Effects of nuts on postprandial glycemia, satiety and hunger sensations in healthy individuals
Abstract
Nuts consumption reduce the risk of coronary heart disease and some studies demonstrate that nuts may also influence glucose metabolism and are not associated with predicted weight gain. The aim of this study was to evaluate the effects of Sorrento nuts, added to a white bread test meal, on postprandial glycemic response in a group of healthy individuals. Furthermore, the satiety and hunger sensations were evaluated.
Ten healthy volunteers, 3 men and 7 women were studied. Three different meals were consumed by each subject: 100 gr of white bread; 100 gr of white bread + 60 gr of nuts, 100 gr of white bread + 40 gr of olive oil + 27 gr of bresaola. The test meals with nuts or olive oil had the same content of total fat.
Blood samples were obtained for glucose analysis before meals and every 30 minutes for 120 minutes after the meal. Bread+nuts had a lower blood glucose response. In particular, at time t60 “Bread+nuts” (72±12 mg/dl) vs “Bread” (91.3±9.1 mg/dl) with p < 0.005, no significant differences with the meal “Bread+oil”. At time t90, “Bread+nuts” (67.8±13.8 mg/dl) vs “Bread” (83.6±7.8 mg/dl) and “Bread+oil” (81.7±14.5 mg/dl) with p < 0.008. Also at time t120 the meal “Bread+nuts” (71.2±8.2 mg/dl) had a significant lower blood glucose response than the two test meals “Bread” (84.4±10.25 mg/dl) and “Bread+oil” (81.6±16.16 mg/dl) with p < 0.05.
Satiety sensation increase after meal bread+nuts, while hunger sensation decrease.
The results of this study show that the consumption of nuts reduces the postprandial glycemic response and increases the satiety sensation.
1Introduction
Postprandial hyperglycemia is a very frequent phenomenon in people with type-1 and type-2 diabetes [1, 2] and it can occur even when overall metabolic control appears to be adequate as assessed by HbA1c [2, 3]. The risk for cardiovascular disease (such as myocardial infarction, stroke) is 3 times higher in diabetic men and 4 times higher in diabetic females than in healthy subjects of the same age and sex, as many studies on different populations confirm. Several studies seem to show a correlation between postprandial glycemic levels and cardiovascular events [4–6].
In the early Nineties, a large population study showed a surprising result: eating a portion of nuts more than once a week appeared to offer protection against heart attack and stroke [7–9]. Regular nut consumption has consistently been demonstrated to reduce risk of heart disease in both men and women in large prospective cohort studies [8–11]. Nuts are rich of both monounsaturated and polyunsaturated fats [12]. Monounsaturated and, in particular, polyunsaturated fats lower LDL-cholesterol. However, even after taking the positive fat profile of nuts into account, it has been found they have a cholesterol-lowering effect [13]. Also because of the apparent benefits of even a modest intake of nuts, it is likely that nuts exert effects beyond the reduction of cholesterol levels.
Nuts are also an energy dense food. This may limit their intake because of concerns about their possible contribution to positive energy balance. However, evidence to date suggests that nuts are not associated with predicted weight gain. Nuts also influence glucose metabolism, reducing postprandial glycemic excursions [8]. The clinical studies [14, 15] that have examined this effect have demonstrated that almonds eaten in combination with carbohydrate-rich meals attenuate the postprandial glycemic response in healthy individuals [14, 16].
This study examined the acute effect of Sorrento nuts ingestion on postprandial glycemic and insulinemic response and on post meal satiety in healthy adults.
2Materials and methods
Results are expressed as mean±SEM. Ten healthy volunteers, 3 men and 7 women, aged between 24 and 30 yrs (27.4±2.23 yrs) were studied.
Subjects with metabolic disorders, smokers and subjects with vitamins, minerals and drugs supplementation which can influence glucose metabolism were excluded from the study.
Participants were instructed to cease all nut consumption at least 1 week before and during the experimental period. Testing took place on 3 mornings 1 week apart and in random order. The day before testing, participants did not consume caffeine or perform moderate-to-intense physical activity and followed a normocaloric diet.
Testing was scheduled at 8:30 a.m. Meals were consumed in 10 minutes. At the start of each test, an arm vein was cannulated and kept patent by a slow infusion of saline, through which 5 ml venous blood sample were obtained. Blood samples were obtained before meals and every 30 minutes for 120 minutes after the meal.
The subjects consumed three different meals (Table 1). The first meal was composed of 100 g of white bread, the second of 100 g of white bread and 60 g of Sorrento nuts, and the third of 100 g of white bread, 40 g of extra virgin olive oil and 27 gr of bresaola. Macronutrients and energy contents of the meals are shown in Table 1. The test meals with nuts and olive oil had the same content of total fat and protein.
