Blood pressure profile and insulin resistance in salt-induced hypertensive rats treated with camel milk
Abstract
BACKGROUND: Hypertension is the most prevalent non-communicable condition in Nigeria and many developing countries; it is recognized as a serious risk factor for cardiovascular diseases and responsible for an estimated 45% of deaths due to heart disease and 51% of deaths due to stroke globally. Several studies testified the health promoting effect of camel milk.
OBJECTIVES: This study was designed to investigate the potentiality of camel milk in the management of hypertension and insulin resistance.
METHOD: Rats were divided into four groups: Group I: control (normal), Group II: salts induced hypertension untreated, Group III: salt-induced hypertension treated with camel milk, Group IV salt-induced hypertension treated with 100 mg/kg Metformin+10 mg/kg Nifedipine. Groups II, III and IV were placed on 8% salt diet for 10 weeks.
RESULT: Significant increase (P < 005) in blood pressure, serum glucose, serum insulin and Homeostasis Model Assessment-Insulin Resistance (HOMA-IR) was observed in Groups II, III and IV compared with control group. Treatment with camel milk lessens the effect of the salt-diet. No significant difference (P > 0.05) was observed between group III and group IV in respect to serum glucose, serum insulin and HOMA-IR. Camel milk supplemented group shows appreciable decrease in SBP and PR from 140 to 121 mmHg and 486 to 384 beats/minute respectively.
CONCLUSION: Camel milk supplementation could be a promising method for the management of hypertension and insulin resistance.
1Introduction
Hypertension is considered to be one of the major public health problems worldwide [1]. It is the most common cardiovascular disease in Black Africans and a major cause of morbidity and mortality among Nigerians [2]. Hypertension is one of the most prevalent non-communicable conditions worldwide; it is responsible for an estimated 45% of deaths due to heart disease and 51% of deaths due to stroke globally [3]. The onset of hypertension is caused by complex interaction between genetic predisposition and environmental factors [4]. Hypertension is associated with the incidence of arthrosclerosis [5]. Previous clinical and epidemiological studies have defined plasma lipoprotein levels such as high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C) and increased total cholesterol (TC) as strong predisposing factors of hypertension [6]. High salt intake is one of the most important environmental risk factor in the pathogenesis of hypertension, which directly causes damage to the vital systems such as central nervous system, renal system and vascular system [7]. Previous studies have shown that there are strong relationships between hypertension and cardiovascular disease [8], with approximately two thirds of stroke and one half of coronary heart disease cases worldwide [9].
It was reported that about 95% of cases of hypertension is of primary type [10]. This kind of hypertension has no identifiable aetiology, and traditionally has been viewed as a consequence of interaction between environmental factors such as sodium intake and genetic factors. Other factors include sedentary life style, visceral obesity, stress and potassium deficiency [11]. All are predisposing factors of essential hypertension characterized by increased peripheral vascular resistance to blood flow Epidemiological studies have shown that, incidence of primary hypertension increases with age, and individuals with relatively high blood pressures at younger ages are at increased risk for the development of primary hypertension [12]. It tends to cluster in families and represents a collection of genetically based diseases or syndromes with several resultants inherited biochemical abnormalities [13] and then may increase risk for cardiac, cerebral, and renal damages. Elevation of renin an enzyme secreted by the kidney, is also a risk factor [14].
The other form of hypertension (secondary hypertension), has an identifiable cause and affecting approximately 5–10% of hypertensive patients [15]. It results from the interplay of several pathophysiological mechanism regulating plasma volume, peripheral vascular resistance and cardiac output, all of which may be increased. Secondary causes include diabetes and Cushing’s syndrome (overproduction of cortisol) [16].
According to the World Health Organization (WHO), approximately 80% of the population in some African and Asian countries and 38% in the Americans depend primarily on complementary and alternative medicine for the prevention, protection and treatment of diseases [17].
Camel milk (CM) is gaining increasing recognition due to its beneficial effects in the control and prevention of multiple health problems [18]. Camel’s milk is an excellent source of well balanced nutrients and also exhibits a range of biological activities that influence digestion, metabolic responses to absorbed nutrients, growth and development of specific organs and resistance to diseases. These biological activities are mainly due to the presence of peptides and protein in milk [19]. Camel milk has been suggested in the management of various diseases [20]. Camel milk has medicinal properties including antibacterial and antiviral activity [21], which may be due to higher concentration of lactoferrin, immunoglobulins, lysozyme and vitamin C which are reported to play a crucial role in the determination of these properties [22]. The milk is used therapeutically against oedema, jaundice, problems of the spleen, tuberculosis, asthma, anaemia, piles and diabetes [23]. Some studies suggested that camel milk, besides being a good nutrient source; it is also an excellent source of components that are involved in some biological activities, one of which is defence against free radicals and reactive oxygen species [24]. It is characterised with low cholesterol, low sugar, high minerals (sodium, potassium, iron, copper, zinc and magnesium), high vitamins (A, B2, C and E) and large concentrations of insulin [25]. The reported health benefits of camel milk justified the great public concern and stimulated our interest to investigate its effects on the management of hypertension.
