Efficacy, Effectiveness, and Safety of Interventions for Von Hippel-Lindau Associated Renal Cell Carcinoma: A Systematic Literature Review
Abstract
Background:
A small proportion of renal cell carcinoma (RCC) are associated with hereditary syndromes such as von Hippel-Lindau disease (VHL) and are commonly treated with surgical interventions. More recently, systemic treatments for VHL-associated RCC have been assessed as an alternative to surgery.
Methods:
A systematic literature review was conducted by searching MEDLINE, EMBASE, and Cochrane Registry of Controlled Trials to collect and interpret published evidence on treatments for VHL-associated RCC patients to better understand the treatment landscape.
Results:
This review identified 32 primary studies, comprised of single-arm clinical trials and real-world studies assessing systemic, surgical, radiological, or image guided ablation interventions. In clinical trials, treatment with sunitinib and pazopanib showed an objective response in 33% and 52% of RCC lesions respectively. For patients treated with belzutifan, 64% of patients showed an objective response, of which 7% were complete response and 57% were partial responses with a 24-month PFS rate of 96%. In real-world studies, treatment with sunitinib, pazopanib, axitinib, and sorafenib showed an objective response in 40%, 0%, 33%, and 25% of RCC lesions respectively, and all the responses were partial. In the studies assessing surgical, radiological, or image guided ablation interventions primary failure/re-intervention rates ranged from 2% to 84%.
Conclusion:
Local procedures are still a mainstay in the management of patients with non-metastatic VHL-associated RCC although multiple procedures incur an increasing rate of complications. A limited number of clinical trials and real-world studies evaluated VEGF-TKIs for the treatment of VHL-RCC, while responses were observed, long term treatment was limited by toxicities.
INTRODUCTION
Renal Cell Carcinoma (RCC) accounts for approximately 3% of all cancers in adults and 85% of malignant kidney tumors; roughly 5% of RCCs have a hereditary basis [1, 2]. There are several histologic subtypes of RCC, most common histologic subtype is clear cell RCC, making up nearly 70% of cases of RCC. The remainder sub-types are called as “non clear cell” or “variant histologies” and encompass papillary, chromophore, translocation, medullary, oncocytoma [1, 3–5]. Most clear cell carcinomas (95%) are sporadic and the remaining 5% are associated with hereditary syndromes like von Hippel-Lindau (VHL) disease [5, 6].
VHL disease is a rare hereditary, autosomal dominant syndrome, with an estimated incidence of 1 in 36,000 people [1, 7, 8]. Individuals with VHL disease are at an increased risk of developing recurrent cysts and lesions that may progress to RCC, specifically with a clear cell histological subtype which is a major cause of death for VHL disease patients [2, 9]. VHL disease is classified into type 1 (the result of a nonsense mutation or deletion is the cause) or type 2 (the result of a missense mutation, based on adrenal involvement) [9]. While families with Type 1 phenotype have a low risk of phaeochromocytoma, this phenotype has a higher risk of developing retinal hemangioblastoma, Central Nervous System (CNS) hemangioblastoma, RCC, and pancreatic neoplasms and cysts. All type 2 phenotypes have phaeochromocytoma, however, Type 2A families have an increased risk of retinal hemangioblastoma, CNS hemangioblastoma, while Type 2B families have an increased risk for retinal hemangioblastomas, CNS hemangioblastomas, RCC, and pancreatic neoplasms and cysts [9, 10]. Type 2 C families experience only phaeochromocytomas, without any additional neoplastic manifestations associated with VHL (i.e. retinal hemangioblastomas, CNS hemangioblastomas, RCC, and pancreatic neoplasms and cysts) [10].
For patients with diagnosed VHL disease, active surveillance is recommended every six to 12 months [9]. Surgical interventions are the most used treatments in patients with VHL disease-associated RCC. Generally, RCC tumors are routinely monitored until the size reaches approximately 3 centimeters before being resected [11]. Several surgical procedures are utilized for treatment, including nephron sparing surgery (NSS), enucleation, and partial or radical nephrectomy [11–13]. Care is taken when applying surgical techniques in order to minimize need for subsequent multiple resections and potential complications. The other treatment options for VHL disease-associated RCC include radiofrequency ablation (RFA), cryosurgery, molecularly targeted therapy [11–13]. Most recently, the United States Food and Drug Administration (US FDA) approved belzutifan, an orally active inhibitor of hypoxia-inducible factor-2alpha (HIF-2α), first in its class for the treatment of VHL disease-associated RCC [14].
We performed a systematic literature review (SLR) to collect and interpret published evidence on the efficacy, effectiveness, and safety of interventions in VHL disease-associated RCC patients to better understand the treatment landscape.
METHODS
Literature searches and eligibility criteria
A systematic literature search was conducted in MEDLINE, EMBASE, and the Cochrane Registry of Controlled Trials (through the OVID portal) on February 10, 2023 (see Tables S1-S3 for search strategies). Conferences from the past three years, including the American Society for Clinical Oncology (ASCO 2020, 2021, and 2022), European Society for Medical Oncology (ESMO for 2020, 2021, and 2022) were also searched. Hand searches were also conducted in clinicaltrials.gov to identify clinical trials that have not been published but are potentially eligible for inclusion.
Studies were included based on the PICOS (Population, Intervention, Comparator, Outcomes, Study design) criteria, as defined in Table S4. In summary, eligible studies included clinical trials (randomized, non-randomized and single-arm) and real-world studies (prospective and retrospective cohorts) reporting efficacy or effectiveness and/or safety of interventions in the treatment of VHL-associated RCC.
Data screening and extraction
All abstracts were screened according to the PICOS criteria. Relevant abstracts were screened again by viewing the full-text study publication to determine a final inclusion status as outlined by the PICOS criteria. Data was then extracted as reported from these studies, including: study characteristics (study design, intervention, geographic location, study design, study duration and period), participant characteristics (age, sex, ECOG, clear cell histology status, VHL disease diagnosis method and VHL type), and outcomes (objective response rate, complete response, partial response, stable disease, progressive disease, primary failure/re-intervention rate, time to surgery, five and ten year repeat surgery rates, five and ten year survival rates, progression free survival, one year progression free survival rate, adverse events, surgical complications).
Study screening and data extraction were performed independently by two reviewers. These reviewers compared their completed work to identify any discrepancies and resolve these through consensus, including a third reviewer if needed. The PRISMA guidelines was used to ensure completeness of all reported items [15].
