Effect of ultrasound treatment on protein content and functional properties of Spirulina powder grown in Algeria
Abstract
BACKGROUND:
There is an important interest to research new protein sources. In this context, Microalgae, particularly Spirulina (Arthrospira platensis), seems to be a good alternative due to its wealthy nutritional composition.
OBJECTIVES:
The aim of this study is to optimize Spirulina functional properties and to extract protein from whole biomass using (RSM).
METHODS:
Ultrasound wad used as pre-treatment to optimize functional properties and to extract proteins from Spirulina powder by isoelectric precipitation. The effect of ultrasound and remaining parameters (pH, temperature, solid to liquid ratio and time) was evaluated by Box-Behnken design. The model was fitted by ANOVA analysis.
RESULTS:
ANOVA analysis showed a significant model (p < 0.05) for functional properties and protein extraction. The protein content of Spirulina powder was found to be 55% (w/w). There is a significant effect of ultrasound on functional properties and protein extraction from Spirulina. The optimum Water Holding Capacity (WHC) was 4.97 g H2O/g Spirulina powder, obtained at pH 4, 50 W power and 5 min sonication. The optimum Oil Holding Capacity (OHC) is 2.3 g H2O/g Spirulina powder, it was obtained at 50°C temperature, 70 w power and 10 min sonication.
CONCLUSION:
Ultrasound has a significant effect on functional properties and protein extraction from Spirulina. Arthrospira platensis grown in Algeria could be incorporated in foodstuff as natural supplement to improve nutritional value and consumer acceptability.
1Introduction
Food safety and nutritional quality were always an important issue for consumer; they are strongly linked to human health. The future food threat is clear, produce more with less resources [1]. Demographic growth, climatic changes, diminishing natural resources (land, water, biological resources) are the most important factors that undermine food security in the world [2].
Nowadays, animal proteins source is the most important in human alimentation. The production of 1 Kg of animal proteins needs 4,9 Kg of vegetal proteins [3]. Thus, there is an important pressure on agriculture by cultures intensification [4]. Also, the consumption of animal proteins is targeted as the cause of several cardiovascular diseases [5]. So, researchers are giving more attention to non-conventional protein sources [6]. During last years, researchers focused on microalgae, especially cyanobateria as an alternative due to high protein content. Cyanobacteria can be found in terrestrial, glaciers, aerial marine; brackish and fresh water environment [7] no competing traditional agriculture in water or land. Among microalgae, Spirulina (Arthrospira platensis) is taking more attention related to its composition. It contains up to 70% proteins, many vitamins, mineral salts, fatty acids, most of amino acids making it a unique vegetarian source of complete protein [8]. Spirulina is autotrophic, it requires only inorganic compounds and light as an energy source for its growth and development [9]. In western countries, algal products have also become popular without the tradition of their usage, Spirulina is often used as supplement to prevent food deficiency in some developed countries [10]. In some developing countries, spirulina is used to fight malnutrition [10]. Nowadays, Spirulina is approved as food for human consumption by many governments and agencies [11].
Also, due to its remarkable protein content, Spirulina can be incorporated to foodstuff to enrichment purpose. Nevertheless, fish taste and green color of Spirulina powder decreased foodstuff acceptability by customers [12]. To overcome this trouble, protein extract from Spirulina is interesting; it could be incorporated to foodstuff instead of whole biomass. In addition to nutritional fact, many of the vegetable proteins require processing to provide a food material having acceptable functional properties, such as emulsification, fat and water absorption, texture modifications, color control and whipping properties, which are attributed primarily to the protein characteristics [13] which improve food acceptability. In consequence, the nutritional value and food acceptability are insured. Recently, some studies revealed that the use of ultrasound increases the concentration of proteins of Spirulina [14]. Another study shows the positive effect of sonication time and sonication energy to recovery protein yield [15]. Also, the application of Response Surface Methodology in the optimization of analytical procedures is very beneficial due to the generation of large amount of information from a small number of experiments [16].
In Algeria, a semi-arid country where 47% of protein intake is imported [17], wide desert land is available and suitable to Spirulina production. It seems to be the alternative to Algeria food threat as it can cover local protein intake submitted to climatic issue (low pluviometry). Spirulina is small scale produced southern Algeria. After collection, it is submitted to sun drying and sold in powder form as food supplement. Some authors studied the incorporation of spirulina in couscous, local traditional dish [18] and in beverage mix with dates [19]. But, the literature on functional properties and protein extraction is still poor. The aim of this study is to optimize functional properties and extract proteins from Spirulina powder grown in Algeria using Response Surface Methodology (RSM) in order to enrich foodstuff.
2Material and methods
2.1Raw material
Spirulina (Arthrospira platensis) powder was provided from southern Algeria (Tamanrasset). After harvest, the material was submitted to sun drying. Moisture content is about 6% sonication time and sonication energy (Table 1).
Table 1
Ranges and levels of independent variables used in Box-Benhken design
| Ranges and levels | |||
| Independent variables | –1 | 0 | +1 |
| Solid to liquid ration (mg/ml) | 1:100 | 3:100 | 5:100 |
| Sonication energy (W) | 30 | 50 | 70 |
| Sonication time (mn) | 4 | 8 | 12 |
2.2Protein extraction
Spirulina powder was added to purified water at different solid to liquid ratio (w/v), sonication time and sonication energy (Table 1). The slurry was stirred until homogeneous solution is obtained. The solution was sonicated using an ultrasonic bath (Fisher bioblock Transonic TI-H ultrasonic bath, 25–45 KHz) settled on the following conditions: Frequency of 25 KHz; sonication energy of 50 W and sonication time of 12 min). A solid to liquid ratio of 1:100 was used and NaOH 1N was added to pH 10. The slurry was then centrifuged (Hettich EBA 8, Germany) at 7000 rpm during 30 mn. After collect of supernatant, TCA 0.3 M was added until pH 3. The solution was centrifuged at 7000 rpm during 30 min; the pellets were collected and neutralized at pH7 with NaOH 1N and preserved to further trials.
2.3Protein content
The protein content was obtained by The Lowry method [20]. The Folin–Ciocalteu reactive (Sharlau, Belgium) was diluted in two volumes of purified water (1:2) and 0.5 mL of the diluted reactive was added to 1.0 mL of sample, previously mixed with 5.0 mL of the reactive “C” [50 volumes of reactive “A” (2.0% Na2CO3 +0.1N NaOH) +1 volume of reactive “B” (1/2 volume of 0.5% CuSO4 5H2O + 1/2 volume of 1.0% C4H4NaO6 4H2O)]. Then, after, samples were stirred for 2 s in a test tube stirrer. Absorbance was measured at 750 nm, 35 min after the start of the chemical reaction at room temperature [21]. Calibration curve is prepared for each assay using Bovine Serum Albumin (BSA) stock solution (200 mg/l). A standard curve is generated with equation form of y = ax+b (Microsoft Excel, 2010), coefficient of correlation (R2) is significant, value is 0.99. The spectrophotometric absorbance was converted to protein concentration using the established calibration curve using the following equation [22].
(1)
where C is the protein concentration (mg/l) obtained from the calibration curve, V is the volume (L) of the lysis buffer used to resuspend the biomass, D is the dilution factor and m is the amount of biomass (mg).
2.4Water holding capacity (WHC)
The WHC of the protein was measured using a modified version of the method of Yuliana et al. [23]. Protein isolate was vigorously mixed with water (1:10, w/w) for 5 min. The slurry was then centrifuged (1500 g, 30 min). The supernatant was weighed and WHC was determined [23].
(2)
where W0 is the weight of the dry sample (g), W1 the weight of the tube plus the dry sample (g) and W2 weight of the tube plus the sediment (g).
