Comparative analysis of chemical composition and in vitro digestibility of Bt versus non-Bt cotton crop residues in Gezira State, Sudan
Abstract
The objective of the experiment was to investigate - and qualify chemical composition of both Bt and non-Bt cotton crop residues (CCR). The in vitro digestibility of each type of CCR was also determined. Week zero represents the period before introduction of animal to graze on Bt-CCR. From each square meter of 14 square meter cotton residues were cut at 10 cm above the ground level. The components of the crop residues in each square meter were separated in to leaves, bolls and tender branches and then weighed freshly and again after drying in an oven at 105°C for 24hours. The results revealed significant differences in chemical composition of whole residues, in vitro digestibility of dry matter and in vitro digestibility of protein in Bt CCR. Crude protein and ash were higher in all components of Bt CCR and crude fiber was higher in bolls only. The in vitro digestibility showed lower levels in Bt CCR than in non-Bt CCR.
1Introduction
Cotton “Gossypium” is the major natural textile fiber crop worldwide. In Sudan, cotton has been grown for centuries. The cotton plant is indigenous and a number of its wild relatives exist in various parts of the country, as well as and the crop production represents a manner to reduce poverty and encouraged the settlement in rural areas. Commercial growing of the crop, however, started in 1867. In 1926 was the official start of functioning of the Gezira Scheme (where cotton crop represents the main cash crop). The bulk of the production, nearly 90% is exported as raw fiber while the other 10% is locally consumed [1]. Due to decline of grazing land, sheep and goats are let loose in the cotton fields for grazing by the farmers and shepherds after harvesting the cotton [2], however in Sudan cows communally grazed on cotton crop residues (CCR).
The cotton harvest leaves several residues in the field, such as stalks, side branches, leaves, bolls, and seeds with adhering cotton lint [3]. The amount of cotton crop residues, known under various names (e.g. cotton straw, cotton sticks, cotton wood) can range from 5 to 7 t/ha [4]. As often happens with crop residues, the quality of the material is highly variable. For instance, CCR are usually dry but some varieties grown under irrigation contain about 30% green leafy foliage even at the time of last picking of cotton [5]. CCR are abandoned as waste, but are often incinerated or ploughed into the soil as they may host insects that can infest the next cotton crop[3, 4, 6]. CCR have been used as organic fertilizers for soil amendment and were shown to improve micro-organism activity and increase seedling growth [3]. Cotton wood (the thicker stems) is used as firewood [7]. In Egypt, cotton stalks are transformed into briquettes for fire [6]. Cotton stalks, leaves and unripe bolls may be grazed or taken to the homestead, chopped and dried for winter feed [7]. CCR are readily browsed by small ruminants. However, they should not be fed to livestock if pesticides residues in the plant are above the maximum residue levels determined by national or supranational authorities (see Potential constraints on the “Nutritional aspects” tab).
Bt cotton has been genetically modified by the insertion of one or more genes from a common soil bacterium, Bacillusthuringiensis (Bt). These genes encode for the production of insecticidal proteins, and thus, genetically transformed plants produce one or more toxins as they grow. The genes that have been inserted into cotton produce toxins that are limited in activity almost exclusively to caterpillar pests (Lepidoptera). However, other strains of Bacillusthuringiensis have genes that encode for toxins with insecticidal activity on some beetles (Coleoptera) and flies (Diptera). Some of these genes are being used to control pests in other crops, such as corn.
The introduction of Biotech cotton in Sudan enhanced cotton productivity and restored cotton as a main cash crop. The increase in hectare age of Bt cotton between 2012 and 2014 is clear evidence that farmer experience was positive in the first year of planting in 2012 and has provided the incentive for a large increase in adoption in 2014 [8].
Several studies showed that the amount of expression of Bt protein in plant is in very minute and its action is limited to certain larval species and may not cause toxicity to mammals [9].
Genetically modified crops as animal feed was used by many authors [10– 14].
