Morphological and molecular effects of phenolic extract from coconut kernel on human prostate cancer cell growth in vitro
Abstract
Coconut is an indispensable ingredient in the diet and traditional medicine of individuals belonging to the Indian subcontinent. Coconut is of high nutritional value owing to the presence of all essential dietary components, viz, saturated fatty acids, arginine rich proteins, fibre and minor components like vitamin E, phytosterols, polyphenols and flavonoids. The polyphenolic content present in coconut kernel is of particular interest due to their numerous reported beneficial effects such as reduction of oxidative stress, combating cancer and in modulating anti-inflammatory pathways. Therefore, in the present study the cytotoxic effect of the polyphenol rich fraction from coconut kernel (CKf) was evaluated in human prostate cancer (DU-145) cells. Individual components present in CKf was determined by LC-MS analysis. It showed that CKf contained several bioactive molecules which have potential anticancer activity viz, coumaric acid, myristin, chlorogenic acid and triterpenoid methyl esters. The cytotoxic effect of CKf at various concentrations (2.5–20 μg/ml) on DU-145 was assessed using MTT assay, AO/EB staining, mitochondrial superoxide/ROS production and changes in intracellular calcium levels, 24 hrs post treatment. Changes in the cell morphology and nucleus were observed using Scanning Electron Microscopy and Confocal microscopy. ROS and mitochondrial superoxide levels was evaluated using DCHF-DA and MitoSOX staining respectively. The impact of ROS on changes in cellular calcium levels was also studied using Fura-2-AM. LDH leakage from C K f treated and control cells were observed colorimetrically. Further, PCR analysis was done to detect changes in mitochondria associated apoptotic gene expression. It was also observed that C K f treatment increased the expression of pro-apoptotic genes - Bax, Bid, Bak and p53 in a dose-dependent manner. Based on the above results, it can be concluded that C K f may be used as a part of a dietary regime for controlling the progression of prostate cancer.
1Introduction
Prostate cancer is the most common non-cutaneous cancer in men. Several scientific studies have examined the relationship between prostate cancer and dietary antioxidants; however, the results of these studies are inconsistent [1–7]. Many dietary components may play a role in the development and progression of prostate cancer [8]. Prostate cancer incidence and mortality differ between countries and between men of different race or ethnicity [9–11], which may partly due to the differences, in dietary patterns [12]. Many plants and oil seeds contain significant amount of unexplored antioxidants and biologically active compounds which show promising anticancer activity [13–20]. Plant foods, especially fruits and vegetables provide a multitude of antioxidants and phytochemicals which have a demonstrable beneficial effect on prostate cancer. Plants have been used in traditional medicines for the treatment of several diseases including cancer and diabetes. In recent years, the interest in oriental medicinal herbs based pharmaceuticals/neutraceuticals has aroused the interest of scientific community as complementary/and or alternative medicines [21]. Several chemotherapeutic drugs derived from plants are used directly as chemotherapeutic agents, such as vinblastine, taxol, camptothecin and podophyllotoxin or for the parent compound for more potent medicines for cancer management [22].
Coconut, a nature’s boon has been influencing the human health for many years in the past and will continue in the future too [23]. Though the saturated fats present in the coconut oil is been blamed for increasing the cholesterol levels in our body, there are few unnoticed components present in it which may prove beneficial to the mankind. Apart from fat, coconut contains water rich in amino acids, especially arginine and glutamic acid, minerals, growth factors and anti diabetic proteins [24]. Other minor components like phytosterols, polyphenols, flavonoids, tocopherols and organic components are also present [25, 26]. This study was designed to evaluate the polyphenol/flavonoids components present in the methanolic fraction and their effect on preventing the growth of prostate cancer cells in in vitro conditions.
2Materials and methods
2.1Materials and reagents
Dwarf×Tall (DxT) variety coconuts were collected from Shornur Panchayat Krishi Bhavan Office, Palakkad, Kerala, India. Minimal Essential Medium (MEM), Fetal bovine serum (FBS), Antibiotic and Antimycotic solution (100X), MTT (3;(4,5;Dimethylthiazol;2yl);2,5;diphenyl tetrazolium bromide), Acridine Orange (AO) (N,N,N’,N”;Tetramethylacridine;3;6;diamine), Ethidium bromide (EB) (3,8;Diamino;5;ethyl;6; phenylphenanthridinium bromide), Trypan blue, DAPI (4’,6;Diamidino;2;phenylindole), RDP Trio Reagent and Lactate dehydrogenase assay kit (EZ countTM) was purchased from Hi;Media Laboratories, India. DCFH;DA (2’,7’;dichlorofluorescin diacetate) was purchased from Sigma-Aldrich, USA. Synthesized oligos for PCR was purchased from Integrated DNA technologies, USA. Revert Aid First Strand cDNA Synthesis Kit was purchased from Thermo Fischer Scientific, USA. Go Taq Green Master Mix was procured from Promega, USA. MitoSOX and Fura-2-AM was procured from Invitrogen, Life Technologies, USA.
2.2Extraction of polyphenol/flavonoid fraction from coconut kernel
Coconut kernel was removed and defatted with petroleum ether (60°–80°C) using a Soxhlet apparatus. The residue obtained after defatting was dried, weighed and exhaustively extracted using 80% methanol. Methanolic extract (CKf) thus obtained was dried in rotary evaporator, weighed and used for further experiments [27].
2.3LC-MS analysis of CKf
Identification of the bioactive compounds present in CKf was analyzed with Waters Xevo G2Q-TOFmassspectrometer with a Waters Acquity H class Ultra Performance Liquid Chromatography (UPLC) system equipped with ACQUITY UPLC™ BEH C18 column (50 mm×2.1 mm×1.7 μm; Waters, Milford, MA, USA) at 30°C. 10 μl of the sample was injected for a total run time of 10 min at a flow rate of 0.3 ml/ min using 0.1% formic acid in water (A) and methanol (B) in gradient program as the mobile phase. The sample was ionized by electrospray ionization (ESI) in negative mode with a capillary voltage at 2.5 kV, sample cone voltage at 30 V and nitrogen was set at 900 L/h and the temperature at 350°C. The cone gas, nitrogen, was set at a flow rate of 50 L/h, and the source temperature was set at 135°C. A full scan analysis ranging from 50 to 1000 m/z was performed. A lock spray ionization source is present along with the waters Q-TOF equipment that performs on line calibration using leucine-enkephalin ([M+H] + m/z 556.2771) for providing accurate and reproducible molecular masses of parent and product ions. The elemental composition was determined by Mass Lynx V 4.1 software.
2.4Cell culture
Human prostate cancer cell line (DU-145) was procured from National Centre for Cell Science (NCCS), Pune, India. The cells were grown and maintained in a humidified incubator at 37°C, 5% CO2 atmosphere in MEM supplemented with 10% fetal bovine serum (FBS) and antibiotics.
2.5In vitro cytotoxic effect of CKf on DU cells: MTT assay
To determine the anti-proliferative effect of CKf, the MTT assay was performed as described earlier [28]. This method is based on conversion of the tetrazolium salt (MTT) to colored formazan by viable, but not dead, cells. DU-145 cells were treated with different concentrations of CKf (0.2 to 10 μg/ml) for 24 h. After the treatment period, the viability of DU-145 cells was determined by adding MTT to the cell cultures to reach a final concentration of 1 mg/mL. After 4 h incubation at 37°C, the dark crystals formed were dissolved by adding to the wells an equal volume of DMSO and the plates were incubated overnight at 37°C and optical densities at 570 nm was measured using a plate reader (Varioskan Flash Microplate reader, Thermo scientific) with a corresponding filter. Data are presented as a percentage of the value obtained from cells incubated in fresh medium only. All experiments were performed in triplicate. The inhibition rate was calculated as follows: Growth inhibition rate(%) = (Acontrol-Adrug/Acontrol)×100.
2.6Determination of apoptosis: AO-EB double staining
To visualize the effect of CKf on apoptosis, the cells were stained using fluorescent Acridine orange-ethidium bromide [29]. DU-145 cells were seeded at a density of 3×104 cells/well and treated with CKf at varying concentrations between 1–10 μg/ml for a period of 24 h at 37°C, 5% CO2. Following incubation, the wells were washed with PBS and AO-EB stain (100 μg/ml each) was added at a ratio of 1:1. The plates were incubated in the dark for 30 min at 37°C. Finally cells were examined at 20X magnification using an Olympus inverted fluorescence microscope.
2.7DAPI staining: Confocal Raman spectra
DU-145 cells were grown and maintained in a humidified incubator at 37°C, 5% CO2 atmosphere in MEM supplemented with 10% FBS and 100X Antibiotic and Antimycotic solution. For the experimental purpose, cells were seeded onto 12-well plate with cover slip (at a density of 4 to 7×104 cells/ml). After 24 h incubation period to allow cell attachment, the cells were treated with various concentrations of CKf, (2.5, 7, 10 μg/ml) and incubated for 24 h. After incubation, cells were washed with PBS and fixed with 10% formaldehyde for 5–10 min. Fixed cells were then washed twice with PBS and permeabilised with Triton-X 100 for 5 min and then subsequently washed 2-3 times in PBS. Cells were then stained with DAPI for 10–15 min, washed twice and mounted onto a slide with mounting solution. Confocal images were taken using NIKON AIR Microscope. Both the bright field and fluorescent images (DAPI) were taken in a 60X magnification.
