Targeted Literature Review of Outcomes to Initial Systemic Therapy for Advanced/Metastatic Non-Clear Cell Renal Cell Carcinoma in Observational Studies
Abstract
Background:
Non-clear cell renal cell carcinoma (nccRCC) is a diverse group of cancers that occurs in approximately 25% of patients with renal cell carcinoma. In the advanced/metastatic setting, survival in all nccRCC subtypes is considered poor, due to the inherent aggressiveness of these cancers, and a lack of effective systemic treatment options. Clinical trials of immune/targeted agents have predominantly focused on patients with ccRCC. There is no globally accepted standard of care for nccRCC; however, recently clinical trials have been initiated in this population.
Objective:
To perform a targeted literature review of published original observational studies reporting common real-world clinical outcomes (real-world overall response rate [rwORR], real-world progression free survival [rwPFS], real-world overall survival [rwOS]) in previously treatment naïve patients with advanced/metastatic nccRCC.
Methods:
A targeted search of MEDLINE and EMBASE was conducted per PRISMA guidelines to identify observational studies in previously treatment naïve patients with advanced/metastatic nccRCC. Publications with adequate information since 2010 and from select conferences since 2020 were considered.
Results:
27 studies across 29 publications were identified. Sample sizes ranged from 7-1,573 across these studies with differences in nccRCC subtypes included and treatments received. Real-world ORR ranged from 0–37%, median rwPFS from 2–17 months, and median rwOS from 3–30 months, across 19, 17, and 24 studies, respectively. These outcomes also varied with receipt/type of treatment and demographic/clinical subgroups with outcomes tending to be worse in patients with papillary RCC compared to chromophobe RCC.
Conclusions:
Clinical outcomes varied, as patient populations, eligible histologies, treatments and methods were heterogeneous.
INTRODUCTION
An estimated 431,288 new cases of kidney cancer were projected worldwide during 2020, with the highest age-standardized incidence rates (per 100,000) reported in Europe (9.5) and North America (12.2) [1]. The majority of kidney cancers are renal cell carcinoma (RCC) and approximately 75% of patients with RCC have clear cell histology with the remainder being non-clear cell RCC (nccRCC) [2]. Factors that have been established as important prognostic determinants of 5-year survival in RCC are tumor stage, grade, local extent of the tumor, presence of regional nodal metastases, and evidence of metastatic disease at presentation [3]. In the advanced/metastatic setting, survival in all subtypes of nccRCC is considered poor, due to the inherent aggressiveness of these cancers, and a lack of effective systemic treatment options [4].
Historically, clinical trials of immune and targeted agents have predominantly focused on patients with clear cell histology. Therefore, and due to limited data in nccRCC patients, the role of various approved agents in the treatment of nccRCC is poorly defined and there is currently no globally accepted standard of care for these patients, and clinical trial participation remains the preferred treatment [3]. A summary of recent prospective clinical trials was described by Brown and colleagues [5]. The purpose of this targeted literature review was to summarize recent publications of observational studies in patients with advanced nccRCC that remain treatment naïve or receive an initial therapy for advanced disease.
MATERIALS AND METHODS
Search strategy
A targeted literature review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [6]. A meta-analysis of the results was out of scope for this study. A search was conducted on the EMBASE and MEDLINE databases by one co-author (S.C.) to identify relevant studies published between January 1 2010 through March 7 2022. A separate manual search of abstracts from the 2022 American Society of Clinical Oncology Genitourinary Symposium (ASCO GU) was conducted using the conference platform on March 14 2022 and the abstracts from the 2020-2021 annual conferences for the American Society of Clinical Oncology (ASCO), the European Society for Medical Oncology (ESMO), and ASCO GU were searched on the EMBASE and MEDLINE databases on March 15 2022. Recent years of conferences were selected to capture new research that may not have yet been published in manuscript form. Keywords used for all database searches include “Kidney Cancer” OR “Renal Cell Carcinoma” AND ("Non-clear cell” OR “Papillary” OR “Chromophobe” OR “Translocation”) AND ("Advanced” OR “Metastatic"). The search was conducted by one author (S.C.) in three stages. In the first stage, duplicate references were removed. In the second stage, titles and abstracts from all unique references selected were screened. In the final stage, the full-text of the remaining publications were screened. For conference abstracts, only the first two phases were utilized to select relevant publications. The other two co-authors (C.L. and M.S.) reviewed the final list of included references to ensure inclusion of eligible studies based on their knowledge of the field.
Exclusion criteria
Only observational studies were considered for this summary. All editorials, reviews, non-clinically focused studies, interventional studies, and non-English language articles were excluded. To narrow the scope of the review, publications without minimum information describing the source population, histology, and treatment of each cohort of treatment-naïve / 1L treated patients with advanced nccRCC or that included patients that had previously received systemic treatment or ccRCC were excluded. In addition, outcomes for subgroups in selected publications without minimum information describing the subgroup were not extracted. The review was limited to manuscripts published between January 1 2010 through March 7 2022 and conference abstracts from ASCO (2020-2021), ESMO (2020-2021), and ASCO GU (2020–2022).
Data extraction and synthesis
From all eligible publications, including conference posters/presentations associated with the selected conference abstracts, the identifiers, design elements, population characteristics with inclusion and exclusion criteria, number of patients overall and in relevant subgroups, treatment intervention(s), clinical outcome definitions and results for overall nccRCC samples, as well as for subgroups, were extracted to a predefined data extraction table. The clinical outcomes of interest included real-world overall response rate (rwORR), real-world progression free survival (PFS), and real-world overall survival (rwOS). For publications that did not report a rwORR, but did report the number of patients with complete and partial response, as well as the total evaluated, a rwORR was calculated for that study population/subgroup. Outcomes between studies and subgroups were qualitatively compared and described. To assist with this qualitative comparison, a base definition for the clinical outcomes of interest was put forth in the protocol and publications that used different definitions were noted. For this targeted literature review, rwORR was defined as the percentage of participants who achieved either a complete response (CR) or partial response (PR). The time from the date of first dose to a) the first of documented disease progression or death due to any cause, and b) death due to any cause, were used for rwPFS and rwOS, respectively. Outcomes between studies were not combined and a meta-analysis was not conducted. All studies were organized by whether or not treatment was administered, treatment class (e.g., mTOR inhibition, tyrosine kinase inhibition, immune checkpoint inhibition, treatment combinations), specific treatment/treatment combinations and nccRCC subtype(s).
RESULTS
A total of 716 abstracts were identified by searching the EMBASE/MEDLINE databases (journals and 2020-2021 ASCO/ASCO GU/ESMO) and reviewing the complete listing of the ASCO GU 2022 RCC abstracts (Fig. 1). After excluding 3 duplicates, 713 abstracts were screened. This screening resulted in 183 full publications for review and 6 conference abstracts selected for inclusion. Of the full publications reviewed, 23 were selected for data extraction and 160 were excluded for the following primary reason: not in scope due to study population (e.g., included ccRCC) or participants were not treatment naïve (n = 127), contained only clinical outcomes and no information on demographic or clinical characteristics for population of interest (n = 15), interventional study design (n = 12), did not report any clinical outcomes of interest (n = 4), not in English (n = 1), and restricted population to select group of patients with only specific sites of metastases (n = 1). Ultimately, twenty-seven unique studies were identified across the 23 full text and 6 conference abstract publications. These are summarized in Table 1.
Fig. 1
Inclusion and exclusion for targeted review of observational studies with outcomes for advanced non-clear cell renal cell carcinoma.
