A randomized clinical trial evaluating a proprietary mixture of Lactobacillus plantarum strains for lowering cholesterol1
Abstract
BACKGROUND: Cardiovascular disease, global leading cause of morbi-mortality, deserve a special attention of efficacious and safe treatments. Development of new principles based on the intestinal microbiome has been revealed as a promising approach.
OBJECTIVE: To assess the effects of a combination of three Lactobacillus plantarum strains on low-density lipoprotein cholesterol (LDL-C) and other lipid parameters in hypercholesterolemic adults.
METHODS: In this double-blind, placebo-controlled, randomized trial, 60 patients (mean age 51.8 y, BMI 26.2 kg/m2, LDL-C 167.5 mg/dL) not receiving lipid-altering treatment were treated either with a L. plantarum-containing probiotic (LpPRO) or placebo (PLBO) single capsule daily for 12 weeks. Lipid and safety parameters were assessed at screening/baseline, 6 and 12 weeks of treatment, and after a 4-week follow-up period.
RESULTS: At 12 weeks, compared to PLBO, the LpPRO group had significantly (p < 0.001) larger reductions in LDL-C (24.4 vs. 9.8 mg/dL), total-C (33.7 vs. 10.6 mg/dL), LDL-C/high-density lipoprotein cholesterol (HDL-C) ratio (0.8 vs. 0.3), oxidized LDL (7.5 vs. 1.0 U/L) and triglycerides (29.1 vs. 4.1 mg/dL). HDL-C was also significantly (p < 0.001) increased in LpPRO vs. PLBO (2.9 vs. 0.4 mg/dL).
CONCLUSIONS: The L. plantarum combination reduced LDL-C and improved other lipid parameters, suggesting its potential for hypercholesterolemia treatment.
1Introduction
Cardiovascular disease (CVD) is a leading cause of morbidity and mortality worldwide [1–6]. Large observational studies and randomized controlled trials demonstrate that elevated low-density lipoprotein cholesterol (LDL-C), total cholesterol (total-C), non-high-density lipoprotein cholesterol (non-HDL-C), and triglycerides (TG) as well as low HDL-C levels, are associated with increased cardiovascular risk [6, 7]. Clinical trials of statin therapy have shown that a 1% reduction in serum cholesterol is associated with approximately a 1% decrease in risk for coronary heart disease (CHD) [6, 8, 9]. Studies of genetic variants that alter cholesterol levels indicate that maintaining lower cholesterol for a longer duration than that of a typical clinical trial yields an even greater reduction in CHD risk, with each 1% reduction in LDL-C attributable to genetic variants being associated with a reduction of 2-3% in CHD event risk [6, 10, 11].
Lifestyle changes, including diet and exercise, are the first line of therapy for individuals with hypercholesterolemia; and pharmacologic agents are recommended for those requiring additional atherogenic cholesterol lowering who are at sufficient risk to justify their use [5, 6]. Dietary adjuncts can play an important role to provide additional cholesterol reduction in those who need additional cholesterol lowering after initial lifestyle changes, whether or not drug therapies are employed [6, 12].
Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” [13–15]. They are regarded as safe for human consumption and are available in a variety of functional foods [16–19].
Some bacterial strains have been shown to reduce lipoprotein cholesterol and triglyceride (TG) levels [20–28]. There are several proposed LDL-C lowering mechanisms of probiotics [27, 29, 30], including: 1) deconjugation of bile acids (via production of bile salt hydrolases) which interferes with the ability of cholesterol to be incorporated into mixed micelles, thus reducing its absorption [30, 31]; deconjugated bile acids may also stabilize ATP binding cassette transporters that are responsible for moving sterols (including cholesterol) out of enterocytes [32, 33]; 2) interference with the bioavailability of cholesterol in the small intestine through cholesterol adsorption into bacterial cell walls [30, 34, 35]; and 3) enhancement of short-chain fatty acid production via colonic fermentation of undigested carbohydrate, which may inhibit cholesterol synthesis and suppress release of free fatty acids from adipose depots, reducing substrate for TG synthesis [30, 36].
Lactobacilli are lactic acid-producing bacteria with reported hypocholesterolemic as well as antiglycemic, antipathogenic, anticarcinogenic, and immunomodulatory properties [37–39]. Lactobacillus plantarum (L. plantarum) is the predominant Lactobacillus species on oral and intestinal human mucosa, and has been shown to survive passage through the human gastrointestinal tract and to establish itself in the intestine after consumption [30, 40]. Bosch et al. 2012 [41] isolated lactic acid bacteria from feces of healthy infants to investigate candidate strains with potential cholesterol-lowering functions. Three L. plantarum strains (CECT 7527, 7528, and 7529) in particular were shown to have the capacity to deconjugate bile acids, assimilate cholesterol, and produce short-chain fatty acids. A mixed culture of these three strains produced better results than individually, and has been given a patent as a hypocholesterolemic agent (EP 2485743 B1) [42]. The safety of these strains has been established in in vitro and in animal models [43]. The aim of the current study was to assess the capacity of this proprietary combination of L. plantarum strains (marketed as AB-Life by AB Biotics, S.A., Barcelona, Spain) to lower LDL-C and other lipoprotein lipid variables in adults with cholesterol levels above the desirable range.
