Gut microbiota and Crohn’s disease
Abstract
INTRODUCTION:
Crohn’s disease (CD) is characterized by a chronic inflammation of the gastrointestinal tract causing abdominal pain, diarrhea, weight loss and systemic symptoms. Although the etiology of this disease is unknown, current knowledge suggests a multifactorial genesis involving genetic, environmental and immunological factors.
EVIDENCE ACQUISITION:
We focused our attention on critical analysis of the recent literature on the role of gut microbiota in inflammatory bowel disease (IBD), by evaluating the differences of composition, functions and role of intestinal flora. In particular, we focused on evidences about the interaction between gut microbiota and pathogenesis of IBD. In this setting, we conducted a PUBMED search for guidelines, systematic reviews (SR) and primary studies.
EVIDENCE SYNTHESIS:
Some data suggest that, in a significant percentage of patients, the microbiota plays an important role in the genesis and maintenance of CD. Probiotic supplementation and antibiotic treatment appear to be a valid therapeutic approach, although the clinical data remain controversial.
CONCLUSIONS:
Despite the exciting and growing research on the role of gut microbiota in IBD, our knowledge remains fairly limited. Further studies are needed to measure the diversity, function and resistance to antibiotics of the intestinal microbiota in CD.
1Introduction
Crohn’s disease [CD] is a life-long and disabling disease. It could affect any portion of the gastrointestinal tract from mouth to anus, with a propensity for the distal small intestine and proximal large bowel [1]. Inflammation is discontinuous along the bowel longitudinal axis and can involve all layers from mucosa to serosa (transmuralphlogosis) [1]. The precise etiology is unknown and therefore a causal therapy is not yet available. Probably CD arise from an interaction between genetic and environmental factors:inparticular, the role of gut microbiota has been highlighted by recent evidences [2].
2Gut microbiota
It is estimated that the human microbiota contains about 1014 cells [3] and most of these cells are found in the gastrointestinal (GI) tract, with a range from 101–103 bacteria per gram of content in the stomach and duodenum to 1011–1012 cells per gram in the large intestine [4]. Currently more than 50 bacterial phyla have been described, although some phylotypes predominate: the Firmicutes, Bacteroidetes and the Actinobacteria [5]. The lower density of microorganisms of the small intestine and their distinct composition may be related to several factors. Various pancreatic secretions, bile and gastric acid found in parts of the small intestine may generate an unfavourable environment for most microorganisms. Additionally, the rapid transit time through the small bowel make the microbes not able to replicate and be stationed in this location.
Moreover, it has been established not only that the microbial composition varies between upper and lower GI tract [6],but also that gut microbiota significantly differs between its composition existing in the lumen and that fixed to imbedded in the mucus layer of the GI tract [7].
The microbial composition of the small intestine differs from that of the colon. For this reason, faecal samples are considered to be more representative of the luminal colonic microbiota than of the microbial residents of the small intestine, while, intraluminal catheter samples and biopsies from locations in the small intestine are regarded as more representative of the small bowel microbiome. Furthermore, stool samples are poor determinants of the microbes associated with the bowel mucosa [8, 9]. Bacteria belonging to the Bacteroidetes and Firmicutesphyla have consistently been demonstrated in microbiome studies where stool samples were used, and smaller amounts of Actinobacteria, Proteobacteria, Fusobacteria, andVerrucomicrobia have also been described [10, 11].
3Physiological role of the gut microbiota
The intestinal microbiome is involved in a wide number of physiological processes, including digestion, metabolism, immune system development and immune response. Moreover, animal studies have suggested that intestinal microbes are implicated in brain development and central nervous system signalling [12]. The digestive enzymes found in the human GI tract are unable to degrade certain carbohydrates such as resistant starch and cellulose; however, intestinal microbes convert these compounds through fermentation, generating metabolites that are favourable to the host. One of the end-products of the fermentation process is short chain fatty acids (SCFAs) that possess anti-inflammatory properties and act as a source of energy. Moreover, the gut microbiota can degrade proteins, which results in various toxic metabolites but also SCFAs. Additionally, intestinal microbes synthesize indispensable amino-acids [13]. Also, certain vitamins are produced by the microbiota, including vitamin K and components of vitamin B [14].
