Date palm fruit extract attenuated oxidative stress induced by two haloacetic acids in Wistar rats
Abstract
BACKGROUND:
Date fruits are the most commonly used part of palms Phoenix dactylifera L. due to their richness in several nutrients and dietary antioxidants. These fruits are widely used in traditional medicine for treatment of various diseases and intoxication.
OBJECTIVE:
This study was undertaken to evaluate the protective effect of dates extract against dichloro (DCA) and trichloroacetic (TCA) acids-induced oxidative stress in blood rats.
METHODS:
Eighty male Wistar rats divided into ten equal groups were given the following treatment for 2 months: Two groups were controls, one treated with aqueous Degla extract (ADE) and other with saline (C). Two groups received 0.5 g/L of TCA or DCA and two other groups received 2 g/L of TCA or DCA in drinking water. Four groups treated with ADE and TCA or DCA at 0.5 and 2 g/L.
RESULTS:
DCA and TCA administration decreased significantly plasma glucose, hemoglobin, total protein, albumin, globulins, calcium and high density lipoprotein levels. While, they significantly increased the levels of plasmatic phosphorus, magnesium, triglyceride, cholesterol, low density lipoprotein and very low density lipoprotein. DCA and TCA treatment induced also significant increase in plasma lipid peroxidation levels and decrease in catalase and glutathione peroxidase activities. In contrast pretreatment with the ADE restored all the parameters cited above to near-normal values.
CONCLUSIONS:
Our investigation revealed that the ADE appeared to be a promising agent for protection against DCA and TCA toxicity.
1Introduction
During the past few years, a particular attention has been attributed to the use of natural plant products as antioxidant intervention to counteract the harmful effect of toxicant exposure. Date palm (phoenix dactylifera L.) is one of the oldest trees dating from 6000 years. The various parts of this plant are widely used in traditional medicine for the treatment of various disorders [1, 2]. Date fruits are the most commonly used part due to their richness in several nutrients (dietary fibers, sugars, vitamins, proteins, fat), beside to their dietary antioxidants. These fruits are consumed at any of the three major stages of maturity such as besser or khalal (fresh, hard ripe, color stage), rutab (crisp to succulent or ripe stage), or tamr (soft pliable, full ripe stage). The information accrued in the past four decades suggest that dates possess diverse medical uses including anti-hyperlipidemic, anti-cancer, gastroprotective, hepatoprotective and nephroprotective activities and thereby serving as an important healthy food in the human diet [1]. In other way, several animal studies have documented the beneficial effects of date fruits against chemically-induced oxidative stress [3–6]. These beneficial effects are attributed to their richness in phytochemical compounds; phenolic acids, sterols, carotenoids, anthocyanins, procyanidins and flavonoids. These compounds are characterized by a strong free radical-scavenging activity and inhibit lipid peroxidation and protein oxidation [7].
Large numbers of environment toxicants have been identified to have potential to generate free radicals in biological system. Dichloroacetic (DCA) and trichloroacetic (TCA) acids are two haloacetic acids identified among the most important toxic disinfectant by-products formed during water chlorination. These two compounds are also used in agriculture as herbicide and fungicide. Numerous studies have shown that these two haloacetates were detected in vegetables, fruits, and grains [8, 9], and can be taken up into foodstuffs from the cooking water [10]. Therefore, human exposure to these compounds can occur via food consumption. DCA and TCA are metabolized via reductive dechlorination pathways, catalyzed by Cytochrome P-450 enzymes, leading to the production of several free radicals that can contribute to the generation of reactive oxygen species (ROS) [11]. These ROS induced lipid peroxidation that causes oxidative damage to DNA, proteins and lipids [11, 12], and consequently causes organ toxicity.
In this study, aqueous date fruits extract, especially at the besser stage (fruit with yellow color), was preferred because this stage was found very rich in many polyphenolics compounds [13, 14] known by their efficacy in the protection from oxidative stress.
The aim of this study was to investigate the protective effect of the aqueous date extract on DCA and TCA- induced oxidative stress in rats’ blood parameters.
2Material and methods
2.1Aqueous date extract preparation
Degla cultivar was collected from the station of Souk Lahad (Kébili, south of Tunisia) at besser stage of maturation. The flesh was manually separated from the pits, crushed and soaked in cold distilled water (1 : 3 ratio, weight to volume) and kept for 48 hours at 4°C [5]. Then the mixture was centrifuged at 4°C for 20 min at 4000 g and the supernatant was collected. The proximate composition and the antioxidant activity of this extract were also summarized in Table 1.
Table 1
Proximate composition* of Degla date palm fruit and its aqueous extract at besser stage
| Component | Amount |
| Date fruit | |
| Carbohydratea | 20.90±0.04 |
| Proteinsb | 3.50±0.12 |
| Asha | 1.58±0.02 |
| Total lipidsa | 0.29±0.03 |
| Saturated fatty acidsc | 48.99±1.45 |
| Monounsaturated fatty acidsc | 40.00±2.15 |
| Polyunsaturated fatty acidsc | 11.00±0.70 |
| Aqueous date fruit extract | |
| Total Phenolicd | 417.71±1.59 |
| Total Flavonoidse | 285.23±1.48 |
| Condensed Tanninse | 73.65±0.30 |
| Polyphenolic compoundsf | |
| Gallic acid | 2.05±0.01 |
| Chlorogenic acid | 2.34±0.06 |
| Protocatechuic acid | 3.06±0.01 |
| Caffeic acid | 4.64±0.03 |
| Syringic acid | 3.02±0.01 |
| m-Hydroxybenzoic acid | 1.93±0.01 |
| Ferulic acid | 5.50±0.02 |
| p-Coumaric acid | 4.71±0.02 |
| m-Coumaric acid | 2.35±0.01 |
| o-Coumaric acid | 1.94±0.01 |
| Phenylacetic acid | 1.52±0.02 |
| Catechin | 2.48±0.02 |
| Antioxidant activity (DPPH (%)) | 89.14±1.09 |
*El Arem et al. (2014a). a% Dry weight. b% Fresh weight. c% of total fatty acid. dmilligrams gallic acid equivalents/100 g fresh weight (mg GAE/100 g FW). emilligrams catechin equivalents/100 g fresh weight (mg CE/100 g FW). fmilligrams/100 g fresh weight (mg/100 g FW).
