Green tea extract attenuates non alcoholic fatty liver disease by decreasing hyperlipidemia and enhancing Superoxide dismutase activity in cholesterol-fed rats
Abstract
BACKGROUND/AIM:
Health benefits of green tea for a wide variety of ailments, including the cancer, heart disease, and liver disease, were reported. It is believed to have beneficial effects in the prevention and treatment of many diseases, one of which is non-alcoholic fatty liver disease (NAFLD). This study inspects the protective effect of green tea against atherosclerosis and NAFLD in comparative approach between curative and preventive models.
MATERIALS AND METHODS:
Twenty four of Wistar rats were studied for 150 days. After 15 days of adaptation period, rats were divided into four groups including normal Group (NG), control Hypercholesterolemic diet Group (CHDG), preventive Group (PG) and curative Group (CG) that followed respectively the following regimens: 1 mL/kg of sunflower oil for 150 days, 1 mL/kg of cholesterol solution prepared at 1.5% (w/v) in sunflower oil span 150 days, 1 ml/kg of cholesterol solution at 1.5% (w/v) in sunflower oil with 3 mL/kg GTLE for 60 days and 1 mL/kg of cholesterol solution at 1.5% (w/v) in sunflower oil for 30 days followed by 3 mL/kg of GTLE for 30 days. These both PG and CG groups were ingested with cholesterol 1.5% (w/v) during remaining period.
RESULTS:
The results showed significant increase, except for NG, during the 30 first days (p≤0.001) in lipid serum profiles including Total Cholesterol (TC), Triacylglycerol (TG) and Low-Density Lipoprotein cholesterol (LDL-c). However, the High-Density Lipoprotein cholesterol (HDL-c) profile decreased during the treatment (p≤0.001). The ingestion of GTLE in treated groups (CG and PG) declined significantly (p≤0.001) in blood lipid concentrations (TC: 67%, TG: 23%, LDL-c: 81.12%) except for the HDL-c that increased up to 15%. The Atherogenic Index (AI) also decreased significantly (p≤0.001) up to 48%, in CG and PG. PG and NG marked same SOD activity values (130.91±7.66 versus 141.31±8.21 U/mL), while CG showed the lowest level. Liver sections were well protected in protective model than curative one.
1Introduction
Tea is one of the most popular beverages worldwide [1]. The Chinese believe that drinking tea promotes good health and longevity [2]. The research identified tea as a Nature’s gift for promoting human health. Although, health benefits have been attributed to tea, especially green tea consumption since in the beginning of its history, scientific investigations of this beverage and its constituents has been underway for less than three decades [3]. The dietary interventions for lowering blood are a major focus in prevention and treatment of cardiovascular diseases [4, 5]. Hence, tea provides a dietary source of bioactive compounds such as polyphenols that helps to prevent a wide variety of diseases such as atherosclerosis, coronary disease, hypercholesterolemia, etc [3]. The chemical compositions of the green tea leaves can be generated via different methods during the drying process. The dried tea preserves more polyphenols, such as catechins compared to others tea processing [2, 6]. The potential health benefits associated with tea consumption have been attributed to the polyphenols anti-oxidative properties. Recently, polyphenols have been found to have a benefic effect on blood lipids in animals [7]. Several authors have reported that polyphenols inhibit intestinal cholesterol absorption and reduce thereby, serum cholesterol concentrations [8, 9]. However, no one studied the hypolipidemia effect after treatment cessation, which represents somehow the originality of the present work.
The present study evaluated the phenolic and flavonoid contents, antioxidant activity and investigated the effects of GTLE (Camellia sinensis L.) and their actions on non-alcoholic hepatic stetosisin cholesterol-fed rats (Wistar strain) (before, during and after treatment cessation by GTLE).
2Materiel and methods
2.1Materials
Green tea bags (BARARI, Sri Lanka) were purchased from a local market. The male Wistar rat strains (Rattus norvegicus) weighing between 120–135 g were obtained from Pasteur Institute in Algiers, Algeria.
2.2Preparation of plant extract
The green tea extract was prepared through the general hot water extraction practice. 10 g of green tea leaves powder was immersed in 100 ml of boiled (100°C) sterile distilled water (autoclaved at 120°C during 20 min) with stirring and in dark for 5 min. The mixture was filtered through Whatman filter (porosity: 0.55 mm) and the extract had been stored at 4°C until analysis.
2.3Determination of total phenolic content
The phenolic content of GTLE was measured by the Folin-Ciocalteu (FC) reagent according to the method used by [10]. Aliquot of 1 mL aqueous extract green tea leaves (GTLAE) or Gallic Acid (0.1 mg/mL) was introduced into 6 test tubes followed by adding 0.1 mL of FC reagent and 0.9 mL of distilled water in each tube. All tubes were stood for 3 min to allow the FC reagent to react with GTE components. 800μL was taken from 7.5% concentrated solution of Na2CO3and 0.4 mL of distilled water was added in each tube for an additional 60 min in order to stabilize the formed blue color. The absorbance of samples and blank (FC-reagent + water) were measured at wavelength of 765 nm. Data were expressed as mg Gallic Acid equivalent (GAE) g–1 of GTLE using the Equation (1).
(1)
C: Total content polyphenols (mg equivalent Gallic acid/g of GTE).
c: Gallic acid concentration (mg/mL).
v: Extract volume (mL).
m: mass of pure GTE (g).
2.4Determination of flavonoids content
The content of flavonoids in plant extracts was determined using spectrophotometric method [11]. A mixture of 1 mL of Aluminum Chloride (AlCl3:2%) and one drop of Acetic Acid were added to 1 mL of GTLE sample and we added the same mixture to 1 mL of standard (Quercetin: 0.1 mg/mL). After 40 min of incubation in dark at room temperature, the absorbance of samples and standard were measured at 415 nm. Data were expressed as mg Quercetin equivalent (QE) g- 1 dry matter (green tea extract) using the Equation (2).
