Only 10 studies invested on zinc alone in the experimental arm from which 4 were completed but none of them had reported their results. Data are summarized in Table 1. Other studies with reported zinc effects on COVID-19 are summarized in Table 2.
Table 1
Clinical trials with zinc+other drugs/dietary supplements for COVID-19
| row | Clinical trial study title | ClinicalTrial.Org identifier | Type of intervention | Zinc dose and duration | Drugs/interventions | Number of patients/Age/Sex |
| 1 | Vitamin D and Zinc Supplementation for Improving Treatment Outcomes Among COVID-19 Patients in India | NCT04641195 | 2×2 factorial randomized double-blind, placebo-controlled trial | zinc gluconate one daily dose of 40 mg for 8 weeks | •Dietary Supplement: Vitamin D3 (cholecalciferol) | 700/18 Years and older/all sex |
| •Dietary Supplement: Zinc (zinc gluconate) | ||||||
| •Dietary Supplement: Zinc (zinc gluconate) &Vitamin D (cholecalciferol) | ||||||
| •placebo | ||||||
| 2 | Placebo Controlled Trial to Evaluate Zinc for the Treatment of COVID-19 in the Outpatient Setting | NCT04621461 | Single Group Assignment/randomized, double-blind, placebo-controlled trial | Zinc Sulfate 220 MG | •Drug: Placebo | 3/30 Years and older/all sex |
| 220 mg once daily for 5 days | •Dietary Supplement: Zinc Sulfate | |||||
| 3 | An Outpatient Study Investigating Non-prescription Treatments for COVID-19 | NCT04621149 | Factorial Assignment/randomized, blinded placebo-controlled trail | 1 liter of filtered water with zinc acetate for 7 days. | •Other: chlorine dioxide | 120/20 Years to 70 Years/all sex |
| •Dietary Supplement: zinc acetate | ||||||
| •Drug: Famotidine | ||||||
| •Other: placebo | ||||||
| •Dietary Supplement: lactoferrin, green tea extract | ||||||
| 4 | Can SARS-CoV-2 Viral Load and COVID-19 Disease Severity be Reduced by Resveratrolassisted Zinc Therapy | NCT04542993 | Single Group Assignment/randomized, blinded placebo-controlled trail | Zinc Picolinate (50 mg PO TID×5 days) | •Dietary Supplement: Zinc Picolinate | 60/18 Years to 75 Years/all sex |
| •Dietary Supplement: Resveratrol | ||||||
| •Dietary Supplement: Zinc Picolinate Placebo | ||||||
| •Dietary Supplement: Resveratrol Placebo | ||||||
| 5 | Trial of Combination Therapy to Treat COVID-19 Infection | NCT0448268 | Parallel Assignment/Double-Blind Randomized Placebo-Controlled Trial | Zinc sulphate for 10 days. (Dose not mentioned) | •Drug: Ivermectin | 30/18 Years to 75 Years/all sex |
| •Drug: Doxycycline Hcl | ||||||
| •Dietary Supplement: Zinc | ||||||
| •Dietary Supplement: Vitamin D3 | ||||||
| •Dietary Supplement: Vitamin C | ||||||
| 6 | Sub-cutaneous Ivermectin in Combination With and Without Oral Zinc: a Placebo Randomized Control Trial on Mild to Moderate COVID-19 Patients | NCT04472585 | parallel Assignment/randomized, controlled, multi-armed, investigator Initiated interventional study | Zinc Sulphate 20mg 3 times a day | •Drug: Ivermectin Injectable Solution | 180/18 Years to 60/all sex |
| •Other: Injectable Placebo | ||||||
| •Drug: Zinc | ||||||
| •Drug: Placebo empty capsule | ||||||
| •Drug: Oral Ivermectin | ||||||
| 7 | The Study of Quadruple Therapy Zinc, Quercetin, Bromelain and Vitamin C on the Clinical Outcomes of Patients Infected With COVID-19 | NCT04468139 | Single Group Assignment/Open labeled Clinical Trial | zinc 50 mg orally daily for 5–10 days | •Drug: Quercetin | 60/18 Years and older/all sex |
| •Dietary Supplement: bromelain | ||||||
| •Drug: Zinc | ||||||
| •Drug: Vitamin C | ||||||
| 8 | Zinc With Chloroquine/Hydroxychloroquine in Treatment of COVID-19 | NCT04447534 | Parallel Assignment/Double-Blind Randomized clinical trail | Zinc tablets (Dose not mentioned) | •Drug: Chloroquine | 200/18 Years and older/all sex |
| •Drug: zinc | ||||||
| 9 | A Study of Hydroxychloroquine and Zinc in the Prevention of COVID-19 Infection in Military Healthcare Workers (COVID-Milit) | NCT04377646 | Parallel Assignment multicenter randomized controlled double blind clinical trial | Zinc 15 mg per day up to 2 months | •Drug: Hydroxychloroquine | 660/18 Years to 65 Years/all sex |
| •Drug: Hydroxychloroquine (placebo) | ||||||
| •Drug: Zinc | ||||||
| •Drug: Zinc (Placebo) | ||||||
| 10 | Coronavirus 2019 (COVID-19)-Using Ascorbic Acid and Zinc Supplementation | NCT04342728 | Single Group Assignment | 50 mg of zinc gluconate to be taken daily at bedtime | •Dietary Supplement: Ascorbic Acid | 214/18 Years and older/all sex |
| Open labeled randomized clinical trail | •Dietary Supplement: Zinc Gluconate | |||||
| •Dietary Supplement: Ascorbic Acid and Zinc Gluconate | ||||||
| •Other: Standard of Care |
Table 2
Studies with reported zinc effects on covid-19 patients
| row | Author | no. of patients/age/sex | Diagnosis/inclusion criteria | regimen | result |
| Case reports | |||||