2.8SEM analysis
DU-145 cells were grown and maintained in a humidified incubator at 37°C under 5% CO2 atmosphere in MEM supplemented with 10% FBS and 100X Antibiotic-Antimycotic solution. For experimental purposes, cells were seeded onto coverslips in 12-well plates at a density of 4–7×104 cells. After a 24 h incubation period to allow for cell attachment, the cells were treated with various concentrations of CKf (2.5, 7, 10 μg/ml) and incubated for 24 h. Cells were then washed with PBS and fixed with 10% formaldehyde for 5–10 min. Fixed cells were washed twice with PBS and then air dried before the samples were processed for SEM analysis.
2.9FTIR; ATR analysis
DU-145 cells (2×104 cells/well) were treated with different concentrations of CKf (2.5, 7, 10 μg/ml) for 24 h. After the incubation period, cells were centrifuged at 1000 rpm for 5 min to remove medium and then fixed in 70% ethanol (EtOH) for 1 h. After centrifugation and washing 2 more times using 70% EtOH, the concentrated cells were applied to aluminium foil and air-dried. ATR-FTIR spectral measurements were performed using a Shimadzu ATR attachment containing a diamond crystal internal reflective element [30].
2.10Ca2 + signalling and mitochondrial membrane potential
The effect of CKf on alterations in intracellular calcium levels were evaluated in DU-145 cells using the ratiometric probe Fura-2-AM. DU-145 cells (2×104cells/well) were treated with different concentrations of CKf (2.5, 7, 10 μg/ml) for 24 h. After the treatment period, culture medium in the wells was replaced with Krebs buffer (1 mM CaCl2; 132 mM NaCl; 4 mM KCl; 1.2 mM Na2HPO4; 1.4 mM MgCl2; 6 mM Glucose;10 mM HEPES, pH 7.4), supplemented with 1 mg/ml bovine serum albumin (BSA) and 5M Fura-2-AM, and incubated at 37°C in the dark for 40 mins. Cells were rinsed twice with Kreb’s buffer following the incubation period and fresh buffer supplemented with BSA minus the probe was added to the wells. The cells were then observed by fluorescence microscopy using the appropriate band-pass filter. (Olympus 1×51) [31].
The electrical potential across the inner mitochondrial membrane of CKf treated DU-145 cells was determined using Rhodamine-123 (R-123), a lipophilic, cationic indicator. DU-145 cells (2×104cells/well in 96-well plates) were treated with different concentrations of CKf for 24 h. After the treatment, cells were rinsed with PBS and fresh media containing R-123 solution (10 μg/ml) was added to the treated wells and the plates were incubated in the dark at 37°C for 20–30 min. Subsequently, the cells were washed twice with PBS and the cell images were taken using a fluorescence microscope (Olympus 1×51) [32].
2.11Levels of ROS: DCFH;DA staining
To determine whether CKf treatment increases the generation of excessive intracellular ROS in DU-145 cells, which may contribute towards cytotoxicity were examined using a fluorescent probe, 2′,7′-dichlorofluorescein diacetate (DCFH-DA) [33]. 96-well plates seeded with DU-145 cells were treated with different concentrations of CKf (2.5, 7, 10 μg/ml) for 24 h. Treated cells were then washed with phosphate buffered saline (PBS) and incubated with fresh DCFH-DA (100 μM) in PBS for 30 min at 37°C, 5% CO2. After incubation, fresh medium without FBS was added to the wells and fluorescence images were taken using an inverted microscope (Olympus 1×51).
2.12Determination of mitochondrial superoxide production: MitoSOX staining
DU-145 cells (2×104) were plated on 96-well plates and incubated overnight to allow for cell attachment. Following treatment with different concentrations of CKf (2.5, 7, 10 μg/ml), cells were labelled with MitoSOX Red (396 μM) in complete medium for 20 mins at 37°C. Post incubation, the cell medium was replaced with fresh medium without the dye and cells were incubated for another 20 mins at 37°C. Images were taken by an inverted microscope using either 10/20X objective lens MitoSOX was excited by laser at 514 nm [34].
2.13LDH leakage assay
Membrane damage in DU-145 cells on treatment with CKf was detected using the EZcountTM LDH Cell Assay Kit, Hi-Media in a one-step reaction. The enzyme in the LDH reagent uses NADH to reduce the dye to a coloured product which can be measured colorimetrically. Briefly, DU-145 cells (2×104) were seeded on a 96-well plate and incubated overnight for adherence. Post adherence, cells were treated with different concentrations of CKf (2.5, 7, 10 μg/ml) for 24 hrs. After the incubation period, lysis solution was added to each well and incubated at 37°C. Post-lysis the LDH reagent was added to all the wells and the plate was incubated at room temperature for 10 min. Reaction was terminated with the addition of a stop solution. Absorbance was read at 580 nm as a main wavelength and at 630 nm for the reference wavelength.
2.14Expression of apoptotic genes by polymerase chain reaction
Total RNA was isolated from DU-145 cells non treated and treated with different concentration of CKf (2.5, 7, 10 μg/ml) for 24 hrs using RDP Trio Reagent, and cDNA was synthesized using Revert Aid First Stand cDNA synthesis Kit, stored at –20°C. Expression of apoptotic genes, Bax, Bid, Bak, and p53 was performed using the following primers: Bax (Forward 5’; GAGAGGTCTTTTTCCGAGTGG; 3’, Reverse 5’; CCTTGAGCACCAGTTTGCTG; 3’), Bak (Forward 5’; GGGTCTATGTTCCCCAGGAT; 3’, Reverse 5’; GCAGGGGTAGAGTTGAGCA; 3’); p53 (Forward 5’; GGCCCACTTCACCGTACTAA; 3’, Reverse 5’; GTGGTTTCAAGGCCAGATGT; 3’) Bid (Forward 5’; CCCACACTGGTGAGACAACT; 3’; Reverse 5’; GTCGTTCTCCATGTCCCTA; 3’). GAPDH was used as an endogenous control. The polymerase chain reaction (PCR) products were analyzed on a 1.5% agarose gel electrophoresis, and band intensity was detected using Gel Doc-EZ imager (BIO-RAD).
2.15Statistical analysis
Experimental results are expressed as mean±S.D of three independent experiments. One-way ANOVA using SPSS-19, IBM Technologies Software was used for statistical analysis, followed by Duncan’s multiple comparison test to assess the significance between groups. Value of p < 0.05 was considered to be statistically significant.
3Results and discussion
3.1CKf shows the presence of biologically active components
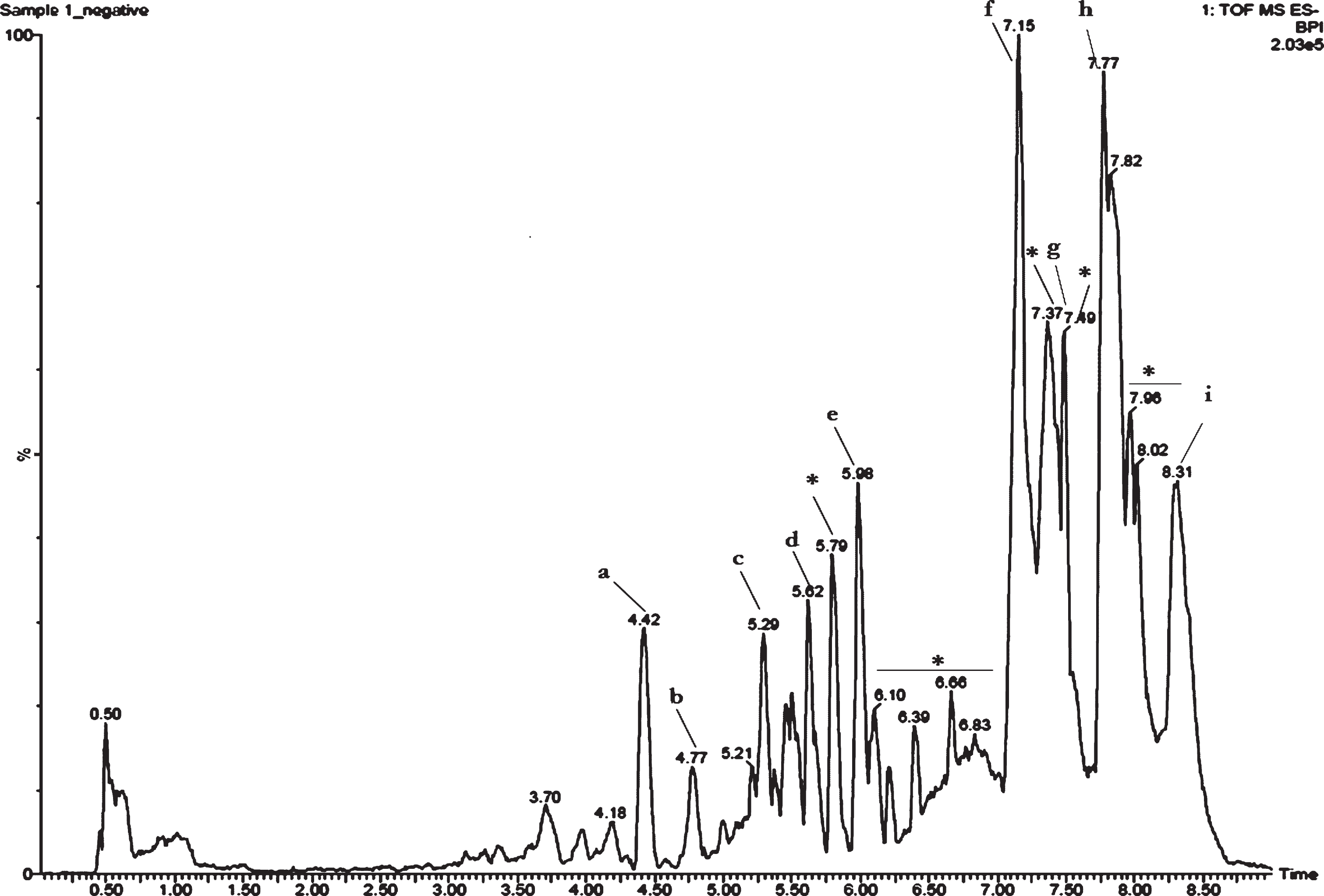
Earlier studies in our laboratory showed that CKf contained polyphenols and flavonoid class of compounds. Further evaluation using LC-MS showed several peaks. Assessment of each peak and the mass obtained was compared with available literature for confirmation of the compounds. Results showed the presence of coumaric acid, caffeic acid, trihydroxy flavone derivatives, chlorogenic acid, myricetin derivative, tetramer of 5-dihydrobenzoic acid, hydroxy cinnamic acid derivative and titerpene methyl esters in CKf (Fig. 1).
