Higher fractional use of Continuous Subcutaneous Insulin Infusion (CSII) is associated with less diabetes related complications: Lessons from long term insulin pump users
Abstract
BACKGROUND:
Several studies have demonstrated that continuous subcutaneous insulin infusion (CSII) is effective in reducing complications in patients with type 1 diabetes (T1DM) in intermediate follow-up; moreover, a recent reports showed cardiovascular mortality reduction in comparison to multiple daily injections (MDI) at 6 years. The aim of this study is to evaluate the long-term outcomes of CSII in a cohort of adult type 1 diabetic patients in term of mortality, complications and life-threatening diseases.
MATERIALS AND METHODS:
This was a retrospective observational study on 141 patients who started CSII before January 2005. Long-term complications, CSII attrition, survival, life-threatening diseases, all mortality causes and the last follow-up HbA1c were recorded.
RESULTS:
The median duration of CSII at the time of analysis was 13 years. Mean age and duration of diabetes at CSII initiation were 38±7 years and 14±10 years, respectively. 11 (7.8%) patients suspended CSII, 5 (3.5%) died (only one –0.7% –for a cardiovascular event). 15 patients (11%) experienced at least one complication related to diabetes. At the last follow-up, mean HbA1c was 56±13 mmol/mol (7.3±1.0%; 36–119 mmol/mol, 5.4–13.0%). Patients who spent more than 55% of their disease duration on CSII developed less events/complications in comparison to patients spending less than 55%. No gender differences were observed.
CONCLUSIONS:
Our data demonstrate that in a clinical setting, long-term (>10 years) CSII treated patients have good glycaemic control at the last visit and a high rate of therapy adherence. The Fractional Time in which patients were treated by CSII is related to low long-term mortality and rate of complications. CSII may be considered an effective and durable therapeutic option for type 1 diabetes early in the course of the disease.
1Background
Type 1 diabetes (T1DM) is related to overall and cardiovascular mortality and morbidity [1–7] and to a high risk of microvascular damage with nephropathy, retinopathy and neuropathy [8, 9]. Data collected from 1997 to 2010 showed that Australian T1DM patients have an estimated loss in life expectancy at birth of 12.2 years compared with the general population [5].
The DCCT [10] (Diabetes Control and Complication Trial) demonstrated that good glycaemic control reduce the risk and progression of complications. DCCT long-term follow-up results (27 years) highlighted an overall mortality reduction in the intensive treatment arm [11]. Intensive therapy reduced the incidence of any cardiovascular disease by 30% (95% CI 7, 48; P = 0.016), and major cardiovascular events (non-fatal myocardial infarction, stroke, or cardiovascular death) by 32% (95% CI23, 56; P = 0.07). These long-term beneficial effects persist up to 30 years.
The Swedish National Diabetes Register demonstrated that from 2002 to 2007 the Life Expectancy at age 20 of Swedish with type 1 diabetes increased by approximately 2 years especially for men. These recent gains have been driven by reduction of cardiovascular mortality, better control of risk factors and by achievement of lower HbA1c levels [12].
A Spanish single centre study [13] showed a low prevalence of diabetic complications in a cohort of 279 patients with T1DM followed for more than 2 decades: diabetic retinopathy was diagnosed in 20.4%, and nephropathy in 11.3% of patients; only 1.3% had Cardiovascular disease. The Authors attributed these results to a specific and regular follow-up program.
In T1DM a higher incidence of cancer and other less frequent autoimmune diseases, such as multiple sclerosis, in comparison to the general population was demonstrated [14–16].
Continuous subcutaneous insulin infusion (CSII) therapy replicates the pancreas function in personalized administration algorithms. This treatment is widely used and is recommended by the most important international guidelines for selected type 1 patients.
Three meta-analyses showed that CSII is more efficient than multiple daily injections (MDI) in reducing HbA1c [17–19] and glycaemic variability both in adults and paediatric patients [20].
CSII therapy was introduced in clinical practice more than 30 years ago, but few long-term data about mortality, complications and incidence of comorbidities have been published.
The National Swedish Registry collected data on 18.168 people with Type 1 diabetes (2441 used CSII) from 2005 and 2012 with a mean follow-up of 6.8 years and demonstrated that the use of insulin pump therapy is associated with lower cardiovascular mortality [21, 22].