2.2Experimental design
Eighty male Wistar rats weighing 180–200 g were housed under standard laboratory conditions with a 12-h light–dark cycle at constant temperature (22±2°C) and humidity (55±5%) and were fed on standard pellet diet and water adlibitum. The animals were handled according to the guidelines of the Tunisian Society for the Care and Use of Laboratory Animals, and were approved by the University of Tunisia Ethical Committee (approval number: FST/LNFP/Pro 152-012). After 2 weeks of acclimation, animals were divided into ten equal groups of eight each. The groups were assigned at random to one of the following treatments: group 1 served as control, group 2 received a daily oral dose (4 ml/kg) of aqueous date extract (ADE), groups 3 to 6 were orally treated with dichloroacetic acid at 0.5 g/l (DCAC1 : 33.78 mg/kg/day), DCA at 2 g/l (DCAC2 : 131.24 mg/kg/day), trichloroacetic acid at 0.5 g/l (TCAC1 : 36.40 mg/kg/day) and TCA at 2 g/l (TCAC2 : 138.26 mg/kg/day) as drinking water, respectively. These doses were based on previous studies that investigated the abilities of DCA and TCA to induce hepatotoxic and hepatocarcinogenic effects in mice and rats when administered at concentrations ranging from 1–5 g/l in the drinking water for 52–75 weeks [15–17]. Groups 7–8 and 9–10 received, respectively, ADE plus DCA at 0.5 or 2 g/l and ADE plus TCA at 0.5 or 2 g/l. All the animals were observed daily for the presence of clinical signs of toxicity during the two months of the study.
2.3Blood collections
After anesthetization of rats by inhalation of diethyl ether, blood was drawn by cardiac puncture and collected into evacuated tubes containing EDTA solution as anticoagulant. Some tubes were used for determination of hemoglobin using a colorimetric cyanomethemoglobin method according to Drabkin and Austin [18]. The remaining tubes were centrifuged (Hettich universal 320R centrifuge) at 4000 g for 15 min at 4°C, and then plasma samples were stored at –20°C in aliquots until analysis.
2.4Plasma biochemical estimation
The glucose, total protein and albumin concentrations of plasma were determined with commercial kits from Biomaghreb Laboratory (Ariana, Tunisia). Globulins concentration was determined as the difference between total protein and albumin. Plasma total bilirubin and plasma contents of triglyceride (TG), total cholesterol (TC), high density lipoprotein (HDL) and low density lipoprotein (LDL) were assayed with commercially available diagnostic kits supplied by Biomaghreb Laboratory (Ariana, Tunisia). Very low density lipoprotein (VLDL) was calculated by dividing the values of TG by a factor of five. Plasma calcium (Ca), phosphorus (P) and magnesium (Mg) levels were measured by spectrophotometer (Shimatzu, UV-1700) using commercial assay kits (Biomaghreb, Tunisia) according to the manufacturer’s directions.
2.5Oxidative stress parameters
2.5.1Plasma lipoperoxidation
Lipid peroxidation (LPO) was evaluated in terms of conjugated diene (CD) which is measured as described by Esterbauer et al. [19]. Results were expressed as μmol hydroperoxides/mg proteins using ɛ= 2.52×104 M–1 cm–1.
2.5.2Antioxidant status
Catalase activity (CAT) was measured according to the method of Aebi [20]. One unit of activity is equal to the μmol of H2O2 destroyed/min/mg proteins. Glutathione peroxidase (GPx) activity was assayed according to the method of Flohe and Gunzler [21]. The activity was expressed as μmol GSH oxidized/min/mg proteins.
2.6Statistical analysis
The data were analyzed using the Statistical Package for Social Sciences (SPSS) program, release 20.0 for Windows (SPSS, Chicago, IL, USA). In each assay, the experimental data represent the mean of eight independent assays±standard deviations. The results were analyzed by ANOVA in order to perform multiple comparisons. Duncan’ test was used to determine any significant differences between analytical parameters of treated and control groups. The statistical significance was set at p < 0.05.
3Results
3.1Biochemical parameters
The administration of DCA and TCA to rats at both concentrations of 0.5 and 2 g/l induced significant changes (p < 0.05) in plasma biochemical compositions as compared to control rats. Data represented in Fig. 1 and Table 2 show that treatment with DCA or TCA engender significant (p < 0.05) decrease of plasma glucose and haemoglobin concentrations as compared to control group. These decreases were more pronounced with the higher DCA concentration. Furthermore, the highest concentration of these two acids induced significant (p < 0.05) decrease of total protein and albumin concentrations and a significant increase of total bilirubin levels as compared to control group. However, only the rats treated with DCA at 2 g/l showed significant decrease in their plasma globulins levels. In addition, the pretreatment of DCA or TCA rats with the ADE maintained the levels of these biochemical parameters to the normal values compared to the control rats and those received ADE alone.