Fig.1
Total ion chromatogram (TIC) of CKf. a-Coumaric acid, b-Caffeic acid, c-Naringenin, d-Naringenin derivative, e-Chlorogenic acid, f-myricetin derivative, g-Tetramer of 5-dihydrobenzoic acid, h-Hydroxy cinnamic acid derivative, i-Titerpene methyl esters. *Unidentified.
3.2CKf is cytotoxic to DU-145 cells
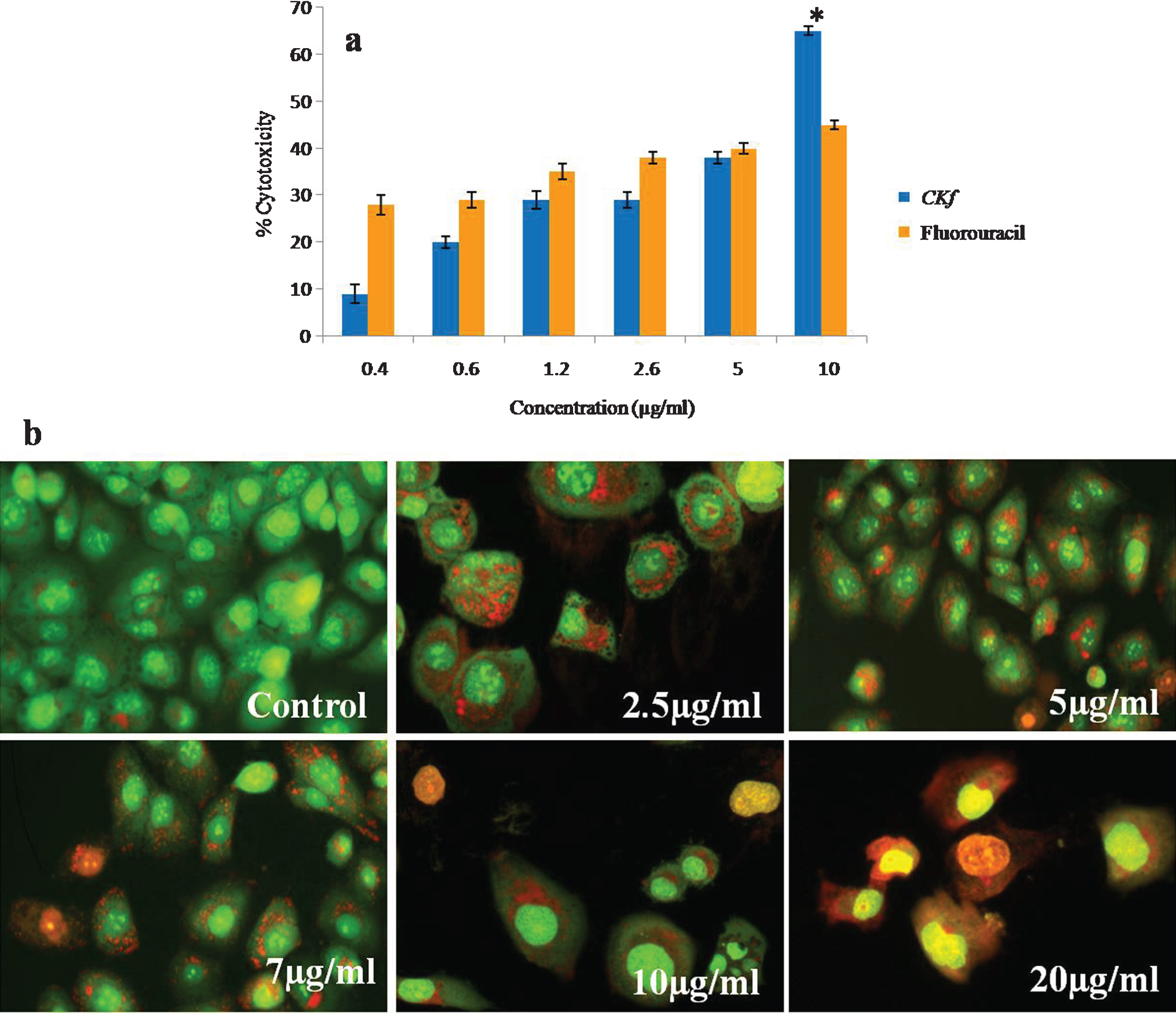
Prostate cancer has the highest mortality rate of all cancers with 94% of patients dying within years of diagnosis. This cancer grows quickly with no effective treatments available, underscoring the importance of finding new therapies. Therefore, the identification and development of alternative medicines for the treatment and prevention of prostate cancer is needed. Dietary sources are a promising source of new therapeutic options, Coconut kernel and oil extracted from it is rich in phytochemicals including phytosterols and polyphenols has many significant medical effects. Though coconut kernel has anti-diabetic and anti-atherosclerotic effect [35–37], there are no published studies on its anti-cancer activity. Therefore, in the present study, we examined the effect of C K f on human prostate cancer cells, DU-145 in vitro. C K f is a methanolic extract containing polyphenols/flavonoids isolated from DxT variety of coconut. The anti-cancer effect of C K f on decreasing the growth rate of cancer cells has been scrutinized at molecular level through following up the expression of relevant mitochondria related genes.The morphological changes inclusive of damaged cell membranes and cell shrinkage, which are features of apoptosis have also been evaluated. Anti-cancer activities of C K f on DU-145 cells at different concentrations were tested using MTT assay. The results showed a significant cytotoxicity in a dose-dependent manner. C K f at 10 μg/ml inhibited growth by 70%, while 5-fluorouracil, an anti-cancer drug could induce cell death at 50% at a similar concentration (Fig. 2). This suggests that C K f is promising in preventing the growth of the prostate cancer cell line under study. Therefore, further studies were done to analyze various processes involved in the C K f mediated cell death.
Fig.2
Cytotoxicy analysis of CKf on DU-145 cells. a) MTT assay; *statistically significant compared to the higher dose of fluorouracil (p < 0.05). b) Acridine orange ethidium bromide staining on DU-145 cells for evaluating apoptosis. Values are expressed as Mean±SD of three independent experiments.
3.3CKf induces apoptosis of DU-145 cells
The results from AO/EB double staining are shown in Fig. 2. From the data it is clear that in 7, 10, 20 μg/mL of CKf treated DU-145 cells, the number of viable cells decreased tremendously. Besides, some cells exhibited typical characteristics of apoptotic cells like plasma membrane blebbing. However, the number of cells stained was larger at higher concentrations of CKf. This indicates that most of the cells were not undergoing cell death which occurs primarily through apoptosis. This effect was comparable with the standard drug 5-fluorouracil. CKf was found to be less toxic towards normal cells, as tested in rat cardiomyocytes, H9c2 cells (unpublished report).
