Administration of repeatedly heated coconut oil alters lipid levels and antioxidant status in cholesterol fed rats
Abstract
BACKGROUND:
Oxidation products generated during repeated heating of cooking oils can participate in the development of cardiovascular diseases.
OBJECTIVE:
The purpose of the study was to investigate the effects of administration of high fat diet with and without cholesterol and to compare the effects of fresh and repeatedly heated coconut oil in rats. The effects of various diets containing coconut oil on lipid levels in serum and tissues, oxidative damage and antioxidant status in tissues were studied.
METHODS:
Coconut oil was heated at 210 ± 10°C for fifteen hours. Male Sprague Dawley rats were divided into four groups of six animals each and were fed the experimental diet for sixty days.
RESULTS:
Results revealed that rats fed high fat – cholesterol enriched diet significantly influenced the lipid levels and antioxidant status in experimental animals. The results indicate that repeated heating of coconut oil increased the lipid levels and oxidative stress in cholesterol fed rats.
CONCLUSIONS:
Study demonstrates that thermally stressed dietary oil increases the atherosclerotic tendency in experimental animals by inducing oxidative stress in addition to those induced by dietary cholesterol.
1Introduction
Atherosclerosis is closely related to the dietary pattern seen among the population, characterized by high intake of energy, total fat and cholesterol. Studies suggest that significant correlation exists between dietary oils, cholesterol levels and atherosclerosis [1]. Reports indicate that compared to normal diet, a high fat diet adversely affects health by increasing atherogenic tendency [2]. Schachinger and Zeiher [3] reported that apart from the traditional risk factors, impaired antioxidant status due to free radical attack is also involved in the development and progression of cardiovascular diseases. Antioxidantdefense mechanism to counteract oxidative damage is compromised by free radicals and as a result, oxidative damage accumulates, leading to cardiovascular diseases. Fruchart and Duriez [4] have reported that free radicals are involved throughout the atherogenic process, beginning from endothelial dysfunction up to the rupture of a lipid-rich atherosclerotic plaque, leading to acute myocardial infarction or sudden death.
The high risk and wide prevalence of coronary atherosclerotic heart disease among the general Indian population is well established and studies suggest that the increased dietary fat intake can be pointed out as one of the main reasons [5]. Replacing the traditional cooking oils like coconut oil condemned to be atherogenic, with refined vegetable oils because of their polyunsaturated fatty acid content, has not curtailed this trend. Coconut oil is unique in that, saturated fatty acids constitute majority of its fatty acids. Due to the predominance of saturated fatty acids, coconut oil is widely believed to increase blood cholesterol and triglyceride levels and predispose to coronary heart disease. On the contrary, the relative abundance of saturated fatty acids in coconut oil helps it in lowering the tendency for undergoing high temperature induced damages, in comparison to unsaturated fatty acids rich cooking oils. Consumption of food items prepared by deep fat frying is a common practice nowadays. In deep frying, oil is heated at high temperature for a long period of time. The common practice of repeatedly using the oil for frying may generate free radicals which are harmful for our health.
In view of these observations, the study was designed to investigate the effects of administration of high fat diet with and without cholesterol and to compare the effects of fresh and repeatedly heated coconut oil in rats. In this paper, we report the result of administration of various diets containing repeatedly heated coconut oil on lipid levels in serum and tissues, oxidative damage and antioxidant status in tissues.
2Materials and methods
2.1Preparation of oil
Coconut oil was purchased from local supermarket. Oil was heated in iron pan using gas stove that was adjusted to maintain the oil temperature at 210 ± 10°C. Oil was heated for 15 hours (three hours per day for five days) with occasional stirring. After heat treatment, the oil samples were cooled and stored.
2.2Animals and experimental design
Male Sprague Dawley rats weighing 150–180 g bred in the departmental animal house were used for the study. The animals were housed individually in polypropylene cages in a room maintained at 25 ± 1°C with 12 hours light and 12 hours dark cycle. All the animal cares and procedures were in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India. The experimental protocol was approved by Institutional Animal Ethics Committee (IAEC) [IAEC-KU-2/2006-2007-BC-TR (11) dated 22/5/2006]. Rats were randomly divided into four groups of six animals each and fed as follows:
1 Group I- 8% unheated coconut oil (UHCO)
2 Group II-15% unheated coconut oil (UHCO)
3 Group III-15% unheated coconut oil (UHCO) + 1% cholesterol
4 Group IV-15% heated coconut oil (HCO) + 1% cholesterol.
8% or 15% oil and 1% cholesterol were mixed with the semi synthetic diet for preparing the experimental diet (Table 1). Animals were maintained on the experimental diet for 60 days. Food intake was recorded daily and body weight of the rats was recorded weekly. After completion of experimental period, rats were fasted overnight and sacrificed. Blood and tissues (liver, heart and kidney) were collected in ice cold containers for various estimations.
Table 1
Composition of the experimental diet
| Ingredients (%) | Group I | Group II | Group III | Group IV |
| Corn starch | 71 | 64 | 63 | 63 |
| Casein | 16 | 16 | 16 | 16 |
| Cholesterol | – | – | 1 | 1 |
| Unheated coconut oil (UHCO) | 8 | 15 | 15 | – |
| Heated coconut oil (HCO) | – | – | – | 15 |
| Salt Mixture * | 4 | 4 | 4 | 4 |
| Vitamin mixture ** | 1 | 1 | 1 | 1 |
*Salt Mixture HMW (g/Kg): Sodium chloride-105, potassium chloride-120, potassium dihydrogen phosphate-310, calcium phosphate-149, calcium carbonate-210, manganese sulphate (anhydrous)-0.2, potassium aluminium sulphate-0.09, magnesium sulphate (anhydrous)-90, ferrous sulphate-14.7, copper sulphate-0.37, sodium fluoride-0.57, potassium iodide-0.05, zinc chloride-15 mg/Kg, cobalt chloride-0.15 mg/Kg. **Vitamin mixture (per 100 g diet): Retinyl palmitate (1000 IU), ergocalciferol(150 IU), α-tocopherol (12 mg), menadione (0.3 mg), thiamine hydrochloride (1.0 mg), riboflavin (1.0 mg), pyridoxine (0.6 mg), niacin (10.0 mg), calcium pantothenate (5.0 mg), inositol (20 mg), folic acid (0.4 mg), vitamin B12 (3.0μg), biotin (20μg) and choline chloride (300 mg).