RESULTS
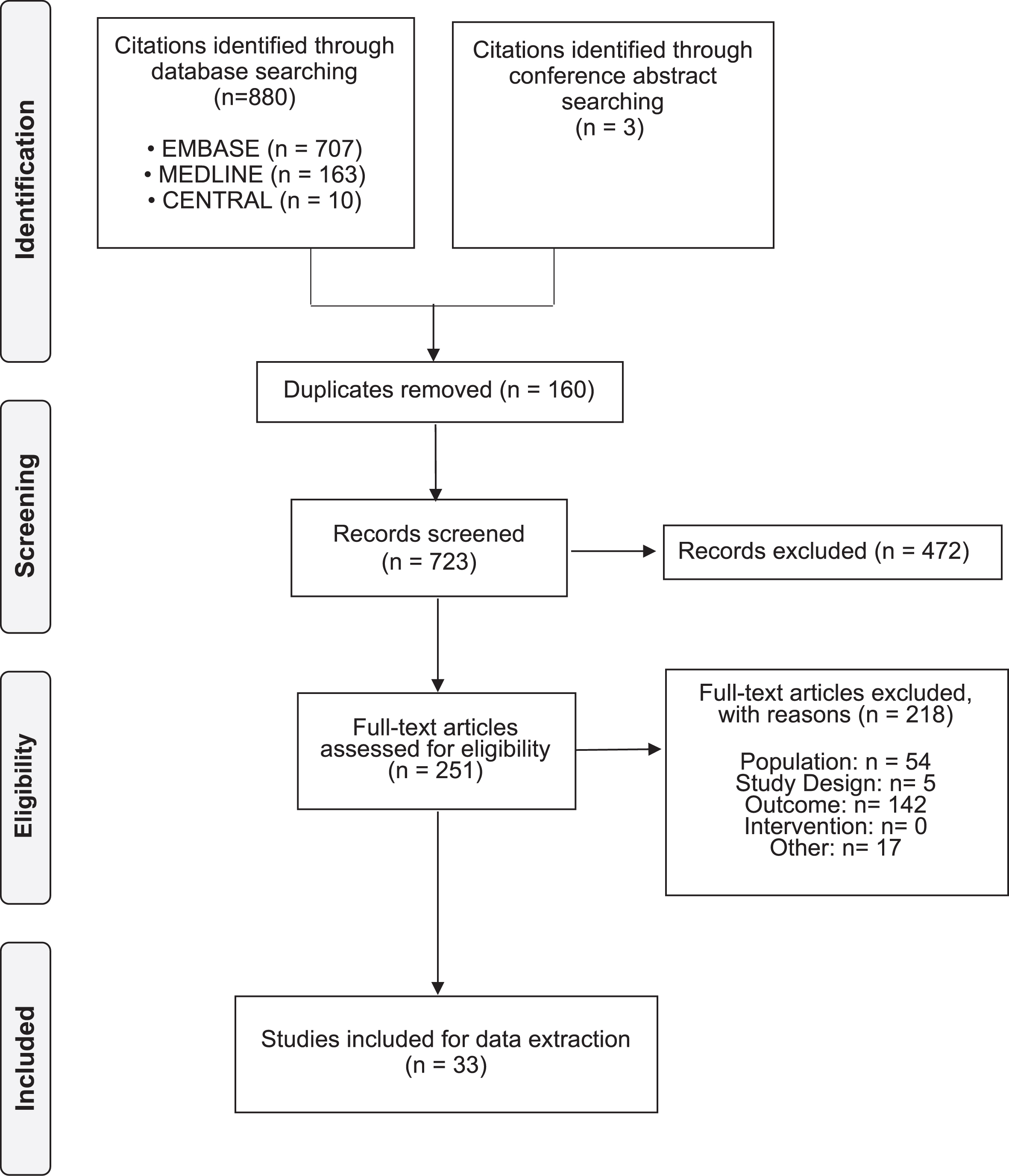
A total of 883 citations were identified through searching the bibliographic databases and conference proceedings. After excluding 160 duplicates, a total of 723 citations were screened, resulting in the inclusion of 33 citations representing 32 unique studies. Study and patient characteristics for all included studies are presented in Table 1.
Table 1
Study and patient characteristics of the included studies
| Study | Study design | Intervention | N | Age (in years), mean/median (range) | Male, n (%) | ECOG 0, n (%) | Clear cell histology, n (%) | VHL type | Prior surgery n (%) |
| Systemic treatments | |||||||||
| Jonasch et al. 2011 [18] | Single-arm trial | Sunitinib 50 mg | 12 | 36 (22, 57)* | – | – | 12 (100) | – | – |
| Jonasch et al. 2018 [17] | Single-arm trial | Pazopanib 800 mg | 32 | 38 (32-42)* | 14 (44) | – | – | – | – |
| LITESPARK-004 [19] | Single-arm trial | Belzutifan 120 mg | 61 | 41 (19-66)* | 32 (52) | 50 (82) | – | 1 (n = 51); 2A (n = 2); 2B (n = 6); missing (n = 2) | – |
| Ma 2019 [20] | Real-world | Sunitinib 50 mg; Sorafenib 400 mg; Axitinib 5 mg; Pazopanib 800 mg | 32 | 41.5 (21-66) | 18 (56) | – | – | – | – |
| Local treatments | |||||||||
| Asthagiri et al. 2010 [25] | Real-world | Stereotactic radiosurgery | 20 | 37.5 (13–67)* | 10 (50) | – | – | – | 15 (75) |
| Capitanio et al. 2021 [26] | Real-world | Surgery | 96 | 38 (32–47)* | 51 (53.1) | – | 96 (100) | – | (90.7) |
| Chan et al. 2022 [27] | Real-world | Image-guided ablation (Radiofrequency ablation, Cryoablation, Irreversible electroporation) | 17 | 43.9 (13.6) | 65 (6) | – | 17 (100) | – | 10 (59) |
| Chang et al. 1998 [28] | Real-world | Linear accelerator-based radiosurgery | 13 | 40 (31–57) | 10 (76.9) | – | – | – | 11 (84.6) |
| Cvek et al. 2022 [29] | Real-world | Stereotactic body radiotherapy | 5 | 22 (18–60)* | 3 (60) | – | – | – | – |
| Eggener et al. 2004 [30] | Real-world | Nephrectomy | 12 | – | 0 (0) | – | – | – | – |
| Frydenberg et al. 1993 [31] | Real-world | Nephrectomy | 19 | 40.3 (15–65) | 8 (73) | – | – | – | 5 (26.32) |
| Goldfarb et al. 1997 [32] | Real-world | Renal transplant | 32 | 36 (19–59) | 23 | – | – | – | 32 (100) |
| Hidaka et al. 2022 [33] | Real-world | Surgery | 58 | 36 (24–41)* | 34 (58.6) | – | – | – | – |
| Hwang et al. 2003 [34] | Real-world | Surgery | 29 | 32 (14, 54) | 14 (48.3) | – | 29 (100) | – | 9 (31) |
| Iwamoto et al. 2011 [35] | Real-world | Radiofrequency Ablation | 3 | 41 | – | – | 3 (100) | – | – |
| Jayanth et al. 2021 [36] | Real-world | Nephron sparing surgery | 17 | 39 (23-41) | 12 (70) | – | 17 (100) | 1 (n = 10); 2B (n = 5); 2 C (n = 2) | – |
| Jilg et al. 2012 [16] | Real-world | Nephron sparing surgery, Nephrectomy, RFA | 54 | 38.5 (18–73)∧ | 24 (44.4) | – | 54 (100) | – | 29 (54) |
| Lund et al. 1994 [37] | Real-world | Nephron sparing surgery | 10 | 33 (23–49)** | – | – | 9 (90) | – | – |
| Kano et al. 2015 [38] | Real-world | Stereotactic radiosurgery | 80 | 38* | 36 (45) | – | – | – | 70 (87.5) |
| Kano et al. 2008 [39] | Real-world | Stereotactic radiosurgery | 13 | 40.2* | 7 (53.8) | – | – | – | 13 (100) |
| Koh et al. 2007 [40] | Real-world | Fractionated external beam radiotherapy | 5 | 31 (25–41) | – | – | – | – | – |
| Morgan et al. 1990 [41] | Real-world | Nephrectomy and/or enucleation | 6 | – | – | – | – | – | – |
| Novick et al. 1992 [42] | Real-world | Nephron sparing surgery | 9 | NR (26–67) | – | – | 9 (100) | – | – |
| Peng et al. 2019 [43] | Real-world | Nephrectomy | 49 | – | 82 (54.67) | – | 72 (100) | 1 (n = 12); 2 (n = 30) | – |
| Persad et al. 1997 [6] | Real-world | Nephrectomy | 11 | 42 (31–62) | 7 (64) | – | – | – | 30 (53.6) |
| Ploussard et al. 2007 [44] | Real-world | Nephron sparing surgery | 18 | 38.5 (24–69) | – | – | (98.8) | – | – |
| Roupret 2003 [45] | Real-world | Nephron sparing surgery | 56 | 37.2** | 26 (46.4) | – | – | – | – |
| Simone et al. 2011 [46] | Real-world | Infratentorial craniospinal radiation therapy (ICSRT) | 7 | ||||||
| Steinbach et al. 1995 [21] | Real-world | Nephron sparing surgery | 65 | 39 (15–67)** | 39 (60) | – | (89) | – | 18 (28) |
| Wessendorf et al. 2021 [47] | Real-world | Radiofreqency ablation | 9 | – | – | – | – | – | – |
