Antioxidant activity and Hypolipidemic effect of Ficus carica leaf and twig extracts in Triton WR-1339-induced hyperlipidemic mice
Abstract
BACKGROUND/AIM:
The hypolipidemic potential of both leaf and twig extracts of Ficus carica on experimental hyperlipidaemia induced by Triton WR-1339, in Swiss albino mice was investigated. In addition, the phenolic, flavonoid and anthocyanin contents of these extracts and their antioxidant activities were determined. These properties may have a synergistic effect on hyperlipidaemia.
MATERIALS AND METHODS:
Leaf and twig samples of F. carica were harvested and collected. The study of antioxidant activity of phenolic compounds was determined by the radical DPPH*, ABTS*+ and FRAP assays. The experimental design was carried out using acute oral toxicity study and Triton model hyperlipidaemia on Swiss albino adult male mice. The animals were observed continuously during the 14 days of the study for any physical signs of toxicity. On the 15th day, the animals were sacrificed by decapitation under anesthesia and the organs were observed for macroscopic pathological lesions. Hyperlipidaemia was induced in the mice by a single intravenous (iv) injection of Triton WR 1339 (300 mg/kg body weight), and the antihyperlipidemic effect of each extract, studied at 150 and 300 mg, was tested by gavage. After 24 hours of administration, serum from blood samples was used to estimate the various parameters of the lipid profile namely TC, TG, LDL and HDL.
RESULTS:
The results of the phenolic and flavonoid compounds of Ficus carica leaves and twigs varied from 12.84 to 19.78 mg gallic acid equivalents (GAE) and 5.02 to 9.72 mg EQ/g dry matter, respectively. The scavenging activity (IC50) against the radical DPPH* and ABTS*+ varied from 346.2 to 461.38 μg/mL and 288.3 to 369.01 μg/mL for twigs and leaves respectively, and from 50.82 to 54.2 μg/mL for FRAP assay. The acute toxicity study showed no mortality and clinical signs of toxicity in the tested doses. The LD50 value of extracts of twigs and leaves of Ficus carica is greater than 5000 mg/kg. The results revealed that the administration of Ficus carica (FC) leaf and twig extracts resulted in a significant (p < 0.05) decline in levels of serum triglycerides, total cholesterol, LDL-c, and VLDL-c, while the serum high-density lipoprotein cholesterol was significantly increased. The decrease rate of the lipid parameters differs significantly (p < 0.05) from the leaf and twig extracts and depends also on the administered dose.
CONCLUSION:
Ficus carica leaf and twig extracts may contain compounds able to lower plasma lipid concentrations, could contribute significantly to the total antioxidant properties and be beneficial in the treatment of hyperlipidaemia.
1Introduction
The evolution of the world and different modern man lifestyles of have sparked the development of new traditions, new behaviours and new diets containing higher calories and fats, which contribute to the emergence and increase of some diseases such as cancers, cardiovascular and metabolic diseases, diabetes, dyslipidemia and obesity [1].
Obesity and overweight are considered hazardous for public health [2]. They are characterized by the uncontrolled increase of adipocytes due to the excessive accumulation of triglycerides in adipose tissue [3]. These lipid dysfunction situations are considered a major factor of most significant risk of hyperlipidaemia occurrence, which results from an increase in cholesterol of both low and high density lipoproteins (LDL and HDL) [4].
The drug treatments of hyperlipidaemia derived from chemical synthesis are often used like fibrates, statins and bile acid sequestrants however, they possess serious side effects [5].
Therefore, there is an urgent need to have drugs with lipid lowering activities with less side effects such as the natural products are one of the best claimed options.
Many plants were selected as domestic remedies for their anti-obesity and antihyperlipidemic activities, including the genus Ficus. It is one of the greatest genus of medicinal plants with about 750 species of woody plants, trees and shrubs [6]. Several therapeutic effects have been attributed to Ficus carica, such as hypoglycemic [7, 8], antioxidant [9], antibacterial and antiviral effects [10–12].
Based upon the above assumptions, our hypothesis was to justify the use of the fig tree (leaf and twig) as a remedy in the treatment of hyperlipidaemia. The aim of the present study was, therefore, to investigate the antihyperlipidemic effect of F. carica leaf and twig extracts in Triton WR-1339 induced Swiss albino mice.
2Materials and methods
2.1Plant material
Leaf and twig samples of F. carica (Taamraouith variety) were harvested in September 2014 (Beni Mlikeche, Bejaia, Algeria). The collected plants were identified by the national center of fruit trees (ITAF of Takriet, Bejaia) and voucher specimens have been deposited at the Herbarium of Algiers University (Algeria). Samples were washed with distilled water and dried in oven at 40 °C. After drying, a first grinding was performed using a copper household mortar, followed by an electrical grinder (IKA WORKS TYPE A11.basic). The obtained powder was passed through a standard 125 μm sieve and only the fraction with particle size <125 μm was used. The powder was stored in airtight bags until use.
2.2Preparation of sample extracts for biochemical assays
Phenolic compounds were extracted using a stirred conventional solvent extraction method. Briefly, fifteen grams of powder were mixed with 300 mL of 80% (v/v) aqueous methanol in a flask and the mixture was kept in a thermostatic water bath at 40 °C, with shaking at a speed of 500 rpm for 12 hours. After this period, the residue was recovered and re-extracted with 150 mL of the same solvent for another 12 hours cycle to complete a total of 24 hours of extraction process. The extracts were combined and concentrated in a rotary evaporator (Buchi R-210 Rotavapor, Switzerland) until the complete elimination of methanol.
Afterwards, the aqueous extract was lyophilized (24 hours) and kept in hermetic amber flasks at –22 °C. Before each trial, the lyophilized extracts were dissolved in distilled water.
2.3Determination of total phenolic content
The total phenolic content (TPC) of the leaf and twig extracts, was determined using the Folin–Ciocalteau assay as reported by Blainski A, Lopes GC and De Mello JCP [13], with slight modifications. Briefly, 500 μL of extract were mixed with 1500 μL of a 10-fold diluted Folin–Ciocalteau reagent. Then, 1500 μL of a sodium carbonate solution (7.5%, w/v) were added. The mixture was incubated in the dark for 90 min with intermittent shaking. Finally, the absorbances of the reaction mixtures were measured at 725 nm (1 cm optical path) against a blank using a UV-Vis spectrophotometer (Shimadzu™ UVmini-1240 Model Spectrophotometer). The phenolic content was calculated as gallic acid equivalents GAE/g on the basis of standard curve of gallic acid. The TPC was expressed as mg of gallic acid equivalents (GAE) per gram of the plant material. The experiment was carried out five times.