2.3Chemical characteristics of oils
The fatty acid composition of oil samples was analyzed in duplicates by gas chromatography (GC). Fatty acids were converted to their methyl esters (FAME) prior to analysis [6]. Shimadzu GC-2010 Gas Chromatograph equipped with Flame Ionization Detector (Shimadzu Corporation, Kyoto, Japan) was used to determine the FAME composition of the tested oils. The column used was capillary column (DB-23, 30 m length, 0.32 mm id wide bore, 0.25μm film thickness, Agilent technologies USA). Nitrogen gas was used as carrier gas with flow rate 30 mL/minute and pressure 180 psi. Fatty acids were identified by comparing their retention time with authentic standards (Sigma, Sydney, Australia). Peroxide value was determined according to AOCS Official method Cd 8-53 [7]. p- Anisidine value was analyzed as described in IUPAC Standard method 2.504 [6]. Total oxidation (TOTOX) value was calculated according to the following equation:
2.4Gain in body weight and absolute organ weight
The body weight of each animal was noted before starting feeding experiment (Initial body weight) and also during experimental period (Final body weight). The absolute weight of organs of respective groups of animals was also recorded. Gain in body weight was calculated as follows:
2.5Biochemical Estimations
Serum samples were analyzed for total cholesterol [8], triglycerides [9] and high density lipoprotein (HDL) cholesterol [10] using diagnostic kits from Agape diagnostics Pvt. Ltd. Kerala, India. Lipids in tissues were extracted by the procedure of Radin [11]. Total cholesterol was estimated in liver and heart by the method of Carr and Drekter [12]. Triglycerides were estimated in tissues by the method of Van Handel and Zilver Smith [13]. Lactate dehydrogenase (LDH) was estimated in serum using enzyme kit purchased from ERBA diagnostics, Germany [14]. Quantitative determination of alkaline phosphatase (ALP) was done using enzyme kit purchased from Dr. Reddy‘s laboratories, Hyderabad, India [15]. Serum glutamate oxaloacetate transaminase (SGOT) and glutamate pyruvate transaminase (SGPT) were assayed using the enzyme kit from CML Biotech (P) Ltd, Ernakulam, India [16]. Malondialdehyde (MDA) was estimated in liver, heart and kidney by the method of Ohkawa et al. [17]. Conjugated dienes were estimated by the method of John and Steven [18]. Superoxide dismutase (SOD) activity in tissues was determined by the method described by Kakkar et al. [19]. Glutathione peroxidase (Gpx) activity was determined in liver, heart and kidney by the method of Lawrence and Burk [20] as modified by Agergaard and Jence [21]. Reduced glutathione (GSH) levels were estimated in the tissues by the method of Moron et al. [22].
2.6Statistical analysis
The results were statistically analyzed with Statistical Package for Social Sciences (SPSS) version 11.5 (SPSS Inc., Chicago, USA). One way analysis of variance (ANOVA) followed by Duncan’s post hoc multiple variance test was used for hypothesis testing. Data were expressed as mean ± SD for six animals in each group. Significance was accepted at p < 0.05.
3Results and discussion
Fatty acid composition of the frying oil is an important factor affecting fried food quality. Gas chromatographic analysis was performed to illustrate the changes in the fatty acid composition of coconut oil. Gas chromatographic analysis of coconut oil (Table 2) revealed significant alterations in the fatty acid composition. Coconut oil has saturated fatty acid content (91.46%), mainly represented by lauric acid (44.17%), myristic acid (20.14%) and palmitic acid (9.38%). MUFA content in coconut oil is represented by oleic acid (6.94%) and PUFA content represented by linoleic acid (1.60%). Fatty acid composition showed that saturation increased and unsaturation decreased as coconut oil was heated repeatedly at elevated temperature. Although the unsaturated fatty acid percentage decrease was absolute reduction possibly due to the formation of scission and cyclic products, the increase in fatty acid concentration was only relative and it resulted from the destruction of some of the unsaturated fatty acid components. The initial degree of unsaturation of the unheated coconut oil was 8.54% which decreased to 2.03% . Saturated fatty acids in unheated coconut oil were 91.46% which relatively increased to 97.97% in the heated oils. Absolute reduction in the percentage of total unsaturated fatty acids is indicated by decrease in the percentage of oleic acid and linoleic acid. Relative increase in the percentage of saturated fatty acids, as indicated by increase in the percentage of lauric acidwas observed after heating. During frying, fatty acids are released and their concentration in the cooking oil increases with repeated use. This may be due to oxidative, thermal and hydrolysis reactions that took place during the period of frying [23]. The decomposition products of these reactions produce off-flavors in the oil. Previous studies demonstrated that thermal oxidation changes the fatty acid profile of the distillable fraction of oxidized corn and olive oil [24]. Decrease in polyunsaturated fatty acids of oils used for frying was reported by Alireza et al. [25] in comparison with fatty acid composition of the same brand of oil before use.
Table 2
Fatty acid composition of coconut oil
| *Fatty acids (%) | UHCO | HCO |
| C8:0 | 8.73 | 9.40 |
| C10:0 | 5.89 | 6.48 |
| C12:0 | 44.17 | 51.95 |
| C14:0 | 20.14 | 20.07 |
| C16:0 | 9.38 | 7.69 |
| C18:0 | 3.15 | 2.38 |
| C18:1 | 6.94 | 1.73 |
| C18:2 | 1.60 | 0.30 |
| SFA | 91.46 | 97.97 |
| MUFA | 6.94 | 1.73 |
| PUFA | 1.60 | 0.30 |
| MUFA+ PUFA (Unsaturation) | 8.54 | 2.03 |
*Given in table is the relative percentage of fatty acids expressed as mean of four estimations. SFA-Saturated fatty acid; MUFA-Monounsaturated fatty acid; PUFA- Polyunsaturated fatty acid.
The stability of cooking oils is measured by estimating primary oxidation products, secondary oxidation products and total oxidation. The primary oxidation products are measured as peroxide value (PV) [26] and the secondary products as p-anisidine value (p-AnV) [27]. These two values can be combined into the totox value [28] which is a measure of the total oxidation. Peroxide value (expressed in mEQ peroxides/Kg oil) of UHCO and HCO were 4.66 ± 0.294 and 8.61 ± 0.542 respectively. p-AnV of UHCO was 2.02 ± 0.127 and for HCO the value was 30.15 ± 1.90. Totox value for UHCO and HCO were 10.24 ± 0.715 and 47.37 ± 2.98 respectively. PV, p-AnV and totox value which measures oxidative changes, were significantly elevated in heated coconut oil in comparison to fresh oil. Increment of peroxide value means the rate of peroxide formation was more than their degradation. Increase in peroxide value and p-anisidine value is summed up in elevated totox value, indicating an overall increase in total oxidation taken place in thermally stressed oil compared to fresh oil.