Furthermore, CSII seems to achieve long-term glycaemic targets better and more easily than MDI: a recent study demonstrated that a cohort of 200 patients with a CSII duration of 6 years had a mean HbA1c value of 64 mmol/mol (8%) statistically lower than baseline [7, 13].
Long-term real-life data on a large cohort of CSII patients outcome are needed.
2Objectives
The primary aim of this retrospective single centre study was to evaluate the overall and cardiovascular mortality and the incidence of macro and microvascular complications, such as cardiovascular events and end-stage renal failure and retinopathy. The occurrence were collected as year since the diagnosis of T1D and evaluated in relation to CSII therapy start and diabetes duration (years).
Secondary objectives were: 1) the correlation between last follow-up HbA1c and the presence of complications; 2) the CSII attrition rate and its causes.
3Materials and methods
3.1Design and subjects
Data of 376 adult T1DM patients in CSII therapy were extracted from the database (MyStar Connect®, Me.Te.Da, S. Benedetto del Tronto, Italy) used at San Camillo Hospital, Rome, Italy. All the 141 patients who consecutively started CSII before January 2005, were included in this retrospective study.
The main reasons to switch to CSII were: poor metabolic control with high HbA1c levels nevertheless MDI intensive protocol (70% of the population), recurrent severe hypoglycaemia (30%). All candidates were evaluated about their motivation to switch from MDI to CSII and educated to insulin pump use with structured individual programs including carbohydrate counting and advanced pump functions instructions.
The last follow-up data had been collected from January 2015 to June 2015 during normal clinical practice or by a phone call, and all data were downloaded from MyStar Connect® database.
The following demographic data were recorded: gender, date of birth, duration of diabetes, age at CSII start, duration of pump therapy, last follow-up visit HbA1c.
Data on survival, micro and macro-vascular events (Table 1), non-diabetes-related life-threatening severe diseases (cancer, multiple sclerosis and dementia) and therapy suspensions were collected.
Table 1
List of complications evaluated
| Macrovascular | Cardiovascular Disease | Myocardial infarction; Coronary Artery Bypass Graft (CABG); Percutaneous Transluminal Coronary Angioplasty (PTCA); Pace Maker and/or Implantable Cardioverter-Defibrillator (ICD) implant; |
| Peripheral Vascular Disease | foot ulcer; Percutaneous Transluminal Angioplasty (PTA); foot/limb major and minor amputation | |
| Sovra-aortic Vascular Disease | Transient Ischemic Attack (TIA); stroke; carotid thromboendoarterectomy (TEA) | |
| Microvascular | Retinopathy | Start of laser therapy for Advanced Retinopathy; |
| Nephropathy | Haemodialysis start; transplantation (kidney; kidney/pancreas) |
All outcomes were evaluated according to gender and to duration of CSII therapy related to diabetes duration.
This retrospective analysis was approved by our local Ethical Committee and a written informed consent was signed by all the patients regarding anonymous use of their clinical data for scientific purposes.
3.2Statistical analysis
Baseline characteristics and clinical data are summarized using appropriate summary statistics. Variables on a continuous scale are described as mean, standard deviation, median and interquartile range, minimum and maximum. Variables on a categorical scale are presented as counts and percentages. Summary statistics are reported with a maximum of 2 decimals, as appropriate. Deaths, complications and suspension are analysed using survival analysis. In case of multiple complications the first complication is considered as the event. Complications are analysed by type and categorized as macrovascular and microvascular. The analysis is supported by a Kaplan Meier curve, indicating the number of patients at risk every 6 years. The exposure time (years) is computed from the starting date of the CSII therapy to the date of the last available follow-up or date of event (death/complication/suspension) [(data end – data in)/30.4]. We defined “Fractional Use” of CSII as the time percentage (years of CSII treatment) of the overall duration of diabetes. Patient population was grouped according to quartiles of Fractional time of use (Quartiles I: <39%, Quartiles II: 39–55%, Quartile III: 55–75%, Quartile IV: >75%) and complications were collected. The Analysis of Variance (ANOVA) was used to check statistical differences among quartiles. A Cox proportional hazards univariate regression model was used for time to complication analysis. The following variables were included in the analysis: Gender, Age, Age at Diabetes, Fractional CSII use and last HbA1c value. Variables were considered statistically significant if lower than 0.05. After testing for proportional hazard assumptions, Cox models were fitted, and hazard ratios (HR) with 95% CI were computed.
