Effects of Nigerian black finger millet (Eleusine coracana L. Gaertn.) seed coat supplementation on streptozotocin-induced diabetic rats
Abstract
The purpose of this study was to evaluate the effect of E. coracana seed coat matter (SCM) - supplemented feed on Streptozotocin (STZ)-induced Wistar albino rats with a view to helping ameliorate the negative impact of diabetes. A total of 25 rats divided into 5 groups of 5 rats each were used in this study. STZ-induced diabetic rats without treatment were used as diabetic control, non-diabetic rats were used as normal control while Metformin (2.5 mg/kg body weight) was used as the standard drug. Other groups are diabetic rats fed with 20% finger millet SCM and diabetic rats fed with 40% finger millet SCM. The experiment was carried out for 6 weeks. The study revealed that there was a clear reduction in the fasting blood glucose level of the SCM-supplemented diabetic and standard drug groups compared to the diabetic control group. The serum albumin, total protein, urea and creatinine levels were significantly improved (P < 0.05) in the SCM-supplemented diabetic groups compared with diabetic control group. There were visible improvements in the weights of rats supplemented with SCM compared to the diabetic control group. The levels of triglycerides, total cholesterol and LDL were also significantly reduced in the SCM-supplemented diabetic groups compared with the diabetic control group. The findings suggest that supplementation of diet with SCM could be helpful in the management of diabetes. However, the small sample size and short observation period imply that more work could be done to validate its potency.
1Introduction
Diabetes mellitus is a well-known carbohydrate metabolism disorder of the endocrine system which is due to an absolute or relative deficiency of insulin secretion, action or both [1]. It is characterised by hyperglycemia and alteration in carbohydrate, protein and lipid metabolism. Indeed, it is a major degenerative disease in the world today, affecting at least 15 million people and having complications which include hypertension, atherosclerosis and microcirculatory disorders [2]. Research has shown that prevalence of diabetes is higher in developed countries than in the developing countries in the mid ‘90 s [3]. At present, howbeit, China, India and the USA have the highest cases of diabetes [4]. More than 100 million people worldwide are affected by diabetes and is predicted that by 2030, it would rise to 366 million [5]. It is estimated that as many as 183 million people are unaware that they are diabetic [6]. A worldwide survey carried out in 2001, shows that diabetes mellitus is affecting nearly 10% of the world population [7]. It is worrying to note that the major part of this numerical increase is expected to occur in developing countries where there is rapid urbanization, nutrition transition, and increasingly sedentary lifestyles [4]. Although there is paucity of data on the prevalence of diabetes in Nigeria and other African countries, available data suggest that diabetes is emerging as a major health problem in Africa, including Nigeria [8]. It is the fourth leading cause of death in the most developed countries and there is substantial evidence that it is epidemic in many developing and newly industrialized nations.
Due to this exponential increase in the incidence of diabetes, there is a rise in demand for food containing complex carbohydrates with high level of dietary fiber and health beneficial phytochemicals since these compounds can improve the condition of sufferers [9].
Eleusine coracana (finger millet) is a cereal that belongs to the family Poaceae, an annual plant widely grown in the arid areas of Africa and Asia. Its diet is known for high sustaining power, low glycaemic response and is usually recommended for diabetics [10]. Indeed, lower incidence of diabetes has been reported in the millet-consuming population [10]. However, most of the phenolics present in millet are concentrated in the seed coat, which is also a rich source of phytates and minerals [11]. These dietary polyphenols and phytates are known for their ability to reduce carbohydrate digestibility and thereby regulate postprandial glycaemic response [12]. However, reports on the ameliorative effects of the millet seed coat supplemented diet against diabetes are scanty. The effect of feeding finger millet on the antioxidant and glycemic status of alloxan-induced diabetes has been reported (13). Indeed, the effect of finger millet seed coat matter (SCM) in Streptozotocin-induced diabetic rats has also been studied in India, Asia [14]. However, to the best of our knowledge, there is no such report on African finger millet. Yet report suggests that climatic differences between geographical regions are known to cause variations in the amount of the active ingredients in plants [15]. This necessitated the need to evaluate the effect of supplementation of finger millet seed coat matter (SCM) from Nigerian Eleusine coracana on Streptozotocin-induced diabetic rats.
2Materials and methods
2.1Chemicals and reagents
All assays kits were from Randox laboratories Ltd. Ardmore, Co. Antrm UK. Streptozotocin (STZ) was purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals and solvents used were of analytical grade.