2Materials and methods
2.1Chemicals and reagents
All the chemicals and reagents used in this study were of analytical grade. Glucose oxidase kit (product of Randox), Insulin assay kit (product of SPI USA) and lipid profile assay kit (product of randox) were used in this work.
2.2Experimental animals
Wistar albino rats of both sexes weighing between 170–220 g were used for the study. The animals were purchased and allowed to acclimatize to the environment for 7 days. All animals were housed in cages (8 rats/ cage), and fed with pelletized growers’ feed (Vital feed, Jos, Nigeria) and allowed access to water ad libitum throughout the experimental period.
2.3Induction of hypertension in rats
The rats were placed on 8% w/w salt diet [26] for 6 weeks, except the control group, and then treated with camel milk for 4 weeks while sustaining salt-diet administration.
2.4Measurement of blood pressure
The baseline blood pressure was measured by tail-cuff method using non-invasive Ugo Basile, series 58500 blood pressure recorder. The average of three readings was taken for each rat and the blood pressure of the rats were monitored throughout the experimental period. Subsequent measurements were done every week.
2.5Collection of milk sample
The milk was collected by cameleer using hand milking from lactating camel (Camelus dromedarius), near Usmanu Danfodiyo University Second Gate at Kwalkwalawa Village, Wammakko Local Gov’t area of Sokoto State, Nigeria. It was collected in a sterile screw jar and kept in a cool container with ice block until transported to the laboratory where it was kept at temperature of –4°C. The pH of the milk was checked every day before administration, to ensure the freshness of the milk.
2.6Grouping of animals
The animals were randomly divided into 4 groups of 8 rats each.
Group I: normal (control group)
Group II: salt-loaded, untreated
Group III: salt-loaded treated with Camel milk (5mls/kg/day) [25]
Group IV: salt-loaded, orally dosed with 100 mg/kg Metformin+10 mg/kg Nifedipine.
2.7Preparation of serum
Twenty four hours after the last treatment and ten hours fasting, the animals were anaesthetised with chloroform vapour and blood samples were collected through cardiac puncture into labelled tubes. The samples collected were allowed to clot and centrifuged at 4000 g for ten minutes. Sera were separated and used in the biochemical analysis
3Biochemical analysis
Insulin was estimated by SPI bio rat insulin enzyme immunoassay kit according to Grassi and Pradelles [27]. The test is based on the competition between unlabelled rat insulin and acetylcholinesterase linked to rat insulin (tracer) for limited specific guinea-pig anti-rat insulin antiserum sites. The complex guinea-pig antiserum-rat insulin binds to the goat anti-guinea-pig antibody that is attached to the well. The plate is then washed and Ellman’s reagent (enzymatic substrate for AChE and chromogen) is added to the wells and the AChE tracer acts on the Ellman’s reagent to form a yellow compound which is determined spectrophotometrically at 405 nm. The intensity of the colour is proportional to the amount of tracer bound to the well and is inversely proportional to the amount of free rat insulin present in the well during the immunological incubation.
Serum glucose was estimated by glucose oxidase method using Randox kit [28].
Insulin resistance index was calculated by Homeostasis Model Assessment- Insulin Resistance (HOMA-IR) as described by Matthews et al. [29]. The equation used is;
Serum total cholesterol (TC) was estimated by enzymatic method using Randox kit [30].
Serum HDL-C was estimated by enzymatic method of Burstein et al. [31] using Randox Kit. Serum Triglyceride was assayed by the method of Tietz [32], using Randox Kit.
Serum LDL-C was calculated using Friedewald formula [33]
Serum VLDL-C was calculated using Friedewald formula [33].
4Data analysis
Data were expressed as mean ± standard deviation of 8 rats in each group. All the biochemical parameters were analysed statistically using one way analysis of variance (ANOVA), using Graph pad instat software (version 5 San Diego, USA). Results were considered statistically significant at p < 005. The data of blood pressure profile (mean ± standard error) was presented in figures.
5Results
The effect of Camel milk supplementation on systolic blood pressure (SBP) and pulse rate (PR) are presented in Figs. 1 and 2 respectively. The result indicated that six weeks administration of 8% w/w salt diet increased the SBP and PR. The elevated SBP and PR were significantly decreased in the group administered with 100 mg/kg Metformin+10 mg/kg Nifedipine, and the group supplemented with camel milk. On the other hand, the salt-loaded untreated group showed a progressive increase of SBP and PR throughout the experimental period.
