Antioxidant capacity and total phenol and flavonoid contents of Teucriumpolium L. grown in Algeria
Abstract
BACKGROUND:
Teucrium polium L. aerial parts are traditionally used in Algerian folk medicine due to its therapeutic properties.
OBJECTIVE:
The present study was designed to evaluate the effect of different extracting solvents (methanol, ethanol, ethyl acetate, chloroform, hexane and water) on antioxidant capacity and total phenol and flavonoid contents of Algerian Teucrium polium aerial parts.
METHODS:
The antioxidant capacity of Teucrium polium extracts was assessed by DPPH (2, 2-diphenyl-1-picrylhydrazyl) and ABTS (2, 2’-azino-bis 3-ethylbenzthiazoline-6-sulfonic acid) free radicals scavenging and reducing power assays. For each extract, the total phenol and flavonoid contents were investigated by the Folin-Ciocalteu and aluminium trichloride methods, respectively.
RESULTS:
All the tested extracts showed an appreciable total phenolic and total flavonoid contents and strong antioxidant capacity. Among the different extracts, the methanolic extract was found to be containing the highest amount of total phenols (206.95±1.82 mg of gallic acid equivalent per gram of extract) and flavonoids (42.16±0.61 mg of quercetin equivalent per gram of extract). The same extract also exhibited a much better reducing power and scavenging capacity against DPPH (IC50 = 6.77±0.15 mg/l) and ABTS (IC50 = 2.79±0.07 mg/l).
CONCLUSION:
These findings suggest that aerial parts of Teucrium polium methanolic extract could be used as natural source of antioxidants in food, pharmaceutical and cosmetic industry.
1Introduction
Oxidative stress is defined in general as excess formation and/or incomplete removal of highly reactive molecules such as reactive oxygen species (ROS) [1]. Free radicals are produced in the human body from normal metabolism or induced by physical and/or chemical factors in the environment [2]. Oxidative radicals are reported to be a potential cause of mutations, damage to lipids, DNA and proteins which in turn lead to certain disorders including cancer [3]. The use of substances with antioxidant ability can be very important in the therapeutic prevention of diseases related to increased oxidative stress, such as cancer, heart disease and aging [4]. Antioxidants that have traditionally been used to inhibit oxidation in foods also quench dreaded free radicals and stop oxidation chains in-vivo, so they have become viewed by many as natures answer to environmental and physiological stress and other disorders [5]. ROS can induce peroxidation of lipids generating secondary oxidants such as heptanol and hexanal, which contributes to oxidative rancidity, deteriorating the flavor of food [6]. The synthetic antioxidants used in the food industry namely, butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), Tertiary-butyl hydroquinone (TBHQ) and propyl gallate (PG) are suspected to be toxic and carcinogenic. Recently, more attention has been given to medicinal plants of therapeutic potentials as antioxidants in reducing free radical induced tissue injury [7]. The antioxidant ability of many plants may be attributed to the presence of phenolic compounds includes phenolic acids (hydroxybenzoic and hydroxycinnamic acids), tannins (hydrolyzable and condensed), and flavonoids [8]. Polyphenols are natural substances capable to neutralise free radicals and reduce the oxidative stress damage on the human body [9]. Antioxidants act as radical scavenger, hydrogen donor, electron donor, peroxide decomposer, singlet oxygen quencher, enzyme inhibitor, synergist, and metal-chelating agents [10]. Therefore, there is an increasing interest in extracting these plant antioxidants and using them as natural antioxidants [11]. Extraction is the first step in analysis polyphenolic, which consists in isolation of phenolic compounds from plant materials [12]. One of the most important factors affecting the extraction efficiency of bioactive compounds from plant materials and their consequent health benefits is the extraction solvent [13].
The genus Teucrium (Lamiaceae) contains more than 340 species spread all over the world, twenty of which are that found abundantly in the flora of Algeria [14]. Teucrium polium L. (Felty germander) called ‘kheyata’ is one of the species from this genus that grows wildly in Algeria. This species was traditionally used in Algerian folk medicine due to its hypoglycemic, hypolipidemic, anti-inflammatory, antibacterial and antioxidant properties [15–19]. Previous studies have described Teucrium polium extracts as a potential source of natural antioxidants and phenolic compounds [20–23]. There is no report in the literature on the impact of different solvents extraction including methanol, ethanol, ethyl acetate, chloroform, hexane and water on antioxidant capacity and amount of total phenol and flavonoid of Algerian Teucrium polium aerial parts.
The main objective of this study was to determine the effect of different extracting solvents on total phenolic and flavonoid contents and antioxidant potency of Teucrium polium aerial parts grown in Algeria. The total phenol and flavonoid contents of the investigated extracts were screened using the Folin-Ciocalteu and aluminium trichloride methods, respectively. The antioxidant capacity of the all extracts was assessed by DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid) free radicals scavenging capacity and reducing power.
2Materials and methods
2.1Plant material
The aerial parts of Teucrium polium were collected at flowering stage from Oum El Bouaghi (east of Algeria) during March 2015. The taxonomic identification of the plant was performed by the botanists of the High National School of Agronomy of Algiers, Algeria. The plant material was air-dried in the shade at room temperature.
2.2Preparation of extracts
Dried and powdered aerial parts (30 g) from Teucrium polium were extracted with 400 ml of six different solvents (methanol, ethanol, ethyl acetate, chloroform, hexane and water) in a Soxhlet apparatus for 6 h. The solvent was evaporated using a rotary evaporator. Each extract was weighed and kept at 4 °C in the dark before analysis.