2.2Identification and preparation of finger millet seed coat matter (SCM)
Seeds of Black Finger Millet (Eleusine coracana) were collected from the Institute for Agricultural Research (IAR), Faculty of Agriculture, Ahmadu Bello University (ABU) Zaria, Kaduna State, Nigeria. The seeds were identified at the Herbarium unit of the Department of Biological sciences ABU, Zaria, with voucher no 2843. Seed coat matter (SCM) was prepared from finger millet according to the protocol described previously by [16]. Twenty percent (20% ) SCM was prepared by adding 400 g of basal diet (Vital Feed Nigeria Ltd) to 100 g of SCM to make 500 g of the mixture, while 40% SCM was prepared by adding 300 g of basal control diet (Vital Feed Nigeria Ltd) to 200 g of SCM to make 500 g of the mixture and was mixed evenly.
2.3Animal collection
Male Wistar Strain albino rats weighing between 150–250 g obtained from the enclosure in the Department of Pharmaceutical Science, ABU Zaria, were used. The rats were kept and maintained in well ventilated cages under ambient environmental conditions. They were maintained on grower’s mash (Vital feeds Nigeria Ltd) and provided with water ad libitum. They were allowed to acclimatize to the laboratory conditions for two weeks before the experiment. Animals described as fasted had been deprived of food for about 12–16 hrs but had been allowed free access to water.
2.4Ethical guidelines
Research experiments were carried out according to the principles of laboratory animal care recommended by the National Institute of Health.
2.5Induction of diabetes
After fasting, diabetes was induced by a single intraperitoneal injection of 55 mg/kg body weight of Streptozotocin (STZ), dissolved in 0.1M fresh cold citrate buffer (pH 4.5) into 12 h fasted rats [17]. The control rats received the vehicle of administration only. The animals were allowed to drink 5% glucose solution overnight to overcome drug-induced hypoglycaemia. After 3 days of STZ post-injection, the blood sugar levels was determined with a glucometer (Acc-cheek Advantage Roche diagnostics GmbH, Germany) and the rats with fasting blood glucose level more than 126 mg/dl (11.1 mmol/L) were considered diabetic hence selected for experimentation.
2.6Animal grouping and treatment
A total of 25 rats randomly divided into 5 groups of 5 rats each were used in this study as follows:
Group 1: Normal control rats that received only the grower’s mash
Group 1: STZ-induced diabetic control rats without treatment (Diabetic control)
Group 2: Diabetic rats fed with 20% finger millet seed coat matter (DE20% )
Group 3: Diabetic rats fed with 40% finger millet seed coat matter (DE40% )
Group 4: Diabetic rats treated daily with Metformin (2.5 mg/kg body weight) daily
Group 5: Non-diabetic without treatment (normal control rats).
Treatments were carried out daily for a period of 42 days.
2.7Biochemical analysis
Fasting blood glucose (FBG) level was determined using the method described by Clark and Lyons [18] using glucometer (Acc-check Advantage.Boche Diagnostic GmbH, Germany) by tail bleeding after fasting the rats for 12 h while weekly body weight was determined using standard analytical weighing balance. The rats were fasted overnight, anesthetized with chloroform and were sacrificed 24 hours after the last treatment. Serum was harvested from the blood and used to assay for aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, total protein, albumin, total cholesterol, HDL-Cholesterol, LDL-Cholesterol, urea, creatinine and triacyglycerols. They were analysed using (Randox laboratories Ltd.) kit according to the manufacturer’s protocol.
2.8Statistical analysis
The analyses were carried out in triplicate for all determinations except where otherwise stated. The results were presented as mean ± standard deviation. The SPSS program (version 17 for Windows, SPSS Inc., Chicago, IL, USA) was used to run the One-way analysis of variance (ANOVA) followed by Duncan Multiple Range tests for multiple comparisons of the mean [19] and values of P less than <0.05 was regarded as statistically significant.
3Results
3.1Body weight changes
Table 1 showed that the relative body weights of the animals. The diabetic control group had the highest weight loss of about 30% . The SCM-supplemented diabetic groups and the standard drug group, however, showed improvement in body weight at the end of the experiment compared to the diabetic control group. However, the standard drug and 20% SCM-supplemented diabetic groups have approximately 8% weight loss while 40% SCM-supplemented group has approximately 10% weight loss.