CCR is usually utilized as a valuable summer feed source in Gezira. This feed source holds about 100,000 animal units for 1– 1.5 months. Genetically modified CCR was first introduced in 2012. Animal owners fear from grazing on this modified crop. However for both genetically modified and non modified CCR, the composition and digestibility is not known. Therefore this research was conducted with two objectives, namely:- 1) to evaluate the chemical composition of both Bt and non-BtCCR using the proximate analysis techniques for crude protein, crude fiber, dry matter and Ash content; 2) to quantify the digestibility of both Bt and non-BtCCR through a standardized in vitro technique, since the digestibility is one of the important measures for the evaluation of CCR for feed purposes.
2Materials and methods
2.1Location of the experiment
Gezira State is located south-west of Khartoum state. The state lies between latitude 32°13′ and 30°15N and longitudes 22°32′ and 20°43′E. It covers an area of about 27545km2 of which around 90% can be utilized for agriculture. It has a virtually flat relief, with slight tilt of the ground sloping gently from south to the north, which made possible the construction of a gravity-based irrigation system that covers part of the Gezira state, was mainly constructed for cotton production and is one of the largest irrigation projects in the world (Gezira scheme). Rainfall is characterized by high degree of spatial and temporal variability of wet and dry decades from season to season as well as within the same season. The state is divided into eight localities.
The experiment was conducted in two localities of Gezira state where cotton crop was cultivated. This included, South Gezira locality (Al- Madina Arab) and Um-Algura locality. In Um-Algura locality, a herd of 25 animal units (AU), 12.6 of Which were milking (18 milking cows) were grazed on non-Bt CCR. In South locality a herd of 22 animal units (AU), 11.2 of Which were milking, (16 milking cows) were grazed on Bt CCR. The lactating cows in the herd of Um- Algura and South Gezira was 50.4% and 51% respectively. The herd in each of the localities was of mixed breeds (local and crosses between local and Friesian cows). CCR yield was estimated by selecting different number of square meter by throwing a rope for fourteen times and making the square meter by the rope itself. This procedure was repeated in each of the five weeks of study. Week zero represents the period before introduction of animal to graze on CCR. From each square meter cotton residues were cut by sickle at 10 cm above the ground level. The components of the crop were separated in to leaves, bolls and tender branches and then weighed freshly and again after drying in an oven at 105°C for 24 h using digital scale. Accordingly percent of each component in both CCR yield and the intake were obtained. The CCR and its component in each week were put in paper envelopes and store in the Department of Animal Production of the Faculty of Agricultural Sciences for chemical analysis and estimation of in vitro digestibility.
2.2Methods of proximate analysis
Proximate analysis of CCR and its components for estimation of dry matter, crude protein, crude fiber, Ash was carried out in the laboratory of Food Science in the faculty of Engineering and Technology, using the procedures of [15].
2.3Moisture content
Moisture content was determined by air-drying of the samples in an oven at 105°C for 24 h. Moisture and dry matter were quantified as follows:
(1)
(2)
Where:
W1 = Sample weight(g)
W2 = Extraction cup
W3 = Extraction cup weight after heating at 105°C.
2.4Ash content
Ash content measured the total in organic matter by high temperature of in cine ration [15]. Approximately 1.0 g of sample was weighed in to a pre-weighed crucible and in cine rated over night at 600°C using aNaberthem muffle furnace. The increase in the final weight of crucible after in cine ration represented the ash and was expressed as percentage of the original sample, calculated as follows:
(3)
Where:
W1 = Sample weight(g)
W2 = Crucible weight(g)
W3 = Crucible weight + ash residue after 600°C.