2.4Determination of total flavonoid content
The content of total flavonoids (TF) was estimated by the AlCl3 method [14]. Briefly, 1.5 mL of extract were added to 1.5 mL of 2% (w/v) methanolic AlCl3 incubated for 1 hour at room temperature. The absorbance was then read at 420 nm (1 cm optical path). The TF was expressed as mg of Quercetin Equivalent (QE) per gram of plant material. The experiment was carried out five times.
2.5Determination of total anthocyanins
Total anthocyanins (TA) content was quantified according to the pH differential method [15]. Absorbance was measured simultaneously at 510 and 700 nm in potassium chloride buffer (pH = 1.0) and sodium acetate buffer (pH = 4.5). The concentration of anthocyanins was obtained using the following equation (Equation 1).
(1)
where:
A is the absorbance A = (A510–A700) pH 1.0-(A510–A700) pH 4.5, DF is the dilution factor, l is the cuvette optical pathlength (1 cm) and m is the weight of the sample (g), ɛ is the molar extinction coefficient of Cyanidine-3-glucoside (26900 L/mol/cm) and M is the molecular weight (449 g/mol).
The total anthocyanin content was expressed as mg Cyanidine-3-glucoside equivalent per 100 g plant materiel.
2.6Determination of reducing power
The reducing power of extracts was determined according to the method of Yen GC and Duh PD [16]. A volume (1 mL) of aqueous extracts was mixed with 2.5 mL of phosphate buffer (0.2 M; pH 6.6) and 2.5 mL of potassium ferricyanide (1%, w/v). The mixture was incubated at 50 °C for 20 min. After incubation, 2.5 mL of trichloroacetic acid (TCA) (10%, w/v) was added to the mixture, and centrifuged at 3000 rpm for 10 min. The upper layer of solution (2.5 mL) was mixed with 2.5 mL of distilled water and 0.5 mL of FeCl3 (1%, w/v). The reducing power was expressed as the absorbance of this mixture measured at 700 nm. The increased absorbance of the reaction mixture indicated increased reducing power
2.7Determination of DPPH radical scavenging activity
The electron donation ability of the obtained aqueous extracts was measured by bleaching of the purple-colored solution of 1, 1-diphenyl-2-picrylhydrazyl radical (DPPH) and was performed according to the method of [17]. A DPPH· solution in methanol (6 10–5 M) was prepared, and 3.9 mL of this solution were mixed with 0.1 mL of extracts and the mixture was incubated in the dark at room temperature for 30 min, and then the decrease in absorbance at 515 nm was recorded.
The antioxidant capacity was expressed as a percentage of inhibition of DPPH· radical (% inhibition of DPPH radical) calculated according to the following equation (Equation 2).
(2)
2.8Determination of ABTS*+ radical scavenging activity
The antioxidant activity of the aqueous extracts was assessed by ABTS*+ assay [18], which is based on the ability of antioxidants to interact with the ABTS*+ radical, decreasing its absorbance at 734 nm. Briefly, a radical solution (7 mM ABTS and 2.45 mM potassium persulfate) was prepared in absolute ethanol and left in the dark at room-temperature for 16 hours before using it in the assay. This solution was then diluted with ethanol to get an absorbance of 0.70±0.02 at 734 nm and equilibrated at 30 °C.
For the antioxidant activity analysis, 990 μl of the diluted radical solution were mixed with 10 μl of the extract at different concentrations and the absorbance was read at 734 nm against ethanol. Antioxidant activity (AOX) was calculated as the percent inhibition of absorbance at 734 nm (Equation 3):
(3)
2.9Experimental design
2.9.1Acute oral toxicity study
The toxicity study was carried out using adult male Swiss albino mice (18 – 23 g) previously described by the OECD guideline (Organization for Economic Cooperation and Development, Guideline 425, adopted on third October, 2008). The overnight fasted mice were divided into nine groups, each group consisted of five animals. The Ficus carica leaf and twig extracts were given separately in various doses (100, 500, 2500, 5000 mg/kg) administered orally with a feeding needle connected to a syringe at stated doses in appropriate volume of distilled water.
In the experiment, the mice were divided into nine groups of five mice each, which were as follows: The first group (1), serving as the control group receives by gavage a saline water (0.9% [w/v] NaCl). The groups (2), (3), (4) and (5) each receives by gavage a single dose of F. carica leaf extract, namely 100, 500, 2500 or 5000 mg/kg of body weight. The groups (6), (7), (8) and (9) receive by gavage a single dose of F. carica twig extract, namely 100, 500, 2500 or 5000 mg/kg of body weight.
The animals were observed for 2 hours continuously and then each hour for 4 hours and finally after every 24 hours up to 14 days for any physical signs of toxicity, such as changes in behavioral responses and also for tremors, convulsion, salivation, diarrhea, lethargy, writhing, gasping, palpitation, sleep, coma, and decreased respiratory rate or mortality.
The animals were fasted overnight and sacrificed by decapitation under anesthesia on the 15th day of the study, and all the organs were observed for gross pathological lesions. The criteria of gross pathological examination were based on the position, shape, size, color, and consistency of the organ.
2.9.2Triton model of hyperlipidaemia
Adult male Swiss albinos mice (average weight 26 g), obtained from Pasteur’s Institute of Algeria (Algiers) were randomly distributed into different experimental groups, housed in transparent plastic cages and were acclimatized for seven days under standard laboratory conditions at 23±2° C and 50±10% room humidity, with a 12 : 12 hours light/dark cycle (from 07 : 00 h to 19 : 00 h). Animals were provided with commercial food pellets and water ad libitum. All experiments were in compliance with the guidelines for the care and use of laboratory animals published by the US National Institute of Health (NIH publication No 85-23, revised 1985) with approval of pharmaceutical group company SAIDAL ethic committee (Algiers, Algeria).
In the experiment, the mice were divided into six groups of five mice each, which were as follows:
The first group (I), serving as the first control group (CG) was administered saline water (0.9% [w/v] NaCl) by gavage; the second (II) hyperlipidaemic control (HG) group received Triton WR-1339 (300 mg/kg body weight; i.p.) and was treated with saline solution by gavage.
The groups (III) and (IV) received Triton WR-1339 (300 mg/kg body weight; i.p.) and gavage with leaf extracts of F. carica (150 and 300 mg/kg body weight, respectively). The groups (V) and (VI) received Triton WR-1339 (300 mg/kg body weight; i.p.) and gavage with twig extracts of F. carica (150 and 300 mg/kg body weight, respectively).