The effect of camel milk supplementation on serum glucose, insulin and HOMA-IR (which defined insulin resistance) in rats with hypertension is presented in Table 1. The result showed statistically significant decrease (P < 0.05) in serum glucose, insulin levels and HOMA-IR of the camel milk supplemented group and drug treated group as compared with salt-loaded untreated group. On the other hand, there was no significant difference (P > 0.05) between camel milk supplemented group, drug treated group and control; in all the parameters analysed.
The results of the lipid profile of salt-induced hypertensive rats were presented in Table 2. Statistical analysis of the results revealed significant decrease (P < 0.05) in TC, TAG, LDL-C and VLDL-C of the group supplemented with camel milk as compared with salt-induced untreated group. On the other hand, serum level of HDL-C of Camel milk supplemented group increased significantly (P < 0.05) when compared with salt-induced untreated group. The results also showed strong similarities (P > 0.05) in TC, TAG, HDL-C, LDL-C and VLDL-C between the group supplemented with camel and control group, so also no statistical significant different (P > 0.05) is observed between the group supplemented with Camel milk and the group treated with 100 mg/kg Metformin+10 mg/kg Nifedipine.
6Discussion
The present study revealed that the 8% salt-diet used; does not only induce hypertension in Wistar albino rats but also associated with insulin resistance. This finding is consistent with the report of Takehide et al. [34]. It was suggested that salt-induced insulin resistance might be attributable to the overproduction of ROS [35]. Our finding confirmed the reports that salt loading to various strains of rats such as Sprague-Dawley rats [36], Wistar rats [37] and Dahl salt-sensitive rats [38], could result to increased mean arterial blood pressure, inhibition of insulin signalling and induces insulin resistance. Dyslipidaemia, a strong predictor of cardiovascular disease, results from increased production of ROS induced by high salt diet. It causes endothelial damage and the loss of physiological vasomotor activity that results from endothelial damage may manifest as increased blood pressure (BP). Therefore, factors like dyslipidaemia that cause endothelial dysfunction may lead to hypertension. Cross-sectional studies have suggested a link between abnormal lipids and hypertension. A few studies have prospectively examined the relationship between plasma lipids and the future development of hypertension [39]. Several studies have indicated a strong relationship between hypertension, dyslipidaemia, insulin resistance and hyperglycaemia [40], all of which are consequences of over production of reactive oxygen species.
Our study also revealed that the high blood pressure observed in the experimental rats due to the administration of 8% salt-diet, was reversed by the camel milk supplementation. The blood pressure lowering effects of camel milk could be attributed to the presence of ACE-inhibitory peptides precursors in the milk. The amino acid sequence was identified as Ala-Ile-Pro-Pro-Lys-Lys-Asn-Gln-Asp [41]. Angiotensin I-converting enzyme (ACE) is a dipeptidyl carboxypeptidase associated with the regulation of blood pressure. ACE inhibition results in a lowering of blood pressure [41]. Al Haj et al. [42], identified ACE-inhibitory precursor peptides from fermented camel milk, and suggested the presence of Tyr, Arg and Pro at their ultimate C-terminal position, making them a possible candidate for ACE- inhibitory activity. These amino acids within the peptides were previously found to have contributed substantially to ACE-inhibitory potency [43]. Other possible mechanism could be due to the presence of antioxidant vitamins and minerals in the milk thereby exhibiting antioxidant activity either by serving as nucleophillic species or scavenge superoxide anions
Insulin resistance and hyperinsulinaemia are good predictors of metabolic syndrome [44]. Our findings indicated a statistically significant (P < 0.05) decrease in insulin levels and insulin resistance in camel milk supplemented as compared with untreated group. Decreased serum insulin and fasting blood glucose levels observed in camel milk supplemented group, reflected increased peripheral response to insulin, as confirmed by the decreased HOMA-IR when compared to salt-induced untreated group. The high concentration of insulin (40 units/l) and insulin-like protein and the immunoglobulin contents of camel milk [45]. Wangoha et al. [46], identified it as a natural product that not only helps glycemic control but also restore the insulin sensitivity, as evidenced in the camel milk-supplemented animals in the present study.