There are reports that amount of food consumed by animals greatly influenced growth responses [29]. Animals fed 15% coconut oil in the diet showed better growth response when compared with rats fed normal diet containing 8% coconut oil (Table 3). This is true when 15% coconut oil is administered both with and without cholesterol in the diet. But no significant difference was observed in body weight gain between high fat fed animals given cholesterol free and cholesterol containing diet. It is reported that when high fat diet is fed to experimental animals, food consumption is more frequently increased and growth performance is improved [30]. In our study, the average body weight gain during experimental period was significantly reduced in animals administered thermally oxidized oil and cholesterol compared with those fed normal, high fat as well as high fat and cholesterol in the diet. The decrease in the body weight gain could be due to the toxic effects of oxidized oil. Edem [31] have reported that oxidized oil results in significant growth retardation compared to fresh oil. Even though no significant difference was observed in liver weight in rats fed with unheated oil, liver weight was increased in rats administered repeatedly heated coconut oil, which is in agreement with the findings of Koch et al. [32].
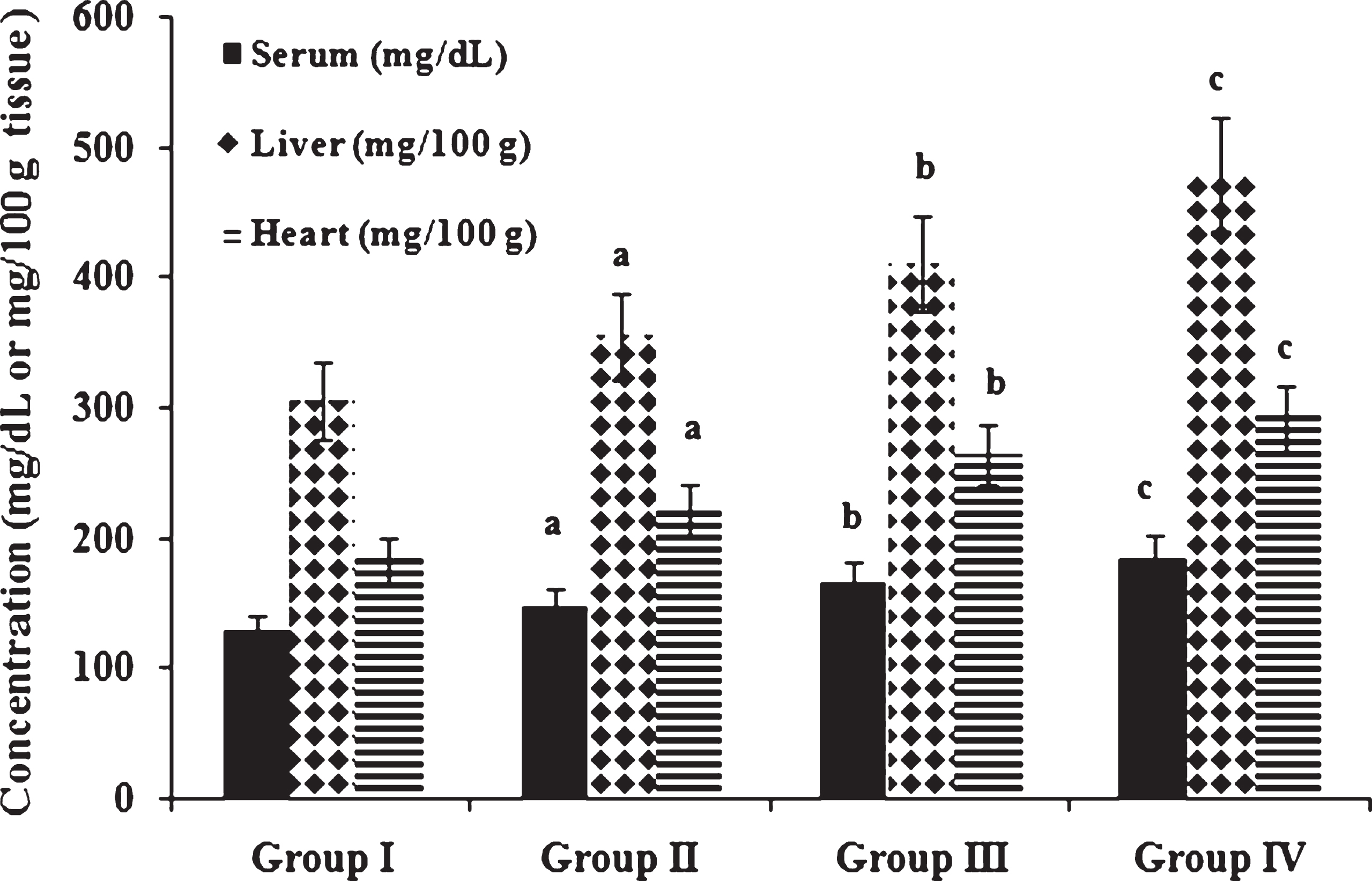
It is observed that feeding repeatedly heated coconut oil produces significant changes in lipid levels in experimental animals. Figure 1 shows total cholesterol concentrations in serum, liver and heart of experimental animals. Concentration of cholesterol was significantly increased in serum, liver and heart of high fat and cholesterol fed animals in comparison to those fed normal diet. Among cholesterol fed animals, those fed heated coconut oil showed increased cholesterol levels in serum and tissues when compared to those fed fresh coconut oil. Narasimhamurthy and Raina [33] have reported elevation of serum total cholesterol, decrease in HDL cholesterol and increase in LDL cholesterol as the adverse effects of oxidized dietary fat administration. Yi-Fa and Yi-Chen [34] have also reported that ingestion of frying oil with cholesterol aggravated hypercholesterolemia. Ramadas and Eshwaran [35] have reported that the quantity and quality of dietary fat and oil intake alter the serum lipid fraction, which play a very important role in many of the diseases. Studies reported that hypercholesterolemia is a major risk factor for atherosclerosis and coronary heart disease [36]. Jensen et al. [37] reported that high fat, high cholesterol diet (atherogenic diet) fed to genetically pre-disposed adult Duroc gilts resulted in an increase in plasma levels of total and LDL cholesterol, which is associated with the generation of fatty streaks in the aorta. Adam et al. [38] have reported that atherosclerotic lesions in humans and animals appear to be related to elevated plasma total cholesterol and decreased HDL cholesterol levels.
Table 3
Initial body weight (g), final body weight (g), gain in body weight (g/rat/60 days) and absolute liver weight (g)
| Groups | Initial body weight | Final body weight | Gain in body weight | Absolute liver weight |
| Group I | 158.36 ± 14.45 | 222.43 ± 20.30 | 64.06 ± 5.85 | 6.87 ± 0.627 |
| Group II | 160.16 ± 14.62 | 230.37 ± 21.02 | 73.64 ± 6.41a | 7.22 ± 0.659 |
| Group III | 171.55 ± 19.37b | 251.43 ± 26.01b | 79.88 ± 6.64 | 8.03 ± 0.732 |
| Group IV | 168.22 ± 14.83 | 234.85 ± 20.91c | 66.63 ± 6.08c | 8.92 ± 0.814c |
Values expressed as mean ± SD of six estimations. aindicates values are significantly different from group I. bindicates values are significantly different from group II. cindicates values are significantly different from group III. Significance accepted at p < 0.05.