Table 1
Composition of the test meals
| Test meals | kcal | Protein (g) | CHO (g) | Fibre (g) | Fat (g) | MUFA (g) | PUFA (g) | SFA (g) |
| White bread (100 g) | 245 | 10.1 | 66.9 g | 9.8 | 2.3 | 0.31 | 0.9 | 0.41 |
| White bread (100 g) + Sorrento Nuts (60 g) | 645 | 19.8 | 70.2 | 15.8 | 41.9 | 5.66 | 28.8 | 3.60 |
| White bread (100 g) + Extra virgin olive | 652 | 19.2 | 66.9 | 9.8 | 43.4 | 30.66 | 4.49 | 7.36 |
| oil (40 g) + Bresaola (27 g) |
CHO, carbohydrates; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids.
Roche/Hitachi cobasc.automated clinical chemistry analyzers were used for glucose quantitative determination, which are based on the enzymatic UV test with hexokinase.
Serum insulin was measured with IMMULITE 2000 insulin, a solid-phase enzyme-labeled chemiluminescent immunometric assay.
Satiety sensation times and hunger recurrence times were also evaluated. For their quantification, an analogue method was adopted: there was a question for each sensation on an isosceles triangle.
The base indicated the maximum of the experienced sensation, the apex indicated the minimum. Subjects were asked to rate each sensation by drawing a line, parallel to the baseline, across the isosceles triangle, every 30 minutes for 120 minutes. The area between the apex and the drawn line represented the intensity of the sensation [17].
Results are expressed as mean±SEM and the analysis of repeated measures variance (ANOVA) was performed to test the differences between the meals. A p < 0.05 was considered significant. The statistical analysis program SPSS was used.
The glycemic index of the test meals was calculated as the incremental blood glucose response of the test meal divided by the corresponding incremental blood glucose response of white bread and expressed as percentage.
GlycemicIndex = (FoodIncremental AUC/ BreadIncremental AUC) * 100.
3Results
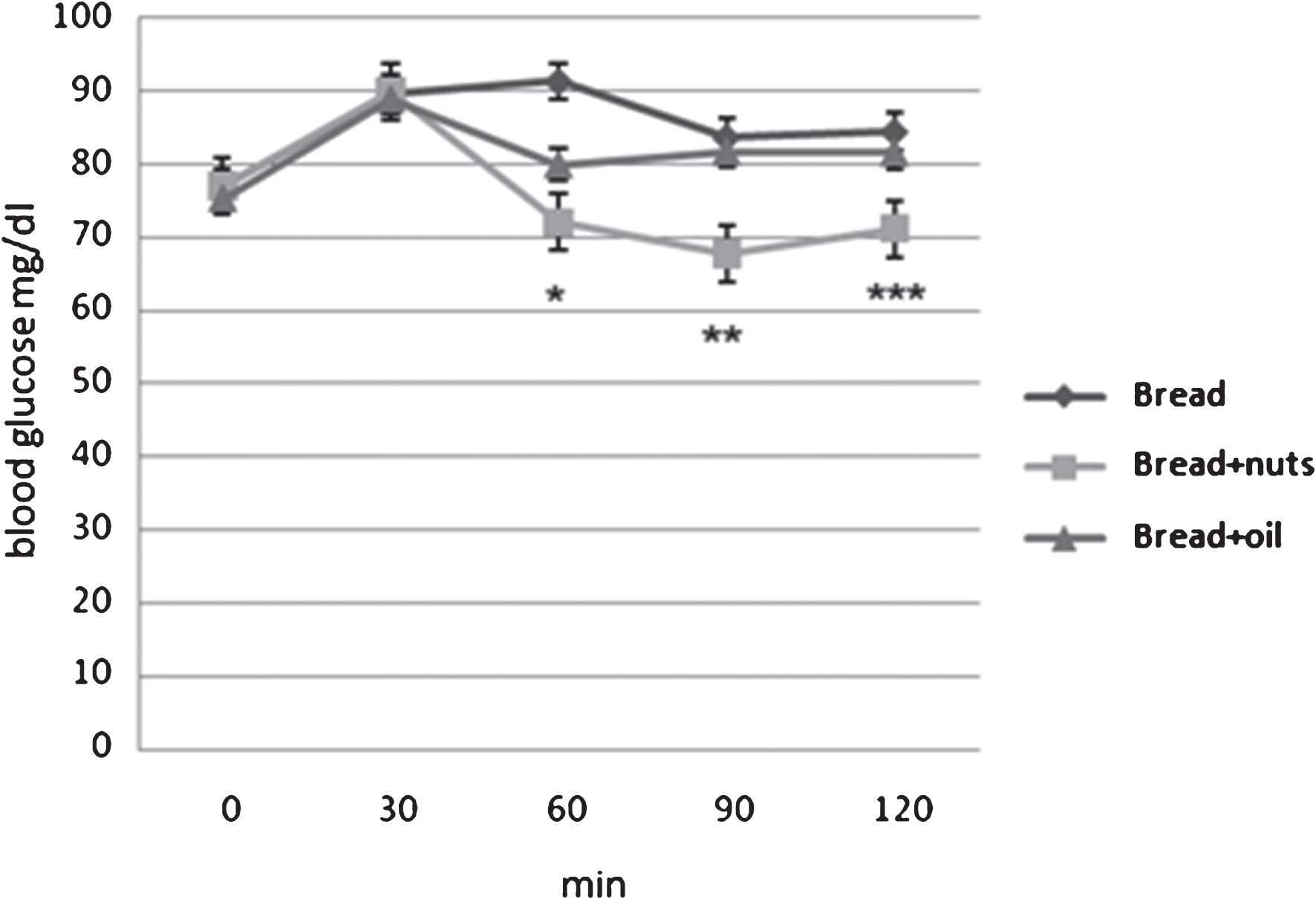
The addition of nuts has reduced the glycemic response to bread. Figure 1 shows the variations in time (min) of glycemia mean levels (mg/dl) after the three meals. The behavior of the glycemic response in the three test meals