Some gut microbes are able to indirectly reduce serum cholesterol through conversion of bile salts, thereby reducing the enterohepatic bile reabsorption and increasing the secretion of bile salts in the faeces [14]. The development and maturation of the human immune system are influenced by both host genetics and environmental elements, including the interaction of microbes with the host. The maturation of gut-associated lymphoid tissue (GALT) (e.g. mesenteric lymph nodes and Peyer’s patches) relies upon post-natal interaction with microbes, which leads to the expansion of lymphoid tissue and formation of germinal centres containing immunoglobulin A (IgA) secreting B cells [15]. Moreover, the intestinal microbiotais also involved in the development of T cells [16].
4Microbiota in Crohn’s disease
Several studies have reported differences between the microbiota of CD patients and healthy subjects. In particular, some authors showed that CD patients had lower amounts of Firmicutesand reduction of Clostridium leptum phylogenetic group [17]. Moreover, the faeces of CD patients seemed to harbour reduced amounts of SCFAs, such as propionic and butyric acid [18]. Willing et al. examined the mucosal microbiota of monozygotic twins concordant or discordant for CD [19] and detected that the amounts of the C. leptum subgroup bacteria andFaecalibacteriumprausnitzii (F.prausnitzii), were significantly lower in mucosal samples from patients with ileal CD than in samples obtained from colonic CD patients and healthy twins. SimilarlySokol et al. confirmed a decrease in F. prausnitzii abundance in stool samples from CD patients [20]. F. prausnitzii is an important member of the normal gut flora and its colonization may prove beneficial to the host. Through bacterial fermentation, F. prausnitzii generates anti-inflammatory metabolic byproducts that may act as a source of energy for the intestinal mucosa and colonocytes (e.g. SCFAs such as formate and butyrate, and d-lactate). Additionally, it may play a role in intestinal immunomodulation and affect the integrity of the epithelial gut barrier [21, 22]. Conversely, it was reported an higher abundance of Enterobacteriaceae in intestinal samples from animals with chronic bowel inflammation and patients [19, 23, 24]. The increase in these bacteria is possibly caused by intestinal inflammation, which may alter the normal gut microbiome and enhance the growth of Enterobacteriaceae [25]. Of particular note are adherent-invasive E. Coli (AIEC) strains, which have been found to be present in higher amount in mucosal samples from CD patients than in samples from healthy individuals, with prevalence of 51.9% and 16.7%, respectively [26].
The role of specific microbes in the pathogenesis of CD has been studied extensively, and two bacteria have been suggested to play a role in CD development: AIEC and Mycobacterium aviumsubspecies paratuberculosis (MAP) [26–28]. Several studies found higher abundance of AIEC in the bowel of CD patients than in control subjects. Furthermore, this bacterium was particularly increased in ileal lesions, compared to colonic lesions, suggesting a specific relationship between AIEC and ileal CD [29, 30]. AIEC may contribute to intestinal inflammation in several ways. The bacterial strain can adhere to carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM6) on host epithelia [31]. CEACAM6 appears to be more expressed in ileal tissue in CD patients compared with non-IBD controls [31]. AIEC strains are able to survive and replicate within intestinal macrophages, where they stimulate the secretion of TNF-α. Moreover, certain AIEC strains have been shown to induce granuloma formation, which is an inflammatory response known to occur in CD [30, 31].
The involvement of MAP in the pathogenesis of CD is controversial. Some studies have shown the presence of this bacterium in tissues from CD patients, others have not [28, 32]. The hypothesis of MAP is the causative agent of CD suggests that antimycobacterial treatment would lead to eradication of the bacterium from the gut, and thereby to the induction of remission. However, antimycobacterial therapy did not induce a long-term improvement of disease in CD patients, thus showing that MAP is not the causative agent of CD [33].
In addition, as above reported, the gut microbiotais associated with several aspects of the human immune system. For instance, the normal resident flora is involved in the development and maturation of GALT, and it is associated with mucosal secretion of immunoglobulins [15, 16]. Furthermore, animal studies have suggested that the microbiota is implicated in the development, regulation and induction of immune cells; including T cells and APCs [34–36]. Additionally, members of the microbiota may influence the integrity of the intestinal epithelial barrier, and produce anti-inflammatory metabolic compounds that are utilized by intestinal epithelia (e.g. SCFAs) [13] and [22]. Also, certain microbes may influence the inflammatory process itself, causing either an increase [37–39] or a decrease [40, 41] in bowel inflammation, and inflammation-associated cancer development [42].