2.3Determination of total phenol content
The total phenol content in the examined plant extracts was determined using Folin-Ciocalteu method described by Singleton et al. [24]. 0.25 ml of extract dissolved in methanol was mixed with 1.25 ml of Folin-Ciocalteu. After 3 min of reaction, 1 ml of solution of sodium carbonate (Na2CO3) at a concentration of 75 g/l was added. The samples were incubated for 30 min in the dark at room temperature. The absorbance was determined using spectrophotometer at 765 nm. Gallic acid was used as standard for the calibration curve. Total phenol content was expressed as milligrams Gallic Acid Equivalent (GAE) per gram of extract.
2.4Determination of total flavonoid content
The flavonoid content in each extract was determined by aluminium trichloride (AlCl3) method as described by Lamaison et al. [25] using quercetine as a reference compound (standard). The sample contained 1 ml of extract dissolved in methanol and 1 ml of solution of aluminium trichloride (2% w/v). The samples were incubated for an hour at room temperature. The absorbance was determined using spectrophotometer at 420 nm. The flavonoid content was expressed as mg of Quercetin Equivalent (QE) per gram of extract.
2.5Antioxidant capacity
2.5.1Determination of DPPH radical scavenging capacity
The radical scavenging capacity of the examined extracts was determined using the DPPH (2,2-diphenyl-1-picrylhydrazyl) assay. All the extracts were tested for the concentration of 1, 3, 5, 10, 20, 50 and 100 mg/l. 25μl of various concentrations of the sample in methanol were added to 975μl of methanolic solution of DPPH (60μM). After 30 min incubation time in darkness at room temperature, the absorbance measurements were recorded at 517 nm. The lower absorbance of the reaction mixture indicates higher free radical scavenging activity. Scavenging capacity of DPPH radical was calculated as follows:
(1)
Where Abs blank is the absorption of the blank sample (t = 0 min) and Abs sample is the absorption of the tested sample (t = 30 min). Absorption of a blank sample containing the same amount of methanol and DPPH solution was acted as the negative control and BHT as the positive control. The IC50 value (concentration of sample providing 50% inhibition) was calculated using the graph plotted the percentage inhibition against concentration of sample.
2.5.2Determination of ABTS radical scavenging capacity
The radical scavenging capacity of the tested extracts against ABTS (2, 2’-azino-bis 3-ethylbenzthiazoline-6-sulfonic acid) radical cation was measured for the evaluation of the antioxidant activity. ABTS• + radical cation was produced by reacting ABTS stock solution with potassium persulfate and allowing the mixture to stand for 12–16 h at 4 °C in the dark before use. The ABTS• + radical cation solution was diluted with methanol to an absorbance of 1±0.02 at 734 nm and equilibrated at 30 °C. Each concentration of the sample in methanol (25μl) was mixed with 1 ml of diluted ABTS• + radical cation solution. After reaction at 30 °C for 7 min, the absorbance at 734 nm was measured. Scavenging capacity of ABTS radical was calculated according to the following formula:
(2)
Where Abs blank is the absorption of the blank (t = 0 min) and Abs sample is the absorption of the tested sample (extracts and standard) (t = 7 min). The ABTS was used as the negative control and trolox as the positive control. The IC50 value was calculated by plotting the percentage inhibition against concentration of sample.
2.5.3Determination of reducing power
The method of Oyaizu [26] was used to determine the capacity of the tested extract and BHT to reduce ferric iron (Fe3 +) to ferrous iron (Fe2 +). The samples (0.125 ml) were mixed with phosphate buffer (2.5 ml, 0.2 mol/l, pH 6.6) and K3Fe(CN)6 (2.5 ml, 1% w/v) and the mixture was incubated for 20 min at 50 °C. 2.5 ml of trichloroacetic acid solution (10%) was added to the mixture, which was then centrifuged at 1500 g for 10 min. The upper layer of solution (2.5 ml) was mixed with distilled water (2.5 ml) and FeCl3 (0.5 ml, 0.1% w/v). Absorbance of all solutions was measured at 700 nm.
2.6Statistical analysis
In this study, all determinations were conducted in triplicate and the data are expressed as the average of three measurements±standard deviation. Statistical analysis was performed using IBM SPSS Statistics 20 software. Statistical comparisons were carried out with oneway analysis of variance (ANOVA) and the probability p value less than 0.05 was considered significant.
3Results and discussion
3.1Extraction yield and total phenol and flavonoid contents
The percentage yield obtained from the aerial parts of Teucrium polium using different solvents extraction is presented in Table 1. The extraction yield of Teucrium polium based on the dry weight of the plant varied from 8.47±0.79 to 23.33±0.61% (g/g) and decreased as follows: methanol > ethanol>water > chloroform > hexane > ethyl acetate. The methanol showed the greatest extraction yield (23.33±0.61% (g/g)) compared to other solvents. These findings are in agreement with those obtained by Zazouli et al. [27], who shown that the methanol was the most appropriate solvent for recovering of the extractable constituents from plants. Variations in recovery percentage of extractable compounds have been probably related to polarity of the extracting solvent. According to Butsat and Siriamornpun [28], the difference in polarities of the extraction solvents might influence the solubility of the chemical constituents in a sample and its extraction yield.