2Materials and methods
2.1Study design
This was a randomized, double-blind placebo-controlled, parallel arm trial conducted at Moncloa Hospital in Madrid, Spain. Subjects were chosen for recruitment by consulting their clinical records and a database of volunteers at the clinical research site. The study included five clinic visits at screening/baseline, the start of the treatment period (week 0), midpoint of the treatment period (week 6), end of the treatment period (week 12), and 4-weeks following the end of the treatment period (week 16). The first subject was enrolled on April 5, 2010, and the last subject completed the study on September 23, 2010. Approval for this study was given by the Institutional Review Board/Independent Ethics Committee of the Hospital Universitario Puerta de Hierro Majadahonda (Madrid, Spain). The study was conducted according to the Declaration of Helsinki, Good Clinical Practice Standards (2000). Subjects were informed of the characteristics of the study both verbally and in writing, and gave informed consent. They were also informed that they could discontinue the study at anytime.
2.2Subjects
Subjects were selected based on results of a detailed medical history, general physical examination, and a blood test. To be included, subjects with less than two major cardiovascular risk factors were required to be aged 18–65 y and have serum total-C 200-300 mg/dL (5.2–7.8 mmol/L) and LDL-C 130–190 mg/dL (3.4–4.9 mmol/L) while not on lipid-altering therapy. For subjects with at least two major cardiovascular risk factors or known atherosclerotic cardiovascular disease, the LDL-C level for eligibility was between 100 and 190 mg/dL (2.6–4.9 mmol/L). Subjects were also required to have body mass index within the range of 19.0–29.9 kg/m2.
Individuals with any of the following were not enrolled in the study: TG >350 mg/dL (3.95 mmol/L), any ischemic cardiovascular event during the previous 6 months, and hypersensitivity to any of the components of the study products. Women who were pregnant or breastfeeding were not enrolled. Use of lipid-altering medications and any other treatment for hypercholesterolemia was not allowed during the 4 weeks prior to the start of the study. Intake of products enriched with plant sterols, drugs with laxative effects, drugs that require close monitoring of levels (e.g., warfarin), or more than four concomitant treatments was also not allowed. Any medications/therapies that the subject was taking were required to be stable for at least 3 months prior to the start of the study.
Subjects were specifically instructed at the start of the study not to begin or change any hormone treatment, and to not significantly change their coffee drinking or alcohol intake habits. Subjects were provided with lifestyle recommendations from the Estrategia Naos, Spanish Strategy for nutrition, physical activity and prevention of obesity [44], but they did not receive a specific diet during the study.
2.3Study products
Using computer-generated random numbers, subjects were assigned to receive either L. Plantarum probiotic (LpPRO) or placebo (PLBO) treatments. Neither the investigators nor the participants knew to which group the participant was assigned. Study products consisted of gelatin capsules of plant origin. One capsule was taken daily with water at breakfast. Compliance with study product consumption was assessed by counting unused capsules returned to the clinic by the participants at study visits.
The composition of the study product capsules is shown in Table 1. The LpPRO product is a proprietary formulation marketed as AB-Life by AB-Biotics S.A. (Barcelona, Spain). The probiotic formula was a mixture of three strains (1 : 1:1) of L. plantarum deposited in the Spanish Type Culture Collection under the following codes: CECT 7527, CECT 7528, and CECT 7529. The industrial production and lyophilization of L. plantarum strains was performed by Sacco S.R.L. (Cadorago, Italy), and the production of the study product capsules was performed by Laboratorios Salvat S.A. (Barcelona, Spain). The capsules contained excipients to ensure the stability of the lyophilized probiotic bacteria. PLBO had exactly the same composition as LpPRO, but without the probiotic. Capsules were stored in a dark, cool, dry place and the level of Lactobacilli in the LpPRO capsules was determined to be relatively constant throughout the trial. The initial level measured when the batch of capsules was produced was 1×1010 colony forming units (CFU)/capsule; when the first participant was enrolled, the concentration was 3.01×109 CFU/capsule; and at the time of the treatment period completion, the level was 1.28×109 CFU/capsule.
2.4Measurements
Fasting blood samples were collected at screening/baseline and weeks 6, 12, and 16 for measurements of serum lipid and lipoprotein cholesterol levels using Analyzer BA400 (Biosystems S.A., Barcelona, Spain); serum oxidized LDL by enzyme-linked immunosorbent assay (ELISA) using the Mercodia® 10-1143-01 kit (Uppsala, Sweden); and serum creatinine, glucose, gamma glutamyl transpeptidase (GGT), glutamate oxaloacetate transaminase (GOT), and glutamate pyruvate transaminase (GPT) using Analyzer BA400. Anthropometrics (body weight and body mass index) were measured at every clinic visit using TANITA BF3000 scales (Tanita Corporation of America, Inc., Arlington Heights, IL, USA). Any adverse events were recorded at the clinic visits by the investigator.
2.5Statistical analyses
A sample size of 30 subjects per group was estimated to provide 80% statistical power to detect an LDL-C reduction of ∼23 mg/dL compared with the placebo response for LpPRO treatment with 5% significance, two-sided, assuming a standard deviation of 30 mg/dL. To ensure adequate representation of both sexes, the difference in participation between men and women was targeted not to exceed 20%.
Statistical analyses were conducted using SPSS for Windows, version 18 software (PASW Statistics, formerly SPSS Inc., Chicago, IL, USA). The primary endpoint variable was the difference between LpPRO and PLBO groups in the change from baseline to week 12 in LDL-C. Because the 12-week timepoint was considered primary, no adjustments to the alpha level were made for multiple comparisons of values at secondary timepoints (weeks 6 and 16). Baseline characteristics were compared between treatment groups using chi-square tests (for qualitative data) and the Student’s t-test (for continuous data). Efficacy and safety variables were analyzed by two-sample t-tests. Statistical significance was defined as a 2-sided p-value <0.05. To discriminate between models assuming equal variances, or not, Levene’s test was used.