| 1 | Finzi et al. [69] | 63-year-old man | COVID-19 | Day 1 : 69 mg Zinc citrate lozenges | Remarkable enhancement of disease after a day of medication. |
| Day2: 207 mg Zinc citrate lozenges | |||||
| Day 3–8 : 184 mg Zinc citrate lozenges | On day 3 he became afebrile. His symptoms continued to improve over the next 10 days | ||||
| 2 | 57-year-old female | COVID-19 | Day 1 : 23–46 mg zinc lozenges daily | Improvement in cough and shortness of breath after a total of 161 mg zinc consumption. | |
| Day 10 : 23 mg lozenge every hour for 5 hours | |||||
| Day 11 : 46 mg zinc lozenges daily | |||||
| Day 12–22 : 115 mg daily | |||||
| 3 | 41-year-old female | COVID-19 | day 9–19 : 138 mg zinc daily | Improvement in PaO2 and fever one day after zinc was initiated | |
| 4 | 26 year-old female | COVID-19 | Week 3 : 150 mg zinc daily for 14 days. | Improvement in cough and body aches and fatigue one day after zinc was initiated | |
| Full recovery 2 weeks later | |||||
| 5 | Sattar et al. [77] | 38-year-old man | COVID-19 | zinc sulfate 220 mg q.d.for 5 days | Improvement 5 days after zinc was initiated |
| 6 | 55-year-old man | COVID-19 | zinc sulfate 220 mg q.d.for 5 days | Improvement 2 days after zinc was initiated | |
| 7 | 74-year-old man | COVID-19 | zinc sulfate 220 mg q.d.for 5 days | Improvement 5 days after zinc was initiated | |
| 8 | Bahloul et al. [78] | 57-year-old woman | pulmonary capillary leak syndrome following COVID-19 | Steroids (dexamethasone 20 mg/day), vitamin C (3 g/day), Hydroxychloroquine(600 mg/day), Azithromycine, Zinc (dose not mentioned), diuretics, Enoxaparine | discharged from ICU within 14 days after medication |
| 9 | 58-year-old man | pulmonary capillary leak syndrome following COVID-19 | Steroids (dexamethasone 20 mg/day), vitamin C (3 g/day), Hydroxychloroquine(600 mg/day), Azithromycine, Zinc (dose not mentioned), diuretics, Enoxaparine | discharged from ICU within 8 days after medication | |
| 10 | Farooqi et al. [79] | 48-year-old male | COVID-19 | Tocilizumab + hydroxychloroquine + azithromycin + zinc (dose not mentioned) | Significant enhancement in both respiratory and overall sate |
| Discharged after 17 days. | |||||
| 11 | Ostojic et al. [80] | three men and two women, age 39.6 + _ 6.9 years | COVID-19 | potassium nitrate (1200 mg), magnesium (200 mg), zinc (50 mg), and citric acid(1000 mg) every 4 hours during 48-hour | Oxygen saturation enhanced 1–7%instantly after treatment and remained above starting points during the 48-hour observation period. No side effects was noticed |
| Retrospective Observational Studies | |||||
| 12 | Capone et al. [72] | 102 patients/average age of 63.2 years/53.9%were male | COVID-19 | Hydroxychloroquine (400 mg Q12 for three doses) + azithromycin (daily/500 mg QD) (G1) in 94.1%patients | Treatment with zinc and vitamin C had no benefits on overall survival in these patients. |
| vitamin C + zinc (dose not mentioned) (G2) in 71.6%of the patients | Treatment with had no benefits on hydroxychloroquine and azithromycin overall survival. | ||||
| Vasopressor support in 82.4%of the patients intravenous immunoglobin in 7.8%of the patients | Treatment with intravenous immunoglobin and treatment anticoagulation showed survival advantages. | ||||
| anticoagulation in virtually 40%of the patients | |||||
| 13 | Carlucci et al. [70] | 932 patients/average age of 62.51 years/74.3%female | COVID-19 | zinc sulphate(220mg capsule containing 50 mg elemental zinc twice daily for 5days) + hydroxychloroquine (400 mg load followed by 200 mg twice daily for 5days) + azithromycin (500 mg once daily) (G1) vs. hydroxychloroquine +azithromycin (G2) | Patients treated with G1 and G2 had no difference in hospitalization period, ventilation length or ICU length. G1 group included more frequent discharged patients. G1 group experienced less ICU admission, ventilation, hospice care than G2 group. After modifying the time of zinc sulfate administration in G1 group, there was still remarkable rise in discharged patients frequency and decrease in mortality and hospice care in comparison to G2. |
| 14 | Finzi et al. [81] | 28 patients/mean age 40/60.71%female | COVID-19 | Zinc gluconate/citrate lozenges (23 mg of elemental zinc, 21 patients) or zinc acetate lozenges (15 mg of elemental zinc, seven patients), at a total dosage of 2–2.5 mg/kg/day for 10 days. | Improvement occurred in all patients after 7 days of treatment. Enhancement in symptoms started on average 1.6 days after zinc supplementation. Due to treatment, 4 patients experienced nausea and 2 patients experienced vomiting. Patients demonstrated better tolerance to zinc gluconate than zinc acetate. |
| 15 | Derwand et al. [82] | 141 patients/average age of 58 years/73%male | COVID-19 | Zinc (dose not mentioned) + low-dose hydroxychloroquine + low-dose azithromycin (G1) vs. control (no treatments) (G2) | Percentage of hospitalized patients in G1 : 2.8% |