2.5Oil holding capacity (OHC)
The OAC of the protein isolate was determined using the method of Yuliana et al. [23]. Protein isolate (0.5 g) was mixed with castor oil (5 cm3) for 1 h at 30°C. The mixture was centrifuged (1500 g, 30 min) and the oil was decanted. The oil trapped mixture was weighed. OAC was expressed as ml of oil trapped per g protein isolate [23].
(3)
where W is the weight of the dry sample (g), V1 is the weight of the tube plus the dry sample (g) and V2 is the weight of the tube plus the sediment (g).
2.6Experimental design
In the present study, a total of 15 experiments were used in the Box-Behnken design (BBD) in order to optimize the both functional properties. For WHC, three parameters, pH, sonication energy and sonication time were optimized by RSM to obtain WHC values by the combination of the three independent variables (Table1). For OHC, the three parameters are temperature, sonication energy and sonication time. The three independent variables are shown in Table 2. The response, for both WHC and OHC was related to the independent variables by the quadratic polynomial model represented as in the following equation:
(4)
where Y is the dependent response, β0, βi, βii and βij are the regression coefficients for intercept, linear, quadratic and interaction terms, respectively; xi and xJ are the independent coded variables.
Table 2
Ranges and levels of independent variables for WHC used in Box-Behnken design
| Ranges and levels | |||
| Independent variables | –1 | 0 | +1 |
| pH | 4 | 7 | 10 |
| Sonication energy (% ) | 30 | 50 | 70 |
| Sonication time (min) | 5 | 10 | 15 |
Table 3
Ranges and levels of independent variables for OHC used in Box-Behnken design
| Ranges and levels | |||
| Independent variables | –1 | 0 | +1 |
| Temperature | 25 | 35 | 50 |
| Sonication energy (% ) | 30 | 50 | 70 |
| Sonication time (min) | 5 | 10 | 15 |
2.7Statistical analysis
The results of the present study were statistically analysed by ANOVA (analysis of variance). Significance of the models was accepted at a level of p < 0.05. The response surfaces were used to determine the optimal conditions of the both functional properties. The statistical software Statistica 10 (Statsoft Inc, USA) was used to those purposes.
3Results and discussion
3.1Protein content
In the present study, the total protein content of Spirulina powder was found to be 55% (w/w) (Table 4). According to Becker [6], the amount of protein present in Spirulina varied between 46 and 62% of its dry weight. This variation was probably related to differences of geographical origin, growing conditions and production methods of Spirulina and more particularly to proteins extraction techniques. In this work, the ANOVA analysis shown that ultrasound is significant with R2 of 0.98. It is confirmed by the curve of predicted versus observed values (Fig. 1). The effect of ultrasound is also confirmed by Pareto Diagram (Fig. 2). The principal parameter affecting extraction is solid to liquid ratio. Further to intercept value from ANOVA analysis, the model mathematical equation would be as follows:
(5)
Where:
LSR: liquid to solid ratio.
Fig.1
Predicted and observed values for protein yield (%) of Arthrospira platensis.
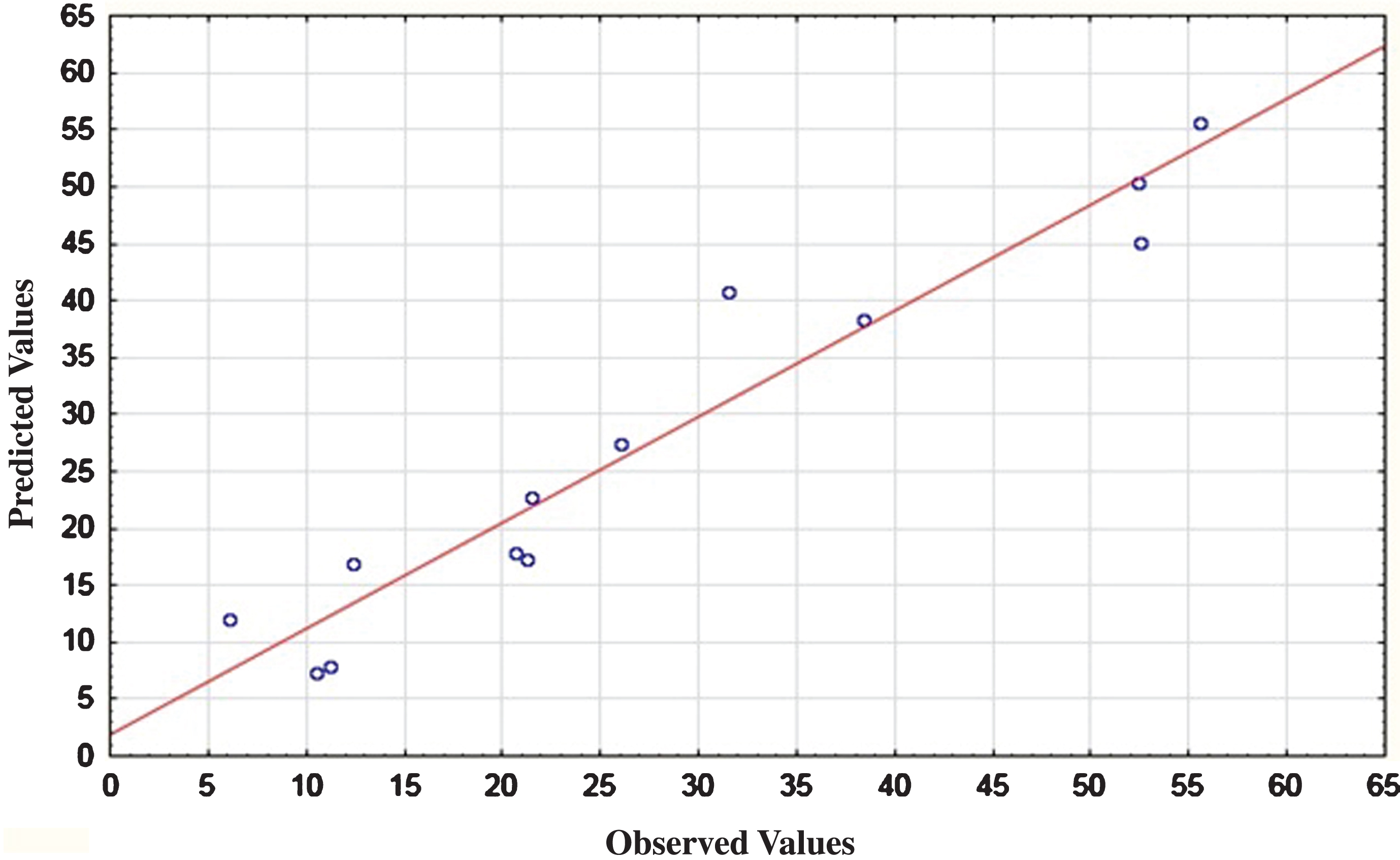
Fig.2
Pareto diagram of Standardized effects (Protein yield).