5Therapeutic potential of microbiota
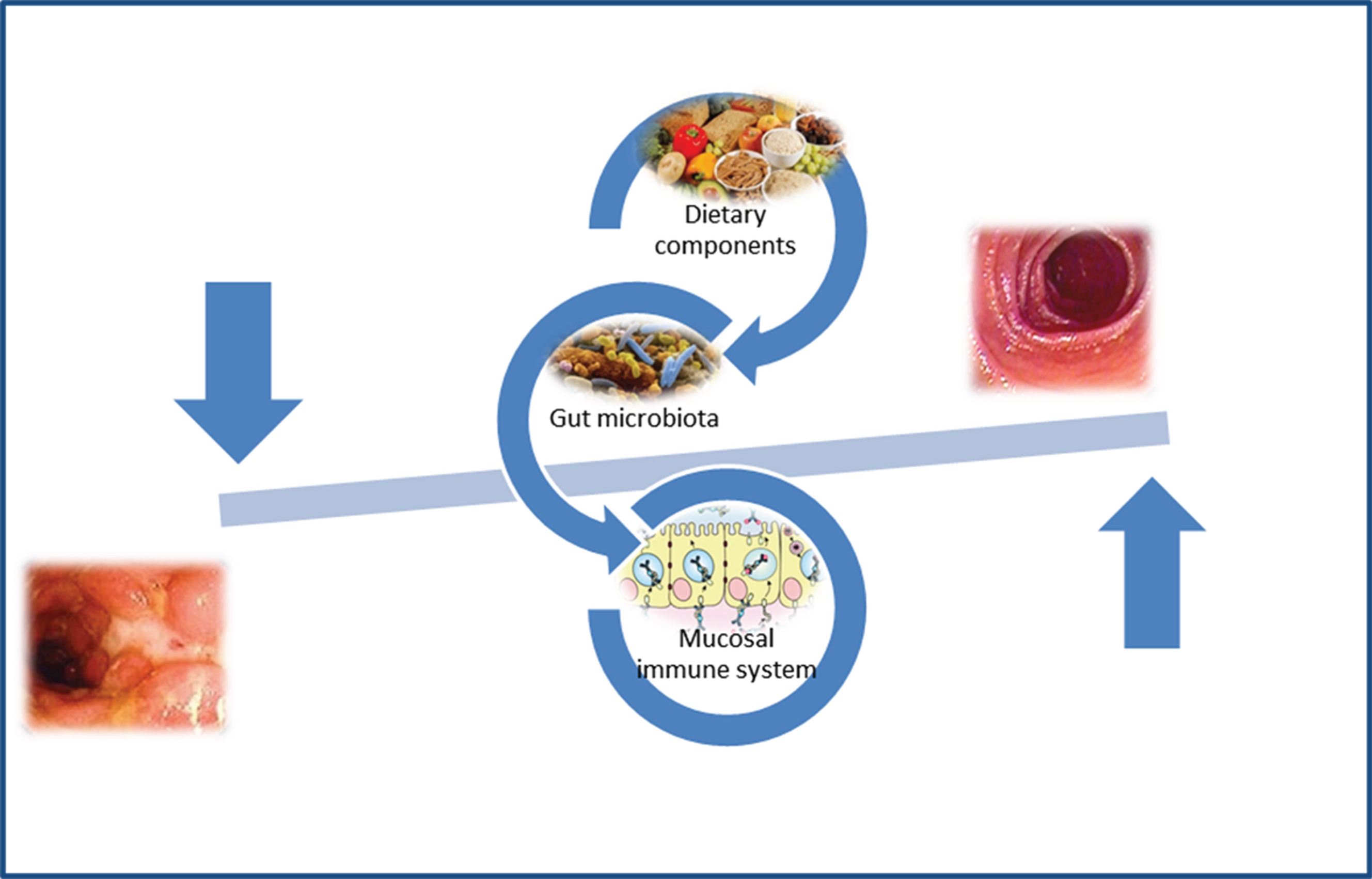
CD patients exhibit alterations in their microbiota function and structure and these microbial alterations are thought to participate in the pathogenesis of the disease (Fig. 1). In this setting, therapies aimed at restoring the CD microbiota into a non-dysbiotic one would prove beneficial effects. Besides antibiotics recent treatment options include the pro- and prebiotics supplementation, and faecal microbiota transplantation.