After 24 h of treatments, animals were anaesthetized with diethyl ether and their blood and livers were collected. The blood samples were immediately centrifuged (3,000 rpm for 10 min) and the plasma was used for lipid analysis using different biochemical kits (Biolabs/Span Kits). The collected livers were weighed and preserved for the histopathological study.
2.9.3Lipid profile analysis
After separation of serum from blood, the various parameters of lipid profile were estimated, including Total Cholesterol (TC), Triglycerides (TG), Low Density Lipoprotein Cholesterol (LDL) and High Density Lipoprotein Cholesterol (HDL). Serum LDL estimation was calculated using Friedwald formula.
2.9.4Histopathological analysis
The liver sections were evaluated for histopathology to assess any architectural changes. The liver was removed, cleaned with saline solution and preserved in 10% formalin for 24 hours. After rinsing with tap water, the sample was divided into equal pieces using transverse portions (thickness, 5 mm). The samples were then dehydrated in increasing ethanol concentrations (70 to 95%), cleared in xylene and embedded in paraffin wax. Histological sections of 4 μm thickness were then performed using a Leica RM2025 rotary microtome. Sections were mounted on glass microscope slides, stained with Haematoxylin and Eosin. They were then examined by light microscopy for conventional morphological evaluation.
2.10Statistical analysis
All data were the mean values from three replicate in each sampling point, and all of the analyses were performed using GraphPad Prism 6 for Windows (version 6.01, 2012). In the in vitro assays, the data expressed as relative values were presented as the mean±SD. A p-value <0.05 was considered significant. The IC50 values were calculated with GraphPad Prism 6.0 under application of the function “log (inhibitor)” after converting the concentrations into their decimal logarithm. For in vivo experiments, each point was repeated at least five times, Data are presented as mean±Standard errors of the means, ANOVA one way was used followed with post Hoc test for multiple comparisons (p < 0.05).
3Results and discussion
3.1Total phenolic, total flavonoid and anthocyanin contents
Table 1 shows the total phenolic, total flavonoid and anthocyanin contents of leaf and twig Ficus carica extracts.
Table 1
Total phenolic content (TPC), total flavonoids (TF) and total anthocyanins (TA) of Ficus carica L leaves and twigs extracts
| TPC (EAG mg/g DM) | TF (EQ mg/g DM) | TA (mg Cy-3-glucoside/100 g DM) | |
| Extracts | Mean±SD | Mean±SD | Mean±SD |
| Leaves | 19.78±1.38a | 9.72±0.09a | 3.22±0.03b |
| Twigs | 12.84±0.29b | 5.02±0.8b | 5.12±0.71a |
Two different letters indicate a significant difference.
The phenolic compound content of both twigs and leaves of Ficus carica ranged from 12.84 to 19.78 mg GAE/g of DM, respectively. The total phenolic contents were significantly higher in the leaves than those of the twigs (p < 0.05).
Our results were higher than those reported by Ghazi et al. [19] on the total phenolic contents of F. carica leaf extracts harvested in Saudi Arabia (9.07±0.33 to 6.29±0.08 mg GAE/g of DM). Konyalıoğlu S, Sağlam H and Kıvçak B [20] and Elansary HO and Elansary DO [21] have studied the phenolic composition of F. carica leaves and found the following rates, 6.909 EAG/g of DM and 69 mg EAG/g, respectively. Although, Allahyari S, Delazar A and Najafi M [22] have reported a very low rate with an average of 0.1229 mg EAG/g of DM. For the phenolic content of the twigs of the same species, the results found by Saoudi M and El Feki A [23] were much higher than those obtained in our study (133±3.50 mg/g EAG/g of DM).
The rate of flavonoids of both Ficus carica twigs and leaves was 5.02 to 9.72 mg of QE/g of DM, respectively. The analysis revealed significant differences and the leaves of Ficus carica contained more flavonoids compounds than the twigs (p < 0.05).
According to Allahyari S, Delazar A and Najafi M [22], and El-Shobaki F, El-Bahay A, Esmail R, El-Megeid AA and Esmail N [24], the flavonoid contents of F. carica leaves were 40.727 mg/g QE of DM and 2.75 mg/g QE of DM, respectively. For the flavonoid contents of Tunisian Ficus stems, the reported values were in the order of 43.25±2.0 mg/g QE of DM [23].
The difference among the values of phenolic compounds and flavonoids of the present study and those reported in literature could be due to several factors including the solvent and the extraction method [25], temperature, solvent-to-solid ratio, time of contact [26]. Indeed, the phenolic and flavonoid contents of a plant material depend on a number of intrinsic (genetic) and extrinsic agro-ecological factors in the plant development area [27]. Exposure to light and preservation technique of the plants may also affect the flavonoid content [28], furthermore, the temperature influences the extraction of a given compound [29].
In this study, the anthocyanin content of both Ficus carica twigs and leaves ranged from 3.22 to 5.12 mg Cy-3-glucoside/100 g of DM, respectively. The assay revealed that the anthocyanin content was significantly (p < 0.05) higher in the twigs than in the leaves.
Anthocyanins are natural water-soluble pigments that contribute significantly to the antioxidant activity and health-promoting effects and the prevention of cancer, coronary heart disease and other degenerative disorders [30–32]. Anthocyanins have been shown to exert benefits on the lipid profile in many animal models [33].
3.2Total antioxidant activity
3.2.1DPPH * radical-scavenging activity
Many studies illustrate the importance given to natural antioxidants in the fields of food processing and medical industry and also to their protective roles against the oxygen reactive species and the correlation between bioactive compounds of plant materials and their antioxidant capacity [34, 35]. The results of this study showed that the inhibition concentration (IC50) of DPPH* scavenging activity of F. carica extracts were ranging from 346.2 to 461.3 μg/mL with significant difference (p < 0.05) (Table 2). The leaf extracts showed a better DPPH* scavenging activity compared to that of the twig extracts. Ghazi F, Rahmat A, Yassin Z, Ramli NS and Buslima NA [19] reported that leaves extracts of F. carica harvested in Saudi Arabia presented percentages of DPPH* scavenging activity ranging from 59.42±1.53% to 63.29±2.51%. The results of the present investigation are in accordance with several previous studies that showed significant correlations (p < 0.05) between the inhibitory power of the radical DPPH* and both total polyphenol (R = 0.71) and flavonoid (R = 0.76) contents [14, 36].