3.4CKf induces morphological and nuclear changes in DU-145 cells
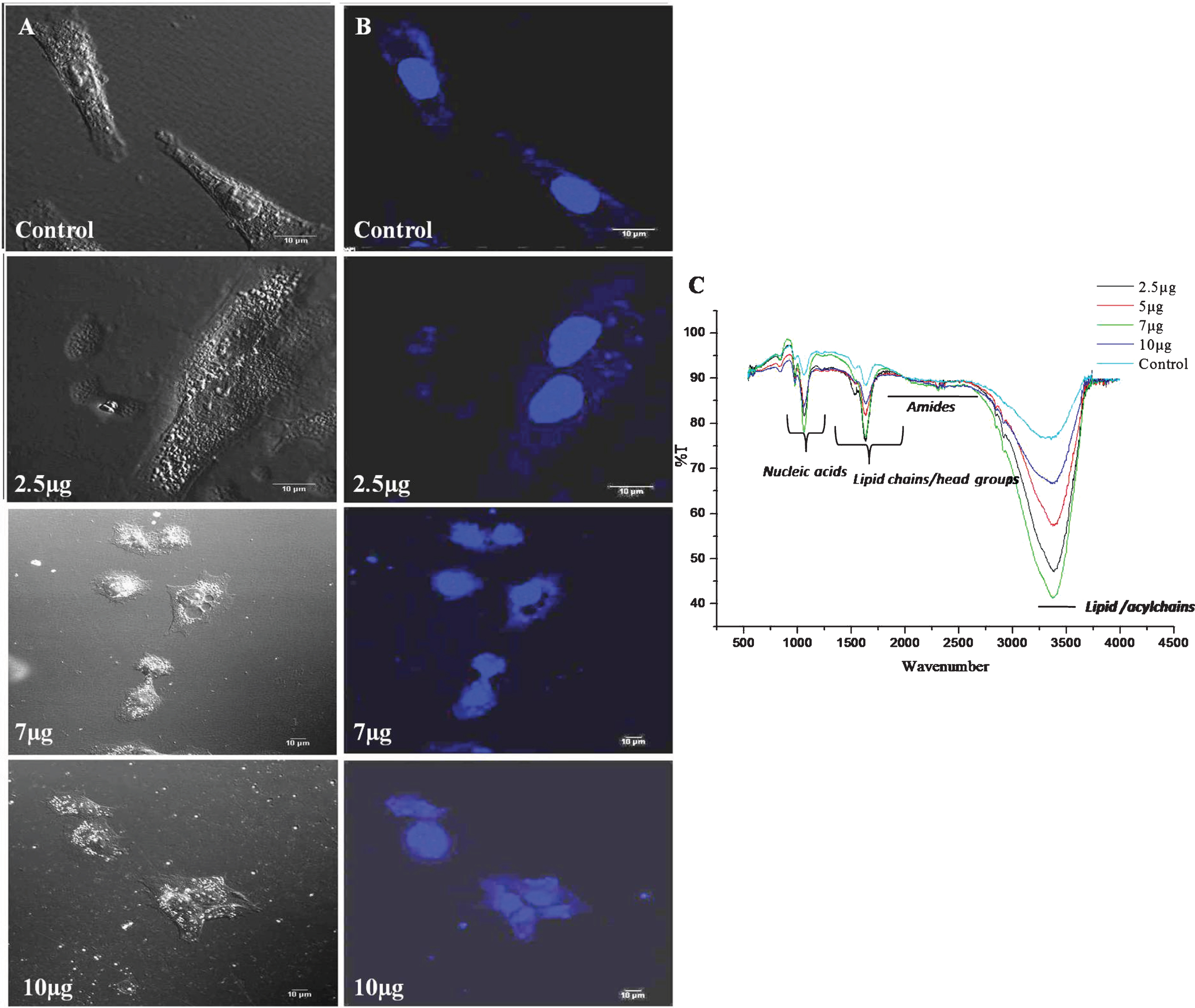
Morphological changes in DU-145 cells exposed to different concentrations of CKf (2–10 μg/ml) for 24 h was observed by SEM (Fig. 3). Contrary to untreated DU-145 cells, which possessed a regular characteristic shape, SEM revealed cell volume shrinkage, membrane blebbing, and membrane cracks in cells treated with CKf. Confocal images of DU-145 cells stained with DAPI showed a reduction in nuclear size, blebbing, apoptotic body formation and chromatin condensation (Fig. 4). Inhibition of proliferation and/or induction of apoptosis in cancer cells are the most important effect of many anti-cancer agents [38]. In the present work, it was found that CKf induced apoptosis in DU-145 cells. FTIR analysis of DU-145 cells treated with CKf showed differential changes in the lipid/acyl chain region (3500–3600 cm–1), nucleic acid region (1500–2000 cm–1) and amide regions (2000–3000 cm–1). These changes indicate the nuclear and cell membrane damage to treated cells (Fig. 4).
Fig.3
SEM analysis for morphological changes of control and CKf treated DU-145 cells. All the experiments were done in triplicate.
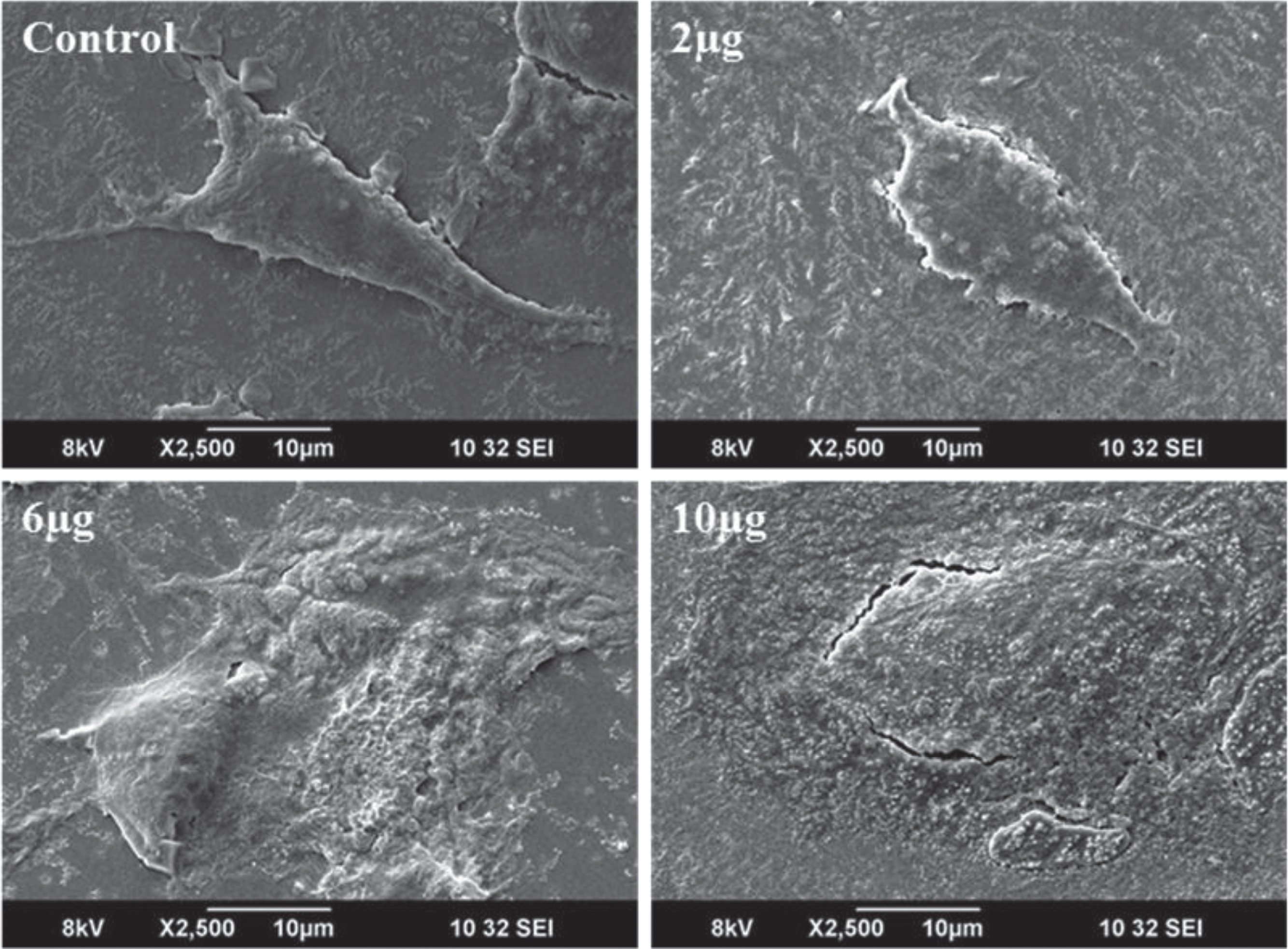
Fig.4
Confocal microscopy and FTIR analysis of control and CKf treated DU-145 cells. A: Normal confocal image B: Confocal image of DAPI stained cells. C: FTIR analysis of control and CKf treated DU-145 cells. All the experiments were done in triplicate.
3.5CKf induces a decrease in mitochondrial membrane potential
One of the most important hallmarks of apoptosis is the depolarisation of the mitochondrial membrane. In addition, mitochondria are the main source of ROS in the cells [39, 40]. Thus, we tested the biological effect of CKf on the mitochondrial membrane potential at different concentration for 24 h. The results showed that 7 and 10 μg/ml of CKf significantly reduced the mitochondrial membrane potential (Fig. 5).
Fig.5
Calcium handling and MMP of CKf treated DU-145 cells. A) MMP B) Fura-2-AM staining, C: Relative fluorescence intensity of Rhodamine-123 and Fura-2-AM stained cells. Values are expressed as Mean±SD of three independent experiments. *Statistically significant compared to control (p < 0.05).
