Post-Metastasectomy Adjuvant Therapy in Patients with Renal Cell Carcinoma: A Systematic Review
Abstract
BACKGROUND:
Pembrolizumab is established as adjuvant therapy for patients with high-risk clear cell renal cell carcinoma (ccRCC) after resection. Patients with completely resected metastatic disease (M1 NED) seem to have greater benefit from adjuvant pembrolizumab in both disease-free survival (DFS) and overall survival (OS); yet, with other agents, adjuvant therapy has not been shown to improve survival. As newer therapies evolve, it is important to understand the efficacy of systemic agents in this patient population.
OBJECTIVE:
We aimed to systematically review available trials investigating adjuvant therapy after metastasectomy in RCC.
METHODS:
Following PRISMA guidelines, we performed a systematic literature search using PubMed and Embase through January 2024. For inclusion, studies were required to include completely resected patients with known metastatic RCC. Patients with only locally advanced and/or regional nodal involvement (N1) alone were excluded. Titles and abstracts were screened to identify articles for full-text, and then a descriptive review was performed.
RESULTS:
A total of 149 articles were initially identified. Ultimately 9 articles published before the end of January 2024 met our inclusion criteria and were included in the analysis. Data were extracted and organized to reflect the role of adjuvant treatment - both targeted therapies as well as immunotherapy in patients who had undergone metastasectomy and rendered M1 NED. With the exception of pembrolizumab, adjuvant therapy in M1 NED was not found to be associated with improved survival.
CONCLUSIONS:
Pembrolizumab appears to benefit M1 NED ccRCC patients after resection even more than other high-risk ccRCC patients. Yet, this same benefit has not been seen with other agents. Future research should focus on trying to establish which M1 NED patients benefit from adjuvant treatment.
INTRODUCTION
Metastasectomy is a key treatment option for patients with oligometastatic renal cell carcinoma (RCC), with the most common sites of non-kidney resection being the lungs, bone, lymph nodes, adrenal glands, pancreas, and liver, respectively [1, 2]. The optimal therapy for patients with metastatic renal cell carcinoma rendered free of disease after resection, often called “M1 NED patients”, is unclear. NCCN guidelines recommend adjuvant pembrolizumab within 1 year of nephrectomy for those patients with metastatic clear cell RCC (ccRCC) following complete resection of disease [3].
In the last four decades, despite sustained effort in the adjuvant setting, only two positive trials have been reported to improve disease-free survival (DFS). Among targeted therapies, the tyrosine kinase inhibitor (TKI) sunitinib was shown to modestly improve DFS, and among immune checkpoint inhibitors (ICIs), pembrolizumab was shown to significantly improve DFS and overall survival (OS) as part of the Keynote 564 trial [4–7]. Other systemic therapy trials have had less favorable results, including those with tyrosine kinase inhibitors (sorafenib, pazopanib, axitinib), and mTOR inhibitor (everolimus) [8–12], as well as those with immune checkpoint inhibitors (atezolizumab, ipilimumab+nivolumab, and nivolumab) [13–15]. Differences in efficacy between these trials have led to speculation regarding the activity of these drugs, specifically in the context of immunotherapy. Even though both pembrolizumab and nivolumab are programmed death-1 (PD-1) inhibitors, the former has been shown to bind to the PD-1 receptor with higher affinity [16]. Further, drugs such as atezolizumab inhibit the programmed death-ligand 1 (PD-L1) receptor instead, and this drug is not used in the context of kidney cancer due to a lack of overall survival benefit in phase-III clinical trials [17]. Other factors include, differences in inclusion criteria (inclusion of non-clear histologies in the IMmotion010 (atezolizumab) trial, duration of adjuvant therapy (six months in the CheckMate-914 trial (ipilimumab+nivolumab) vs. one year in KEYNOTE-564 trial (pembrolizumab).
Among adjuvant-treated patients, those with completely resected metastatic disease – i.e., M1 NED patients - are at a uniquely higher risk for progression. Within Keynote 564, the M1 NED subgroup had the lowest DFS HR for pembrolizumab compared to placebo in a sub-group analysis, suggesting that this sub-group may benefit most from adjuvant therapy [4]. The NCCN recommendation in favor of adjuvant pembrolizumab is based on these results.
While several adjuvant studies, including S-TRAC, EVEREST, and Checkmate 914, did not include the M1NED high-risk subgroup [7, 12, 13]. Several others did. in this review, we sought to systematically provide a review of the existing trials of systemic therapy after complete resection of metastatic RCC.