(2)
X: amount of flavonoids (mg of Quercetin equivalent/g of GTE)
A: Absorbance of GTLE
A0: Absorbance quercetin solution
m: mass of GTLE (mg)
m0: mass of quercetin solution.
2.5In vitro antioxidant activity assay
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of the test sample was determinate by UV-Vis spectrophotometer (Optizen 2120UV, Korea) according to the method of Fernandez-Orozco et al. [12], with Ascorbic Acid standard of DPPH scavenger. 10μL of extract solution or AcAwas mixed with 1 mL of DPPH methanolic solution (0.004%). The mixture was then sheltered for 30 min in dark at ambiant temperature about 25°C. Samples were mesured at 517 nm. The obtained results were expressed as percentage of DPPH inhibition according to the Equation (3).
(3)
AA: Antioxidant activity
A0: Initial absorbance;
At: Absorbance at time t.
2.6Dietary treatment experiment
A total of 24 Wistar rats (120–135 g) were quarantined for a period of 15 days before the experiment execution, in order to adapt animals to local environment. They were kept individually in cages, maintained on standard diet (AIN 93 M) and water ad-libitum, with an alternating 12 h light-dark cycle. Animal experimentation was conducted for 150 days. During all this experiment period, animals were avoided to be subjected to any kind of stress.
To study the effects of green tea on blood lipid level, the animals (Rattus norvegicus) were randomly divided into four experimental groups consisting of six animals (n = 6) each as follows:
Normal Group (NG): Oral feeding (gavage) twice-daily of 1 mL/kg of sunflower oil for 150 days, this free-cholesterol oil was chosen due to its capacity to dissolve cholesterol and its weak scent.
Control Hypercholesterolemic diet Group (CHDG): Oral feeding twice-daily of 1 mL/kg of cholesterol solution prepared at 1.5% (w/v) in sunflower oil span 150 days.
Preventive Group (PG): Oral feeding twice-daily of 1 mL/kg of cholesterol solution prepared at 1.5% (w/v) in sunflower oil enriched with 3 mL/kg green tea extract for 60 days. The animals had been orally ingested with cholesterol (1.5% in sunflower oil (w/v)) for the remaining period (until 150 days).
Curative Group (CG): Oral feeding twice-daily of 1 mL/kg of cholesterol solution prepared at 1.5% (w/v) in sunflower oil for 30 days followed by treatment with green tea extract (3 mL/kg) for 30 days. The animals had been orally ingested with cholesterol (1.5% (w/v)) for the remaining period (until 150 days).
The curative model was ingested firstly with cholesterol high diet to induce hyperlipidemia then followed by treatment using GTLE for the next 30 days, whereas in preventive model both high cholesterol diet and GTLE treatment were ingested simultaneously during 60 days.
2.7Ethics
All in vivo experimental procedures and the protocols employed on rats were approved according to the regulations established by the Laboratory of Veterinary Histology, National School of Veterinary Sciences of Algiers, Algeria following the recommendations of the European Pharmacopoeia 8.0 under reference number 215/2013.
2.8Collection of plasma and liver
Rats were fasted overnight but had free access to water in order to measure serum biochemical parameters. Blood samples were obtained from the retro-orbital venous plexus using sterile Pasteur pipettes (Sigma-Aldrich, Germany) and directly collected in heparinized tubes (in the 1st, 30th, 60th and 150th days of experiment). The plasma was then separated from blood samples by centrifugation (2000×g for 10 min.). Samples were stored at – 70°C until biochemical analysis assayed in duplicate for each animal. Liver was removed from each animal, cleaned with sterilized saline water and weighed, and then prepared for histological analysis.
2.9Assay of plasma lipid parameters
The plasma lipid parameters including TC, TG, HDL-c, LDL-c and VLDL-c were analyzed using express chemistry analyzer (COBAS C311, Roche Hitachi, Germany). This analyzer is automated, software-controlled for clinical chemistry analysis based on photometric tests. All biochemical measurements were carried out using plasma prepared as mentioned in previous title.
The Atherogenic index (AI) was calculated according Hamafi et al. [13] following the next formula:
AI = (Total cholesterol - HDL - Cholesterol)/HDL - Cholesterol
2.10Determination of Superoxide dismutase (SOD)
The SOD activity was estimated strictly according to the SOD kit assay (RANDOX, United Kingdom), where methodology procedure was performed strictly according to the kit specifications. The SOD activity was expressed as unit per milliliter of serum (U/mg, protein).
2.11Histological analysis of liver sections
The histological study of liver’s rats was realized following the methodology described by Delafield [14]. In brief, livers was fixed in 10% buffered formalin and dehydrated in alcohol, samples were cleared with xylol and entrenched in paraffin blocks. Sections of 3μm thickness approximately were immersed in Harris haematoxylin and eosin for histological screening. Images were acquired using a light microscope (LeicaStore Miami, Coral Gables FL) fitted with an Optika digital-Camera system (Optika, DM 1000, Italy).
2.12Statistical analysis
Biochemical screenings were carried out in duplicate for each rat (six rats in each group) and results were presented as mean value±standard deviation of twelve repetitions. The data were subjected to One-way ANOVA and differences between groups were determined by Duncan’s multiple range tests. Statistical analysis was accomplished using statistical package for the social sciences 16.0 software for Windows (SPSS Inc., Chicago, IL, USA). Differences at p≤0.05 were considered to be significant.