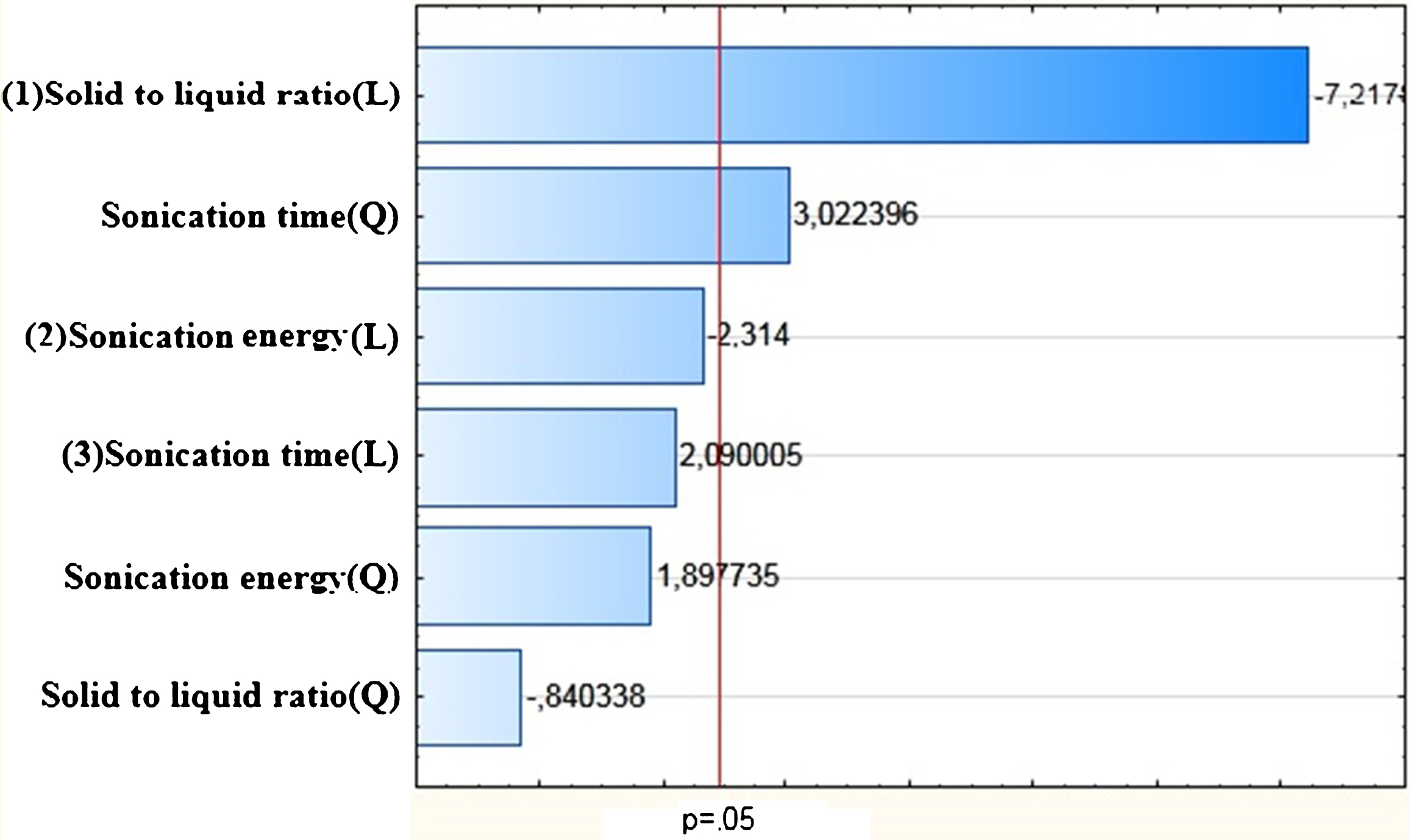
Table 4
Box Behnken Design for protein extraction response for Arthrospira platensis
| Runs | Liquid to solid ratio | Sonication energy | Sonication time | Protein yield (%) |
| 1 | –1 | –1 | 0 | 55,541 |
| 2 | –1 | 0 | –1 | 31,482 |
| 3 | –1 | 0 | 1 | 52,370 |
| 4 | –1 | 1 | 0 | 52,464 |
| 5 | 0 | –1 | –1 | 20,619 |
| 6 | 0 | –1 | 1 | 26,044 |
| 7 | 0 | 0 | 0 | 37.27 |
| 8 | 0 | 0 | 0 | 38,405 |
| 9 | 0 | 1 | –1 | 10,542 |
| 10 | 0 | 1 | 1 | 12,365 |
| 11 | 1 | –1 | 0 | 21,489 |
| 12 | 1 | 0 | –1 | 11,230 |
| 13 | 1 | 0 | 1 | 21,262 |
| 14 | 0 | 0 | 0 | 39.68 |
| 15 | 1 | 1 | 0 | 6,062 |
Those results were higher than those found by Lupatini et al. (38% (w/w)) [24] and Safi et al. [14] (47% (w/w)). This may be due to use of ultrasound as a pre-treatment before protein extraction. The effect of ultrasound on protein content was confirmed by Show et al. [25], by increasing the penetration of solvent into cellular material. Those results were similar to literature as the sonication increase protein content of Arthrospira platensis [13]. For microalgae Nannochloropsis, higher protein content is observed after sonication [26].
3.2Functional properties
Recently, many researchers were using a combination of ultrasound and response surface methodology (RSM) in order to optimized the process variables in their works [27–30]. Even though a food is nutritionally wealth and balanced, its acceptability could be poor and, thus, rejected by consumers. Improving functional properties of food is of great interest to fill this gap. In this context, optimization of functional properties of Spirulina powder using RSM was investigated.
3.2.1Water holding capacity
This property is important in the formulation of many foods [31]. The water holding capacity (WHC) of foods can be defined as the ability to hold its own and added water during the application of forces, pressing, centrifugation or heating [32]. WHC plays a major role in the formation of food texture, especially in comminuted meat products and baked doughs. Water retention of plant proteins used as additives in various foods influences quality characteristics of the finished food products [33].
3.2.1.1Model fitting.
Effects of the three extractions parameters, namely pH, sonication energy and sonication time were determined by Box-Behnken Design. As shown in Pareto Diagram (Fig. 3), the effect of each parameter was determined, the sonication time is the most influent parameter in quadratic effect, but there is no significant effect of sonication energy and pH. The statistical analysis of the model and significance of each parameter was determined by F-test and p-value. The corresponding parameters would be more significant if the absolute F-value becomes greater and the p-value becomes smaller [34]. The model is significant as p-value is less than 0.05 and F-value very high. The determination coefficient (R2) of the model is 0.93 which confirmed the good fit of the model (Table 5). The deviation between predicted and observed values is very low (Fig. 4). The pH has no significance on WHC, this result is similar to study of Benelhadj et al. [35] where higher WHC is obtained at pH10 due to the increase of bound water further to the increase of polarity and electric charge. Maximum value of WHC observed is 4.97 g H2O/g powder while minimum value is 3.86 g H2O/g powder (Table 6). In similar study, the WHC of spirulina flour was 2,2 g H2O/g spirulina, which shows the effect of ultrasound [36], and maize flour and wheat flour blend [37]. Also, WHC obtained in this study is higher than legumes flour [38], and soya meal [36]. The presence of polysaccharides at 15% in Spirulina powder may explain WHC high value. Higuerra-Barraza et al. [39] concluded that 20 min of ultrasound resulted in an increase of the proportion of water trapped with the myofibrillar of proteins.
Fig.3
Pareto Diagram on Standardized effects (WHC).