METHODS
Search strategy
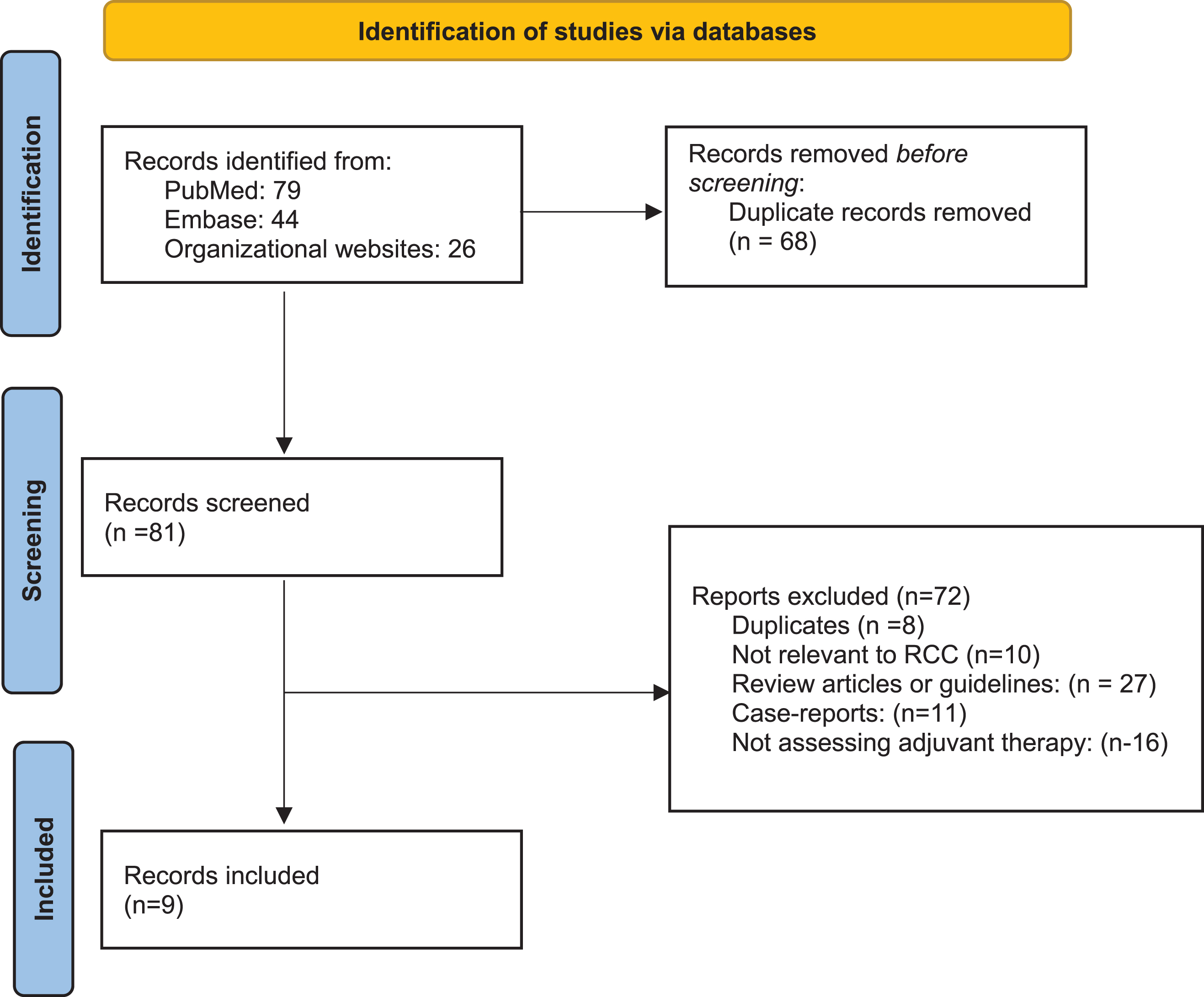
A systematic literature search was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [18]. A search was performed in electronic databases, including PubMed/MEDLINE and Scopus. The search terms included (Renal cell carcinoma OR kidney cancer) AND (adjuvant AND tyrosine kinase inhibitor OR sunitinib OR sorafenib OR pazopanib OR axitinib OR everolimus OR immune checkpoint OR nivolumab OR ipilimumab OR pembrolizumab OR avelumab OR atezolizumab OR IL2) AND (metastasectomy or M1NED). An additional manual search was performed to identify published abstracts from the European Society of Medical Oncology and the American Society of Clinical Oncology (ASCO) annual meetings. We excluded non-English articles, articles without original data (e.g. review articles or editorials), and repeated publications on the same cohort. In multiple publications on the same trial, we reported the most recent publication with the longest follow-up. Ultimately, 9 papers were included in the final review, as shown in Fig. 1.