3.2Blood glucose levels changes
From Fig. 1, as the week passed by, there was gradual reduction in the blood glucose level in SCM-supplemented diabetic groups (40% and 20% SCM) when compared to the diabetic control group. Among the SCM-supplemented diabetic groups 20% SCM showed higher ameliorative effect than 40% SCM and standard drug (2.5 mg/kg bw Metformin).
3.3Attenuation of liver enzymes activities
From Table 2, there was significant (P < 0.05) difference in serum AST activity between the diabetic control group and the SCM-supplemented diabetic groups (20% and 40% SCM). There was no significant difference (P > 0.05) in serum AST activity between the group treated with 2.5 mg/kg bw of Metformin and SCM-supplemented diabetic groups. The diabetic control group showed a significantly higher (P < 0.05) ALT activity when compared with both the SCM-supplemented diabetic groups and standard drug groups.
3.4Improved serum lipid profile and total proteins
The levels of cholesterol in the diabetic control group is significantly (P < 0.05) different from the SCM-fed diabetic rats as shown in Table 3. There was no significant (P > 0.05) difference among the SCM-fed diabetic groups (20% and 40% SCM). There was significant reduction (P < 0.05) in serum triglyceride levels in the experimental control groups fed with 20% and 40% SCM when compared with the diabetic control group. However, no significant (P > 0.05) difference was observed among the SCM-fed diabetic groups. Serum HDL concentration as presented in Table 3 showed a significant reduction (P < 0.05) in diabetic control group when compared with the SCM-fed and Metformin treated diabetic groups. There was no significant difference (P > 0.05) in serum HDL among the experimental groups supplemented with 20% and 40% SCM. The group treated with Metformin showed significant difference (P < 0.05) compared with the diabetic control group. However, the LDL level was significantly reduced (P < 0.05) in the SCM-fed and Metformin treated diabetic groups when compared with the diabetic control group.
In Table 4, there was significant increase (P < 0.05) in the serum total protein concentration in diabetic supplemented groups and standard drug group compared to the diabetic control group at the end of the 6 weeks experimental period. There was no significant (P > 0.05) difference between the group treated with Metformin and the SCM-fed diabetic groups. There was significant (P < 0.05) improvement in serum albumin level in the SCM-fed diabetic groups when compared with the diabetic control group.
3.5Amelioration of kidney pathology
Table 5 shows the serum creatinine and urea levels in the various groups of rats in the study. There is no significant (P > 0.05) difference in the levels of creatinine in diabetic rats fed with varying concentrations of SCM. However, there was significant (P < 0.05) reduction in serum creatinine concentration among the experimental groups fed with different concentrations of finger millet SCM compared with the diabetic control group. There was no significant (P > 0.05) difference between the groups treated with standard drug (2.5 mg/kg b.w of Metformin) and the groups fed with varying concentrations of SCM. There was significant (P < 0.05) reduction in urea concentration in the experimental groups fed with 20% and 40% SCM when compared with the diabetic control group. Among the diabetic experimental group fed with different concentration, there was no significant (P > 0.05) difference. The diabetic group treated with 2.5 mg/kg b.w of Metformin also showed significant reduction compared to the diabetic control group.
4Discussion
When rats are injected with Streptozotocin, they provide an animal model of insulin-dependent diabetes mellitus [20]. The intraperitoneal administration of STZ (60 mg/kg body weight) selectively destroys the insulin secreting β-cells of the pancreas by breaking the DNA strand, resulting in decreased endogenous insulin release, causing activation of poly(ADP-ribose) polymerase (PARP) resulting in reduction of cellular NAD+ and cell death [21]. The antihyperglycemic effects of the SCM-supplemented diabetic rats possibly represent increase in insulin secretion by regeneration of damaged pancreatic β-cells in STZ-induced diabetic rats [22]. The antihyperglycaemic influence of the millet SCM observed in the present study is in conformity with Hegde et al. [13], who observed 36% and Shobana et al. [14] who observed 39% reduction in blood glucose levels in Alloxan and Streptozotocin-induced diabetic rats maintained on the millet whole meal-incorporated diet. This could be attributed to the synergistic effect of the phenolic compounds present in the millet seed coat.