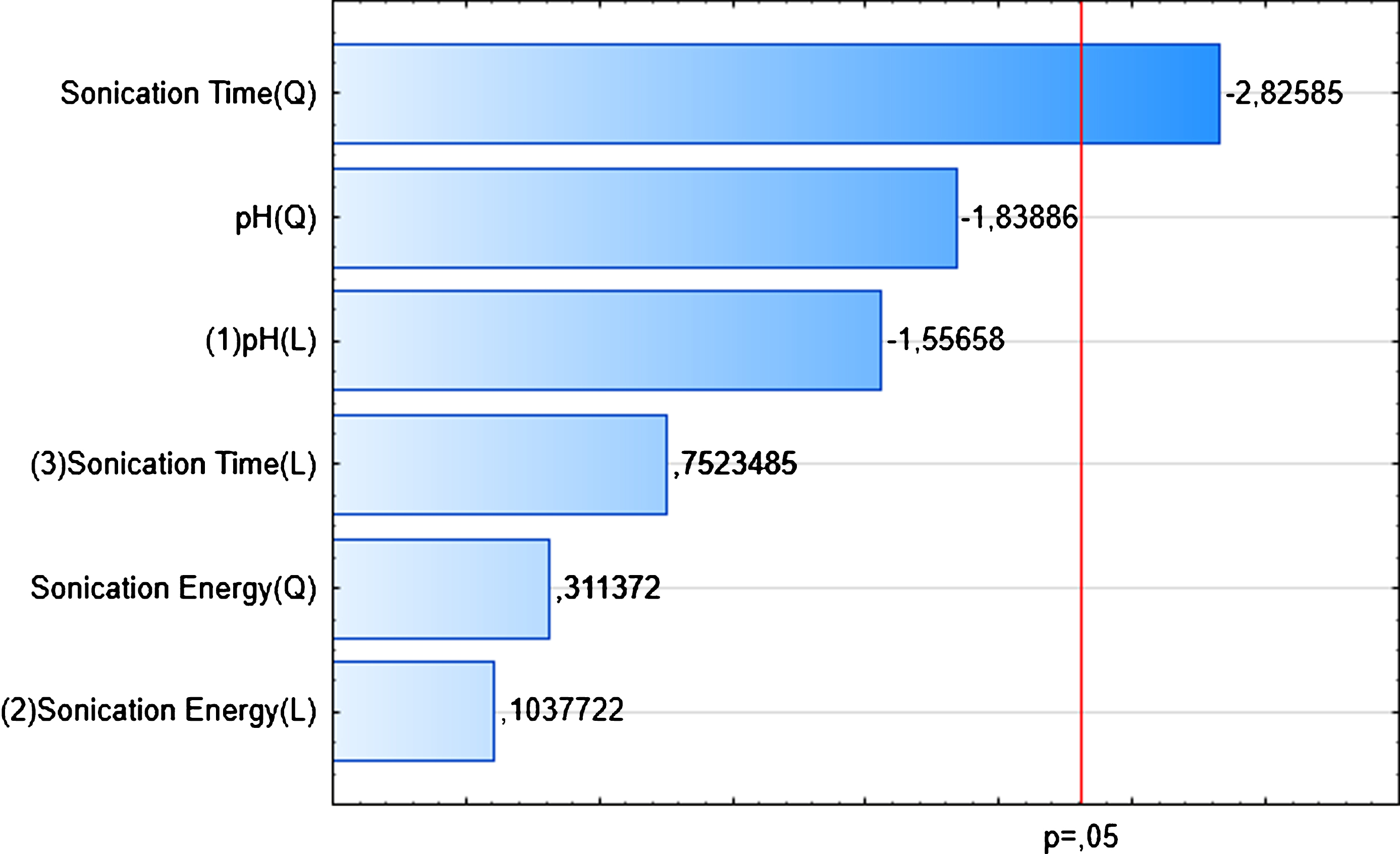
Table 5
Responses model fitting
| Coefficients | WHC | OHC |
| R2 | 0.93 | 0.85 |
| R2 adj. | 0.82 | 0.58 |
| P | 0.01 | 0.1 |
Fig.4
Predicted and observed values for WHC (g H2O/g powder) of Arthrospira platensis.
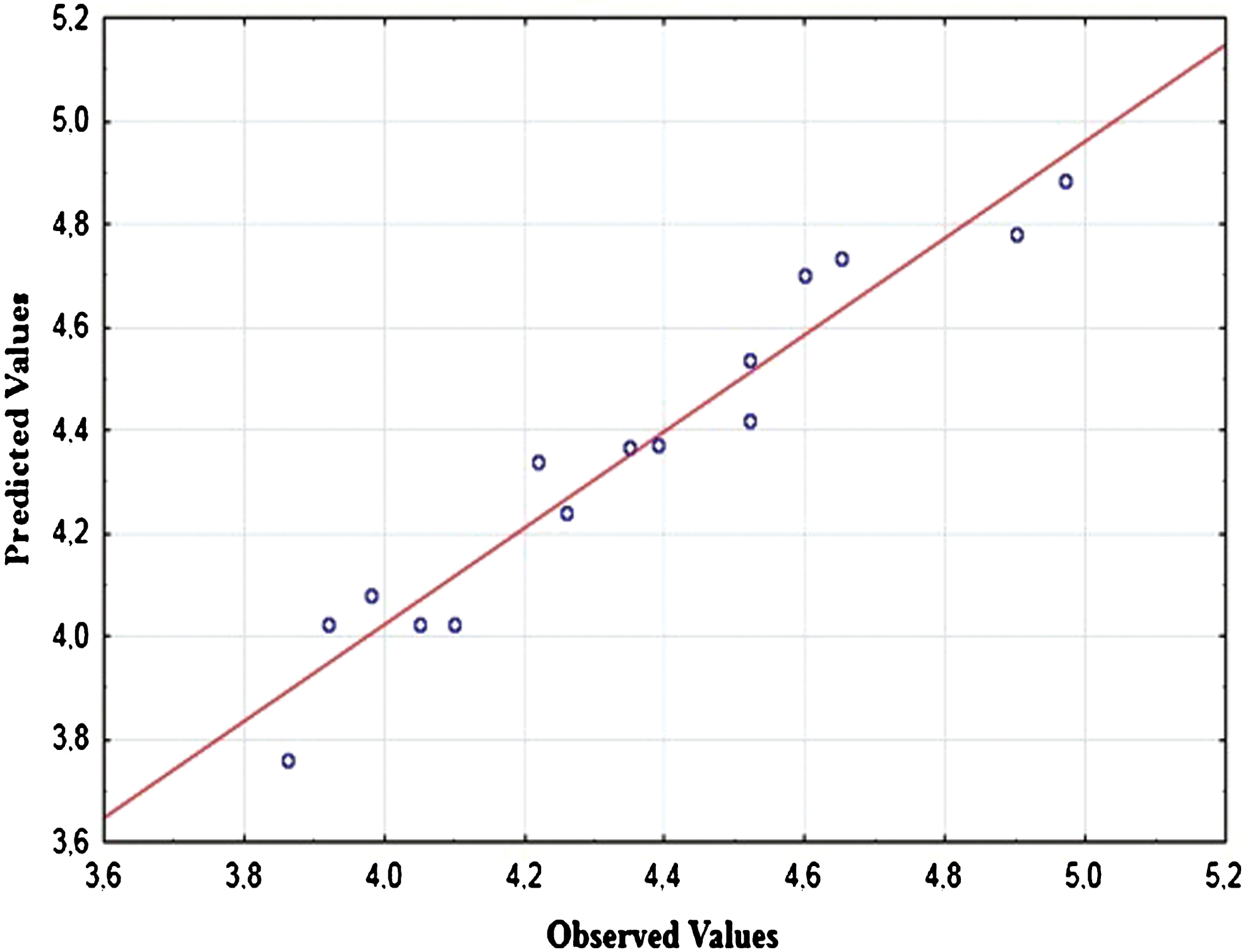
Table 6
Box-Behnken design and observed values of WHC
| Runs | pH | Energie | Temps | WHC |
| 1 | −1 | 0 | +1 | 4,97 |
| 2 | 0 | 0 | 0 | 3,92 |
| 3 | −1 | 0 | 1 | 4,9 |
| 4 | 1 | 0 | ‒1 | 4,22 |
| 5 | −1 | −1 | 0 | 4,6 |
| 6 | 1 | 0 | 1 | 4,65 |
| 7 | 0 | 1 | −1 | 4,26 |
| 8 | 0 | 1 | 1 | 4,52 |
| 9 | 0 | −1 | 1 | 4,35 |
| 10 | 0 | 0 | 0 | 4,05 |
| 11 | 1 | 1 | 0 | 4,52 |
| 12 | 0 | 0 | 0 | 4,1 |
| 13 | −1 | 1 | 0 | 3,98 |
| 14 | 0 | −1 | −1 | 4,39 |
| 15 | 1 | −1 | 0 | 3,86 |
3.2.1.2Analysis of surface response.