Fig. 1
PRISMA flowchart.
RESULTS
Description of results
A PRISMA flow-diagram for the selection process of included studies is provided in Fig. 1. The initial online search yielded 149 articles of which 9 were included in final analysis. Table 1 summarizes the basic findings of these articles. Three articles were randomized trials of adjuvant immunotherapy in high-risk patients including an M1 NED subgroup, two articles were randomized trials of adjuvant TKI specifically in M1 NED patients, two articles were retrospective series (one of multiple TKI therapies and one of interferon alpha era treatments), one article was a randomized trial of high-dose IL-2, and one article was a non-randomized trial of a novel RCC vaccine.
Table 1
Studies describing the role of adjuvant systemic therapy in M1 NED patients
| Immunotherapy Trials | |||||||
| Study | Study Drug | Control Arm | Histological Inclusion | Pathologic Inclusion | Median Follow-up | No. of patients (Overall and M1 NED) | Outcome in M1 NED patients (And for overall study when others included) |
| Keynote-564 [4–6] | Pembrolizumab 200 mg every 3 weeks for up to 1 year | Placebo | ccRCC+/- sarcomatoid component | pT2 (grade 4 or sarcomatoid) or≥pT3 (any grade) pTxN1 or M1 NED (within 1 year of nephrectomy) | 57.2 months | 989 total 57 M1 NED | Disease Free Survival M1 NED Subgroup HR (0.40 0.20–0.81) [Overall HR (0.72 0.59–0.87)] Overall Survival M1 NED Subgroup HR 0.51 (0.15–1.75) [Overall HR 0.62 (0.44–0.87)] |
| Immotion-010 [15] | Atezolizumab 1,200 mg every 3 weeks for 1 year | Placebo | ccRCC or RCC with sarcomatoid component | pT2 (gr4) or pT3a (gr 3 or gr 4) or≥pT3b (any grade) or pTxN1 or M1 NED (within 1 year of nephrectomy) | 44.7 months | 773 total 108 M1 NED - 84 metachronous resection - 24 synchronous resection | Disease Free Survival M1 NED Subgroup HR 0.93 (0.58–1.49) [Overall HR 0.93 (0.75–1.15)] |
| PROSPER [14] | Nivolumab 480 mg IV q4 weeks 1 doses preoperatively, and up to 9 monthly doses post-operatively | Observation | Any histology (80% ccRCC) | pT2-4 or M1 NED (capable of being rendered NED within 12 weeks of surgery) | 16 months | 779 total 28 M1 NED | Disease Free Survival M1 NED Subgroup HR 0.89 (0.31–2.57) [Overall 0.97 (0.74–1.28)] |
| Targeted Therapy/Tki | |||||||
| Study | Study Drug | Control Arm | Histological Inclusion | Pathologic Inclusion | Median Follow-up | No. of patients (Overall and M1 NED) | Outcome in M1 NED patients (And for overall study when others included) |
| E2810 [22] | Pazopanib 800 mg daily for 1 year | Placebo | ccRCC | M1 NED only | 30 months | 129 M1 NED | Disease Free Survival HR 0.85 (0.55–1.31) |
| RESORT [21] | Sorafenib 400 mg twice daily for 1 year | Observation | ccRCC | M1 NED only | 38 months | 69 M1 NED | Disease Free Survival HR 1.35 (0.72–2.54) |
| Park et al Retrospective series [23] | Immediate Adjuvant TKI Therapy (Sunitinib, Sorafenib, or Pazopanib) | Retrospective control with deferred treatment | Any histology (90% ccRCC) | M1 NED only | 12 months | 53 patients - 19 received immediate post-op TKI - 34 received delayed TKI | Disease Free Survival (immediate vs. delayed TKI) HR 0.418 (0.118–0.859) Cancer-specific Survival (immediate vs. delayed TKI) HR 0.640 95% CI (0.258–2.093) |
| CYTOKINES | |||||||
| Study | Study Drug | Control Arm | Histological Inclusion | Pathologic Inclusion | Median Follow-up | No. of patients (Overall and M1 NED) | Outcome in M1 NED patients (And for overall study when others included) |
| Cytokine Working Group Randomized Trial [19] | High-dose bolus IL2 – q8 hours days 1–5 and 15–19 for maximum 28 doses. | Observation | RCC (majority were ccRCC) | pT3b-4 or N1-N3 or M1 NED | 22 months | 69 total 25 M1 NED | Disease Free Survival No formal HR, though not significant for M1 NED or overall cohort, 0.43 and 0.49. Overall Survival No formal HR, though not significant for M1 NED or overall cohort, 0.91 and 0.29. |