In addition, salt-loaded rats had elevated levels of plasma total cholesterol (TC), triglycerides (TG), low density lipoprotein-C (LDL-C), very low density lipoprotein-C (LDL-C), and significant decreased (P < 0.05) in high density lipoprotein cholesterol (HDL-C). This could be attributed to increase concentration of sodium in circulation which in turn activates sympathetic nervous system [47] as well as increased signalling through the mineral corticoid receptors (MR) [48]. These may lead to increase production of reactive oxygen species which result to oxidative stress, and finally contribute to aetiopathology of dyslipidaemia [49]. Treatment with camel milk restored the above mentioned changes and improved them towards normal levels in the experimental rats. The obvious amelioration of the hyperlipidemia and dyslipidaemia in the camel milk supplemented group is in agreement with previous reports which stated that fresh and fermented camel milk contains Bifidobacteria, which lower plasma lipids in rats administered with a high-cholesterol diet [50, 51]. The hypolipidemic effect of camel milk could be due to its high content of L-carnitine, which decreases cholesterol absorption [52, 53]. Another possible mechanism is that camel milk may alter the PPAR alpha/SREBP1 ratio, as mentioned in the work conducted by Ziamajidi, et al. [54], which led to enhanced activity of the fat-metabolizing enzymes and hormones, resulting in increased caloric loss or decreased fat storage.
7Conclusion
The present study confirmed the health promoting effect of camel milk as evident of normalizing the elevated blood pressure and improvement of insulin sensitivity in salt–induced hypertensive rats.
References
[1] | Wolf-Maier K , Cooper RS , Banegas JR , Giampaoli S , Hense H , Joffres M , et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. J Amer Med Ass. (2003) ;289: :2363–2369. |
[2] | Cooper RS , Rotimi C . Establishing the epidemiologic basis for prevention of cardiovascular diseases in Africa. Ethn Dis. (1993) ;3: :13–22. |
[3] | Bello M . Nigerians wake up to high blood pressure. Bulletin of the World Health Organization. (2013) ;91: :242–243. |
[4] | Nwanjo HU . Efficacy of aqueous extract of Vernonia amydalina in plasma lipoprotein and oxidative status in diabetic rats. Nig J Physiol Sci. (2005) ;20: (1-2):39. |
[5] | Hayman LL . Abnormal blood lipids: Is it environment or is it geness? J. Cardiovasc Nurs. (2000) ;4: :39–49. |
[6] | Austin MA , Croto Y , Lenfant C , Tyroler HA . The hypertriglyceridaemia: Risk managements and epidemiology. AMJ Cardiol. (1991) ;68: :72A–75A. |
[7] | Theophile G . Antioxidant effects and the therapeutic mode of action of calcium channel blockers in hypertension and atherosclerosis. Philos Trans R Soc Lond Biol sci. 360: (1464):2259–2272. |
[8] | Joint National Committee (JNC) on PreventionDetection, evaluation and treatment of high blood pressure: The JNC 7th report. JAMA. (2003) ;289: :2560–2572. |
[9] | WHO. World health report; Reducing risk, promoting healthy life World Health Organisation, Geneva Switzerland. (2002) ;7–14. |
[10] | Gareth B , Gregory YHL , Eoin OB . The pathophysiology of hypertension. BMJ. (2001) ;322: :912. |
[11] | David BY . Potassium depletion and diastolic dysfunction. Ann N Y AcadSci. (2006) ;1083: :77–110. |
[12] | Schiffrin EL . Reactivity of small blood vessels in hypertension: Relation with structural changes. Hypertension. (1992) ;19: :II–9. |
[13] | Carretero OA , Oparil S . Essential Hypertension Part I: Definition and aetiology. Circulation. (2000) ;101: (3):329–335. |
[14] | Segura J , Ruilope LM . Obesity, Essential hypertension and renin angiotensin system. Public Health Nutr. (2007) ;10: (10A):1151–1155. |
[15] | Carter AR , Zhou ZH , Calhoun DA , Bubien JK . Hyperactive ENaC identifies hypertensive individuals amenable to amiloride therapy. Am J Physiol Cell Physiol. (2001) ;281: :C1413–1421. |