2.5Crude protein (CP)
The Kjeldahl method according to [15] was used for the determination of CP in duplicate, as follows: 150 mg sample was digested in concentrated sulphuric acid and two Kjeltabs performed as catalyst. Then ammonia from the digestion process was released after reaction with 40% sodium hydroxide and distilled using FOSS Kjeltec2200, trapped in 4% boric acid and quantified by titration against 0.1 M hydrochloric acid– titration was operated manually and run according to the operation manual from the manufacturer. Crudeproteinwasestablishedbymultiplyingthetotalnitrogenwiththeconversion factorof6.25.Crudeproteinwascalculatedas follows:
(4)
(5)
Where:
S = HCL titration for sample
B = HCL titration for blank
N = Neutrality for HCL
2.6Crude fiber (CF)
About 0.6 g of defatted sample in a pre- weighed Scintaglass crucible was used for CF determination usingacid -base hydrolysis. The crucible was fitted to the Fiber tec 2022 Fiber cap and run according to the manufacturer’s operating instructions. Hydrolysed and oven- dried sample was later ashed in the muffle furnace at 600°C for 4 h and CF in the defatted sample expressed as a percentage of the original undefatted sample. CF was determined as follows:
(6)
Where:
W1 = Capsule weight (g)
W2 = Sample weight (g)
W3 = Capsule weight after 130°C
W4 = Empty crucible weight
W5 = Crucible weight after 600°C.
2.7Determination of in vitro digestibility
The technique for determination of conventional in vitro digestibility complied with the [16] modification of the [17] two-stage procedure. Twenty- four 50-mL Nalgene tubes were placed in a rack. Subsequently, 0.5 g of experimental samples were added to each of 20 tubes, 0.5 g samples from laboratory standards (grass hay) were added to 2 tubes and 2 tubes were used as blanks for the experiments. In each tube, 35 mL of a buffer– inoculums mixture as described by [18] was added under purging with CO2 and caped tightly with a rubber stopper/gas-release port [16]. Samples were incubated for 48 h in a water bath at 39°C, followed by further digestion in an acid– pepsin solution containing 6.6 g/L pepsin (Fisher Scientific, Pittsburgh, PA, USA) and 0.1 N hydrochloric acid (35 mL of acid– pepsin solution was added to each tube) for 48 h in water bath at 39°C. All tubes were mixed by swirling (Vortex Genie-2 Mixer, VWR Scientific, West Chester, PA, USA) them at 2, 4, 20, and 28 h after adding the buffer– inoculums and at 2, 4, and 6 h after adding acid– pepsin. After completion of the digestion, contents were filtered into pre-weighed standard coarse fritted disk Gooch crucibles under mild vacuum, dried at 100 C for 12 h, weighed for determination of DM, placed in a muffle furnace at 525°C for at least 12 h, and reweighed to complete calculation.
2.8Statistical analysis
Statistical analysis were performed using SPSS. To compare the means t-test was used.
3Results and discussion
As presented in Table 1, the chemical composition of Bt and non-BtCCR, showed that the DM in leaves, bolls and tender branches were not significantly different. In this study the DM of Bt and non Bt leaves was 92 and 91.5% respectively. This higher DM is mainly attributed to that, the leaves were collected from CCR about one month after water withdrawal. While [19, 20] found a DM of 27% in fresh leaves. The ash content was significantly higher in Bt leaves(p < 0.001) than non-Bt ones, and only the Ash content observed in non-Bt leaves fell within the range reported previously [19, 20]. The CP content of leaves was significantly different between Bt and non-BtCCR, and had the same trend of CP in other parts of CCR, the protein was higher in Bt-CCR compared to non-Bt CCR. However, the CP in leaves in BtCCR (9.9%) and in non-BtCCR (8.0%) in this study was lower than reported by [19, 20] (lower limit of the range of % 12.1– 12.3). The FC of leaves was significantly higher in non-BtCCR (11.0) leaves than in BtCCR (7.9%), and the data were in the range reported by [19, 20]. Also ash content of Bt and non-Bt CCR in tender branches was not significantly different in this study. However, estimate of ash in cotton straw was not available in the literature cited for this study. While ash of bolls in Bt and non-Bt CCR was significantly higher in BtCCR. However, estimate of chemical composition of bolls and tender branches as a separate agronomical part of CCR was lacking in the literature. The CP of bolls and tender branches in Bt and non Bt CCR were highly significantly different and had the same trend of CP of leaves. The protein was higher in BtCCR compared to non-Bt CCR in both bolls and tender branches. However, the CP of bolls and tender branches in this result of 11.2 and 7.3 in Bt and 6.6 and 6.1% in non-Bt CCR respectively, only CP of bolls in BtCCR was out of the range of 3.5– 9.6% reported previously [4, 21– 26]. The Fiber of Bt and non-Bt CCR in bolls was not significantly different. However, this result of 53.7% in Bt and 44.2% in Non-Bt CCR in this study was in the range of 43.0– 64.9% reported previously [4, 21– 26]. While fiber of Bt and non-BtCCR in tender branches was significantly higher in non-BtCCR. However, the result of this study for both Bt and non-BtCCR was in the range of 43.0– 64.9% reported by [4, 21– 26]. However, there are estimate of chemical composition in poor roughages such as straws, but only few reports on chemical composition of leaves of CCR were found.