| Kwak et al. Retrospective series [20] | Multiple historical interventions: IFN-alpha, IL-2, 5-FU | Untreated control | RCC ( 80% ccRCC) | M1 NED only | 22 months | 93 patients, 70 systemically treated patients, 23 observed patients | Overall survival HR 1.54 (0.765–3.115) |
| MISCALLANEOUS | |||||||
| Study | Study Drug | Control Arm | Histological Inclusion | Pathologic Inclusion | Median Follow-up | No. of patients (Overall and M1 NED) | Outcome in M1 NED patients (And for overall study when others included) |
| Rausch UroRCC Peptide Vaccine [24] | Synthetic multi-peptide vaccine | Retrospective contemporary control | RCC (not specified) | M1 NED only | 56 months | 83 patients 19 treated patients M1 NED (vs 44 controls) | Overall survival HR 0.19 (0.05–0.69) |
Among the trials identified, only Keynote 564 established a survival advantage with pembrolizumab in the overall cohort, an advantage that appeared more pronounced within the M1 NED subgroup. DFS hazard ratio (HR) was reported as 0.40 (95% CI: 0.20–0.82) in M1 NED patients in comparison to 0.72 (95% CI: 0.59–0.87) for the overall cohort [6]. A more pronounced HR for OS was also observed for the M1 NED subgroup for pembrolizumab but with the 95% CI crossing unity: HR 0.51 (95% CI: 0.15–1.75) in M1 NED subgroup compared to 0.62 (95% CI: 0.44–0.87) for the overall subgroup [5].
Despite the impressive results of pembrolizumab, within the other large adjuvant immunotherapy trial that also included an M1 NED sub-group, IMmotion 010, the same trend was not observed with adjuvant atezolizumab [15]. The M1 NED subgroup within IMmotion 010 had a DFS HR of 0.93 (95% CI: 0.58–1.49), similar to the DFS HR of the overall study, 0.93 (95% CI: 0.75–1.15). Similarly, in the other large adjuvant immunotherapy trial, PROSPER, which included neoadjuvant priming with one dose of nivolumab followed by adjuvant nivolumab after nephrectomy, the M1 NED group did not seem to uniquely benefit from adjuvant therapy. In fact, the M1 NED subgroup DFS HR within PROSPER was 0.89 (95% CI: 0.31–2.57), compared to the overall DFS HR of 0.97 (95% CI: 0.74–1.28). However, it is worth noting that within PROSPER, the number of patients in the M1 NED subgroup was small, with only 28 total patients (4% of the overall sample). Previously conducted trials and retrospective series using cytokines such as interleukin-2 (IL2) or interferon-a also did not show significant improvement in DFS when administered adjuvantly in the post-metastasectomy setting [19, 20]. (Table 1)
The two trials that evaluated adjuvant TKIs after metastasectomy include RESORT (comparing sorafenib to observation in 76 patients pretreated with nephrectomy and undergoing radical metastasectomy) [21] and the ECOG-ACRIN trial E2810 (comparing pazopanib to placebo in 129 patients with NED following metastasectomy) [22]. Both were negative trials, with a trend towards worse overall survival with pazopanib and worse relapse-free survival with sorafenib, suggesting a very limited role for adjuvant TKIs in patients with resected metastatic disease. A retrospective series conducted by Park et al., compared outcomes of 53 metastatic RCC patients who underwent metastasectomy after preoperative targeted therapy [23]. Of these, 34 patients (64.1%) stopped targeted therapy after metastasectomy, and 19 patients (35.9%) continued targeted therapy. They found DFS to be better in patients who received immediate postoperative targeted therapy, although the cancer-specific mortality was not significantly different between the two groups.