[16] | Dodt C , Wellhöner JP , Schütt M , Sayk F . [Glucocorticoids and hypertension] (in German) Der Internist (2009) ;50: (1):36–41. |
[17] | National Centre for Complementary and Alternative Medicine (NCCAM) What Is Complementary and Alternative Medicine? National Institutes of Health. (2012) ). |
[18] | Korish AA , Arafah MM . Camel milk ameliorates steatohepatitis, insulin resistance and lipid peroxidation in experimental non-alcoholic fatty liver disease. BMC Complementary and Alternative Medicine. (2013) ;13: :264. |
[19] | Quita SM , Kurdi LAF . Antigenotoxic and anticytotoxic effect of camel milk in mice treated with cisplatin Saudi. Journal of Biological Sciences. (2010) ;17: (I2):159–166. |
[20] | Kergoat M , Gespach C , Rosselin G , Portha B . Evaluation of in Vivo Insulin Action and Glucose Metabolism in Milk-Fed Rats Bioscience Reports (1992) ;12: :273–280. |
[21] | El-Ouardy KI , Mohamed MPC , Lorenzo FB, Paula SS , Nadia AJ . Antimicrobial activities of the bacteriocin-like substances produced bylacticacid bacteria isolated from moroccan dromedary milk African. Journal of Biotechnology. (2011) ;10: :10447–10455. |
[22] | Konuspayeva G . Physico-chemical and biochemical variability of milk of big Camelidae (Camelusbactrianus, Camelus dromedaries and Hybrids) in Kazakhstan du lait des grandiscamelides (Camelusbactrianus, Camelus dromedaries and Hybrids) (2007) ;12: :56. |
[23] | Knoess KH . Milk production of the dromedary Proceeding of the IFS Symposium Camels, (SC’79). Sudan. (1979) ;23: :201–214. |
[24] | Badriah A . Effect of Camel milk on Blood Glucose, Cholesterol, Triglyceride and Liver Enzymes Activities in Female Albino Rats World Applied Sciences Journal. 17. (2012) 11 1394–1397. |
[25] | Khalid G , Al-Fartosi1 , Alyaa M , Mohammed AA , Murtda HH . The role of Camel’s milk against some oxidant-antioxidant markers of male rats treated withCCl4. International Journal of Research in Pharmaceutical and Biomedical Sciences. (2012) ;3: (1):385–389. |
[26] | Tian N , Thrasher KD , Grudy PD , Hughson MD Jr , Manning RD . Antioxidants treatments prevents renal damage dysfunction and reduces arterial pressure in salt sensitivity hypertension. Hypertension. (2015) ;459: :34–39. |
[27] | Grassi J , Pradelles P . Compounds labelled by the acetylcholinesterase of elecrophorus electricus Its preparation process and its use as a tracer or marquer in enzyme- immunological determinations United States. (1991) ;22. |
[28] | Trinder P . Determination of blood glucose in blood using glucose oxidase with an alternative oxygen acceptor. Annals of Clin Biochem. (1969) ;6: :24–25. |
[29] | Matthews DR , Hosker JP , Rudenski AS , Naylor BA , Treacher DF , Turner RC . Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. (1985) ;28: :412–419. |
[30] | Allain CC , Poon LS , Chan CSG , Richmond W , Fu PC . Enzymatic determination of total serum cholesterol. Clinical Chemistry. (1974) ;20: :470. |
[31] | Burstein M , Scholnick HR , Morfin R . Rapid method for the isolation oflipoproteins from human serum by precipitation with polyanions. J. Lipid Res. (1970) ;11: :583–595. |
[32] | Tietz NW . Serum triglyceride determination. In: Clinical guide to laboratory tests, second edition, W.B. Saunders Co, Philadelphia, USA. (1990) ;554–556. |
[33] | Friedewald WT , Levy RI , Fredrickson DS . Estimation of LDL-C in plasma without the use of the preparative ultracentrifuge. Clinical Chemistry. (1972) ;18: (6):499–502. |
[34] | Takehide O , Tomoichiro A , Katsuyuki A , Hideyuki S , Motonobu A , et al. High-Salt Diet Enhances Insulin Signaling and Induces Insulin Resistance in Dahl Salt Sensitive Rats Hypertension (2002) ;40: :83–89. |
[35] | Toshiro F . Insulin resistance and salt-sensitive hypertension in metabolic syndrome. Nephrol Dial Transplant. (2007) ;22: (11):3102–3107. |
[36] | Niu T , Kristina DT , Paul DG , Michael DH , Davis RM . Antioxidant Treatment prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitivity hypertension. Hypertension. (2005) ;45: :934–939. |