Statistical tests were based on a two-sided significance level of 0.05.
For statistical analysis, SAS 9.3 for Windows (SAS Inst. Inc., Cary, NC) was used.
4Results
141 Patients (45% males) were followed for a median (IQR) follow-up of 13 (11–16) years (mean 14±4 years).
Mean age at diabetes diagnosis was 23±14 years.
The mean age at pump therapy start was 38±14 years and the mean number of years without CSII was 14±10 years.
At last follow-up, mean HbA1c was 7.3% ±1.0% (5.4–13.0%) [56±13 mmol/mol (36–119 mmol/mol)].
Mortality –5 (3.5%) deaths occurred: 1 due to kidney transplantation complication, 1 to HIV infection, 2 to cancer and 1 to heart failure. A Kaplan Meier survival curve describes 95.8% (90.0–98.2%) survival (IC) at 15 years; no significant gender difference was found (Fig. 1).
Diabetes related complications –15 patients (11%) experienced at least one complication with a median (IQR) time of 12 (10–15) years to first occurrence. 10/141 (7%) patients experienced a macrovascular event, whilst 5/141 (4.2%) experienced a microvascular one (Table 2).
Fig.1
Survival curves: overall and for gender.
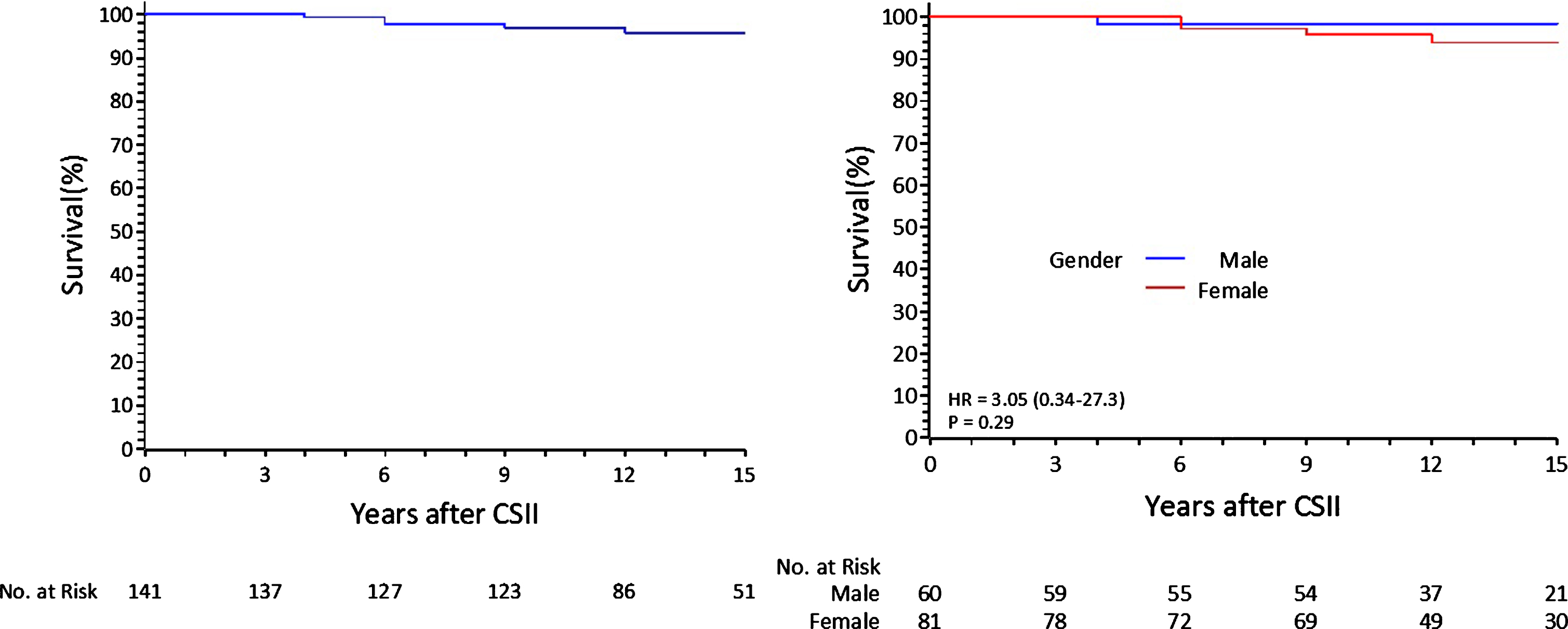
Table 2
Diabetes complications
| Complication | Total (N = 15) |
| Macrovascular | 10 (67%) |
| Cardiovascular Disease | 6 (40%) |
| Peripheral Vascular Disease | 1 (7%) |
| Sovra-aortic Vascular Disease | 3 (20%) |
| Microvascular | 5 (33%) |
| Nephropathy | 2 (13%) |
| Retinopathy | 3 (20%) |
Patient population grouped according to quartiles of Fractional Time of CSII use demonstrated that more is the time spent with an insulin pump more is the freedom from complications (p = 0.026) (Fig. 2).