A recent phase 1/2 clinical trial identified 19 patients with no residual disease after metastasectomy for mRCC, who were treated with a patient-specific multi-peptide vaccine [24]. Median OS for patients receiving adjuvant UroRCC was not reached (mean: 112.6 months, 95% CI: 92.1–133.1 vs. 57.96 months; 95% CI: 92.1–133.1 in the control group) and remained significant after adjustment for Memorial Sloan Kettering Cancer Center risk groups, age, and metastatic sites, thus making this a promising strategy warranting further study.
DISCUSSION
Among resected RCC patients, those with completely resected metastatic RCC have demonstrated systemic disease and have a high risk of recurrence. Many adjuvant therapy trials have not included these M1 NED patients. The more pronounced DFS HR of the small M1 NED subgroup within Keynote 564 suggested this patient population had a more pronounced benefit from adjuvant immunotherapy. Despite the impressive results of pembrolizumab, other adjuvant immunotherapy trials, IMmotion 010 and PROSPER did not show similar results. While an argument can be made for these trials having smaller subgroups, the IMmotion 010 had a higher proportion of M1 NED patients (14% versus 6% in Keynote 564), and one would have expected to see similar results between the two studies. The discrepant results may reflect differences in the agents used, pembrolizumab acting on PD-1 vs atezolizumab acting on PDL-1, especially as atezolizumab in combination with bevacizumab has not demonstrated OS benefit in metastatic ccRCC [17]. This has previously been demonstrated in a pan-tumor meta-analysis where, anti–PD-1 drugs were shown to be clinically superior to anti-PD-L1 drugs and has been proven using molecular analyses [25]. PROSPER, another clinical trial that involved neoadjuvant priming with nivolumab followed by adjuvant nivolumab after nephrectomy, was negative, though it included only 28 total patients with M1NED, thus making it difficult to draw meaningful conclusions. Prior to the advent of immune-checkpoint inhibitors, other cytokine based therapies such as interleukin-2 (IL2), interferon (IFN)-α had been the standard of care based on improved survival in metastatic RCC [26]. However, subsequent evaluation in the adjuvant setting did. Not show clinical benefit and these drugs were not adopted for use after nephrectomy or complete metastasectomy [20].
The two trials that evaluated targeted therapies, specifically tyrosine kinase inhibitors [RESORT (sorafenib) and the ECOG-ACRIN trial E2810 (pazopanib)] were specifically designed for patients who had undergone metastasectomy. They were both negative trials, suggesting a limited role for TKIs in resected metastatic disease. Mechanistically, it maybe because immunotherapy, which has been shown to yield prolonged complete responses in a subset of patients with gross metastatic disease, may also be more likely to eradicate micrometastases as compared to TKIs.
In addition to the studies presented in this systematic review, there are ongoing trials that include M1NED patients. The pending LITESPARK-022 trial, comparing pembrolizumab plus belzutifan or pembrolizumab plus placebo, will provide unique insights into whether the addition of belzutifan to pembrolizumab will yield extra benefit in this patient population [27].
The major unanswered question of adjuvant therapy in RCC is identifying which patients are cured with surgery alone and which patients are at high risk for recurrence. Clinical models exist, but predictive biomarkers are not yet established. Tissue-based genomic and transcriptomic biomarkers that may be associated with the risk of recurrence are being investigated [28]. Additionally, circulating tumor DNA, which has shown promise in other cancers, is being investigated to detect minimal residual disease in RCC. Currently, however, RCC is considered a low-shedding tumor, and circulating tumor DNA is often only detected in those patients with larger burden of disease [29, 30]. Ongoing efforts to develop more sensitive assays should aim to circumvent this shortcoming.
Another important aspect of adjuvant treatment in resectable RCC is that it should maximize the proportion of patients cured without adding undue burden. It is important to consider that in the Keynote 564 trial, the rate of discontinuation of drug due to any adverse event was higher in the pembrolizumab arm (21.1% vs. 2.2%). Patients in the pembrolizumab arm experienced a higher incidence of serious adverse events of any cause (20.7% vs. 11.5%), as well as drug-related high-grade adverse events (18.6% vs. 1.2%) [5]. Thus inclusion of medical side effects of treatment as well as the financial toxicity is important to consider, as adjuvant pembrolizumab has only been found to be cost-effective in the highest-risk patients [31].