Fig.1
Effect of aqueous date extract on the plasma glucose levels of rats treated with DCA and TCA at 0.5 or 2 g/L. Data are reported as the mean±SD of eight animals in each group. Bars not sharing a common superscript letter (a–d) differ significantly at p < 0.05. C, control; ADE, aqueous date extract; DCAC1, dichloroacetic acid at 0.5 g/L; DCAC2, dichloroacetic acid at 2 g/L, TAC1, trichloroacetic acid at 0.5 g/L, TAC2, trichloroacetic acid at 2 g/L. *Significant difference (p < 0.05) compared to control group.
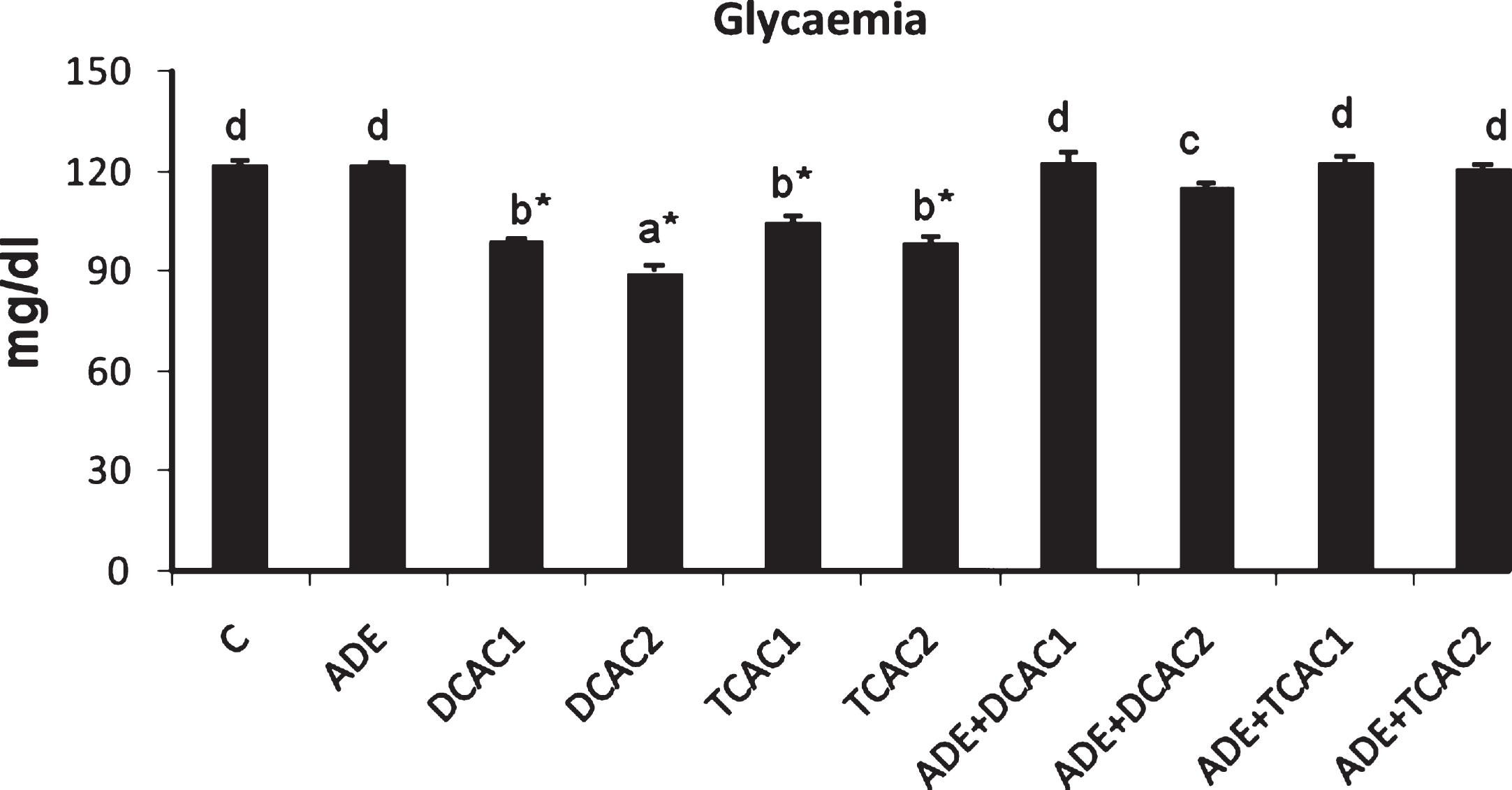
Table 2
Effect of the ADE on plasma hematological and biochemical parameters in rats treated with two different concentrations of DCA and TCA
| Total proteins | Albumin | Globulins | Total Bilirubin | Hemoglobin | |
| (g/dl) | (g/dl) | (g/dl) | (mg/ml) | (g/dl) | |
| C | 7.93±0.25d | 3.80±0.09b | 4.13±0.29b | 10.44±0.25a | 14.83±0.26e |
| ADE | 7.95±0.23d | 3.83±0.11b | 4.12±0.31b | 10.65±0.70a | 14.84±0.24e |
| DCAC1 | 7.04±0.19ab* | 3.59±0.03ab | 3.45±0.19ab | 11.91±0.39ab | 13.23±0.09bc* |
| DCAC2 | 6.60±0.24a* | 3.49±0.03a* | 3.11±0.22a* | 12.42±0.81ab* | 12.73±0.23ab* |
| TCAC1 | 7.32±0.29abcd | 3.66±0.06ab | 3.66±0.27ab | 11.48±0.52a | 13.61±0.34cd* |
| TCAC2 | 7.10±0.19abc* | 3.51±0.07a* | 3.59±0.22ab | 13.82±0.78b* | 12.01±0.32a* |
| ADE+DCAC1 | 7.81±0.14cd | 3.81±0.03b | 4.00±0.14b | 10.90±0.69a | 14.33±0.30de |
| ADE+DCAC2 | 7.52±0.20bcd | 3.78±0.04b | 3.74±0.20ab | 10.58±0.80a | 14.13±0.26de |
| ADE+TCAC1 | 7.98±0.23d | 3.83±0.13b | 4.15±0.26b | 10.56±0.90a | 14.73±0.19e |
| ADE+TCAC2 | 7.90±0.29d | 3.79±0.09b | 4.11±0.30b | 10.79±0.52a | 14.46±0.35e |
Data are expressed as means±SD (n = 8 rats per group). Values not sharing a common letter (a–e) differ significantly at p < 0.05. ADE: aqueous date extract, DCAC1: Dichloroacetic acid at 0.5 g/L, DCAC2: Dichloroacetic acid at 2 g/L, TAC1: Trichloroacetic acid at 0.5 g/L, TAC2: Trichloroacetic acid at 2 g/L. *Significant difference (p < 0.05) compared to control group.
The present data showed also that treatment with DCA or TCA induced significant (p < 0.05) change of plasma minerals levels (Table 3). This change results in reduction of calcium level in groups treated with both concentrations of DCA and the higher concentration of TCA as compared to control group. In contrary, groups received TCA at both concentrations and that received the higher concentration of DCA present significant (p < 0.05) increase in magnesium levels in comparison to control group. However, only animals received DCA or TCA at 2 g/l showed significant (p < 0.05) increase of phosphorus levels when compared to control animals. These perturbations of serum mineral levels were restored to normal values in rats pretreated with ADE.
Table 3
Effect of the ADE on plasma minerals levels in rats treated with two different concentrations of DCA and TCA
| Calcium | Phosphorus | Magnesium | |
| (mmol/l) | (mmol/l) | (mmol/l) | |
| C | 0.73±0.10c | 1.94±0.06a | 0.60±0.01c |
| ADE | 0.73±0.10c | 1.93±0.06a | 0.62±0.05c |
| DCAC1 | 0.63±0.05ab* | 2.05±0.08abc | 0.54±0.03b |
| DCAC2 | 0.58±0.05a* | 2.29±0.10bc* | 0.49±0.05a* |
| TCAC1 | 0.68±0.10bc | 1.97±0.10ab | 0.54±0.03b* |
| TCAC2 | 0.55±0.04a* | 2.35±0.13c* | 0.51±0.06a* |
| ADE+DCAC1 | 0.72±0.02c | 1.92±0.11a | 0.61±0.03c |
| ADE+DCAC2 | 0.70±0.02bc | 1.96±0.09ab | 0.58±0.03c |
| ADE+TCAC1 | 0.73±0.05c | 1.89±0.09a | 0.63±0.02c |
| ADE+TCAC2 | 0.68±0.06bc | 1.98±0.08ab | 0.60±0.03c |
Data are expressed as means±SD (n = 8 rats per group). Values not sharing a common letter (a–c) differ significantly at p < 0.05. ADE: aqueous date extract, DCAC1: Dichloroacetic acid at 0.5 g/L, DCAC2: Dichloroacetic acid at 2 g/L, TAC1: Trichloroacetic acid at 0.5 g/L, TAC2: Trichloroacetic acid at 2 g/L. *Significant difference (p < 0.05) compared to control group.
Furthermore, the present study showed that DCA and TCA induced significant (p < 0.05) increase of plasma triglyceride and very low density lipoprotein concentrations in groups treated with both concentrations of these haloacids. The cholesterol and low density lipoprotein concentrations were increased in groups receiving the highest concentration of DCA and TCA, while high density lipoproteins level was significantly decreased (p < 0.05) only in group treated with the concentration of 2 g/l of DCA or TCA compared to control group (Table 4). Results revealed that there were no significant changes in the lipid profile of rats treated with ADE alone, while the presence of ADE with DCA or TCA could alleviate the adverse effects of these two compounds.