| Percentage of hospitalized patients in G2 : 15.4% | |||||
| Mortality in G1 : 0.7% | |||||
| Mortality in G2 : 3.4% | |||||
| Fewer hospitalizations was noticeable in G1 group. | |||||
| 16 | Yao et al. [5] | 242 patients/average age of 68 years (G1 : 65)/83%female | COVID-19 | zinc sulfate (a total daily dose of 440 mg (100 mg elemental zinc)) (G1) vs. control (G2) | Percentage of patients with moderate symptoms in G1 : 20.4% |
| patients with severe symptoms in G1 : 54.1% | |||||
| patients with critical state in G1 : 25.5% | |||||
| patients with moderate symptoms in G2 : 30.4% | |||||
| patients with severe symptoms in G2 : 45.7% | |||||
| patients with critical state in G2 : 23.9% | |||||
| Zinc sulfate was not related to decrease in mortality in hospital. Zinc did not significantly cause any survival advantage in this study. | |||||
| 17 | Atakla et al. [83] | 36 patients/average age of 9.66±1.32 years/44.44%female | COVID-19 | Azithromycin (15 mg/kg) + zinc (dose not mentioned) in all patients | All children eventually recovered from COVID-19. |
| 94.44%of the patients received chloroquine sulfate (5 mg/kg) 5.55%of the patients who had HIV received antiviral medication | |||||
| Randomized Controlled Trials | |||||
| 18 | Patel et al. [8] | 33 patients/average age of 61.08 years/63.63%male | COVID-19 (hospitalized, oxygen saturationof 94%or less) | Large dose of intravenous zinc (elemental zinc concentration 0.24 mg/kg/day for a maximum of 7 days) vs. control | The study did not attain its target participation. No serious treatment-related adverse events. |
| 19 | Abd-Elsalam et al. [11] | 191 patients/average age of 43.56 years/60.73%male | COVID-19 | Hydroxychloroquine(400 mg twice daily on the first day, then 200 mg twice daily for 5 days) (G1) vs. hydroxychloroquine+zinc (zinc sulfate 220 mg (50 mg of elemental zinc) twice daily)(G2) | Adding zinc to hydroxychloroquine did not cause enhanced efficacy regarding recovery, ventilation demand, and mortality. |
| 20 | Abdelmaksoud et al. [9] | 134 patients/average age of 52.05 years/41.8 %female | COVID-19 (patients with anosmia and/or hyposmia) | Zinc (220 mg zinc sulfate equivocal to 50 mg elemental zinc twice daily) vs. control (no zinc) | The smell/taste abilities in the patients who received zinc recovered remarkably faster in the control (p < 0.001). There was no significant difference between zinc-receiving patients and control in the length of overall COVID-19 recovery. The zinc state of patients did not indicate an important role in causing hyposmia and/or anosmia or COVID-19 intesity. |
| 21 | Thomas et al. [7] | 214 patients/average age of 45.2years/61.7%female | COVID-19 (received outpatient care) | High-dose zinc gluconate (50 mg of zinc gluconate at bedtime) (G1) | Days required for 50%decrease in symptoms in G1 : 5.9 |
| High-dose Ascorbic Acid (8000 mg of ascorbic acid to be divided over 2–3 times per day with meals) (G2) | Days required for 50%decrease in symptoms in G2 : 5.5 | ||||
| Large dose of Zinc gluconate and large dose of Ascorbic Acid (G3) | Days required for 50%decrease in symptoms in G3 : 5.5 | ||||
| Usual care with no supplements (G4) | Days required for 50%decrease in symptoms in G4 : 6.7 | ||||
| The study was discontinued owing to no remarkable difference between treatment groups’ outcomes in terms of the duration of reduction of 50%of symptoms. There was no significant difference concerning length of total resolution of symptoms. | |||||
| Non Randomized Controlled Trial | |||||
| 22 | Elalfy et al. [84] | 113 patients/average age of 37.7 years/53.98%female | COVID-19 (mild and early moderate cases) | Nitazoxanide (500 mg rapid release formula/6 h) + ribavirin(1200 mg (400 mg divided doses)) + ivermectin (up to 90 Kg (200–300 μg/kg), 90–120 kg (300–400 μg/kg), more than 120 kg (30 mg fixed dose)) + Zinc (30 mg twice daily) (G1) vs. symptomatic treatment (G2) | 7th day viral clearance percentage of nasopharynx in G1 : 58.1% |
| 7th day viral clearance percentage of nasopharynx in G2 : 0% | |||||
| 15th day viral clearance percentage of nasopharynx in G1 : 73.1% | |||||
| 15th day viral clearance percentage of nasopharynx in G2 : 13.7% | |||||
| 15th day cumulative clearance percentage in G1 : 88.7% | |||||
| 15th day cumulative clearance percentage in G2 : 13.7% | |||||
| G1 treatment took a shorter period of time to clear COVID-19 from nasopharynx of the patients than G2. | |||||
It has been seen that Zinc addition may decrease hospital stay and accelerate recovery in children with Lower respiratory tract infection [65]. Another double blind trial has demonstrated a faster recovery of pneumonia in children with zinc supplementation. Moreover, the study mentioned reduced antibiotic resistance with zinc administration [66].