Table 2
IC50 (μg/mL) values of both leaves and twigs extracts on FRAP assay and scavenging activity against the ABTS*, + radical and DPPH*, expressed as mean±SD (n = 9)
| IC50 (μg/ml) | |||
| Extract | FRAP | ABTS*, + | DPPH* |
| Leaves | 50.82±6. 86b | 288.3±4.98b | 346.2±22.07b |
| Twigs | 54.2±6. 47a | 369.01±11.51a | 461.38±3.74a |
The DPPH*scavenging activity of plant extracts has been attributed to the presence of phenolic compounds which easily yield protons for reduction [37–39] while the anti-radical power is influenced by several factors: the extraction method, the nature and concentration solvent, the temperature and the time of extraction [40, 41].
3.2.2ABTS*+ assay
The cation radical of 2,2’-azino bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) is stable in its free form. It is formed by oxidation of the colorless ABTS*+ with potassium persulfate [18]. The results (Table 2) indicated that the IC50 (μg/mL) values of both leaf and twig extracts of F. carica on scavenging activity against the ABTS*+ radical varied significantly (p < 0.05) from 288.3 to 369.01 μg/mL. In contrast, the leaf extracts showed a better ABTS*+ scavenging activity compared to that of the twigs. Several studies have shown that the ABTS*+ scavenging activity is strongly influenced by the extraction solvent, which could modify the quantity and quality of the extracted antioxidants [42].
Manian R, Anusuya N, Siddhuraju P and Manian S [43] reported that phenolic compounds with high molecular weights have more ability to capture free ABTS *+ radicals.
3.2.3FRAP (Ferric reducing/antioxidant power) assay
This test is used to determine the ability of the extracts to give electrons [44], or to give a hydrogen atom, which allows them to act on the peroxidation of lipids as primary and secondary antioxidant [45]. It is based on the ability of extracts to reduce ferric ions (Fe3 +) to ferrous ions (Fe2 +). The reduction of ferric ions is accompanied by a color change from yellow to green. The intensity of the color depends on the reduction potential of the compounds present in the reaction medium [46]. The results given in Table 2 show that the IC50 (μg/mL) values on FRAP assay varied significantly (p < 0.05) from 50.8 to 54.2 for leaf and twig extracts, respectively. According to Shi Y-X, Xu Y-K, Hu H-B, Na Z and Wang W-H [47], the reducing power of F. carica leaves harvested in China was about 33% at a concentration of 0.25 mg/mL. In this study, the significant correlation between the reducing power of both F. carica leaves and twigs extracts and the total phenolics (R = 0.82) and flavonoids (R = 0.58) contents was observed.
3.3Acute toxicity studies
The acute toxicity study of the tested doses showed that animals have no visible signs of acute toxicity during the first hours and after 14 days of observation for all extracts. As there were no mortality and clinical signs of toxicity in the tested doses, and the LD50 values of Ficus carica twig and leaf extracts are greater than 5000 mg/kg.
An old traditional habit uses the leaves and young twigs of F. carica for feeding farm animals like: goats, cattles and sheep in the region (Algeria), which confirms the non-toxicity of the leaves and twigs.
3.4Anti hyperlipidaemic effect of F. carica extracts
Hyperlipidaemia is known as a major risk factor in the development of cardiovascular and coronary heart diseases, which are strongly related to metabolic imbalances of lipid and plasma lipoproteins [48–50]. The antihyperlipidemic effect of F. Carica leaf and twig extracts, at different doses (150–300 mg/kg) in experimental models of hyperlipidaemia, was investigated.
Intravenous injection of Triton WR 1339 in experimental animals results in a progressive increase in the concentration of lipids in the blood. Its action is believed to be, at least in part, due to the capacity of the detergent to associate with triglycerides in the plasma in such a way to reduce their rate of hydrolysis by the enzymes, clearing factor lipase or lipoprotein lipase, and so to interfere with their uptake from the circulation by the extra-hepatic tissues [51]. Triton WR-1339, also known as Tyloxapol, was used for the first time to exploit its ability to block lipid clearance by measuring the rates of triglycerides and cholesterol synthesis. It is an investigator tool which studies the metabolism of fats and the metabolic correlation between plasma lipoproteins [52, 53]. Blood lipid parameters were determined after 24 hours of injection of Triton WR-1339 to mice. The results are expressed as mean of five essays±standard errors.
3.4.1Effect of oral administration of F. carica extracts on serum lipids
Recently, a number of clinical studies suggest that the increased risk of coronary heart disease is associated with a high serum concentration of TC, LDL-C and triglycerides. The abnormally high concentration of serum lipids is mainly due to the increase in the mobilization of free fatty acids from the peripheral depots [54]. On the other hand, low serum concentration of HDL-C is also responsible for coronary heart disease [54]. Table 3 showed the effect of administration of F. Carica (FC) leaf and twig extracts at 150–300 mg/kg body weight on serum lipids of hyperlipidaemic mice after 24 hours of Triton WR-1339 injection.
Table 3
Effect of leaves and twigs Ficus carica extracts on plasma lipid parameters in Triton WR-1339-induced hyperlipemia in mice
| Groups | Total cholestérol (mg/dL) | Triglycerides (mg/dL) | HDL-c (mg/dL) | LDL-c (mg/dL) | VLDL-c (mg/dL) |
| Control group (CG) | 106.93±5.01f | 98.6±2.83e | 38.16±1.19e | 49.05±3.06e | 19.71±3.11e |
| Hyperlipidaemic control (HG) | 200.35±6.71a | 207.74±2.18a | 35.19±2.03f | 123.61±0.59a | 41.54±4.18a |
| Treated groups with leaves extract at 150 mg/kg | 144.09±4.77d | 141.16±1.67c | 48.54±0.62c | 67.32±3.2c | 28.23±3.43c |
| Treated groups with leaves extract at 300 mg/kg | 137.22±3.48e | 127.21±4.05d | 55.82±1.40a | 55.97±1.47d | 25.44±3.24d |
| Treated groups with twigs extract at 150 mg/kg | 157.23±3.78b | 150.81±1.61b | 47.02±1.02d | 80.04±1.72b | 30.16±2.81b |
| Treated groups with twigs extract at 300 mg/kg | 149.56±3.31c | 139.05±3.89c | 50.00±1.48b | 67.75±3.85c | 27.80±1.79c |
Data are presented as mean of fives replicates±Standard errors of the means, ANOVA one way was used followed with post Hoc test for multiple comparisons, P < 0.05. HDL-C: High-density lipoprotein cholesterol; LDL-C: low density lipoprotein cholesterol. Different letters indicate a significant difference (P < 0.05).