Table 4
Effect of the ADE on plasma lipid and lipoprotein (mmol/L) profiles of rats treated with two different concentrations of DCA and TCA
| Triglycerides | Cholesterols | HDL-C | LDL-C | VLDL-C | |
| C | 0.56±0.007a | 2.46±0.21a | 0.91±0.24c | 1.30±0.19a | 0.11±0.01a |
| ADE | 0.56±0.07a | 2.45±0.40a | 0.92±0.20c | 1.28±0.45a | 0.11±0.01a |
| DCAC1 | 0.89±0.14c* | 3.01±0.57a | 0.77±0.15abc | 1.83±0.60ab | 0.19±0.01c* |
| DCAC2 | 1.24±0.15d* | 4.23±0.48b* | 0.62±0.22a* | 3.04±0.71c* | 0.25±0.01d* |
| TCAC1 | 0.84±0.08bc* | 2.93±0.60a | 0.84±0.17bc | 1.70±0.71ab | 0.17±0.02bc* |
| TCAC2 | 1.15±0.25d* | 3.24±0.42a* | 0.68±0.25ab* | 2.03±0.49b* | 0.23±0.01d* |
| ADE+DCAC1 | 0.57±0.04a | 2.50±0.18a | 0.93±0.05c | 1.32±0.11a | 0.11±0.01a |
| ADE+DCAC2 | 0.69±0.13ab | 2.90±0.76a | 0.89±0.09c | 1.69±0.63ab | 0.14±0.01ab |
| ADE+TCAC1 | 0.57±0.12a | 2.45±0.95a | 0.93±0.20c | 1.26±0.74a | 0.11±0.01a |
| ADE+TCAC2 | 0.63±0.01a | 2.63±0.25a | 0.90±0.09c | 1.43±0.32ab | 0.12±0.02a |
Data are expressed as means±SD (n = 8 rats per group). Values not sharing a common letter (a–d) differ significantly at p < 0.05. ADE: aqueous date extract, DCAC1: Dichloroacetic acid at 0.5 g/L, DCAC2: Dichloroacetic acid at 2 g/L, TAC1: Trichloroacetic acid at 0.5 g/L, TAC2: Trichloroacetic acid at 2 g/L. *Significant difference (p < 0.05) compared to control group.
3.2Estimation of lipid peroxidation and the enzymatic antioxidant activity
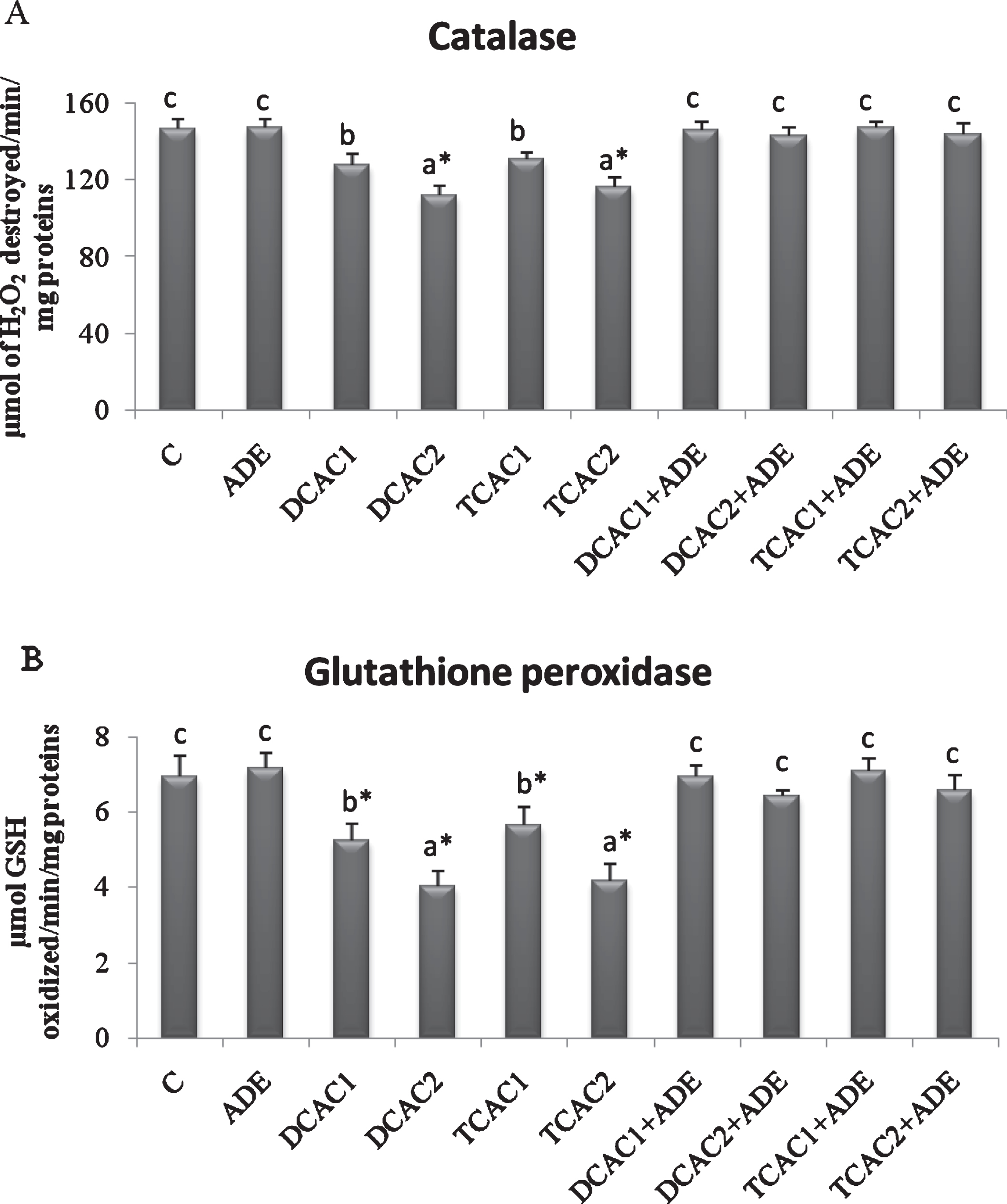
The effect of subchronic exposure to DCA or TCA and the treatment with ADE on the rats’ plasma lipid peroxidation, and CAT and GPx antioxidant activities are shown in Figs. 2 and 3. At Both concentrations, DCA and TCA decreased significantly (p < 0.05) CAT activity. While, only at the higher concentration (2 g/l) DCA and TCA increased significantly (p < 0.05) the plasma lipid peroxidation, as indicated by the higher amount of CD, and decreased GPx activity as compared to control group. However, pretreatment with the ADE of DCA and TCA treated groups significantly reduced CD and increased CAT and GPx activities in comparison to DCA and TCA groups (Fig. 2).