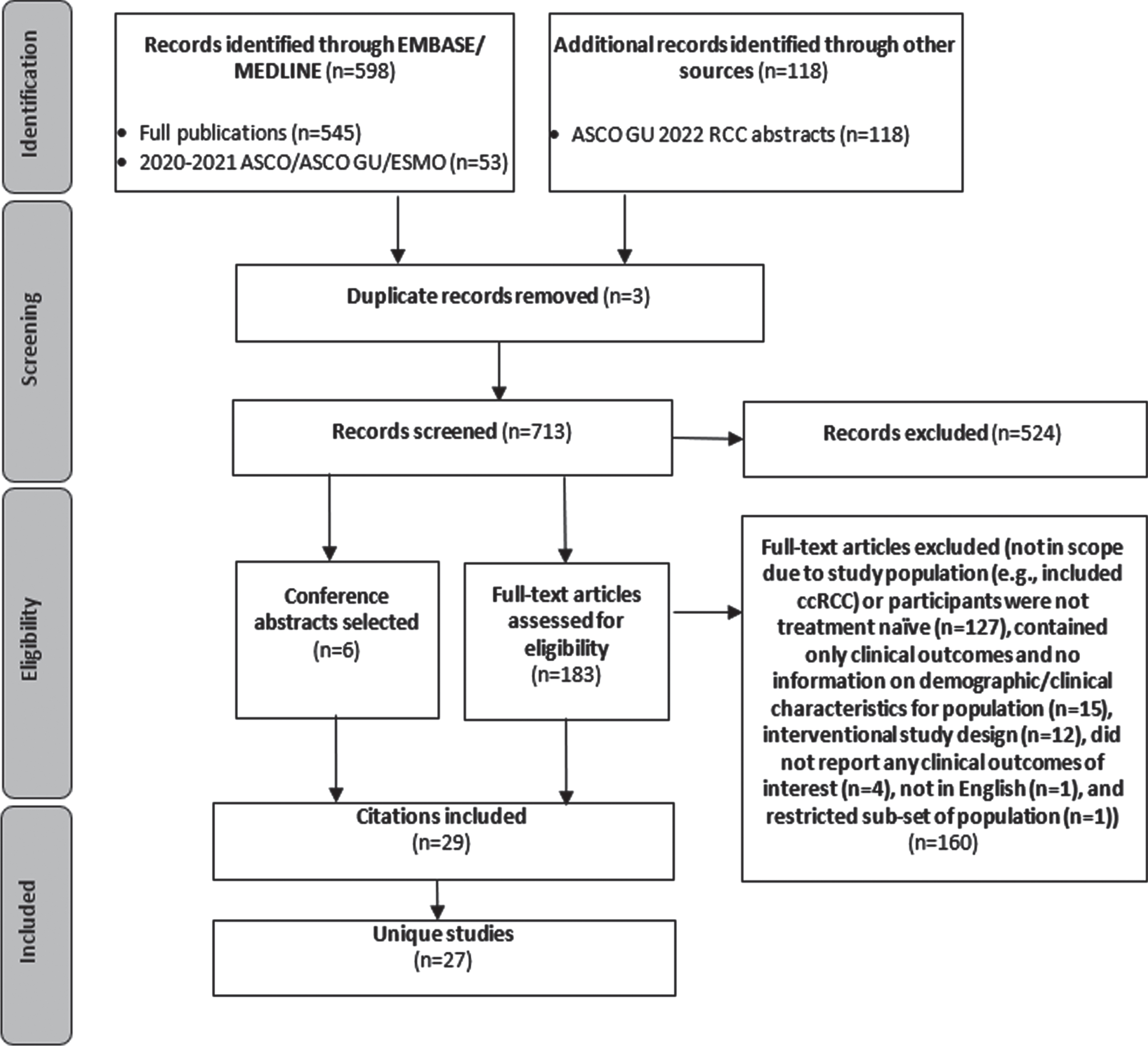
Table 1
Real-world ORR, PFS, and OS in observational studies of nccRCC
| Study Identifier | Population (N) | 1L Treatment (%) | Histological subtype (%) | Real-world clinical outcomes | ||
| rwORR (%) | rwPFS, months (median [95% CI]) | rwOS, months (median [95% CI]) | ||||
| Luzzago S., 2021 [7] | Not treated (n = 350) | 0% treated | 35.1% pRCC, 3.7% chRCC, 4.9% collecting duct, 56.3% sarcomatoid | NR | NR | 3 (2–4) ‡ |
| Only systemic treated (n = 387) | 100% systemically treated (type NR) | 41.9% pRCC, 4.9% chRCC, 5.4% collecting duct, 47.8% sarcomatoid | NR | NR | 7 (6–8) ‡ | |
| Only CN treated (n = 396) | 0% systemically treated | 39.4% pRCC, 8.8% chRCC, 6.3% collecting duct, 45.5% sarcomatoid | NR | NR | 9 (7–12) ‡ | |
| CN and systemic treated (n = 440) | 100% systemically treated (type NR) | 32.7% pRCC, 7.7% chRCC, 8.6% collecting duct, 50.9% sarcomatoid | NR | NR | 13 (11–15) ‡ | |
| Rosiello G., 2021 [8] | All patients (n = 585) | 52% treated (type NR) | 100% pRCC | NR | NR | 13 (NR) |
| Non-Hispanic Caucasian patients (n = 479) | 56% treated (type NR) | 72.2% pRCC, 14.8% chRCC, 12.9% collecting duct | NR | NR | 11 (9–14) | |
| Luzzago S., 2020 [9] | Non-Hispanic African American patients (n = 183) | 48% treated (type NR) | 85.8% pRCC, 6.0% chRCC, 8.2% collecting duct | NR | NR | 16 (12–24) |
| Hispanic patients (n = 77) | 44% treated (type NR) | 61.0% pRCC, 16.9% chRCC, 22.1% collecting duct | NR | NR | 10 (7–14) | |
| Ged Y., 2019 [10] | All patients (n = 109) | 52% no treatment, 2% EVE, 5% EVE + BEV, 3% IFN, 6% PAZ, 1% SOR, 21% SUN, 1% SUN + BEV, 3% SUN + EVE, 2% SUN + Gemcitabine, 5% TEM | 100% chRCC | NR | NR | 25 (12–33) ‡ |
| Sarcomatoid patients (n = 29) | 41% no treatment, 7% EVE + BEV, 7% IFN, 7% PAZ, 3% SOR, 21% SUN, 7% SUN + Gemcitabine, 7% TEM | 100% chRCC | NR | NR | 7.5 (NR) ‡ | |
| Nonsarcomatoid patients (n = 80) | 56% no treatment, 3% EVE, 4% EVE + BEV, 1% IFN, 6% PAZ, 21% SUN, 1% SUN + BEV, 4% SUN + EVE, 4% TEM | 100% chRCC | NR | NR | 38.0 (NR) ‡ | |
| All patients (n = 86) | 23% no treatment, 2% EVE, 3% PAZ, 8% SOR, 52% SUN, 5% TEM, 6% Other | 100% pRCC | NR | NR | 11.2 (NR) ‡ | |
| Stenman M., 2019 [11] | Treated patients (n = 66) | 3% EVE, 5% PAZ 11% SOR 68% SUN, 6% TEM, 8% Other | 100% pRCC | NR | NR | 15.8 (NR) ‡ |
| No systemic treatment (n = 20) | 0% treated | 100% pRCC | NR | NR | 3.4 (NR) ‡ | |
| Martin A., 2019 [12] | All patients (n173) | 8.1% no treatment, 1% EVE, 6% PAZ, 5% SOR, 51% SUN, 10% TEM, 7% Chemotherapy, 6% Cytokine, 4% Local treatment, 2% Other | 55.5% pRCC, 13.9% chRCC, 6.9% uRCC, 23.1% sarcomatoid, 0.6% oncocytoma | 23.2% * [n = 142] | 7.1 (5.1–9.1) [n = 157] | 17.9 (15.0–20.9) ‡ |
| Ito K., 2018 [13] | All patients (n = 33) | 9% no treatment, 12% IFN, 3% IFN + IL2 + Uracil + Tegafur, 18% SOR, 42% SUN, 15% TEM | 100% pRCC | NR | NR | 16.4 (NR) ‡ |
| TKI / mTORi treated patients (n = 25) | 24% SOR, 56% SUN, 20% TEM | 100% pRCC | NR | 5.1 (NR) | 22.4 (NR) ‡ | |
| Colomba E., 2017 [14] | All treated patients (N = 61) | 3.3% BEV combo, 11.5% EVE, 1.6% IFN + BEV, 3.2% PAZ, 8.2% SOR, 65.7% SUN, 6.7% TEM | 100% chRCC | 25.0% [n = 52] | NR | 20.8 (11.6–35.2) |
| Anti-angiogenic treated (n = 50) | 4% BEV combo, 2% IFN + BEV, 4% PAZ, 10% SOR, 80% SUN | 100% chRCC | 28.9% [n = 45] | NR | 22.9 (17.8–49.2) | |
| mTORi treated (n = 11) | 63.6% EVE, 36.4% TEM | 100% chRCC | 0% [n = 7] | NR | 3.2 (2.3-NE) | |
| Bando Y., 2022 [15] | All treated patients (n = 33) | 30.3% IPI + NIV, 3.0% PAZ, 6.1% SOR, 33.3% SUN, 2.7% TEM | 42.4% pRCC, 3.0% chRCC, 12.1% translocation, 12.1% collecting duct, 21.2% uRCC, 9.1% spindle cell | 12.5% * [n = 32] | 4.5 (NR) | 12.6 (NR) |
| IPI+NIV treated (n = 10) | 100% IPI + NIV | 30.0% pRCC, 10.0% chRCC, 20.0% translocation, 30.0% uRCC, 10.0% spindle cell | 30.0% [n = 10] | 3.5 (NR) | 19.6 (NR) | |
| TKI / mTORi treated (n = 23) | 47.8% SUN, 8.7% SOR, 4.3% PAZ, 39.1% TEM | 47.8% pRCC, 8.7% translocation, 17.4% collecting duct, 17.4% uRCC, 8.7% spindle cell | 4.5% [n = 22] | 4.7 (NR) | 10.6 (NR) | |
| Ishihara H., 2021 [16] | All treated patients (n = 38) | 3% AXI, 3% Cytokine, 21% PAZ, 18% SOR, 37% SUN, 18% TEM | 66% pRCC, 3% chRCC, 8% translocation, 5% collecting duct, 5% uRCC, 8% mucinous tubular & spindle cell, 3% spindle cell, 3% tubulocystic | 13% | NR | 15.4 (12.4–23.8) |