was similar and characterized by a temporary increase, followed by a decrease towards basal levels. The data obtained show at time t 60 a glycemic increase for the meal “Bread+nuts” (72±12 mg/dl) lower than the meal “Bread” (91.3±9.1 mg/dl) with p < 0.005, no significant differences with the meal “Bread+oil” (79.9±3.4 mg/dl). At time t90, the increase results significantly lower after the intake of the meal “Bread+nuts” (67.8±13.8 mg/dl) than the meals “Bread” (83.6±7.8 mg/dl) and “Bread+oil” (81.7±14.5 mg/dl) with p < 0.008. Also at time t120 the meal “Bread+nuts” (71.2±8.2 mg/dl) had a significant lower blood glucose response respect the two test meals “Bread” (84.4±10.25 mg/dl) and “Bread+oil” (81.6±16.16 mg/dl) with p < 0.05. No differences are reported between test meal Bread and Bread+oil.
Fig.1
Glycemic responses to the three meals. *Bread+nuts vs Bread p = 0.002. **Bread+nuts vs Bread, Bread+oil p = 0.014. ***Bread+nuts vs Bread, Bread+oil p = 0.05.
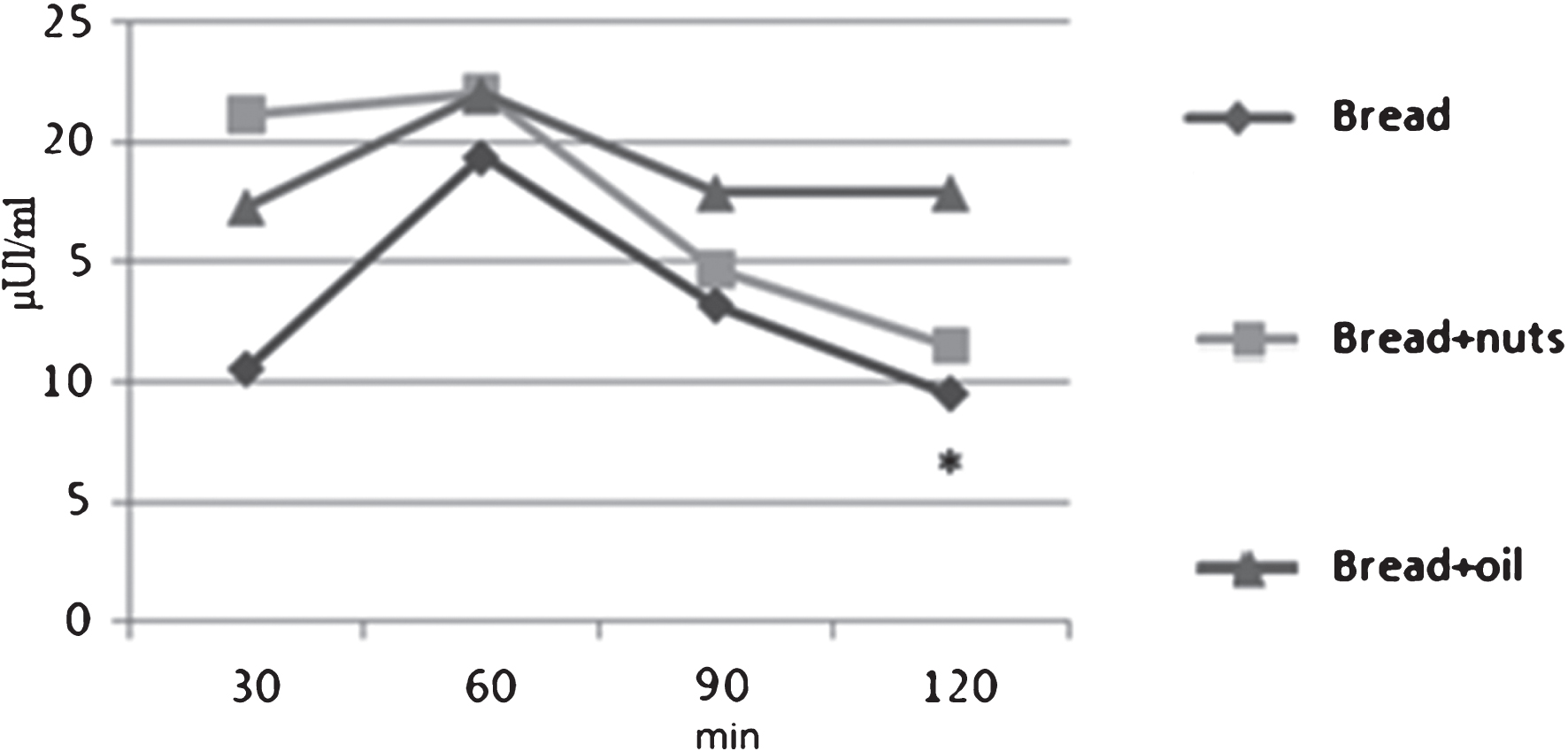