This systematic review has several limitations. As has been shown in previous studies, prognosis of patients with kidney cancer is dependent on additional clinical factors such as metastatic sites involved, and time since nephrectomy [32, 33]. However, granular details regarding sites of metastases resected, number of lesions subjected to metastasectomy, and interval since nephrectomy were not available in the papers cited from clinical trials in this review and should be a topic of a future analysis if such details could be made available. While searching literature for evidence, we found that some studies that did not report numerical data on survival and in the context of M1NED patients. Therefore, due to lack of data, a merged analysis of outcomes was not possible. Moreover, this being a systematic review, data was collected in a retrospective manner, and was thus prone to errors. There are also prior studies to suggest that there can be a publication bias associated with systematic reviews, in that prior studies with only significant results may get over-selected when gathering evidence [34].
Further studies to evaluate the role of adjuvant systemic therapy in M1NED patients should focus on the incorporation of biomarkers to identify patients at the highest risk of relapse, whether in the form of minimal residual disease assessment from circulating tumor DNA analysis or refined molecular techniques, such as transcriptomic signatures. Further, the inherent risk of subjecting these patients to adverse events from these systemic drugs should be considered when designing future trials for this patient population that may have been cured with metastasectomy alone.
CONCLUSION
No adjuvant TKI trial has shown improvement in DFS for M1 NED patients with ccRCC. The markedly lower DFS HR for the M1 NED subgroup for pembrolizumab within Keynote 564 has not been replicated in other immunotherapy trials using nivolumab and atezolizumab. The Chekmate-914 trial, that tested the combination of nivolumab plus ipilimumab and did not include M1NED patients, was speculated to be negative due to the lack of oligometastatic disease patients when compared to the pembrolizumab trial; the present review however points out that trials that had included a similar population were also negative. The conflicting results within adjuvant immunotherapy trials suggest more research is needed to determine which patients are at highest risk of relapse and would thus benefit most from adjuvant immunotherapy, specifically pembrolizumab.
ACKNOWLEDGMENTS
This work is supported in part by the NCI Cancer Center Support Grant P30 CA093373 (PNL) and NCI K08 CA273542 (SG)
FUNDING
This work is supported in part by the NCI Cancer Center Support Grant P30 CA093373 (PNL) and NCI K08 CA273542 (SG).
AUTHOR CONTRIBUTIONS
SM, PNL and SG contributed to the conception, performance and interpretation of data review for this paper.
CONFLICTS OF INTEREST
PNL is co-Editor-in-Chief of this journal and SG is an Editorial Board Member of this journal, but they have not been involved in the peer-review process of this paper, nor had access to any information regarding its peer-review. SM has no conflicts of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author.
REFERENCES
[1] | Lyon TD , Roussel E , Sharma V , et al. International multi-institutional characterization of the perioperative morbidity of metastasectomy for renal cell carcinoma. European Urology Oncology. (2023) ;6: (1):76–83. doi: 10.1016/j.euo.2022.11.003 |
[2] | Meyer CP , Sun M , Karam JA , et al. Complications after metastasectomy for renal cell carcinoma—a population-based assessment. European Urology. (2017) ;72: (2):171–174. doi: 10.1016/j.eururo.2017.03.005 |
[3] | Motzer RJ , Jonasch E , Agarwal N , et al. Kidney Cancer, Version 3.2022. J Natl Compr Canc Netw. (2022) ;20: (1):71–90. doi: 10.6004/jnccn.2022.0001 |