| Yousef et al. 2019 [48] | Real-world | Surgery | 20 | – | 15 (75) | – | – | – | – |
| Yao et al. 2002 [49] | Real-world | Nephrectomy with adjuvant postoperative interferon and/or chemotherapy | 78 | – | – | – | – | – | 56 (60) |
*Median age was reported; **age at diagnosis; ∧Calculated (mean age was 37 years in males (range 18–66 years) and 40 years in females (range 21–73 years). Abbreviations: ECOG, Eastern Cooperative Oncology Group; N, number of patients; RFA, radiofrequency ablation; VHL, Von Hippel-Landau.
Of the 32 unique studies, three studies were single-arm clinical trials that assessed systemic treatments in patients with VHL disease-associated RCC: belzutifan 120 mg once daily until disease progression or intolerable toxicity in LITESPARK-004. In Jonasch et al. 2011, sunitinib 50 mg was given once daily for 28 days with a 14-day break between the cycles for up to four cycles. In Jonasch et al. 2018, pazopanib 800 mg was given daily for 28 days of treatment up to six cycles of treatment (Table 1). The mean/median age at treatment initiation/diagnosis was around 40 years, and the proportion of men included in these studies was around 50%. Only LITESPARK-004 reported data on ECOG status, with 82% of patients with a score of 0 (Table 1).
Systemic treatments for VHL disease-associated RCC
Overall response rate
Response rates were reported in all three clinical trials (Table 2). Jonasch et al. 2011 and Jonasch et al. 2018 reported response by lesions whereas LITESPARK-004 reported response by patients. In a study by Jonasch et al. 2011, sunitinib treatment showed a partial response in 33% of RCC lesions, stable disease in 56% of RCC lesions, and progressive disease in 11% of RCC lesions. In Jonasch et al. 2018 study, pazopanib treated patients reported an objective response of 52% in RCC lesions, with 3% of lesions exhibiting complete response and 49% of RCC lesions exhibiting partial response. In patients treated with belzutifan (LITESPARK-004), 64% patients showed an objective response, with 7% (n = 4) of patients achieving complete response and 57% (n = 35) of patients achieving partial response. The median duration of response was not reached with belzutifan treatment, the median time to response was 11.1 months (range: 2.7–30.5), the other two studies with systemic treatments did not report data on median duration and median time to response. One real-world study assessed the use of tyrosine kinase inhibitors (TKI). Ma et al. 2019 assessed the use of sunitinib, sorafenib, axitinib, and pazopanib and reported response by lesions. Treatment with sunitinib showed a partial response in 40% of RCC lesions, stable disease in 33% of RCC lesions, and progressive disease in 27% of RCC lesions. Sorafenib treated individuals showed a partial response in 25% of RCC lesions, stable disease in 42% of RCC lesions, and progressive disease in 33% of RCC lesions. Patients treated with axitinib showed a partial response in 33% of RCC lesions and stable disease in 67% of RCC lesions. Treatment with pazopanib showed no response in any RCC lesions and stable disease in 100% of RCC lesions. No complete responses were reported in any of the TKIs (Table 2).
Table 2
Response outcomes in patients treated with systemic interventions
| N | ORR (%) | CR (%) | PR (%) | SD (%) | PD (%) | |
| Clinical trials | ||||||
| Belzutifan | ||||||
| RCC (LITESPARK-004) [50] | 61 | 64 | 7 | 57 | – | – |
| Pazopanib | ||||||
| RCC* (Jonasch et al. 2018) [17] | 59 | 52 | 3 | 49 | 47 | 0 |
| Sunitinib | ||||||
| RCC* (Jonasch et al. 2011) [18] | 18 | 33 | 0 | 33 | 56 | 11 |
| Real-world studies | ||||||
| Sunitinib | ||||||
| RCC* (Ma et al. 2019) [20] | 15 | 40 | 0 | 40 | 33 | 27 |
| Sorafenib | ||||||
| RCC* (Ma et al. 2019) [20] | 12 | 25 | 0 | 25 | 42 | 33 |
| Axitinib | ||||||
| RCC* (Ma et al. 2019) [20] | 6 | 33 | 0 | 33 | 67 | 0 |
| Pazopanib | ||||||
| RCC* (Ma et al. 2019) [20] | 3 | 0 | 0 | 0 | 100 | 0 |
*Outcomes reported for number of lesions meeting criteria. Abbreviations: CR, Complete response; ORR, Overall response rate; PD, Progressive disease; PR, Partial response; SD, Stable disease.
Progression-free survival
The 24-month PFS rate was 96% in patients treated with belzutifan. PFS for patients receiving sunitinib and pazopanib were not reported (Table 3).