Fig.1
Concentration of cholesterol in serum and tissues. Values expressed as mean ± SD of six estimations. aindicates values are significantly different from group I. bindicates values are significantly different from group II. cindicates values are significantly different from group III. Significance accepted at p < 0.05.
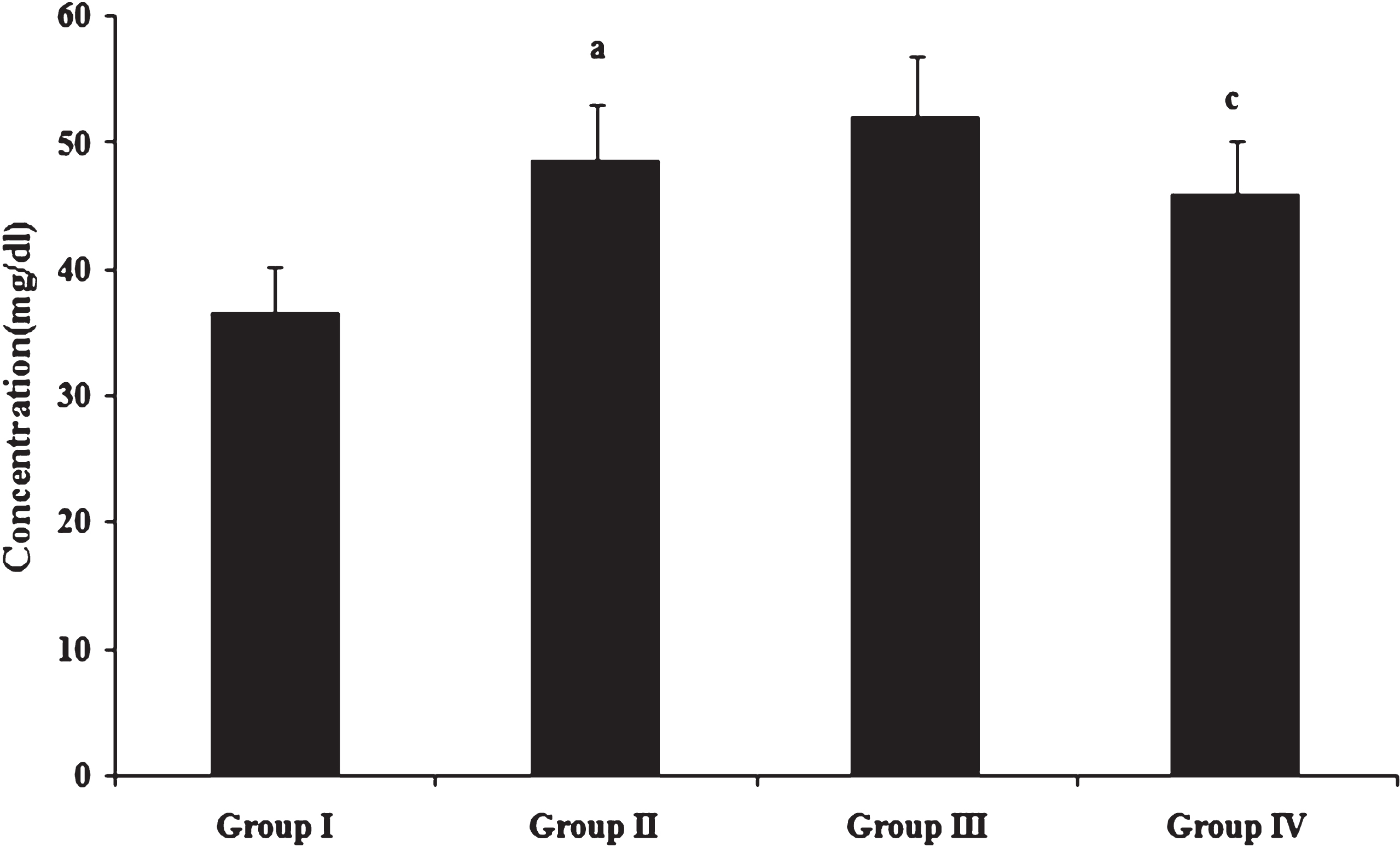
HDL scavenges cholesterol from the blood and tissues and delivers it to the liver where it is processed for excretion. Serum HDL cholesterol concentration (Fig. 2) was significantly increased in high fat and cholesterol fed animals compared to those fed normal diet. No significant difference was observed in HDL cholesterol levels between high fat and high fat as well as cholesterol fed animals. Among cholesterol fed animals, those fed with heated coconut oil showed significantly decreased HDL cholesterol concentration in comparison to fresh coconut oil fed animals. Independent association of low levels of HDL cholesterol with increased risk for cardiovascular disease has been well established through epidemiological studies such as the Framingham Heart Study in the United States [39] and the PROCAM study in Europe [40]. Assmann and Gotto [41] observed that the relation between HDL cholesterol and atherosclerosis is an inverse one. This relationship is supported by the potential anti atherogenic properties of HDL cholesterol, including its mediation of reverse cholesterol transport, in which cholesterol from peripheral tissues is returned to the liver for excretion in the bile.
Fig.2
Concentration of HDL cholesterol. Values expressed as mean ± SD of six estimations. aindicates values are significantly different from group I. cindicates values are significantly different from group III. Significance accepted at p < 0.05.
Triglycerides are important since they influence lipid deposition and clotting mechanisms. Triglycerides in serum, liver and heart (Table 4) were significantly increased in high fat and cholesterol fed animals compared to those fed normal diet. Among cholesterol fed animals, those fed heated coconut oil showed higher triglycerides concentration in comparison to those fed fresh coconut oil. Accumulated biological evidence related to triglycerides, atherosclerosis and thrombosis has indicated an independent role for triglycerides in coronary heart disease risk [42]. Rueda-Clausen et al. [43] reported that consumption of deep-fried palm oil increased serum triglyceride levels in humans which are in agreement with the results observed in this study. Adam et al. 38] have reported that there was an increasing trend in serum total cholesterol, LDL cholesterol, triglycerides and decreasing trend in HDL cholesterol in animals fed heated oil and cholesterol.
Table 4
Concentration of triglycerides in serum and tissues
| Group I | Group II | Group III | Group IV | |
| Serum (mg/dL) | 8.42 ± 0.768 | 10.24 ± 0.934a | 11.95 ± 1.04b | 15.03 ± 1.25c |
| Liver (mg/100 g) | 283.69 ± 28.69 | 353.03 ± 33.87a | 416.89 ± 36.28b | 473.64 ± 47.90c |
| Heart (mg/100 g) | 46.97 ± 4.51 | 54.42 ± 4.97a | 64.19 ± 5.34b | 89.52 ± 8.17c |
Values expressed as mean ± SD of six estimations. aindicates values are significantly different from group I. bindicates values are significantly different from group II. cindicates values are significantly different from group III. Significance accepted at p < 0.05.