Table 1
Extraction yield, total phenol and flavonoid contents of Teucrium polium extracts
| Extracts | Yield (% (g/g)) | Total phenols (mg GAE/g) | Total Flavonoids (mg QE/g) |
| Methanol | 23.33±0.61a | 206.95±1.82a | 42.16±0.61a |
| Ethanol | 21.97±0.28b | 157.42±4.12c | 34.46±2.24c |
| Ethyl acetate | 8.47±0.79e | 113.23±3.54d | 5.74±0.19f |
| Chloroform | 10.62±1.15d | 102.36±2.83e | 12.83±1.92e |
| Hexane | 9.80±0.43de | 83.7±0.15f | 23.65±0.60d |
| Water | 20.15±0.85c | 184.84±2.25b | 38.95±1.13b |
Data were expressed as mean of three measurements±standard deviations. Values with different letters in the same experiment and same column differ significantly at p < 0.05.
Being plant secondary metabolites, the phenolic compounds (simple phenolics, phenolic acids, anthocyanins, hydroxycinnamic acid derivatives and flavonoids) are very important judging from the virtue of their antioxidant capacities by chelating redox-active metal ions, inactivating lipid free radical chains, and avoiding the hydroperoxide conversions into reactive oxyradicals [29, 30]. The total phenol and flavonoid contents of different Teucrium polium extracts were estimated through the Folin-Ciocalteu and aluminium trichloride (AlCl3) methods, respectively. The amounts of total phenolic and flavonoid present in various Teucrium polium extracts (methanol, ethanol, ethyl acetate, chloroform, hexane and water) are given in Table 1. The Total phenol content was expressed as mg Gallic Acid Equivalent (GAE) per gram of extract (the equation of calibration curve: y = 0.0107x + 0.0065, R2 = 0.9993). Total phenol content in different tested extracts was ranged between 83.7±0.15 and 206.95±1.82 mg of GAE/g and decreased in the order of methanol > water > ethanol > ethyl acetate > chloroform > hexane. The total flavonoid content in extracts was expressed as mg of Quercetin Equivalent (QE) per gram of extract (y = 0.0343x + 0.0058, R2 = 0.9999). The flavonoid content of examined extracts was came in a range from 5.74±0.19 to 42.16±0.61 mg of QE/g and decrease in the following order: methanol > water > ethanol > hexane > chloroform > ethyl acetate. The results of the present study indicated a wide variation of the total phenol and flavonoid contents in the different Teurium polium extracts (Table 1). This could have been due to solubility of phenolic compounds in the extracting solvent. Our data showed that methanolic extract from Teucrium polium aerial parts exhibited the highest amount of total phenols and flavonoids with values of 206.95±1.82 mg of GAE/g and 42.16±0.61 mg of QE/g, respectively. These findings were in good agreement with previous reports, which also found that methanol was the most effective solvent in extracting phenolic components from plants [20, 31]. The recovery of phenols from plant materials is influenced by solubility of the phenolic compounds in the solvent used for the extraction process [27]. Addai et al. [31] have been reported that with increase in solvent polarity, total phenol and total flavonoid content increased in extract. Phenolic compounds are often extracted in higher amounts in more polar solvents [32]. According to Khorasani Esmaeili et al. [33], a possible justification would be due to the formation of complexes by a part of phenolic compounds with carbohydrates and proteins, which are more extractable in methanol than in other solvents that have been emphasized in this study.
3.2Antioxidant capacity
In the present study, the in vitro antioxidant capacity of the all Teucrium polium extracts was assessed by DPPH, ABTS and reducing power assays. The BHT and trolox were used as reference standards.
The DPPH assay was the most commonly method used for determination of scavenging activity of antioxidants [34]. The DPPH free radical scavenging capacities of the six tested extracts are shown in Fig. 1. All the examined extracts were able to reduce the stable free radical of DPPH to the yellow-colored diphenylpicrylhydrazine radical due to donation of a hydrogen atom. All the extracts showed a dose-dependent radical scavenging capacity. The IC50 values (concentration required for 50% inhibition) of each extract are presented in Table 2. Lower IC50 value indicates higher scavenging capacity of the extracts. All the Teucrium polium extracts revealed various DPPH scavenging capacities, with an IC50 values ranged from 6.77±0.15 to 9.90±0.04 mg/l. According to Adaramola and Onigbinde [35], different extractability of antioxidant constituents by extraction solvents due to variations in the polarity of the solvents may be responsible for the differences in the antioxidant capacity of extracts in different solvents. The DPPH scavenging capacity of the tested extracts was found to be decrease in the following order: methanol > ethanol > ethyl acetate > water > chloroform > hexane. As shown in Table 2, all the examined extracts exhibited higher scavenging capacity than the synthetic standard BHT (IC50 = 27.99 mg/l). The methanol extract showed the greatest DPPH radical scavenging capacity with an IC50 value of 2.79±0.07 mg/l.
Fig.1
DPPH radical scavenging capacity of Teucrium polium extracts. Met: Methanol, Eth: Ethanol, Ethyl ac: Ethyl acetate, Chl: Chloroform, Hex: Hexane and Wat: water. Data were expressed as mean of three measurements. Values differ significantly at p < 0.05.