Fig.2
Effect of aqueous date extract on the plasma diene conjugated levels of rats treated with DCA and TCA at 0.5 or 2 g/L. Data are reported as the mean±SD of eight animals in each group. Bars not sharing a common superscript letter (a–c) differ significantly at p < 0.05. C, control; ADE, aqueous date extract; DCAC1, dichloroacetic acid at 0.5 g/L; DCAC2, dichloroacetic acid at 2 g/L, TAC1, trichloroacetic acid at 0.5 g/L, TAC2, trichloroacetic acid at 2 g/L. *Significant difference (p < 0.05) compared to control group.
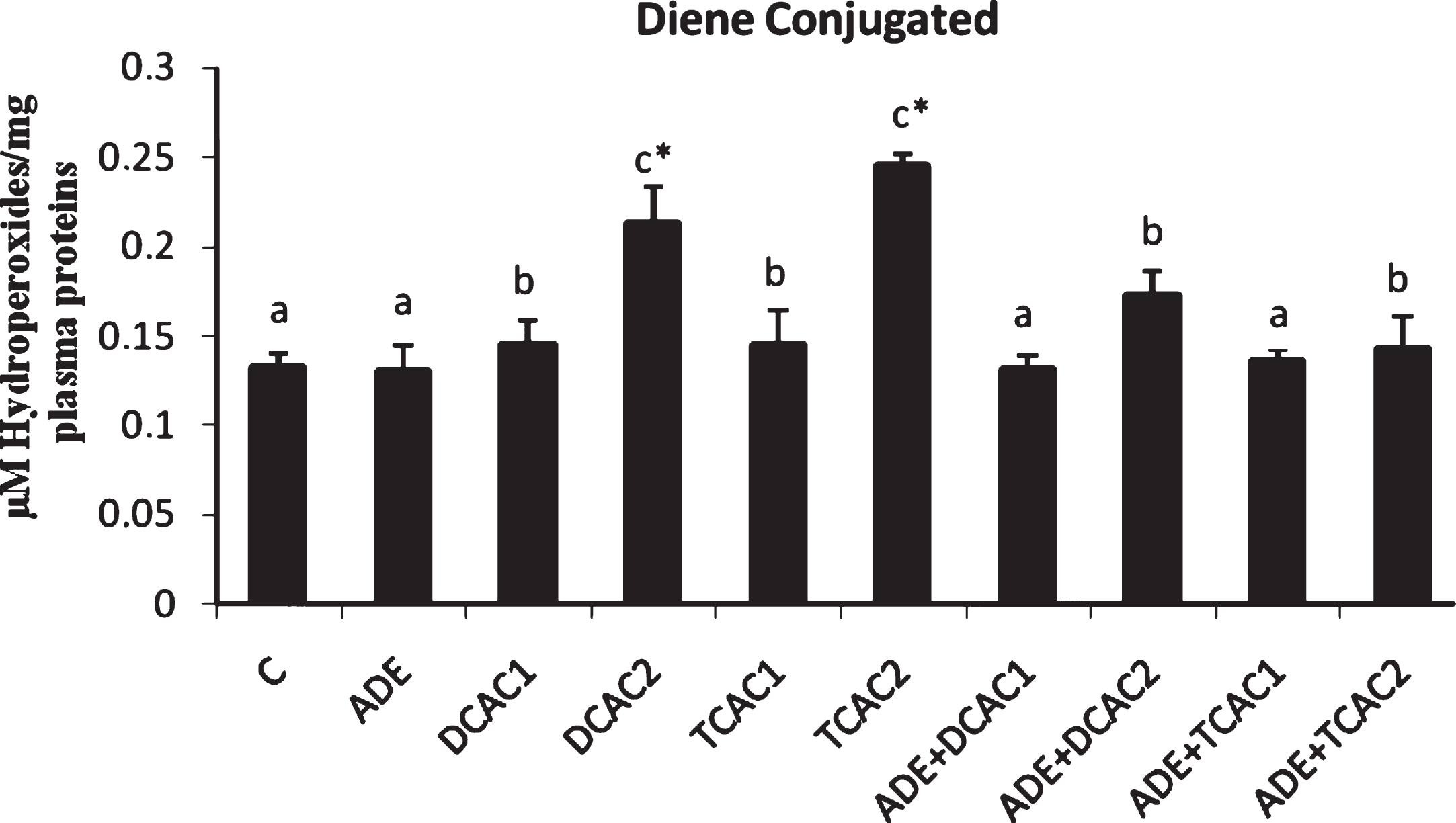
Fig.3
Effect of aqueous date extract on the plasma antioxidant activity of catalase (A) and glutathione peroxidase (B) of rats treated with DCA and TCA at 0.5 or 2 g/L. Data are reported as the mean±SD of eight animals in each group. Bars not sharing a common superscript letter (a–c) differ significantly at p < 0.05. C, control; ADE, aqueous date extract; DCAC1, dichloroacetic acid at 0.5 g/L; DCAC2, dichloroacetic acid at 2 g/L, TAC1, trichloroacetic acid at 0.5 g/L, TAC2, trichloroacetic acid at 2 g/L. *Significant difference (p < 0.05) compared to control group.
4Discussion
The alteration in plasma biochemical parameters and lipid peroxidation level are indicative of organs dysfunction. Significant restoration of these parameters was observed in animals pre-treated with ADE offering protection against DCA and TCA toxicity in rats.
Blood glucose concentration is known to depend on the ability of the liver to absorb or produce glucose. The liver performs its glucostatic function owing to its ability to synthesize or degrade glycogen according to the needs of the organism, as well as via gluconeogenesis [22]. The decline of blood glucose level indicates that rats became hypoglycemic due to DCA and TCA intoxications, and this effect was more marked with DCA treatment. In fact, the action of DCA on carbohydrate metabolites may be attributed to an increased utilization of pyruvate through activation of the pyruvate dehydrogenase resulting in a decrease in the release of gluconeogenic precursors from peripheral tissues [23]. In the same way, the obtained result showed that TCA has effect similar to that of DCA which can suggest that TCA can affect intermediary metabolism. Similar results are found by Davis [24] in female rats treated with TCA and those reported by previous studies using other xenobiotics [3, 25]. This hypoglycemia was corrected with the ADE that may be attributed to their high content of sugar, since about 80–85% of the sugar is sucrose at the besser stage of maturation, which can provide rapid energy [26]. Additionally, the corrective effect of ADE may be mediated through stimulating insulin synthesis and/or increasing secretion of pancreatic β cells of Langerhans [25, 27] that could be attributed to its richness on polyphenolic compounds [28]. Similar results were found by Ahmed et al. [3] in thioacetamide intoxicated rats.
Our study indicated also that administration of DCA and TCA to rats reduced significantly plasma total protein, albumin and globulins levels. The reduction in plasma total protein, particularly albumin, could be attributed to changes in protein and free amino acid metabolism and their synthesis in the liver [29]. Moreover, the observed declines in total protein level could be attributed in part to increased renal protein loss or in other part to the damaging effect of DCA and TCA on liver cells, as confirmed by the increased activities of AST and ALT in plasma as demonstrated in ours previous findings [5, 6]. Our results are in accord with those reported by previous studies [30, 31]. The pretreatment with the ADE increased these parameters to control values. This effect of ADE could be associated with an amelioration of liver function and regulation of protein biosynthesis attributed principally to their content of essential amino acids with nutritional and healthy values [31]. It was revealed that proteins and amino acids are very important nutrients and they play a major role in the synthesis of microsomal detoxifying enzymes which help detoxify toxicants that enter into the animal’sbody.