The effect of sonication time and pH on WHC of Spirulina flour is significant both in quadratic and linear effect (Fig. 5a). The increase of sonication time increases WHC, while the decrease of pH increases WHC. Figure 5b shows the effect of sonication time and sonication energy, we observe the quadratic effect of sonication time, but there is no effect of sonication energy on WHC. In Fig. 5c, we can see the quadratic effect of pH, WHC increases for values less than pH 4. From regression analysis values (Table 7), we can estimate mathematical model equation:
(6)
Where:
ST: Sonication time.
SE: Sonication energy.
Fig.5
Response surface plots of WHC versus, (a) Sonication time and pH (b) Sonication time and sonication energy (c) Sonication energy and pH.
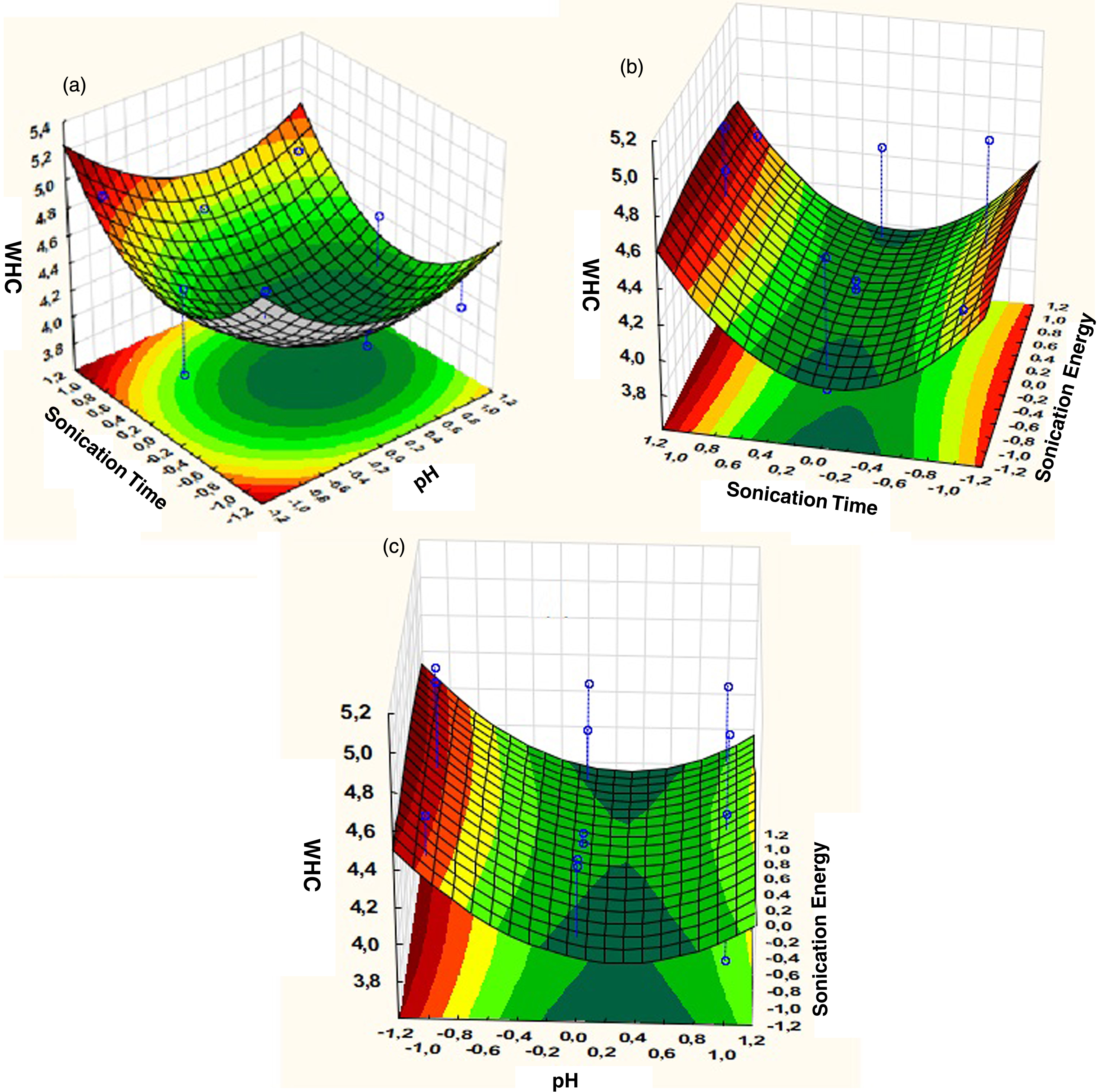
Fig.6
Pareto Diagram on Standadized effects (OHC).
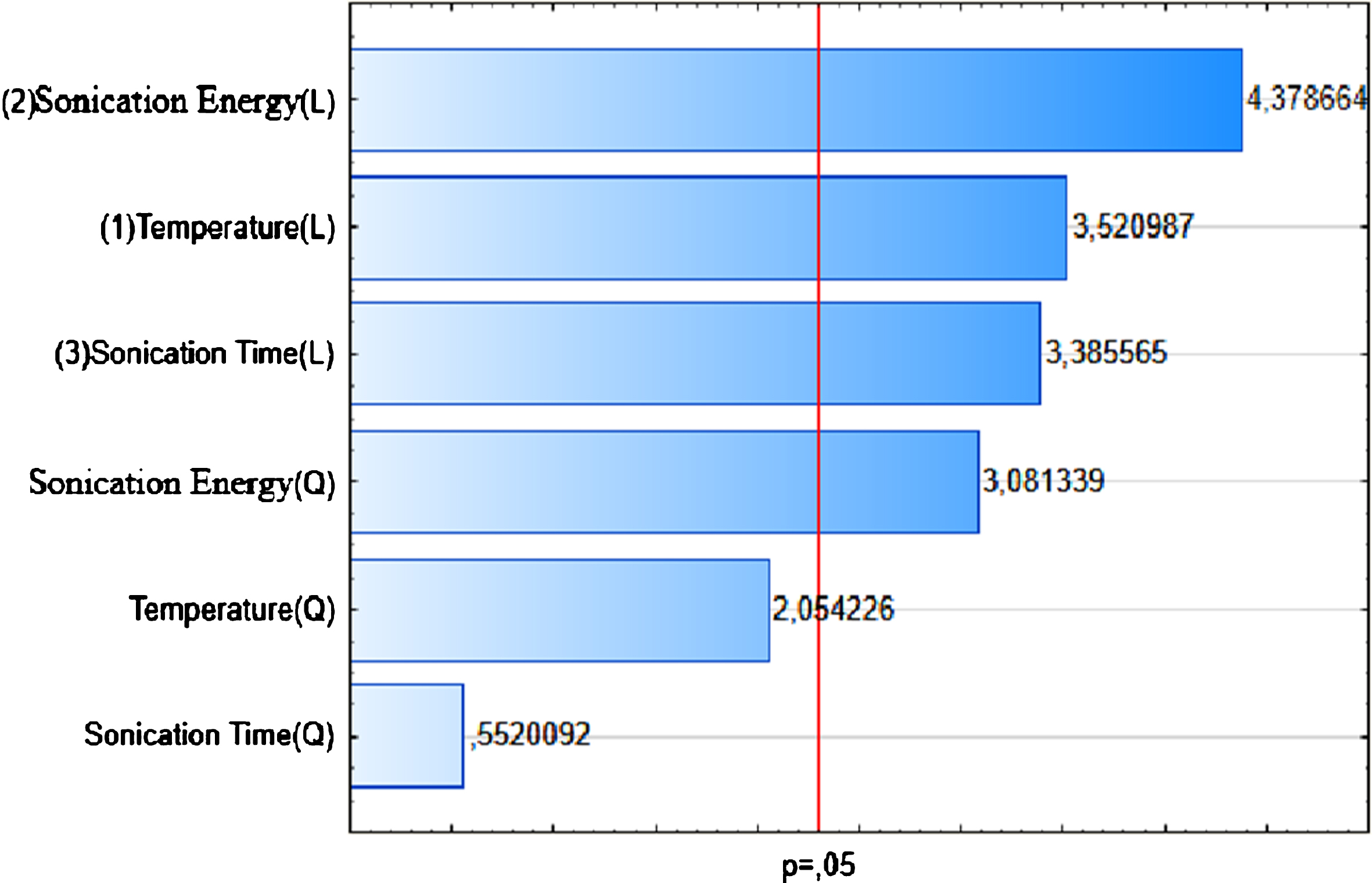
Table 7
WHC regression analysis
| Response WHC | |||||
| Source of variation | Sum of squares | Freedom Degree | Mean square | Fc | Probability Fc |
| Regression | 1.53 | 9 | 0.17 | 8.57 | 0.014 |
| Residual | 0.099 | 5 | 0.019 | ||
| Total | 1.629 | 14 | 0.189 | ||
3.2.2Oil holding capacity
Measurements of fat binding may provide data for selecting a protein raw material. Many properties of foods involve the interactions of proteins and lipids: formation of emulsions, fat emulsification in meats, fat entrapment in sausage batters, flavor absorption, and dough preparation [33]. High oil holding capacity is desirable for use in the cold meat industry, particularly for sausages where the protein can bridge the fat and water.