Fig.1
Gut microbiota and pathogenesis of Crohn’s disease.
6Prebiotics and probiotics
Prebiotics are non-digestible dietary components that promote the growth of beneficial intestinal microbes (i.e. probiotics like Bifidobacteria and Lactobacilli), and include compounds such as bran, inulin, oligosaccharides and fructooligosaccharides (FOS) [43]. Several studies have investigated the use of pre- and probiotics in CD patients. In a placebo-controlled trial in active CD, FOS failed to demonstrate significant discrepancies in the faecal proportions of Bifidobacteria between patients receiving FOS and patients given placebo [44]. In a double-blind, randomized trial, some authors investigated the level of post-operative recurrence of disease after Lactobacillus johnsonii LA1 (L. johnsonii LA1) administration for 6 months in 98 CD patients [45]. After 6 months of probiotic administration, no statistically significant differences were observed in endoscopic recurrence between the placebo group and the group receiving L. johnsonii LA1. Similarly, Prantera et al. compared Lactobacillus rhamnosus GG to placebo in 45 CD patients, reporting no significant differences in rate of disease recurrence between the two groups [46].
A few trials have conducted to evaluate the use of synmbiotics (synergistic combination of a probiotic and prebiotic) in CD [47, 48]. Steel et al. conducted a randomized, double-blind placebo-controlled trial involving 35 patients with active CD, using a synbiotic comprising Synergy 1 and Bifidobacterium longum [47]. Synbiotic determined significant improvement in clinical outcomes, with reductions in both Crohn’s disease activity indices (p = 0.020) and histological scores (p = 0.018). Instead, Chermesh et al evaluated the role of Synbiotic 2000 (containing 4 probiotic species and 4 prebiotics) in 30 patients with CD, in order to assess the role in preventing post operative recurrence of disease [48]. No difference in either clinical or endoscopic relapse rate was found between patients treated with Synbiotic 2000 or placebo.
As discussed above, the use of pre- and probiotics and synbioticsappears to be of little benefit in the treatment of CD; however, scientific data on their application and effect in this disease are limited, so further investigations are needed in order to determine the real role of these products in the treatment of CD.
7Fecal microbiota transplantation
Faecalmicrobiota transplantation (FMT) wasfirst used 1958 in 4 cases of pseudomembranous colitis, thought to be caused by Micrococcus pyogenes (i.e. Staphylococcus) [49]. Since dysbiosis significantly increases the risk of C. difficile overgrowth in the intestine, transplantation of a non-dysbiotic intestinal flora has been suggested to reverse this alteration, resulting in resolution of infection [50]. Thereafter, several systematic reviews have reported promising results of FMT in C. difficile infection [51, 52]. FMT has been also suggested to represent a novel treatment option for CD but limited data exist on the efficacy of this therapy [52–54]. Faecal samples are collected from healthy donors; stool samples are then diluted and filtered, and should be administered within 6 h of collection [55]. Various modalities of transplantation exist, and faecal substance may be infused by upper GI endoscopy, colonoscopy, retention enema or nasogastric and nasojejunal tubes [55]. A recent systematic review have investigated the use of FMT in CD patients, describing 133 patients with IBD (53 with CD, 77 with ulcerative colitis (UC), and 3 with undefined IBD) receiving FMT [53]. While 57% of the patients received transplantation as a treatment alternative to refractory IBD, the remaining of patients was transplanted with faecal microbes due to C. difficile infection. In general, reports on patient outcome after FMT were incomplete, and studies used different parameters to stratify patients, making data analysis difficult. However, included studies showed a reduction or resolution of symptoms in 71% of patients following transplantation, with comparable results among CD and UC patients. Furthermore, certain studies reported adverse reactions to FMT, including vomiting and diarrhea, fever and transient elevation of C-reactive protein [52, 53]. Although data on FMT in CD are limited, initial findings indicate that this procedure is relatively safe and that it may be a favourable addition to current CD treatment. Colman and Rubin performed a systematic review and meta-analysis in order to evaluate the efficacy of FMT as treatment for patients with IBD [56]. Eighteen studies were included (122 patients: 79 affected by UC, 39 with CD and 4 with IBD unclassified). Subgroup analyses showed clinical remission in 60.5% of subjects with CD (95% CI 28.4%–85.6%, p = 0.05; I2 = 37%). Suskind at al. conducted a prospective open-label study and evaluated 9 patients with CD, aged 12 to 19 years, with mild-to-moderate disease activity (defined using Pediatric Crohn’s Disease Activity Index (PCDAI) [57]. They received FMT by nasogastric tube with follow-up evaluations at 2, 6, and 12 weeks. The mean PCDAI score improved with patients having a baseline of 19.7±7.2, with improvement at 2 weeks to 6.4±6.6 and at 6 weeks to 8.6±4.9. Based on PCDAI, 7 of 9 patients were in remission at 2 weeks and 5 of 9 patients were in remission at 6 and 12 weeks.