| Started treatment 2008–2011 (n = 12) | 50% SOR, 25% SUN, 25% TEM | 83% pRCC, 8% collecting duct, 8% mucinous tubular & spindle cell | NR | NR | 12.1 (7.96–15.4) | |
| Started treatment 2012–2018 (n = 26) | 4% AXI, 4% Cytokine, 31% PAZ, 4% SOR, 42% SUN, 15% TEM | 58% pRCC, 4% chRCC, 12% translocation, 4% collecting duct, 8% uRCC, 8% mucinous tubular & spindle cell, 4% spindle cell, 4% tubulocystic | NR | NR | 25.0 (13.3-NE) | |
| Staehler M., 2020 [17] | Outcome cohort (n = 82) | NR | 100% pRCC | 25% * [n = 55] | 5.4 (4.1–9.2) | 12.0 (8.1–20.0) |
| Poprach A., 2019 [18] | TKI treated patients (n = 93) | 83.9% SUN, 16.1% PAZ | 94% pRCC, 6% chRCC | 15.7% | 6.5 (2.5–10.5) | 22.0 (14.6–29.4) |
| Agarwala V., 2018 [19] | Treated patients (n = 40) | 17.5% EVE, 20% PAZ, 35% SOR, 22.5% SUN, 5% best supportive care | 62.5% pRCC, 12.5% chRCC, 2.5% translocation, 7.5% other, 15% sarcomatoid | 15% | NR | 11.7 (NR) |
| Graham J., 2019 [20] | All treated patients (n = 353) | 8.8% PAZ, 10% SOR, 55% SUN, 16% TEM, 11% other | 100% pRCC | NR | NR | 13.2 (12.0–16.1) |
| Treated patients who also underwent CN (n = 244) | 7.8% PAZ, 13% SOR, 55% SUN, 13% TEM, 12% other | 100% pRCC | 12% | 5.1 (NR) | 16.3 (NR) | |
| Treated patients without a CN (n = 109) | 11% PAZ, 2.8% SOR, 54% SUN, 23% TEM, 9.1% other | 100% pRCC | 5.9% | 3.4 (NR) | 8.6 (NR) | |
| Kim J.K., 2019 [21,22] | Treated patients (n = 156) | 1% AXI, 10% EVE, 10% PAZ, 6% SOR, 34% SUN, 30% TEM, 9% cytokines | 59.6% pRCC, 12.8% chRCC, 5.8% translocation, 11.5% collecting duct, 10.3% uRCC | NR | 5.00 (4.00–6.00) | NR |
| Yan X., 2022 [23] | Treated patients (n = 45) | 13% AXI, 11% PAZ, 33% SOR, 31% SUN, 12% NR | 100% translocation | NR | 7.4 (4.5–8.8) | 17.9 (12.4–24.4) |
| Laramee S., 2022 [24] | TKI treated patients (n = 204) | 15% PAZ, 81% SUN, 4% other | 33.3% pRCC, 13.2% chRCC, 1.0% translocation, 1.0% collecting duct, 36.3% uRCC, 15.2% other | 17% | NR | NR |
| mTORi treated patients (n = 19) | 26% EVE, 74% TEM | 47.4% pRCC, 15.8% chRCC, 26.3% uRCC, 10.5% other | 5% | NR | NR | |
| ICI treated patients (n = 42) | 71% IPI + NIV, 26% PEM + AXI, 3% ICI monotherapy- type NR | 28.6% pRCC, 7.1% chRCC, 40.5% uRCC, 23.8% other | 37% | NR | NR | |
| Graham J., 2021 [25] | ICI based treated (n = 65) | 21.5% ATE + BEV, 20.0% NIV mono, 30.8% NIV + IPI, 20.0% PEM mono, 7.7% other | 40% pRCC, 18.5% chRCC, 6.2% translocation, 9.2% collecting duct, 24.6% uRCC, 1.5% missing | 25.0% | NR | 28.6 (NR) |
| TKI monotherapy treated (n = 924) | 1.3% Cabozantinib, 18.5% PAZ, 1.7% Savolitinib, 7.8% SOR, 68.4% SUN, 2.3% other | 50.0% pRCC, 12.4% chRCC, 4.2% translocation, 2.1% collecting duct, 18.2% uRCC 13.1% missing | 17.8% | NR | 19.2 (NR) | |
| mTORi monotherapy treated (n = 186) | 24.2% EVE, 75.8% TEM | 60.2% pRCC, 14.5% chRCC, 2.7% translocation, 2.7% collecting duct, 13.4% uRCC, 10.8% missing | 5.8% | NR | 12.6 (NR) | |
| Cancel M., 2021 [26] | SUN treated (n = 107) | 100% SUN | 100% pRCC | 10% [n = 94] | 5.5 (5.1–6.0) | 16.0 (10.8–21.1) |
| EVE treated (n = 31) | 100% EVE | 100% pRCC | 17% [n = 29] | 6.2 (3.2–9.2) | 20.3 (14.6–26.0) | |
| Lee I.H., 2020 [27] | TEM treated patients (n = 74) | 100% TEM | 37.8% pRCC, 17.6% chRCC, 4.1% translocation, 1.4% collecting duct, 25.7% uRCC, 9.5% other, 4.1% sarcomatoid | 8.2% | 3 (NR) | 8 (NR) |
| Lee J.B., 2019 [28] | TEM treated patients (n = 44) | 100% TEM | 54% pRCC, 25% chRCC, 2% translocation, 5% collecting duct, 14% other | 11% [n = 35] | 7.6 (5.0–10.2) | 17.6 (0–39.1) |
| Bonadio R.C., 2019 [29] | SUN treated (n = 16) | 100% SUN | 50.0% pRCC, 25.0% chRCC, 12.5% uRCC, 12.5% sarcomatoid | 8.3% [n = 12] | 6.6 (NR) | 30.4 (NR) |
| PAZ treated (n = 37) | 100% PAZ | 32.4% pRCC, 2.7% chRCC, 10.8% translocation, 40.5% uRCC, 13.5% sarcomatoid | 8.7% [n = 23] | 4.9 (NR) | 8.7 (NR) | |
| Buti S., 2017 [30] | PAZ treated patients (n = 37) | 100% PAZ | 51% pRCC, 24% chRCC, 3% translocation, 22% uRCC | 27% * | 15.9 (5.9–25.8) | 17.3 (11.5–23.0) |
| Keizman D., 2016 [31] | SUN treated patients (n = 36) | 100% SUN | 100% chRCC | 28% * | 10 (SD 9; range 1–44) | 26 (SD 10; range 1–75) |
| Shi H.-Z., 2015 [32] | SUN treated patients (n = 37) | 100% SUN | 67.6% pRCC, 5.4% chRCC, 21.6% uRCC, 5.4% spindle cell | 13.5% | 6 (3.6–8.4) | 9 (6.9–11.1) |
| Tachibana H., 2021 [33] | IPI + NIV treated patients (n = 7) | 100% IPI + NIV | 100% pRCC | 14.2% | 2.4 (NR) | NE |