In agreement with previous studies [30, 33, 34] we have found that TCA and DCA increased plasma bilirubin level. It have been shown that hyperbilirubinemia is a very sensitive test to substantiate the functional integrity of the liver and severity of necrosis which increases the binding, conjugation and excretory capacity of hepatocytes that is proportional to the erythrocyte degeneration rate [35]. This hyperbilirubinemia was significantly decreased in ADE pretreated groups indicating that bilirubin was rapidly and selectively taken up into the liver as a function of healthy hepatocytes membrane maintained with the ADE.
The significant decreases in hemoglobin level in DCA and TCA treated groups observed in this study might be due to the inhibitory effect of these two compounds of many steps of heme biosynthesis and glycolysis in rats, as the result of haloacetates exposure [30, 36]. Interestingly, the ADE normalized the hemoglobin level that might be associated in part to their minerals content especially iron, carry 2 mg/100 g dry weight of fruits [37], that being a component of hemoglobin inside the red blood cells, determines the oxygen-carrying capacity of the blood. Additionally to their ascorbic acid content that plays an important role in iron absorption and its transport. Increases in hemoglobin concentration further support the use of dates in folk medicine against anemia [38]. Furthermore, the ADE normalized the plasma metal levels showing their ability to stabilize membrane permeability that was associated with cellular injury [39].
The enhancement of plasma triglyceride level in DCA and TCA treated groups might be a result of an imbalance between the rate of synthesis and the rate of release of triglyceride by the parenchyma cells into the systemic circulation [40]. In addition, the significant increase in total cholesterol, triglyceride and LDL, and the significant decrease in HDL in the intoxicated rats may indicate hepatopathy, cardiac damage as well as renal failure, which could be probably due to free radical-induced oxidative damage. Indeed, DCA has been shown as a noncompetitive inhibitor of the rate-limiting microsomal enzyme in cholesterol biosynthesis, hydroxymethylglutaryl CoA reductase [41]. Similarly, Acharya et al. [42] have shown that TCA induced rise in rats’ serum cholesterol and triglyceride levels. These authors suggested that the lipid profile changes was consistent with the onset of hepatomegaly, which would increase the energy demands of the liver and activate succinate dehydrogenase, leading to increased oxidative phosphorylation and mobilization of lipids. Many studies have shown the lipids lowering effects of ADE [27, 43, 44]. These effects may be explained by different mechanisms: First, date fruits contain very low amounts of fat. Second, date fruits contain an appreciate amount of dietary fiber (6.8%), which may have significant hypocholesterolemic activity by reducing the absorption of cholesterol and reabsorption of bile acids in the intestinal lumen or by producing, after fermentation, a series of short-chain fatty acids that may inhibit hepatic cholesterol biosynthesis. In fact, the effect of fiber on lowering-cholesterol level was proved in previous study conducted in healthy subjects supplemented with strawberries [45]. Third, the phytochemicals such as phytosterols and phytoestrogens present in date fruits might significantly reduced hypercholesterolemia [38]. Moreover, secondary plant metabolites such as flavonoids, saponins and polyphenolics from polar extracts of date fruits may be responsible for this antihyperlipidemic activity. In fact, flavonoid compounds may augment the activity of lecithin acyl transferase (LCAT), which regulates blood lipids. LCAT plays a key role in the incorporation of free cholesterol into HDL and transferring it back to VLDL and LDL which are taken back later in liver cells [44]. Furthermore, due do their phytochemical compositions the ADE may normalize the triglycerides levels by increasing the endothelium bound lipoprotein lipase activity that hydrolyses the triglyceride into fatty acids [44]. In fact, Eid et al. [14] showed that date fruits contain important amount of proanthocyanidins at besser stage, and the lowering effect of proanthocyanidins in triglyceride, total cholesterol, LDL-cholesterol and enhancement of HDL-cholesterol in animal studies has been demonstrated [46, 47]. Additionally, the ability of proanthocyanidins to inhibit the expression of 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase, HMG-CoA synthetase and other enzymes involved in cholesterol biosynthesis has been demonstrated [48]. Additionally date fruits contain vitamin C that was known as a strong inhibitor of LDL oxidation, a recognized factor in the pathogenesis and progression of human atherosclerosis, a scavenger of free radicals and other reactive species, and prevent their interaction to oxidize LDL [45, 49].
The antioxidants enzymes (SOD, GPx and CAT) limit the effects of oxidant molecules on tissues and are active in the defense against oxidative cell injury by means of their being free radical scavengers. These enzymes work together to eliminate ROS where small deviations in physiological concentrations may have a dramatic effect on the resistance of cellular lipids, proteins and DNA to oxidative damage. Lipid peroxidation has been suggested as one of the molecular mechanisms involved in organochlorine, carbamate or organophosphorous pesticide [50]. Among the variety of reactions that characterize the oxidative damage of lipid molecules, the unstaurations rearrangement forming conjugated dienes is an accepted indicator of the propagative phase associated with the free radical–induced lipid peroxidation [51]. Our results showed an important increase of conjugated dienes in plasma of groups treated specially with the highest concentration of DCA and TCA, indicating lipid peroxidation resulting from exposure to DCA and TCA. Several in vitro and in vivo studies have shown the capability of date fruit extract to scavenge superoxide and hydroxyl radicals and also to inhibit lipid peroxidation and proteins oxidation [38, 52]. In our study the amelioration of the CAT and GPx activities and the preventive effect of the ADE against the formation of diene conjugate radical could be attributed to their richness in vitamins, especially the water soluble vitamins (B-complex and C). These vitamins act as co-enzymes to assist the working of every cell in our body [32]. Furthermore, the protective effect of ADE may be attributed to their richness in antioxidant compounds such as carotenoids, phenolic acids, proanthocyanidins, catechin, flavonoids, glutathione and tocopherols [38, 52]. It was demonstrated that flavonoids can be incorporated in cells plasma membranes, which becomes more ordered and therefore enhances their stability. The localization of flavonoids in the plasma membranes could strictly hinder the diffusion of free radicals, and thereby decreases resulting damage [53]. Recently, polyphenols have been shown to offer an indirect antioxidant protection by activating endogenous defense systems, mainly by modulating the expression of some antioxidant enzyme genes [54]. In fact, the coordination of the endogenous and exogenous antioxidant response is achieved, at least in part, through antioxidant responsive elements, which are found in the promoters of many of the chemical- and oxidative stress-induced genes [54]. In particular, polyphenols have been shown to activate the AMPK pathway that leads to the modulation of Nfr2, a transcription factor that controls many genes involved in antioxidant defense [54–56].