3Results
Sixty subjects were randomized to receive either the LpPRO (n = 30) or PLBO (n = 30) treatments and all completed the full length of the study, including the 4-week follow-up after the completion of the treatment period (Fig. 1). Demographic and clinical characteristics were similar between treatment groups (Table 2). Overall, 43.3% of the subjects were female, and subjects had a mean age of 51.8±7.2 years and mean body mass index (BMI) of 26.2±2.5 kg/m2. Mean±standard deviation baseline serum LDL-C and total-C levels were 167.5±20.5 mg/dL (4.3±0.5 mmol/L) and 250.0±27.5 mg/dL (6.5±0.7 mmol/L), respectively. There has been no participant from poor compliance or noncompliance
The serum lipid profile and serum oxidized LDL concentrations at baseline and changes from baseline to 6, 12, and 16 weeks for LpPRO and PLBO treatment groups are shown in Table 3. There were no statistically significant differences between LpPRO and PLBO groups in any of the lipid and lipoprotein lipid parameters measured at baseline. After 6 weeks of treatment, LDL-C, total-C, the LDL-C/HDL-C ratio, and oxidized LDL were reduced from baseline in both treatment groups, but there were no significant differences in the responses between LpPRO and PLBO. The LDL-C concentration at baseline and 6, 12, and 16 weeks is shown in Fig. 2. The TG concentration was reduced from baseline at 6 weeks in the LpPRO (but not PLBO) group, but the PLBO and LpPRO responses to treatment were not significantly different. The HDL-C concentration was increased from baseline at 6 weeks in both treatment groups, but there was no difference in responses between groups at this time point.
After 12 weeks of treatment, LDL-C decreased in both the LpPRO and PLBO groups, but the reduction in the LpPRO group was significantly larger (24.4±11.8 mg/dL [0.6±0.3 mmol/L] vs. 9.8±5.3 mg/dL [0.3±0.1 mmol/L], p < 0.001 between groups) (Fig. 2). The reduction in LDL-C persisted to the end of the 4-week follow-up period of no treatment (–20.5±12.1 mg/dL [–0.5±0.3 mmol/L] vs. –9.5±6.1 mg/dL [–0.2±0.2 mmol/L], for LpPRO and PLBO, respectively; p < 0.001 between groups). Total-C, the LDL-C/HDL-C ratio, and oxidized LDL responded similarly to LDL-C in that concentrations were reduced from baseline at both weeks 12 and 16 in the LpPRO and PLBO groups, and the reduction from baseline in the LpPRO group was significantly larger than the PLBO response (p < 0.001 for all).
The HDL-C concentration at weeks 12 and 16 was increased from baseline in the LpPRO group, but not the PLBO group, and the difference in responses between groups was statistically significant at both time points (p < 0.001). The TG concentration was decreased from baseline at both 12 and 16 weeks in the LpPRO group, but not the PLBO group, and the difference in responses between groups was statistically significant at both time points (p < 0.05 for both).
Body weight and BMI decreased significantly from baseline in both treatment groups throughout the treatment and follow-up periods, but the changes were not statistically significantly different between groups. In general, glucose, creatinine, GOT, GPT, and GGT concentrations remained stable and were within physiological limits at all timepoints and there were no significant differences between treatment groups in changes from baseline. There were no treatment-related adverse events reported to the investigators.
4Discussion
The results of this study demonstrate that ingestion of a proprietary combination of three strains of L. plantarum by 60 men and women with hypercholesterolemia, produced an 8.4% greater LDL-C reduction (the difference between groups in terms of percentual changes compared to baseline values computed for each subject), compared to placebo, after 12 weeks of ingestion, and significantly altered the concentrations of total-C (–9.0%), HDL-C (+5.5%), the LDL-C/HDL-C ratio (–12.8%), TG (–9.0%), and oxidized LDL (–11.3%). The magnitude of the LDL-C reduction is similar to that shown for recommended dietary interventions such as plant sterols and viscous fibers [4, 6, 12, 45].
Previous studies of the effects of probiotic products on lipoprotein lipids and other cardiovascular disease risk factors have produced mixed results, depending on the probiotic organisms and strains evaluated [23, 25–28]. In a meta-analysis of 13 randomized, controlled trials of the effects of probiotics on lipoprotein lipids in subjects with normal to mildly elevated cholesterol levels, Guo et al. 2011 [26] reported pooled estimates for control-adjusted effects for LDL-C of –4.9 mg/dL (95% confidence interval –9.9 to –2.9 mg/dL), for total-C of –6.4 mg/dL (–7.9 to –1.9 mg/dL), for HDL-C of –0.11 mg/dL (–1.9 to –1.69 mg/dL), and for TG of –3.95 mg/dL (–10.32 to –2.42 mg/dL).
The lipid responses to LpPRO were not maximal after 6 weeks, and did not reach statistical significance compared with PLBO until the 12-week time point. These findings are consistent with the hypothesis that it requires several weeks of administration for the metabolic effects of probiotics to become evident, presumably because time is required for the probiotic strains to become established in the intestine. It is also notable that no material narrowing of the differences between treatment groups was evident at the conclusion of the 4-week post-treatment follow-up period, suggesting that shifts in gut microbiota were maintained for at least this length of time after the probiotic was discontinued.