Hypertriglyceridemia and hypercholesterolemia are the most common lipid abnormalities in diabetes [23] and the abnormal high concentration of serum lipids in diabetic animals are mostly due to an increased mobilisation of free fatty acids from peripheral fat depots [24]. The ability of the SCM-supplemented diabetic rats, in this study, to significantly reduce serum TC, TG and LDL and increase HDL significantly could be beneficial in preventing diabetic complications like coronary heart diseases and atherosclerosis in diabetic conditions.
Loss of body weight is a major consequence of diabetes in rats [25] and it could be due to dehydration and catabolism of fats and protein [26]. The improved body weight of SCM-supplemented diabetic rats, therefore, suggests preventive effect of the supplement on breakdown of structural proteins.
Elevated level of serum urea and creatinine observed in the diabetic control compared to normal control is an indication of impaired renal function in the diabetic control which may be caused by abnormal glucose regulation or elevated glucose and glycated protein tissue levels [26]. Urea and creatinine lowering observed in the SCM-fed groups therefore, showed protective effect on the kidney.
Elevated serum AST and ALT levels in diabetes may be due to liver dysfunction [27]. Therefore, increased level of AST and ALT in the diabetic control suggests leakage of enzymes from the liver cytosol into the blood stream and represents the toxicity of STZ in the liver. Diabetic rats supplemented with SCM showed significant reduction in both enzymes which represents the protective effect of the SCM on the liver in diabetic conditions.
In diabetic condition, reduction in protein and albumin levels may be due to proteinuria, albuminuria or increased protein metabolisms which are clinical markers of diabetic nephropathy [28]. The improvement in the levels of protein and albumin in SCM-supplemented diabetic rats could be due to increase in insulin mediated amino acid uptake, enhancement of protein synthesis and/or inhibition of protein degradation as suggested by Almdal and Vilstrup [29]. The improvement in protein and albumin levels, as well as reduction in urea and creatinine level when treated with various concentration of the seed coat matter is consistent with Shobana et al. [14] and Hegde et al. [13].
5Conclusion
The present animal study revealed that supplementation of animals’ diet with finger millet SCM has antihyperglycemic and antihyperlipidemic effect. It also suggests that the supplementation has ability to protect sensitive organs such as liver and kidney thereby making the SCM a viable alternative for the management of diabetes and its associated complications.
References
1 | Wais M, Nazish I, Samad A, Beg S, Abusufyan S, Ajaj SA, Aqil M(2012) Herbal drugs for diabetic treatment: An updated review of patentsRecent Pat on Antiinfect Drug Discov7: 1535910.2174/157489112799829701 |
2 | Ogbonnia SO, Odimegwu JI, Enwuru VN(2008) Evaluation of hypoglycemic and hypolipidemic effects of ethanolic extracts of Treculia africana Decne and Bryophyllum pinnatum Lam. and their mixture on Streptozotocin (STZ)-induced diabetic ratsAfr J Biotechnol7: 1525352539 |
3 | King H, Aubert RE, Herman WH(1998) Global burden of diabetes, -Prevalence, numerical estimates and projectionsDiabetes Care21: 914141431 |
4 | Hu FB(2011) Globalization of Diabetes: The role of diet, lifestyle, and genesDiabetes Care34: 12491257 |
5 | Ponnusamy S, Ravindran R, Zinjarde S, Bhargava S, Kumar AR(2011) Evaluation of Traditional Indian Antidiabetic Medicinal Plants for Human Pancreatic Amylase Inhibitory Effect In Vitro Evid-Based Complement Alternat Med11010.1155/2011/515647 |
6 | International Diabetic Federation, IDF The Global Burden, 5th Edu. IDF Diabetes Atlas, United State, 2011 |
7 | World Health Organisation, WHO Screening for Type 2 Diabetes. WHO/NMH/MNC/03.1, 2003; 1-48 |
8 | Mbanya JC, Bonicci F, Nagan K(1996) Guidelines for the Management of NIDDM in Africa. A consensus documentNovo NordiskGreek135 |
9 | Shobana S, Malleshi NG(2007) Preparation and functional properties of decorticated finger millet (Eleusine coracana)J Food Eng79: 252953810.1016/j.jfoodeng.2006.01.076 |