| ORACLE, 2021 [34] | IPI + NIV treated patients (n = 32) | 100% IPI + NIV | NR | 28.1% | 13.6 (NR) | 19.2 (10.4–24.7) |
| ICI + TKI treated patients (n = 19) | NR breakdown of AXI+avelumab, AXI+PEM, BEV+ATE | NR | 31.6% | 16.8 (NR) | 12.4 (8.4–24.7) | |
| EVE + LEN treated patients (n = 5) | 100% EVE+LEN | NR | 0% | 2.1 (NR) | 7.9 (2.5–23.9) | |
‡Outcome definition differed from base definition. *Calculated based on data reported in publication (ORR = CR+PR). Abbreviations: ATE: atezolizumab; AXI: axitinib; BEV: bevacizumab; CB: clinical benefit; CI: confidence interval; CN: cytoreductive nephrectomy; CR: complete response; DC: disease control; DOR: duration of response; EVE: everolimus; ICI: immune checkpoint inhibitor; IFN: interferon-α; IL2: Interleukin-2; IPI: ipilimumab; IQR: interquartile range; ITT: intention to treat; mTORi: mammalian target of rapamycin inhibitors; NE: not evaluable (per publication); NIV: nivolumab; NR: Not reported; PAZ: pazopanib; PEM: pembrolizumab; rwORR: real-world overall response rate; rwOS: real-world overall survival; rwPFS: real-world progression free survival; SOR: sorafenib; SUN: sunitinib; TEM: temsirolimus; TKI: tyrosine kinase inhibitors.
Across the identified observational studies, sample size ranged from 7 to 1,573 patients and all but one [13] utilized a secondary data study design. When reported, the most common method for evaluating tumor response was RECIST (17/28). The clinical outcomes of interest are reported for each publication in Table 1 and the distribution of these clinical outcomes across the identified publications are reported in Table 2. Differences were noted in the start of follow-up for both rwOS and rwPFS with some studies using diagnosis rather than start of treatment as the index date for these clinical outcomes. Results that utilized diagnosis or deviated from the base definition are denoted in Table 1. Overall, rwORR ranged from 0–37% (Fig. 2), median rwPFS from 2–17 months (Fig. 3), and median rwOS from 3–30 months (Fig. 4), across 19, 17, and 24 studies, respectively (Table 2). The majority of studies (14/19) reported a rwORR of 25% or less, most publications (12/17) reported a median rwPFS of 8 months or less, and for many (21/24) the median rwOS was 25 months or less. When limited to studies that used outcome definitions that aligned with the base definition of the clinical outcomes as described in the prior ‘Data extraction and synthesis’ section, the minimum in the range of median rwOS increased from 0 to 8 months and the maximum was unchanged. Different definitions for rwORR were not reported in the selected publications and the range for median rwPFS was not impacted when limiting to publications using a definition similar to the protocol definition. Some studies also reported results for subgroups of study samples; for subgroups with adequate demographic and clinical information within the publication, the reported results are also included in Table 1.
Table 2
Range of rwORR, rwPFS, and rwOS in observational studies of nccRCC overall and by initial therapy class and histological subtype
| Population Characteristic | Range of rwORR (%) (Number of unique studies/ unique cohorts) | Range of median rwPFS, months (Number of unique studies/ unique cohorts) | Range of median rwOS, months (Number of unique studies/ unique cohorts) |
| All publications | 0–37% (19) | 2–17 (17) | 3–30 (24) |
| By initial therapy class | |||
| No drug treatment | NR | NR | 3–9 (2/3) |
| Only ICI drug(s) | 14–37% (4/4) | 2–4 (4/4) | 20–29 (4/4) |
| Only mTORi drug(s) | 0–17% (6/6) | 3–8 (6/6) | 3–20 (6/6) |
| Only VEGF-TKI drug(s) | 8–29% (10/11) | 5–16 (10/11) | 9–30 (10/11) |
| By nccRCC histological subtype | |||
| Papillary RCC | 6–25% (4/6) | 2–6 (5/7) | 3–22 (5/8) |
| Chromophobe RCC | 0–29% (2/3) | 10 (1/1) | 3–38 (3/5) |
Abbreviations: ICI: immune checkpoint inhibitor; mTORi: mammalian target of rapamycin inhibitors; NR: Not reported; rwORR: real-world overall response rate; rwOS: real-world overall survival; rwPFS: real-world progression free survival; TKI: tyrosine kinase inhibitors.
Fig. 2
rwORR of Observational studies (overall and subgroups) by treatment/sample size.
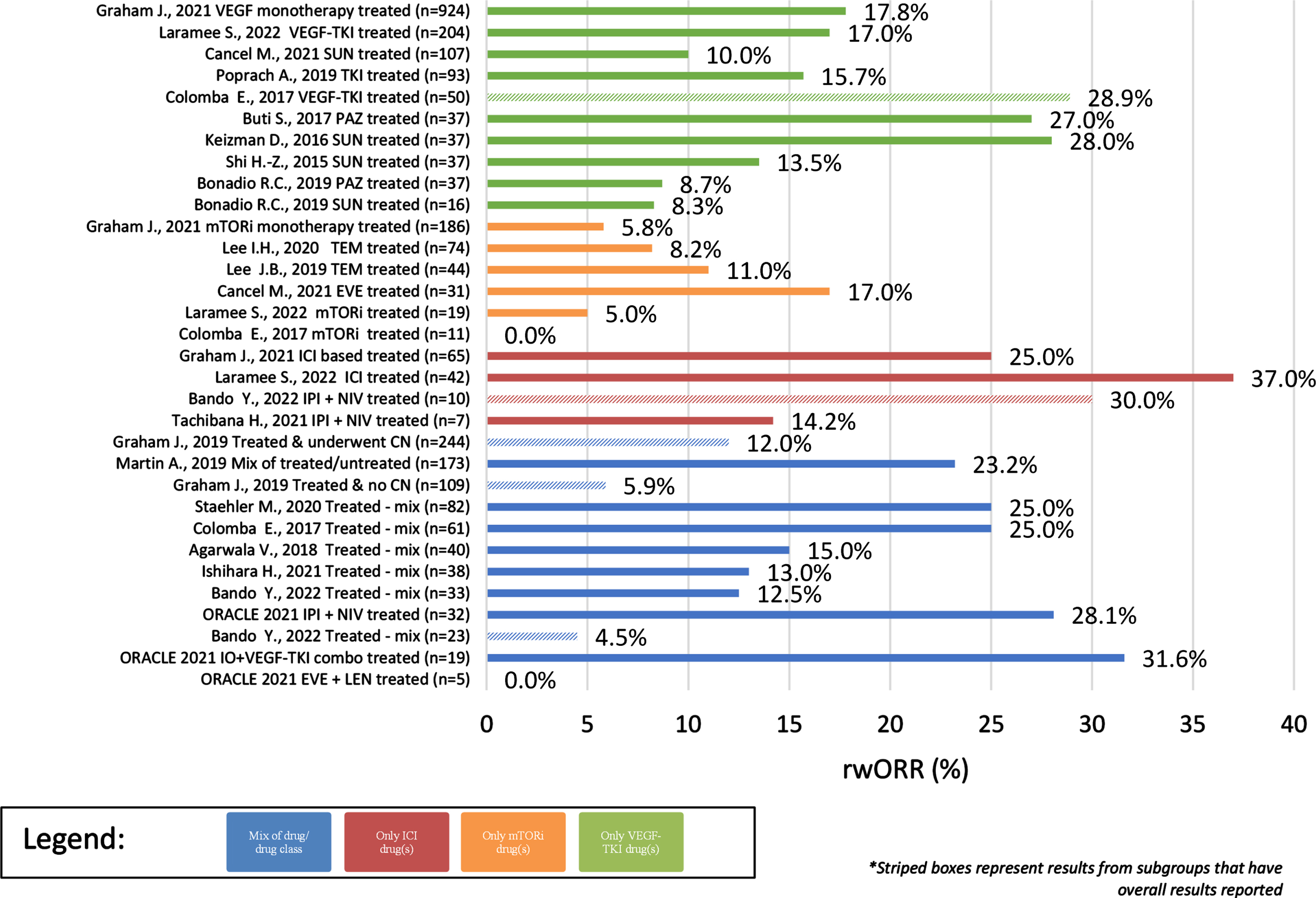
Fig. 3
Median rwPFS of Observational studies (overall and subgroups) by treatment/sample size.