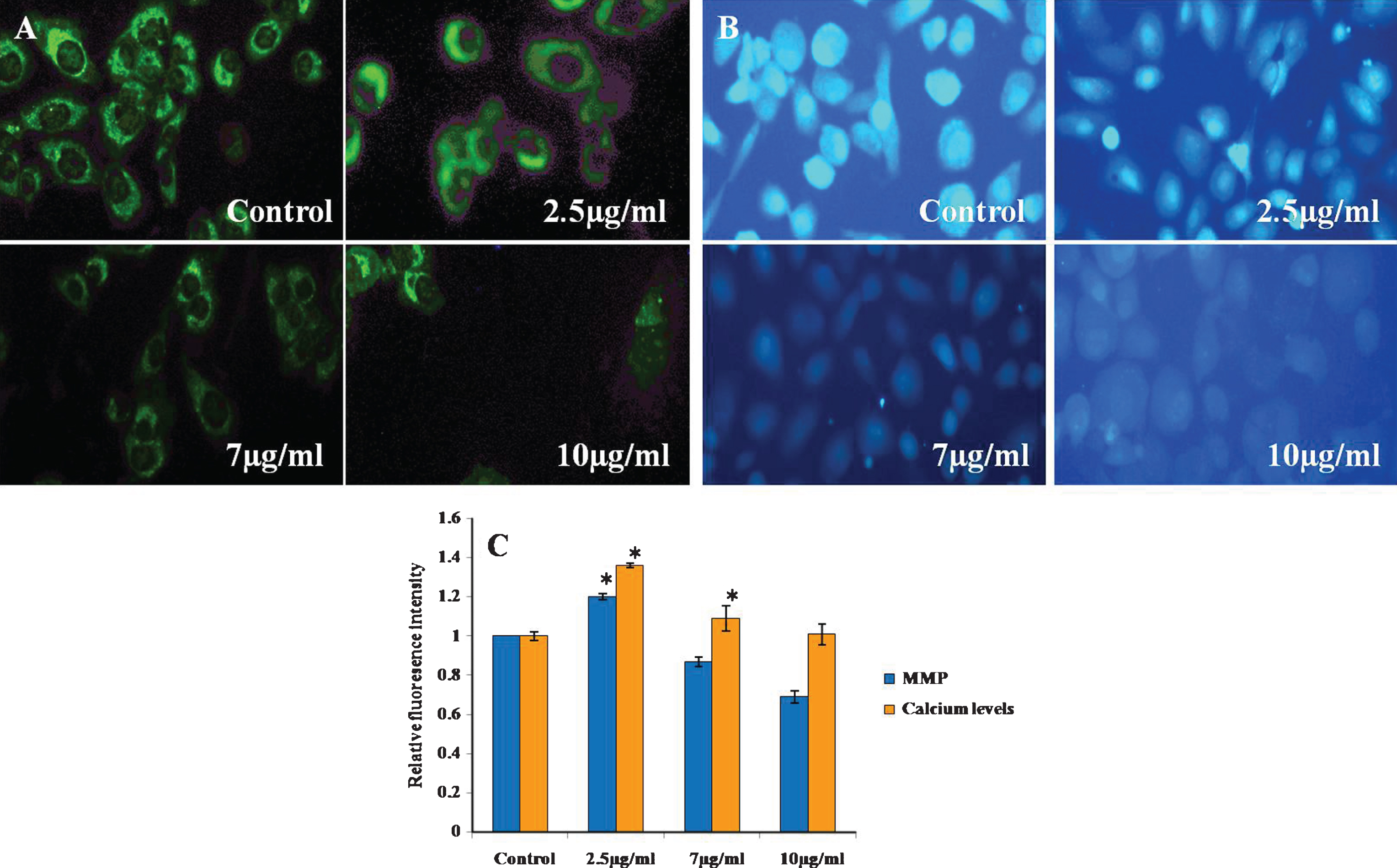
3.6CKf did not affect intracellular calcium levels in DU-145 cells
Since Ca2 + signalling is involved in apoptosis, the modulation of intracellular Ca2 + levels in CKf treated DU-145 cells were recorded. It was found that there was no significant increase in intracellular Ca2 + was detected after CKf stimulation. This indicates that CKf induces cell death through a mechanism independent of calcium (Fig. 5). Mitochondria are central players in cellular Ca2 + signalling by buffering cellular Ca2 + signals [41]. It is widely known that Ca2 + displays growth-inhibiting and differentiation-promoting activities in a variety of normal and malignant cells [42]. In the present experiment, intracellular Ca2 + did not show any significant change in CKf treated DU-145 cells. From these results it may be speculated that CKf is not of any effect on endoplasmic reticulum (ER), which is the major source of intracellular calcium mobilisation or there may not be any effect on the influx of extracellular Ca2 +. These results suggest that CKf exerts a selective biological effect on mitochondria and ER physiology. Though Ca2 + shows to be essential for inducing apoptosis, CKf induced apoptosis may be Ca2 + independent.
3.7CKf triggers an increase in reactive oxygen species production: Lowers antioxidant master switch, Nrf-2 gene expression
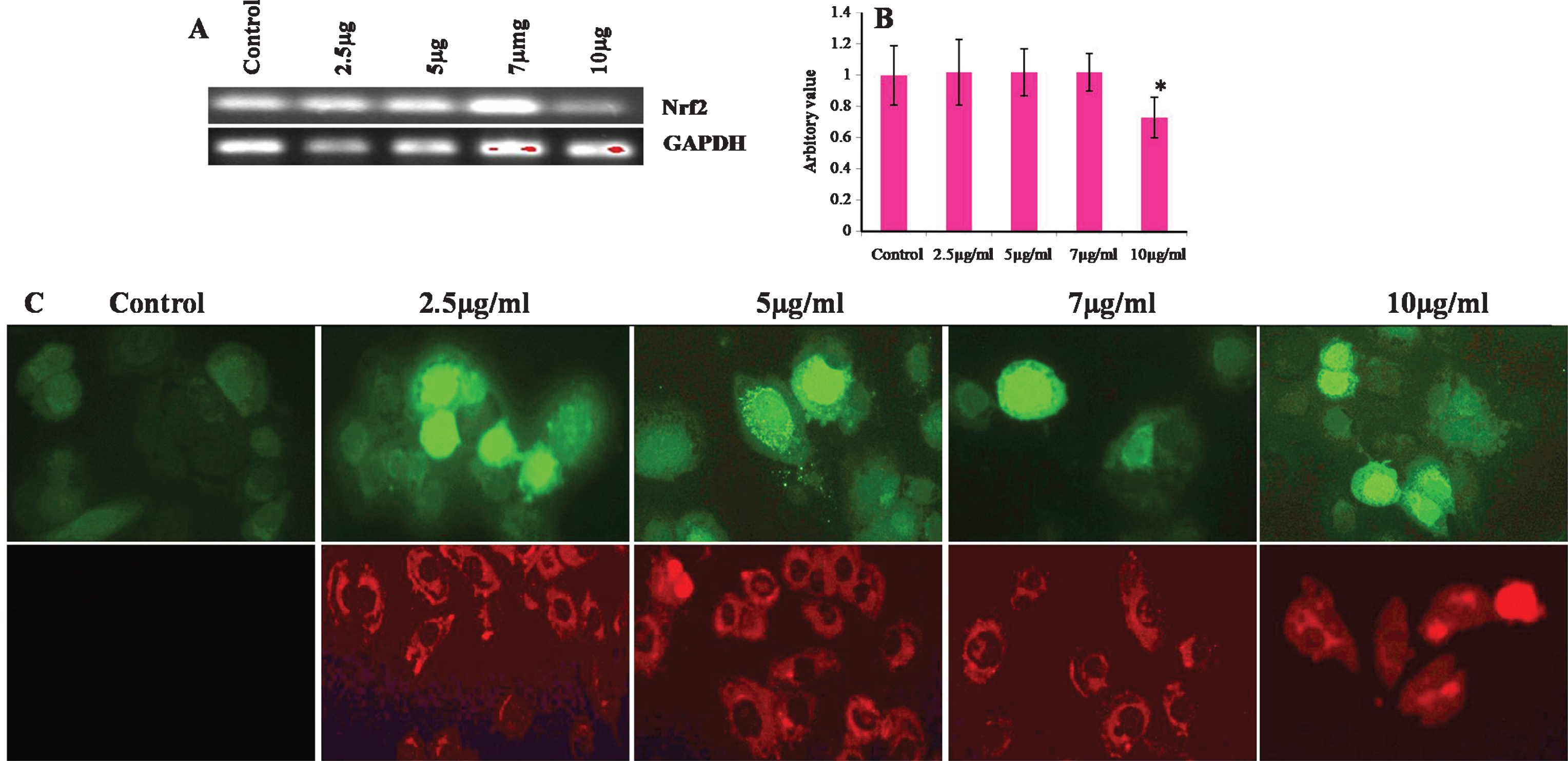
Considering the results shown in Fig. 6, we examined whether CKf could trigger intracellular ROS increase and mitochondrial superoxide production using DCFH-DA as a specific ROS probe and MitoSOX as superoxide probe. In fact, 5, 7 and 10 μg/ml of CKf induced a significant increase in ROS levels and superoxide production in 24 h. Nrf-2, a transcription factor acts as antioxidant master switch inducing several antioxidant enzymes to protect cells from harmful oxidants. Our experimental results showed that 10 μg/ml of CKf showed maximum amount of ROS produced and reduced the levels of Nrf-2 significantly compared to control and lower concentrations, indicative of severe intracellular stress. Changes in the intracellular level of ROS have been reported to play an important role in the early step of apoptosis, anticipating loss of the mitochondrial membrane potential and release of the apoptotic-inducing factors and enhanced expressions of mitochondria associated apoptotic gene [43]. Studies using several cells lines have proved that chemically induced ROS production is responsible for apoptosis in different types of cancer [44–49]. In our cell model, DU-145 cells, CKf significantly increased ROS and mitochondrial superoxide production in a dose-dependent manner with a decrease in mitochondrial membrane potential. Mitochondrial trans-membrane potential is often used as an indicator of metabolic health as well as cellular viability. Any disruption to the membrane potential is detrimental to cell growth and function which may result in apoptotic phenomenon [50, 51]. The present study underlines that CKf induce cell death in DU-145 cells in the same manner involving mitochondria and ROS.
Fig.6
Effect of different concentrations of CKf on ROS and mitochondrial superoxide production and Nrf-2 gene expression in control and treated DU-145 cells. A: Gel image of the Nrf-2 gene expression in control and CKf treated DU-145 cells B: Band intensities of each gene. C: Panel above shows the florescence image of CKf treated DU-145 cells after staining with DCFH-DA, Panel below shows the fluorescence image of CKf treated DU-145 cells after staining with MitoSOX. * Statistically significant compared to control (p < 0.05). All the experiments were repeated three times.