Oxidative stress as measured by the concentration of peroxidation products namely MDA and conjugated dienes in liver, heart and kidney is shown in Figs. 3 & 4. Peroxidation products were significantly increased in high fat and cholesterol fed animals in comparison to those fed normal diet. Among cholesterol fed groups, those fed heated coconut oil showed higher concentration of peroxidation products than those fed fresh coconut oil. Previous studies have reported that oxidized fatty acids in the diet play a significant role in lipoprotein oxidation [44]. An imbalance between reactive oxygen species and antioxidant reserve results in oxidative stress as indicated by the accumulation of peroxidation products. Further, evidences have shown that oxidative stress in turn can contribute to the progression of atherosclerosis [45].
Fig.3
Concentration of MDA. Values expressed as mean ± SD of six estimations. aindicates values are significantly different from group I. bindicates values are significantly different from group II. cindicates values are significantly different from group III. Significance accepted at p < 0.05.
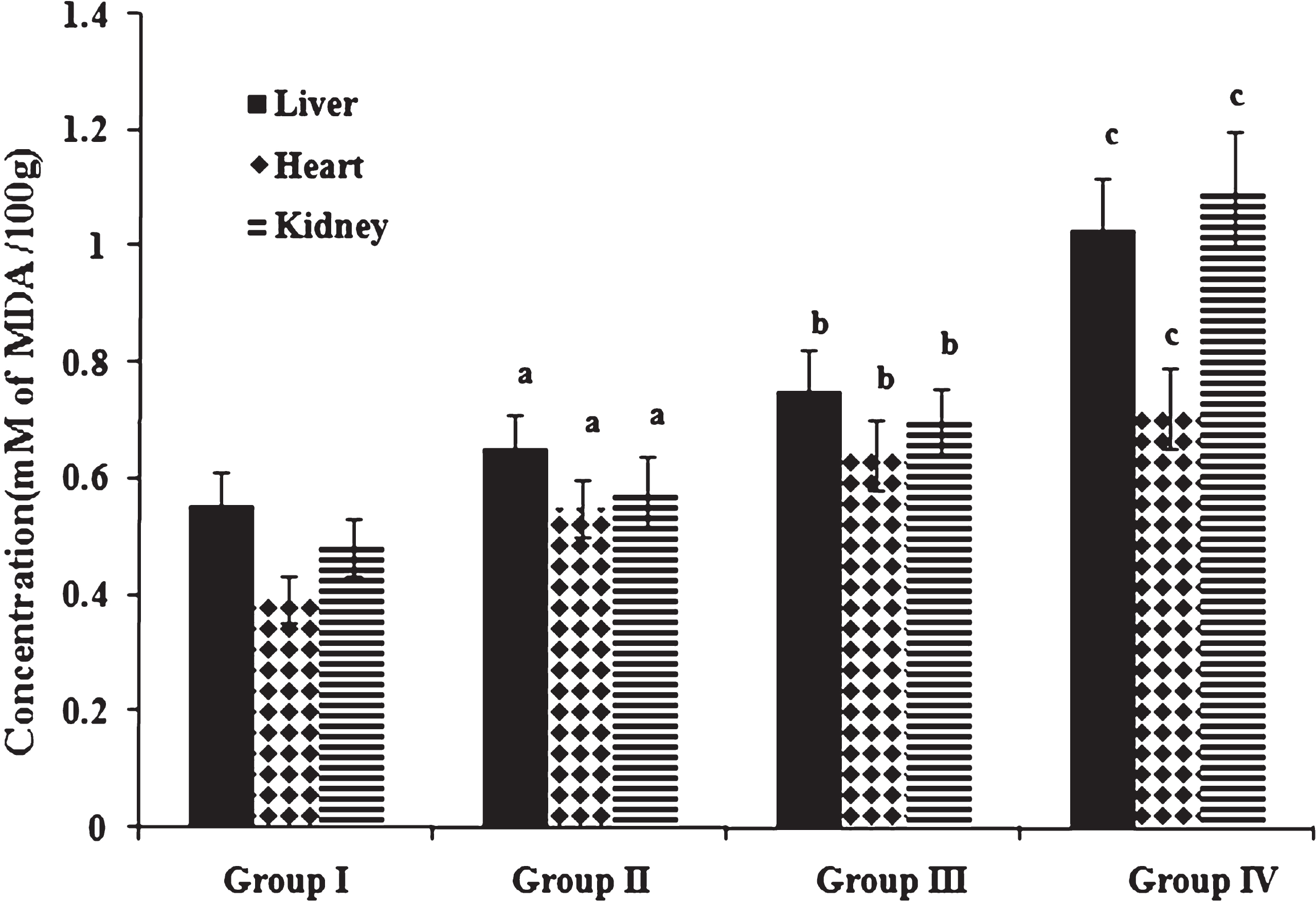
Fig.4
Concentration of conjugated dienes. Values expressed as mean ± SD of six estimations. aindicates values are significantly different from group I. bindicates values are significantly different from group II. cindicates values are significantly different from group III. Significance accepted at p < 0.05.