3Results and discussion
3.1Total phenolic, flavonoid contents and antioxidant capacity assays
The GTLE is derived from dried leaves of C. sinensis (38.9% w/w as yield) and contains mainly natural tea polyphenols. The active ingredients of green tea contain polyphenols (295 mg GAE/g of GTLE and flavonoids (256 mg QE/g of GTLE). According to Mukhtar et al. [15] and Sasazuki et al. [16], phenolic fraction represents between 30 to 40% of the extractable solids of dried GTLE together alkaloids (such as caffeine and theobromine), carbohydrates, tannins and minerals (such as fluoride and aluminium). The DPPH antioxidant assay noted that this extract had very strong antioxidant capacity (70%). According to Pekal and Pyrzynska [17], tea infusions recorded a high percentage of inhibition (75%). It was reported that phenolic compounds (especially known as catechins) were associated with antioxidant activity (highly positive relationship) and play an important role in stabilizing lipid peroxidation within the cell membrane and the prevention of coronary diseases [18–20]. Thus, Green tea preparations contain other phenolic fractions such as flavonols predominated by quercetin with few quantities of kaempferol and myricetin [21]. These bioactive molecules can ameliorate antioxidant potential in the plasma exhibiting favorable effects on atherosclerosis [22]. In addition, polyphenols prevent the generation of free radicals by inactivating the existing enzymes (MDA and SOD) responsible to their generation or by increasing the enzymatic antioxidant activity, probably via the induction of protein molecule biosynthesis [23, 24].
3.2Effect on blood lipid profiles (Before, during and after treatment cessation)
Hyperlipidemia has a close relationship to atherosclerosis and coronary heart diseases. A high level of plasma cholesterol has been ranked as one of the major risk factors contributing to the incidence and severity of coronary heart disease [25, 26]. Results of lipid profile levels are shown in Fig. 1.
Fig.1
Effect of GTLE on lipid parameters of hypercholesterolemic rats throughout the experiment; Total cholesterol (TC), Triacylglycerol (TG), High-density lipoprotein cholesterol (HDL-c), low density-lipoprotein cholesterol (LDL-c), very-low-density-lipoprotein cholesterol (VLDL-c) and atherogenic index (AI). NG: normal group, CHDG: Control Hypercholesterolemic diet Group, CG: curative group, PG: preventive group Values were±SD given in (mg/dL) for n = 6 rats. *Significantly different from normal group (NG) (P≤0.05); + significantly different from hypercholesterolemic diet control (CHDG) (P≤0.05). Significantly different values are represented by different letters (a-c) (P < 0.05).
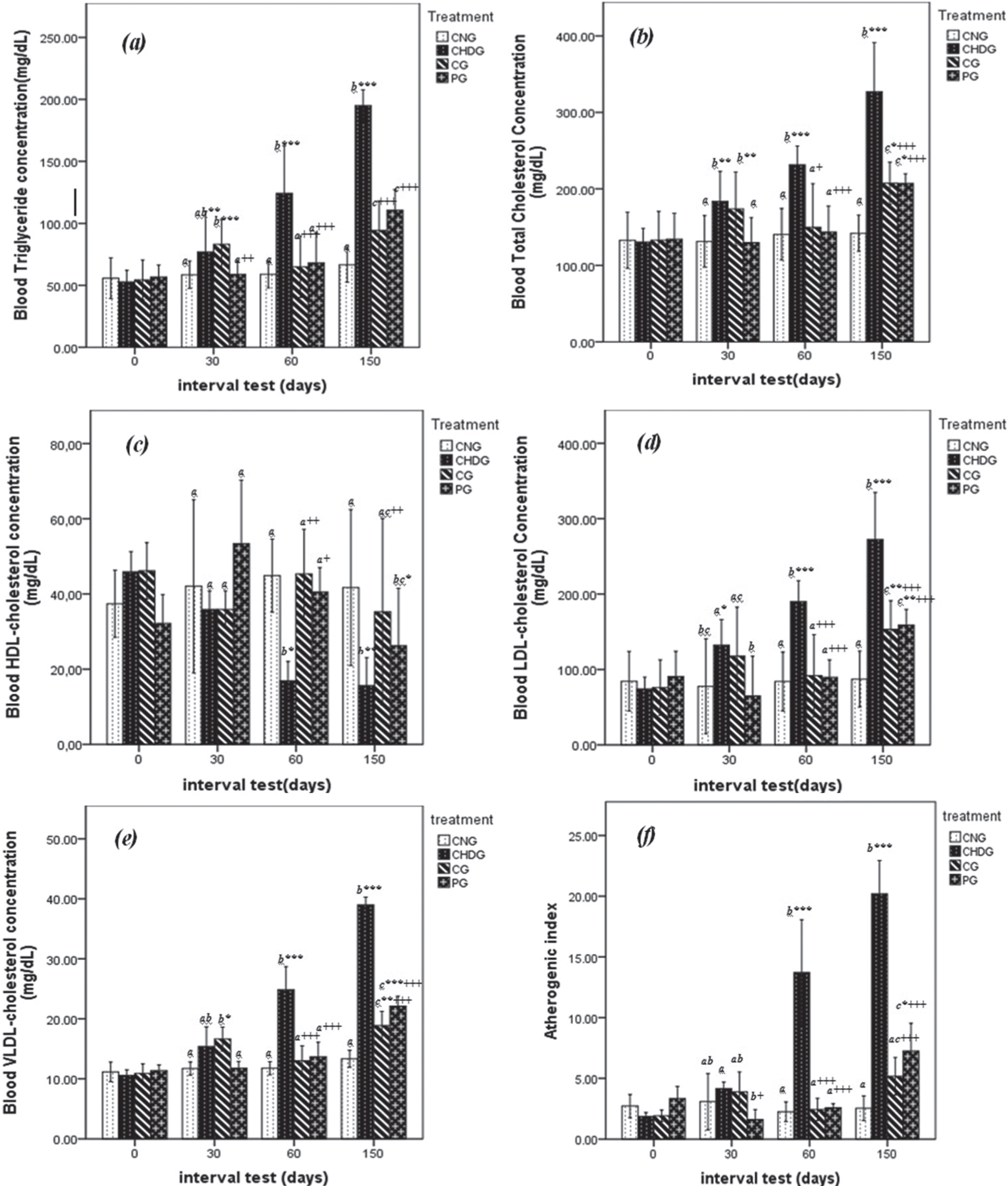
In the beginning of current study (1st day of experiment), all five parameters (TG, TC, LDL-c, HDL-c and VLDL-c) measured present similar amounts (p > 0.05) between animals of CNG, CHDG, CG and PG groups after two weeks adaptation period.