The effects of probiotics to enhance fermentation and thus increase the colonic production of short-chain fatty acids may help to explain the TG-lowering effect for LpPRO in this study, and the associated HDL-C raising effect. Changes in TG and HDL-C are often inversely related because a reduction in TG reduces the exchange of TG on TG-rich lipoprotein particles for cholesteryl ester from HDL (and LDL) particles, catalyzed by the enzyme cholesteryl ester transfer protein [46, 47]. Circulating short-chain fatty acids activate adipose tissue G-protein coupled receptors [48, 49], which suppress the release of free fatty acids from adipose tissues into the circulation [48, 50, 51]. Thus, the availability of substrate for hepatic production of TG is decreased [46]. Lowering the circulating level of free fatty acids may also increase insulin sensitivity, and enhance the ability of insulin to activate lipoprotein lipase, thus enhancing TG clearance [52].
Although some studies in animals, and a limited number in humans, have shown anti-inflammatory and anti-oxidant effects of probiotics [53, 54], the reduction of oxidized LDL with LpPRO is a novel finding. Elevated levels of oxidized LDL have been shown to be associated with increased risk for the development and progression of atherosclerosis [55]. Oxidized fatty acids and proteins within LDL particles may stimulate unregulated uptake of LDL particles by macrophages within the arterial wall and by monocytes in circulation, which enhances pro-inflammatory and oxidative processes [56]. Thus, a reduction in oxidized LDL is theoretically favorable. However, to date, insufficient evidence exists from intervention studies to fully understand the clinical implications of reducing oxidized LDL [55]. Additional research is needed to better define the mechanistic links and clinical implications of the reduced level of oxidized LDL observed with LpPRO administration.
Measures of safety (including anthropometric characteristics and biochemical laboratory tests) indicated that LpPRO was safe and well tolerated by the subjects. There were no adverse events related to the study products. Changes in safety laboratory values did not differ between treatment groups and were all within normal physiological limits.
This study was not designed to evaluate the mechanisms of the cholesterol-lowering effect of LpPRO, nor was it designed to determine whether lowering cholesterol levels with ingestion of LpPRO would result in reduced cardiovascular disease event risk. Results from a clinical trial of the drug ezetimibe, which reduces cholesterol absorption, showed that the reduction in LDL-C produced by this mechanism lowered cardiovascular disease event risk [57]. This provides suggestive evidence that reducing cholesterol absorption through the use of dietary adjuncts that lower cholesterol absorption, e.g., with probiotics, plant sterols and viscous fibers, might also reduce CVD event risk, although this remains to be directly demonstrated in randomized, controlledtrials.
5Conclusions
The results of this double-blind, placebo-controlled, parallel arm study indicate that consumption of a probiotic product comprised of a proprietary mixture of three strains of L. plantarum was safe, well tolerated, and reduced levels of LDL-C, total-C, oxidized LDL, and TG significantly more than PLBO, while also significantly raising HDL-C, in men and women with hypercholesterolemia.
Acknowledgments
We thank Ana Jurczynska for her technical assistance during this study. We also thank Marco Puma Duque for assisting in the manuscript preparation. This study received external funding from the Ministry of Education and Science of Spain (PTQ05-02-02782), the CDTI-Neotec Project IDI-2006-0244 ‘Development of probiotic products with specific effects’ and the CDTI-PID Project IDI-20101629 ‘Clinical Assays of AB LIFE, AB 13.1 and AB FORTIS’. M. C. F. prepared the manuscript; T. L. and J. M. C. conducted the research; J. C. conducted the research, designed the study and performed the statistical analysis. All authors read and approved the final manuscript. J. C. and M. C. F. are employed by AB-BIOTICS, SA and report a conflict of interest. All other contributors have no conflicts of interest to report.
References
[1] | Nichols M , Townsend N , Scarborough P , Rayner M . Cardiovascular disease in Europe: Epidemiological update. European Heart Journal. (2013) ;34: :3028–34. DOI: 10.1093/eurheartj/eht356 |