Table 3
Survival outcomes
| Study | Intervention | N | Median TTS, months (95% CI) | 5-year, repeat surgery rate, % | 10-year, repeat surgery rate, % | Median OS, months (95% CI) | 5-year, survival, % | 10-year, survival, % | Median PFS, months (95% CI) | 12-month, PFS rate, % | 24-month, PFS rate, % | 5-year, PFS, % | 10-year, PFS, % |
| Systemic treatments | |||||||||||||
| Jonasch et al. 2011 [18] | Sunitinib 50 mg | 12 | 27 (0.8-5.6) | – | – | – | – | – | – | – | 91 | 83 | 61 |
| Jonasch et al. 2018 [17] | Pazopanib 800 mg | 32 | – | – | – | – | – | – | – | – | – | – | – |
| LITESPARK-004 [19] | Belzutifan 120 mg | 61 | – | – | – | – | – | – | – | – | 96 | – | – |
| Ma et al. 2019 [20] | Sunitinib 50 mg; Sorafenib 400 mg; Axitinib 5 mg; Pazopanib 800 mg | 32 | – | – | – | – | – | – | – | – | – | – | – |
| Local treatments | |||||||||||||
| Asthagiri et al. 2010 [25] | Stereotactic radiosurgery | 20 | 2.7 years (0.8–5.6) | – | – | – | – | – | – | – | – | – | – |
| Capitanio et al. 2021 [26] | Surgery | 96 | – | – | – | – | 96 | 92.5 | – | – | – | 88.3 | 70.7 |
| Chan et al. 2022 [27] | Image-guided ablation (Radiofrequency ablation, Cryoablation, Irreversible electroporation) | 17 | – | – | – | – | 100 | 90 | – | – | – | – | – |
| Chang et al. 1998 [28] | Linear accelerator-based radiosurgery | 13 | – | – | – | – | – | – | – | – | – | – | – |
| Cvek et al. 2022 [29] | Stereotactic body radiotherapy | 5 | – | – | – | – | – | – | – | – | – | – | – |
| Eggener et al. 2004 [30] | Nephrectomy | 12 | – | – | – | – | – | – | – | – | – | – | – |
| Frydenberg et al. 1993 [31] | Nephrectomy | 19 | – | – | – | – | – | – | – | – | – | – | – |
| Goldfarb et al. 1997 [32] | Renal transplant | 32 | – | – | – | – | 65 | – | – | – | – | – | – |
| Hidaka et al. 2022 [33] | Surgery | 58 | – | – | – | – | – | – | – | – | – | – | – |
| Hwang et al. 2003 [34] | Surgery | 29 | – | – | – | – | – | – | – | – | – | – | – |
| Iwamoto et al. 2011 [35] | Radiofrequency Ablation | 3 | – | – | – | – | – | – | – | – | – | – | – |
| Jayanth et al. 2021 [36] | Nephron sparing surgery | 17 | – | – | – | – | – | – | – | – | – | – | – |
| Jilg et al. 2012 [16] | NSS, Nephrectomy, RFA | 54 | 149.6 | 21 | 42 | – | 96.5 | 82.5 | – | – | – | – | – |
| Lund et al. 1994 [37] | Nephron sparing surgery | 10 | – | – | – | – | – | – | – | – | – | – | – |
| Kano et al. 2015 [38] | Stereotactic radiosurgery | 80 | – | – | – | – | 82 | 77 | – | – | – | – | – |
| Kano et al. 2008 [39] | Stereotactic radiosurgery | 13 | 55 (8–153) | – | – | – | 90.9 | 77.9 | – | 97.4 | – | – | 97.4 |
| Koh et al. 2007 [40] | Fractionated external beam radiotherapy | 5 | – | – | – | – | 100 | – | – | – | – | – | – |
| Morgan et al. 1990 [41] | Nephrectomy and/or enucleation | 6 | – | – | – | – | – | – | – | – | – | – | – |
| Novick et al. 1992 [42] | Nephron sparing surgery | 9 | – | – | – | – | – | – | – | – | – | – | – |
| Peng et al. 2019 [43] | Nephrectomy | 49 | – | – | – | – | – | – | – | – | – | – | – |
| Persad et al. 1997 [6] | Nephrectomy | 11 | – | – | – | – | – | – | – | – | – | – | – |
| Ploussard et al. 2007 [44] | Nephron sparing surgery | 18 | – | 23.1 | 63.4 | – | – | 93.8 | – | – | – | – | – |
| Roupret et all 2003 [45] | Nephron sparing surgery | 56 | – | – | – | – | 100 | 67 | – | – | – | – | – |
| Simone et al. 2011 [46] | Infratentorial craniospinal radiation therapy | 7 | – | – | – | – | 71.4 | – | – | – | – | – | – |
| Steinbach et al. 1995 [21] | Nephron sparing surgery | 65 | – | – | – | – | 87 | 68 | – | – | – | – | – |
| Wessendorf et al. 2021 [47] | Radiofreqency ablation | 9 | – | – | – | NR | – | – | NR | – | – | – | – |
| Yousef et al. 2019 [48] | Surgery | 20 | – | – | – | – | – | – | – | – | – | – | – |
| Yao et al. 2002 [49] | Nephrectomy with adjuvant postoperative interferon and/or chemotherapy | 78 | – | – | – | – | – | – | – | – | – | – | – |
Abbreviations: NR, Not reached; NSS, nephron sparing surgery; OS, Overall survival; PFS, Progression-free survival; RFA, radiofrequency ablation; TTS, Time to surgery.
Toxicities
In patients receiving belzutifan, 85% of patients continued treatment with no dose reduction due to adverse events. The most common grade 3 toxicities experienced in patients treated with belzutifan was anemia (8%), hypertension (8%), and fatigue (5%). One patient each (2%) experienced a grade 4 adverse event (retinal detachment) and grade 5 adverse event (acute toxic effects of fentanyl), however, both were determined to be unrelated to treatment. Treatment discontinuation due to toxicity was observed in one patient (2%) due to grade 1 dizziness.
In patients receiving pazopanib, 32% of patients continued treatment with no dose reduction due to adverse events. The most common grade 3 toxicities experienced in patients treated with pazopanib were an increase in aspartate aminotransferase (10%) and an increase in alanine aminotransferase (10%). Grade 4 and 5 toxicities included an increase in alanine aminotransferase (grade 4 : 3%) and a nervous system bleed due to head trauma from a fall (grade 5 : 3%). Treatment related serious adverse events included appendicitis and gastritis (one patient each). A total of 23% of patients discontinued pazopanib treatment due to toxicity for transaminitis (grade 3/4), back pain (grade 2), diarrhea and fatigue (grade 2), and abdominal pain, fatigue, and diarrhea (grade 2).
In patients receiving sunitinib, 33% of patients continued treatment with no dose reduction due to adverse events. The most common grade 3 toxicities experienced in patients treated with sunitinib included fatigue (33%), neutropenia (26%), hand–foot syndrome (13%), and nausea (13%). No sunitinib treated patients experienced grade 4 or 5 toxicities. Treatment with sunitinib resulted in one patient (7%) discontinuing due to toxicity (neutropenia).
Local treatments for VHL disease-associated RCC
Three real-world studies assessed image guided ablation techniques, seven studies assessed radiation-based therapies, and the remaining 18 studies assessed surgical interventions.
Primary failure/re-intervention rate
In the three studies assessing image guided ablation techniques the median duration of follow up was 79 months in Chan et al. 2022 and 20.3 months in Iwamoto et al. 2011. The mean follow up was 34 months for Wessendorf et al. 2021. Chan et al. 2022 assessed a combination of image guided ablation techniques (i.e., radiofrequency ablation, cryoablation, irreversible electroporation), whereas Wessendorf et al. 2021 and Iwamoto et al. 2011 exclusively assessed radiofrequency ablation. The primary failure/re-intervention rate was 5.9% in Chan et al. 2022 and was not reported in Wessendorf et al. 2021 and Iwamoto et al. 2011 (Table 4).