8Conclusions
A growing number of patients are diagnosed with IBD worldwide. The incidence is increasing, especially in newly industrialized countries, probably due to the relatively recent westernization of these societies. The recognition of several susceptibility genes and environmental risk factors has focused on the intestinal microbiota, that appear to play an essential role in disease pathogenesis, probably because of inappropriate interactions with the gut innate immune system. Initial findings regarding microbiota modulation are promising and several studies are elaborating on whether or not it can be useful in the treatment of CD. As numerous disease conditions appear to be related to the intricate microbial communities residing in human body, the future of this “new field” of medicine is certainly interesting and stimulating.
References
[1] | Khanna S , Raffals LE . The microbiome in Crohn’s disease: Role in pathogenesis and role of microbiome replacement therapies. Gastroenterol Clin North Am. (2017) ;46: (3):481–92. |
[2] | Pascal V , Pozuelo M , Borruel N , Casellas F , Campos D , Santiago A , Martinez X , Varela E , Sarrabayrouse G , Machiels K , Vermeire S , Sokol H , Guarner F , Manichanh C . “A microbialsignature for Crohn’s disease. Gut. (2017) ;66: (5):813–22. |
[3] | Sekirov I , Russell SL , Antunes LC , Finlay BB . Gut microbiota in health and disease. Physiol Rev. (2010) ;90: :859–904. |
[4] | Orel R , Kamhi Trop T . Intestinal microbiota, probiotics and prebiotics in inflammatory bowel disease. World J Gastroenterol. (2014) ;20: (33):11505–24. |
[5] | Dethlefsen L , McFall-Ngai M , Relman DA . An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. (2007) ;449: :811–18. |
[6] | Zoetendal EG , Rajilic-Stojanovic M , de Vos WM . High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota. Gut. (2008) ;57: :1605–15. |
[7] | Swidsinski A , Loening-Baucke V , Lochs H , Hale LP . Spatial organization of bacterial flora in normal and inflamed intestine: A fluorescence in situ hybridization study in mice. World J Gastroenterol. (2005) ;11: :1131–40. |
[8] | Frank DN , St Amand AL , Feldman RA , Boedeker EC , Harpaz N , Pace NR . Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. (2007) ;104: :13780–5. |
[9] | Zoetendal EG , Raes J , van den Bogert B , Arumugam M , Booijink CC , Troost FJ , Bork P , Wels M , de Vos WM , Kleerebezem M . The human small intestinal microbiota is driven by rapid uptake and conversion of simple carbohydrates. ISME J. (2012) ;6: :1415–26. |
[10] | Eckburg PB , Bik EM , Bernstein CN , Purdom E , Dethlefsen L , Sargent MM , Gill SR , Nelson KE , Relman DA . Diversity of the human intestinal microbial flora. Science. (2005) ;308: :1635–58. |
[11] | Turnbaugh PJ , Hamady M , Yatsunenko T , Cantarel BL , Duncan A , Ley RE , Sogin ML , Jones WJ , Roe BA , Affourtit JP , Egholm M , Henrissat B , Heath AC , Knight R , Gordon JI . A core gut microbiome in obese and lean twins. Nature. (2009) ;457: :480–4. |
[12] | Dore J , Simren M , Buttle L , Guarner F . Hot topics in gut microbiota. United Eur Gastroenterology J. (2013) ;1: :311–8. |
[13] | Rajilic-Stojanovic M . Function of the microbiota. Best Pract Res Clin Gastroenterol. (2013) ;27: :5–16. |
[14] | Ramakrishna BS . Role of the gut microbiota in human nutrition and metabolism. J Gastroenterol Hepatol. (2013) ;28: :9–17. |