For postprandial incremental plasma insulin levels, it is possible to observe a significant difference at 120 min between the meal “Bread” (9.3±5.5 μUl/ml) and the meal “Bread+oil” (17.9±8.4 μUl/ml) with p = 0.03, with no differences between bread and bread+nut. (Fig. 2).
Fig.2
Incremental insulinemic response to the three meals. *Bread vs Bread+oil p = 0.03.
No significant differences are observed between incremental insulin AUC to the Bread (1322.4±483.2) μUl/ml(min); Bread + nut (1592.6±597) μUl/ml(min); Bread + oil (1455.7±833.1) μUl/ml(min).
Results show a more rapid recurrence of hunger after the intake of test meal of bread and bread+oil, and a slower recurrence after the meal containing Sorrento nuts.
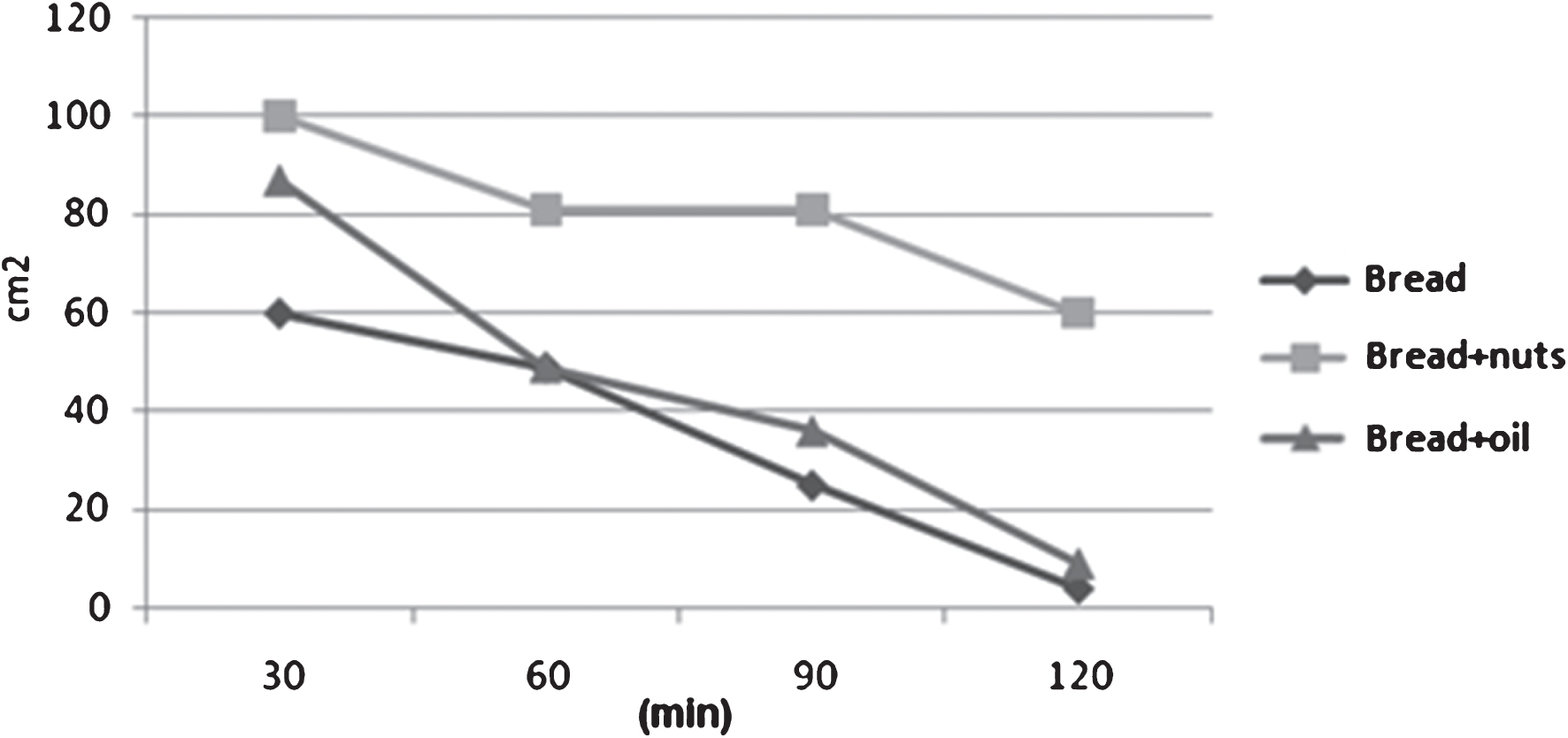
The variation in the time of satiety sensation after the different meals presents only in the first phases of postprandial period a stronger fullness sensation for the meal “Bread+nuts” (Figs. 3-4).
Fig.3
Hunger sensation after the three different meals.
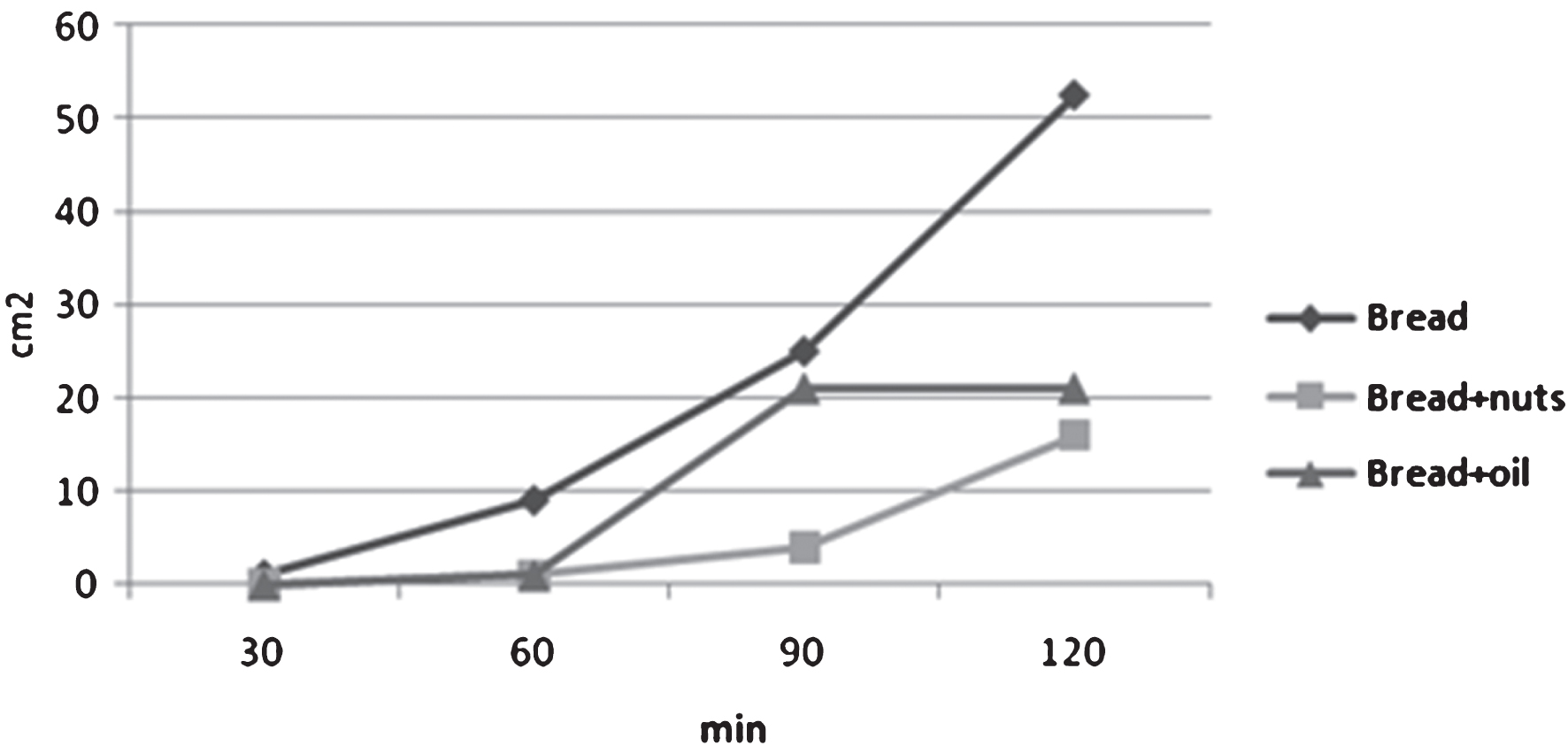
Fig.4
Variation in the time of the satiety sensation after the three meals.