5Conclusion
From the present results, it can be concluded that exposure of animals to DCA and TCA is capable of inducing marked hazardous alterations in lipid peroxidation, antioxidant enzymes activity and some biochemical parameters marker of organs dysfunction. The aqueous date extract showed their capability to alleviate the harmful effect of these two compounds. Further studies are needed to explore the potential protective effect of date fruits in other organs and to understand the mechanism of action of the active compounds of these fruits.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Acknowledgments
The authors would like to thank Mr. Yahia SALAH for his assistance.
References
[1] | Baliga MS , Baliga BRV , Kandathil SM , Bhat HP , Vayalil PK . A review of the chemistry and pharmacology of the date fruits (Phoenix dactylifera L.). Food Res Int. (2011) ;44: (7):1812–22. |
[2] | Rahmani AH , Aly SM , Ali H , Babiker AY , Srikar S , Khan AA . Therapeutic effects of date fruits (Phoenix dactylifera) in the prevention of diseases via modulation of anti-inflammatory, anti-oxidant and anti-tumour activity. Int J Clin Exp Med. (2014) ;7: (3):483–91. |
[3] | Ahmed MB , Hasona NA , Selemain HA . Protective effects of extract from dates (Phoenix Datylifera L.) and ascorbic acid on thioacetamide induced hepatotoxicity in rats. Iran J Pharm Res. (2008) ;7: (3):193–201. |
[4] | Saafi EB , Louedi M , Zakhama AF , Najjard MF , Hammami M , Achour L . Protective effect of date palm fruit extract (phoenix dactylifera L. ) on dimethoate induced-oxidative stress in rat liver. Exp Toxicol Pathol. (2011) ;63: (5):433–41. |
[5] | El Arem A , Ghrairi F , Lahouar L , Thouri A , Saafi EB , Ayed A , et al. Hepatoprotective activity of date fruit extracts against dichloroacetic acid-induced liver damage in rats. J Funct Foods. (2014) ;9: :119–30. |
[6] | El Arem A , Saafi EB , Ghrairi F , Thouri A , Zekri M , Ayed A , et al. Aqueous date fruit extract protects against lipid peroxidation and improves antioxidant status in the liver of rats subchronically exposed to Trichloroacetic acid. J Physiol Biochem. (2014) ;70: (2):451–64. |
[7] | Scalbert A , Manach C , Morand C , Remesy C . Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. (2005) ;45: (4):287–306. |
[8] | Reimann S , Grob K , Frank H . Chloroacetic acids in rainwater. Environ Sci Technol. (1996) ;30: (7):2340–4. |
[9] | Raymer J , Pellizari E , Childs B , Briggs K , Shoemaker J . Analytical methods for water disinfection byproducts in foods and beverages. J Expo Anal Environ Epidemiol. (2000) ;10: (6):808–15. |
[10] | Wu WW , Benjamin MM , Korshin GV . Effects of thermal treatment on halogenated disinfection byproducts in drinking water. Water Res. (2001) ;35: (15):3545–50. |
[11] | Larson JL , Bull RJ . Metabolism and lipoperoxidative activity of trichloroacetate and dichloroacetate in rats and mice. Toxicol Appl Pharmacol. (1992) ;115: (2):268–77. |
[12] | Austin EW , Parrish JM , Kinder DH , Bull RJ . Lipid peroxidation and formation of 8-hydroxydeoxyguanosine from acute doses of halogenated acetic acids. Fund Appl Toxicol. (1996) ;31: (1):77–82. |
[13] | El Arem A , Saafi EB , Mechri B , Lahouar L , Issaoui M , Hammami M , et al. Effects of the ripening stage on phenolic profile, phytochemical composition and antioxidant activity of date palm fruit. J Agric Food Chem. (2012) ;60: (44):10896–902. |
[14] | Eid NMS , Al-Awadi B , Vauzour D , Oruna-Concha MJ , Spencer JPE . Effect of cultivar type and ripening on the polyphenol content of date palm fruit. J Agric Food Chem. (2013) ;61: (10):2453–60. |
[15] | Bull RJ , Sanchez IM , Nelson MA , Larson JL , Lansing AJ . Liver tumor induction in B6C3F1 mice by dichloroacetate and dichloroacetate. Toxicol. (1990) ;63: (3):341–59. |
[16] | Davis M . Subacute toxicity of trichloroacetic acid in male and female rats. Toxicol. (1990) ;63: (1):63–72. |
[17] | Mather GG , Exon JH , Koller LD . Subchronic 90 day toxicity ofdichloroacetic and trichloroacetic acid in rats. Toxicol. (1990) ;64: (1):71–80. |
[18] | Drabkin DL , Austin JH . Spectrophotometric studies. I. Spectrophotometric constants for common hemoglobin derivatives in human, Dog, and rabbit blood. J Biol Chem. (1932) ;98: :719–33. |
[19] | Esterbauer H , Striegl G , Puhl H , Rotheneder M . Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radic Res Commun. (1989) ;6: (1):67–75. |
[20] | Aebi H . Catalase in vitro. Methods Enzymol. (1984) ;105: :121–6. |
[21] | Flohe L , WA Gunzler . Assays of glutathione peroxidase. Methods Enzymol. (1984) ;105: (1):114–210. |
[22] | Ahmed OM , Abdel-Hamid H , Bastway M , Hasona NA . Antihyperglycemic effects of Plantago Ispaghula seeds aqueous extract in diabetic and hypercholesterolemic rats. J Egypt Ger Soc Zool. (2006) ;51A: :371–93. |
[23] | Stacpoole PW , Henderson GN , Yan Z , James M . Pharmacokinetics, metabolism and toxicology of dichloroacetate. Drug Metab Rev. (1998) ;30: (3):499–539. |
[24] | Davis M . Subacute toxicity of trichloroacetic acid in male and female rats. Toxicol. (1990) ;63: (1):63–72. |
[25] | Al-Sultan SI , El-Bahr SM . Effect of aqueous extract of fenugreek (trigonella foenum-graecum L.) on selected biochemical and oxidative stress biomarkers in rats intoxicated with carbon tetrachloride. Int J Pharmacol. (2015) ;11: (1):43–9. |
[26] | Chao CCT , Krueger RR . The date palm (phoenix dactylifera L.): Overview of biology, uses, and cultivation. Hortscience. (2007) ;42: (5):1077–82. |
[27] | Koohi V , Ostovar A , Kheirkhah HRA , Asadzadeh K . The effect of date palm consumption on the serum levels of glucose, triglyceride, cholesterol, HDL, VLDL, and LDL in rat’s serum with experimental diabetes. Int J Biosci. (2014) ;5: (5):43–51. |
[28] | Edirisinghe I , Burton-Freeman B . Anti-diabetic actions of berry polyphenols –review on proposed mechanisms of action. J Berry Res. (2016) ;6: (2):237–50. |