At the second phase of treatment (30th days), rats of CG receiving hypercholesterolemic diet for 30 days (then treated with green tea until 60th day), mark high levels of lipid profiles with significant differences at p < 0.05 compared to animals of PG (Fig. 1a– e), where TG = 90.93±6.24 versus 57.79±7.17 mg/dL, TC = 173.75±24.12 versus 130.00±16.13 mg/dL, HDL-c = 37.96±0.23 versus 62.01±18.14 mg/dL, LDL-c = 131.31±21.51versus 57.21±36.41 mg/dL and VLDL-c attained 18.19±1.25 against 11.56±1.43 mg/dL in CG and PG groups respectively. There is no significant difference (p > 0.05) between lipid profile values of CG rats and those of positive control (CHDG) at this step. Also, there is no significant difference in the plasma lipid profiles between animals of preventive and normal control groups (p > 0.05) (see Fig. 1).
As can be seen from Fig. 1(a– e), rats of PG receiving hypercholesterolemic diet mixed with green tea during 60 days possessed similar values of TG, TC, LDL-c, HDL-c compared to animals of CNG, whereas the VLDL-c parameter was slightly higher at p < 0.05 in PG group (14.63±2.32 against 12.37±0.85 mg/dL).
At the next phase of experiment (60th day), levels of TG were similar (p > 0.05) in CNG, CG and PG given respectively 61.89±4.16, 57.49±5.86 and 73.04±11.54 mg/dL. These values represent approximatively half of TG concentration in rats none treated with green tea (CHDG) showing 115.57±9.81 mg/dL, the reduction rate in CG from 30 day to 60 day was 28.96%. TC showed also importance reduction in CG, where green tea intake decreased this parameter from 87.75±4.12 (30th day) to 75.02±8.40 mg/dL in the 60th day, this observation proclaim about 14.50% of TC declining and ranked this group in same class with PG (71.78±6.90 mg/dL) and NG (70.66±11.81 mg/dL) (p > 0.05) (Fig. 1a, b).
As shown in Fig. 1e, VLDL-c followed same kinetic of TG and TC, where green tea administration reduced this biochemical factor in CG from 18.19±1.25 to 11.50±1.18 mg/dL (36.78% of reduction), this value is statistically similar to the PG (14.63±2.32 mg/dL). As regards the atherogenic index, it was also very high significantly (P < 0.001) reduced (Fig. 1f) by green tea extract up to 21% for CG and 36% for PG, respectively; although, the modeling group (CHDG) was in contrast significantly increased by 47%.
After treatment cessation (60th– 150th day), no difference was observed in the levels serum lipid profiles (p > 0.05) of CG and PG as shown in Fig. 1, except for HDL-c that decreased significantly (p < 0.01) where the values of CG and PG were 35.27±9.99 and 26.23±6.15 mg/dL compared to modeling group (15.67±2.97 mg/dL).
From observations mentioned above, it is clearly that green tea consumption exhibited a remarkable reduction of risk factors of atherosclerosis in rats of PG group compared to groups of animals none treated. Many reports have been indicated the protective effect of green tea intake in atherosclerosis, the studies by Kavantzas et al. [22] and Tijburg et al. [27] realized on hyper-cholesterolemic rabbits showed the positive action of green tea on reduction of biomarkers of atherosclerosis. Our study was realized in comparative approach between curative and preventive model, raison of the prolongation of experiments to the second step of 30 days, where hypercholesterolemic diet was stopped for CG group and replaced by green tea treatment.
Some Studies indicated that the health beneficial effects of green tea due to either the individual or the synergistic action of the phenolic components and that they have been attributed mainly to the catechins, epigallocatechin-3-gallate (EGCG), epicatechin (EC), epigallocatechin (EGC) and epicatechingallate (ECG) [28, 29]. As well as, the flavonoids enhance the lecithin Acyl-transferase (LCAT) by increasing their activity, which regulates blood lipids [30]. LCAT plays a key role in the incorporation of free cholesterol into HDL (this may increase HDL) and its transfer back to VLDL and LDL, which are later returned in liver cells [31].
However, the increase in HDL-C observed in this study, might be due to the stimulation of pre-β HDL-C and reverse cholesterol transport as demonstrated by previous study [32]. High HDL-c levels could potential contribute to its anti-atherogenic properties, including its capacity to inhibit LDL oxidation and protect endothelial cells from the cytotoxic effects of oxidized LDL [33]. The anti-atherogenic effect GTL extract found in our study might be due to the presence of polyphenols antioxidants (catechin and its derivatives), which play a crucial role in reducing blood cholesterol in rats [26]. Green tea leaves extract treated animals (CG, PG) also showed a decrease the atherogenic index (48%) with respect of hypercholesterolemic group (CHDG), which is generally believed to be beneficial since the HDL level is inversely correlated with coronary heart disease and, the reduction in this ratio is considered as an anti-atherosclerotic factor [33]. The further work should identify the main effective components based on this result.