[2] | Fuster V . Global burden of cardiovascular disease: Time to implement feasible strategies and to monitor results. J Am Coll Cardiol. (2014) ;64: (5):520–2. DOI: 10.1016/j.jacc.2014.06.1151 |
[3] | Gielen S , Landmesser U . The Year in Cardiology Cardiovascular disease prevention. Eur Heart J [Internet]. (2014) ;35: (5):307–12. DOI: 10.1093/eurheartj/eht551. |
[4] | Grundy SM , Arai H , Barter P , Bersot TP , Betteridge DJ , Carmena R , et al. An international atherosclerosis society position paper: Global recommendations for the management of dyslipidemia – Full report. J Clin Lipidol. (2014) ;8: (1):29–60. DOI: 10.1016/j.jacl.2013.12.005 |
[5] | Stone NJ , Robinson JG , Lichtenstein AH , Bairey Merz CN , Blum CB , Eckel RH , et al. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. (2014) ;63: (25 PART B):2889–934. DOI: 10.1016/j.jacc.2013.11.002 |
[6] | Jacobson TA , Ito MK , Maki KC , Orringer CE , Bays HE , Jones PH , et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: Part 1 – executive summary. J Clin Lipidol [Internet]. Mosby, Inc.; (2015) ;9: (2):129–69. DOI: 10.1016/j.jacl.2015.02.003. Available from: http://dx.doi.org/10.1016/j.jacl.2014.07.007 |
[7] | Chapman MJ , Ginsberg HN , Amarenco P , Andreotti F , Borén J , Catapano AL , et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: Evidence and guidance for management. European Heart Journal. (2011) ;32: :1345–261. DOI: 10.1093/eurheartj/ehr112 |
[8] | Law MR , Wald NJ , Thompson SG . By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease? BMJ. (1994) ;308: (6925):367–72. DOI: 10.1136/bmj.308.6925.367 |
[9] | Law MR , Wald NJ , Rudnicka AR . Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: Systematic review and meta-analysis. BMJ [Internet]. (2003) ;326: (7404):1423. DOI: 10.1136/bmj.326.7404.1423. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=162260&tool=pmcentrez&rendertype=abstract |
[10] | Brown MS , Goldstein JL . Lowering LDL— Not Only How Low, but how long? Science (80-). (2006) ;311: (5768):1721–3. DOI: 10.1126/science.1125884 |
[11] | The Myocardial Infarction Genetics Investigators Consortium. Inactivating Mutations in NPC1L1 and Protection from Coronary Heart Disease. N Engl J Med [Internet]. (2014) ;(12Nov14):11. DOI: 10.1056/NEJMoa1405386. Available from: http://www.nejm.org/doi/abs/10.1056/NEJMoa1405386 |
[12] | Jacobson TA , Maki KC , Orringer C , Jones P , Kris-Etherton P , Sikand G , et al. National lipid association recommendations for patient-centered management of dyslipidemia: Part 2. J Clin Lipidol [Internet]. Elsevier Inc; (2015) ;9: (6):S1–122.e1. DOI: http://dx.doi.org/10.1016/j.jacl.2015.09.002. Available from: http://www.sciencedirect.com/science/article/pii/S1933287415003803 |
[13] | Reid G , Jass J , Sebulsky MT , McCormick JK . Potential uses of probiotics in clinical practice. Clinical Microbiology Reviews. (2003) ;16: :658–72. DOI: 10.1128/CMR.16.4.658-672.2003 |
[14] | WHO. World Health Organization. Food and Agriculture Organization of the United Nations. Report of the Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacter. (2006) . DOI: 10.1186/1471-2180-14-112 |
[15] | Prakash S , Tomaro-Duchesneau C , Saha S , Cantor A . The gut microbiota and human health with an emphasis on the use of microencapsulated bacterial cells. Journal of Biomedicine and Biotechnology. (2011) :981214. DOI: 10.1155/2011/981214 |
[16] | Marteau BP . Safety aspects of probiotic products. Scand J Nutr. (2001) ;45: :22–4. |
[17] | Chettipalli ND , Santosh P , Raj P . Evaluation of the various uses of microorganisms with emphasis on probiotics. J Microb Biochem Technol [Internet]. (2011) ;03: (04):1–7. DOI: 10.4172/1948-5948.R1-004. Available from: http://www.omicsonline.org/1948-5948/JMBT-R1-004.digital/JMBT-R1-004.html |
[18] | Nagpal R , Behare PV , Kumar M , Mohania D , Yadav M , Jain S , et al. Milk, milk products, and disease free health: An updated overview. Crit Rev Food Sci Nutr [Internet]. (2012) ;52: (4):321–33. DOI: 10.1080/10408398.2010.500231. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22332596 |
[19] | Nagpal R , Kumar A , Kumar M , Behare PV , Jain S , Yadav H . Probiotics, their health benefits and applications for developing healthier foods: A review. FEMS Microbiology Letters. (2012) ;334: :1–15. DOI: 10.1111/j.1574-6968.2012.02593.x |
[20] | Gilliland SE , Nelson CR , Maxwell C . Assimilation of cholesterol by Lactobacillus acidophilus. Appl Environ Microbiol. (1985) ;49: (2):377–81. |
[21] | Danielson AD , Peo ER , Shahani KM , Lewis AJ , Whalen PJ , Amer MA . Anticholesteremic property of Lactobacillus acidophilus yogurt fed to mature boars. J Anim Sci. (1989) ;67: (4):966–74. |