In a recent study in Japan on 62 patients with COVID-19, the severity of the disease was significantly related to low serum zinc levels. According to this study, prolonged hypozincemia may increase the severity of COVID-19 [67].
In another study on 47 patients with COVID-19, the fasting zinc levels were significantly reduced compared to controls. In zinc deficient patients, the chance of experiencing complications, hospital stay and mortality were much higher (with the Odds Ratio of 5.54) [68]. Furthermore, in a study by Finzi et al., a 24 hours supplementation of high dose zinc (115–207 mg daily) showed significant improvement of symptoms in all 4 studied patients [69].
A retrospective study by Carlucci et al. in 2020 showed that addition of 50 mg zinc sulfate twice daily for 5 days to hydroxychloroquine can decrease the mortality rate in patients who were not treated in ICU [70]. As explained previously, there are reports mentioning the role of chloroquine as an ionophore for Zn. Therefore the amount of zinc absorption by the lysosome is intensified. As a result, the cytotoxicity of chloroquine is boosted when combined with zinc supplements [10].
Although most of studies supporting zinc supplementation in COVID treatment, there were several studies showing contradictory results.
A result of a recent published clinical trial in Egypt that conducted on 191 COVID-19 patients treated with hydroxychloroquine or chloroquine with or without zinc sulfate supplementation, did not show any significant difference in the mortality, the necessity of mechanical ventilation and clinical improvement between cases and controls [11].
A retrospective study by Yao et al. did not find a significant association in survival of hospitalized patients with COVID-19 who were treated with zinc sulfate compared to control group [5].
In the study performed by Natarajan et.al, the viral load reduction was compared between two groups. The first group used a mixture of 15 herbal drugs, known to have anti-pyretic, anti-inflammatory, and anti-bacterial effects. The second group were given daily oral tablets of zinc (100 mg) and vitamin C (60,000 IU).The results showed significant decrease in the CT value of RT-PCR in both groups, but the mean difference in the CT value was more notable in the herbal group [71].
In the retrospective chart review of 102 critically ill COVID-19 positive patients performed by Capone et al, patients treated with vitamin C plus zinc had no overall survival impact in comparison to the intravenous immunoglobulin (IVIG) and anticoagulation group [72]. This may be caused by the patients’ status when invested since zinc is more beneficial in mild illnesses.
Despite various studies mentioned about zinc supplementations in the treatment of COVID-19 patients, there are still insufficient data explaining the exact mechanism of its effects.
It is important to mention that high dose zinc usage for a long period can damage lymphocyte and neutrophil functions and disturb the immune system responses [73]. The adverse effects of high doses of zinc consumption are anemia, genitourinary tract complications, and consequences of copper deficiency [74, 75]. However, to support immune system, it seems that it is better for cancer patients to receive zinc supplement for up to three months [75].
It should be considered that gastrointestinal absorption, diet, abnormal excretion and drug interactions can influence the effect of zinc. As explained by Das RR, the benefits of zinc supplements take around 100 hours to be notable. Thus in acute scenarios there is not enough time for an adequate response [76]. This may explain the different responses seen in patients.
The present study has some limitations. First, the reviewed articles only obtained from PUBMED database. Second, most of related clinical trials has not been published yet; so, certain effect of zinc therapy on COVID-19 treatment cannot be determined.
6Conclusion
In conclusion, findings of the most reviewed articles approved the effect of zinc supplementation on reducing the chance of complication, length of hospital stay and mortality rate of COVID-19. Although there is still not enough evidence, it seems that zinc is beneficial as a supplementory treatment for COVID-19 especially in zinc deficient patients. Zinc inhibts viral replication and excess cytokyine relase, thereby protects against lung damage. It can also inhance anti-viral drug effects causing shorter duration of disease specially in milder cases. Considering that gastrointestinal absorption, diet, abnormal excretion and drug interactions can influence the effect of zinc, for future trials, we recommend to match these factors in the study groups. We also recommend performing cohort studies in order to determine preventive role of zinc in COVID-19.