Table 4
Surgical outcomes
| Study | Intervention | N | Primary failure/re-intervention rate, % |
| Asthagiri et al. 2010 [25] | Stereotactic radiosurgery | 20 | 2% |
| Capitanio et al. 2021 [26] | Surgery | 96 | 8 years: 50% |
| Chan et al. 2022 [27] | Image-guided ablation (Radiofrequency ablation, Cryoablation, Irreversible electroporation) | 17 | 3 years: 5.9% |
| Chang et al. 1998 | Linear accelerator-based radiosurgery | 13 | – |
| Cvek et al. 2022 [29] | Stereotactic body radiotherapy | 5 | – |
| Eggener et al. 2004 [30] | Nephrectomy | 12 | 3.2 years: 25% |
| Frydenberg et al. 1993 [31] | Nephrectomy | – | – |
| Goldfarb et al. 1997 [32] | Renal transplant | 32 | 9% |
| Hidaka et al. 2022 [33] | Surgery | – | – |
| Hwang et al. 2003 [34] | Surgery | 29 | 1.8 years: 28% |
| Iwamoto et al. 2011 [35] | Radiofrequency ablation | – | – |
| Jayanth et al. 2021 [36] | Nephron sparing surgery | 13 | 6.5 years: 53% |
| Jilg et al. 2012 [16] | NSS, Nephrectomy, RFA | 54 | 5.6 years: 55% |
| Lund et al. 1994 [37] | Nephron sparing surgery | 10 | 3.25 years: 40% |
| Kano et al. 2015 [38] | Stereotactic radiosurgery | 80 | 1 year: 7%; 3 years: 21%; 5 years: 43%; 10 years: 84% |
| Kano et al. 2008 [39] | Stereotactic radiosurgery | 13 | 3.6 years: 80% |
| Koh et al. 2007 [40] | Fractionated external beam radiotherapy | 5 | 40% |
| Morgan et al. 1990 [41] | Nephrectomy and/or enucleation | – | – |
| Novick et al. 1992 [42] | Nephron sparing surgery | 9 | 5 years: 77.7% * |
| Peng et al. 2019 [43] | Nephrectomy | – | – |
| Persad et al. 1997 [6] | Nephrectomy | 11 | 45.5% |
| Ploussard et al. 2007 [44] | Nephron sparing surgery | 18 | 2 years: 14.5%; 5 years: 45.6%; 10 years: 83.7% |
| Roupret et al. 2003 [45] | Nephron sparing surgery | 56 | 13 years: 46.4% |
| Simone et al. 2011 | Infratentorial craniospinal radiation therapy | – | – |
| Steinbach et al. 1995 [21] | Nephron sparing surgery | 49 | 51% |
| Wessendorf et al. 2021 [47] | Radiofrequency ablation | – | – |
| Yao et al. 2002 [49] | Nephrectomy | – | – |
| Yousef et al. 2019 [48] | Surgery | 20 | 4.1 years: 65% * |
*Number of tumours, Abbreviations: NR, Not reached; NSS, nephron sparing surgery; RFA, radiofrequency ablation.
In the seven studies assessing radiation-based therapies, the median duration of follow-up ranged from 50.1 months (Kano et al. 2008) to 114 months (Kano et al. 2015). The median time to next surgery was reported in two studies with median reported times of 32 months (Asthagiri et al. 2010) and 55 months (Kano et al. 2015). The primary failure/re-intervention rate in radiation-based therapies ranged from 2% (Asthagiri et al. 2010) to 84% (Kano et al. 2015).
In the 18 studies assessing surgical interventions, the median duration of follow-up ranged from 21 months (Hwang et al. 2003) to 100 months (Ploussard et al. 2007). Jilg et al. 2012 was the only study that reported data on time to next surgery with a median time of 149.6 months [16]. The primary failure/re-intervention rate in surgery studies ranged from 9% (Goldfarb et al. 1997) to 83.7% (Ploussard et al. 2007). Two surgery studies determined the primary failure/re-intervention rate by number of tumors instead of by number of patients (Table 4).
Surgical complications
Complications due to surgery were reported in ten studies (Table 5). Operative mortality was not observed in any of the studies. In the study by Jilg et al. 2012, nearly 50% patients reported hemorrhage, 11% patients reported retroperitoneal hematoma, 30% reported urinary leak, 7% reported perioperative hemodialysis, and 7% reported bleeding. In the study by Jayanth et al. 2021, 3% patients reported renal failure and urinary leakage. In the study by Hwang et al. 2003, 3% patients reported renal failure and urinary leakage. In the study by Hidaka et al. 2022, 1.7% patients reported hemorrhage and 2.7% reported stroke. In the study by Lund et al. 1994, 10% patients reported pleural effusion, bowel obstruction, pneumonia, and postoperative coagulopathy. Ploussard et al. 2007 reported a blood transfusion requirement in 22% of the patients and urinary fistula in 17% of patients. Steinbach et al. 1995 reported a patient for each of the following complications: blood transfusion, hemorrhage, pneumonia, renal failure, and retroperitoneal hematoma. Wessendorf et al. 2021 reported a patient for each of the following complications: asymptomatic pneumothorax, hemorrhage, skin burn, urinary bladder tamponade, and urinary tract infection.
Table 5
Surgical complications
| Study | Hidaka et al. 2022 [33] | Hwang et al. 2003 [34] | Iwamoto et al. 2011 [35] | Jayanth et al. 2021 [36] | Jilg et al. 2012 [16] | Lund et al. 1994 [37] | Novick et al. 1992 [42] | Ploussard et al. 2007 [44] | Steinbach et al. 1995 [21] | Wessendorf et al. 2021 [47] |
| Intervention | Surgery | Surgery | RFA | NSS | NSS, RFA or nephrectomy | NSS | NSS | NSS | NSS | NSS |
| N (%) | 58 | 29 | 3 | 17 | 54 | 10 | 9 | 18 | 65 | 9 |
| Asymptomatic pneumothorax | – | – | – | – | – | – | – | – | – | 1 (11.1) |
| Atelectasis | – | – | – | – | – | – | – | – | 1 (2) | – |
| Blood transfusion | – | – | – | – | – | – | – | 4 (22) | – | – |
| Bowel obstruction (small) | – | – | – | – | – | 1 (10) | – | – | – | – |
| Hematuria and urine leak in preparation for RFA | – | – | – | – | – | – | – | – | – | 1 (11.1) |
| Hemorrhage/coagulopathy | 1 (1.7) | – | – | 1 (7) | 28 (49) | 1 (10) | – | – | 1 (2) | – |
| Hemorrhage (post-op) | – | – | – | – | 1 (2) | – | – | – | – | – |
| Hemorrhagic shock (post-op hemorrhage) | – | – | – | – | 3 (6) | – | – | – | – | – |
| Operative mortality | 0 | 0 | 0 | – | 0 | 0 | 0 | 0 | 0 | – |
| Perioperative hemodialysis | – | – | – | 1 (7) | – | – | – | – | – | – |
| Pleural effusion | – | – | – | – | 1 (2) | 1 (10) | – | – | – | – |
| Pneumonia | 0 | 1 (3) | – | – | – | 1 (10) | – | – | 1(2) | – |
| Pseudomembranous colitis | – | 1 (3) | – | – | – | – | – | – | – | – |
| Renal failure (acute) | – | 1 (3) | – | – | 2 (4) | – | – | 0 | 1 (2) | – |
| Retroperitoneal hematoma | – | – | – | – | 5 (11) | – | – | – | 1 (2) | – |
| Skin burn | – | – | – | – | – | – | – | – | – | 1 (11.1) |
| Stroke | 2 (2.7) | – | – | – | – | – | – | – | – | – |
| Ureteropelvic junction obstruction following RFTA | – | – | – | – | 1 (2) | – | – | – | – | – |
| Urinary bladder tamponade and hematuria | – | – | – | – | – | – | – | – | – | 1 (11.1) |
| Urinary fistula | – | – | – | – | – | – | 3 (17) | – | – | |
| Urinary leakage | – | 1 (3) | – | 4 (30) | 1 (2) | – | – | – | – | – |
| Urinary tract infections and hematuria | – | – | – | – | – | – | – | – | – | 1 (11.1) |
| Urinoma | – | – | – | – | 4 (8) | – | – | – | – | – |
| Wound infection | – | – | – | – | 2 (4) | – | – | – | – | – |
Abbreviations NSS, nephron sparing surgery; RFA, radiofrequency ablation; RFTA, radiofrequency thermal ablation.