From the results of the current study, it is noticeable that the consumption of green tea in hypercholesterolemic rats has demonstrated potential beneficial effect on the biochemical risk factors of atherosclerosis in both protective and curative models. However, the curative system was observed to possess the high favorable effect, particularly in HDL-c and LDL-c parameters.
3.3Effect on the major marker of oxidative stress (SOD)
The results obtained in the SOD activity levels in plasma from baseline (1st day) to the treatment cessation of experiments (60th day) are cited in Table 1. As seen from Table 1, at baseline, animals in four groups presented similar values of SOD activity. However, at middle phase (30th day), SOD activity presented similar values (p > 0.05) between PG (135.04±6.70 U/mL) and NG (143.79±5.39 U/mL) with significant difference in comparison to CG and CHDG groups showing 78.89±9.01 and 83.14±7.56 U/mL respectively.
Table 1
Superoxide dismutase activity levels in the plasma (Unit/mL) in hypercholesterolemic-diet rats
| Groups | 00 day | 30 day | 60 day |
|---|---|---|---|
| CNG | 146.13±06.71a | 143.79±05.39a | 141.31±08.21a |
| CHDG | 157.67±05.51a | 83.14±07.56b | 72.48±06.02b |
| CG | 143.41±09.45a | 78.89±09.01b | 101.09±04.31c |
| PG | 153.09±08.33a | 135.04±06.70a | 130.91±07.66a |
a-c: values (mean±standard deviation, n = 12) in the same column sharing different letters are signi cantly different (P < 0.05). CNG: control normal group, CHDG: control high diet group, CG: curative group, PG: preventive group.
As summarized in Table 1, green tea consumption increased SOD activity in CG rats with 28.14% in the second month of experiment to attain 101.09±4.31 U/mL (60th day). However, these rats did not reach PG value (130.91±7.66 U/mL) presenting similarity (p > 0.05) with NG presenting 141.31±8.21 U/mL. The positive control showed the lowest activity with 72.48±6.02 U/mL.
The SOD activity is qualified as first lines of defense against oxygen-derived free radicals when his amount exceeds the normal physiologic level [34], it possess also the capability to quench advanced oxidative stress caused by hyperlipidemia [35] known by their harmful effects on human health, especially as risk developing of cancer and heart diseases [36].
The antioxidant enzyme SOD enters in biochemical reactions to catalyze and accelerate the dismutation of superoxide anion into hydrogen peroxide, which further reacts to produce water or oxygen [34]. This enzyme can interfere sequentially with Glucose-6-phosphate dehydrogenase to convert free radicals to non-radical species in order to inhibit their damaging effects against tissues and molecules such as proteins, lipids and nucleic acid [37, 38].
3.4Liver histopathology
The liver plays a central role in the maintenance of systemic lipid homeostasis, this organ is highly susceptible to reactive oxygen species (ROSs) damage [39]. Non-alcoholic fatty liver disease (NAFLD) ranging from simple steatosis that might progress to non-alcoholic steatohepatitis (NASH), advanced fibrosis, and cirrhosis affects approximately 20–30% of the population in developed countries [40]. These pathologies are commonly characterized by accumulation of triglycerides within hepatocytes in the form of macro- and micro-vesicles of lipid with a benign prognosis and a subsequent NASH-related inflammation [25].
Histological examinations of liver sections are shown in Fig. 2(a–f). At the treatment cessation the NG sections showed intact histological structure (Fig. 2a). CHDG (Fig. 2b) liver examination showed swelling and vacuolization in the endothelial cells with fibrosis and hyalinosis and inflammatory cells infiltration and few fibroblastic cells proliferation. The CG (Fig. 2c) showed sections with less damage effects comparatively to CHDG (Fig. 2b) justified by the regeneration action under GTLE intake, whereas the PG (Fig. 2d2) showed sections with high resemblances to intact architecture of NG (Fig. 2a). After treatment cessation, the CHDG (Fig. 2d1) showed advanced symptoms of damages especially in vacuolization, the CG (Fig. 2e) showed stable situation in comparison to its examination at treatment cessation whereas the PG (Fig. 2f) showed intact structures relatively comparable to NG (Fig. 2a).
Fig.2
Effects of GTLAE on morphological features of rats’ livers during experiment. Hematoxylin eosin coloration. Optic microscopy; 40x magnification. At treatment cessation: (a) NG, (b) CHDG, (c) CG, (d2) PG; After treatment cessation: (d1) CHDG, (e) CG, (f) PG.