Acknowledgments
The authors have no acknowledgments.
Author’s contribution
All authors contributed equally to this project.
Conflict of interests
There is no conflict of interests.
Funding/support
This study did not receive any funds or financial support.
References
[1] | Khoshnevisasl P , Sadeghzadeh M , Sadeghzadeh S . A Review of COVID-19 in Children. Journal of Comprehensive Pediatrics. (2020) ;11: (3). |
[2] | Aghdam MK , Sadeghzadeh M , Sadeghzadeh S , Namakin K . Challenges in a child with asthma and COVID-19. New Microbes New Infections. (2020) ;37: :100740. |
[3] | WHO COVID-19 Dashboard. Geneva: World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2021. |
[4] | Rahmanzade R , Rahmanzadeh R , Hashemian SM , Tabarsi P . Iran’s Approach to COVID-19: Evolving Treatment Protocols and Ongoing Clinical Trials. Front Public Health. (2020) ;8: :551889. |
[5] | Yao JS , Paguio JA , Dee EC , Tan HC , Moulick A , Milazzo C , et al. The Minimal Effect of Zinc on the Survival of Hospitalized Patients With COVID-19: An Observational Study. Chest. (2021) ;159: (1):108–11. |
[6] | Cheng RZ . Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? Medicine in Drug Discovery. (2020) ;5: :100028. |
[7] | Thomas S , Patel D , Bittel B , Wolski K , Wang Q , Kumar A , et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA network open. (2103) ;4: (2):e210369–e. |
[8] | Patel O , Chinni V , El-Khoury J , Perera M , Neto AS , McDonald C , et al. A pilot double-blind safety and feasibility randomizedcontrolled trial of high-dose intravenous zinc in hospitalizedCOVID-19 patients. J Med Virol. (2021) ;93: (5):3261–7. |
[9] | Abdelmaksoud AA , Ghweil AA , Hassan MH , Rashad A , Khodeary A , Aref ZF , et al. Olfactory Disturbances as Presenting Manifestation Among Egyptian Patients with COVID-19: Possible Role of Zinc. Biol Trace Elem Res. (2021) ;19: :1–8. |
[10] | Rahman MT , Idid SZ . Can Zn Be a Critical Element in COVID-19 Treatment? Biol Trace Elem Res. (2021) ;199: (2):550–8. |
[11] | Abd-Elsalam S , Soliman S , Esmail ES , Khalaf M , Mostafa EF , Medhat MA , et al. Do Zinc Supplements Enhance the Clinical Efficacy of Hydroxychloroquine?: a Randomized, Multicenter Trial. Biol Trace Elem Res. (2021) ;199: (10):3642–6. |
[12] | Mossink JJBN . Prevention, Health. Zinc as nutritional intervention and prevention measure for COVID–19 disease. BMJ Nutrition, Prevention Health. (2020) ;3: (1):111–7. |
[13] | Hambidge KM , Miller LV , Krebs NFJIJfV . Research N. Physiological requirements for zinc. Physiological requirements for zinc. International Journal for Vitamin. (2011) ;81: (1):72–8. |
[14] | Solomons NW , Jacob RJTAJoCN . Studies on the bioavailability of zinc in humans: effects of heme and nonheme iron on the absorption of zinc. The American Journal of Clinical Nutrition. (1981) ;34: (4):475–82. |
[15] | McDowell LR . Minerals in animal and human nutrition. 2nd edition Amsterdam: Elsevier Science BV; (2003) .p. 660. |
[16] | Roohani N , Hurrell R , Kelishadi R . Schulin RJJorimstojoIUoMS. Zinc and its importance for human health: An integrative review. Journal of research in medical sciences. (2013) ;18: (2):144–57. |
[17] | KrebsNF. Overview of zinc absorption and excretion in the human gastrointestinal tract. The Journal of nutrition. (2000) ;130: (5):1374S–7S. |
[18] | Mayor-Ibarguren A , Busca-Arenzana C , Robles-Marhuenda Á . AHypothesis for the Possible Role of Zinc in the ImmunologicalPathways Related to COVID-19 Infection. Front Immunol. (2020) ;11: :1736. |
[19] | Sekler I , Sensi SL , Hershfinkel M , Silverman WF . editors, Mechanism and regulation of cellular zinc transport. Molecular Medicine. (2007) ;13: (7-8):337–43. |
[20] | Hambidge M , Krebs NFJAron . Interrelationships of key variables of human zinc homeostasis: relevance to dietary zinc requirements (2001) ;21: (1):429–52. |
[21] | Andriollo-Sanchez M , Hininger-Favier I , Meunier N , Toti E , Zaccaria M , Brandolini-Bunlon M , et al. Zinc intake and status in middle-aged and older European subjects: the ZENITH study. European Journal of Clinical Nutrition. (2005) ;59: (2):S37–S. |
[22] | Maywald M , Wessels I , Rink LJIjoms . Zinc signals and immunity. International Journal of Molecular Sciences. (2017) ;18: (10):2222. |