DISCUSSION
This SLR identified a total of 32 primary studies, of which three were clinical trials assessing pharmacological interventions (belzutifan, sunitinib, pazopanib), one was a real-world study assessing VEGF-TKIs (sunitinib, pazopanib, sorafenib, axitinib). The remaining 28 were real-world studies assessing surgical, radiological, or image guided ablation interventions. All clinical trials with systemic therapies reported data on response rates, one study reporting outcomes by patients and the other two studies reporting outcomes by lesions. In clinical trials, treatment with sunitinib and pazopanib showed an objective response in approximately 30% and 50% of lesions respectively, and the majority were partial responses. The number of lesions assessed in patients treated with sunitinib was lower (n = 18) than the number of lesions assessed in patients treated with pazopanib (n = 59) [17, 18]. Treatment with belzutifan showed an objective response in 64% of patients with RCC lesions. In patients treated with belzutifan, the 24-month PFS rate was nearly 100%, and PFS was not reported for patients treated with pazopanib and sunitinib [17–19]. Real-world studies, with sunitinib and pazopanib treatment were only in part consistent with the clinical trial experience with these agents. The ORR of sunitinib was 40% whereas, pazopanib was 0% [20]. Differences in sample size may account for these differences in response rates. However, in the real-world study only three lesions were assessed in patients treated with pazopanib [20]. In the studies where local treatments were included, where reported, the 5-year repeat surgery rate was around 20% and the 5-year overall survival was around 90% for patients undergoing surgery.
Fig. 1
PRISMA flow diagram of study selection.
Currently, surgery remains a mainstay in the management of patients with non metastatic VHL disease-associated RCC as it reduces the risk of distant metastasis, however, it has acute and long-term complications. These patients require multiple tumor removals throughout their lifetime, which are limited by post-operative scarring and multiple procedures incur in increasing rates of complications. The re-intervention or failure rates observed in this work for the surgical procedures ranged from 9% to 84% indicating that in a majority of the patients multiple procedures are required. Multiple resections and surgeries may also result in progressive renal function deterioration and diminished quality of life, along with increased morbidity and mortality for each surgery. Jilg et al. 2012 found the NSS approach to be practical but highlighted the technical skill requirement and difficulty of repeat NSS, implementing a limit of up to three repeated NSS [16]. Complication rates for the first NSS was 53.6%, 44.4% for the second, and increased to 67% with a rise in the number of severe complications [16]. Steinbach et al. 1995 also reported better cancer-specific survival rates for patients undergoing NSS compared to radical nephrectomies [21]. Also, the cost of VHL-related surgeries will be an important factor to be considered in the management of patients with VHL-disease associated RCC. In a study by Wang et al., from the patients included in the LITESPARK-004 trial, the annual cost of VHL-related surgeries was US $20,499 before the initiation of belzutifan treatment, while after the initiation of belzutifan the annual cost of VHL-related surgeries was US $956. The corresponding annual costs for VHL-related surgical complications before and after belzutifan treatment were US $36,760 and US $1,580, showing a clear decrease in the costs related to surgeries and treatment of surgical complications post belzutifan initiation [22]. This highlights the importance of finding less invasive systemic therapies that reduce the surgical burden in these patients and carry a lower incidence of morbidity and mortality.
As identified in this work, several systemic therapies have been studied in the patients with VHL-associated tumors, however, except belzutifan none of the therapies have been approved in the treatment of VHL disease. In the belzutifan study by Jonasch et al, the authors reported that patients included in the trial had undergone several surgical or ablative procedures before study entry, however, after initiation of belzutifan only three patients needed an intervention directed at a neoplasm associated with VHL disease [19]. Studies evaluating tyrosine kinase inhibitors showed some promise in the treatment of VHL associated tumors, however, these treatments resulted in adverse events that led to treatment discontinuations and dose reductions [23, 24]. In the pazopanib study by Jonasch et al, treatment discontinuation due to adverse events was 23% and only 32% patients received full dose of pazopanib with acceptable safety [17]. In contrast, in the belzutifan study by Jonasch et al, only 2% patients discontinued treatment due to adverse events, and 85% patients received full dose of belzutifan [19].
Overall, the clinical evidence base assessing VHL patients is limited and sparse making it challenging to generalize outcomes and apply them to current clinical practice. The lack of evidence also points to the underreporting of outcomes including adverse events, failure or re-intervention rates and surgical complications (only 10 of the 18 surgical studies reported surgical complications). Furthermore, there is an underreporting of baseline characteristics for study participants, including VHL disease type, as Type 1 and 2B patients are shown to have a higher risk of RCC.
There are several strengths of the SLR approach. First, by systematically reviewing the literature, all available relevant evidence is pulled and condensed into one document, making the review of such evidence a much easier task for clinicians and decision makers. Our SLR did not identify any previously conducted systematic reviews of VHL associated RCC, therefore this evidence can serve as an important resource for future research purposes. Limitations of this SLR include that it was restricted to published data only. There is potentially a degree of publication bias present since some clinical trials fail to be published while others are published in abstract form, but not as full reports and thus will present limited information. Publication of outcomes data was incomplete, absent, or disparate between studies making it challenging to interpret the data. Another important limitation of this SLR is that the number of patients in some studies is very small.
CONCLUSION
In non-metastatic VHL-associated RCC patients, local treatments are still the mainstay. A limited number of clinical trials and real-world studies evaluated VEGF-TKIs for the treatment of VHL-RCC, while responses were observed, long term treatment was limited by toxicities. In the absence of head-to-head comparison of systemic therapies studied in the VHL disease, this review highlights the importance of belzutifan approved by FDA based on a high response rate and manageable toxicity, being the only approved treatment in this indication as a treatment choice. Belzutifan might serve as an alternative treatment option to surgical interventions and other systemic therapies, mainly due to its low and manageable toxicity profile and promising efficacy when compared to other systemic therapies studied in this population. Belzutifan treatment can be helpful in reducing the tumor size therefore leading to fewer indications of local treatments for the patients and can prevent the decrease in the overall quality of life by avoiding the surgical complications in the patients with VHL disease. This review also highlights the unmet need for more treatments that are less invasive to treat these patients.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
Funding for this work was provided by Merck & Co., Inc.