3.8CKf treated cells showed leakage of LDH
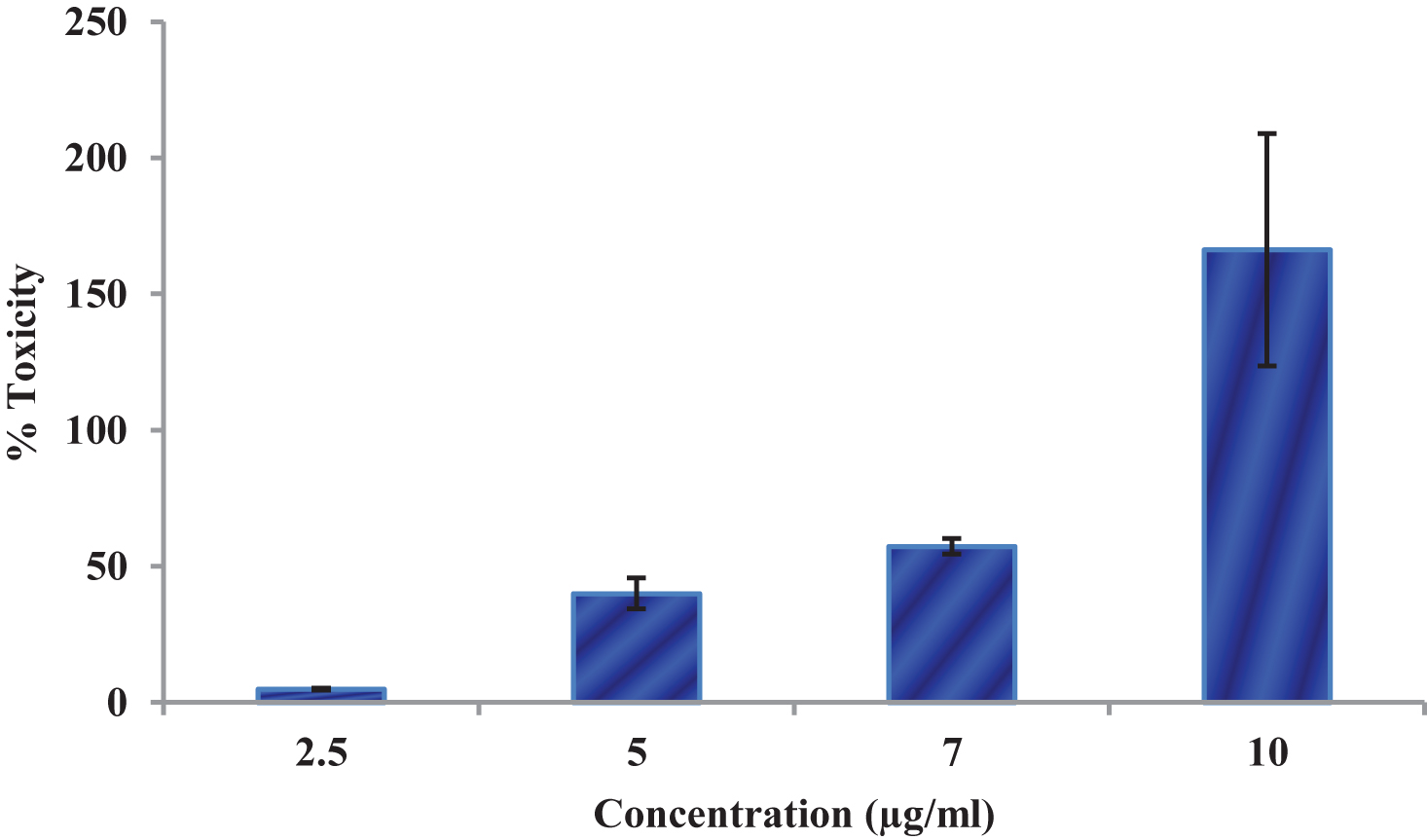
Figure 7 shows the effect of CKf on LDH leakage in DU-145 cells. The LDH leakage assay is a simple, reliable and fast cytotoxicity assay based on the measurement of lactate dehydrogenase activity in the extracellular medium. LDH, a cytoplasmic enzyme is released when the cell membrane is damaged [52]. The cell membrane damage of DU-145 cells after the treatment with CKf extract was measured by the release of LDH. The control cells and lower concentrations of CKf showed lower levels of LDH in the extracellular medium, while a concentration of 10 μg/ml showed significantly higher levels of LDL in the extracellular medium. This observation was supported by the confocal and SEM images.
Fig.7
LDH Leakage levels in control and CKf treated DU-145 cells. Values are expressed as Mean±SD of three independent experiments. * Statistically significant compared to control cells (p < 0.05).
3.9CKf modulates mitochondria associated gene expression in DU-145 cells
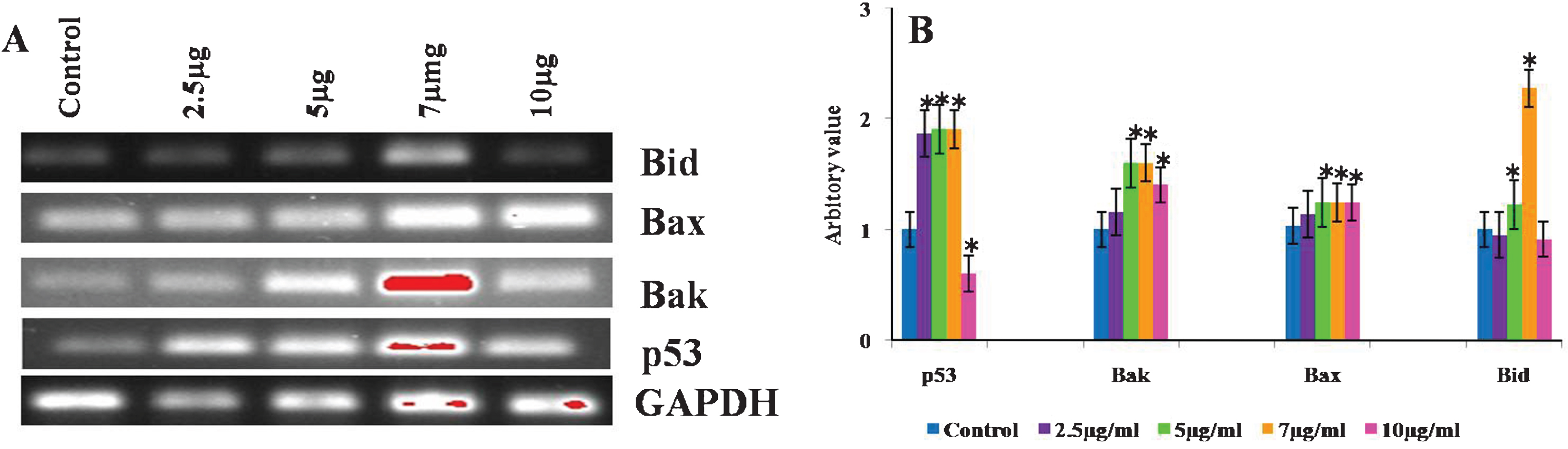
Reverse transcriptase PCR analysis was done to evaluate the expression of pro-apoptotic genes, Bax, Bak, Bid as well as p53 analysed to determine the role of mitochondria in CKf induced cell death in DU-145 cells. Bax, Bak and p53 were found to be increased in all treated cells compared to control cells. The expression of Bid showed differential changes in control and treated DU-145 cells. Bid expression of 7 μg treated cells were found to be higher, while 5 μg dose showed slight increase. At higher concentrations, Bid levels in DU-145 cells were found to be lowered (Fig. 8). Bax and Bak, pro-apoptotic genes necessary for the execution of the apoptotic program. Several works have emphasized the role of Bax in both cancer progression and/or resistance to chemo- or radiotherapy-induced apoptosis in human and in animal models [53]. Studies have shown that Bax, a pro-apoptotic gene, induces apoptosis by increasing the activity of Caspase-3 mediated through p53. The activation of Bax results in mitochondrial disruption and subsequent release of Cytochrome-c through the outer mitochondrial membrane into the cytosol. Inside the cytosol, Cytochrome-c associates with Apoptotic Protease Activating Factor-1 and activates Caspase-9 which, in turn, triggers the activation of Caspase-3 [54]. It is clear from our experiments that the mRNA levels of Bax and p53 are significantly increased with increased concentration of CKf indicating the role of mitochondria in cell death.
Fig.8
Effect of CKf of different mitochondria associated gene expression in control and treated DU-145 cells. The experiment was repeated three times. A: Gel imaged of the PCR products B: Band intensities of each gene.
CKf was showed to contain several biologically active components as revealed by the LC-MS data. LC-MS data showed that CKf contained myricetin, hydroxycinnamic acids, coumaric acid, caffeic acid, chlorogenic acid, and triterpene methyl esters etc as active components. The presence of caffeic acid, p-coumaric acid, ferulic acid, and (±) catechin was already reported in virgin coconut oil, but the spectrum of polyphenolic compounds in kernel has not been isolated or studied so far [55]. p-Coumaric acid is the abundant isomer of cinnamic acid and also widely found in edible plants such as peanuts, tomatoes, carrots etc. p-Coumaric acid is reported to have antitumor and anti-mutagenic activities [56, 57]. It was shown that p-Coumaric acid inhibited the growth of colon cancer cells by inducing apoptosis through ROS-mitochondrial pathway [58]. Myricetin is a flavanol found in various berries, herbs, and walnuts. Previous studies have demonstrated that myricetin has anti-cancer effects against several types of cancer, including hepatocarcinoma, skin carcinoma, and pancreatic cancer [59, 60]. Myricetin increased the BCL2-associated X protein/B-cell lymphoma 2 ratio and induced the release of apoptosis-inducing factor from mitochondria of colon cancer cells [61]. Chlorogenic acid is shown to have significant anti-tumor activity by affecting changes in the gene expression by significantly upregulating the responsive genes (CaN, NFATC2, NFATC2ip, and NFATC3) involved in immune pathways as well as the IL-2R and IFN-γ to promote activation and proliferation of T cells, macrophages, and NK cells, thus enhancing their surveillance and killing abilities, further suppressing the growth rate of tumor cells [62]. Accumulated data show that triterpenoids exhibit a broad spectrum of anti-cancer properties, including anti-proliferative, anti-metastatic and anti-angiogenic activities mediated through androgen receptor, nuclear factor-kappa B, activator protein-1, p53 and 14-3-3 [63].