Fig.2
Freedom from complications according to quartiles of time spent in CSII.
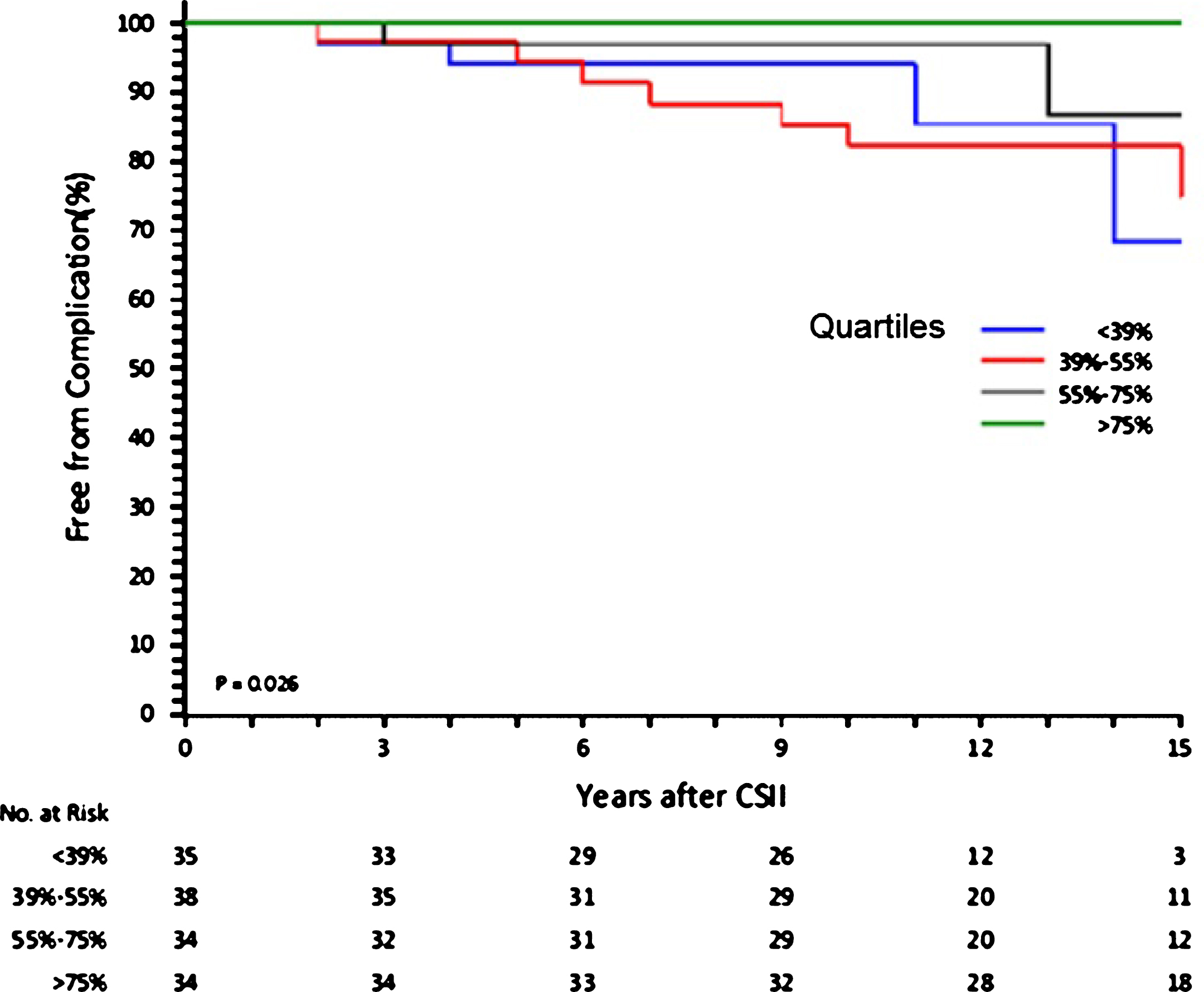
The group with the lowest Fractional Use of CSII had the longest duration of disease and started CSII at an older age (Table 3). The longer duration of diabetes of this group might have compounded the results, while the other three groups shared similar CSII starting age and final HbA1c levels. Therefore the first quartile group was not used for the univariate Cox analysis.