Table 1
Percent chemical composition of Bt- cotton and non Bt-cotton crop residues at week graze
| % | Leaves | Bolls | Tender Branches | |||||||||
| Non-Bt | Bt | SD | Sig | Non-Bt | Bt | SD | Sig | Non-Bt | Bt | SD | Sig | |
| DM | 91.53 | 92.38 | 0.410 | 0.079 | 91.38 | 91.88 | 0.728 | 0.146 | 90.97 | 92.18 | 0.4000 | 0.116 |
| Ash | 14.40 | 18.20 | 0.400 | 0.008 | 7.72 | 10.07 | 0.653 | 0.001 | 5.50 | 6.49 | 0.720 | 0.118 |
| Protein | 8.013 | 9.616 | 1.56 | 0.005 | 6.56 | 11.15 | 0.728 | 0.000 | 6.09 | 7.293 | 0.456 | 0.015 |
| Fiber | 11.02 | 7.880 | 0.800 | 0.000 | 44.24 | 53.71 | 1.523 | 0.067 | 64.20 | 43.30 | 1.550 | 0.000 |
The in vitro DM digestibility from Bt and non-BtCCR in weeks 1, 2 and 3 were significantly different (Table 2). Non-BtCCR showed higher digestibility (60, 56 and 54% in weeks 1,2 and 3) compared to Bt CCR (46, 41.3 and 38.7% in weeks 1, 2 and 3), although a clear variation throughout the weeks was observed. This may be due to the feed selection according to its botanical composition.
Table 2
Percent In vitro digestibility DM from Bt cotton and non-Bt cotton crop residues
| Week | Non Bt | Bt | SD | Sig. |
| 1 | 60.00 | 46.00 | 2.150 | *** |
| 2 | 56.00 | 41.33 | 0.420 | *** |
| 3 | 54.00 | 38.67 | 0.430 | *** |
| SD | 2.123 | 3.145 | ||
| Level of sig | *** | *** |
The in vitro DM digestibility of week1 of both Bt and non Bt-CCR in this study where the intake could be more than 50% leaves was lower than that of 89.7% OM digestibility reported by [19, 20] for fresh cotton crop leaves. While only the digestibility of non-Bt CCR in week 1 was comparable to that of 64% OM digestibility reported by [19, 20] for cotton straw. However, the Bt-CCR digestibility in the same week was much lower than that reported by [20]. When the digestibility of CCR in the present study was compared with 54% of sorghum straw (common roughage in Sudan) reported by [27], it is clear that the digestibility of non-Bt CCR in all weeks was equal or higher than that of [27]. While that of Bt CCR at all weeks was lower than that of sorghum straw reported by the same authors. The reasons for reduced digestibility of Bt CCR in this study is unclear, despite of its higher contents of leaves compared to non Bt-CCR.