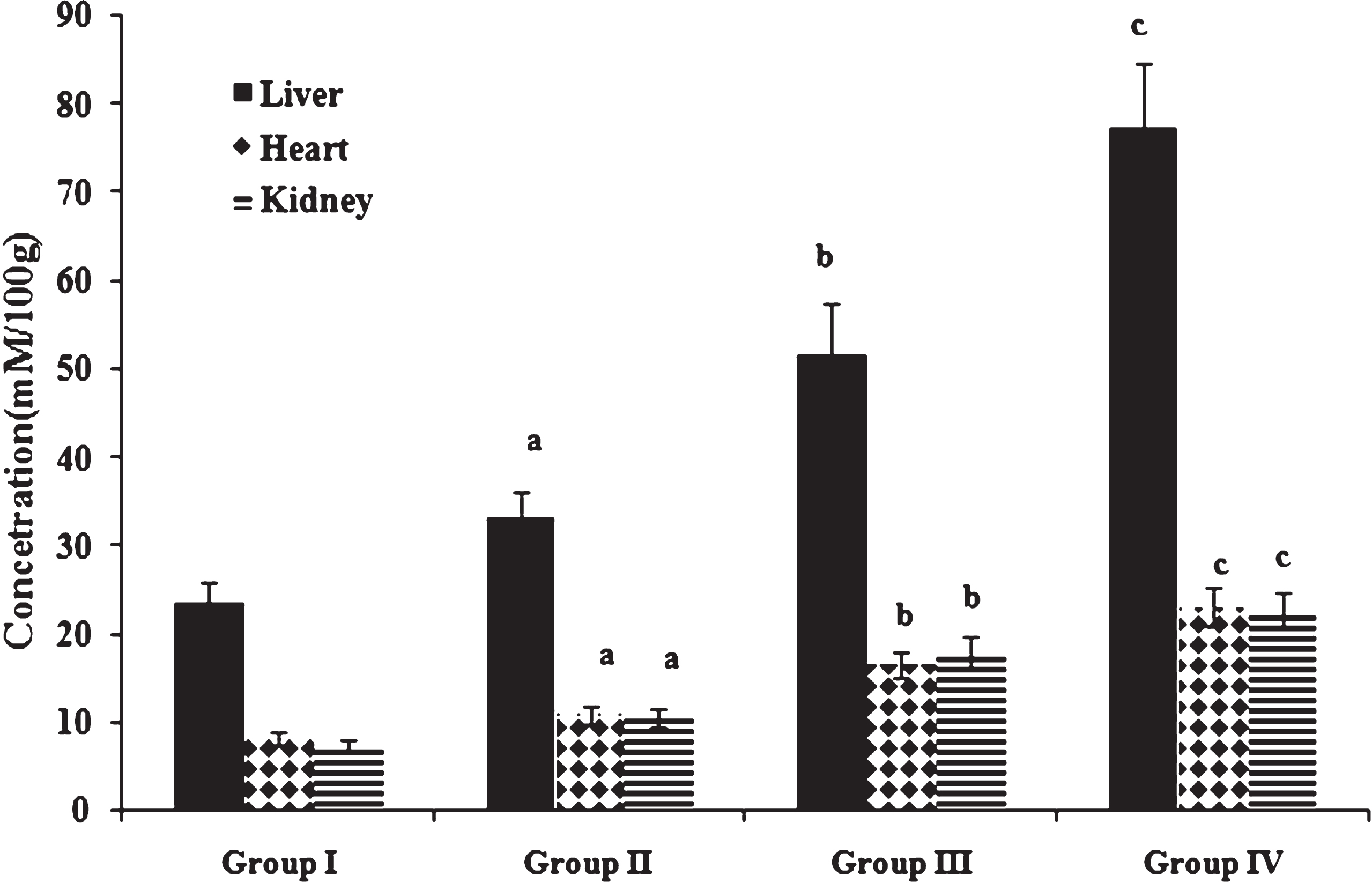
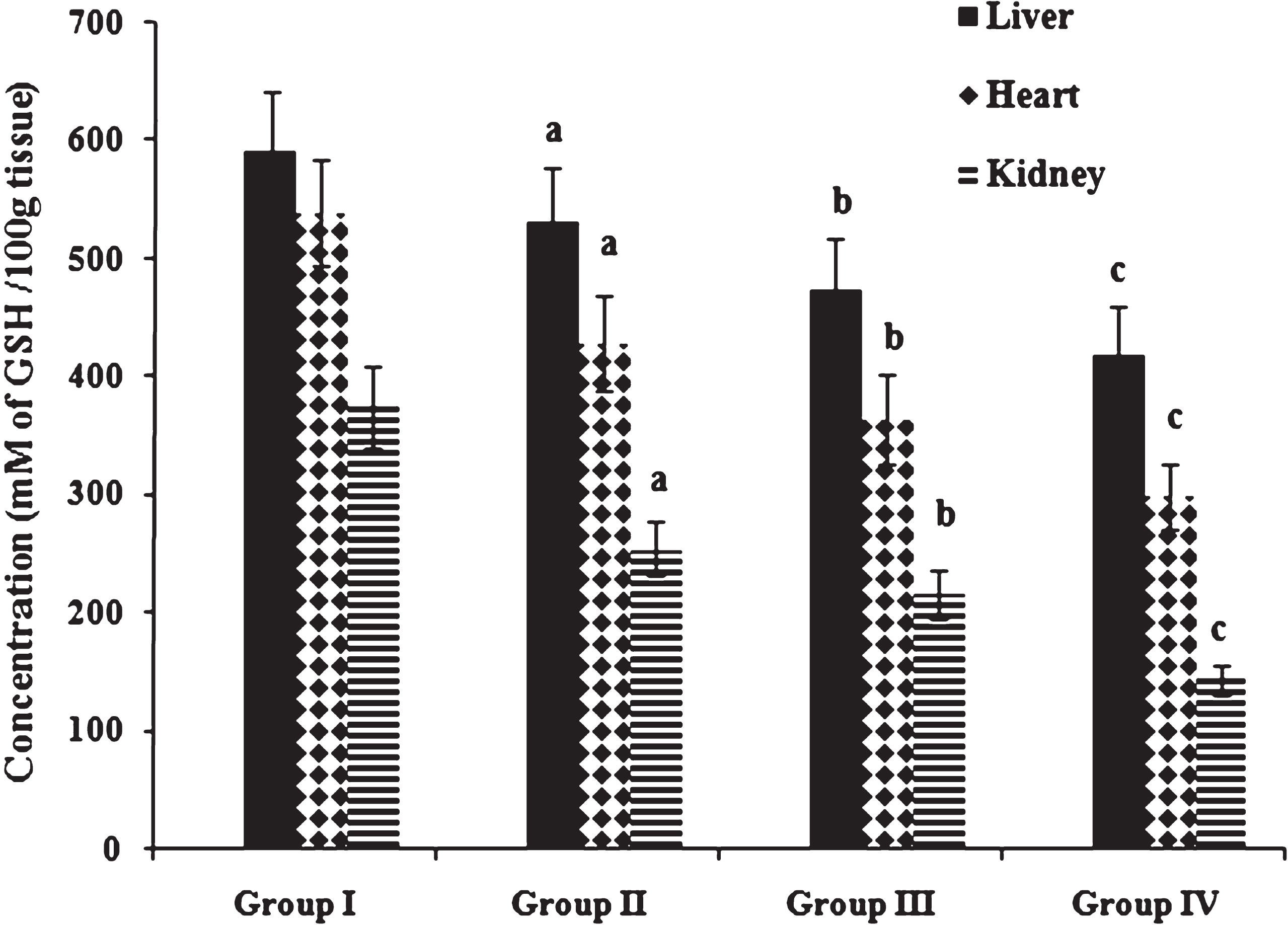
Table 5 shows the activities of antioxidant enzymes SOD and GPx in liver, heart and kidney. Compared with normal diet fed animals given coconut oil, antioxidant enzymes SOD and GPx and reduced gluthathione content were decreased in animals fed high fat and cholesterol diet. Antioxidant status was further compromised by heated coconut oil administration as indicated by depression in antioxidant enzyme activities. Concentration of reduced glutathione (Fig. 5) in liver, heart and kidney was significantly decreased in high fat and cholesterol fed animals given coconut oil when compared to normal diet fed rats given coconut oil. Administration of heated coconut oil in comparison to fresh coconut oil significantly decreased the concentration of reduced glutathione in cholesterol fed animals. Abdelhalim and Moussa [46] have reported that hypercholesterolemia may produce reactive oxygen species and other free radicals which increase TBARS and decrease SOD and GPx enzymes activities.