After 24 hours of triton injection, a significant (p < 0.05) increase in cholesterol, triglycerides, LDL-c and VLDL-c was recorded accompanied by a decrease in HDL-c. According to Mohammadi H, Abdelouahed E, Hassar M, Bouchrif B, Qarbal B, Dahbi F, Hilal L and Ghalim N [55] the increase in cholesterol and plasma triglycerides due to the injection of Triton WR-1339 mainly results from an increased secretion of VLDL-c by the liver accompanied by a significant reduction of catabolism of VLDL- c and LDL-c.
Triton is known to induce hyperlipidaemia in two phases: Phase I is believed to be due to increased hepatic cholesterol biosynthesis through triton’s interference with the tissue uptake of plasma lipids [53], while Phase II involves triton’s interference with cholesterol excretion and metabolism [56, 57].
Administration of Ficus carica (FC) leaf and twig extracts resulted in a significant decline in levels of serum triglycerides, total cholesterol, LDL-c, and VLDL-c, while the serum high-density lipoprotein cholesterol was significantly increased.
The results revealed that the rate of decrease of the lipid parameters differs significantly (p < 0.05) from the leaf and twig extracts which was dose-dependent. The study showed that increasing doses of the extracts caused significant (p < 0.05) reductions in the serum levels of triglycerides, total cholesterol, LDL-c, and VLDL-c in a dose-related fashion, while the reverse was observed on serum HDL-c.
According to Frick et al. [58], the treatment with fibrates, a widely used class of lipid-modifying agents, resulted in a significant decrease in plasma triglycerides (79%) and is usually associated with a decrease in low density lipoprotein (LDL), cholesterol (11%), and an increase in high density lipoprotein (HDL)-cholesterol concentrations (27%).
The results of this study also showed that treatment with leaf and twig extracts of Ficus carica resulted in a reduction of triglyceride concentration (from 32.08 to 37.29% for leaves and from 27.38 to 33.03% for twigs) and cholesterol levels (from 28.08 to 31.5% for the leaves and from 21.34 to 25.16% for the twigs), and an increase in the HDL concentrations (from 37.91 to 58.5% for the leaves and from 33.5 to 42% for the twigs).
The significant decrease in plasma concentration of cholesterol, triglycerides, VLDL-c and LDL-c and the increase of HDL-c levels after administration of increasing doses (150 and 300 mg/kg) of the FC leaf and twig extracts suggest that the anti-cholesterol activity of the extracts may result from a rapid catabolism of lipids in liver receptors for their final elimination as bile acids [59]. The flavonoids are known to low serum lipids by inhibiting the activity of HMG-CoA reductase and up-regulating the hepatic expression of peroxisome alpha and gamma proliferators [60].
Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R and Kinae N [61] proved inverse correlation between quercetin intake and plasma total cholesterol concentration and plasma LDL-cholesterol concentration.
Moreover, epidemiological studies have provided evidence that a high consumption of phenolic compounds is inversely related to the risk of cardiovascular diseases [62, 63].
In vivo studies (on animal and on human) showed that flavonoids have antidiabetic, antihyperlipidemic and antioxidant properties which are associated with their ability to decrease blood glucose level, decrease plasma total cholesterol concentration and enhance antioxidant system [33, 61, 64].
Thus, the presence of hypolipidaemic phytocomponents like flavonoids in Ficus carica could account for the observed bioactivity. These results are in accordance with those found by Khanna A, Rizvi F and Chander R [59], when studying the antihyperlipidaemic activity of Phyllanthus niruri extracts in hyperlipidaemic rats.
Adeneye AA, Adeyemi OO and Agbaje EO [53] and Girija K, Lakshman K, Udaya C, Sachi GS and Divya T [65], have studied the effect of Hunteria umbellate and three species of Amaranthus extracts on Triton WR 1339-hyperlipidaemia induced in rats and have shown that extracts of these plants decreased significantly the total cholesterol, triglycerides, LDL-c and VLDL-c levels and increased HDL-c rates in plasma.
3.4.2Histopathological effect of oral treatment with 300 mg/kg of FC in Triton WR-1339 induced hyperlipidaemic mice
The (Fig. 1a, 1b, 1c and 1d) showed respectively, a sectional representations of the liver with haematoxylin/eosin’ (HE×400) performed after 24 hours of processing, Lot I (normal control), Lot II (hyperlipidaemic control), Lot III and IV (treated with the respective doses of 300 mg/kg of both leaf and twig extracts of F. carica).
Fig.1
(a) Sectional representations (400×magnification) of untreated liver with hematoxylin/eosin performed after 24 hours of processing. Lot I (normal control). (CV): congestive vein. (b) Sectional representations (400×magnification) of pre-treated, triton-induced hyperlipidaemic mice liver with hematoxylin/eosin performed after 24 hours of processing. Lot II (control hyperlipidaemic) showing a massive distribution of lipids within hepatocytes (HP) which allows intrahepatic provision of these lipids in the form of vacuoles (LV). Presence of an infiltrate with neutrophils (arrows). (c) Sectional representations (400×magnification) of pre-treated, triton-induced hyperlipidaemic and a dose of 300 mg/kg from leaf extracts mice liver with hematoxylin/eosin performed after 24 hours of processing. Lot III showing a small lipid vacuoles (SLV) and others optically (LVO) empty which are present in hepatocytes. (d) Sectional representations (400×magnification) of pre-treated, triton-induced hyperlipidaemic and a dose of 300 mg/kg from twig extracts mice liver with hematoxylin/eosin performed after 24 hours of processing. Lot IV showing a small lipid vacuoles (SLV) and others optically (LVO) empty which are present in hepatocytes.