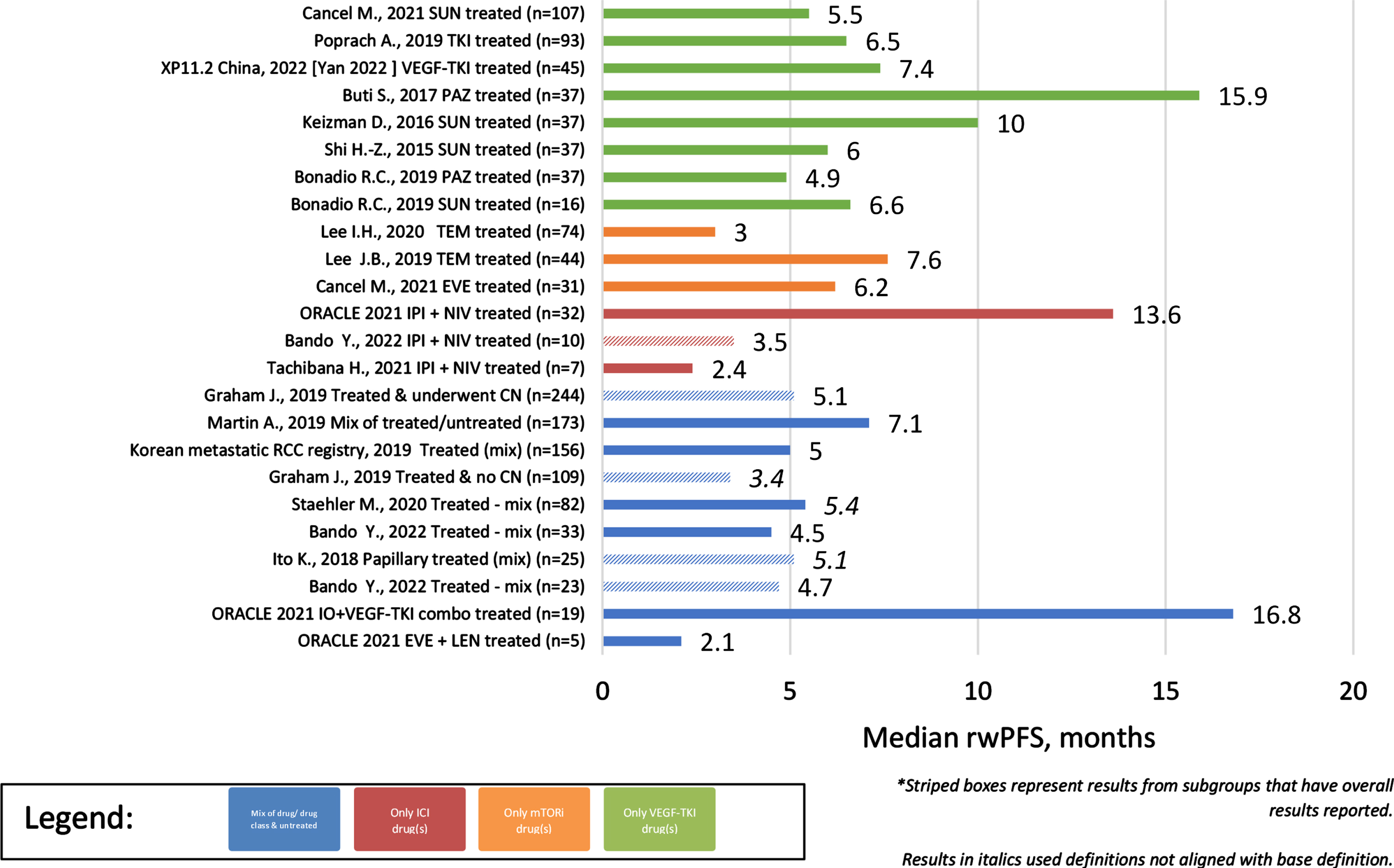
Fig. 4
Median rwOS of Observational studies (overall and subgroups) by treatment/sample size.