3.2.2.1Fitting the model.
Effect of the three extraction parameters: temperature, sonication time and sonication energy were determined by Box-Behnken Design. The effect of each parameter is determined by Pareto Diagram (Fig. 3). The sonication energy is the most influent parameter in linear effect, followed by temperature and sonication time in linear effect. Also, we notice a quadratic effect of sonication energy. The significance of the model is confirmed by F-test and p-value. Coefficient of determination R2 is 0.85 confirming the good fit of the model (Table 5). Figure 7 shows a small difference between predicted and observed values, coefficient of variation is very low (2,1%). The values of OHC are significant as a measure of hydrophobic capacity of proteins. Maximum OHC value obtained is 2.3 ml H2O/g Spirulina powder, while minimum value is 1.97 ml H2O/g Spirulina powder (Table 8). It is slightly higher than the value obtained by Devi and Venkatarman [36] due to the influence of ultrasound which enhance protein release. Also, OHC values obtained in this study are higher than full fat maize flour (1.07) and wheat flour (0.7) [37]. The high protein content in Spirulina explain this difference, protein side chain hydrophobic group increases and more protein oil complexes are generated [40]. This approach in confirmed in the study on several whole legumes flour where for protein content ranging between 22% and 28%, the OHC is between 0,93 g H2O/g legume flour and 1,38 g H2O/g legume flour [38].
Fig.7
Predicted and observed values for OHC (g H2O/g powder) of Arthrospira platensis.
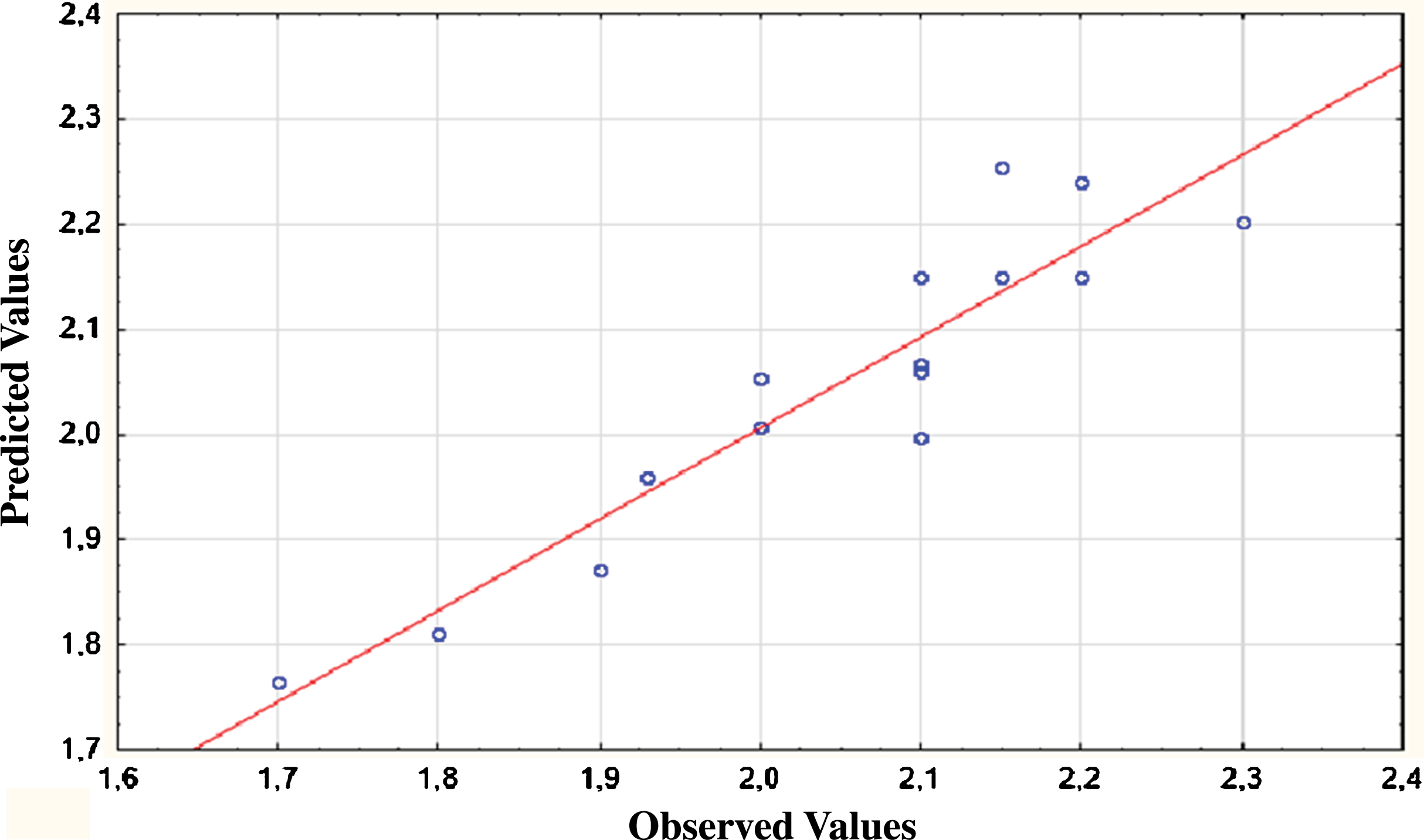
Table 8
Box-Behnken design and observed values of OHC
| Runs | Temperature | Sonication time | Sonication energy | OHC |
| 1 | 0 | 0 | 0 | 2,1 |
| 2 | 0 | 0 | 0 | 2,2 |
| 3 | 1 | 0 | 1 | 2,15 |
| 4 | 1 | 0 | – 1 | 2,1 |
| 5 | – 1 | – 1 | 0 | 1,7 |
| 6 | – 1 | 1 | 0 | 2 |
| 7 | 1 | 1 | 0 | 2,3 |
| 8 | 0 | – 1 | – 1 | 1,8 |
| 9 | 0 | 0 | 0 | 2,15 |
| 10 | – 1 | 0 | 1 | 2,1 |
| 11 | 1 | – 1 | 0 | 1,93 |
| 12 | 0 | 1 | 1 | 2,2 |
| 13 | 0 | – 1 | 0 | 2,1 |
| 14 | 0 | 1 | – 1 | 2 |
| 15 | – 1 | 0 | – 1 | 1,9 |
3.2.2.2Surface response analysis.