The assessment of the glycemic index calculated as the incremental area under the glycemic response curve after the intake of bread+nut and bread+oil shows a lower value (43%) for the meal bread+nuts than meal bread+oil (Table 2).
Table 2
Incremental blood glucose area and Glycemic index
| “Bread” | “Bread+Nuts” | “Bread+Oil” | |
| Glucose AUC mg×min/dl | 1130±334 | 350±60 | 832±104 |
| Glycemic index | 100 | 30.9 | 73.7 |
4Discussion
This study evaluates the acute effects of nuts on postprandial blood glucose and satiety. The consumption of ”bread+nuts” resulted in a lower postprandial glycemic response and greater satiety. Nuts are one of the natural plant foods richest in fat. However, the fatty acid composition of nuts is beneficial because the saturated fatty acid (SFA) content is low and nearly half of the total fat content is polyunsaturated fat and monounsaturated fatty acids MUFA (oleic acid) [12]. With regard to walnuts, it must be underlined that they have the highest content in linolenic acid of all edible plants [18]. Nuts are also an excellent source of protein (approximately 25% of energy) and are rich sources of flavonoids, and L-arginine [19]. As this aminoacid is the precursor of the endogenous vasodilator, nitric oxide (NO) [20], nut intake might help improve vascular reactivity. Nuts also are a good source of dietary fiber, which ranges from 4 to 11 g per 100 g of nuts, and in standard servings provide 5–10% of daily fiber requirements [21].
Among the constituents of nuts there are significant amounts of essential micronutrients that are associated with an improved health status when consumed at doses beyond those necessary to prevent deficiency states. Nuts contain sizeable amounts of folate [18, 22], a B-vitamin necessary for normal cellular function that plays an important role in detoxifying homocysteine [18, 23]. Nuts are also rich sources of phytates, antioxidant vitamins (e.g., tocopherols) and phenolic compounds, necessary to protect the germ from oxidative stress and preserve the reproductive potential of the seed, but also bioavailable after consumption and capable of providing a significant antioxidant load [23].
Four prospective studies conducted in the US have reported a beneficial effect of nut consumption on CHD incidence after follow-ups ranging from six to 18 years of large cohorts of previously healthy subjects [9–26]. A pooled analysis of these studies shows that subjects in the highest intake group for nut consumption had a 37% reduction in multivariable-adjusted risk of fatal CHD [26]. Importantly, a dose-response relationship between nut consumption and reduced CHD mortality rates was reported for all four studies, strengthening the causal link. Of particular note are the results of the Physicians’ Health Study [25], where the inverse association between nut consumption and total CHD mortality was primarily due to a reduction in sudden cardiac death.
In addition to cardiac outcomes, the Nurses’ Health Study also ascertained the incidence of type-2 diabetes, a major risk factor for CHD, by frequency of nut and peanut butter intake during a 16-year follow-up [27]. Nut consumption was inversely associated with risk of type-2 diabetes. Considering only lean women (BMI <25 kg/m2), a 45% risk reduction was observed in those consuming nuts five times or more per week. Consumption of peanut butter was also inversely associated with type-2 diabetes in women consuming peanut butter more than four times a week (equivalent to ≥15 ounce of peanuts per week) compared with those who never or almost never ate peanut butter.
In the present study, nuts have been shown to reduce the glycemic response to a meal, even when compared to one balanced for energy and macronutrient profile (bread+olive oil). This may relate to high unsaturated fat content of nuts, and their unique physical structure. Nuts could improve the release of the incretin hormone GLP-1 as proposed by Cassady who postulated that the high unsaturated fat and protein content of nuts would stimulate GLP-1 secretion [28].