[22] | De Smet I , De Boever P , Verstraete W . Cholesterol lowering in pigs through enhanced bacterial bile salt hydrolase activity. Br J Nutr [Internet]. (1998) ;79: (2):185–94. DOI: 10.1079/BJN19980030. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9536863 |
[23] | Agerholm-Larsen L , Bell ML , Grunwald GK , Astrup A . The effect of a probiotic milk product on plasma cholesterol: A meta-analysis of short-term intervention studies. Eur J Clin Nutr [Internet]. (2000) ;54: (11):856–60. DOI: 10.1038/sj.ejcn.1601104. Available from: www.ebscohost.com |
[24] | Liong MT , Shah NP . Bile salt deconjugation ability, bile salt hydrolase activity and cholesterol co-precipitation ability of lactobacilli strains. Int Dairy J. (2005) ;15: (4):391–8. DOI: 10.1016/j.idairyj.2004.08.007 |
[25] | Del Piano M , Morelli L , Strozzi GP , Allesina S , Barba M , Deidda F , et al. Probiotics: From research to consumer. Dig Liver Dis. (2006) ;38: (Suppl.2). DOI: 10.1016/S1590-8658(07)60004-8 |
[26] | Guo Z , Liu XM , Zhang QX , Shen Z , Tian FW , Zhang H , et al. Influence of consumption of probiotics on the plasma lipid profile: A meta-analysis of randomised controlled trials. Nutrition, Metabolism and Cardiovascular Diseases. (2011) ;21: :844–50. DOI: 10.1016/j.numecd.2011.04.008 |
[27] | Dirienzo DB . Effect of probiotics on biomarkers of cardiovascular disease: Implications for heart-healthy diets. Nutr Rev. (2014) ;72: (1):18–29. DOI: 10.1111/nure.12084 |
[28] | Ettinger G , MacDonald K , Reid G , Burton JP . The influence of the human microbiome and probiotics on cardiovascular health. Gut Microbes [Internet]. (2014) ;5: (6):719–28. DOI: 10.4161/19490976.2014.983775. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4615746&tool=pmcentrez&rendertype=abstract |
[29] | Ooi LG , Liong MT . Cholesterol-lowering effects of probiotics and prebiotics: A review of in Vivo and in Vitro Findings. Int J Mol Sci. (2010) ;11: (6):2499–22. DOI: 10.3390/ijms11062499 |
[30] | Kumar M , Nagpal R , Kumar R , Hemalatha R , Verma V , Kumar A , et al. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Experimental Diabetes Research. (2012) ;2012. DOI: 10.1155/2012/902917 |
[31] | Sanders TA . Food production and food safety. Bmj. (1999) ;318: (7199):1689–93. |
[32] | Johnson BJH , Lee JY , Pickert A , Urbatsch IL . Bile acids stimulate atp hydrolysis in the purified cholesterol transporter ABCG5/G8. Biochemistry. (2010) ;49: (16):3403–11. DOI: 10.1021/bi902064g |
[33] | Jones ML , Tomaro-Duchesneau C , Martoni CJ , Prakash S . Cholesterol lowering with bile salt hydrolase-active probiotic bacteria, mechanism of action, clinical evidence, and future direction for heart health applications. Expert Opin Biol Ther. (2013) ;13: (5):631–42. DOI: 10.1517/14712598.2013.758706 |
[34] | Kimoto H , Ohmomo S , Okamoto T . Cholesterol removal from media by lactococci. J Dairy Sci [Internet]. (2002) ;85: (12):3182–88 DOI: 10.3168/jds.S0022-0302(02)74406-8. Available from: http://dx.doi.org/10.3168/jds.S0022-0302(02)74406-8 |
[35] | Lye HS , Rahmat-Ali GR , Liong MT . Mechanisms of cholesterol removal by lactobacilli under conditions that mimic the human gastrointestinal tract. Int Dairy J. (2010) ;20: (3):169–75. DOI: 10.1016/j.idairyj.2009.10.003 |
[36] | Gunness P , Gidley MJ . Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food Funct. (2010) ;1: (2):149–55 DOI: 10.1039/c0fo00080a |
[37] | Pereira DIA , Gibson GR . Effects of consumption of probiotics and prebiotics on serum lipid levels in humans. Crit Rev Biochem Mol Biol. (2002) ;37: (4):259–81. DOI: 10.1080/10409230290771519 |
[38] | Kumar M , Kumar A , Nagpal R , Mohania D , Behare P , Verma V , et al. Cancer-preventing attributes of probiotics: An update. Int J Food Sci Nutr. (2010) ;61: (5):473–96. DOI: 10.3109/09637480903455971 |
[39] | Shiby VK , Mishra HN . Fermented milks and milk products as functional foods–a review. Crit Rev Food Sci Nutr [Internet]. (2013) ;53: (5):482–96. DOI: 10.1080/10408398.2010.547398. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23391015 |
[40] | Ranganathan N , Friedman EA , Tam P , Rao V , Ranganathan P , Dheer R . Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: A 6-month pilot scale trial in Canada. Curr Med Res Opin. (2009) ;25: (8):1919–30. DOI: 10.1185/03007990903069249 |
[41] | Bosch M , Rodriguez M , Garcia F , Fernández E , Fuentes MC , Cuñé J . Probiotic properties of Lactobacillus plantarum CECT and CECT isolated from faeces of healthy children. Lett Appl Microbiol. (2012) ;54: (3):240–6. DOI: 10.1111/j.1472-765X.2011.03199.x |