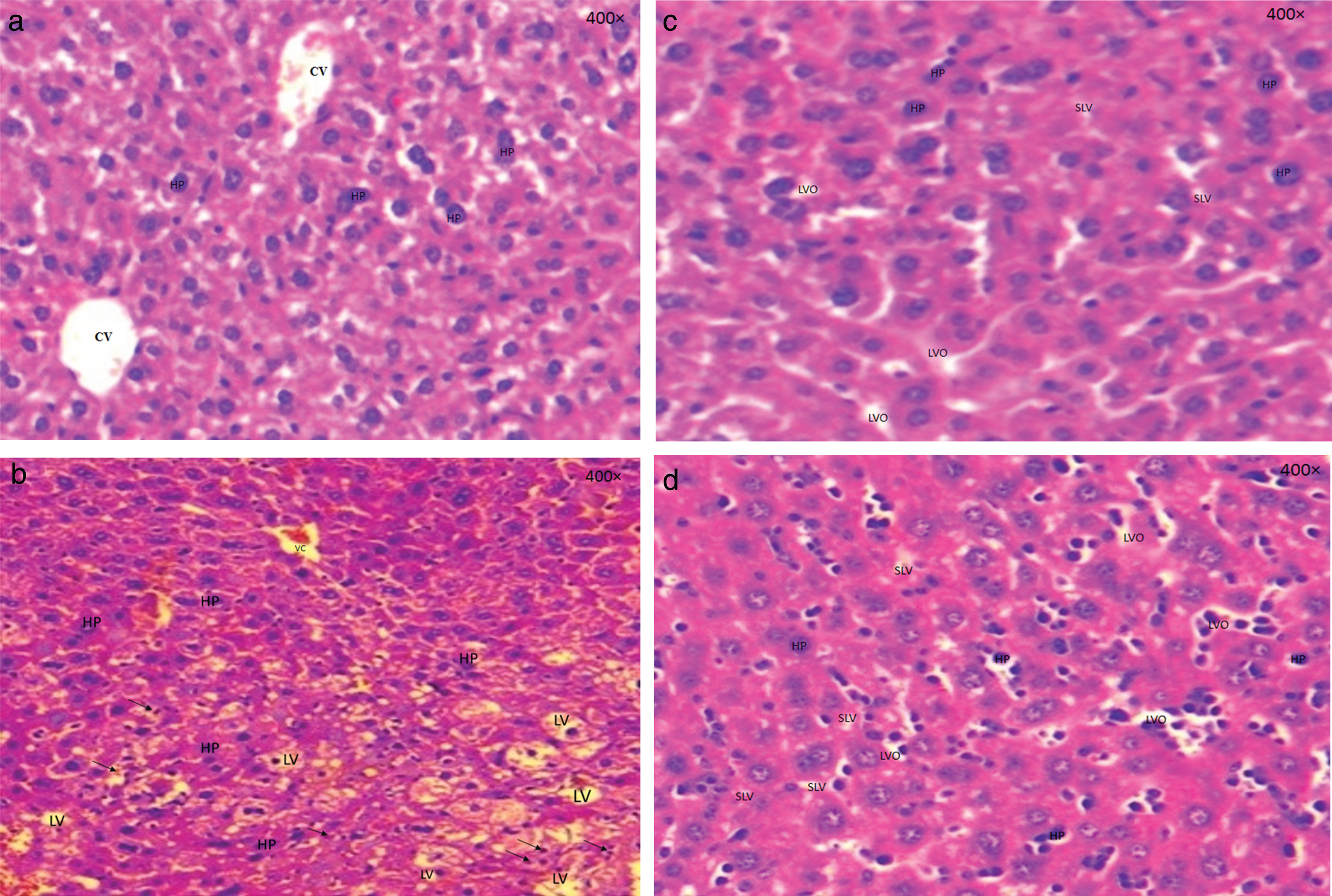
Figure 1a shows a sectional representation of untreated liver of control mice group (Lot I), which reveals a normal appearance without liver modification. The hepatocytes are organized around Centro-lobular vein receiving the blood from the liver parenchyma.
The sectional representation of the mice liver in Lot II (hyperlipidaemia) (Fig. 1b) shows that the Triton WR-1339 induced a massive distribution of lipids within hepatocytes, which allows intrahepatic provision of these lipids in the form of vacuoles. Moreover, we noted the appearance of congestive veins compared to those observed in the normal liver architecture in Lot I control. On Lot II, an inflammatory reaction is mainly observed which was not observed in the other Lots.
Figure 1c and 1d showed a sectional representations of mice liver of Lot III and IV respectively. A manifestation of small lipid vacuoles distributed in the hepatocytes were observed, which means that this dose (300 mg/kg from both leaf and twig extracts) reduced the volume of vacuoles in small persistent lipid vacuoles and has a lower rate compared with Lots II and IV.
These figures showed small lipid vacuoles and others optically empty, which are present in hepatocytes compared to Lot I. These vacuoles are present with a lower rate in comparison with Lot II (hyperlipidaemic control).
The results of this study showed clearly that leaf and twig extracts of F. carica, have an effect on lipid accumulation on the liver and act as antihyperlipidaemic agents.
4Conclusion
The results of this study showed that Ficus carica is a promising anti-oxidant and antihyperlipidaemic agent and has therapeutic potentials in the prevention of hyperlipidaemia induced by Triton WR-1339 on Swiss albinos mice and have mediated effects on triglyceride and cholesterol biosynthesis. These results indicate that the F. carica leaf and twig extracts have a lipid-lowering effect and are effective in lowering the risk of outbreak of fatty liver. Clarification of mechanisms generating these activities needs more investigations. In other numerous studies, the role of natural compound effects in experimental hyperlipidaemia has been reported in vitro and in vivo but there is little information about the use of these compounds in treating obesity in humans and the mechanism of their action. Thus, it will be a wide-open spectrum of biological and clinical studies.
Conflict of interest
We confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgments
Authors are thankful to the members of the Faculty of Natural Sciences and Life, and Faculty of Medicine, University of Bejaia- Algeria.