Table 3
Patients characteristics according to the quartiles of time spent in CSII
| Quartiles | <39% | 39% –55% | 55% –75% | >75% |
| (Fractional CSII use) | (N = 35) | (N = 38) | (N = 34) | (N = 34) |
| Age at CSII start (years) | 46.2 | 35.8 | 37.4 | 32.2 |
| Age at Diabetes Onset (years) | 17.3 | 19.8 | 27.8 | 28.8 |
| Duration of diabetes (years) | 41.1 | 29.5 | 25.6 | 19.6 |
| Last HbA1c % | 7.2±1.0 | 7.3±0.8 | 7.2±0.9 | 7.5±1.4 |
| (mmol/mol) | (55±11) | (56±9) | (55±10) | (58±16) |
From the univariate Cox analysis, time spent in CSII split in quartiles is significantly associated to the risk of complication. Patients on CSII treatment for less than 55% of the duration of diabetes, are 1.6-times more likely to experience a complication (HR = 1.64, IC(1.33–20.00), p < 0.05) (Table 4).
Table 4
Predictors of first complication –univariate Cox regression
| Parameter | N | HR (95% CI) | p-value |
| Gender | 106 | 0.33 (0.09–1.29) | 0.112 |
| Age | 106 | 1.01 (0.97–1.06) | 0.600 |
| Age at Diabetes | 106 | 0.99 (0.95–1.04) | 0.709 |
| Fractional CSII use (<55%) | 106 | 1.64 (1.33–20.0) | 0.018 |
| HbA1c | 92 | 1.01 (0.57–1.77) | 0.983 |
CSII attrition –11 (7.8%) patients stopped CSII therapy after a mean of 13±5 years: 8 for psychological-social reasons (listlessness, unsatisfactory quality of life), 2 for loss of self-management capability (post-stroke and dementia) and 1 was in remission post renal/pancreas transplantation. No patient reported serious subcutaneous problems (e.g. lipodystrophy, skin fibrosis) leading to therapy suspension.
There were 14 (10)% non-diabetes-related life-threatening severe pathologies: 3 multiple sclerosis, 9 cancers and 2 cases of dementia.
5Discussion
Several retrospective analysis of long term CSII use have been published lately but the present study seems to report the longest follow-up, with a median duration of 13 years and assesses outcomes beyond glycaemic control.
In Italy the first guidelines on the CSII indication had been published in 2009 [23]. So previously, the most common clinical indications for the adoption of CSII in this T1DM cohort were poor metabolic control with high HbA1c levels despite MDI intensive therapy, recurrent hypoglycaemia and excess of glucose variability. A secondary indication was the need of therapy flexibility for better quality of life (QoL).
The therapy adherence was 92.2%. This rate is higher than what reported in literature (7.8 vs. 10% attrition rate) [21, 22]. This is likely due to the high patient satisfaction demonstrated by the CSII users QoL scores obtained in the Equality study [24], that included the majority of our patients.
Additional factors contributed to the successful and sustainable effects of CSII therapy. The first one was the presence of a dedicated team composed by endocrinologists, nurses, dieticians and psychologists that have been following all patients since the start of CSII therapy and helping them to be motivated, as well as the implementation of a structured educational program [25, 26].
The second reason has been related to the total reimbursement of CSII covered by the National Health Care System for the last two decades.
Our cohort consists mostly of patients who started CSII in adulthood (mean age 37.9 years) with a mean diabetes duration of 14.6±10.5 years. Mortality rate was 3.5% : 4 patients deaths were not directly related to diabetes, and only one patient died for post-ischemic dilated cardiomyopathy already present before CSII initiation. In an Australian registry, life expectancy reduction was 12.2 years comparing the general age matched population [5].