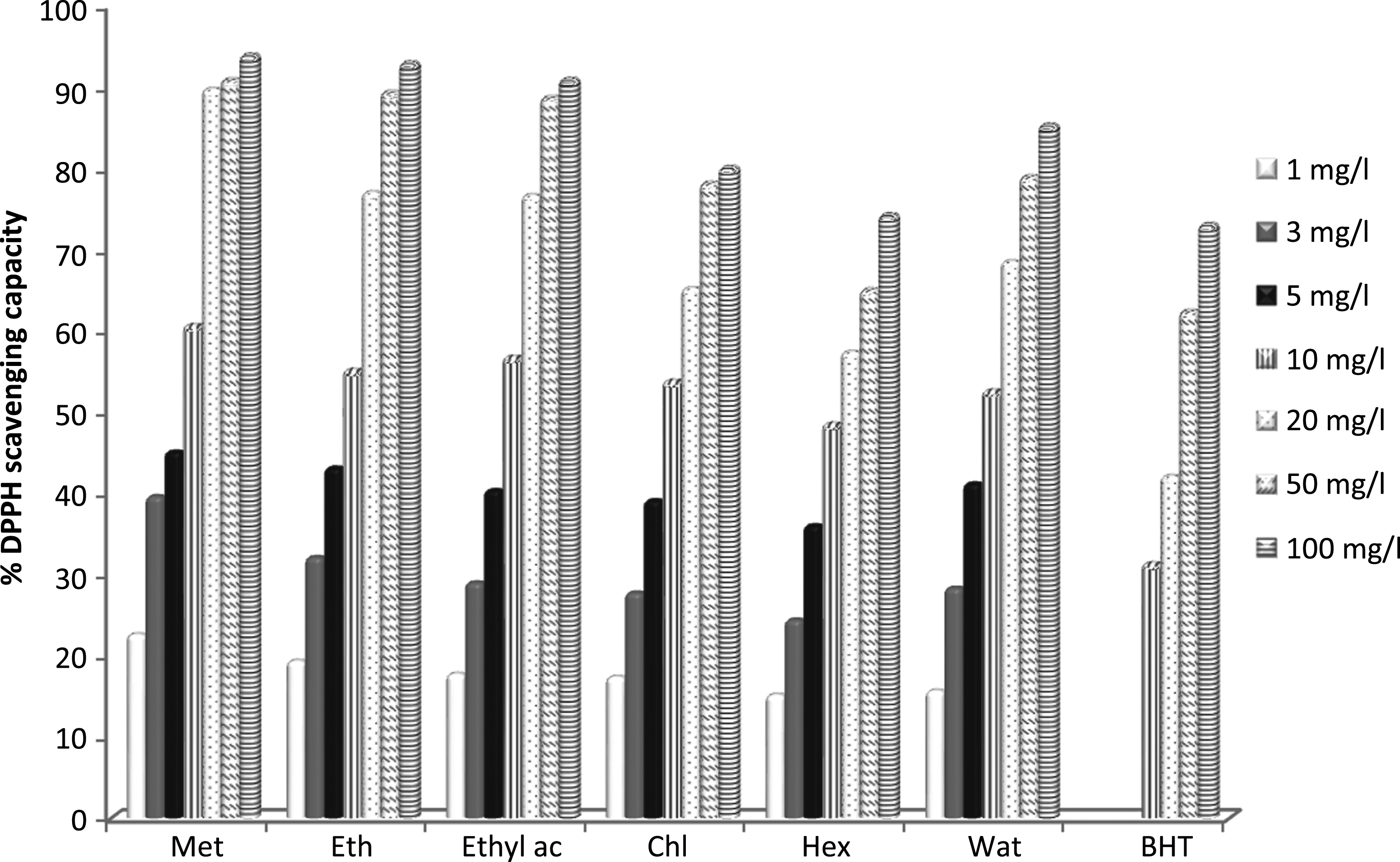
Table 2
IC50 values in DPPH and ABTS radicals scavenging capacity
| Extracts | DPPH IC50 (mg/l) | ABTS IC50 (mg/l) |
| Methanol | 6.77±0.15f | 2.79±0.075b |
| Ethanol | 8.01±0.069e | 3.49±0.1c |
| Ethyl acetate | 8.02±0.07e | 3.82±0.02d |
| Chloroform | 8.61±0.32c | 4.60±0.08e |
| Hexane | 9.90±0.04b | 6.90±0.05f |
| Water | 8.37±0.28d | 3.68±0.25cd |
| BHT | 27.99±0.0a | – |
| Trolox | – | 0.82±0.0a |
Data were expressed as mean of three measurements±standard deviations. Values with different letters in the same experiment and same column differ significantly at p < 0.05.