[23] | Cardozo LF , Mafra DJNDT . Don’t forget the zinc. Nephrology Dialysis Transplantation. (2020) ;35: (7):1094–8. |
[24] | Prasad AS , Miale A Jr , Farid Z , Sandstead H , Schulert AJJoL , Medicine C . Zinc metabolism in patients with the syndrome of iron deficiency anemia, hepatosplenomegaly, dwarfism, and hypogonadism. Journal of Laboratory. Clinical Medicine. (1963) ;61: (4):537–49. |
[25] | Sadeghzadeh M , khoshnevisAsl P , Koosha A , NorouziPakdel M . The relation between serum zinc level and febrile seizures in children admitted to Zanjan Valie-Asr hospital. J Adv Med Biomed Res. (2011) ;19: (74):17–24. |
[26] | Sadeghzadeh M , Nabi S , Khoshnevisasl P , Mousavinasab N . The Correlation between cerebrospinal fluid and levels of serum zinc and calcium in children with febrile seizure. Journal of Comprehensive Pediatrics. (2013) ;3: (5):179–183. |
[27] | Brown KH , Peerson JM , Rivera J , Allen LH . Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: a meta-analysis of randomized controlled trials. The American Journal of Clinical Nutrition. (2002) ;75: (6):1062–71. |
[28] | Kelishadi R , Alikhassy H , Amiri M . Zinc and copper status in children with high family risk of premature cardiovascular disease. Annals of Saudi Medicine. (2002) ;22: (5-6):291–4. |
[29] | Müller O , Becher H , van Zweeden AB , Ye Y , Diallo DA , Konate AT , et al. Effect of zinc supplementation on malaria and other causes of morbidity in west African children: randomised double blind placebo controlled trial. Bmj. (2001) ;322: (7302):1567. |
[30] | Kelishadi R , Hashemipour M , Adeli K , Tavakoli N , Movahedian-Attar A , Shapouri J , et al. Effect of zinc supplementation on markers of insulin resistance, oxidative stress, and inflammation among prepubescent children with metabolic syndrome. J Metabolic Syndrome. (2010) ;8: (6):505–10. |
[31] | Hashemipour M , Kelishadi R , Shapouri J , Sarrafzadegan N , Amini M , Tavakoli N , et al. Effect of zinc supplementation on insulin resistance and components of the metabolic syndrome in prepubertal obese children. Hormones. (2009) ;8: (4):279–85. |
[32] | Shankar AH , Genton B , Baisor M , Paino J , Tamja S , Adiguma T , et al. The influence of zinc supplementation on morbidity due to Plasmodium falciparum: a randomized trial in preschool children in Papua New Guinea. The American Journal of Tropical Medicine Hygiene. (2000) ;62: (6):663–9. |
[33] | Kumar A , Kubota Y , Chernov M , Kasuya H . Potential role of zinc supplementation in prophylaxis and treatment of COVID-19. Med Hypotheses. 2020;144. |
[34] | Lanke K , Krenn B , Melchers W , Seipelt J , Van Kuppeveld FJ . PDTC inhibits picornavirus polyprotein processing and RNA replication by transporting zinc ions into cells. Journal of General Virology. (2007) ;88: (4):1206–17. |
[35] | Wessels I , Rolles B , Rink L . The Potential Impact of Zinc Supplementation on COVID-19 Pathogenesis. Front Immunol. (2020) ;11: :1712. |
[36] | Woodworth BA , Zhang S , Tamashiro E , Bhargave G , Palmer JN , Cohen NA , et al. Zinc increases ciliary beat frequency in a calcium-dependent manner. American Journal of Rhinology. (2010) ;24: (1):6–10. |
[37] | Darma A , Ranuh RG , Merbawani W , Setyoningrum RA , Hidajat B , Hidayati SN , et al. Zinc supplementation effect on the bronchial cilia length, the number of cilia, and the number of intact bronchial cell in zinc deficiency rats. The Indonesian Biomedical Journal. (2020) ;12: (1):78–84. |
[38] | Read SA , Obeid S , Ahlenstiel C , Ahlenstie lG . The role of zinc in antiviral immunity. Advances in Nutrition. (2019) ;10: (4):696–710. |
[39] | Gao H , Dai W , Zhao L , Min J , Wang F . The role of zinc and zinc homeostasis in macrophage function. Journal of Immunology Research. (2018) ;6: ;2018:6872621. |
[40] | Gasmi A , Noor S , Tippairote T , Dadar M , Menzel A , Bjørklund G . Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic. Clin Immunol. 2020;215. |
[41] | Haryanto B , Suksmasari T , Wintergerst E , Maggini S . Multivitamin supplementation supports immune function and ameliorates conditions triggered by reduced air quality. Vitam Miner. (2015) ;4: :1–15. |
[42] | Gasmi A , Tippairote T , Mujawdiya PK , Peana M , Menzel A , Dadar M , et al. Micronutrients as immunomodulatory tools for COVID-19 management. Clin Immunol. (2020) ;220: :108545. |