During the follow-up, macro-vascular events incidence was 7%, lower than the fatal and non-fatal cardiovascular events incidence (10.5%) reported in the 7-year follow-up of the National Sweden Registry [12], while the incidence of microvascular complications was 4.2%: it is not assessed in other studies.
Unique to our adult cohort was the correlation between the Fractional Use of CSII and the lower rates of diabetes related complications. The survival analysis showed that long-standing insulin pump users had less events compared to those who spent a shorter time in CSII. This result has been confirmed by a Cox Univariate analysis that demonstrated a 1.6-fold higher risk of complications in CSII subjects treated for less than 55% of the duration of their diabetes. This observation is consistent with a study on children and adolescents [27–29] demonstrating reduced incidence of complications when CSII started earlier in the course of the disease. No differences in terms of HbA1c was shown at the last follow-up among the four groups in accordance to what has been demonstrated in the Swedish Registry [21]. The cancer incidence (9 cases out of 141, 6.38%) was in accordance with literature data [14].
6Limitations
The limitation of this retrospective analysis is the lack of full data set in regards to baseline glycaemia and glucose variability due to the unavailability of electronic data management systems, that were introduced lately. The limited availability of other clinical data such as microalbuminuria, blood pressure, lipid levels, that cannot be retrieved from the database must be considered as challenges of this study.
7Conclusions
This single centre retrospective analysis collected consecutively real life data of all patients followed for more than 13 years, with complete morbidity and mortality data.
Results suggested that long-term CSII therapy in well selected and motivated patients was associated with low mortality and complication rate, high adherence and good glycaemic control. Higher Fractional Use of CSII was associated with a decrease of micro and macrovascular complications. The next step of the research will be the study of the cardiovascular risk factors(hypercholesterolemia, hypertension, smoking habit) prevalence in our cohort and the match with a T1DM MDI treated patients group.
Therefore insulin pump therapy might represent an efficient treatment for T1DM long-term management and could help to prevent cardiovascular events and progression to the end stage of microvascular complications.
Intervention studies are needed to investigate the long term benefits of the CSII therapies both in terms of mortality and glycaemic control.
Conflict of interest
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The authors have no competing interests to declare.
References
[1] | Soedamah-Muthu SS , Fuller JH , Mulnier HE , Raleigh VS , Lawrenson RA , Colhoun HM . All-cause mortality rates in patients with type 1 diabetes mellitus compared with a non-diabetic population from the UK general practice research database, 1992–1999. Diabetologia. (2006) ;49: (4):660–6. |
[2] | Shankar A , Klein R , Klein BEK , Moss SE . Association between glycosylated hemoglobin level and cardiovascular and all-cause mortality in type 1 diabetes. Am J Epidemiol. (2007) ;166: (4):393–402. |
[3] | Pambianco G , Costacou T , Ellis D , Becker DJ , Klein R , Orchard TJ . The 30-year natural history of type 1 diabetes complications: The Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes. (2006) ;55: (5):1463–9. |
[4] | Secrest AM , Becker DJ , Kelsey SF , LaPorte RE , Orchard TJ . All-cause mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes: The Allegheny County type 1 diabetes registry. Diabetes Care. (2010) ;33: (12):2573–9. |
[5] | Huo L , Harding JL , Peeters A , Shaw JE , Magliano DJ . Life expectancy of type 1 diabetic patients during 1997–2010: A national Australian registry-based cohort study. Diabetologia. (2016) ;59: (6):1177–85. |
[6] | Nielsen NM1 , Westergaard T , Frisch M , Rostgaard K , Wohlfahrt J , Koch-Henriksen Melbye M , Hjalgrim H . Type 1 diabetes and multiple sclerosis: A Danish population-based cohort study. Arch Neurol. (2006) ;63: (7):1001–4. |
[7] | Orchard TJ , Costacou T , Kretowski A , Nesto RW . Type 1 diabetes and coronary artery disease. Diabetes Care. (2006) ;29: (11):2528–38. |