[37] | Kagota S , TamashiroA , Yamaguchi Y , Sugura R , Kuno T , Nakamura K , Kunitomo M . Downregulation of vascular soluble guanylate cyclaseinduced by high salt intake in spontaneously hypertensive rats. Br JPharmacol. (2001) ;134: :737–744. |
[38] | Ogihara T , Asano T , Ando K . High-salt diet enhances insulin signaling and induces insulin resistance in Dahl salt sensitive rats. Hypertension. (2002) ;40: :83–89. |
[39] | Haffner SM , Valdez RA , Hazuda HP . Prospective analysis of the insulin resistance syndrome (syndrome X). Diabetes. (1992) ;41: :715–722. |
[40] | Bergman RN , Kim SP , Hsu IR . Abdominal obesity: Role in the pathophysiology of metabolic disease and cardiovascular risk. Am J Med. (2007) ;120: :S3–8. |
[41] | Quan S , Tsuda H , Miyamoto T . Angiotensin I-converting enzyme inhibitory peptides in skim milk fermented with Lactobacillus helveticus 130B4 from Camel milk in InnerMongolia. China Journal of the Science of Food and Agriculture. (2008) ;2688–92. |
[42] | Al Haj OA , Al Kanhal HA . Compositional, technological and nutritional aspectsof dromedary. Camel milk In Dairy J. (2010) ;20: :811–821. |
[43] | Jang A , Lee M . Purification and identification of angiotensin converting enzyme inhibitory peptides from beef hydrolysates. Meat Science. (2005) ;69: :653–661. |
[44] | Sironi AM , Gastaldelli A , Mari A . Visceral fat in hypertension: Influence on insulin resistance and beta-cell function. Hypertension. (2004) ;44: :127–133. |
[45] | Alhaj OA , Brückner H , Al-Khalifa AS . Identification of ACE-inhibitoryprecursor peptides from fermented camel milk (Camelus dromedarius) produced byLactobacillus helveticus or Lactobacillus acidophilus using HPLC MS. In Dairy J. (2012) ;22: :98–103. |
[46] | Wangoha J , Faraha Z . Puhana.:Iso-electric focusing of Camel milk proteins. Int Dairy J. (1998) ;8: :617–621. |
[47] | Pawloski-Dahm CM , Gordon FJ . Increased dietary salt sensitizes vasomotor neurons of the rostral ventrolateral medulla. Hypertension. (1993) ;22: :929–933. |
[48] | Stas S . Mineralocorticoid receptor blockade attenuates chronic overexpression of the renin–angiotensin–aldosterone system stimulation of reduced nicotinamide adenine dinucleotide phosphate oxidase and cardiac remodeling. Endocrinology. (2007) ;148: :3773–3780. |
[49] | Fujita T . Mineralocorticoid receptors, salt-sensitive hypertension, and metabolic syndrome. Hypertension. (2010) ;55: :813–818. |
[50] | Elayan AA , Sulieman AM , Saleh FA . The hypocholesterolemic effect of gariss and gariss containing bifido bacteria in rats fed on a cholesterol-enriched diet. Asian J Biochem. (2008) ;3: :43–47. |
[51] | Mohamed BE , Idam NZ . Effect of Camel milk on plasma lipid profile ofhypercholesteremic rats. OJVRTM. (2011) ;15: :314–317. |
[52] | Alhomida AS , Junaid MA , A-Jafari AA . Total, free, short-chain and long-chain acyl carnitine levels in Arabian Camel milk (Camelus dromedarius). J Ocul PharmacolTher. (1997) ;13: :381–387. |
[53] | Karanth J , Jeevaratnam K . Effect of dietary lipid, carnitine and exercise on lipid profile in rat blood, liver and muscle. Indian J Exp Biol. (2009) ;47: :748–753. |
[54] | Ziamajidi N , Khaghani S , Hassanzadeh G , Vardasbi S , Ahmadian S , Nowrouzi A , Ghaffari SM , Abdirad A . Amelioration by chicory seed extract of diabetes- and oleic acid-induced non-alcoholic fatty liver disease (NAFLD) /non-alcoholic steatohepatitis (NASH) via modulation of PPARα and SREBP-1. Food Chem Toxicol. (2013) ;58: :198–209. |
Figures and Tables
Fig.1
Effect Camel milk supplementation on systolic blood pressure of salt- loaded rats. SBP: Systolic blood pressure, Group I; control, Group II: salt-loaded untreated, **group III: salt-loaded treated with Camel milk (5 mls/kg b·w/day from weeks 6–10), **Group IV: salt-loaded orally dosed with 100 mg/kg Metformin + 10 mg/kg Nifedipine; from weeks 6–10.