[4] | Choueiri TK , Tomczak P , Park SH , et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. New England Journal of Medicine. (2021) ;385: (8):683–694. doi: 10.1056/NEJMoa2106391 |
[5] | Choueiri TK , Tomczak P , Park SH , et al. Overall survival results from the phase 3 KEYNOTE-564 study of adjuvant pembrolizumab versus placebo for the treatment of clear cell renal cell carcinoma (ccRCC). JCO. (2024) ;42: (4_suppl):LBA359–LBA359. doi: 10.1200/JCO.2024.42.4_suppl.LBA359 |
[6] | Powles T , Tomczak P , Park SH , et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Oncology. (2022) ;23: (9):1133–1144. doi: 10.1016/S1470-2045(22)00487-9 |
[7] | Ravaud A , Motzer RJ , Pandha HS , et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. New England Journal of Medicine. (2016) ;375: (23):2246–2254. doi: 10.1056/NEJMoa1611406 |
[8] | Eisen T , Frangou E , Oza B , et al. Adjuvant Sorafenib for Renal Cell Carcinoma at Intermediate or High Risk of Relapse: Results From the SORCE Randomized Phase III Intergroup Trial. J Clin Oncol. (2020) ;38: (34):4064–4075. doi: 10.1200/JCO.20.01800 |
[9] | Gross-Goupil M , Kwon TG , Eto M , et al. Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: results from the phase III, randomized ATLAS trial. Annals of Oncology. (2018) ;29: (12):2371–2378. doi: 10.1093/annonc/mdy454 |
[10] | Haas NB , Manola J , Uzzo RG , et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. (2016) ;387: (10032):2008–2016. doi: 10.1016/S0140-6736(16)00559-6 |
[11] | Motzer RJ , Haas NB , Donskov F , et al. Randomized Phase III Trial of Adjuvant Pazopanib Versus Placebo After Nephrectomy in Patients With Localized or Locally Advanced Renal Cell Carcinoma. J Clin Oncol. (2017) ;35: (35):3916–3923. doi: 10.1200/JCO.2017.73.5324 |
[12] | Ryan CW , Tangen CM , Heath EI , et al. Adjuvant everolimus after surgery for renal cell carcinoma (EVEREST): a double-blind, placebo-controlled, randomised, phase 3 trial. The Lancet. (2023) ;402: (10407):1043–1051. doi: 10.1016/S0140-6736(23)00913-3 |
[13] | Motzer RJ , Russo P , Grünwald V , et al. Adjuvant nivolumab plus ipilimumab versus placebo for localised renal cell carcinoma after nephrectomy (CheckMate 914): a double-blind, randomised, phase 3 trial. The Lancet. (2023) ;401: (10379):821–832. doi: 10.1016/S0140-6736(22)02574-0 |
[14] | Allaf M , Kim SE , Harshman LC , et al. LBA67 Phase III randomized study comparing perioperative nivolumab (nivo) versus observation in patients (Pts) with renal cell carcinoma (RCC) undergoing nephrectomy (PROSPER, ECOG-ACRIN EA8143), a National Clinical Trials Network trial. Annals of Oncology. (2022) ;33: :S1432–S1433. doi: 10.1016/j.annonc.2022.08.072 |
[15] | Pal SK , Uzzo R , Karam JA , et al. Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increased risk of recurrence following resection (IMmotion010): a multicentre, randomised, double-blind, phase 3 trial. The Lancet. (2022) ;400: (10358):1103–1116. doi: 10.1016/S0140-6736(22)01658-0 |
[16] | Rofi E , Del Re M , Arrigoni E , et al. Clinical pharmacology of monoclonal antibodies targeting PD-1 axis in urothelial cancers. Critical Reviews in Oncology/Hematology. (2020) ;154: :102891. doi: 10.1016/j.critrevonc.2020.102891 |
[17] | Motzer RJ , Powles T , Atkins MB , et al. Final Overall Survival and Molecular Analysis in IMmotion151, a Phase 3 Trial Comparing Atezolizumab Plus Bevacizumab vs Sunitinib in Patients With Previously Untreated Metastatic Renal Cell Carcinoma. JAMA Oncology. (2022) ;8: (2):275–280. doi: 10.1001/jamaoncol.2021.5981 |
[18] | Page MJ , McKenzie JE , Bossuyt PM , et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) ;372: :n71. doi: 10.1136/bmj.n71 |
[19] | Clark JI , Atkins MB , Urba WJ , et al. Adjuvant High-Dose Bolus Interleukin-2 for Patients With High-Risk Renal Cell Carcinoma: A Cytokine Working Group Randomized Trial. JCO. (2003) ;21: (16):3133–3140. doi: 10.1200/JCO.2003.02.014 |