In the light of our observations with Cocos nucifera and previous reports which link the presence of dietary polyphenols with reduced oxidative stress and improved health benefits, it wouldn’t be wrong to suggest that there exists a need for the identification, development and promotion of polyphenol enriched foods as an essential part of our daily diet. This proposal however does raise some very significant questions. One of them being the criterion for selection of these super-foods on the basis of their antioxidant potential. Oxidant scavenging is a broad term and all bioactive molecules wouldn’t be equally effective in scavenging the various types of free radicals generated which includes superoxides, singlet oxygen, hydroxyl radical and peroxynitrites. Previous study reported the significance of ORACMR5, a battery of tests which determine the antioxidant potential of various foods and the differences encountered in processed vs unprocessed samples [64, 65]. Such food profiles might be helpful when assigning a polyphenol rich diet to counter oxidative stress and related ailments. Again, several studies using single phytochemicals in oxidant-scavenging studies generally use concentrations which cells will never be exposed to when the compound is administered orally or otherwise to a human being. Systemic studies in live animal models cannot be considered as completely foolproof alternatives to a human body due to the existing differences in drug absorption, metabolism and scavenging properties. On the positive side, it is entirely possible that the beneficial effect exerted by one component on a system might be enhanced or rendered much more effective when multiple plant components are involved. Phytochemicals are also being evaluated as candidates useful against self-renewal of cancer stem cells when looking at possible anticancer strategies [66].
4Conclusion
Polyphenols containing fraction from coconut kernel (CKf) was found to prevent the growth of human prostate cancer cells, DU-145 in vitro through the ROS-mitochondria mediated apoptosis. The cytotoxic activity of the extract could be attributed to a combinatorial effect of multiple polyphenolic compounds which have been identified and their possible role in altering cellular redox levels. In vitro experiments prove without a doubt that the extract has a significant impact on mitochondrial viability and in the expression of relevant genes. These results suggest that CKf can be considered as a part of the dietary plan in using nutraceuticals when preventing prostate cancer. The discussion of our present work can be enhanced by evaluating the impact of administration of CKf on a suitable tumor bearing animal model. Effect of polyphenols on the tumor-cell antioxidant system will be determined through western blot experiments and consequently the mode of cell death will be elucidated.
Conflict of interest
The authors declare no conflict of interests.
Acknowledgments
This study was supported by the grant from the Coconut Development Board, Ministry of Agriculture, and Government of India (Grant No. 1345/2011). The authors are grateful to School of Biosciences, Mahatma Gandhi University and Department of Biotechnology, Government of India, for the excellent research facilities supported through the prestigious DBT-BUILDER programme (BT/PR4800/INF/22/152/2012 Dated March 22, 2012).
References
[1] | Lodi A , Saha A , Lu X , Wang B , Sentandreu E , Collins M , Kolonin MG , DiGiovanni J , Tiziani S . Combinatorial treatment with natural compounds in prostate cancer inhibits prostate tumor growth and leads to key modulations of cancer cell metabolism. npj Precision Oncology. (2017) ;1: :18. |
[2] | Russell LH , Mazzio E , Badisa RB , Zhu ZP , Agharahimi M , Millington DJ , Goodman CB . Differential cytotoxicity of triphala and its phenolic constituent gallic acid on human prostate cancer LNCap and normal cells. Anticancer Research. (2011) ;31: :3739–45. |
[3] | Lall RK , Syed DN , Adhami VM , Khan MI , Mukhtar H . Dietary polyphenols in prevention and treatment of prostate cancer. International Journal of Molecular Sciences. (2015) ;16: :3350–76. |
[4] | Tan J , Chen B , He L , Tang Y , Jiang Z , Yin G , Wang J , Jiang X . Anacardic acid (6-pentadecylsalicylic acid) induces apoptosis of prostate cancer cells through inhibition of androgen receptor and activation of p53 signalling. Chinese Journal of Cancer Research. (2012) ;24: :275–83. |
[5] | Sanderson JT , Clabault H , Patton C , Lassalle-Claux G , Jean-François J , Paré AF , Hébert MJ , Surette ME , Touaibia M . Antiproliferative, antiandrogenic and cytotoxic effects of novel caffeic acid derivatives in LNCaP human androgen-dependent prostate cancer cells. Bioorganic & Medicinal Chemistry. (2013) ;21: :7182–93. |
[6] | Tolba MF , Esmat A , Al-Abd AM , Azab SS , Khalifa AE , Mosli HA , Abdel-Rahman SZ , Abdel-Naim AB . Caffeic acid phenethyl ester synergistically enhances docetaxel and paclitaxel cytotoxicity in prostate cancer cells. IUBMB Life. (2013) ;65: :716–29. |
[7] | Vanella L , Di Giacomo C , Acquaviva R , Barbagallo I , Cardile V , Kim DH , Abraham NG , Sorrenti V . Apoptotic markers in a prostate cancer cell line: Effect of ellagic acid. Oncology Reports. (2013) ;30: :2804–10. |
[8] | Hori S , Jabbar T , Kachroo N , Vasconcelos JC , Robson CN , Gnanapragasam VJ . Outcomes and predictive factors for biochemical relapse following primary androgen deprivation therapy in men with bone scan negative prostate cancer. Journal of Cancer Research and Clinical Oncology. (2011) ;137: :235–41. |
[9] | Zhang L , Yang BX , Zhang HT , Wang JG , Wang HL , Zhao XJ . Prostate cancer: An emerging threat to the health of aging men in Asia. Asian Journal of Andrology. (2011) ;13: :574–8. |
[10] | Grönberg H . Prostate cancer epidemiology. The Lancet. (2003) ;361: :859–64. |
[11] | Kheirandish P , Chinegwundoh F . Ethnic differences in prostate cancer. British Journal of Cancer. (2011) ;105: :481–5. |
[12] | Willis MS , Wians FH . The role of nutrition in preventing prostate cancer: A review of the proposed mechanism of action of various dietary substances. Clinica Chimca Acta. (2003) ;330: :57–83. |
[13] | Prakash J , Gupta SK . Chemopreventive activity of Ocimum sanctum seed oil. Journal of Ethnopharmacology. (2000) ;72: :29–34. |
[14] | Mohansrinivasan V , Devi C , Deori M , Biswas A , Naine S . Exploring the anticancer activity of grape seed extract on skin cancer cell Lines A431. Brazilian Archives of Biology and Technology. (2015) ;58: :540–6. |
[15] | Fantini M , Benvenuto M , Masuelli L , Frajese GV , Tresoldi I , Modesti A , Bei R . In vitro and in vivo anti tumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: Perspectives on cancer treatment. International Journal of Molecular Sciences. (2015) ;16: :9236–82. |
[16] | Karna P , Gundala SR , Gupta MV , Shamsi SA , Pace RD , Yates C , Narayan S , Aneja R . Polyphenol-rich sweet potato greens extract inhibits proliferation and induces apoptosis in prostate cancer cells in vitro and in vivo. Carcinogenesis. (2011) ;32: :1872–80. |
[17] | Priego S , Feddi F , Ferrer P , Mena S , Benlloch M , Ortega A , Carretero J , Obrador E , Asensi M , Estrela JM . Natural polyphenols facilitate elimination of HT-29 colorectal cancer xenografts by chemoradiotherapy: A Bcl-2-and superoxide dismutase 2-dependent mechanism. Molecular Cancer Therapeutics. (2008) ;7: :3330–42. |
[18] | Cai Y , Luo Q , Sun M , Corke H . Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sciences. (2004) ;74: :2157–84. |
[19] | Owen RW , Giacosa A , Hull WE , Haubner R , Spiegelhalder B , Bartsch H . The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. European Journal of Cancer. (2000) ;36: :1235–47. |
[20] | Seeram NP , Adams LS , Henning SM , Niu Y , Zhang Y , Nair MG , Heber D . In vitro anti proliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. The Journal of Nutritional Biochemistry. (2005) ;16: :360–7. |
[21] | Kaefer CM , Milner JA . In: Benzie IFF, Wachtel-Galor S, editors. Herbal Medicine: Biomolecular and Clinical Aspects. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis. (2011) . |
[22] | Cragg GM , Newman DJ . Natural products: A continuing source of novel drug leads. Biochim Biophysics Acta. (2013) ;1830: :3670–95. |
[23] | Debmandal M , Mandal S . Coconut (Cocos nucifera L.: Arecaceae): in health promotion and disease prevention. Asian Pacific Journal of Tropical Medicine. (2011) ;4: :241–7. |
[24] | Salil G , Nevin KG , Rajamohan T . Arginine rich coconut kernel protein modulates diabetes in alloxan treated rats. Chemco Biological Interactions. (2011) ;189: :107–11. |