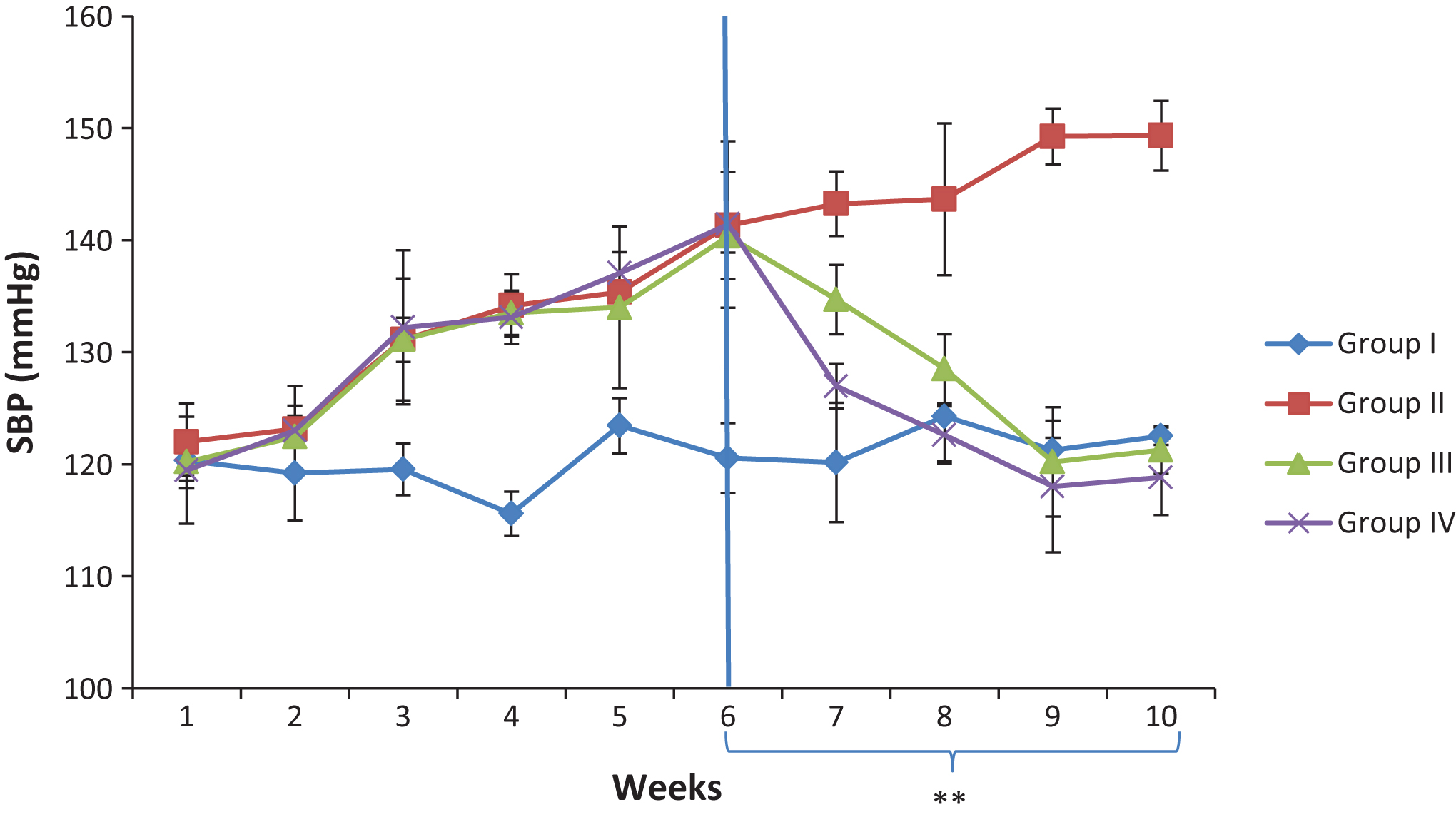
Fig.2
Effect of Camel milk supplementation on pulse rate of salt loaded rats. Group I; control, Group II: salt-loaded untreated, **group III: salt-loaded treated with Camel milk (5 mls/kg b·w/day; from weeks 6–10), **Group IV: salt-loaded orally dosed with 100 mg/kg Metformin + 10 mg/kg Nifedipine; from weeks 6–10.
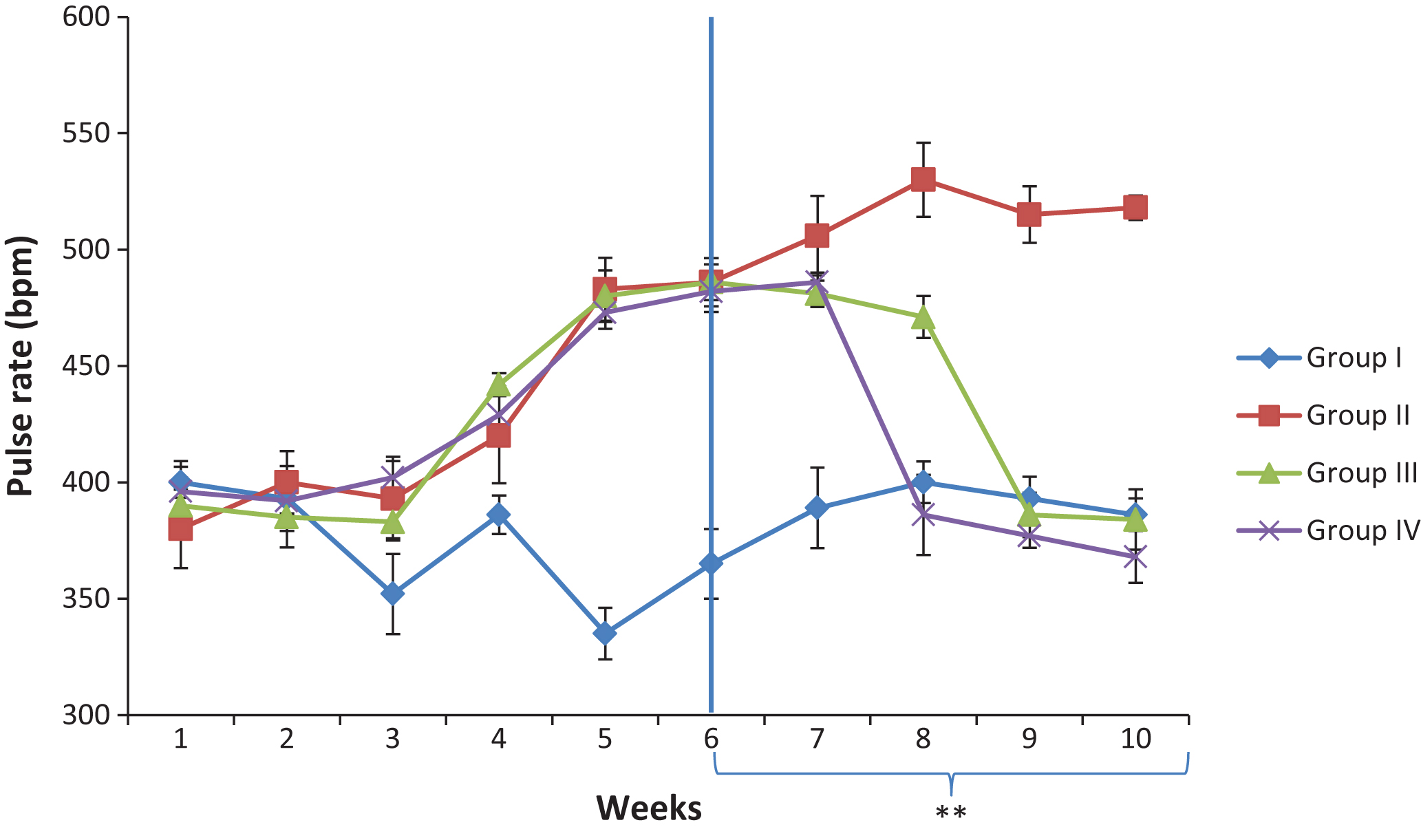
Table 1
Effect of camel milk supplementation on serum glucose, insulin and HOMA-IR in salt-induced hypertensive rats
| Parameters | Groups | |||
| I | II | III | IV | |
| [Gluc.] (mmol/L) | 5.10 ± 0.76a | 8.90 ± 1.30b | 4.22 ± 0.80ac | 4.57 ± 0.64ac |
| [Insulin] (μU/ml) | 7.63 ± 1.66a | 25.07 ± 3.72b | 8.63 ± 1.53ac | 6.70 ± 0.56ac |
| HOMA_IR | 1.77 ± 0.62a | 10.16 ± 3.96b | 1.62 ± 0.52ac | 1.37 ± 0.42ac |
HOMA-IR; Homeostasis Model Assessment- Insulin Resistance, Gluc - Glucose. Group I; control, Group II: salt-loaded untreated, group III: salt-loaded treated with Camel milk (5mls/kg b.w/day; from weeks 6-10), Group IV: salt-loaded orally dosed with 100 mg/kg Metformin+10 mg/kg Nifedipine; from weeks 6–10. values are expressed as mean ± S.D of eight replicates. Mean value having different superscript letters in rows are significantly different (p < 0.05).