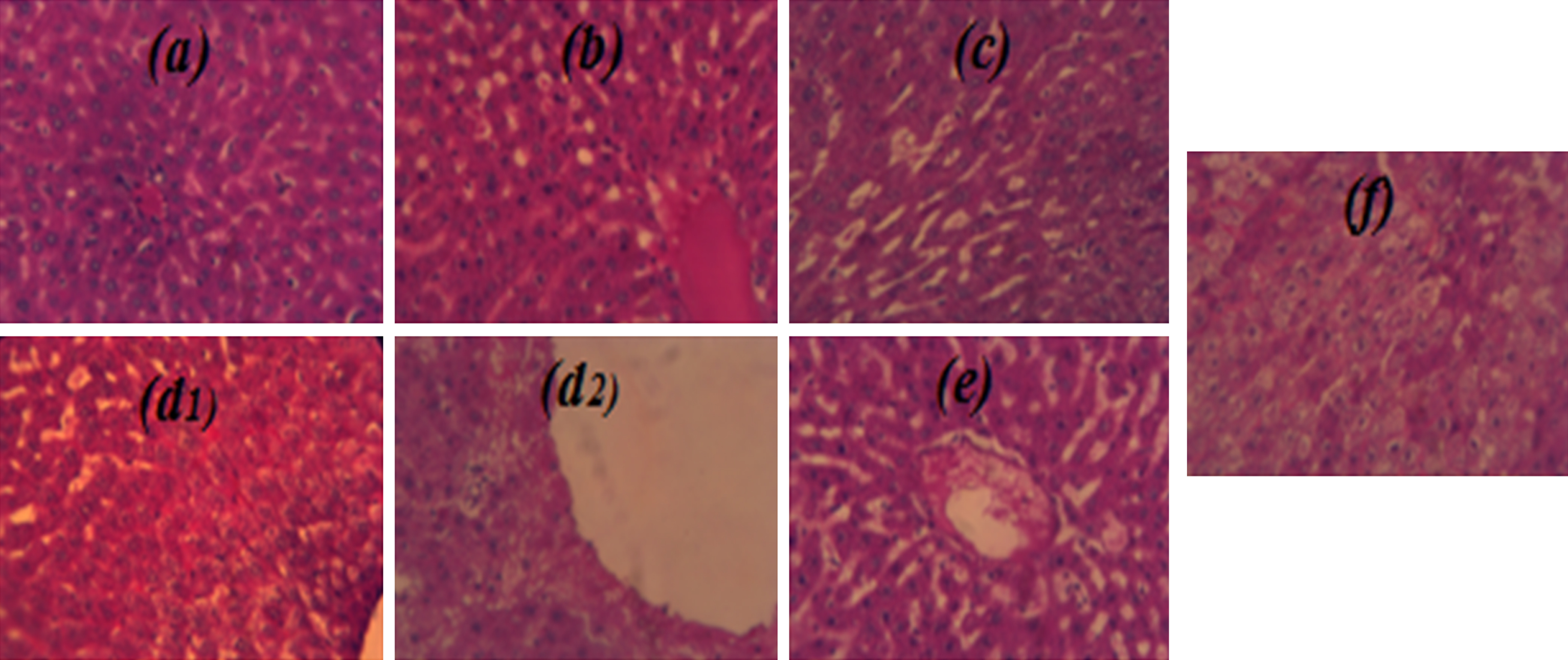
As can be seen from Fig. 2, it is clear that the green tea intake in hypercholesterol-diet rats has a beneficial effect on the liver. The histological study of liver did not reveal remarkable changes in structure of the central vein and surrounding hepatocytes between rats of NG (Fig. 2a) and of those of PG (Fig. 2d2 and f). However, regeneration of hepatocytes near to the central vein was noticed in rats of CG (Fig. 2c) due to green tea intake after 30 day of hypercholesterol-diet. The liver sections of CHDG show abnormality structure and produced inflammatory changes in cells’ infiltration (Fig. 2b) compared to NG (Fig. 2a).
After treatment cessation (150 day), the liver sections obtained from all treated animals (fed-cholesterol diet) showed different inflammations such as hepatocytes degeneration with steatotic tissues, and Mallory bodies were formed in hyperlipidemia rats (Fig. 2d1; Fig. 2d2), which was also the case with curative and preventive groups (Fig. 2e; Fig. 2f).
The positive result observed during liver’s examination could be attributed to the antioxidant properties of GTLE that reduce the lipid peroxidation which in can restore the integrity of the cell membrane and ameliorate their permeability. Since the oxidative damage as the fundamental mechanism of metals toxicity occurs mainly through production of different ROS during the reactions and reacts with biological molecules, eventually cause membranes and tissues damages [41].
Green tea polyphenols as dietary antioxidants in human health and disease might protect against oxidative stress caused by ROS which could lead to damage in biological molecules, such as proteins and DNA. It was observed, in the current study, that GTLE intake decreased significantly SOD, this antioxidant enzyme as well as glutathione peroxidase (GPx) and catalase (CAT) play a vital role in getting rid of these oxidants and preventing cellular injury [42]. In addition, liver enzymes activity is considered as a biochemical parameter to evaluate the function and damage of kidney and liver [43].
The research on green tea shows its potencies to prevent oxidative damage and improve oxidant-antioxidant balance. These beneficial effects are attributed to the potential ability of the phenolic compounds to reduce, counteract or also repair damage resulting from oxidative stress and inflammation associated with diseases conditions [44].
Besides the direct role of phenolic compounds as antioxidants, these secondary metabolites can repress the activation of redox sensitive transcription factors playing the role of mediators in inflammatory reactions [45]. Green tea and its catechins components, mainly represented by epigallocatechin (EGC), epicatechin gallate (ECG), and epigallocatechin gallate (EGCG), can exert their actions directly at tissue and/or cellular levels [46]. In addition, diverse natural flavor-rich tea constituents such as terpenes, oxygenated terpenes, sesquiterpenes and organic acids may have contribution in diseases related to reactive oxygen species [47], and can play a protective effect on liver by decreasing accumulation of hepatic fat [48].
The effect of green tea on NAFLD is attributed essentially to its large amounts of catechins that possess a potent antioxidant activity shown to be 25 to 100 times more powerful than vitamins C and E, as well as minerals that function as co-factors in antioxidant enzymes: zinc, selenium and manganese [49, 50]. So, the results obtained with Wistar rats in the current study would imply that regular intake of green tea may play remarkable effects on the biochemical risk factors atherosclerosis and NAFLD for the hypercholesterolemic regimens due essentially to the modern industrial foods and eating habits.
4Conclusion
The current study confirms the protective role of green tea intake in against atherosclerosis on hypercholesterol-fed rats. The results indicated equality effects between curative and preventive models on all biochemical risk factors of atherosclerosis, except for HDL-c and LDL-c levels shown better-quality in curative system. However, liver’s tissues were mostly prevented in prevention type with enhancing SOD activity.