[15] | Sommer F , Backhed F . The gut microbiota—masters of host development and physiology. Nat Rev Microbiol. (2013) ;11: :227–38. |
[16] | Mazmanian SK , Liu CH , Tzianabos AO , Kasper DL . An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. (2005) ;122: :107–18. |
[17] | Manichanh C , Rigottier-Gois L , Bonnaud E , Gloux K , Pelletier E , Frangeul L , Nalin R , Jarrin C , Chardon P , Marteau P , Roca J , Dore J . Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. (2006) ;55: :205–11. |
[18] | Takaishi H , Matsuki T , Nakazawa A , Takada T , Kado S , Asahara T , Kamada N , Sakuraba A , Yajima T , Higuchi H , Inoue N , Ogata H , Iwao Y , Nomoto K , Tanaka R , Hibi T . Imbalance in intestinal microflora constitution could be involved in the pathogenesis of inflammatory bowel disease. Int J Med Microbiol. (2008) ;298: :463–72. |
[19] | Willing B , Halfvarson J , Dicksved J , Rosenquist M , Järnerot G , Engstrand L , Tysk C , Jansson JK . Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn’s disease. Inflamm Bowel Dis. (2009) ;15: :653–60. |
[20] | Sokol H , Seksik P , Furet JP , Firmesse O , Nion-Larmurier I , Beaugerie L , Cosnes J , Corthier G , Marteau P , Doré J . Low counts of Faecalibacteriumprausnitzii in colitis microbiota. Inflamm. Bowel Dis. (2009) ;15: :1183–9. |
[21] | Miquel S , Martin R , Rossi O , Bermudez-Humaran LG , Chatel JM , Sokol H , Thomas M , Wells JM , Langella P . Faecalibacteriumprausnitzii and human intestinalhealth. Curr Opin Microbiol. (2013) ;16: :255–61. |
[22] | Galecka M , Szachta P , Bartnicka A , Lykowska-Szuber L , Eder P , Schwiertz A . Faecalibacteriumprausnitzii and Crohn’s disease—is there any connection? Pol J Microbiol. (2013) ;62: :91–5. |
[23] | Honneffer JB , Minamoto Y , Suchodolski JS . Microbiota alterations in acute and chronic gastrointestinal inflammation of cats and dogs. World J Gastroenterol. (2014) ;20: :16489–97. |
[24] | Morgan XC , Tickle TL , Sokol H , Gevers D , Devaney KL , Ward DV , Reyes JA , Shah SA , LeLeiko N , Snapper SB , Bousvaros A , Korzenik J , Sands BE , Xavier RJ , Huttenhower C . Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. (2012) ;13: :R79. |
[25] | Lupp C , Robertson ML , Wickham ME , Sekirov I , Champion OL , Gaynor EC , Finlay BB . Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. (2007) ;2: :119–29. |
[26] | Martinez-Medina M , Aldeguer X , Lopez-Siles M , González-Huix F , López-Oliu C , Dahbi G , Blanco JE , Blanco J , Garcia-Gil LJ , Darfeuille-Michaud A . Molecular diversity of Escherichia coli in the human gut: New ecological evidence supporting the role of adherent-invasive E. coli (AIEC) in Crohn’s disease. Inflamm Bowel Dis. (2009) ;15: :872–82. |
[27] | Masip L , Veeravalli K , Georgiou G . The many faces of glutathione in bacteria. Antioxid Redox Signal. (2006) ;8: :753–62. |
[28] | Frank DN , Robertson CE , Hamm CM , Kpadeh Z , Zhang T , Chen H , Zhu W , Sartor RB , Boedeker EC , Harpaz N , Pace NR , Li E . Disease phenotype and genotype are associated with shifts in intestinal-associated microbiota in inflammatory bowel diseases. Inflamm Bowel Dis. (2011) ;17: :179–84. |