[8] | Jensen T , Borch-Johnsen K , Kofoed-Enevoldsen A , Deckert T . Coronary heart disease in young type 1 (insulin-dependent) diabetic patients with and without diabetic nephropathy: Incidence and risk factors. Diabetologia. (1987) ;30: :144–8. |
[9] | Orchard TJ , Olson JC , Erbey JR , et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 0-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications; Study. Diabetes Care. (2003) ;26: :1374–9;3. |
[10] | Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Intensive Diabetes Treatment and Cardiovascular Outcomes in Type 1 Diabetes: The DCCT/EDIC Study 30-Year Follow-up. Diabetes Care (2016) ;39: (5):686–93. |
[11] | Nathan DM , Cleary PA , Backlund JY , et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. (2005) ;353: :2643. |
[12] | Petrie D , Lung TWC , Rawshani A , et al. Recent trends in life expectancy for people with type 1 diabetes in Sweden. Diabetologia. (2016) ;59: :1167–76. |
[13] | Amor AJ , Cabrer M , Giménez M , et al. Clinical status of a cohort of patients with type 1 diabetes diagnosed more than 2 decades before. Results of a specific clinical follow-up program. Endocrinol Nutr. (2016) :pii: 1575-0922(16)30039-0. |
[14] | Harding JL , Shaw JE , Peeters A , et al. Cancer risk among people with type 1 and type 2 diabetes: Disentangling true associations, detection bias, and reverse causation. Diabetes Care. (2015) ;38: (2):264–70. |
[15] | Livingstone SJ , Looker HC , Hothersall EJ , et al. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish Registry Linkage Study. PLoS Med. (2012) ;9: :e1001321. |
[16] | Orchard TJ , Nathan DM , Zinman B , et al. Association Between 7 Years of Intensive Treatment of Type 1 Diabetes and Long-term Mortality. JAMA. (2015) ;313: (1):45–53. |
[17] | Misso ML , Egberts KJ , Page M , O’Connor D , Shaw J . Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev. (2010) ;(1):CD005103. |
[18] | Jeitler K , Horvath K , Berghold A , et al. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: Systematic review and meta-analysis. Diabetologia. (2008) ;51: :941–51. |
[19] | Monami M , Lamanna C , Marchionni N , et al. CSII versus MDI in type 1 diabetes: A meta-analysis. Acta Diabetol. (2010) ;47: :77–81. |
[20] | Chimenti EM , de la Morena LH , Vaquero PM , Saez-de-Ibarra L , Dominguez MG , Sanchez LF . Assessing glycaemic variability with continuous glucose monitoring system before and after continuous subcutaneous insulin infusion in people with Type 1 diabetes. Diabetes Res Clin Pract. (2010) ;90: :e57–9. |
[21] | Steineck I , Cederholm J , Eliasson B , Rawshani A , Eeg-Olofsson K , Svensson AM , Zethelius B , Avdic T , Landin-Olsson M , Jendle J , Gudbjörnsdóttir S . The Swedish National Diabetes Register Insulin pump therapy, multiple daily injections, and cardiovascular mortality in 18.168 people with type 1 diabetes: Observational study. BMJ. (2015) ;350: :h3234. |
[22] | Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol (1995) ;75: :894–903. |
[23] | Standard italiani per la cura del diabete mellito SID-AMD. Linee Guida e raccomandazioni. (2009) , Ed. Infomedica. |
[24] | Nicolucci A , Maione A , Franciosi M , Amoretti R , Busetto E , Capani F , Bruttomesso D , Di Bartolo P , Girelli A , Leonetti F , Morviducci L , Ponzi P , Vitacolonna E . Quality of life and treatment satisfaction in adults with Type 1 diabetes: A comparison between continuous subcutaneous insulin infusion and multiple daily injections. Diabet Med. (2008) ;25: (2):213–20. |
[25] | Bruttomesso D . Selezione dei pazienti. La terapia insulinica con microinfusore. Eco edizioni internazionali. (2006) . |
[26] | Hayward RA , Reaven PD , Wiitala WL , et al. VADT Investigators. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2015) ;372: :2197–206. |
[27] | Eeg-Olofsson K , Cederholm J , Nilsson PM , et al. Glycemic control and cardiovascular disease in 7454 patients with type 1 diabetes: An observational study from the Swedish National Diabetes Register (NDR). Diabetes Care. (2010) ;33: :1640–6. |
[28] | Brancato D , Fleres M , Aiello V , Saura G , Scorsone A , Ferrara L , Provenzano G , Di Noto A , Spano L , Provenzano V . The effectiveness and durability of an early insulin pump therapy in children and adolescents with type 1 diabetes mellitus. DTT. (2014) ;16: (11). |
[29] | Tamborlane WV , Beck RW , Bode BW , et al. Juvenile diabetes research foundation continuous glucose monitoring study grouContinuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. (2008) ;359: :1464–76. |