Animal rights
All experimental procedures were authorized by the Institutional Animal Care Committee of the National Administration of Algerian Higher Education and Scientific Research. Ethical approval number: 98-11 law of August 22, 1998. Also, the study protocol was approved by the Ethics Committee of the Algerian Ministry of Public Health and conformed to the principles outlined in the declaration of Helsinki.
Conflict of interest
All authors declare no conflicts of interest.
Acknowledgments
The authors would like to acknowledge the financial support of the Algerian Agency for the Research & Development in Health (PNR nos. 208/ANDRS/2011 and 41/ANDRS/2011) from the National Administration of Algerian Higher Education and Scientific Research (DGRSDT). The authors also would like to thank Ms. Asma Belazzouz for her help.
References
[1] | Chan EWC , Soh EY , Tie PP , Law YP . Antioxidant and antibacterial properties of green, black, and herbal teas of Camellia sinensis . Pharmacogn Res. (2011) ;3: :266–72. |
[2] | Yang CS , Maliakal P , Meng X . Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. (2002) ;42: :25–54. |
[3] | Khan N , Mukhtar H . Tea polyphenols for health promotion. Life Sci. (2007) ;81: :519–33. |
[4] | Lewis SJ . Prevention and treatment of atherosclerosis: A practitioner's guide for 2008. Am J Med. (2009) ;122: (1):38S–50S. |
[5] | La Rosa JC . Low density lipoprotein cholesterol reduction: The end is more important than the means. Am J Cardiol. (2007) ;100: (2):240–2. |
[6] | Ju J , Lu G , Lambert JD , Yang CS . Inhibition of carcinogenesis by tea constituents. Semin Cancer Biol. (2007) ;17: :395–402. |
[7] | Ruiz-Roso B , Quintela JC , De la Fuente E , Haya J , Pérez-Olleros L . Insoluble carob fiber rich in polyphenols lowers total and LDL cholesterol in hypercholesterolemic sujects. Plant Foods Hum Nutr. (2010) ;65: (1):50–6. |
[8] | Bursill CA , Roach PD . A green tea catechin extract upregulates the hepatic low-density lipoprotein receptor in rats. Lipids Health Dis. (2007) ;42: :625–5. |
[9] | Kobayashi M , Ikeda I . Chapter 48-Modulation of Intestinal Cholesterol Absorption by Dietary Tea Polyphenols. In: Aguiar O , editors. Polyphenols in Human Health and Disease. (2014) . p. 625–38. |
[10] | Nait Bachir Y , Zafour A , Medjkane M . Formulation of stable microcapsules suspensions content Salvia officinalis extract for its antioxidant activity preservation. J Food Process Preserv. (2018) ;42: (2):e13446. |
[11] | Quettier DC , Gressier B , Vasseur J , Dine T , Brunet C , Luyckx M , Cazin M , Cazin JC , Bailleul F , Trotin F . Phenolic compounds and antioxidant activities of buckwheat (FagopyrumesculentumMoench) hulls and flour. The J Ethnopharmacol. (2000) ;2: :35–42. |
[12] | Fernandez-Orozco R , Roca M , Gandul-Rojas B , Gallardo-Guerrero L . DPPH-scavenging capacity of chloroplastic pigments and phenolic compoundsof olive fruits (cv.Arbequina) during ripening. J Food Compos Anal. (2011) ;24: :858–64. |
[13] | Harnafi H , Caid HS , Bouanani NH , Aziz M , Amrani S . Hypolipemic activity of polyphenol-rich extracts from Ocimumbasilicum in triton WR–induced hyperlipidemic mice. Food Chem. (2008) ;108: :205–12. |
[14] | Delafield F . Haematoxylin and Eosin for General Staining. Staining of the animal Tissues Practical and Theoretical. Oxford: Oxford University Press, (1984) . |
[15] | Mukhtar H , Ahmad N . Green tea in chemoprevention of cancer. Toxicol Sci. (1999) ;52: :111–7. |
[16] | Sasazuki SM , Inoue T , Miura M , Iwasaki S . Plasma tea polyphnols and gastric cancer risk: A case-control study nested in large populaion-based prospective study in Japan. Cancer Epidemiology, Biomarkers & Prevention. (2008) ;17: :343–51. |
[17] | Pekal A , Pyrzynska PDP . Comparison of the antioxidant properties of commonly consumed commercial teas. Int J Food Pro. (2012) ;15: :5. |
[18] | Yen GC , Duh PD , Tsai CL . Relationship between antioxidant activity and maturity of peanut hulls. J Agric Food Chem. (1993) ;48: (41):67–70. |
[19] | Velioglu YS , Mazza G , Gao L , Oomah BD . Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J Agric Food Chem. (1998) ;46: :4113–7. |
[20] | Parr A , Bolwell GP . Phenols in the plant and in man: The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J Sci Food Agric. (2000) ;80: :985–1012. |
[21] | Matsubara S , Rodriguez-Rmaya DB . Conteúdo de miricetina, quercetina e kaempferol em chás comercializados no Brasil (Myciretin, quercetin and kaempterol contents in teas commercialized in Brazil). Ciênc Tecnol Aliment. (2006) ;26: (2):380–5. |
[22] | Kavantzas N , Chatziioannou A , Yanni AE , Tsakayannis D , Balafoutas D . Effect of green tea on angiogenesis and severity of atherosclerosis in cholesterol-fed rabbit. Vas Pharmcol. (2006) ;44: :461–3. |