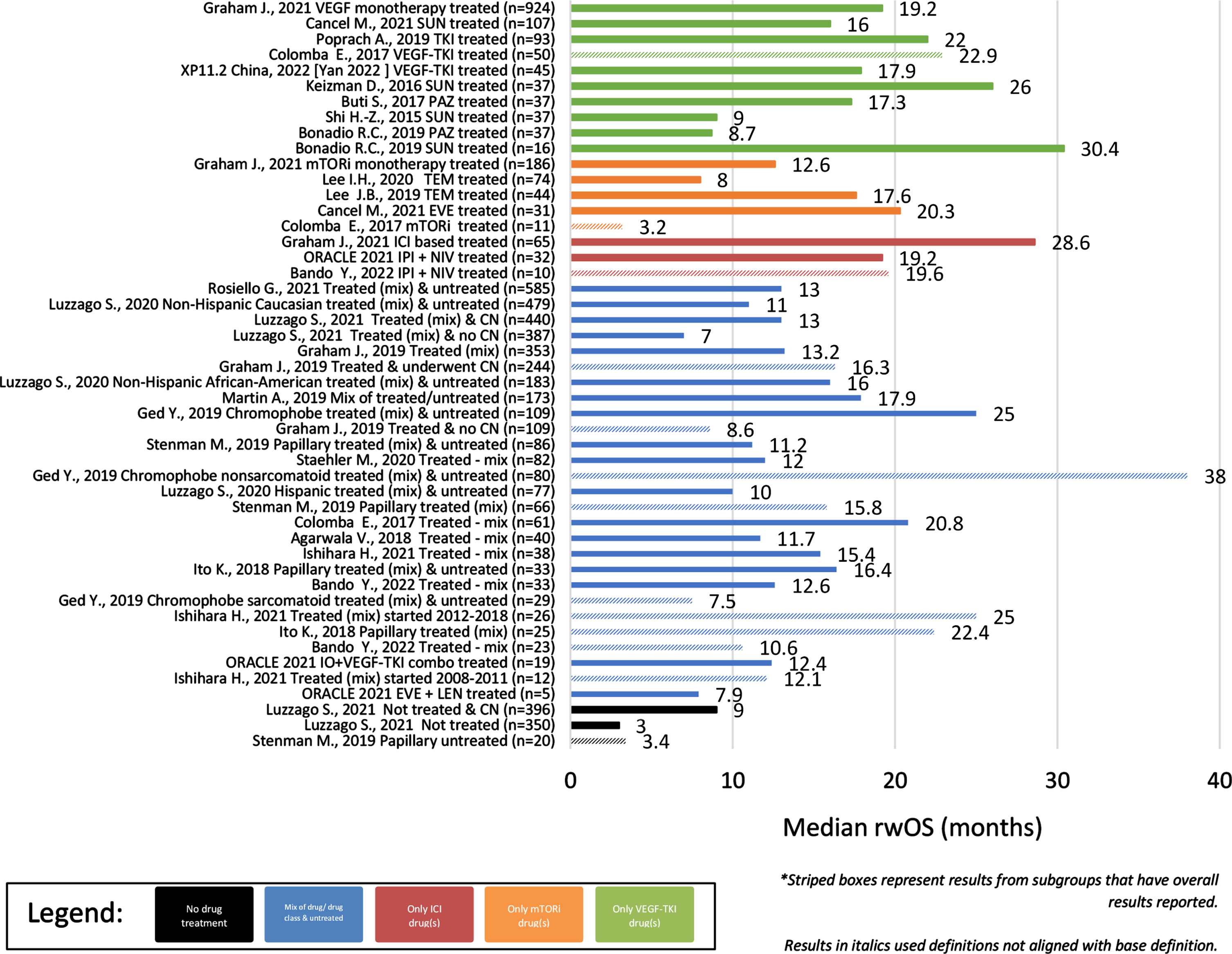
In cohorts/subgroups without any systemic treatment (n = 2 unique studies –3 unique cohorts), only median rwOS was reported, ranging from 3–9 months since diagnosis with advanced/metastatic disease (Table 2). Cohorts and subgroups comprised of those that did not receive systemic therapy had a less favorable range of median rwOS than those that were treated. Comparing study results for cohorts/subgroups treated with a single treatment class, the ranges of clinical outcomes were more favorable for those treated with a tyrosine kinase inhibitor (TKI) or an immune checkpoint inhibitor (ICI)-based therapy than a mammalian target of rapamycin inhibitor (mTORi). For cohorts/subgroups focusing on TKI treatment(s) (n = 10 unique studies –11 unique cohorts), rwORR ranged from 8–29%, median rwPFS from 5–16 months, and median rwOS from 9–30 months. Limiting to TKI monotherapies (n = 8 unique studies –9 unique cohorts) had minimal impact on only the rwORR range, decreasing the maximum to 28% . For cohorts/subgroups focusing on ICI treatment(s) (n = 4 unique studies/cohorts), rwORR ranged from 14–37%, median rwPFS from 2–4 months, and median rwOS from 20–29 months. None of the identified observational study publications included clinical outcomes for ICI monotherapy. Of cohorts/subgroups focusing on mTORi treatment(s) (n = 6 unique studies/cohorts), rwORR ranged from 0–17%, median rwPFS from 3–8 months, and median rwOS from 3–20 months.
Across the observational cohorts/subgroups focusing on a single drug, rwORR ranged from 8–28% in patients treated with sunitinib (n = 4 unique studies/cohorts), 9–27% in patients treated with pazopanib (n = 2 unique studies/cohorts), 8–11% in patients treated with temsirolimus (n = 2 unique studies/cohorts), and was 17% in the single subgroup comprised solely of patients treated with everolimus. In these same studies, median rwPFS ranged from 6–10 months in patients treated with sunitinib, 5–16 months in patients treated with pazopanib, 3–8 months in patients treated with temsirolimus, and was 6 months in the single subgroup of patients treated with everolimus. Median rwOS ranged in these same studies from 9–30 months in patients treated with sunitinib, 9–17 months in patients treated with pazopanib, 8–18 months in patients treated with temsirolimus, and was 20 months in the single subgroup of patients treated with everolimus.
The range of rwORR in patients comprised of those treated with a single drug combination was 14–30% in patients treated with ipilimumab + nivolumab (n = 3 unique studies/cohorts) and was 0% in the single subgroup that focused on patients treated with everolimus + lenvatinib. Median rwPFS ranged from 2–14 months in patients treated with ipilimumab + nivolumab and was 2.1 months in the single subgroup of patients treated with everolimus + lenvatinib. Median rwOS ranged from 19-20 months in patients treated with ipilimumab + nivolumab and was 8 months in the single subgroup of patients treated with everolimus + lenvatinib.
Clinical outcomes also differed per histological subtype (Table 2), including longer survival in patients without sarcomatoid features. In cohorts/subgroups of solely patients with papillary RCC, rwORR ranged from 6–25% (4/6), median rwPFS from 2–6 months (5/7), and median rwOS from 3–22 months (5/8). Of those unique studies/cohorts with papillary RCC that received treatment, median rwOS ranged from 9–22 months (4/7). Of those unique studies/cohorts solely comprised of patients with chromophobe RCC, rwORR ranged from 0–29% (2/3), median rwPFS was 10 months in the single study/cohort reporting it, and median rwOS ranged from 3–38 months (3/5). Overall, outcomes of patients with papillary RCC tended to be worse than patients with chromophobe RCC. Only 1 study reported results stratified by presence of sarcomatoid features, specifically median rwOS, with those without sarcomatoid features overall having longer survival than those with sarcomatoid features (38 vs 7.5 months).
DISCUSSION
While observational studies were identified in this review that focused on clinical outcomes in 1L eligible patients with advanced/metastatic nccRCC, heterogeneity in the patient populations and treatments included, as well as study design used, were found to contribute to an incomplete understanding of the clinical outcomes of the advanced/metastatic nccRCC population as a whole. This heterogeneity in study population was also noted in a recent systematic literature review that focused solely on clinical trials evaluating 1L treatment in metastatic nccRCC [5]. Overall, Brown et al. reported that the range of ORR and PFS was wide, favoring TKI and ICI-based combination regimens, which was also noted in this review. While clinical outcomes varied with patient demographics, histologic subtype and choice of treatment (e.g., ICI vs TKI, monotherapy vs combination therapy), no studies controlled for variability in population characteristics. Additionally, there were inconsistencies in defining the histologic subtypes included within nccRCC (e.g. ccRCC with sarcomatoid features) and in defining the clinical endpoints (e.g. rwPFS and rwOS). As such, interpretation of these differences is limited given the variance in the populations within/across studies and the heterogeneity of study designs. Therefore, comparisons of historical controls with future studies, such as those ongoing trials in patients with nccRCC, must be carefully selected to ensure similarity of patient characteristics and methodologies/outcome definitions.
This literature review focused solely on patients with treatment naïve advanced/metastatic nccRCC that either remained without treatment or received front line treatment in the observational setting. There have been a number of notable studies, including clinical trials, which focused on patients with advanced/metastatic nccRCC treated in this/other settings [35–40]. The KEYNOTE-427 cohort B Phase II study (NCT02853344) evaluated pembrolizumab monotherapy in untreated patients with advanced nccRCC (n = 165) and reported an ORR of 26.7%, median PFS of 4.2 months, and median OS of 28.9 months [37]. An additional, notable Phase II trial, PAPMET (NCT02761057), focused on patients with metastatic papillary RCC that had received up to one prior treatment (n = 147; 7% received ≥1 prior systemic regimen in the advanced/metastatic setting) that were randomized to one of four different treatment arms (sunitinib vs cabozantinib vs crizotinib vs savolitinib) and reported ranges of clinical outcomes across the treatment arms for ORR (0–23%), median PFS (2.8–9.0 months), and median OS (11.7–20.0 months) [38]. The CheckMate 374 Phase IIIb/IV study (NCT02596035) evaluated nivolumab monotherapy in patients with nccRCC that had received up to 3 prior systemic therapies (n = 44; 34.1% received ≥1 prior systemic regimen in the advanced/metastatic setting) and reported an ORR of 13.6%, median PFS of 2.2 months, and median OS of 16.3 months [36]. Early results from KEYNOTE-B61 Phase II study evaluating pembrolizumab plus lenvatinib in a single arm trial show promise of such ongoing research with a reported ORR of 47.6% in 82 patients with 24 or more weeks of follow-up [40]. Despite inclusion of previously treated patients within their nccRCC cohorts, the outcomes reported for each of these studies were within range of those observational studies identified as part of this literature review.