10 | Shobana S, Sreerama YN, Malleshi NG(2009) Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: Mode of inhibition of α-glucosidase and pancreatic amylaseFood Chem115: 41268127310.1016/j.foodchem.2009.01.042 |
11 | Shobana S, Meera MS, Malleshi NG(2006) Major nutrients and phytochemical contents of the finger millet fractionsProceedings of the Souvenir, 18th Indian Convention of Food Scientists and Technologists, AFST(I). Hyderabad, India26 |
12 | Thompson LU, Button CL, Jenkins DJA(1987) Phytic acid and calcium effect in vitro rate of navy bean starch digestion and blood glucose response in humansAm J Clin Nutr46: 3467473 |
13 | Hegde PS, Rajasekaran NS, Chandra TS(2005) Effects of the antioxidant properties of millet species on oxidative stress and glycemic status in alloxan-induced ratsNutr Res25: 121109112010.1016/j.nutres.2005.09.020 |
14 | Shobana S, Harsha MR, Platel K, Srinivasan K, Malleshi NG(2010) Amelioration of hyperglycaemia and its associated complications by finger millet (Eleusine coracana L.) seed coat matter in Streptozotocin-induced diabetic ratsBr J Nutr104: 121787179510.1017/S0007114510002977 |
15 | Lale NES. Stored Product Entomology and Acarology in Tropical Africa. Mole Publications, Maiduguri, Nigeria. 2002 |
16 | Chethan S, Malleshi NG(2007) Finger millet polyphenols: Optimization of extraction and the effect of pH on their stabilityFood Chem105: 286287010.1016/j.foodchem.2007.02.012 |
17 | Burcelin R, Eddouks M, Maury J, Kande J, Assan R, Girard J(1995) Excessive glucose production, rather than Insulin resistance, account for hyperglycemia in recent onset Streptozotocin-diabetic ratsDiabetologia38: 328329010.1007/BF00400632 |
18 | Clark LJr, Lyons C(1962) Blood glucose determinationAn NY Acad Sci102: 29 |
19 | Duncan RC, Knapp RG, Miller MC(1977) Test of hypothesis in population meansIn: Introductory Biostatistics for the health sciencesJohn Wiley and Sons IncNY7196 |
20 | Rajiv Gandhi G, Ignacimuthu S, Gabriel Paulraj M(2011) Solanum torvum Swartz. fruit containing phenolic compounds shows antidiabetic and antioxidant effects in Streptozotocin-Induced diabetic ratsFood Chem Toxicol49: 27252733 |
21 | Bolzan AD, Bianchi MS(2003) Genotoxicity of StreptozotocinMutat Res512: 121134 |
22 | Okpe O, Ibrahim S, Njoku GC, Ndidi US, Atabo S(2014) Pancreatic islet regeneration and some liver biochemical parameters of leaf extracts of Vitex Doniana in normal and Streptozotocin-induced diabetic albino ratsAsian Pac J Trop Biomed4: 2124130 |
23 | Al-Shamaony L, al-Khazraji SM, Twaij HA(1994) Hypoglycaemic effect of Artemisia herba alba II - Effect of a valuable extract on some blood parameters in diabetic animalsJ Ethnopharmacol43: 16717110.1016/0378-8741(94)90038-8 |
24 | Al-Logmani AS, Zari TA(2009) Effects of Nigella sativa L. and Cinnamomum zeylanicum Blume oils on some physiological parameters in streptozotocin-induced diabetic ratsBoletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas8: 28696 |
25 | Ramachandran S, Naveen KR, Rajinikanth B, Akbar M, Rajasekaran A(2012) Antidiabetic, antihyperlipidemic and in vivo antioxidant potential of aqueous extract of Anogiessus latifolia bark in type 2 diabetic ratsAsian Pac J Trop Disease2: 2S596S602 |
26 | Lal SS, Sukla Y, Singh A, Andriyas EA, Lall AM(2009) Hyperuricemia, high serum urea and hypoproteinemia are the risk factor for diabetesAsian J Med Sci1: 23334 |
27 | Rao GM, Morghom LO, Kabur MN, Ben Mohmud BM, Ashibani K(1989) Serum glutamic oxaloacetic transaminase (GOT) and glutamate pyruvate transaminase (GPT) levels in diabetes mellitusIndian J Med Sci5: 118122doi: 06/1989; 43(5):118-21 |
28 | Kaleem M, Medha P, Ahmed QU, Asif M, Bano B(2008) Beneficial effects of Annona squamosa extract in streptozotocin-induced diabetic ratsSingapore Med J49: 10800 |
29 | Almdal JP, Vilstrup H(1988) Strict insulin therapy normalizes organ nitrogen contents and the capacity of urea nitrogen synthesis in experimental diabetes in ratsDiabetologia31: 114118 |
Figures and Tables
Fig.1
Effect of dietary finger millet seed coat matter (SCM) on fasting blood glucose levels of STZ-induced diabetic Wistar rats. DE, diabetic experimental; DC, diabetic control; NC, normal control; STD DRG, standard drug (Metformin).