References
[1] | Ahima RS . Obesity epidemic in need of answers. Gastroenterology. (2006) ;131: :991. |
[2] | Visscher TL , Seidell JC . The public health impact of obesity. Annual Review of Public Health. (2001) ;22: :355–75. |
[3] | Bocquier A , Boullu-Ciocca S , Verger P , Oliver C . Obésité: Oú en sommes-nous? La presse Médicale. (2006) ;35: :270–6. |
[4] | Rifkind BM , Segal P . Lipid Research Clinics Program reference values for hyperlipidemia and hypolipidemia. Jama. (1983) ;250: :1869–72. |
[5] | Chattopadhyaya R , Pathak D , Jindal DP . Antihyperlipidemic agents: A review. Indian Drugs. (1996) ;33: :85–97. |
[6] | Joseph B , Raj SJ . Pharmacognostic and phytochemical properties of Ficus carica Linn–An overview. International Journal of PharmTech Research. (2011) ;3: :8–12. |
[7] | Perez C , Canal J , Torres M . Experimental diabetes treated with ficus carica extract: Effect on oxidative stress parameters. Acta Diabetologica. (2003) ;40: :3–8. |
[8] | Serraclara A , Hawkins F , Perez C , Domínguez E , Campillo JE , Torres MaD . Hypoglycemic action of an oral fig-leaf decoction in type-I diabetic patients. Diabetes Research and Clinical Practice. (1998) ;39: :19–22. |
[9] | Çalişkan O , Polat AA . Phytochemical and antioxidant properties of selected fig (Ficus carica L.) accessions from the eastern Mediterranean region of Turkey. Scientia Horticulturae. (2011) ;128: :473–8. |
[10] | Asadi F , Pourkabir M , Maclaren R , Shahriari A . Alterations to lipid parameters in response to fig tree (Ficus carica) leaf extract in chicken liver slices. Turkish Journal of Veterinary and Animal Sciences. (2006) ;30: :315–8. |
[11] | Perez C , Canal JR , Campillo JE , Romero A , Torres MD . Hypotriglyceridaemic activity of Ficus carica leaves in experimental hypertriglyceridaemic rats. Phytotherapy Research. (1999) ;13: :188–91. |
[12] | CAMPILLO J , Torres M , Dominguez E , Romero A , Perez C . Ficus-carica leaf administration reduces hypertriglyceridemia in streptozocin diabetic rats. In Diabetologia. springer verlag 175 fifth ave, New York, NY 10010; (1994) :A213. |
[13] | Blainski A , Lopes GC , De Mello JCP . Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium Brasiliense L. Molecules. (2013) ;18: :6852–65. |
[14] | Solomon A , Golubowicz S , Yablowicz Z , Grossman S , Bergman M , Gottlieb HE , Altman A , Kerem Z , Flaishman MA . Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.). Journal of Agricultural and Food Chemistry. (2006) ;54: :7717–23. |
[15] | Sutharut J , Sudarat J . Total anthocyanin content and antioxidant activity of germinated colored rice. International Food Research Journal. (2012) ;19. |
[16] | Yen GC , Duh PD . Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. Journal of Agricultural and Food Chemistry. (1994) ;42: :629–32. |
[17] | Brand-Williams W , Cuvelier M-E , Berset C . Use of a free radical method to evaluate antioxidant activity. LWT-Food science and Technology. (1995) ;28: :25–30. |
[18] | Re R , Pellegrini N , Proteggente A , Pannala A , Yang M , Rice-Evans C . Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine. (1999) ;26: :1231–7. |
[19] | Ghazi F , Rahmat A , Yassin Z , Ramli NS , Buslima NA . Determination of total polyphenols and nutritional composition of two different types of Ficus carica leaves cultivated in Saudi Arabia. Pakistan Journal of Nutrition. (2012) ;11: :1061–5. |
[20] | Konyalıoğlu S , Sağlam H , Kıvçak B . α-tocopherol, flavonoid, and phenol contents and antioxidant activity of Ficus carica. leaves. Pharmaceutical Biology. (2005) ;43: :683–6. |
[21] | Elansary HO , Elansary DO . Genetic diversity and biochemical activity of leaves and fruits of main Ficus Sgrown in Egypt. J Hortic Sci Ornament Plants. (2013) ;5: :30–6. |
[22] | Allahyari S , Delazar A , Najafi M . Evaluation of general toxicity, anti-oxidant activity and effects of Ficus carica leaves extract on ischemia/reperfusion injuries in isolated heart of rat. Advanced Pharmaceutical Bulletin. (2014) ;4: :577. |
[23] | Saoudi M , El Feki A . Protective role of Ficus carica stem extract against hepatic oxidative damage induced by methanol in male Wistar rats. Evidence-Based Complementary and Alternative Medicine. (2011) ;2012: . |
[24] | El-Shobaki F , El-Bahay A , Esmail R , El-Megeid AA , Esmail N . Effect of figs fruit (Ficus carica L.) and its leaves on hyperglycemia in alloxan diabetic rats. World Journal of Dairy & Food Sciences. (2010) ;5: :47–57. |
[25] | Naczk M , Shahidi F . Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. Journal of Pharmaceutical and Biomedical Analysis. (2006) ;41: :1523–42. |
[26] | Pinelo M , Rubilar M , Jerez M , Sineiro J , Núñez MJ . Effect of solvent, temperature, and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. Journal of Agricultural and Food Chemistry. (2005) ;53: :2111–7. |
[27] | Lamperi L , Chiuminatto U , Cincinelli A , Galvan P , Giordani E , Lepri L , Del Bubba M . Polyphenol levels and free radical scavenging activities of four apple cultivars from integrated and organic farming in different Italian areas. Journal of Agricultural and Food Chemistry. (2008) ;56: :6536–46. |
[28] | Rawel HM , Meidtner K , Kroll J . Binding of selected phenolic compounds to proteins. Journal of Agricultural and Food Chemistry. (2005) ;53: :4228–35. |
[29] | Montealegre RR , Peces RR , Vozmediano JC , Gascueña JM , Romero EG . Phenolic compounds in skins and seeds of ten grape Vitis vinifera varieties grown in a warm climate. Journal of Food Composition and Analysis. (2006) ;19: :687–93. |
[30] | Ivanovic J , Tadic V , Dimitrijevic S , Stamenic M , Petrovic S , Zizovic I . Antioxidant properties of the anthocyanin-containing ultrasonic extract from blackberry cultivar “Čačanska Bestrna”. Industrial Crops and Products. (2014) ;53: :274–81. |
[31] | Dai J , Patel JD , Mumper RJ . Characterization of blackberry extract and its antiproliferative and anti-inflammatory properties. Journal of Medicinal Food. (2007) ;10: :258–65. |
[32] | Duchnowicz P , Broncel M , Podsędek A , Koter-Michalak M . Hypolipidemic and antioxidant effects of hydroxycinnamic acids, quercetin, and cyanidin 3-glucoside in hypercholesterolemic erythrocytes (in vitro study). European Journal of Nutrition. (2012) ;51: :435–43. |
[33] | Qin Y , Xia M , Ma J , Hao Y , Liu J , Mou H , Cao L , Ling W . Anthocyanin supplementation improves serum LDL-and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. The American Journal of Clinical Nutrition. (2009) ;90: :485–92. |