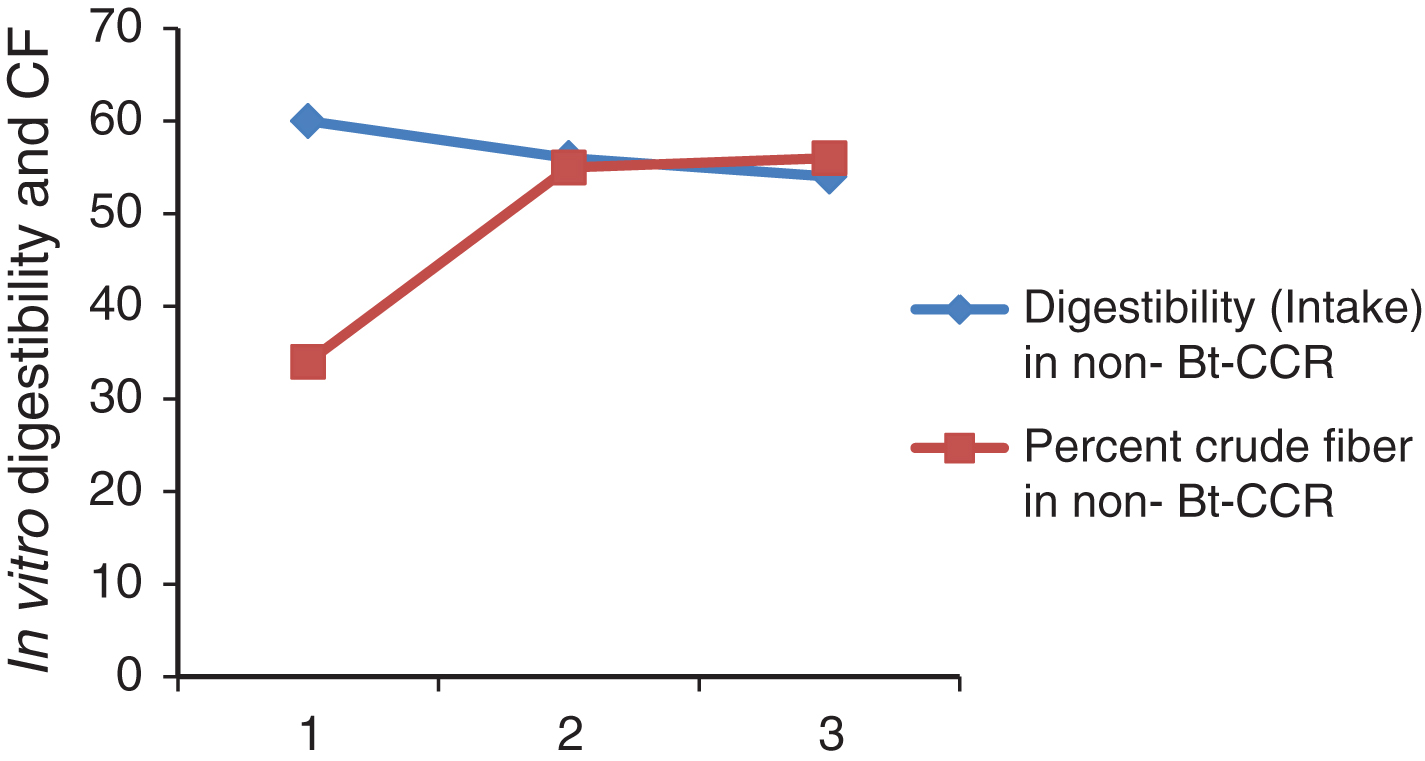
In vitro protein digestibility in Bt and non Bt-CCR in weeks 1, 2 and 3 were highly significantly different (Table 3). The digestibility was significantly higher in non Bt CCR in week 1. While in the other weeks, it was significantly higher in Bt CCR. This result from Bt and non-Bt CCR in this study was higher than that of 41.9 % CP digestibility reported for sorghum straw by [27]. However, digestibility of CP from CCR was not cited at least in the available literature. It is clear from the Table (4) that, the digestibility was not affected by CF content at least in BtCCR in this study (Fig. 1) and (Fig. 2). However, it may be indicated that, CF in non-BtCCR is more digestible than the CF of BtCCR (where the intake in the last week contained greater amount of tender branches).