AUTHOR CONTRIBUTIONS
Conception and design of the study: CB, MS
Acquisition, analysis, or interpretation of data: CB, KY, LG, JL
Writing: CB, KY, LG, JL
Supervision: EJ, CB, MS
CONFLICT OF INTEREST
CB, KY, LG, JL has no conflict of interest to disclose. MS is currently an employee at Merck. EJ is an Editorial Board Member of this journal but was not involved in the peer-review process of this paper, nor had access to any information regarding its peer-review. EJ has received research funding from Arrowhead, Merck, and NiKang, as well as honoraria from Aveo, Aravive, Calithera, Eisai, Exelixis, Ipsen, Merck, NiKang, Novartis, Pfizer, and Takeda.
DATA AVAILABILITY
The data for the systematic literature review, search strategies and PICOS criteria is available upon request.
SUPPLEMENTARY MATERIAL
[1] The systematic literature review search strategies and PICOS criteria is available in the supplementary materials.
Supplementary material can be found here: https://dx.doi.org/10.3233/KCA-230021.
REFERENCES
[1] | Zhang L , Xu B , Wang Y , Liu C , Lu K , Huang Y et al. Advanced renal cell carcinoma associated with von Hippel-Lindau disease: A case report and review of the literature. Oncology Letters. (2015) ;10: (2):1087–90. |
[2] | Kim E , Zschiedrich S Renal cell carcinoma in von Hippel-Lindau Disease - From tumor genetics to novel therapeutic strategies. Front Pediatric. 2018;6. |
[3] | Alliance VF The VHL Handbook: What You Need to Know About VHL: A Reference Handbook For People With von Hippel-Lindau Disease, Their Families, and Support Personnel. Boston, MA, VHL Family Alliance. 2014. |
[4] | Muglia VF , Prando A Renal cell carcinoma: histological classification and correlation with imaging findings. Radiol Bras. 2015;48. |
[5] | Lopez-Beltran A , Scarpelli M , Montironi R , Kirkali Z . 2004 WHO classification of the renal tumors of the adults. European Urology. 2006;49. |
[6] | Persad RAP JL , Sharma SD , Haq A , Doyle PT Surgical management of the renal manifestations of von Hippel-Lindau disease: a review of a United Kingdom case series. Br J Urol. (1997) ;80: (3):392–6. |
[7] | Gläsker S , Vergauwen E , Koch CA , Kutikov A , Vortmeyer AO Von Hippel-Lindau Disease: Current Challenges and Future Prospects. Onco Targets Ther. (2020) ;13: :5669–90. |
[8] | Findeis-Hosey JJ , McMahon KQ , Findeis SK Hereditary Cancer Syndromes in Children: Von Hippel–Lindau Disease. Journal of Pediatric Genetics. (2016) ;5: (2):116. |
[9] | Ashouri K , Mohseni S , Tourtelot J , Sharma P , Spiess PE Implications of Von Hippel-Lindau Syndrome and Renal Cell Carcinoma. Journal of Kidney Cancer VHL. 2015;2. |
[10] | Lonser RR , Glenn GM , Walther M , Chew EY , Libutti SK , Linehan WM et al. von Hippel-Lindau disease. Lancet. (2003) ;361: (9374):2059–67. |
[11] | Network NCC. NCCN Clinical Practive Guidelines in Oncology (NCCN Guidlines): Kidney Cancer: National Comprehensive Cancer Network; 2020 [Version 1.2021:[ |
[12] | Institute NC. Renal Cell Cancer Treatment (PDQ®)–Health Professional Version: National Institute of Health; 2020 [updated July 22, 2020. |
[13] | Hsieh JJ , Le VH , Oyama T , Ricketts CJ , Ho TH , Cheng EH Chromosome 3p loss–orchestrated VHL, HIF, and epigenetic deregulation in clear cell renal cell carcinoma. Journal of Clinical Oncology. (2018) ;36: (36):3533. |
[14] | FDA U. [Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-belzutifan-cancers-associated-von-hippel-lindau-disease. |
[15] | Page MJ , McKenzie JE , Bossuyt PM , Boutron I , Hoffmann TC , Mulrow CD et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) ;372: :n71. |
[16] | Jilg CAN HP , Glasker S , Schafer O , Leiber C , Ardelt PU , Schwardt M , Schultze-Seemann W . Nephron sparing surgery in von Hippel-Lindau associated renal cell carcinoma; clinicopathological long-term follow-up. Fam Cancer. (2012) ;11: (3):387–94. |
[17] | Jonasch E , McCutcheon IE , Gombos DS , Ahrar K , Perrier ND , Liu D et al. Pazopanib in patients with von Hippel-Lindau disease: a single-arm, single-centre, phase 2 trial. Lancet Oncol. (2018) ;19: (10):1351–9. |
[18] | Jonasch EM IE , Waguespack SG , Wen S , Davis DW , Smith LA , Tannir NM , Gombos DS , Fuller GN , Matin SF . Pilot trial of sunitinib therapy in patients with von Hippel-Lindau disease. Ann Oncol. (2011) ;22: (12):2661–6. |
[19] | Jonasch E , Donskov F , Iliopoulos O , Rathmell WK , Narayan VK , Maughan BL et al. Belzutifan for Renal Cell Carcinoma in von Hippel–Lindau Disease. New England Journal of Medicine. (2021) ;385: (22):2036–46. |
[20] | Ma K , Hong B , Zhou J , Gong Y , Wang J , Liu S et al. The Efficacy and Safety of Tyrosine Kinase Inhibitors for Von Hippel-Lindau Disease: A Retrospective Study of 32 Patients. Front Oncol. (2019) ;9: :1122. |
[21] | Steinbach FN AC , Zincke H , Miller DP , Williams RD , Lund G , Skinner DG , Esrig D , Richie JP , DeKernion JB , Marshall F , Marsh CL Treatment of renal cell carcinoma in von Hippel-Lindau disease: A multicenter study. Journal of Urology. (1995) ;153: (6):1812–6. |
[22] | Wang L , Bensimon AG , Sundaram M , Xu R , Lai Y , Liu Y et al. Burden of surgeries and surgical complications in patients with Von Hippel Lindau (VHL) disease before and after treatment with belzutifan. Journal of Clinical Oncology. (2023) ;41: (6_suppl):733. |
[23] | Oudard S , Elaidi R , Brizard M , Le Rest C , Caillet V , Deveaux S et al. Sunitinib for the treatment of benign and malignant neoplasms from von Hippel-Lindau disease: A single-arm, prospective phase II clinical study from the PREDIR grouOncotarget (2016) ;7: (51):85306–17. |
[24] | Pilié P , Hasanov E , Matin SF , Woodson AHH , Marcott VD , Bird S et al. Pilot study of dovitinib in patients with von Hippel-Lindau disease. Oncotarget. (2018) ;9: (34):23390–5. |
[25] | Asthagiri AR , Mehta GU , Zach L , Li X , Butman JA , Camphausen KA et al. Prospective evaluation of radiosurgery for hemangioblastomas in von Hippel-Lindau disease. Neuro Oncol. (2010) ;12: (1):80–6. |