[43] | Maggini S , Beveridge S , Sorbara P , Senatore G . Feeding the immune system: the role of micronutrients in restoring resistance to infections. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition Natural Resources. (2008) ;3: (098):1–21. |
[44] | Berg K , Bolt G , Andersen H , Owen TC . Zinc potentiates the antiviral action of human IFN-α tenfold. Journal of Interferon Cytokine Research. (2001) ;21: (7):471–4. |
[45] | Novick S , Godfrey J , Pollack R , Wilder HR . Zinc-induced suppression of inflammation in the respiratory tract, caused by infection with human rhinovirus and other irritants. Medical Hypotheses. (1997) ;49: (4):347–57. |
[46] | Novick S , Godfrey J , Godfrey N , Wilder HJMh . How does zinc modify the common cold? Clinical observations and implications regarding mechanisms of action. Med Hypotheses. (1996) ;46: (3):295–302. |
[47] | Kaltenberg J , Plum LM , Ober-Blöbaum JL , Hönscheid A , Rink L , Haase H . Zinc signals promote IL-2-dependent proliferation of Tcells. European Journal of Immunology. (2010) ;40: (5):1496–503. |
[48] | Perry DK , Smyth MJ , Stennicke HR , Salvesen GS , Duriez P , Poirier GG , et al. Zinc is a potent inhibitor of the apoptotic protease, caspase-3 a novel target for zinc in the inhibition of apoptosis. Journal of Biological Chemistry. (1997) ;272: (30):18530–3. |
[49] | von Pein JB , Stocks CJ , Schembri MA , Kapetanovic R , Sweet MJ . An alloy of zinc and innate immunity: Galvanising host defence against infection. Cellular Microbiology. 2020:e13268. |
[50] | Kumar M , Al Khodor S . Pathophysiology and treatment strategies for COVID-19. J Transl Med. (2020) ;18: (1):353. |
[51] | Wiersinga WJ , Rhodes A , Cheng AC , Peacock SJ , Prescott HC . Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease (COVID-19): A Review. JAMA. (2020) ;324: (8):782–93. |
[52] | Turner AJ , Hiscox JA , Hooper NM . ACE from vasopeptidase to SARS virus receptor. Trends in Pharmacological Sciences. (2004) ;25: (6):291–4. |
[53] | Doboszewska U , Wlaź P , Nowak G , Młyniec K . Targeting zincmetalloenzymes in coronavirus disease 2019. Br J Pharmacol. (2020) ;177: (21):4887–4898. |
[54] | Te Velthuis AJ , van den Worm SH , Sims AC , Baric RS , Snijder EJ , van Hemert MJ . Zn2+inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathogens. (2010) ;6: (11):e1001176. |
[55] | Han Y-S , Chang G-G , Juo C-G , Lee H-J , Yeh S-H , Hsu JT-A , et al. Papain-like protease 2 (PLP2) from severe acute respiratory syndrome coronavirus (SARS-CoV): expression, purification, characterization, and inhibition. Biochemistry. (2005) ;44: (30):10349–59. |
[56] | Law HK , Cheung CY , Ng HY , Sia SF , Chan YO , Luk W , et al. Chemokine up-regulation in sars-coronavirus–infected, monocyte-derived human dendritic cells. Blood. (2005) ;106: (7):2366–74. |
[57] | Pedersen SF , Ho YC . SARS-CoV-2: a storm is raging. The Journal of Clinical Investigation. (2020) ;130: (5). |
[58] | Cheung CY , Poon LL , Ng IH , Luk W , Sia S-F , Wu MH , et al. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. Journal of Virology. (2005) ;79: (12):7819–26. |
[59] | Tayyib NA , Ramaiah P , Alsolami FJ , Alshmemri MS . Immunomodulatory Effects of Zinc as a Supportive Strategies for COVID-19. Journal of Pharmaceutical Research International. 2020:14-22. |
[60] | Skalny AV , Rink L , Ajsuvakova OP , Aschner M , Gritsenko VA , Alekseenko SI , et al. Zinc and respiratory tract infections: Perspectives for COVID19. International Journal of Molecular Medicine. (2020) ;46: (1):17–26. |
[61] | Sargsyan K , Lin C-C , Chen T , Grauffel C , Chen Y-P , Yang W-Z , et al. Multi-Targeting of Functional Cysteines in Multiple Conserved SARS-CoV-2 Domains by Clinically Safe Zn-ejectors. ChemRxiv. (2020) ; 23;11: (36):9904–9. |
[62] | Skalny AV , Rink L , Ajsuvakova OP , Aschner M , Gritsenko VA , Alekseenko SI , et al. Zinc and respiratory tract infections:Perspectives for COVID-19. (2020) ;46: (1):17–26. |
[63] | Abbehausen C . Zinc finger domains as therapeutic targets for metal-based compounds–an update. Metallomics. (2019) ;11: (1):15–28. |
[64] | de Almeida Brasiel PG . The key role of zinc in elderly immunity: A possible approach in the COVID-19 crisis. Clinical Nutrition ESPEN. (2020) ;38: :65–6. |