Table 2
Serum Lipid profile of salts- induced hypertensive rats treated with camel milk
| Parameters (mg/dl) | Groups | |||
| I | II | III | IV | |
| TC | 96.53 ± 6.03a | 177.33 ± 5.89b | 94.00 ± 5.03ac | 103.88 ± 6.76ac |
| TAG | 101.56 ± 7.33a | 174.56 ± 6.57b | 100.07 ± 5.44ac | 113.34 ± 7.22ac |
| HDL-C | 48.67 ± 5.55a | 28.33 ± 5.77b | 53.67 ± 3.43ac | 56.55 ± 3.45ac |
| LDL-C | 27.55 ± 3.72a | 114.09 ± 7.80b | 20.52 ± 4.99ac | 23.07 ± 3.98ac |
| VLDL-C | 20.31 ± 3.20a | 34.91 ± 2.89b | 20.01 ± 2.04ac | 23.78 ± 2.88ac |
TC- total cholesterol, TG- triglyceride, HDL-C- high density lipoprotein- cholesterol, LDL-C- low density lipoprotein-cholesterol, VLDL-C- very low density lipoprotein-cholesterol, Group I; control, Group II: salt-loaded untreated, group III: salt-loaded treated with Camel milk (5 mls/kg b.w/day; from weeks 6–10) Group IV: salt-loaded orally dosed with 100 mg/kg Metformin+10 mg/kg Nifedipine; from weeks 6–10. Values are expressed as mean ± S.D of eight replicates. Mean value having different superscript letters in rows are significantly different (p < 0.05).