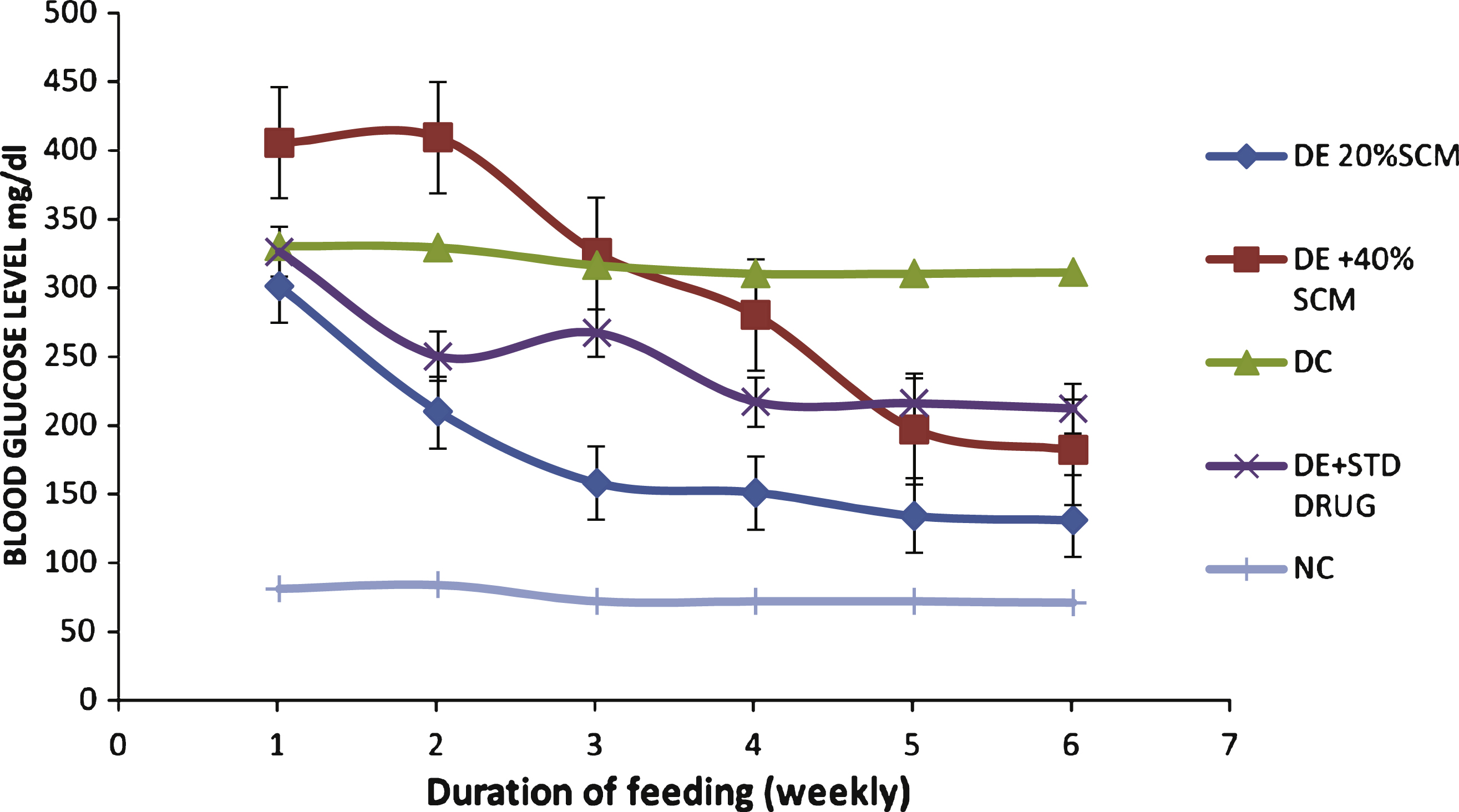
Table 1
Effect of dietary finger millet seed coat matter (SCM) on body weights of STZ-induced diabetic rats
| Treatment Group | Final Weight (g) | Initial weight (g) | Change in weight (% ) |
| NC | 217 ± 0.47 | 190 ± 0.36 | ↑14.21 |
| DC | 120 ± 0.93 | 150 ± 0.93 | ↓30.00 |
| DE+Metformin | 139 ± 8.05 | 152 ± 0.68 | ↓8.50 |
| DE+20% SCM | 119 ± 9.17 | 130 ± 0.67 | ↓8.40 |
| DE+40% SCM | 128 ± 9.19 | 143 ± 4.03 | ↓10.40 |
Values are Mean ± SD (n = 5). DE, diabetic experimental; DC, diabetic control; NC, normal control.