[42] | Google Patents. Lactobacillus plantarum strains as hypocholesterolemic agents. EP 2485743 B1. http://www.google.com/patents/EP743B1?cl=en |
[43] | Bosch M , Fuentes MC , Audivert S , Bonachera MA , Peiró S , Cuñé J . Lactobacillus plantarum CECT and Probiotic candidates to reduce cholesterol levels. J Sci Food Agric. (2014) ;94: (4):803–9. DOI: 10.1002/jsfa.6467 |
[44] | Estrategia Naos, Spanish Strategy for nutrition physical activity and prevention of obesity. Retrieved from http://wwwn.naos.aesan.msssi.gob.es/naos/ficheros/estrategia/NAOS_Strategy.pdf |
[45] | National Cholesterol Education Program (NCEP) Expert Panel. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Arch Intern Med [Internet]. (2002) (6):284. DOI: 10.1001/archinte.1991.00400060019005. Available from: https://www.nhlbi.nih.gov/guidelines/cholesterol/atp3_rpt.htm |
[46] | Packard CJ . Triacylglycerol-rich lipoproteins and the generation of small, dense low-density lipoprotein. Biochem Soc Trans. (2003) ;31: (Pt 5):1066–9. DOI: 10.1042/BST0311066 |
[47] | Szapary PO , Rader DJ . The triglyceride-high-density lipoprotein axis: An important target of therapy? American Heart Journal. (2004) ;148: :211–21. DOI: 10.1016/j.ahj.2004.03.037 |
[48] | Sleeth ML , Thompson EL , Ford HE , Zac-Varghese SEK , Frost G . Free fatty acid receptor 2 and nutrient sensing: A proposed role for fibre, fermentable carbohydrates and short-chain fatty acids in appetite regulation. Nutr Res Rev. (2010) ;23: (1):135–45. DOI: 10.1017/S0954422410000089 |
[49] | Kimura I , Inoue D , Hirano K , Tsujimoto G . The SCFA receptor GPR43 and energy metabolism. Frontiers in Endocrinology. (2014) ;5. DOI: 10.3389/fendo.2014.00085 |
[50] | Robertson MD , Currie JM , Morgan LM , Jewell DP , Frayn KN . Prior short-term consumption of resistant starch enhances postprandial insulin sensitivity in healthy subjects. Diabetologia. (2003) ;46: (5):659–65. DOI: 10.1007/s00125-003-1081-0 |
[51] | Robertson MD , Bickerton AS , Dennis AL , Vidal H , Frayn KN . Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr. (2005) ;82: (3):559–67. |
[52] | Ginsberg HN , Zhang YL , Hernandez-Ono A . Regulation of plasma triglycerides in insulin resistance and diabetes. Arch Med Res [Internet]. (2005) ;36: (3):232–40. DOI: 10.1016/j.arcmed.2005.01.005. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15925013 |
[53] | National Osteoporosis Foundation. Fast facts. Accessed 24 Jan ((2014) ) [Internet]. Available from: http://www.nof.org/connect/get-the-facts |
[54] | Carvalho BM , Abdalla Saad MJ . Influence of Gut microbiota on subclinical inflammation and insulin resistance. Mediators of Inflammation. (2013) ;2013. DOI: 10.1155/2013/986734 |
[55] | Parthasarathy S , Raghavamenon A , Garelnabi MO , Santanam N . Oxidized low-density lipoprotein. Methods Mol Biol. (2010) ;610: (1):403–17. DOI: 10.1007/978-1-60327-029-8_24 |
[56] | Steinberg D , Witztum JL . Oxidized low-density lipoprotein and atherosclerosis. Arterioscler Thromb Vasc Biol. (2010) ;30: (12):2311–6. DOI: 10.1161/ATVBAHA.108.179697 |
[57] | Cannon CP , Blazing MA , Giugliano RP , McCagg A , White JA , Theroux P , et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med [Internet]. (2015) ;372: (25):2387–97. DOI: 10.1056/NEJMoa1410489. Available from: http://www.nejm.org/doi/abs/10.1056/NEJMoa1410489\nhttp://www.ncbi.nlm.nih.gov/pubmed/26039521 |
Figures and Tables
Fig.1
Disposition of subjects throughout the study. Abbreviations: LpPRO = Lactobacillus plantarum-containing probiotic, PLBO = placebo.
Fig.2
Mean (standard deviation) LDL-C concentrations (mg/dL) at each timepoint for subjects receiving LpPRO or PLBO capsules. *p < 0.001 between treatments from the unpaired t-test. To convert mg/dL to mmol/L (SI unit) for cholesterol, multiply by 0.02586. Abbreviations: LpPRO = Lactobacillus plantarum-containing probiotic, PLBO = placebo.
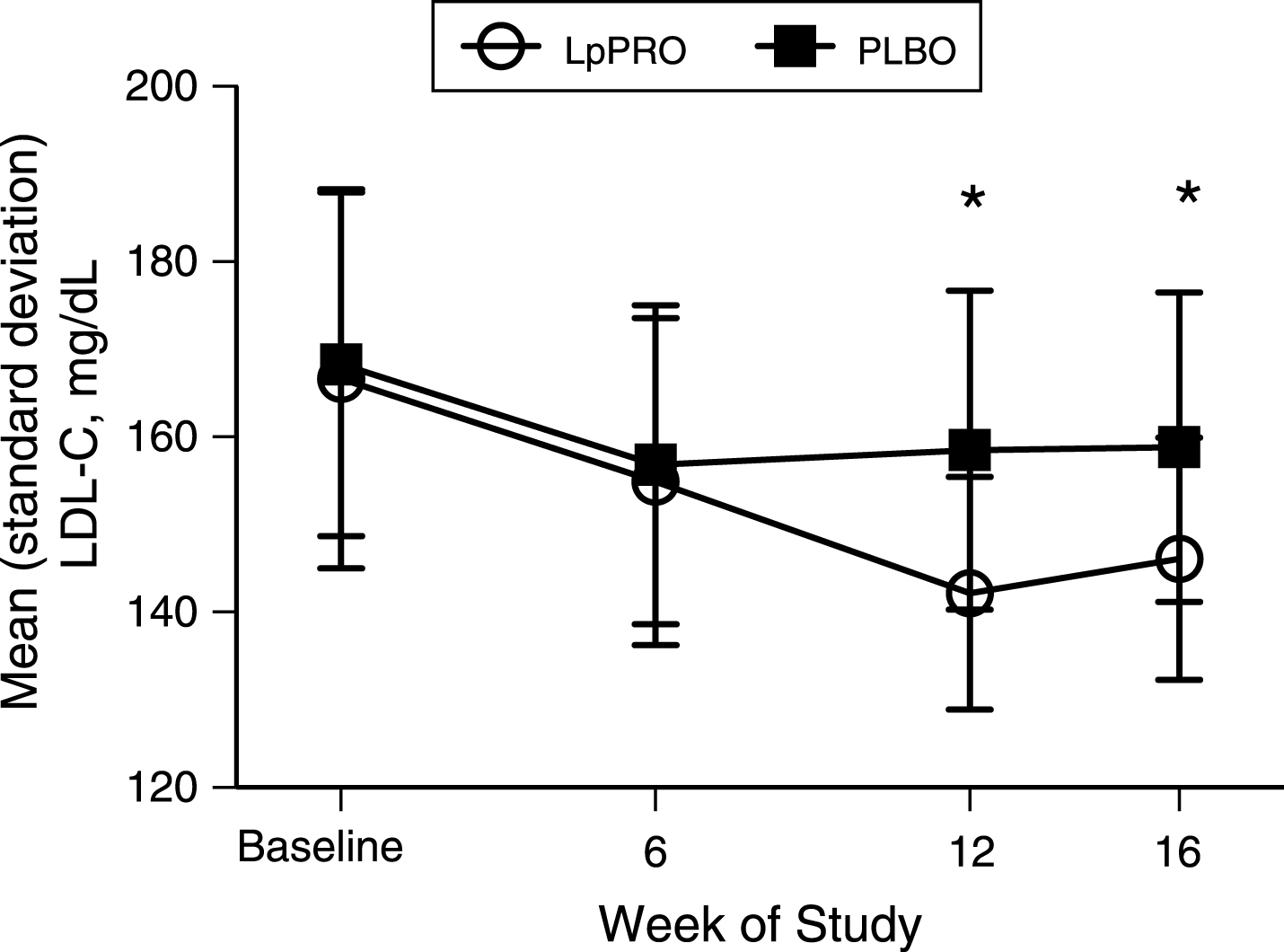
Table 1
Composition of study product capsules
| Component | LpPRO Capsules | PLBO Capsules |
| Per Capsule (mg) | ||
| Probiotic mixture of Lactobacillus plantaruma | 100.0 | 0.0 |
| Corn starch | 0.0 | 100.0 |
| Microcrystalline cellulose, pH 10.2 | 71.1 | 71.1 |
| Talc | 2.6 | 2.6 |
| Magnesium stearate | 1.3 | 1.3 |
aMixture contained a combination of 3 strains of Lactobacillus plantarum (1 :1:1) at >1.2×109 colony forming units. Abbreviations: LpPRO = Lactobacillus plantarum-containing product, PLBO = placebo.