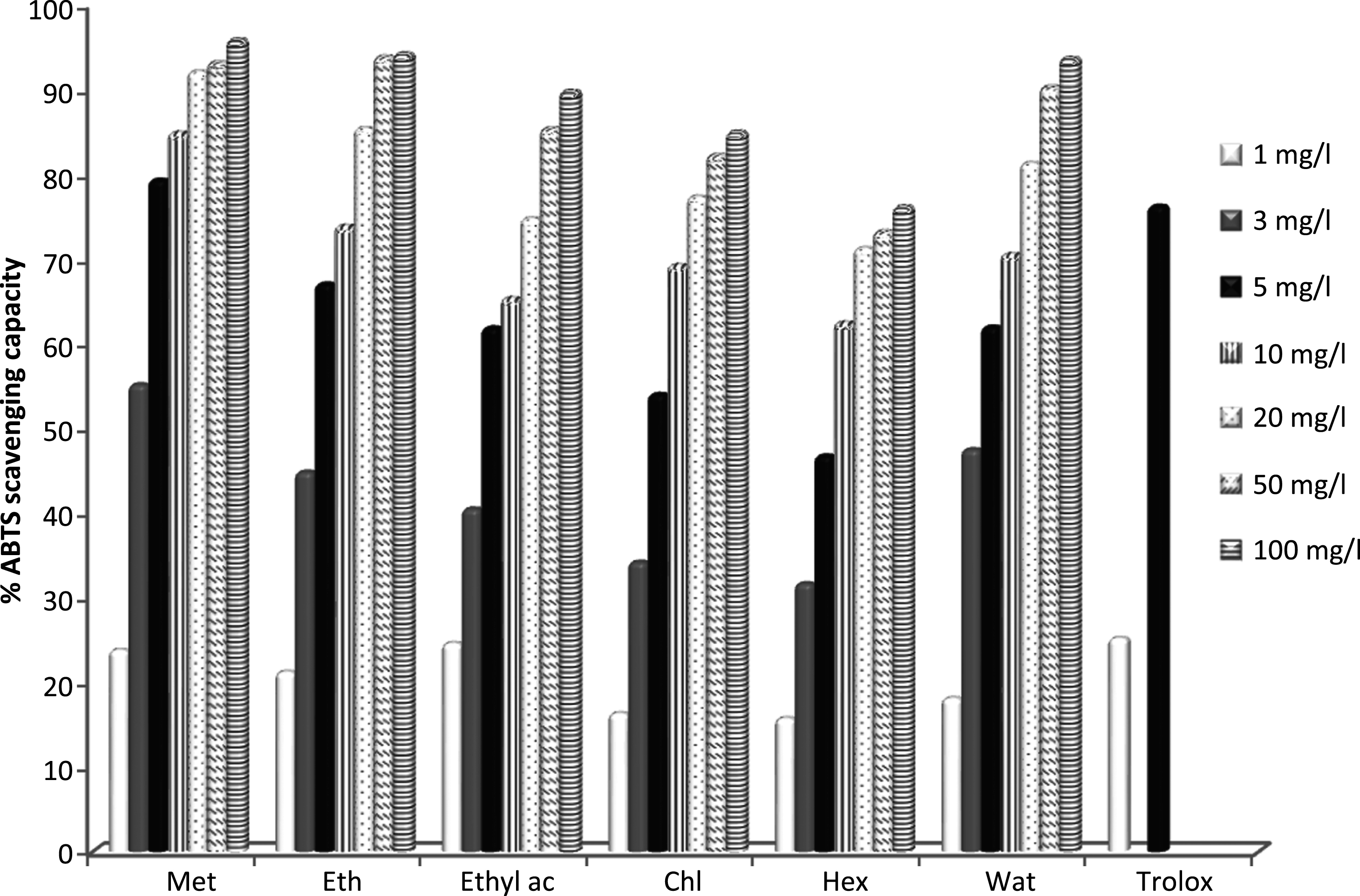
The ABTS free radical scavenging assay was used to test in vitro the antioxidant capacity of different Teucrium polium extracts. The antioxidants of tested extracts react with the stable ABTS+ free radical cation (deep blue/green colour) and convert it to its colorless neutral form, by their hydrogen donating ability to ABTS. The IC50 values in the ABTS radical scavenging capacity assay of the different extracts are given in Table 2. All the examined extracts exhibited a concentration-dependent inhibition of the ABTS radicals as shown in Fig. 2. The investigated extracts denoted differential radical scavenging capacity against ABTS radical cation, with an IC50 values ranked between 2.79±0.07 and 6.90±0.05 mg/l. All the examined extracts showed lower scavenging capacity compared to the synthetic antioxidant trolox (IC50 = 0.82 mg/l). However, at a concentration of 5 mg/l, the methanolic extract exhibited a comparable ABTS free radical scavenging ability with standard trolox. The ABTS scavenging capacity of investigated Teucrium polium extracts was found effective in this order methanol > ethanol > water > ethyl acetate > chloroform > hexane. Among the six tested extracts, the methanol extract exhibited the greatest radical scavenging capacity (IC50 = 2.79±0.07 mg/l) when it reacted with the ABTS free radicals.
Fig.2
ABTS radical scavenging capacity of Teucrium polium extracts. Met: Methanol, Eth: Ethanol, Ethyl ac: Ethyl acetate, Chl: Chloroform, Hex: Hexane and Wat: water. Data were expressed as mean of three measurements. Values differ significantly at p < 0.05.
The results of DPPH and ABTS free radicals scavenging assay revealed that the methanolic extract present the best antioxidant potential. This could be attributed to the presence of high amount of total phenols in the methanolic extract (Table 1). There are some studies in the literature that report a high linear correlation between antioxidant capacity and total phenolic content [36–38]. Viuda-Martos et al. [39] have been reported that phenolic content can be used as an important indicator of antioxidant capacity of plants and can be used as a preliminary screen for any product when intended to be used as a natural source of antioxidants in functional foods. Phenolics possess one or more aromatic rings, extended conjugated aromatic system to delocalize an unpaired electron, and one or more hydroxyl groups that are prone to donate a hydrogen atom or an electron to a free radical; therefore, they have ideal structure for free radical scavenging capacities [40].