[65] | Rerksuppaphol S , Rerksuppaphol L . A randomized controlled trial of zinc supplementation in the treatment of acute respiratory tract infection in Thai children. Pediatric Reports. (2019) ;11: (2):15–20. |
[66] | Qasemzadeh MJ , Fathi M , Tashvighi M , Gharehbeglou M , Yadollah-Damavandi S , Parsa Y , et al. The effect of adjuvant zinc therapy on recovery from pneumonia in hospitalized children: a double-blind randomized controlled trial. Scientifica. (2014) ; 12: ;2014. |
[67] | Yasui Y , Yasui H , Suzuki K , Saitou T , Yamamoto Y , Ishizaka T , et al. Analysis of the predictive factors for critical illness of COVID-19 during treatment-Relationship between serum zinc level and critical illness of COVID-19. International Journal of Infectious Diseases. (2020) ;1: (100):230–6. |
[68] | Jothimani D , Kailasam E , Danielraj S , Nallathambi B , Ramachandran H , Sekar P , et al. COVID-19: Poor outcomes in patients with Zinc deficiency. International Journal of Infectious Diseases. (2020) ;1: (100):343–9. |
[69] | Finzi E . Treatment of SARS-CoV-2 with high dose oral zinc salts: A report on four patients. Int J Infect Dis. (2020) ;99: :307–9. |
[70] | Carlucci PM , Ahuja T , Petrilli C , Rajagopalan H , Jones S , Rahimian J . Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. 2020:jmm001250. |
[71] | Natarajan S , Anbarasi C , Sathiyarajeswaran P , Manickam P , Geetha S , Kathiravan R , et al. Kabasura Kudineer (KSK), a poly-herbal Siddha medicine, reduced SARS-CoV-2 viral load in asymptomatic COVID-19 individuals as compared to vitamin C and zinc supplementation: findings from a prospective, exploratory, open-labeled, comparative, randomized controlled trial, Tamil Nadu, India. Trials. (2021) ;22: (1):1–11. |
[72] | Capone S , Abramyan S , Ross B , Rosenberg J , Zeibeq J , Vasudevan V , et al. Characterization of Critically Ill COVID-19 Patients at a Brooklyn Safety-Net Hospital. Cureus. (2020) ;12: (8):e9809. |
[73] | Pal A , Squitti R , Picozza M , Pawar A , Rongioletti M , Dutta AK , et al. Zinc and COVID-19: basis of current clinical trials (2020) ;19: :1–11. |
[74] | Saper RB , Rash R . Zinc: an essential micronutrient. American Family Physician. (2009) ;79: (9):768. |
[75] | Derouiche S . Zinc Supplementation Prevents the Complications of COVID-19 Infection in Cancer Patients. Asian Pacific Journal of Cancer Care. (2020) ;5: (S1):137–41. |
[76] | Das RR . Zinc in acute bronchiolitis: is it really ineffective? Iran J Pediatr. (2011) ;21: (3):409–10. |
[77] | Sattar Y , Connerney M , Rauf H , Saini M , Ullah W , Mamtani S , et al. Three cases of COVID-19 disease with colonic manifestations. (2020) ;115: (6):948–50. |
[78] | Bahloul M , Ketata W , Lahyeni D , Mayoufi H , Kotti A , Smaoui F , et al. Pulmonary capillary leak syndrome following COVID-19 virusinfection. J Med Virol. (2021) ;93: (1):94–6. |
[79] | Farooqi F , Dhawan N , Morgan R , Dinh J , Nedd K , Yatzkan G , et al. Treatment of severe COVID-19 with tocilizumab mitigates cytokine storm and averts mechanical ventilation during acute respiratory distress: a case report and literature review. Tropical Medicine. (2020) ;5: (3):112. |
[80] | Ostojic SM , Milovancev A , Drid P , Nikolaidis A . Oxygen saturation improved with nitrate-based nutritional formula in patients with COVID-19. Journal of International Medical Research. (2021) ;49: (4):03000605211012380. |
[81] | Finzi E , Harrington A . Zinc treatment of outpatient COVID-19: Aretrospective review of 28 consecutive patients. J Med Virol. (2021) ;93: (5):2588–90. |
[82] | Derwand R , Scholz M , Zelenko V . COVID-19 outpatients: early risk-stratified treatment with zinc plus low-dose hydroxy chloroquine and azithromycin: a retrospective case series study. International Journal of Antimicrobial Agents. (2020) ;56: (6):106214. |
[83] | Atakla HG , Noudohounsi MMUD , Salami AY , Sacca H , Houinato AG , Barry MC , et al. COVID-19 infection in pediatric subjects: study of 36 cases in Conakry. The Pan African Medical Journal. (2020) ;37: (Suppl 1):42. |
[84] | Elalfy H , Besheer T , El-Mesery A , El-Gilany AH , Soliman MAA , Alhawarey A , et al. Effect of a combination of nitazoxanide,ribavirin, and ivermectin plus zinc supplement (MANS. NRIZ study) onthe clearance of mild COVID-19. J Med Virol. (2021) ;93: (5):3176–83. |