Table 5
Activities of antioxidant enzymes
| Parameters | Group I | Group II | Group III | Group IV |
| SOD | ||||
| Liver | 13.39 ± 1.22 | 11.44 ± 1.04a | 6.01 ± 0.548b | 4.15 ± 0.378c |
| Heart | 16.80 ± 1.53 | 14.79 ± 1.35a | 12.54 ± 1.14b | 5.12 ± 0.467c |
| Kidney | 13.66 ± 1.25 | 12.24 ± 1.07a | 9.94 ± 1.01b | 7.88 ± 0.842c |
| GPx | ||||
| Liver | 0.676 ± 0.056 | 0.541 ± 0.047a | 0.429 ± 0.046b | 0.184 ± 0.018c |
| Heart | 0.975 ± 0.089 | 0.860 ± 0.078a | 0.714 ± 0.065b | 0.575 ± 0.052c |
| Kidney | 0.912 ± 0.083 | 0.755 ± 0.069a | 0.511 ± 0.047b | 0.310 ± 0.028c |
SOD, Superoxide dismutase (Units/mg protein); GPx, Glutathione peroxidase (x 10–2 Units/mg protein). Values expressed as mean ± SD of six estimations. aindicates values are significantly different from group I. bindicates values are significantly different from group II. cindicates values are significantly different from group III. Significance accepted at p < 0.05.
Fig.5
Concentration of reduced glutathione. Values expressed as mean ± SD of six estimations. aindicates values are significantly different from group I. bindicates values are significantly different from group II. cindicates values are significantly different from group III. Significance accepted at p < 0.05.
Serum toxicity marker enzymes (Table 6) namely ALP, LDH, SGOT and SGPT were significantly increased in high fat and cholesterol fed animals in comparison to those fed normal diet. Among cholesterol fed animals, toxicity marker enzyme activities were markedly elevated in heated coconut oil fed animals in comparison to those fed fresh oil. Aruna et al. [47] suggested that the increased activities of toxicity markers in thermally oxidized oil fed animals may be due to increased oxidative stress induced by lipid oxidation products. Increased lipid peroxidation products and compromised antioxidant defense mechanism may be associated with the elevated levels of toxicity marker enzymes.
Table 6
Serum toxicity marker enzymes
| Group I | Group II | Group III | Group IV | |
| ALP | 9.45 ± 0.862 | 14.50 ± 1.32a | 24.12 ± 2.20b | 30.38 ± 2.77c |
| LDH | 232.59 ± 23.52 | 281.17 ± 25.66a | 361.50 ± 32.99b | 487.95 ± 44.53c |
| SGOT | 22.40 ± 2.05 | 26.30 ± 2.40a | 29.61 ± 2.70b | 37.57 ± 3.43c |
| SGPT | 21.06 ± 1.92 | 27.77 ± 2.53a | 32.84 ± 3.00b | 39.68 ± 3.62c |
ALP, Alkaline phosphatase (KA Units/L); LDH, Lactate dehydrogenase (IU/L); SGOT, Serum glutamate oxaloacetate transaminase (IU/L); SGPT, Serum glutamate pyruvate transaminase (IU/L). Values expressed as mean ± SD of six estimations. aindicates values are significantly different from group I. bindicates values are significantly different from group II. cindicates values are significantly different from group III. Significance accepted at p < 0.05.
4Conclusions
From the results, it is evident that high fat – cholesterol enriched diet had significant influence on the lipid levels and antioxidant status in experimental animals. In conclusion, repeated heating of coconut oil leads to increased lipid levels and lipid peroxidation in cholesterol fed rats. The results indicate that thermally stressed oils increase the atherosclerotic tendency in experimental animals by inducing oxidative stress.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Acknowledgments
Financial assistance from University Grants Commission (UGC), India in the form of Research Fellowship in Science for Meritorious Students (RFSMS) – Junior Research Fellowship (JRF) to Ms. Chinu Chacko is gratefully acknowledged.
References
[1] | Bavelaar FJ , Beynen AC . The relation between diet, plasma cholesterol and atherosclerosis in pigeons, quails and chickens. International Journal of Poultry Science. (2004) ;3: :671–84. |
[2] | Sommerville LJ , Kelemen SE , Ellison SP , England RN , Autieri MV . Increased atherosclerosis and vascular smooth muscle cell activation in AIF-1 transgenic mice fed a high-fat diet. Atherosclerosis. (2012) ;220: :45–52. |
[3] | Schächinger V , Zeiher AM . Atherogenesis- recent insights into basic mechanisms and their clinical impact. Nephrol Dial Transplant. (2002) ;17: :2055–64. |
[4] | Fruchart JC , Duriez P . Free radicals and atherosclerosis. In: Rice-Evans CA and Burdon RH (eds) Free Radical Damage and its Control. Elsevier Science. (1994) ; pp. 257–81. |
[5] | Goyal A , Yusuf S . The burden of cardiovascular disease in the Indian subcontinent. Indian J Med Res. (2006) ;124: :235–44. |
[6] | International Union of Pure and Applied Chemistry (IUPAC). ((1987) ) Standard Method 2.301. Preparation of fatty acid methyl ester. In: Paquot C and Hautefenne A (eds.). Standard Methods for the Analysis of Oils, Fats and Derivatives. 7th ed. Blackwell Scientific, Oxford, London. |
[7] | AOCS. AOCS official method Cd 8-53: Peroxide value-Acetic acid –Chloroform method. In: Official Methods and Recommended Practices of the American Oil Chemists’ Society. AOCS press, Champaign, (1999) . |
[8] | Allain CC , Poon LS , Chan CS , Richmond W , Fu PC . Enzymatic determination of total serum cholesterol. Clin Chem. (1974) ;20: :470–5. |
[9] | Buccolo G , David H . Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. (1973) ;19: :476–82. |
[10] | López Virella MF , Stone P , Ellis S , Colwell JA . Cholesterol determination in high density lipoprotein separated by three different methods. Clin Chem. (1977) ;23: :882–4. |
[11] | Radin NS . Meth Enzymol. (1981) ;72: :5–7. |
[12] | Carr JJ , Drekter IJ . Simplified rapid technique for the extraction and determination of serum cholesterol without saponification. Clin Chem. (1956) ;2: :353–68. |
[13] | Van Handel E , Zilversmit DB . Micro method for direct determination of serum triglycerides. J Lab Clin Med. (1957) ;50: :152–7. |
[14] | Henry RT , Chiamori N , Goiub OJ , Berkman S . Methods of enzymatic analysis. Am J Clin Path. (1960) ;34: :381–90. |