More than 30 years ago, Hunt and Stubbs [29] observed that there was equal slowing in the gastric emptying of isoenergetic amounts of fat, protein, and carbohydrate, and indicated that added energy and not the type of macronutrient was what determined gastric emptying. In 2004, Westphal et al [30] reported a positive correlation between increased energy and gastric-emptying time after feeding on meals that differed in macronutrient and energy content. In our study, the meals “bread+nuts” and “bread+oil” are isoenergetic, therefore another mechanism must be sought for the different glycemic responses. Nuts are rich in phytates and quercetin and Lo Piparo et al. [31] demonstrated that flavonoids bind to the active site of amylase, exerting varyingdegrees of enzyme inhibition. Based on in vitro studies, the inhibitory action of phytate was considered to result from the binding of calcium required as a cofactor for amylase enzyme activity. Nuts are also rich in fiber and magnesium and, in several clinical studies, high-fiber diets decreased insulin demand among patients with type 2 diabetes [32, 33].
Satiety basically means how satisfied a person feels after eating a certain food, and how long it makes him feel full. Knowing which foods have a high satiety factor can have obvious advantages to people who want to lose weight. With a few exceptions, human feeding trials have shown that nut ingestion moderates appetite postprandial. Specifically, the inclusion of almonds and peanuts suppresses hunger and desire to eat and increases fullness ratings after ingestion. Our data confirm these observations. The underlying mechanisms for the appetitive effects of nut consumption are not well understood due to a paucity of studies on the issue.
In conclusion, these data demonstrate that the ingestion of Sorrento nuts lowered postprandial glycemic by 43% in healthy individuals and increased satiety.
Conflict of interest
None to report.
Acknowledgments
We would like to thank M.D’Anna and G. Bucciero for their kind collaboration. We thank all partecipants for their kind participation in the study.
References
[1] | Akbar DH . Sub-optimal postprandial blood glucose level in diabetics attending the outpatient clinic of a University Hospital. Saudi Med J. (2003) ;24: (10):1109–12. |
[2] | Bonora E , Corrao G , Bagnardi V , Ceriello A , Comaschi M , Montanari P , Meigs JB . Prevalence and correlates of postprandial hyperglycaemia in a large sample of patients with type 2 diabetes mellitus. Diabetologia. (2006) ;49: (5):846–54. |
[3] | Erlinger TP , Brancati FL . Post challenge hyperglycemia in a national sample of U.S. adults with type 2 diabetes. Diabetes Care. (2001) ;24: (10):1734–8. |
[4] | Liu S , Willett WC , Stampfer MJ , Hu FB , Franz M , Sampson L , Hennekens CH , Manson JE . A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J ClinNutr. (2000) ;71: (6):1455–61. |
[5] | de Vegt F , Dekker JM , Ruhé HG , Stehouwer CD , Nijpels G , Bouter LM , Heine RJ . Hyperglycaemia is associated with all-cause andcardiovascular mortality in the Hoorn population: The Hoorn Study. Diabetologia. (1999) ;42: (8):926–31. |
[6] | Hanefeld M , Fischer S , Julius U , Schulze J , Schwanebeck U , Schmechel H , Ziegelasch HJ , Lindner J . Risk factors for myocardialinfarction and death in newly detected NIDDM: The DiabetesIntervention Study, 11-year follow-uP. Diabetologia. (1996) ;39: (12):1577–83. |
[7] | Kendall CW , Josse AR , Esfahani A , Jenkins DJ . Nuts, metabolic syndrome and diabetes. Br J Nutr. (2010) ;104: (4):465–73. |
[8] | Kendall CW , Esfahani A , Josse AR , Augustin LS , Vidgen E , Jenkins DJ . The glycemic effect of nut-enriched meals in healthy and diabetic subjects. NutrMetabCardiovasc Dis. (2011) ;21: (Suppl 1):S34–9. |
[9] | Fraser GE , Sabaté J , Beeson WL , Strahan TM . A possibleprotective effect of nut consumption on risk of coronary heartdisease. The Adventist Health Study. Arch Intern Med. (1992) ;152: (7):1416–24. |