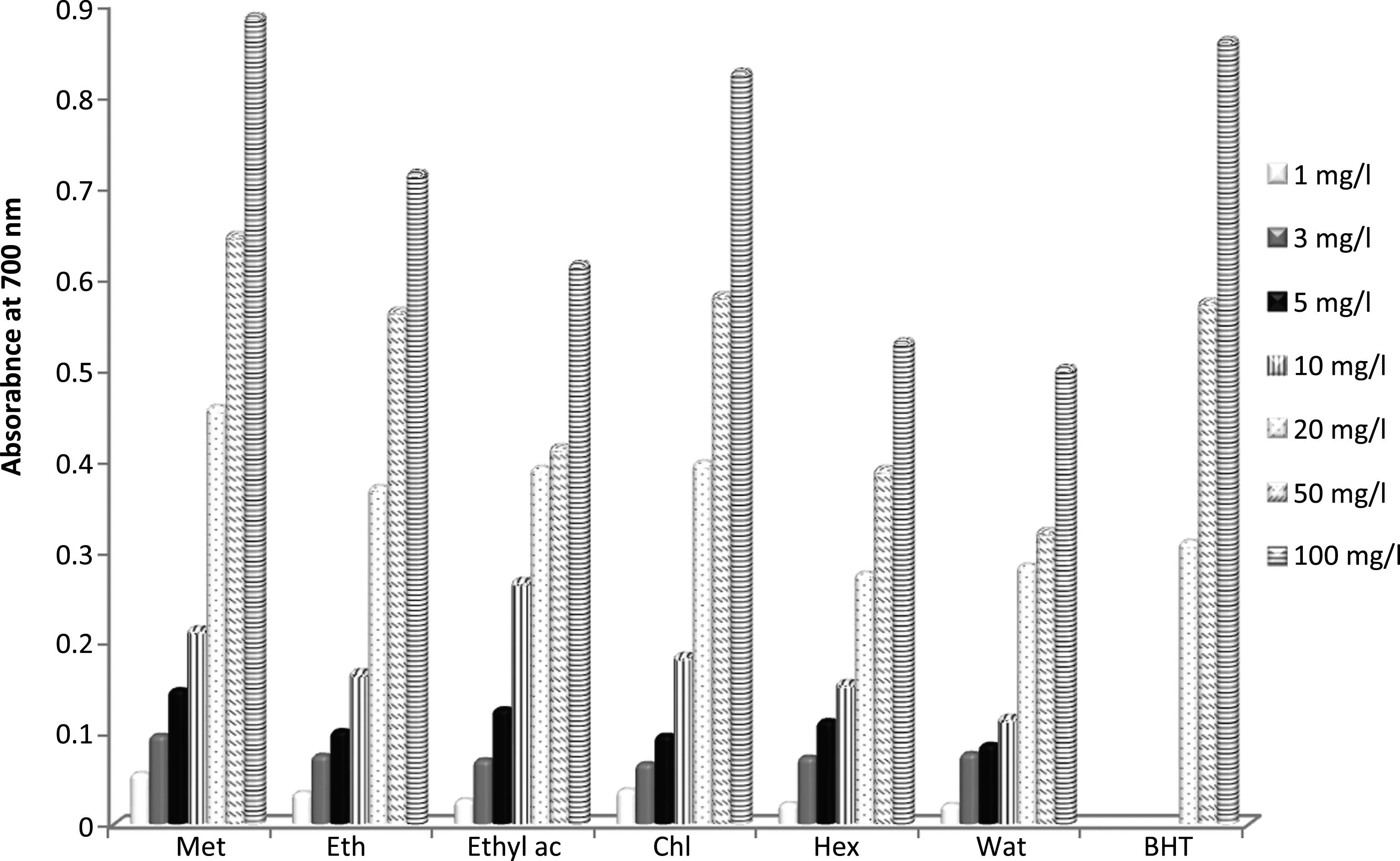
The different Teucrium polium extracts were also subjected to screening for their possible in vitro antioxidant capacity by reducing power assay. Reducing power is widely used to evaluate the antioxidant capacity of plant extracts [41]. In the reducing power assay, the presence of antioxidants in plant extracts causes the reducing of ferric iron (Fe3 +) to ferrous iron (Fe2 +) by giving away an electron. The reductive abilities of different Teucrium polium extracts are shown in Fig. 3. All the examined extracts exhibited good reducing power capability. Ferric reducing power increases with the increase in concentration of the extract. The antioxidant capacity of the examined extracts by reducing power assay can be ranked in the following order: methanol > chloroform > ethanol > ethyl acetate > hexane > water. The methanolic extract showed the strongest reducing power ability in comparison to other extracts and standard BHT (Fig. 3). These results were in agreement with those found by Pavithra and Vadivukkarasi [42] on leaves from Kedrostis foetidissima, which reported that methanolic extract exhibited highest reducing power ability when compared to other extracts.
Fig.3
Reducing power ability of Teucrium polium extracts. Met: Methanol, Eth: Ethanol, Ethyl ac: Ethyl acetate, Chl: Chloroform, Hex: Hexane and Wat: water. Data were expressed as mean of three measurements. Values differ significantly at p < 0.05.
4Conclusion
In conclusion, results of this study demonstrated that the type of solvents used significantly affected the extraction efficiency of bioactive compounds and antioxidant potency of Teucrium polium aerial parts grown in Algeria. Variations in phenolic compounds content and antioxidant capacity between extracts were probably related to polarity of the extracting solvent. All the examined extracts contained appreciable contents of total phenolic and flavonoid and exhibited good antioxidant potential. Among the six tested extracts, methanolic extract was found to have the strongest antioxidant capacity when was assessed by DPPH, ABTS and reducing power assays. The obtained data also showed that methanol was the most efficient solvent for extraction of the highest amount of total phenolic and flavonoid from Teucrium polium aerial parts. Thereby, it can be said that antioxidant capacity of the methanolic extract was probably due to the presence of high content of phenolic compounds which possess ideal structural chemistry for free radical scavenging capacity. Our results revealed that Teucrium polium methanolic extract could be considered as good source of natural antioxidants capable to reduce oxidative stress damage.