[29] | Darfeuille-Michaud A , Boudeau J , Bulois P , Neut C , Glasser AL , Barnich N , Bringer MA , Swidsinski A , Beaugerie L , Colombel JF . High prevalence of adherent-invasive Escherichia coli-associated with ileal mucosa in Crohn’s disease. Gastroenterology. (2004) ;127: :412–21. |
[30] | Baumgart M , Dogan B , Rishniw M , Weitzman G , Bosworth B , Yantiss R , Orsi RH , Wiedmann M , McDonough P , Kim SG , Berg D , Schukken Y , Scherl E , Simpson KW . Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn’s disease involving the ileum. ISME J. (2007) ;1: :403–18. |
[31] | Barnich N , Carvalho FA , Glasser AL , Darcha C , Jantscheff P , Allez M , Peeters H , Bommelaer G , Desreumaux P , Colombel JF , Darfeuille-Michaud A . CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J Clin Invest. (2007) ;117: :1566–74. |
[32] | Sartor RB . Does Mycobacterium avium subspecies paratuberculosis cause Crohn’s disease? Gut. (2005) ;54: :896–8. |
[33] | Selby W , Pavli P , Crotty B , Florin T , Radford-Smith G , Gibson P, Mitchell B, Connell W, Read R, Merrett M, Ee H, Hetzel D, Antibiotics in Crohn’s Disease Study Group. Two-year combination antibiotic therapy with clarithromycin, rifabutin, and clofazimine for Crohn’s disease. Gastroenterology. (2007) ;132: :2313–9. |
[34] | Ivanov II , Atarashi K , Manel N , Brodie EL , Shima T , Karaoz U , Wei D , Goldfarb KC , Santee CA , Lynch SV , Tanoue T , Imaoka A , Itoh K , Takeda K , Umesaki Y , Honda K , Littman DR . Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. (2009) ;139: :485–98. |
[35] | Mazmanian SK , Round JL , Kasper DL . A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. (2008) ;453: :620–5. |
[36] | Ocvirk S , Sava IG , Lengfelder I , Lagkouvardos I , Steck N , Roh JH , Tchaptchet S , Bao Y , Hansen JJ , Huebner J , Carroll IM , Murray BE , Sartor RB , Haller D . Surface-associated lipoproteins link Enterococcus faecalis virulence to colitogenic activity in IL-10-deficient mice independent of their expression levels. PLoS Pathog. (2015) ;11: :e1004911. |
[37] | Buffie CG , Pamer EG . Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol. (2013) ;13: :790–801. |
[38] | Clarke TB , Davis KM , Lysenko ES , Zhou AY , Yu Y , Weiser JN . Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. (2010) ;16: :228–31. |
[39] | Benson A , Pifer R , Behrendt CL , Hooper LV , Yarovinsky F . Gut commensal bacteria direct a protective immune response against Toxoplasma gondii. Cell Host Microbe. (2009) ;6: :187–96. |
[40] | Hörmannsperger G , Clavel T , Hoffmann M , Reiff C , Kelly D , Loh G , Blaut M , Hölzlwimmer G , Haller D . Posttranslational inhibition of proinflammatory chemokine secretion in intestinal epithelial cells: Implications for specific IBD indications. J Clin Gastroenterol. (2010) ;44: :S10–5. |
[41] | Ho J , Kurtz CC , Naganuma M , Ernst PB , Cominelli F , Rivera-Nieves J . A CD8+/CD103 high T cell subset regulates TNF-mediated chronic murine ileitis. J Immunol. (2008) ;180: :2573–80. |
[42] | Couturier-Maillard A , Secher T , Rehman A , Normand S , De Arcangelis A , Haesler R , Huot L , Grandjean T , Bressenot A , Delanoye-Crespin A , Gaillot O, Schreiber S, Lemoine Y, Ryffel B, Hot D, Núñez G, Chen G, Rosenstiel P, Chamaillard M. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J Clin Invest. (2013) ;123: :700–11. |
[43] | Foxx-Orenstein AE , Chey WD . Manipulation of the gut microbiota as a novel treatment strategy for gastrointestinal disorders. Am J Gastroenterol. (2012) ;1: :41–6. |