[15] | Kind PRN , King EJ . Estimation of plasma phosphate by determination of hydrolyzed phenol with amino antipyrine. J Clin Pathol. (1954) ;7: :322–6. |
[16] | Reitman S , Frankel S . A colorimetric method for the determination of serum glutamic oxaloacetic acid and glutamic pyruvate transaminase. Am J Clin Pathol. (1957) ;28: :56–63. |
[17] | Ohkawa H , Ohisha N , Yagi K . Assay of lipid peroxides in animal tissues by Thiobaribituric acid reaction. Anal Biochem. (1979) ;5: :351–8. |
[18] | John AB , Steven DA . Microsomal lipid peroxidation. In: Meth Enzymol. Academic Press, New York, (1978) ;52: :302–10. |
[19] | Kakkar P , Das B , Viswanathan PN . A modified spectrophotometric assay of superoxide dismutase. Ind J Biochem Biophys. (1984) ;2: :130–2. |
[20] | Lawrence RA , Burk RF . Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. (1976) ;71: :952–8. |
[21] | Agergaard N , Jense PT . Procedure for blood glutathione peroxidasedetermination in cattle and swine. Acta Vet Scand. (1982) ;23: :515–27. |
[22] | Moron MS , Depierre JW , Mannervik B . Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. (1979) ;582: :67–78. |
[23] | Velasco J , Marmesat S , Dobarganes MC . Chemistry of frying. In: Serpil Sahin and Servet Gulum Sumnu (eds.). Advances in Deep-Fat Frying of Foods. CRC Press. (2008) ; pp. 33–56. |
[24] | Suleiman AE-RM , El-Makhzangy A , Ramadan MF . Antiradical performance and physicochemical characteristics of vegetable oils upon frying of French fries: A preliminary comparative study. J Food Lipids. (2006) ;13: :259–76. |
[25] | Alireza S , Tan CP , Hamed M , Che Man YB . Effect of frying process on fatty acid composition and iodine value of selected vegetable oils and their blends. International Food Research Journal. (2010) ;17: :295–302. |
[26] | Stier RF . The measurement of frying oil quality and authenticity. In: Rossell JB . (ed.) Frying – Improving Quality. Woodhead Publishing, Cambridge, England. (2001) ; pp. 165–93. |
[27] | Matthaus B . Utilization of high-oleic rapeseed oil for deep-fat frying of French fries compared to other commonly used edible oils. Eur J Lipid Sci Technol. (2006) ;108: :200–11. |
[28] | Mariod A , Matthaus B , Eichner K , Hussein I. H . Frying quality and oxidative stability of two unconventional oils. J Am Oil Chem Soc. (2006) ;83: :529–38. |
[29] | Albanes D . Caloric intake, body weight, and cancer: A review. Nutr Cancer. (1987) ;9: :199–217. |
[30] | Lim PK , Boey PL , Ng WK . Dietary palm oil level affects growth performance, protein retention and tissue vitamin E concentration of African catfish, Clarias gariepinus. Aquaculture. (2001) ;202: :101–12. |
[31] | Edem DO . Haematological and histological alterations induced in rats by palm oil containing diets. Eur J Sci Res. (2009) ;32: :405–18. |
[32] | Koch A , König B , Spielmann J , Leitner A , Stangl GI , Eder K . Thermally oxidized oil increases the expression of insulin induced genes and inhibits activation of sterol regulatory element-binding protein-2 in rat liver. J Nutr. (2007) ;137: :2018–23. |
[33] | Narasimhamurthy K , Raina PL . Long term feeding effects of heated and fried oils on lipids and lipoproteins in rats. Mol Cell Biochem. (1999) ;195: :143–53. |
[34] | Lu Yi-Fa , Lo Yi-Chen . Effect of deep frying oil given with and without dietary cholesterol on lipid metabolism in rats. Nutr Res. (1995) ;15: :1783–92. |
[35] | Ramadas SV , Eshwaran PP . Consumption pattern of fats and oils and serum lipid profile of selected adults- Part I. Indian J Nutr Diet. (2000) ;37: :47–8. |
[36] | Huxley R , Lewington S , Clarke R . Cholesterol, coronary heart disease and stroke: A review of published evidence from observational studies and randomized controlled trials. Semin Vasc Med. (2002) ;2: :315–23. |
[37] | Jensen TW , Mazur MJ , Pettigew JE , Perez-Mendoza VG , Zachary J , Schook LB . A cloned pig model for examining atherosclerosis induced by high fat, high cholesterol diets. Anim Biotechnol. (2010) ;21: :179–87. |
[38] | Adam SK , Soelaiman IN , Umar NA , Mokhtar N , Mohamed N , Jaarin K . Effects of repeatedly heated palm oil on serum lipid profile, lipid peroxidation and homocysteine levels in a post-menopausal rat model. Mcgill J Med. (2008) ;11: :145–51. |
[39] | Dawber TR , Meadors GF , Moore FEJr . Epidemiological approaches to heart disease: The Framingham Study. Am J Public Health Nations Health. (1951) ;41: :279–86. |
[40] | National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Final report. Circulation. (2002) ;106: :3143–421. |
[41] | Assmann G , Gotto AM . Atherosclerosis: Evolving vascular biology and clinical implications. HDL cholesterol and protective factors in atherosclerosis. Circulation. (2004) ;109: :III-8–III-14. |
[42] | Iso H , Naito Y , Sato S , Kitamura A , Okamura T , Sankai T , Shimamoto T , Iida M , Komachi Y . Serum triglycerides and risk of coronary heart disease among Japanese men and women. Am J Epidemiol. (2001) ;153: :490–9. |
[43] | Rueda-Clausen CF , Silva FA , Lindarte MA , Villa-Roel C , Gomez E , Gutierrez R , Cure-Cure C , López-Jaramillo P . Olive, soybean and palm oils intake have a similar acute detrimental effect over the endothelial function in healthy young subjects. Nutr Metab Cardiovasc Dis. (2007) ;17: :50–7. |
[44] | Staprãns I , Rapp JH , Pan XM , Kim KY , Feingold KR . Oxidized lipids in the diet are a source of oxidized lipid in chylomicrons of human serum. Arterioscler Thromb. (1994) ;14: :1900–5. |
[45] | Stocker R , Keaney JFJr . Role of oxidative modifications in atherosclerosis. Physiol Rev. (2004) ;84: :1381–478. |
[46] | Abdelhalim MA , Moussa SA . Biochemical changes of hemoglobin and osmotic fragility of red blood cells in high fat diet rabbits. Pak J Biol Sci. (2010) ;13: :73–7. |
[47] | Aruna K , Rukkumani R , Sureshvarma P , Menon VP . Role of an aminothiazole derivative on ethanol- and thermally oxidized sunflower oil induced toxicity. Pol J Pharmacol. (2004) ;56: :233–40. |