We observe the linear and quadratic effect of sonication energy, OHC increases when sonication energy increases, the same influence is observed for temperature in quadratic effect (Fig. 8a). Sonication time has a linear effect, when sonication time increases, OHC increases (Fig. 8b). In Fig. 8c, we can observe the effect of sonication energy, both in linear and quadratic effect, while sonication time is significant only in linear effect. The model mathematical equation could be determined from regression analysis values (Table 9), and is as follows:
(7)
T: Temperature (°C)
SE: Sonication Energy (%)
Fig.8
Response surface plots of OHC versus, (a) Sonication energy and temperature (b) Sonication time and temperature (c) Sonication time and sonication energy.
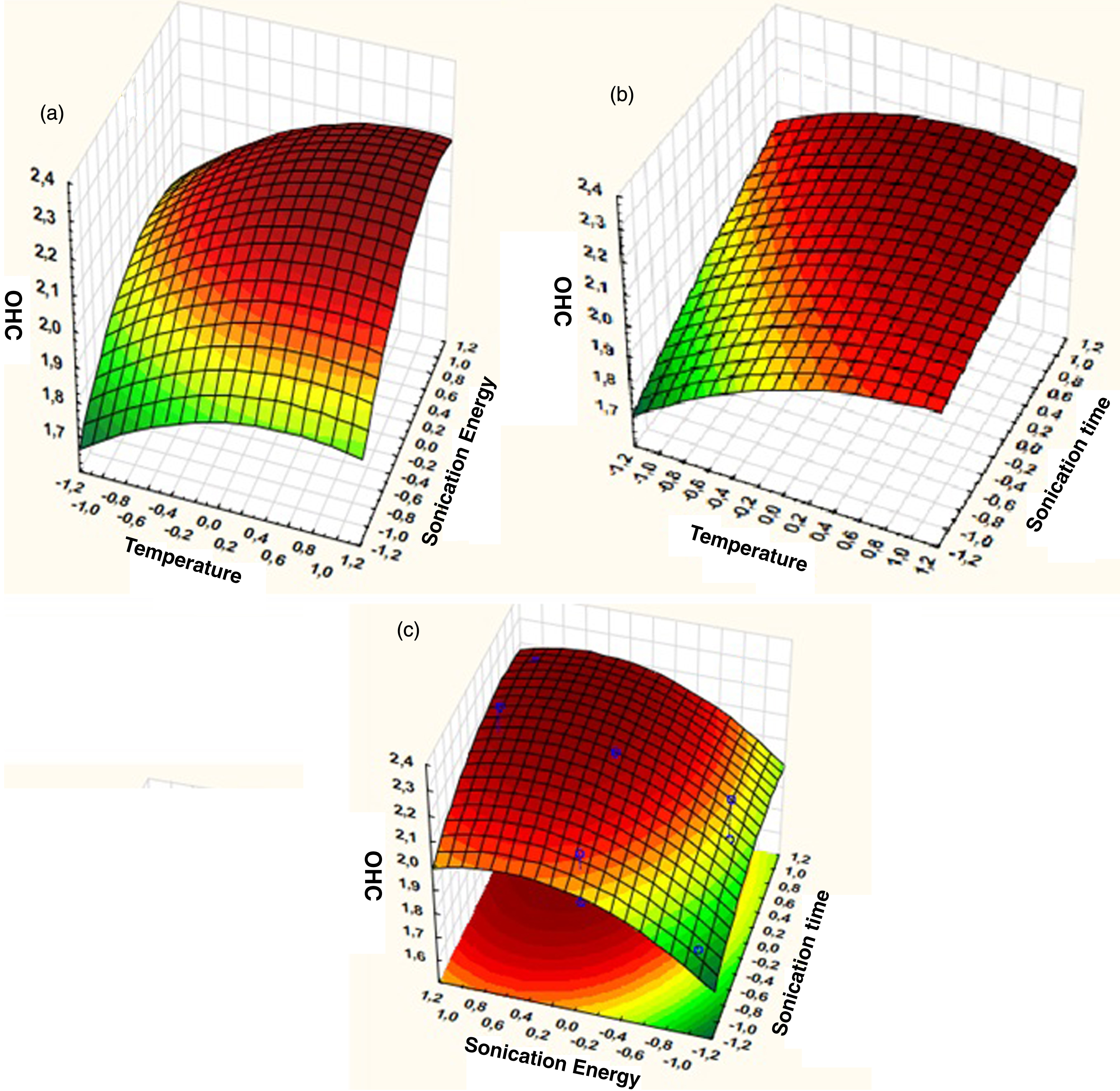
Table 9
OHC regression
| Response OHC | |||||
| Source of variation | Sum of squares | Freedom Degree | Mean square | Fc | Probability Fc |
| Regression | 0.31 | 9 | 0.03 | 3.18 | 0.1 |
| Residual | 0.054 | 5 | 0.01 | ||
| Total | 0.364 | 14 | 0.04 | ||
4Conclusion
Recently, there is a growing interest to search natural protein sources as an alternative to unhealthy animal proteins in the food industry. In this context, Spirulina (Arthrospira platensis) has attracted a lot of interest due to its interesting nutritional properties. The present work demonstrated that response surface methodology was a good tool to determine optimal conditions of Arthrospira platensis functional properties. Furthermore, the use of ultrasound as pretreatment contributed to improve protein content, water holding capacity (WHC) and oil holding capacity (OHC) of Spirulina powder. Indeed, the WHC and OHC have been increased up to 4.97 g H2O/g Spirulina powder and 2.3 ml H2O/g Spirulina powder respectively. Those values were obtained at pH 4, 70 W sonication power and 10 min for WHC, temperature of 50°C, 30 W sonication power and 8 min. The high protein content of Spirulina powder tested in the present study demonstrated the positive effect of ultrasound on protein extraction. The protein yield obtained is 55% at liquid to solid ratio of 1:100, 50W sonication energy and 12 min sonication time. The good functional properties of Algerian Spirulina powder may be attributed to its high protein content. Thus, Arthrospira platensis incorporation in foodstuff will improve nutritional value and consumer acceptability.