[34] | Clarkson PM , Thompson HS . Antioxidants: What role do they play in physical activity and health? The American Journal of Clinical Nutrition. (2000) ;72: :637s–46s. |
[35] | Knekt P , Kumpulainen J , Järvinen R , Rissanen H , Heliövaara M , Reunanen A , Hakulinen T , Aromaa A . Flavonoid intake and risk of chronic diseases. The American Journal of Clinical nutrition. (2002) ;76: :560–8. |
[36] | Veberic R , Colaric M , Stampar F . Phenolic acids and flavonoids of fig fruit (Ficus carica L.) in the northern Mediterranean region. Food Chemistry. (2008) ;106: :153–7. |
[37] | Soare JR , Dinis TC , Cunha AP , Almeida L . Antioxidant activities of some extracts of Thymus zygis. Free Radical Research. (1997) ;26: :469–78. |
[38] | Wong PY , Kitts DD . Studies on the dual antioxidant and antibacterial properties of parsley (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts. Food Chemistry. (2006) ;97: :505–15. |
[39] | Benković V , Orsolić N , Knežević AH , Ramić S , Đkić D , Bašić I , Kopjar N . Evaluation of the radioprotective effects of propolis and flavonoids in gamma-irradiated mice: The alkaline comet assay study. Biological and Pharmaceutical Bulletin. (2008) ;31: :167–72. |
[40] | Jalili A , Alipour S , Sadegzadeh A . Antioxidant and antiradical activities of phenolic extracts from Juglanse regia hulls and shells. Int Res J Plant Sci. (2011) ;1: :282–9. |
[41] | Lim D-H , Choi D , Choi O-Y , Cho K-A , Kim R , Choi H-S , Cho H . Effect of Astragalus sinicus L. seed extract on antioxidant activity. Journal of Industrial and Engineering Chemistry. (2011) ;17: :510–6. |
[42] | Floegel A , Kim D-O , Chung S-J , Koo SI , Chun OK . Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. Journal of Food Composition and Analysis. (2011) ;24: :1043–8. |
[43] | Manian R , Anusuya N , Siddhuraju P , Manian S . The antioxidant activity and free radical scavenging potential of two different solvent extracts of Camellia sinensis (L.) O. Kuntz, Ficus bengalensis L. and Ficus racemosa L. Food Chemistry. (2008) ;107: :1000–7. |
[44] | Duh P-D , Tu Y-Y , Yen G-C . Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat). LWT-Food Science and Technology. (1999) ;32: :269–77. |
[45] | Yen G-C , Chen H-Y . Antioxidant activity of various tea extracts in relation to their antimutagenicity. Journal of Agricultural and Food Chemistry. (1995) ;43: :27–32. |
[46] | Zou Y , Lu Y , Wei D . Antioxidant activity of a flavonoid-rich extract of hypericum perforatum L. in Vitro. Journal of Agricultural and Food Chemistry. (2004) ;52: :5032–9. |
[47] | Shi Y-X , Xu Y-K , Hu H-B , Na Z , Wang W-H . Preliminary assessment of antioxidant activity of young edible leaves of seven Ficus species in the ethnic diet in Xishuangbanna, Southwest China. Food Chemistry. (2011) ;128: :889–94. |
[48] | Zarzecki MS , Araujo SM , Bortolotto VC , de Paula MT , Jesse CR , Prigol M . Hypolipidemic action of chrysin on Triton WR-1339-induced hyperlipidemia in female C57BL/6 mice. Toxicology Reports. (2014) ;1: :200–8. |
[49] | Nelson RH . Hyperlipidemia as a risk factor for cardiovascular disease. Primary Care: Clinics in Office Practice. (2013) ;40: :195–211. |
[50] | Bauer JE . Lipoprotein-mediated transport of dietary and synthesized lipids and lipid abnormalities of dogs and cats. Journal of the American Veterinary Medical Association. (2004) ;224: :668–75. |
[51] | Hall JA , Gradin JL , Andreasen CB , Wander RC . Use of a nonionic detergent (Triton WR 1339) in healthy cats to assess hepatic secretion of triglyceride. American Journal of Veterinary Research. (2000) ;61: :941–50. |
[52] | Patil UK , Saraf S , Dixit V . Hypolipidemic activity of seeds of Cassia tora Linn. Journal of Ethnopharmacology. (2004) ;90: :249–52. |
[53] | Adeneye AA , Adeyemi OO , Agbaje EO . Anti-obesity and antihyperlipidaemic effect of Hunteria umbellata seed extract in experimental hyperlipidaemia. Journal of Ethnopharmacology. (2010) ;130: :307–14. |
[54] | Ahmed I , Lakhani MS , Gillett M , John A , Raza H . Hypotriglyceridemic and hypocholesterolemic effects of anti-diabetic Momordica charantia (karela) fruit extract in streptozotocin-induced diabetic rats. Diabetes Research and Clinical Practice. (2001) ;51: :155–61. |
[55] | Mohammadi H , Abdelouahed E , Hassar M , Bouchrif B , Qarbal B , Dahbi F , Hilal L , Ghalim N . Glycaemic control, HbA1c, and lipid profile in children with type 1 diabetes mellitus. Eur J Sci Res. (2009) ;29: :289–94. |
[56] | Banerjee A , Vaghasiya R , Shrivastava N , Padh H , Nivsarkar M . Anti-hyperlipidemic effect of carcia papaya L in sprague dawley rats. Nigerian Journal of Natural Products and Medicine. (2006) ;10: :69–72. |
[57] | Vogel H , Vogel W . Anti-atherosclerotic activity: Triton-induced hyperlipidaemia. Drug discovery and evaluation; Pharmacological Assays. (1997) :606. |
[58] | Frick MH , Elo O , Haapa K , Heinonen OP , Heinsalmi P , Helo P , Huttunen JK , Kaitaniemi P , Koskinen P , Manninen V . Helsinki Heart Study: Primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. New England Journal of Medicine. (1987) ;317: :1237–45. |
[59] | Khanna A , Rizvi F , Chander R . Lipid lowering activity of Phyllanthus niruri in hyperlipemic rats. Journal of Ethnopharmacology. (2002) ;82: :19–22. |
[60] | Sharma B , Balomajumder C , Roy P . Hypoglycemic and hypolipidemic effects of flavonoid rich extract from Eugenia jambolana seeds on streptozotocin induced diabetic rats. Food and Chemical Toxicology. (2008) ;46: :2376–83. |
[61] | Arai Y , Watanabe S , Kimira M , Shimoi K , Mochizuki R , Kinae N . Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. The Journal of Nutrition. (2000) ;130: :2243–50. |
[62] | Fuhrman B , Lavy A , Aviram M . Consumption of red wine with meals reduces the susceptibility of human plasma and low-density lipoprotein to lipid peroxidation. The American Journal of Clinical Nutrition. (1995) ;61: :549–554. |
[63] | Tijburg L , Mattern T , Folts J , Weisgerber U , Katan M . Tea flavonoids and cardiovascular diseases: A review. Critical Reviews in Food Science & Nutrition. (1997) ;37: :771–85. |
[64] | Jung U , Kim H , Lee J , Lee M , Kim H , Park E , Kim H , Jeong T , Choi M . Naringin supplementation lowers plasma lipids and enhances erythrocyte antioxidant enzyme activities in hypercholesterolemic subjects. Clinical Nutrition. (2003) ;22: :561–8. |
[65] | Girija K , Lakshman K , Udaya C , Sachi GS , Divya T . Anti–diabetic and anti–cholesterolemic activity of methanol extracts of three species of Amaranthus. Asian Pacific Journal of Tropical Biomedicine. (2011) ;1: :133–8. |