Table 3
Percent in vitro digestibility protein from Bt-cotton and non-Bt cotton crop residues
| Week | Non Bt | Bt | SD | Sig. |
| 1 | 61.00 | 54.00 | 0.834 | *** |
| 2 | 47.33 | 59.00 | 1.234 | *** |
| 3 | 46.00 | 48.50 | 0.900 | * |
| SD | 5.276 | 4.078 | ||
| Level of sig | *** | *** |
±SD mean Standard deviation.
Table 4
Percent crude fiber content and the digestibility of total intake and crude protein in CCR
| Week | Bt | Non-Bt | ||||
| 1 | 2 | 3 | 1 | 2 | 3 | |
| Digestibility DM | 46 | 48.33 | 38.67 | 60 | 56 | 54 |
| Protein digestibility | 54 | 59 | 48.50 | 61 | 47.33 | 46 |
| Percent crude fiber | 29 | 50.16 | 43.3 | 34 | 55.33 | 55.99 |
Fig.1
In vitro digestibility and CF of DM Bt-CCR.
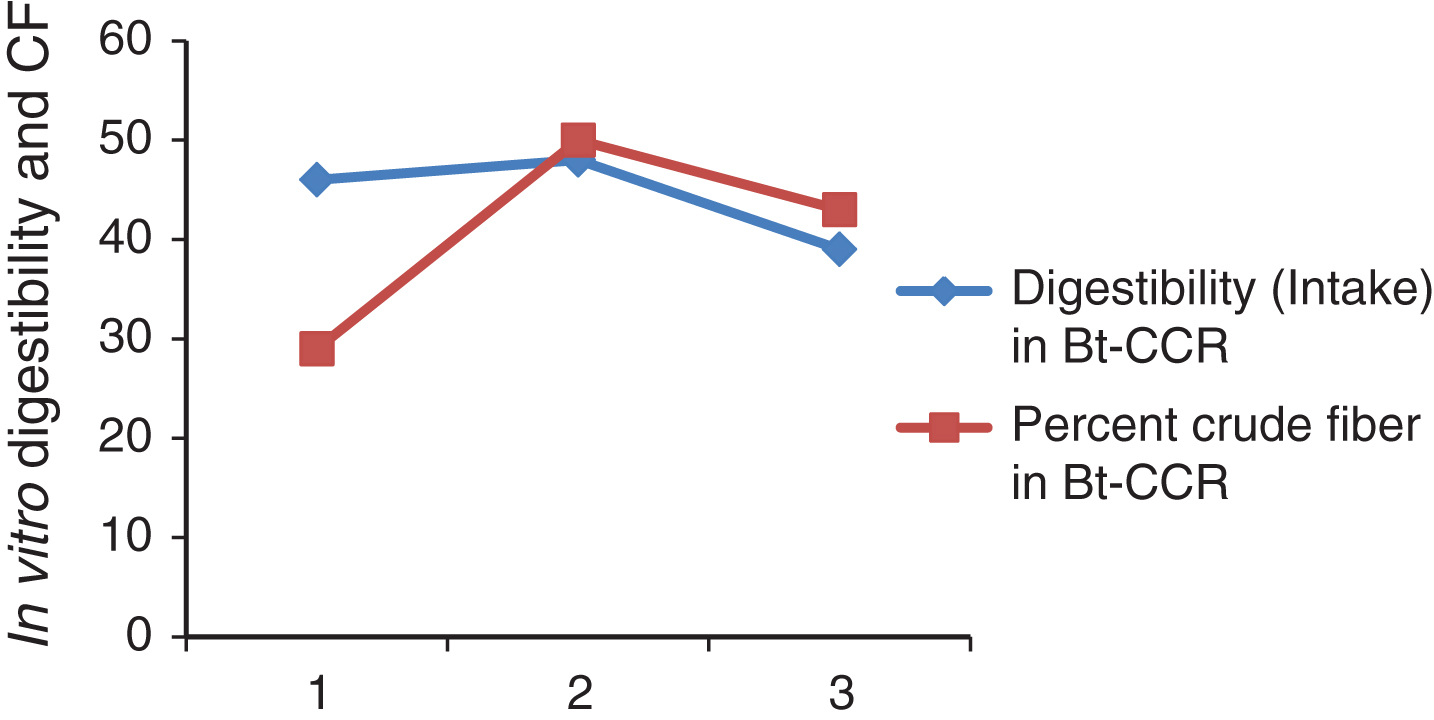
Fig.2
In vitro digestibility and CF of DM of non-Bt-CCR.