[23] | Galleano M , Verstraeten SV , Oteiza PI , Fraga CG . Antioxidant actions of flavonoids: Thermodynamic and kinetic analysis. Arch Biochem Biophys. (2010) ;501: :23–30. |
[24] | Lambert JD , Elias RJ . The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch Biochem Biophys. (2010) ;501: :65–72. |
[25] | Lidofsky SD . Nonalcoholic fatty liver disease diagnosis, relation to metabolic syndrome and approach to treatment. Curr Diabetes Re. (2008) ;8: :25–30. |
[26] | Setorki M , Asgary S , Eidi A , Rohani AH , Khazaei M . Acute effects of vinegar intake on some biochemical risk factors of atherosclerosis in hypercholesterolemic rabbits. Lipids Health Dis. (2010) ;28: :1–10. |
[27] | Tijburg LBM , Wiseman SA , Meijer GW , Weststrate JA . Effects of green tea, black tea and dietary lipophylic antioxidants on LDL oxidizability and atherosclerosis in hypercholesterolemic rabbits. Atherosclerosis. (1997) ;135: :37–47. |
[28] | Yang CS , Landau JM . Effects of tea consumption nutrition health. J Nut. (2000) ;130: :2409–12. |
[29] | Yoo CL , Su PQ , Zhou XX . Study on effect and mechanism of Scutellariabaicalensis stem-leaf total flavonoid in regulating lipid metabolism. Zhongguo Zhong Yao ZA Zhi. (2008) ;33: (9):1046–56. |
[30] | Dobiásová M , Frohlich J . Advances in understanding of the role of lecithin cholesterolacyltransferase (LCAT) in cholesterol transport. Clinica Chimica Acta. (1999) ;286: :257–71. |
[31] | Raederstorff DG , Schlachter MF , Elste V , Weber P . Effect of EGCG on lipid absorption and plasma lipid levels in rats. J Nut Biochem. (2003) ;14: :326–32. |
[32] | Bruno RS , Dugan CE , Smyth JA , DiNatale DA , Koo SI . Green tea extract protects leptin-deficient, spontaneously obese mice from hepatic steatosis and injury. J Nut. (2008) ;138: :323–31. |
[33] | Vogiatzi G , Tousoulis D , Stefanadis C . The role of oxidative stress in atherosclerosis. Hellenic J Cardiol. (2009) ;50: :402–9. |
[34] | Zheng W , Huang LZ , Zhao L , Wang B , Xu HB , Wang GY , Wang ZL , Zhou H . Superoxide dismutase activity and malondialdehyde level in plasma and morphological evaluation of acute severe hemorrhagic shock in rats. Am J Emerg Med. (2008) ;26: :54–8. |
[35] | Assman G , Nofer J . Athropometric protective effects of high density lipoproteins. Annu Rev Med. (2003) ;54: :321–41. |
[36] | Mukhtar M , Ahmad N . Tea polyphenols: Prevention of cancer and optimizing health. Am J Clin Nutr. (2000) ;71: (6):1698–702. |
[37] | Liu CM , Zheng YL , Lu J , Zhang ZF , Fan SH . Quercetin protects rat liver against lead-induced oxidative stress and apoptosis. Environ Toxicol Pharmacol. (2010) ;29: :158–66. |
[38] | Avti PK , Kumar S , Pathak CM , Vaiphei K , Khanduja KL . Smokeless tobacco impairs the antioxidant defense in liver, lung, and kidney of rats. Toxicol Sci. (2006) ;89: :547–53. |
[39] | Clark JM . The epidemiology of nonalcoholicfattyliverdisease in adults. J Clin Gastroenterol. (2006) ;40: (3):5–10. |
[40] | Schreuder TC , Verwer BJ , VanNieuwkerk CM , Mulder CJ . Nonalcoholic fatty liver disease: An overview of current insights in pathogenesis, diagnosis and treatment. World J Gastroenterol. (2008) ;14: :2474–86. |
[41] | Vuillaume M . Reduced oxygen species, mutation, induction and cancer initiation. Mutat Res. (1987) ;186: :43–72. |
[42] | Urso ML , Clarkson PM . Oxidative stress, exercise, and antioxidant supplementation. Toxicol. (2003) ;189: :41–54. |
[43] | Marques C , Meireles M , Norberto S , Leite J , Freitas J , Pestana D , Faria A , Calhau C . High-fat diet-induced obesity Rat model: A comparison between Wistar and Sprague-Dawley Rat. ADIPOCYTE. (2016) ;5: (1):11–21. |
[44] | Crespy V , Williamson G . A review of the health effects of green tea catechins in in vivo animal models. J Nutr. (2004) ;134: :3431–40. |
[45] | Faber JL . Mechanism of cell injury by activated oxygen species. Environ Health Perspect. (1994) ;102: :17–24. |
[46] | He YH , Kies C . Green and black tea consumption by humans: Impact on polyphenols concentrations on feces, blood and urine. Plant Foods Hum Nutr. (1994) ;46: :221–9. |
[47] | Brown JE , Khodr H , Hider RC , Rice-Evans CA . Structural dependence of flavonoid interactions with Cu2 + ions: Implications for their antioxidant properties. Biochem J. (1998) ;330: (Pt3):1173–8. |
[48] | Adriene RL , Rosemary GFAP , Sheila AA , Márcio GZ , Fernanda BAP , Stella MSD . Effect of decaffeination of green and roasted coffees on the in vivo antioxidant activity and prevention of liver injury in rats. Rev Bras Farmacogn. (2013) ;23: (3):506–12. |
[49] | Al-Awaida W , Akash M , Aburubaiha Z , Talib WH , Shehadeh H . Chinese green tea (Lung Chen) consumption reduces oxidative stress, inflammation and tissues damage in smoke exposed rats. Iran J Basic Med Sci. (2014) ;17: :740–6. |
[50] | Terao J , Kawai Y , Murota K . Vegetable flavonoids and cardiovascular disease. Asia Pac J Clin Nutr. (2008) ;17: :291–3. |