[25] | Nevin KG , Rajamohan T . Wet and dry extraction of coconut oil: Impact on lipid metabolic and antioxidant status in cholesterol coadministered rats. Canadian Journal of Physiology and Pharmacology. (2009) ;87: :610–6. |
[26] | Nevin KG , Rajamohan T . Beneficial effects of virgin coconut oil on lipid parameters and in vitro LDL oxidation. Clinical Biochemstry. (2004) ;37: :830–5. |
[27] | Dhanyakrishnan R , Sunitha MC , Prakashkumar B , Sandya S , Nevin KG . Polyphenolic extract from coconut kernel modulates apoptotic genes, reactive oxygen species production, and prevents proliferation of human colon cancer cell line. International Journal of Clinical and Experimental Physiology. (2016) ;3: :113–321. |
[28] | Mosmann T . Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods. (1983) ;65: :55–63. |
[29] | Baskić D , Popović S , Ristić P , Arsenijević NN . Analysis of cycloheximide-induced apoptosis in human leukocytes: Fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biology International. (2006) ;30: :924–32. |
[30] | Cui L , Butler HJ , Martin-Hirsch PL , Martin FL . Aluminium foil as a potential substrate for ATR-FTIR, transflection FTIR or Raman spectrochemical analysis of biological specimens. Analalytical Methods. (2016) ;8: :481–7. |
[31] | Wu GS , Guo JJ , Bao JL , Li XW , Chen XP , Lu JJ , Wang YT . Anti-cancer properties of triterpenoids isolated from Ganoderma lucidum–a review. Expert Opinion in Investigational Drugs. (2013) ;228: :981–92. |
[32] | Wang Y , Zhao X , Gao X , Nie X , Yang Y , Fan X . Development of fluorescence imaging-based assay for screening cardioprotective compounds from medicinal plants. Analytica Chimica Acta. (2011) ;702: :87–94. |
[33] | Warleta F , Quesada CS , Campos M , Allouche Y , Beltran G , Gaforio JJ . Hydroxytyrosol protects against oxidative DNA damage in human breast cells. Nutrients. (2011) ;3: :839–57. |
[34] | Passos JF , Saretzki G , Ahmed S , Nelson G , Richter T , Peters H , Wappler I , Birket MJ , Harold G , Schaeuble K , Birch-Machin MA . Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS One Biology. (2007) ;5: :e110. |
[35] | Lima EB , Sousa CN , Meneses LN , Ximenes NC , Júnior S , Vasconcelos GS , Lima NB , Patrocínio MC , Macedo D , Vasconcelos SM . Cocos nucifera (L.)(Arecaceae): A phytochemical and pharmacological review. Brazilian Journal of Medical and Biological Research. (2015) ;48: :953–64. |
[36] | Mini S , Rajamohan T . Influence of coconut kernel protein on lipid metabolism in alcohol fed rats. Indian Jouranl of Experimental Biology. (2004) ;42: :53–7. |
[37] | Salil G , Rajamohan T . Hypolipidemic and antiperoxidative effect of coconut protein in hypercholesterolemic rats. (2001) ;39: :1028–34. |
[38] | Lowe SW , Lin AW . Apoptosis in cancer. Carcinogenesis. (2000) ;21: :485–95. |
[39] | Thannickal VJ , Fanburg BL . Reactive oxygen species in cell signaling. American Journal of Physiology. (2000) ;279: :L1005–28. |
[40] | Torres M , Forman HJ . Redox signaling and the MAP kinase pathways. Biofactors. (2003) ;17: :287–96. |
[41] | Eager KR , Roden LD , Dulhunty AF . Actions of sulfhydryl reagents on single ryanodine receptor Ca (2+)-release channels from sheep myocardium. Amarican Journal of Physiology. (1997) ;272: :C1908–18. |
[42] | Lamprecht SA , Lipkin M . Chemoprevention of colon cancer by calcium, vitamin D and folate: Molecular mechanisms. Nature Reviews of Cancer. (2003) ;3: :601–14. |
[43] | Cheung F , Kwokpingho CH , Chan AS . Superoxide anion is involved in the early apoptosis mediated by Gleditsia sinensis fruit extract. International Journal of Molecular Medicine. (2004) ;13: :909–13. |
[44] | Lin YT , Yang JS , Lin SY , Tan TW , Ho CC , Hsia TC , Chiu TH , Yu CS , Lu HF , Weng YS , Chung JG . Diallyl disulfide (DADS) induces apoptosis in human cervical cancer Ca Ski cells via reactive oxygen species and Ca2+-dependent mitochondria-dependent pathway. Anticancer Research. (2008) ;28: :2791–9. |
[45] | Zhang R , Humphreys I , Sahu RP , Shi Y , Srivastava SK . In vitro and in vivo induction of apoptosis by capsaicin in pancreatic cancer cells is mediated through ROS generation and mitochondrial death pathway. Apoptosis. (2008) ;13: :1465–78. |
[46] | Qian X . Glibenclamide exerts an antitumor activity through reactive oxygen species–c-jun NH (2)-terminal kinase pathway in human gastric cancer cell line MGC-803. Biochemical Pharmacology. (2008) ;76: :1705–15. |
[47] | Xiao D , Powolny AA , Singh SV . Benzyl isothiocyanate targets mitochondrial respiratory chain to trigger reactive oxygen species-dependent apoptosis in human breast cancer cells. Journal of Biological Chemistry. (2008) ;283: :30151–63. |
[48] | Pan MH , Gao JH , Lai CS , Wang YJ , Chen WM , Lo CY , Wang M , Dushenkov S , Ho CT . Antitumor activity of 3, 5, 4′-trimethoxystilbene in COLO 205 cells and xenografts in SCID mice. Molecular Carcinogenesis. (2008) ;47: :184–96. |
[49] | Marchetti P , Castedo M , Susin SA , Zamzami N , Hirsch T , Macho A , Haeffner A , Hirsch F , Geuskens M , Kroemer G . Mitochondrial permeability transition is a central coordinating event of apoptosis. Journal of Experimental Medicine. (1996) ;184: :1155–560. |
[50] | Zamzami N , Marchetti P , Castedo M , Decaudin D , Macho A , Hirsch T , Susin SAPetit PX . Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. Journal of Experimental Medicine. (1995) ;182: :367–77. |
[51] | Finkel T , Holbrook NJ . Oxidants, oxidative stress and the biology of ageing. Nature. (2000) ;408: :239–47. |
[52] | Saad B , Dakwar S , Said O , Abu-Hijleh G , Battah FA , Kmeel A , Aziazeh H . Evaluation of medicinal plant hepatotoxicity in co-cultures of hepatocytes and monocytes. Evidence Based Complementary and Alternative Medicine. (2006) ;3: :93–8. |
[53] | Cartron PF , Julin P , Oliver L , Martin S , Meflah L , Vallette FM . Nonredundant role of Bax and Bak in Bid-mediated apoptosis. Molecular and Cellular Biology. (2003) ;23: :4701–12. |
[54] | Cain K , Brown DG , Langlais C , Cohen GM . Caspase activation involves the formation of the aposome, a large (∼700 kDa) caspase-activating complex. Journal of Biological Chemistry. (1999) ;274: :22686–92. |
[55] | Seneviratne KN , Dissanayake SDM . Variation of phenolic content in coconut oil extracted by two conventional methods. International Journal of Food Science and Technology. (2008) ;43: :597–602. |
[56] | Ferguson LR , Lim IF , Pearson AE , Ralph J , Harris PJ . Bacterial antimutagenesis by hydroxycinnamic acids from plant cell walls. Mutation Research. (2003) ;542: :49–58. |
[57] | Kroon PA , Williamson G . Hydroxycinnamates in plants and food: Current and future perspectives. Journal of Science Food and Agriculture. (1999) ;79: :355–61. |
[58] | Jaganathan SK , Supriyanto E , Mandal M . Events associated with apoptotic effect of p-coumaric acid in HCT-15 colon cancer cells. World Journal of Gastroenterology. (2013) ;19: :7726–34. |
[59] | Shiomi K , Kuriyama I , Yoshida H , Mizushina Y . Inhibitory effects of myricetin on mammalian DNA polymerase, topoisomerase and human cancer cell proliferation. Food Chemistry. (2013) ;139: :910–8. |
[60] | Phillips PA , Sangwan V , Borja-Cacho D , Dudeja V , Vickers SM , Saluja AK . Myricetin induces pancreatic cancer cell death via the induction of apoptosis and inhibition of the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Cancer Letters. (2011) ;308: :181–8. |
[61] | Kim ME , Ha TK , Yoon JH , Lee JS . Myricetin induces cell death of human colon cancer cells via BAX/BCL2-dependent pathway. Anticancer Research. (2014) ;2: :701–6. |
[62] | Kang TY , Yang HR , Zhang J , Li D , Lin J , Wang L , Xu X . The studies of chlorogenic Acid antitumor mechanism by gene chip detection: The immune pathway gene expression. Journal of Analytical Methods in Chemistry. (2013) ;9: :2013. |
[63] | Wu JR , Liou SF , Lin SW , Chai CY , Dai ZK , Liang JC , Chen J , Yeh JL . Lercanidipine inhibits vascular smooth muscle cell proliferation and neointimal formation via reducing intracellular reactive oxygen species and inactivating Ras-ERK1/2 signalling. Pharmacological Research. (1999) ;59: :48–56. |
[64] | Prior RL , Sintara M , Chang T . Multi-radical (ORACMR5) antioxidant capacity of selected berries and effects of food processing. Journal of Berry Research. (2016) ;6: :159–73. |
[65] | Morita M , Naito Y , Yoshikawa T , Niki E . Antioxidant capacity of blueberry extracts: Peroxyl radical scavenging and inhibition of plasma lipid oxidation induced by multiple oxidants. Journal of Berry Research. (2017) :1–9. (Preprint). |
[66] | Pistollato F , Giampieri F , Battino M . The use of plant-derived bioactive compounds to target cancer stem cells and modulate tumor microenvironment. Food and Chemical Toxicology. (2015) ;75: :58–70. |