3.1Conclusions
This research evaluated the chemical composition and in vitro digestibility of dry matter and crude protein of Bt compared to non-Bt cotton crop residues in Gezira State, Sudan. Based on the study findings it could be concluded that:
The Crude Protein content of Bt and non-BtCCR in leaves, bolls and tender branches was significantly different (P < 0.01) and had the same trend of ash; the protein was higher in Bt-CCR compared to non-Bt-CCR in all of CCR components. While the DM in leaves, bolls and tender branches were not significantly different. Also BtCCR had higher fiber in bolls when compared to non-BtCCR. BtCCR had higher fiber in leaves and tender branches when compared to non-Bt CCR.
The in vitro digestibility of DM of BtCCR was lower when compared to non-BtCCR. The BtCCR had higher CP in vitro when compared to non-BtCCR.
3.2Recommendations
More investigations are needed to elucidate the reasons of BtCCR effects on feed intake and the in vitro digestibility characteristics.
References
[1] | Elfadil Abdelrahman Babiker. Report on Sudan Cotton Research and Production Scenarios: Challenges, Achievements and Prospects. Agricultural research corporation-Sudan. (2009) . |
[2] | Reiser R , Fu HC . The mechanism of gossypol detoxification by ruminant animals. J Nutr. (1962) ;76: :215–8. [PubMed]). |
[3] | Huang Wuren , Bai Zhihui , Hoefel Daniel , Hu Qing , Lv Xin , Zhuang Guoqiang , Xu Shengjun , Qi Hongyan , Zhang Hongxun . Effects of cotton straw amendment on soil fertility and microbial communities. Front Envir Sci Eng. (2012) ;6: (3):336–49. |
[4] | Silanikove N , Levanon D . Cotton straw: Composition, variability and effect of anaerobic preservation. Biomass. (1986) ;9: :101–12. |
[5] | Narasa Reddy GV , Raj Reddy M . Effect of ammonia treatment and processing of whole cotton plants as sole source of roughage in complete feeds for growing cross-bred calves. Anim Feed Sci Technol. (1985) ;13: (1/2):93–102. |
[6] | El Saeidy E . Renewable Energy in Agriculture in Egypt: Technological fundamentals of briquetting cotton stalks as a biofuel. Dissertation, Humboldt-Universität zu Berlin, Landwirtschaftlich-Gärtnerische Fakultät. (2004) . |
[7] | Suttie JM . Hay and straw conservation for small-scale farming and pastoral conditions. FAO Plant Production and Protection Series No. 29, FAO, Rome. (2000) . |
[8] | James Clive. Global Status of Commercialized Biotech/GM Crops: 2014. ISAAA Brief No. 49. ISAAA: Ithaca, New York. Food and Agriculture Organization of the United Nations. (2014) . |
[9] | Betz FS , Hammond BG , Fuchs RL . Safety and advantages of Bacillus thuringiensis-protected plants to control insect pests. Regular Toxicology Pharmacology. (2000) ;32: :156–73. [PubMed]). |
[10] | Donkin Shwan S . Performance of dairy cattle fed specialty corn hybrids. Paper presented at the Purdue Forage day. (2000) . |
[11] | Singhal KK , Tyagi AK , Rajput YS , Singh M , Perez T , Hartnell GF . Effect of feeding cottonseed produced from Bollgard (BG II) cotton on feed intake, milk production and composition in lactating crossbred cows. Proc. 12th AAAP Cong., Busan, South Korea. (2006) ;757. |
[12] | Calsamiglia S , Hernangez B , Hartnell GF , Phipps R . Effect of corn silage derived from genetically modified variety containing two trans genes on food intake, milk production and composition and absence of detectable transgenic deoxyribonucleic acid in milk in Holstein dairy cows. J Dairy Sci. (2007) ;90: :4718–23. |