[26] | Capitanio U , Rosiello G , Erdem S , Rowe I , Kara O , Roussel E et al. Clinical, surgical, pathological and follow-up features of kidney cancer patients with Von Hippel-Lindau syndrome: novel insights from a large consortium. World J Urol. (2021) ;39: (8):2969–75. |
[27] | Chan VW , Lenton J , Smith J , Jagdev S , Ralph C , Vasudev N et al. Multimodal image-guided ablation on management of renal cancer in Von-Hippel-Lindau syndrome patients from 2004 to 2021 at a specialist centre: A longitudinal observational study. Eur J Surg Oncol. (2022) ;48: (3):672–9. |
[28] | Chang SD , Meisel JA , Hancock SL , Martin DP , McManus M , Adler JR Jr . Treatment of hemangioblastomas in von Hippel-Lindau disease with linear accelerator-based radiosurgery. Neurosurgery. (1998) ;43: (1):28–34; discussion -5. |
[29] | Cvek J , Knybel L , Reguli S , Lipina R , Hanzlikova P , Silhán P et al. Stereotactic radiotherapy for spinal hemangioblastoma –disease control and volume analysis in long-term follow up. Rep Pract Oncol Radiother. (2022) ;27: (1):134–41. |
[30] | Eggener SER JN , Smith ND , Nadler RB , Kontak J , Flanigan RC , Waters WB , Picken M , Campbell SC Renal tumors in young adults. Journal of Urology. (2004) ;171: (1):106–10. |
[31] | Frydenberg MM RS , Zincke H . Conservative renal surgery for renal cell carcinoma in von Hippel-Lindau’s disease. Journal of Urology. (1993) ;149: (3):461–4. |
[32] | Goldfarb DA , Neumann HP , Penn I , Novick AC . Results of renal transplantation in patients with renal cell carcinoma and von Hippel-Lindau disease. Transplantation. (1997) ;64: (12):1726–9. |
[33] | Hidaka T , Ikawa F , Michihata N , Onishi S , Matsuda S , Ozono I et al. Perioperative Surgical Risks in Patients With Hemangioblastomas: A Retrospective Nationwide Review in Japan. World Neurosurg. (2023) ;170: :e21–e7. |
[34] | Hwang JJU EM , Pavlovich CP , Pautler SE , Libutti SK , Linehan WM , Walther MM Surgical management of multi-organ visceral tumors in patients with von Hippel-Lindau disease: a single stage approach. Journal of Urology. (2003) ;169: (3):895–8. |
[35] | Iwamoto YK H , Yamakado K , Soga N , Arima K , Takeda K , Sugimura Y Management of renal tumors in Von Hippel-Lindau disease by percutaneous CT fluoroscopic guided radiofrequency ablation: preliminary results. Fam Cancer. (2011) ;10: (3):529–34. |
[36] | Jayanth ST , Mukherjee P , George AJP , Chandrasingh J , Nirmal TJ , Mukha RP et al. Outcomes of nephron sparing surgery and cortical sparing adrenalectomy in the management of Von Hippel–Lindau syndrome. African Journal of Urology. (2021) ;27: (1):150. |
[37] | Lund GOF B , Curtis MA , Williams RD Conservative surgical therapy of localized renal cell carcinoma in von Hippel-Lindau disease. Cancer. (1994) ;74: (9):2541–5. |
[38] | Kano H , Shuto T , Iwai Y , Sheehan J , Yamamoto M , McBride HL et al. Stereotactic radiosurgery for intracranial hemangioblastomas: a retrospective international outcome study. J Neurosurg. (2015) ;122: (6):1469–78. |
[39] | Kano H , Niranjan A , Mongia S , Kondziolka D , Flickinger JC , Lunsford LD The role of stereotactic radiosurgery for intracranial hemangioblastomas. Neurosurgery. (2008) ;63: (3):443–50; discussion 50-1 |
[40] | Koh ES , Nichol A , Millar BA , Ménard C , Pond G , Laperriere NJ . Role of fractionated external beam radiotherapy in hemangioblastomaof the central nervous system. Int J Radiat Oncol Biol Phys. (2007) ;69: (5):1521–6. |
[41] | Morgan WRZ Progression and survival after renalconserving surgery for renal cell carcinoma: experience in 104 patients and extended followup. Journal of Urology. (1990) ;144: (4):852–7; discussion 7-8. |
[42] | Novick ACSSB Long-term followup after nephron sparing surgery for renal cell carcinoma in von Hippel-Lindau disease. Journal of Urology. (1992) ;147: (6):1488–90. |
[43] | Peng XCJ , Wang J , Peng S , Liu S , Ma K , Zhou J , Hong B , Zhou B , Zhang J , Cai L , Gong K Natural history of renal tumours in von Hippel-Lindau disease: A large retrospective study of Chinese patients. Journal of Medical Genetics. 2019. |
[44] | Ploussard GDS , Ferlicot S , Ples R , Rocher L , Richard S , Benoit G Local recurrence after nephron-sparing surgery in von Hippel-Lindau disease. Urology. (2007) ;70: (3):435–9. |
[45] | Roupret M , Hopirtean V , Mejean A , Thiounn N , Dufour B , Chretien Y et al. Nephron sparing surgery for renal cell carcinoma and von Hippel-Lindau’s disease: a single center experience. J Urol. (2003) ;170: (5):1752–5. |
[46] | Simone CB 2nd, Lonser RR , Ondos J , Oldfield EH , Camphausen K , Simone NL Infratentorial craniospinal irradiation for von Hippel-Lindau: a retrospective study supporting a new treatment for patients with CNS hemangioblastomas. Neuro Oncol. (2011) ;13: (9):1030–6. |
[47] | Wessendorf J , König A , Heers H , Mahnken AH Repeat Percutaneous Radiofrequency Ablation of T1 Renal Cell Carcinomas is Safe in Patients with Von Hippel-Lindau Disease. Cardiovasc Intervent Radiol. (2021) ;44: (12):2022–5. |
[48] | Yousef A , Rutkowski MJ , Yalcin CE , Eren OC , Caliskan I , Tihan T Sporadic and Von-Hippel Lindau disease-associated spinal hemangioblastomas: institutional experience on their similarities and differences. J Neurooncol. (2019) ;143: (3):547–52. |
[49] | Yao MYM , Kishida T , Nakaigawa N , Baba M , Kobayashi K , Miura T , Moriyama M , Nagashima Y , Nakatani Y , Kubota Y , Kondo KI VHL tumorsuppressor gene alterations associated with good prognosis insporadic clear-cell renal carcinoma. Journal of the National CancerInstitute. (2002) ;94: (20):1569–75. |
[50] | Srinivasan R , Iliopoulos O , Rathmell WK , Narayan V , Maughan BL , Oudard S et al. LBA69 Belzutifan, a HIF-2α Inhibitor, for von Hippel-Lindau (VHL) disease-associated neoplasms: 36 months of follow-up of the phase II LITESPARK-004 study. Ann Oncol. (2022) ;33: :S1433–S4. |