[20] | Kwak C , Park YH , Jeong CW , Lee SE , Ku JH . No role of adjuvant systemic therapy after complete metastasectomy in metastatic renal cell carcinoma? Urologic Oncology: Seminars and Original Investigations. (2007) ;25: (4):310–316. doi: 10.1016/j.urolonc.2006.08.022 |
[21] | Procopio G , Apollonio G , Cognetti F , et al. Sorafenib Versus Observation Following Radical Metastasectomy for Clear-cell Renal Cell Carcinoma: Results from the Phase 2 Randomized Open-label RESORT Study. European Urology Oncology. (2019) ;2: (6):699–707. doi: 10.1016/j.euo.2019.08.011 |
[22] | Appleman LJ , Puligandla M , Pal SK , et al. Randomized, double-blind phase III study of pazopanib versus placebo in patients with metastatic renal cell carcinoma who have no evidence of disease following metastasectomy: A trial of the ECOG-ACRIN cancer research group (E2810). JCO. (2019) ;37: (15_suppl):4502–4502. doi: 10.1200/JCO.2019.37.15_suppl.4502 |
[23] | Park YH , Jung JW , Lee BK , et al. Targeted therapy after complete resection of metastatic lesions in metastatic renal cell carcinoma. International Journal of Urology. (2015) ;22: (2):153–157. doi: 10.1111/iju.12662 |
[24] | Rausch S , Gouttefangeas C , Hennenlotter J , et al. Results of a Phase 1/2 Study in Metastatic Renal Cell Carcinoma Patients Treated with a Patient-specific Adjuvant Multi-peptide Vaccine after Resection of Metastases. European Urology Focus. (2019) ;5: (4):604–607. doi: 10.1016/j.euf.2017.09.009 |
[25] | Duan J , Cui L , Zhao X , et al. Use of Immunotherapy With Programmed Cell Death 1 vs Programmed Cell Death Ligand 1 Inhibitors in Patients With Cancer: A Systematic Review and Meta-analysis. JAMA Oncology. (2020) ;6: (3):375–384. doi: 10.1001/jamaoncol.2019.5367 |
[26] | Vogelzang NJ , Stadler WM . Kidney cancer. The Lancet. (1998) ;352: (9141):1691–1696. doi: 10.1016/S0140-6736(98)01041-1 |
[27] | Choueiri TK , Bedke J , Karam JA , et al. LITESPARK-022: A phase 3 study of pembrolizumab+belzutifan as adjuvant treatment of clear cell renal cell carcinoma (ccRCC). JCO. (2022) ;40: (16_suppl):TPS4602–TPS4602. doi: 10.1200/JCO.2022.40.16_suppl.TPS4602 |
[28] | Vasudev NS , Scelo G , Glennon KI , et al. Application of Genomic Sequencing to Refine Patient Stratification for Adjuvant Therapy in Renal Cell Carcinoma. Clin Cancer Res. (2023) ;29: (7):1220–1231. doi: 10.1158/1078-0432.CCR-22-1936 |
[29] | Geertsen L , Koldby KM , Thomassen M , Kruse T , Lund L . Circulating Tumor DNA in Patients with Renal Cell Carcinoma. A Systematic Review of the Literature. European Urology Open Science. (2022) ;37: :27–35. doi: 10.1016/j.euros.2021.12.006 |
[30] | Smigelski M , Sudhaman S , Nagpal S , et al. P Utility of circulating tumor (ct)DNA testing for molecular residual disease (MRD) detection and treatment response monitoring in patients (pts) with renal cell carcinoma (RCC). Annals of Oncology. (2023) ;34: :S1027. doi: 10.1016/j.annonc.2023.09.1138 |
[31] | Sharma V , Wymer KM , Joyce DD , et al. Cost-effectiveness of Adjuvant Pembrolizumab After Nephrectomy for High-risk Renal Cell Carcinoma: Insights for Patient Selection From a Markov Model. J Urol. (2023) ;209: (1):89–98. doi: 10.1097/JU.0000000000002953 |
[32] | Dudani S , de Velasco G , Wells JC , et al. Evaluation of Clear Cell, Papillary, and Chromophobe Renal Cell Carcinoma Metastasis Sites and Association With Survival. JAMA Network Open. (2021) ;4: (1):e2021869. doi: 10.1001/jamanetworkopen.2020.21869 |
[33] | Stellato M , Santini D , Verzoni E , et al. Impact of Previous Nephrectomy on Clinical Outcome of Metastatic Renal Carcinoma Treated With Immune-Oncology: A Real-World Study on Behalf of Meet-URO Group (MeetUro-7b). Front Oncol. (2021) ;11: :682449. doi: 10.3389/fonc.2021.682449 |
[34] | Owens JK . Systematic reviews: Brief overview of methods, limitations, and resources. Nurse Author & Editor. (2021) ;31: (3-4):69–72. doi: 10.1111/nae2.28 |