Table 2
Demographic and other baseline characteristics of subjects assigned to LpPRO or PLBO treatmentsa
| Characteristic | LpPRO (n = 30) | PLBO (n = 30) |
| Sex (male/female) | 19/11 | 15/15 |
| Age, y | 51.2 (7.7) | 52.3 (6.7) |
| Systolic blood pressure (mm Hg) | 114.4 (15.7) | 115.8 (15.1) |
| Diastolic blood pressure (mm Hg) | 71.0 (8.4) | 71.9 (10.0) |
| Heart rate (bpm) | 74.3 (8.5) | 76.7 (9.5) |
aValues, except for sex, are means (standard deviation). There were no significant differences between treatment groups at baseline assessed by an unpaired t-test. Abbreviations: LpPRO = Lactobacillus plantarum-containing product, PLBO = placebo.
Table 3
Serum lipid profile and serum oxidized LDL at baseline and changes from baseline to weeks 6, 12, and 16a
| Parameter | LpPRO (n = 30) | PLBO (n = 30) | P-valueb |
| LDL-C, mg/dL | |||
| Baseline | 166.6 (21.6) | 168.3 (19.6) | NS |
| Δ Week 6 | –11.7 (5.5) | –11.5 (5.4) | NS |
| Δ Week 12 | –24.4 (11.8) | –9.8 (5.3) | <0.001 |
| Δ Week 16 | –20.5 (12.1) | –9.5 (6.1) | <0.001 |
| Total-C, mg/dL | |||
| Baseline | 247.4 (31.3) | 252.6 (4.3) | NS |
| Δ Week 6 | –14.5 (11.0) | –13.3 (13.0) | NS |
| Δ Week 12 | –33.7 (18.1) | –10.6 (13.1) | <0.001 |
| Δ Week 16 | –27.1 (17.5) | –9.7 (15.2) | <0.001 |
| HDL-C, mg/dL | |||
| Baseline | 44.2 (6.9) | 46.3 (10.3) | NS |
| Δ Week 6 | 0.5 (1.2) | 0.4 (1.0) | NS |
| Δ Week 12 | 2.9 (2.1) | 0.4 (1.7) | <0.001 |
| Δ Week 16 | 2.8 (2.1) | 0.3 (1.9) | <0.001 |
| LDL-C/HDL-C ratio | |||
| Baseline | 3.9 (0.8) | 3.8 (0.9) | NS |
| Δ Week 6 | –0.3 (0.1) | –0.3 (0.2) | NS |
| Δ Week 12 | –0.8 (0.3) | –0.3 (0.2) | <0.001 |
| Δ Week 16 | –0.7 (0.3) | –0.2 (0.2) | <0.001 |
| TG, mg/dL | |||
| Baseline | 179.8 (70.9) | 189.2 (73.6) | NS |
| Δ Week 6 | –12.2 (30.4) | –7.0 (47.6) | NS |
| Δ Week 12 | –29.1 (39.5) | –4.1 (44.6) | <0.05 |
| Δ Week 16 | –33.3 (47.0) | –6.5 (52.2) | <0.05 |
| Oxidized LDL, U/L | |||
| Baseline | 54.7 (11.0) | 56.4 (10.0) | NS |
| Δ Week 6 | –3.3 (1.0) | –3.7 (1.0) | NS |
| Δ Week 12 | –7.5 (3.6) | –1.0 (2.0) | <0.001 |
| Δ Week 16 | –6.4 (3.5) | –0.8 (2.5) | <0.001 |
aValues are means (standard deviation). To convert mg/dL to mmol/L (SI unit) for cholesterol, multiply by 0.02586; to convert mg/dL to mmol/L (SI unit) for TG, multiply by 0.00129. bP-values represent differences between treatment groups from an unpaired t-test. Abbreviations: HDL-C = high-density lipoprotein cholesterol, LDL = low-density lipoprotein, LDL-C = low-density lipoprotein cholesterol, LpPRO = Lactobacillus plantarum-containing product, NS = not statistically significant, PLBO = placebo, TG = triglycerides, total-C = total cholesterol.