[13] | Steinke K , Guerther P , Paul V , Wiedemmann S , Ettle T , Albrecht C , Meyes HH , Spiekers H , Scwarz FJ . Effect of long term feeding of genetically modified corn (event MON810) on the performance of lactating dairy cows. J Anim Physiol Anim Nutr (Berl). (2010) ;94: (5):185–93. |
[14] | Calabro Tudisco S , Cutrignelli MI , Moniello G , Grossi M , Mastellone V , Lombardi P , Pero MF , Infascelli F . Genetically modified soybean in goat diet: Influence on kid performance. Small Ruminant Research. (2015) ;126: (supplement: 1):67–74. |
[15] | Association of Official Analytical Chemists. (AOAC). Official Methods of Analysis (1990) . |
[16] | Galyean ML . Laboratory Procedures in Animal Nutrition Research, 12th ed. West Texas A and M University, Division of Agriculture and Texas A and M Research and Extension Center, Amarillo, TX, USA (1997) . |
[17] | Tilley JMA , Terry RA . A two-stage technique for the in vitro digestion of forage crops. J Br Grassl Soc. (1963) ;18: :104–11. |
[18] | Marten GC , Barnes RF . Prediction of energy digestibility of forages with in vitro rumen fermentation and fungal enzyme systems. In: Pigden W.G. , Balch C.C. , Graham M. (Eds.), Standardization of Analytical Methodology for Feeds. International Research Center, Ottawa, Ontario, Canada, (1980) ;61–71. |
[19] | Mecha I , Adegbola TA . Chemical composition of some southern Nigeria forage eaten by goats. In: Browse in Africa, the current state of knowledge. Le Houérou H.N. (ed.), ILCA, Addis Ababa, Ethiopia, (1980) 303–6. |
[20] | CIRAD (1991). Laboratory data 1963–1991. |
[21] | BenGhedalia D , Shefet G , Miron J . Effect of ozone and ammonium hydroxide treatments on the composition and in-vitro digestibility of cotton straw. J Sci Food Agric. (1980) ;31: (12):1337–42. |
[22] | Flachowsky G , Guther G , Wolf I , Lohnert HJ , Legel S , Hennig A . Investigations on the digestibility and food intake of differently treated cotton straw from Egypt. Beitragezur Tropischen Land wirtschaft und Veterinary Medicine. (1981) ;19: (4):447–54. |
[23] | Silanikove N . Effect of CaO- or NaOH-hydrogen peroxide treatments on the composition and in vitro digestibility of cotton straw. Bioresour Technol. (1994) ;48: (1):71–3. |
[24] | Kirubanath K , Narsimha Reddy D , Nagalakshmi D . Effect of processing cotton straw based complete diet with expander extruder on performance of crossbred calves. Asian-Australasian Journal of Animal Sciences. (2003) ;16: (11):1572–6. |
[25] | Grewal RS , Saijpaul S , Kaushal S . Effect of cotton stems addition on the chemical composition and in sacco dry matter digestibility of pearl millet silage. Asian-Aust J Anim Sci. (2003) ;16: (12):1722–4. |
[26] | Hamza AS , Mohammad TF , El Tahan AAH , El Shannawy MM . Effect of combining two biological treatments on chemical composition, digestibility and feeding values of cotton stalks fed to sheeEgypt J Sheep, Goat and Desert Anim Sci (2006) ;1: (1):187–97. |
[27] | ElObied GH , Ali JA . Optimum concentrate supplement to sorghum straw to reduce live weight loss in calves during summer season in Sudan. I International Journal of Agricultural Sciences. (2013) ;3: (9):016–021. |