Although the literature review was comprehensive and utilized a pre-specified approach, the findings may be incomplete as systematic literature review was not undertaken nor was a meta-analysis performed. Additionally, publication bias is an inherent limitation of any literature review. Selection bias may also be a limitation as some publications were excluded if the characteristics and outcomes for patients with nccRCC were not reported for this subgroup but instead were reported as part of the overall RCC population.
Overall, this literature review demonstrates that the response to available treatments for nccRCC in the real-world setting is still relatively low across the majority of observational studies. While Brown et al. (5) provided an overview of clinical outcomes for patients with nccRCC, it was limited to the interventional setting. This review expands upon what was previously reported to complete the snapshot of clinical outcomes in patients with advanced/metastatic nccRCC. These collectively emphasize the continued need for research to identify treatments that improve the prognosis of patients with advanced/metastatic nccRCC.
ACKNOWLEDGMENTS
The authors have no acknowledgements.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
SC, MS, and CL attest to substantially contributing to the conception, design or planning of the study; and/or the acquisition or analysis of the data and/or interpretation of the results; and substantially contributed to the drafting of the manuscript and/or critically reviewing or revising it for important intellectual content; and reviewed the final version of the manuscript to be submitted and am in agreement with its content and submission; and had access to all the relevant study data and related analyses, and vouch for the completeness and accuracy of the data presented.
CONFLICT OF INTEREST
SC and MS are employees of Merck & Co., Inc. SC holds personal stock in Merck & Co., Inc. and Organon & Co. MS holds personal stock in Merck & Co., Inc. In the past 36 months, CL has received: research funds to his institute from AstraZeneca, BMS, Calthera, Eisai, Eli Lilly, Exelixis, and Merck, consulting fees from Aveo, BMS, Exelixis, Eisai, Merck, Pfizer, EMD Serono, and Cardinal Health, payment or honoraria from AiCME for a CME event and from Ideology Health, Intellisphere, Medscape, and Research to Practice for educational events. CL is on the Medical Steering Committee for the Kidney Cancer Association.
REFERENCES
[1] | Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. https://gco.iarc.fr/today. Accessed January 27, 2022. |
[2] | Padala SA , Barsouk A , Thandra KC , Saginala K , Mohammed A , Vakiti A , Rawla P , Barsouk A . Epidemiology of renal cell carcinoma. World Journal of Oncology. (2020) ;11: (3):79. |
[3] | National Comprehensive Cancer Network. Kidney Cancer (Version 4.2022; Updated December 21, 2021). http://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf. Accessed January 31, 2022. |
[4] | Motzer RJ , Bacik J , Mariani T , Russo P , Mazumdar M , Reuter V . Treatment outcome and survival associated with metastatic renal cell carcinoma of non–clear-cell histology. Journal of Clinical Oncology. (2002) ;20: (9):2376–2381. |
[5] | Brown JR , Calaway A , Castle E , Garcia J , Barata PC . Systematic review of treatment of metastatic non-clear cell renal cell carcinoma. Kidney Cancer. (2022) ;6: (1):53–68. |
[6] | Moher D , Altman DG , Liberati A , Tetzlaff J . PRISMA statement. Epidemiology. (2011) ;22: (1):128. |
[7] | Luzzago S , Palumbo C , Rosiello G , Knipper S , Pecoraro A , Mistretta FA , Tian Z , Musi G , Montanari E , Soulières D , Shariat SF . Association between systemic therapy and/or cytoreductive nephrectomy and survival in contemporary metastatic non–clear cell renal cell carcinoma patients. European Urology Focus. (2021) ;7: (3):598–607. |
[8] | Rosiello G , Palumbo C , Knipper S , Pecoraro A , Luzzago S , St-Hilaire PA , Tian Z , Capitanio U , Montorsi F , Shariat SF , Saad F . Comparison of survival outcomes in patients with metastatic papillary vs. clear-cell renal cell carcinoma: A propensity-score analysis. World Journal of Urology. (2021) ;39: (2):461–472. |
[9] | Luzzago S , Palumbo C , Rosiello G , Knipper S , Pecoraro A , Nazzani S , Tian Z , Musi G , Montanari E , Shariat SF , Saad F . Racial and ethnic differences in survival in contemporary metastatic renal cell carcinoma patients, according to alternative treatment modalities. Cancer Causes & Control. (2020) ;31: (3):263–272. |
[10] | Ged Y , Chen YB , Knezevic A , Casuscelli J , Redzematovic A , DiNatale RG , Carlo MI , Lee CH , Feldman DR , Patil S , Hakimi AA . Metastatic chromophobe renal cell carcinoma: Presence or absence of sarcomatoid differentiation determines clinical course and treatment outcomes. Clinical Genitourinary Cancer. (2019) ;17: (3):e678–e688. |
[11] | Stenman M , Staehler M , Szabados B , Sandström P , Laurell A , Lindskog M , Harmenberg U . Metastatic papillary renal cell carcinoma in the era of targeted therapy–a retrospective study from three European academic centres. Acta Oncologica. (2019) ;58: (3):306–312. |
[12] | Martín A , Puente J , Pinto A , Gajate P , Gordoa TA , Grande E , Herrero A , Maximiano C , Garrido M , Gallegos I , Villalobos ML . Real-world outcome of 173 metastatic non-clear cell renal cell carcinoma (nccRCC) cases: The experience of the center group for genitourinary tumors. Kidney Cancer. (2019) ;3: (1):41–50. |
[13] | Ito K , Mikami S , Tatsugami K , Masumori N , Shinohara N , Kondo T , Nakanishi S , Nagashima Y , Eto M , Kamba T , Kuroda N . Clinical outcomes in patients with metastatic papillary renal-cell carcinoma: A multi-institutional study in Japan. Clinical Genitourinary Cancer. (2018) ;16: (6):e1201–e1214. |
[14] | Colomba E , Le Teuff G , Eisen T , Stewart GD , Fife K , Larkin J , Biondo A , Pickering L , Srinivasan A , Boyle H , Derosa L . Metastatic chromophobe renal cell carcinoma treated with targeted therapies: A Renal Cross Channel Group study. European Journal of Cancer. (2017) ;80: :55–62. |
[15] | Bando Y , Furukawa J , Okamura Y , Hara T , Terakawa T , Nakano Y , Fujisawa M . Comparative efficacy of combination therapy of ipilimumab plus nivolumab for non-clear cell renal cell carcinoma. Anticancer Research. (2022) ;42: (2):973–979. |
[16] | Ishihara H , Tachibana H , Takagi T , Yoshida K , Kondo T , Tanabe K . Effect of improved systemic therapy on patient survival in metastatic non-clear-cell renal cell carcinoma. International Journal of Urology. (2021) ;28: (5):605–607. |
[17] | Staehler M , Goebell PJ , Müller L , Emde TO , Wetzel N , Kruggel L , Jänicke M , Marschner N , RCC-Registry Group (Tumour Registry of Advanced Renal Cell Carcinoma).Rare patients in routine care: Treatment and outcome in advanced papillary renal cell carcinoma in the prospective German clinical RCC-registry. International Journal of Cancer. (2020) ;146: (5):1307–1315. |