References
[1] | Schneider UA , Havlík P , Schmid E , Valin H , Mosnier A , Obersteiner M , et al. Impacts of population growth, economic development, and technical change on global food production and consumption. Agricultural Systems (2011) ;104: (2):204-15. |
[2] | Wu S-H , Ho C-T , Nah S-L , Chau C-F. Global hunger: A challenge to agricultural, food, and nutritional sciences. Critical Reviews in Food Science and Nutrition. (2014) ;54: (2):151-62. |
[3] | Guéguen J , Walrand S , Bourgeois O. Les protéines végétales: Contexte et potentiels en alimentation humaine. Cahiers de Nutrition et de Diététique. (2016) ;51: (4):177-85. |
[4] | De Boer J , Aiking H. On the merits of plant-based proteins for global food security: Marrying macro and micro perspectives. Ecological Economics. (2011) ;70: (7):1259-65. |
[5] | Boffetta P , Couto E , Wichmann J , Ferrari P , Trichopoulos D , Bueno-de-Mesquita HB , et al. Fruit and vegetable intake and overall cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). Journal of the National Cancer Institute. (2010) ;102: (8):529-37. |
[6] | Becker EW. Micro-algae as a source of protein. Biotechnology Advances. (2007) ;25: (2):207-10. Epub 2007/01/02. |
[7] | Dixit RB , Suseela M. Cyanobacteria: Potential candidates for drug discovery. Antonie Van Leeuwenhoek. (2013) ;103: (5):947-61. |
[8] | Mahajan A , Ahluwalia A. Effect of processing on functional properties of Spirulina protein preparations. African Journal of Microbiology Research. (2010) ;4: (1):055-60. |
[9] | Pignolet O , Jubeau S , Vaca-Garcia C , Michaud P. Highly valuable microalgae: Biochemical and topological aspects. Journal of Industrial Microbiology & Biotechnology. (2013) ;40: (8):781-96. |
[10] | Simpore J , Zongo F , Kabore F , Dansou D , Bere A , Nikiema J-B , et al. Nutrition rehabilitation of HIV-infected and HIV-negative undernourished children utilizing spirulina. Annals of Nutrition and Metabolism. (2005) ;49: (6):373-80. |
[11] | Finamore A , Palmery M , Bensehaila S , Peluso I. Antioxidant, immunomodulating, and microbial-modulating activities of the sustainable and ecofriendly spirulina. Oxidative Medicine and Cellular Longevity. (2017) ;2017. |
[12] | Falquet J , Hurni J. J. Spiruline: Aspects nutritionnels: Flamant vert; (1986) . |
[13] | Ogunwolu SO , Henshaw FO , Mock H-P , Santros A , Awonorin SO. Functional properties of protein concentrates and isolates produced from cashew (Anacardium occidentale L.) nut. Food Chemistry. (2009) ;115: (3):852-8. |
[14] | Safi C , Ursu AV , Laroche C , Zebib B , Merah O , Pontalier P-Y , et al. Aqueous extraction of proteins from microalgae: Effect of different cell disruption methods. Algal Research. (2014) ;3: :61-5. |
[15] | Lupatini AL , de Oliveira Bispo L , Colla LM , Costa JAV , Canan C , Colla E. Protein and carbohydrate extraction from S. platensis biomass by ultrasound and mechanical agitation. Food Res Int. (2017) ;99: (Pt 3):1028-35. Epub 2017/09/04. |
[16] | Bezerra MA , Santelli RE , Oliveira EP , Villar LS , Escaleira LA. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. (2008) ;76: (5):965-77. |
[17] | Akçay S , Karasoy A. Remittances and calorie consumption nexus in Algeria. International Migration. (2017) ;55: (4):103-17. |
[18] | Doumandji A , Boutekrabt L , Saidi NA , Doumandji S. Etude de l’impact de l’incorporation de la spiruline sur les propriétés nutritionnelles, technologiques et organoleptiques du couscous artisanal. Revue « Nature & Technologie» n. (2012) ;6: :41. |
[19] | Benahmed Djilali A , Benamara S , Saidi N , Meksoud A. Preliminary characterization of food tablets from date (Phoenix dactylifera L.) and spirulina (Spirulina sp.) powders. Powder Technology. (2011) ;208: (3):725-30. |
[20] | Slocombe SP , Ross M , Thomas N , McNeill S , Stanley MS. A rapid and general method for measurement of protein in micro-algal biomass. Bioresource Technology. (2013) ;129: :51-7. |
[21] | Barbarino E , Lourenço SO. An evaluation of methods for extraction and quantification of protein from marine macro-and microalgae. Journal of Applied Phycology. (2005) ;17: (5):447-60. |
[22] | Lopez CV , Garcia Mdel C , Fernandez FG , Bustos CS , Chisti Y , Sevilla JM. Protein measurements of microalgal and cyanobacterial biomass. Bioresource Technology. (2010) ;101: (19):7587-91. Epub 2010/05/25. |
[23] | Yuliana M , Truong CT , Huynh LH , Ho QP , Ju Y-H. Isolation and characterization of protein isolated from defatted cashew nut shell: Influence of pH and NaCl on solubility and functional properties. LWT - Food Science and Technology. (2014) ;55: (2):621-6. |
[24] | Lupatini AL , de Oliveira Bispo L , Colla LM , Costa JAV , Canan C , Colla E. Protein and carbohydrate extraction from S. platensis biomass by ultrasound and mechanical agitation. Food Research International. (2016) . |
[25] | Show KY , Lee DJ , Tay JH , Lee TM , Chang JS. Microalgal drying and cell disruption–recent advances. Bioresource Technology. (2015) ;184: :258-66. Epub 2014/12/04. |
[26] | Gerde JA , Montalbo-Lomboy M , Yao L , Grewell D , Wang T. Evaluation of microalgae cell disruption by ultrasonic treatment. Bioresource Technology. (2012) ;125: :175-81. |
[27] | Aydar A , Bağdatlioğlu N , Köseoğlu O. Effect of ultrasound on olive oil extraction and optimization of ultrasound-assisted extraction of extra virgin olive oil by response surface methodology (RSM). Grasas y Aceites. (2017) ;68: (2):189. |
[28] | Ait Chaouche SF , Mouhouche F , Hazzit M , Ferradji A. Optimization of Extraction Yield of Algerian Mentha pulegium L. Essential Oil by Ultrasound-Assisted Hydrodistillation using Response Surface Methodology. Research Journal of Phytochemistry. (2017) ;11: (3):142-9. |
[29] | Li H-Z , Zhang Z-J , Hou T-Y , Li X-J , Chen T. Optimization of ultrasound-assisted hexane extraction of perilla oil using response surface methodology. Industrial Crops and Products. (2015) ;76: :18-24. |
[30] | Ali A , Lim XY , Chong CH , Mah SH , Chua BL. Optimization of ultrasound-assisted extraction of natural antioxidants from Piper betle using response surface methodology. LWT. (2018) ;89: :681-8. |
[31] | Kinsella JE , Melachouris N. Functional properties of proteins in foods: A survey. Critical Reviews in Food Science & Nutrition. (1976) ;7: (3):219-80. |
[32] | Zayas JF. Water holding capacity of proteins. Functionality of proteins in food: Springer; (1997) . pp. 76-133. |
[33] | Zayas JF. Functionality of proteins in food: Springer Science & Business Media; (2012) . |
[34] | Tan E-S , Ying-Yuan N , Gan C-Y. A comparative study of physicochemical characteristics and functionalities of pinto bean protein isolate (PBPI) against the soybean protein isolate (SPI) after the extraction optimisation. Food Chemistry. (2014) ;152: :447-55. |
[35] | Benelhadj S , Fejji N , Degraeve P , Attia H , Ghorbel D , Gharsallaoui A. Properties of lysozyme/Arthrospira platensis (Spirulina) protein complexes for antimicrobial edible food packaging. Algal Research. (2016) ;15: :43-9. |
[36] | Devi MA , Venkataraman L. functional properties of protein products of mass cultivated blue-green alga Spirulina platensia. Journal of Food Science. (1984) ;49: (1):24-7. |
[37] | Shad MA , Nawaz H , Noor M , Ahmad HB , Hussain M , Choudhry MA. Functional properties of maize flour and its blends with wheat flour: Optimization of preparation conditions by response surface methodology. Pak J Bot. (2013) ;45: (6):2027-35. |
[38] | Du S-K , Jiang H , Yu X , Jane J-L. Physicochemical and functional properties of whole legume flour. LWT-Food Science and Technology. (2014) ;55: (1), 308-13. |
[39] | Higuera-Barraza O , Del Toro-Sanchez C , Ruiz-Cruz S , Márquez-Ríos E. Effects of high-energy ultrasound onthe functional properties of proteins. Ultrasonics Sonochemistry. (2016) ;31: :558-62. |
[40] | Jiang L , Wang J , Li Y , Wang Z , Liang J , Wang R , et al. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Research International. (2014) ;62: :595-601. |