[10] | Sabaté J , Fraser GE , Burke K , Knutsen SF , Bennett H , Lindsted KD . Effects of walnuts on serum lipid levels and blood pressure innormal men. N Engl J Med. (1993) ;328: (9):603–7. |
[11] | Sabaté J , Ang Y . Nuts and health outcomes: New epidemiologicevidence. Am J Clin Nutr. (2009) ;89: (5):1643S–8. |
[12] | Ros E , Mataix J . Fatty acid composition of nuts–implications for cardiovascular health. Br J Nutr. (2006) ;96: (Suppl 2):S29–35. |
[13] | Mukuddem-Petersen J , Oosthuizen W , Jerling JC . A systematic review of the effects of nuts on blood lipid profiles in humans. J Nutr. (2005) ;135: (9):2082–9. |
[14] | Jenkins DJ , Kendall CW , Josse AR , Salvatore S , Brighenti F , Augustin LS , Ellis PR , Vidgen E , Rao AV . Almonds decrease postprandial glycemia, insulinemia, and oxidative damage in healthy individuals. J Nutr. (2006) ;136: (12):2987–92. |
[15] | Johnston CS , Buller AJ . Vinegar and peanut products as complementary foods to reduce postprandial glycemia. J Am Diet Assoc. (2005) ;105: (12):1939–42. |
[16] | Josse AR , Kendall CW , Augustin LS , Ellis PR , Jenkins DJ . Almonds and postprandial glycemia–a dose-response study. Metabolism. (2007) ;56: (3):400–4. |
[17] | Porrini M , Crovetti R , Testolin G , Silva S . Evaluation of satietysensations and food intake after different preloads. Appetite. (1995) ;25: :17–30. |
[18] | Hepburn FN , Exler J , Weihrauch JL . Provisional tables on the content of omega-3 fatty acids and other fat components of selected foods. J Am Diet Assoc. (1986) ;86: (6):788–93. |
[19] | Brufau G , Boatella J , Rafecas M . Nuts: Source of energy and macronutrients. Br J Nutr. (2006) ;96: (Suppl 2):S24–8. |
[20] | Huynh NN , Chin-Dusting J . Amino acids, arginase and nitric oxide in vascular health. Clin Exp Pharmacol Physiol. (2006) ;33: (1-2):1–8. |
[21] | Salas-Salvadó J , Bulló M , Pérez-Heras A , Ros E . Dietaryfibre, nuts and cardiovascular diseases. Br J Nutr. (2006) ;96: (Suppl 2):S46–51. |
[22] | Welch GN , Loscalzo J . Homocysteine and atherothrombosis. N Engl J Med. (1998) ;338: (15):1042–50. |
[23] | Blomhoff R , Carlsen MH , Andersen LF , Jacobs DR Jr. . Health benefits of nuts: Potential role of antioxidants. Br J Nutr. (2006) ;96: (Suppl 2):S52–60. |
[24] | Hu FB , Stampfer MJ , Manson JE , Rimm EB , Colditz GA , Rosner BA , Speizer FE , Hennekens CH , Willett WC . Frequent nut consumption and risk of coronary heart disease in women: Prospective cohort study. BMJ. (1998) ;317: (7169):1341–5. |
[25] | Albert CM , Gaziano JM , Willett WC , Manson JE . Nut consumption and decreased risk of sudden cardiac death in the Physicians’ Health Study. Arch Intern Med. (2002) ;162: (12):1382–7. |
[26] | Kelly JH Jr , Sabaté J . Nuts and coronary heart disease: Anepidemiological perspective. Br J Nutr. (2006) ;96: (Suppl 2):S61–7. |
[27] | Jiang R , Manson JE , Stampfer MJ , Liu S , Willett WC , Hu FB . Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA. (2002) ;288: (20):2554–60. |
[28] | Cassady BA , Hollis JH , Fulford AD , Considine RV , Mattes RD . Mastication of almonds: Effects of lipid bioaccessibility, appetite, and hormone response. Am J Clin Nutr. (2009) ;89: (3):794–800. |
[29] | Hunt JN , Stubbs DF . The volume and energy content of meals as determinants of gastric emptying. J Physiol. (1975) ;245: (1):209–25. |
[30] | Westphal S , Kästner S , Taneva E , Leodolter A , Dierkes J , Luley C . Postprandial lipid and carbohydrate responses after the ingestion of a casein-enriched mixed meal. Am J ClinNutr. (2004) ;80: (2):284–90. |
[31] | Lo Piparo E , Scheib H , Frei N , Williamson G , Grigorov M , Chou CJ . Flavonoids for controlling starch digestion: Structural requirements for inhibiting human alpha-amylase. J Med Chem. (2008) ;51: (12):3555–61. |
[32] | Rivellese A , Riccardi G , Giacco A , et al. Effect of dietary fibre on glucose control and serum lipoproteins in diabetic patients. Lancet. (1980) ;2: :447–50. |
[33] | Anderson JW , Gustafson NJ , Bryant CA , Tietyen-Clark J . Dietary fiber and diabetes: Acomprehensive review and practical application. J Am Diet Assoc. (1987) ;87: :1189–97. |