[29] | Yeragi SG , Rana AM , Koli VA . Effect of pesticides on protein metabolism of Mud skipper Boleophthalmus Dussumieri. J Ecotoxicol Environ Monit. (2003) ;13: (3):211–4. |
[30] | Celik I , Temur A . Determination hematotoxic and hepatotoxic effects of trichloroacetic acid at sublethal dosage in rats. Food Chem Toxicol. (2009) ;47: (6):1324–6. |
[31] | Karami-Mohajeri S , Abdollahi M . Toxic influence of organophosphate, carbamate, and organochlorine pesticides on cellular metabolism of lipids, proteins, and carbohydrates: A systematic review. Hum Exp Toxicol. (2010) ;30: (9):1119–40. |
[32] | Nasir MU , Hussain S , Jabbar S , Rahid F , Khalid N , Mehmood A . A review on the nutritional content, functional properties and medicinal potential of dates. Sci Lett. (2015) ;3: (1):17–22. |
[33] | Katz R , Tai CN , Diener RM , McConnell RF , Semonick DE . Dichloroacetate, sodium: 3-month oral toxicity studies in rats and dogs. Toxicol Appl Pharmacol. (1981) ;57: (2):273–87. |
[34] | Abdel-Hamid NM , Fawzy MA , El-Moselhy MA . Evaluation of hepatoprotective and anticancer properties of aqueous olive leaf extract in chemically induced hepatocellular carcinoma in rats. Am J Med Med Sci. (2011) ;1: (1):15–22. |
[35] | Singh B , Saxena AK , Chandan BK , Anand KK , Suri OP , Suri KA , et al. Hepatoprotective activity of verbenalin on experimental liver damage in rodents. Fitoterapia. (1998) ;69: (2):134–40. |
[36] | Cicmanec JL , Condie LW , Olson GR , Wang SR . 90-Day toxicity study ofdichloroacetate in dogs. Fund Appl Toxicol (1991) ;17: (2):376–89. |
[37] | Rastegar S , Rahemi M , Baghizadeh A , Gholami M . Enzyme activity and biochemical changes of three date palm cultivars with different softening pattern during ripening. Food Chem. (2012) ;134: (3):1279–86. |
[38] | Vayalil PK . Date fruits (phoenix dactylifera Linn): An emerging medicinal food. Crit Rev Food Sci Nutr. (2012) ;52: (3):249–71. |
[39] | El-kholy TA , Hassanen NHM , Abbas HY . Protection of the Mushroom (shiitake “ Lentinus-edodes) against Carbon-tetrachloride-induced renal injury in rats. Life Sci J. (2013) ;10: (1):1701–8. |
[40] | Agbor GA , Oben JE , Nkegoum B , Takla JP , Ngogang JY . Hepatoprotective activity of Hibiscus cannabinus (Linn.) against carbon tetrachloride and paracetamol induced liver damage in rats. Pak J Biol Sci. (2005) ;8: (10):1397–401. |
[41] | Stacpoole PW . The pharmacology of dichloroacetate. Metabolism. (1989) ;38: (11):1124–44. |
[42] | Acharya S , Mehta K , Rodriguez S . Administration of subtoxic doses of t-butyl alcohol and trichloroacetic acid male Wistar rats to study the interactive toxicity. Toxicol Lett. (1995) ;80: (1-3):97–104. |
[43] | Hasan NS , Amon ZH , Nor AI , Arapoc DJ , Azlan A . The role of dates (phoenix dactylifera) aqueous extract in improving the plasma lipid profiles of diet-induced hypercholesterolemic rabbits. Res J Biol Sci. (2010) ;5: (9):632–7. |
[44] | Vembu S , Sivanasan D , Prasanna G . Effect of phoenix dactylifera on high fat diet induced obesity. J Chem Pharma Res. (2012) ;4: (1):348–52. |
[45] | Alvarez-Suarez J M , Giampieri F , Tulipani S , Casoli T , Di Stefano G , González-Paramás AM , Santos-Buelga C , Busco F , Quiles JL , Cordero MD , Bompadre S , Mezzetti B , Battino M . One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J Nutr Biochem. (2014) ;25: (3):289–94. |
[46] | Blade C , Arola L , Salvado MJ . Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Mol Nutr Food Res. (2010) ;54: (1):37–59. |
[47] | Ahmed AF , Al-Qahtani JH , Al-Yousef HM , Al-Said MS , Ashour AE , Al-Sohaibani M , et al. Proanthocyanidin-rich date seed extract protects against chemically induced hepatorenal toxicity. J Med Food. (2015) ;185: (3):280–9. |
[48] | Del Bas JM , Ricketts ML , Vaque M , Sala E , Quesada H , Ardevol A , et al. Dietary procyanidins enhance transcriptional activity of bile acid activated FXR in vitro and reduce triglyceridemia in vivo in a FXR-dependent manner. Mol Nutr Food Res. (2009) ;53: (7):805–14. |
[49] | Kaliora AC , Dedoussis GVZ , Schmidt H . Dietary antioxidants in preventing atherogenesis. Atherosclerosis. (2006) ;187: (1):1–17. |
[50] | Attia AM , Nasr HM . Dimethoate-induced changes in biochemical parameters of experimental rat serum and its neutralization by black seed (nigella sativa L.) oil. Slovak J Anim Sci. (2009) ;42: (2):87–94. |
[51] | Rolando HM , Mauricio D , Veronica L , Fernando LB , Lucia Y , Susana V , et al. Balance between oxidative damage and proliferative potential in an experimental rat model of CCL4–induced cirrhosis: Protective role of adenosine administration. Hepatol. (1997) ;26: (5):1100–10. |
[52] | Zhang CR , Aldosari SA , Vidyasagar PSPV , Nair KM , Nair MG . Antioxidant and anti-inflammatory assays confirm bioactive compounds in Ajwa date fruit. J Agric Food Chem. (2013) ;61: (24):5834–40. |
[53] | Chaudhuri S , Banerjee A , Basu K , Sengupta B , Sengupta PK . Interaction of flavonoids with red blood cell membrane lipids and proteins: Antioxidant and antihemolytic effects. Int J Biol Macromol. (2007) ;41: (1):42–48. |
[54] | Gasparrini M , Giampieri F , Alvarez-Suarez JM , Mazzoni L , Forbes-Hernandez TY , Quiles JL , Bullon P , Battino M . AMPK as a new attractive therapeutic target for disease prevention: The role of dietary compounds AMPK and disease prevention. Curr Drug Targets. (2016) ;17: (8):1–25. |
[55] | Giampieri F , Alvarez-Suarez JM , Battino M . Strawberry and human health: Effects beyond antioxidant activity. J Agric Food Chem. (2014) ;62: (18):3867–76. |
[56] | Giampieri F , Alvarez-Suarez JM , Gasparrini M , Forbes-Hernandez TY , Afrin S , Bompadre S , Rubini C , Zizzi A , Astolfi P , Santos-Buelga C , Gonzalez-Paramas AM , Quiles JL , Mezzetti B , Battino M . Strawberry consumption alleviates doxorubicin-induced toxicity by suppressing oxidative stress. Food Chem Toxicol. (2016) ;94: :128–37. |