References
[1] | Boumerfeg S , Baghiani A , Djarmouni M , Ameni D , Adjadj M , Belkhiri F , et al. Inhibitory Activity on Xanthine Oxidase and Antioxidant Properties of Teucrium polium L. Extracts. Chinese Medicine. (2012) ;03: (01):30–41. |
[2] | Alimpic A , Oaldje M , Matevski V , Marin PD , Duletic-Lausevic S . Antioxidant activity and total phenolic and flavonoid contents of Salvia amplexicaulis Lam. extracts. Archives of Biological Sciences. (2014) ;66: (1):307–16. |
[3] | Rehan T , Tahira R , Rehan T , Bibi A , Naeemullah M . Screening of Seven Medicinal Plants of Family Lamiaceae for Total Phenolics, Flavonoids and Antioxidant Activity. Pakhtunkhwa J Life Sci. (2014) ;2: (03/04):107–17. |
[4] | Araújo KSdS , Neto DLdS , Mariano SMB . A Systematic Review of the Antioxidant Activity of Apiculture Products in Brazil, (2017) . doi:10.5772/66756 |
[5] | Husain N , Kumar A . Reactive Oxygen Species and Natural Antioxidants: A Review. Advances in Bioresearch. (2012) ;3: (4):164–75. |
[6] | Chaouche TM , Haddouchi F , Ksouri R , Atik-Bekkara F . Evaluation of antioxidant activity of hydromethanolic extracts of some medicinal species from South Algeria. J Chin Med Assoc. (2014) ;77: (6):302–7. |
[7] | Khettaf A , Belloula N , Dridi S . Antioxidant activity, phenolic and flavonoid contents of some wild medicinal plants in southeastern Algeria. African Journal of Biotechnology. (2016) ;15: (13):524–30. |
[8] | Do QD , Angkawijaya AE , Tran-Nguyen PL , Huynh LH , Soetaredjo FE , Ismadji S , Ju YH . Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. (2014) ;22: (3):296–302. |
[9] | Hussain T , Tan B , Yin Y , Blachier F , Tossou MCB , Rahu N . Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Medicine and Cellular Longevity. (2016) ; pp. 9. |
[10] | Lobo V , Patil A , Phatak A , Chandra N . Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. (2010) ;4: (8):118–26. |
[11] | Padalia H , Poptani R , Chanda S . Evaluation of in Vitro Antioxidant Properties of Solvent Extracts of Selected Medicinal Plants and Their Synergistic Efficacy. Journal of Herbs, Spices & Medicinal Plants. (2017) :1–13. |
[12] | Boeing JS , Barizão ÉO , E Silva BC , Montanher PF , de Cinque Almeida V , Visentainer JV . Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: Application of principal component analysis. Chemistry Central Journal. (2014) ;8: (1):48. |
[13] | Ngo TV , Scarlett CJ , Bowyer MC , Ngo PD , Vuong QV . Impact of Different Extraction Solvents on Bioactive Compounds and Antioxidant Capacity from the Root of Salacia chinensis L. Journal of Food Quality. (2017) ;1–8. |
[14] | Quézel P , Santa S . Nouvelle flore de l’Algérie et des régions désertiques méridionales. Éditions du Centre National de la Recherche Scientifique: Paris, France. (1963) ;2: :783. |
[15] | Rasekh HR , Khoshnood MJ , Kamalinejad M . Hypolipidemic effects of Teucrium polium in rats Fitoterapia. (2001) ;72: :937–9. |
[16] | Esmaeili MA , Yazdanparast R . Hypoglycaemic effect of Teucrium polium: Studies with rat pancreatic islets. Journal Ethnopharmacology. (2004) ;95: :27–30. |
[17] | De Marino S , Festa C , Zollo F , Incollingo F , Raimo G , Evangelista G , et al. Antioxidant activity of phenolic and phenylethanoid glycosides from Teucrium polium L. Food Chemistry. (2012) ;133: (1):21–8. |
[18] | Djabou N , Lorenzi V , Guinoiseau E , Andreani S , Giuliani MC , Desjobert JM , Bolla JM , Costa J , Berti L , Luciani A , Alain Muselli A . Phytochemical composition of Corsican Teucrium essential oils and antibacterial activity against foodborne or toxi-infectious pathogens. Food Control. (2013) ;30: :354–63. |
[19] | Sharififar F , Dehghan-nudeh GH , Mirtajaldini M . Major flavonoids with antioxidant activity from Teucrium polium L. Food Chemistry. (2009) ;112: :885–8. |
[20] | Stankovic MS , Neda Niciforovic N , Mihailovic V , Topuzovic M , Solujic S . Antioxidant activity, total phenolic content and flavonoid concentrations of different plant parts of Teucrium polium L. subspolium. Acta Societatis Botanicorum Poloniae. (2012) ;81: (2):117–22. |
[21] | Belmekki N , Bendimerad N , Bekhechi C , Fernandez X . Chemical analysis and antimicrobial activity of Teucrium polium L. essential oil from Western Algeria. Journal of Medicinal Plants Research. (2013) ;7: (14):897–902. |
[22] | Bakari S , Ncir M , Felhi S , Hajlaoui H , Saoudi M , Gharsallah N , et al. Chemical composition and in vitro evaluation of total phenolic, flavonoid, and antioxidant properties of essential oil and solvent extract from the aerial parts of Teucrium polium grown in Tunisia. Food Science and Biotechnology. (2015) ;24: (6):1943–9. |