Table 2
Effect of finger millet seed coat matter (SCM) on the activity of ALT, AST and ALP in STZ-induced diabetic rats
| Treatment Group | AST (U/l) | ALT (U/l) | ALP (U/l) |
| NC | 20.66 ± 0.64a | 36.81 ± 1.03a | 57.67 ± 4.33a |
| DC | 26.64 ± 1.33b | 47.67 ± 1.86c | 84.33 ± 4.91c |
| DE+20% SCM | 22.00 ± 1.15a | 42.00 ± 1.53b | 65.67 ± 1.76b |
| DE+40% SCM | 21.33 ± 0.66a | 40.33 ± 1.45ab | 63.33 ± 6.23ab |
| DE+Metformin | 22.00 ± 1.15a | 41.67 ± 0.88b | 71.00 ± 2.08b |
Values are Mean ± SD (n = 5). Different superscripts signify difference in significance at P < 0.05 down the column. DE, diabetic experimental; DC, diabetic control; NC, normal control.
Table 3
Effect of finger millet seed coat matter (SCM) on the lipid profiles of STZ-induced diabetic rats
| Treatment group | TC (mg/dl) | TG (mg/dl) | HDL-c (mg/dl) | LDL-c (mg/dl) |
| NC | 203.33 ± 25.17a | 63.33 ± 6.67a | 63.33 ± 8.82b | 127.34 ± 15.02a |
| DC | 270.00 ± 26.46b | 100.00 ± 11.15b | 43.33 ± 3.33a | 206.67 ± 20.82c |
| DE+20% SCM | 226.67 ± 30.55a | 77.00 ± 14.80a | 70.33 ± 15.60b | 140.94 ± 11.99b |
| DE+40% SCM | 226.67 ± 25.17a | 73.33 ± 12.03a | 74.33 ± 14.33b | 139.67 ± 8.44b |
| DE+Metformin | 200.00 ± 20.00a | 60.00 ± 11.55a | 60.00 ± 5.77b | 128.00 ± 11.92a |
Values are Mean ± SD (n = 5). Different superscripts signify difference in significance at P < 0.05 down the column. DE, diabetic experimental; DC, diabetic control; NC, normal control.
Table 4
Effect of finger millet seed coat matter on serum concentration of total protein and albumin in STZ-induced diabetic rats
| Treatment group | Total protein (g/l) | Albumin (g/l) |
| NC | 69.33 ± 3.48b | 38.33 ± 2.73c |
| DC | 42.33 ± 5.17a | 26.00 ± 1.15a |
| DE+20% SCM | 64.33 ± 2.03b | 32.00 ± 1.00b |
| DE+40% SCM | 67.33 ± 2.40b | 39.33 ± 2.40c |
| DE+Metformin | 73.66 ± 1.45bc | 41.00 ± 0.58c |
Values are Mean ± SD (n = 5). Different superscripts signify difference in significance at P < 0.05 down the column. DE, diabetic experimental; DC, diabetic control; NC, normal control.
Table 5
Effect of Finger millet seed coat matter on the level of creatinine and urea in STZ-induced diabetic rats
| Treatment Group | Urea (nM/mg protein) | Creatinine (mg/dl) |
| NC | 1.83 ± 0.09a | 0.55 ± 0.05a |
| DC | 5.40 ± 0.12c | 2.60 ± 0.01c |
| DE+20% SCM | 3.63 ± 0.03b | 1.20 ± 0.06b |
| DE+40% SCM | 3.50 ± 0.10b | 1.47 ± 0.06b |
| DE+Metformin | 3.33 ± 0.24b | 1.23 ± 0.33b |
Values are Mean ± SD (n = 5). Different superscripts signify difference in significance at P < 0.05 down the column. DE, diabetic experimental; DC, diabetic control; NC, normal control.