[44] | Benjamin JL , Hedin CR , Koutsoumpas A , Ng SC , McCarthy NE , Hart AL , Kamm MA , Sanderson JD , Knight SC , Forbes A , Stagg AJ , Whelan K , Lindsay JO . Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn’s disease. Gut. (2011) ;60: :923–9. |
[45] | Marteau P , Lémann M , Seksik P, Laharie D, Colombel JF, Bouhnik Y, Cadiot G, Soulé JC, Bourreille A, Metman E, Lerebours E, Carbonnel F, Dupas JL, Veyrac M, Coffin B, Moreau J, Abitbol V, Blum-Sperisen S, Mary JY. Ineffectiveness of Lactobacillus johnsonii LA1 for prophylaxis of postoperative recurrence in Crohn’s disease: A randomised, double blind, placebo controlled GETAID trial. Gut. (2006) ;55: :842–7. |
[46] | Prantera C , Scribano ML , Falasco G , Andreoli A , Luzi C . Ineffectiveness of probiotics in preventing recurrence after curative resection for Crohn’s disease: A randomised controlled trial with Lactobacillus GG Gut. (2002) ;51: :405–9. |
[47] | Steed H , Macfarlane GT , Blackett KL , Bahrami B , Reynolds N , Walsh SV , Cummings JH , Macfarlane S . Clinical trial: The microbiological and immunological effects of synbiotic consumption - a randomized double-blind placebo-controlled study in active Crohn’s disease. Aliment Pharmacol Ther. (2010) ;32: (7):872–83. |
[48] | Chermesh I , Tamir A , Reshef R , Chowers Y , Suissa A , Katz D , Gelber M , Halpern Z , Bengmark S , Eliakim R . Failure of Synbiotic to prevent postoperative recurrence of Crohn’s disease. Dig Dis Sci. (2007) ;52: (2):385–9. Epub Jan 9. |
[49] | Eiseman B , Silen W , Bascom GS , Kauvar AJ . Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. (1958) ;44: :854–9. |
[50] | Khanna S , Pardi DS . Clostridium difficile infection: New insights into management. Mayo Clin Proc. (2012) ;87: :1106–17. |
[51] | Kassam Z , Lee CH , Yuan Y , Hunt RH . Fecalmicrobiota transplantation for Clostridium difficile infection: Systematic review and meta-analysis. Am J Gastroenterol. (2013) ;108: :500–8. |
[52] | Brandt LJ , Aroniadis OC , Mellow M , Kanatzar A , Kelly C , Park T , Stollman N , Rohlke F , Surawicz C . Long-term follow-up of colonoscopic faecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. (2012) ;107: :1079–87. |
[53] | Ianiro G , Bibbo S , Scaldaferri F , Gasbarrini A , Cammarota G . Fecal microbiota transplantation in inflammatory bowel disease: Beyond the excitement. Medicine. (2014) ;93: :e97. |
[54] | Kelly CR , Kahn S , Kashyap P , Laine L , Rubin D , Atreja A , Moore T , Wu G . Update on fecal microbiota transplantation Indications, methodologies, mechanisms, and outlook. Gastroenterology. (2015) ;149: (1):223–37. |
[55] | Smits LP , Bouter KE , de Vos WM , Borody TJ, Nieuwdorp M. Therapeutic potential of fecalmicrobiota transplantation. Gastroenterology. (2013) ;145: :946–53. |
[56] | Colman RJ , Rubin DT . Fecal microbiota transplantation as therapy for inflammatory bowel disease: A systematic review and meta-analysis. J Crohns Colitis. (2014) ;8: (12):1569–81. |
[57] | Suskind DL , Brittnacher MJ , Wahbeh G , Shaffer ML , Hayden HS , Qin X , Singh N , Damman CJ , Hager KR , Nielson H , Miller SI . Fecal microbial transplant effect on clinical outcomes and fecal microbiome in active Crohn’s disease. Inflamm Bowel Dis. (2015) ;21: (3):556–63. |