[18] | Poprach A , Rumanová K , Lakomý R , Chloupková R , Staník M , Pokrivcak T , Kiss I , Slaby O , Studentova H , Melichar B , Juracek J . Tyrosine kinase inhibitors in the first-line treatment for metastatic nonclear cell renal carcinoma: A retrospective analysis of a national database. In Urologic Oncology: Seminars and Original Investigations. (2019) ;37: (4):294–-e1).Elsevier. |
[19] | Agarwala V , Ramaswamy A , Joshi A , Patil VM , Noronha V , Menon S , Popat BP , Nilesh S , Prabhash K . Treatment outcomes of metastatic nonclear cell renal cell carcinoma: A single institution retrospective analysis. South Asian Journal of Cancer. (2018) ;7: (04):226–230. |
[20] | Graham J , Wells JC , Donskov F , Lee JL , Fraccon A , Pasini F , Porta C , Bowman IA , Bjarnason GA , Ernst DS , Rha SY . Cytoreductive nephrectomy in metastatic papillary renal cell carcinoma: Results from the international metastatic renal cell carcinoma database consortium. European Urology Oncology. (2019) ;2: (6):643–648. |
[21] | Kim JK , Kim SH , Song MK , Joo J , Seo SI , Kwak C , Jeong CW , Song C , Hwang EC , Seo IY , Lee H . Application of the international metastatic renal cell carcinoma database consortium and Memorial Sloan Kettering Cancer Center risk models in patients with metastatic non-clear cell renal cell carcinoma: A multi-institutional retrospective study using the Korean metastatic renal cell carcinoma registry. Cancer Research and Treatment: Official Journal of Korean Cancer Association. (2019) ;51: (2):758–768. |
[22] | Kim JK , Kim SH , Song MK , Joo J , Seo SI , Kwak C , Jeong CW , Song C , Hwang EC , Seo IY , Lee H . Survival and clinical prognostic factors in metastatic non-clear cell renal cell carcinoma treated with targeted therapy: A multi-institutional, retrospective study using the Korean metastatic renal cell carcinoma registry. Cancer Medicine. (2019) ;8: (7):3401–3410. |
[23] | Yan X , Zhou L , Li S , Wu X , Cui C , Chi Z , Si L , Tang B , Li C , Mao L , Wang X . Systemic therapy in patients with metastatic Xp11. 2 translocation renal cell carcinoma. Clinical Genitourinary Cancer. 2022. |
[24] | Laramee S , Ghosh S , Kollmannsberger CK , Hansen AR , Wood L , Soulieres D , Canil CM , Saleh R , Castonguay V , Bjarnason GA , Basappa NS . Effectiveness of first-line ther- apy in patients with advanced non-clear renal cell carcinoma (nccRCC). |
[25] | Graham J , Wells C , Dudani S , Gan CL , Donskov F , Lee JL , Kollmannsberger CK , Pal SK , Beuselinck B , Hansen AR , North SA . Effectiveness of first-line immune check- point inhibitors (ICI) in advanced non-clear cell renal cell carcinoma (ccRCC). |
[26] | Cancel M , Fromont G , Blonz C , Chevreau C , Rioux-Leclercq N , Laguerre B , Oudard S , Gross-Goupil M , Gravis G , Goldwasser F , Rolland F . Everolimus or sunitinib as first-line treatment of metastatic papillary renal cell carcinoma: A retrospective study of the GETUG group (Groupe d’Etude des Tumeurs Uro-Génitales). European Journal of Cancer. (2021) ;158: :1–1. |
[27] | Lee IH , Kang BW , Kim JG , Bae WK , Ki MS , Park I , Jo JC , Kim JY , Koh SA , Lee KH , Cho YY . Comparison of three risk stratification models for non-clear cell renal cell carcinoma patients treated with temsirolimus as first-line therapy. The Korean Journal of Internal Medicine. (2020) ;35: (1):185. |
[28] | Lee JB , Park HS , Park S , Lee HJ , Kwon KA , Choi YJ , Kim YJ , Nam CM , Cho NH , Kang B , Chung HC . Temsirolimus in Asian metastatic/recurrent non-clear cell renal carcinoma. Cancer Research and Treatment: Official Journal of Korean Cancer Association. (2019) ;51: (4):1578–1588. |
[29] | Bonadioa RC , Velho PI , Marta GN , Nardo M , Souza MC , Muniz DQ , Bezerra RO , Bispo RK , Faraj SF , Bastos DA , Dzik C . Real-world evidence on first-line treatment for metastatic renal cell carcinoma with non-clear cell and sarcomatoid histologies: Are sunitinib and pazopanib interchangeable? ecancermedicalscience. 2019;13. |
[30] | Buti S , Bersanelli M , Maines F , Facchini G , Gelsomino F , Zustovich F , Santoni M , Verri E , De Giorgi U , Masini C , Morelli F . First-line PAzopanib in NOn–clear-cell renal cArcinoMA: The Italian retrospective multicenter PANORAMA study. Clinical Genitourinary Cancer. (2017) ;15: (4):e609–e614. |
[31] | Keizman D , Sarid D , Lee JL , Sella A , Gottfried M , Hammers H , Eisenberger MA , Carducci MA , Sinibaldi V , Neiman V , Rosenbaum E . Outcome of patients with metastatic chromophobe renal cell carcinoma treated with sunitinib. The Oncologist. (2016) ;21: (10):1212–1217. |
[32] | Shi HZ , Tian J , Li CL . Safety and efficacy of sunitinib for advanced non-clear cell renal cell carcinoma. Asia-Pacific Journal of Clinical Oncology. (2015) ;11: (4):328–333. |
[33] | Tachibana H , Kondo T , Ishihara H , Fukuda H , Yoshida K , Takagi T , Izuka J , Kobayashi H , Tanabe K . Modest efficacy of nivolumab plus ipilimumab in patients with papillary renal cell carcinoma. Japanese Journal of Clinical Oncology. (2021) ;51: (4):646–653. |
[34] | Kilari D , Szabo A , Ghatalia P , Rose TL , Weise N , Tucker MD , Nelson AA , Dong H , Hester D , Acharya L , Jain RK . Outcomes with novel combinations in non-clear cell renal cell carcinoma (nccRCC): ORACLE study. |
[35] | Lee CH , Voss MH , Carlo MI , Chen YB , Zucker M , Knezevic A , Lefkowitz RA , Shapnik N , Dadoun C , Reznik E , Shah NJ . Phase II trial of cabozantinib plus nivolumab in patients with non–clear-cell renal cell carcinoma and genomic correlates. Journal of Clinical Oncology. (2022) ;40: (21):2333–2341. |
[36] | Vogelzang NJ , Olsen MR , McFarlane JJ , Arrowsmith E , Bauer TM , Jain RK , Somer B , Lam ET , Kochenderfer MD , Molina A , Doshi G . Safety and efficacy of nivolumab in patients with advanced non–clear cell renal cell carcinoma: Results from the phase IIIb/IV CheckMate 374 study. Clinical Genitourinary Cancer. (2020) ;18: (6):461–468. |
[37] | McDermott DF , Lee JL , Ziobro M , Suarez C , Langiewicz P , Matveev VB , Wiechno P , Gafanov RA , Tomczak P , Pouliot F , Donskov F . Open-label, single-arm, phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced non–clear cell renal cell carcinoma. Journal of Clinical Oncology. (2021) ;39: (9):1029. |
[38] | Pal SK , Tangen C , Thompson IM , Balzer-Haas N , George DJ , Heng DY , Shuch B , Stein M , Tretiakova M , Humphrey P , Adeniran A . A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: A randomised, open-label, phase 2 trial. The Lancet. (2021) ;397: (10275):695–703. |
[39] | Koshkin VS , Barata PC , Zhang T , George DJ , Atkins MB , Kelly WJ , Vogelzang NJ , Pal SK , Hsu J , Appleman LJ , Ornstein MC . Clinical activity of nivolumab in patients with non-clear cell renal cell carcinoma. Journal for Immunotherapy of Cancer. (2018) ;6: (1):1–7. |
[40] | Albiges L , Gurney HP , Atduev V , Suárez C , Duran MC , Pook D , Tomczak P , Barthelemy P , Lee JL , Nalbandian T , Stus V . O Phase II KEYNOTE-B61 study of pembrolizumab (Pembro)+ lenvatinib (Lenva) as first-line treatment for non-clear cell renal cell carcinoma (nccRCC). Annals of Oncology. (2022) ;33: :S1204–S1205. |