[23] | Dridi A , Hadef Y , Bouloudani L . Determination of Total Phenol, Flavonoid, Antioxidant and Antimicrobial Activity of Methanolic Extract of Teucrium polium L. in Algerian East. International Journal of Pharmacognosy and Phytochemical Research. (2016) ;8: (10):1566–70. |
[24] | Singleton VL , Ortofer R , Lamuela-Raventos RM . Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin- Ciocalteu reagent. In: Packer L. (ed). Methods in Enzymology. Orlando. Academic Press. (1999) . |
[25] | Lamaison JLC , Carnet A . Teneurs en principaux flavonoïdes des fleurs de Crataegeus monogyna Jacq et de Crataegeus laevigata (Poiret D. C) en fonction de la végétation. Pharmaceutica Acta Helvetiae. (1990) ;65: :315–20. |
[26] | Oyaizu M . Studies on product of browning reaction prepared from glucose amine. Japan Journal of Nutrition. (1986) ;44: :307–15. |
[27] | Zazouli S , Chigr M , Jouaiti A . Effect of polar and nonpolar solvent on total phenolic and antioxidant activity of roots extracts of Caralluma europaea. Der Pharma Chemica. (2016) ;8: (11):191–6. |
[28] | Butsat S , Siriamornpun S . Antioxidant capacities and phenolic compounds of the husk, bran and endosperm of Thai rice. Food Chemistry. (2010) ;119: (2):606–13. |
[29] | Adebiyi OE , Olayemi FO , Ning-Hua T , Guang-Zhi Z . In vitro antioxidant activity, total phenolic and flavonoid contents of ethanol extract of stem and leaf of Grewia carpinifolia. Beni-Suef University Journal of Basic and Applied Sciences. (2017) ;6: (1):10–4. |
[30] | Nabi NG , Shrivastava M . Phytochemical Screening and Antioxidant Activity of Ethanol Extract of Psoralea corylifolia seeds. UK Journal of Pharmaceutical and Biosciences. (2017) ;5: (2):01–07. |
[31] | Addai ZR , Abdullah A , Mutalib SA . Effect of extraction solvents on the phenolic content and antioxidant properties of two papaya cultivars. Journal of Medicinal Plants Research. (2013) ;7: (46):3354–9. |
[32] | Iloki-Assanga SB , Lewis-Lujan LM , Lara-Espinoza CL , Gil-Salido AA , Fernandez-Angulo D , Rubio-Pino JL , et al. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum. BMC Res Notes. (2015) ;8: :396 2015/09/02.13. |
[33] | Khorasani Esmaeili A , Mat Taha R , Mohajer S , Banisalam B . Antioxidant Activity and Total Phenolic and Flavonoid Content of Various Solvent Extracts from In Vivo and In Vitro Grown Trifolium pratense L. (Red Clover). Biomed Res Int. (2015) ;2015: :643285. Epub 2015/06/13. |
[34] | Ait Chaouche FS , Mouhouche F , Hazzit M and Ferradji A . Optimization of extraction yield of Algerian Mentha pulegium L. essential oil by ultrasound-assisted hydrodistillation using response surface methodology. Res J Phytochem. (2017) ;11: :142–9. |
[35] | Adaramola B , Onigbinde A . Influence of extraction technique on the mineral content and antioxidant capacity of edible oil extracted from ginger rhizome. Chem Int. (2017) ;3: (1). |
[36] | Sadeghi Z , Valizadeh J , Shermeh OA , Akaberi M . Antioxidant activity and total phenolic content of Boerhavia elegans (choisy) grown in Baluchestan, Iran. Avicenna Journal of Phytomedicine. (2015) ;5: (1):1. |
[37] | Qasim M , Aziz I , Rasheed M , Gul B , Ajmal khan M . effect of extraction solvents on polyphenols and antioxidant activity of medicinal halophytes. Pak J Bot. (2016) ;48: (2):621–7. |
[38] | Al-Rimawi F , Abu-Lafi S , Abbadi J , Alamarneh AAA , Sawahreh RA , Odeh I . Analysis of Phenolic and Flavonoids of Wild Ephedra alata Plant Extracts by Lc/Pda and Lc/Ms and Their Antioxidant Activity. African Journal of Traditional, Complementary and Alternative Medicines. (2017) ;14: (2):130–41. |
[39] | Viuda-Matros M , Ruiz NY , Fernandez LJ , Perez JA . Spices as functional foods: A review. Journal of Critical Reviews in Food Science and Nutrition. (2011) ;51: (1):13–28. |
[40] | Skrovankova S , Misurcova L , Machu L . Antioxidant Activity and Protecting Health Effects of Common Medicinal Plants. Advances in Food and Nutrition Research. (2012) ;67: :76–139. |
[41] | Raman ST , Ganeshan AP , Chen C , Jin C , Li SH , Chen HJ , Gui Z . In vitro and In vivo antioxidant activity of flavonoid extracted from mulberry fruit (Morus alba L.). Phcog Mag. (2016) ;12: :128–33. |
[42] | Pavithra K , Vadivukkarasi S . Evaluation of free radical scavenging activity of various extracts of leaves from Kedrostis foetidissima (Jacq) Cogn. Food Science and Human Wellness. (2015) ;4: (1):42–6. |




