Fibrinogen Levels in Patients with Metastatic Renal Cell Carcinoma Treated with Nivolumab: Results of a Multicenter Prospective Trial
Abstract
Background:
Introduction of immune checkpoint inhibitors in the standard of care for metastatic renal cell carcinoma (mRCC) requires robust but yet simple biomarkers to predict efficacy of immunotherapy.
Objective:
The aim of this study was to evaluate the association between fibrinogen levels and efficacy of second-line therapy with nivolumab in mRCC.
Methods:
This is a prospective multicenter biomarker study. Fibrinogen levels were measured one week prior to second-line nivolumab therapy and six times monthly. A high fibrinogen level was defined as ≥5 g/L. Patients were divided into two cohorts: high (H) and normal (N) fibrinogen levels. The primary endpoint was overall survival (OS).
Results:
The median OS was 31.5 months (95% confidence interval [CI], 27.9 to 35.1) in cohort N vs. 20.9 months (95% CI, 18.1 to 23.7) in cohort H (hazard ratio [HR], 0.39; 98.5% CI, 0.21 to 0.7; P = 0.002). The median progression-free survival was 9.4 months (95% CI, 5.5 to 14.1) in cohort N and 4.0 months (95% CI, 2.9 to 5.1) in cohort H (HR, 0.65; 95% CI, 0.51 to 0.72; P < 0.001). The objective response rate was higher in N cohort (33% vs. 17% ; P = 0.012). No statistically significant changes of fibrinogen concentration during nivolumab therapy were found.
Conclusion:
The study demonstrated an association of hyperfibrinogenemia with worse clinical outcomes of second-line nivolumab monotherapy in patients with mRCC. Further validation of fibrinogen as a predictive biomarker for immunotherapy efficacy in patients with mRCC is warranted.
INTRODUCTION
Currently, there are no validated predictive biomarkers for efficacy of immunotherapy in patients with metastatic renal cell carcinoma (mRCC) [1]. At the same time, checkpoint inhibitors are widely used in the first and subsequent lines of systemic therapy [2]. Nivolumab was the first checkpoint inhibitor to be approved for the treatment of patients with mRCC progressing on prior antiangiogenic therapy [3]. The choice of therapy is increasingly being supported by predictive risk models such as the International Metastatic RCC Database Consortium (IMDC) model, although these models have not been designed to predict response to therapy.
It is well known that hyperfibrinogenemia is associated with the risk of cardiovascular diseases and thrombosis [4]. Several studies have found a dose effect with an increased risk of myocardial infarction and death in patients with the elevated plasma fibrinogen levels [5]. Also, other diseases, such as infections and autoimmune diseases, can lead to an increase in fibrinogen levels [6–8]. During the systemic inflammatory process, high concentrations of cytokines appear, which are a trigger for the development of coagulopathy.
In a number of coagulation-related studies, elevated fibrinogen levels were found in patients with renal cell carcinoma (RCC), lung and liver cancers [9–11]. Hyperfibrinogenemia correlated with more advanced disease [9]. The coagulation system is known to overlap with the immune system. Tissue factor elevation and abnormal coagulability have previously been shown to be negative predictors of overall survival (OS) in mRCC patients treated with cytokines. The impact of hyperfibrinogenemia on the clinical outcomes in patients with mRCC treated with checkpoint inhibitors is unknown. It can be assumed that levels of fibrinogen confer a more inflammatory environment, which may affect efficacy and safety of immunotherapy.
In this prospective multicenter biomarker study, we evaluated the association between fibrinogen levels and the efficacy of second-line therapy with nivolumab in metastatic clear-cell RCC.
METHODS
Patients
Eligible patients were required to have verified metastatic clear-cell renal cell carcinoma resistant to the first-line targeted therapy, measurable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1), ≤3 IMDC risk factors, ECOG PS 0–2, acceptable blood count (absolute granulocyte count ≥1500/mm3, platelets ≥100 000/mm3 and ≤400 000/mm3), adequate renal (measured or calculated creatinine clearance >60 ml/min) and hepatic (bilirubin ≤1.5-fold the upper limit of normal) functions, no anticoagulation therapy, and age 40–65 years. Patients with untreated CNS metastases and uncontrolled medical conditions (such as unstable angina pectoris, recent myocardial infarction, symptomatic congestive heart failure, acquired or inherited bleeding disorders, or thrombosis) were ineligible for this trial. Additionally, patients with a history of cardiovascular disease, coagulopathy i.e. blood clots, autoimmune diseases and COVID-19 within the past 6 months were not included in the study.
The clinical trial protocol and the informed consent form were approved by the institutional review boards of participating clinical sites (study approval number: KCRB112018). The study was done in accordance with the Declaration of Helsinki, and applicable local regulatory requirements and laws. Patients provided written informed consent before study initiation.
Trial design and endpoints
In this prospective multicenter cohort study, fibrinogen levels were measured one week prior to the second-line nivolumab therapy and then monthly (up to 6 months). High fibrinogen level was defined as ≥5 g/L. Patients were divided into two cohorts: high (cohort H) and normal (cohort N) fibrinogen levels. Matching technique was used to achieve balanced distribution of patient characteristics (number of IMDC risk factors and ECOG PS). All patients were treated with nivolumab (intravenously, 240 mg or 480 mg every 2 or 4 weeks) until disease progression or unacceptable toxicity.
The primary endpoint was OS in cohorts H and N. Secondary endpoints included progression-free survival (PFS), objective response rate (ORR), and a fibrinogen levels during therapy. Tumor measurements were assessed by computed tomography (CT) at screening and repeated every 12 weeks. Additional CT scans were obtained as warranted to confirm response (no sooner than 4 weeks and no later than 6 weeks after the first observation of response), or whenever the disease progression was suspected from clinical evaluation and/or symptoms. ORR and disease progression were assessed according to RECIST version 1.1. This study did not investigate the safety of standard treatment with nivolumab in both cohorts.
Statistical analysis
The Kaplan-Meier method was used to estimate the OS and PFS. ORR and corresponding 95% confidence intervals (CIs) were calculated using the Clopper-Pearson method. A post-hoc analysis of the effects of clinically relevant baseline features –sex, ECOG PS, prior nephrectomy, prior targeted agent, and individual IMDC risk factors –on OS was performed with univariable and multivariable models sequentially for each intent-to-treat treatment (ITT) cohort. Each factor was first analyzed individually in the univariable analysis. Baseline factors associated with OS at P < 0.05 in the univariable model were entered into a full Cox proportional hazards multivariable regression model. Descriptive statistics (mean, median, and proportion) were used to summarize baseline patient characteristics and treatment features. All statistical analyses were carried out using IBM SPSS Statistics Base v22.0 (SPSS, Inc., Chicago, IL, USA).
Sample size was based on the conditional estimation. Based on previously reported 3-year OS rate of 39% in the Checkmate 025 study [12], and assumption that the corresponding rate will be 29% in the cohort with a high level of fibrinogen and 59% in the cohort with a normal fibrinogen level, we estimated a sample size of eighty-four patients to be enrolled in the study (α= 0.05; β= 0.02).
RESULTS
Patient characteristics
The study design and patient flow are illustrated in Fig. 1. Eighty-four patients with mRCC scheduled for the second-line therapy with nivolumab were prospectively enrolled between November 2018 and January 2021. The demographic and clinical characteristics of these subjects were balanced between the two cohorts (Table 1). The median age at the time of initiation of therapy with nivolumab was 58 (range, 39–65) and 62 (44–65) years in cohorts H and L, respectively. The cohorts were predominantly composed of males (71%, 60 males vs. 24 females). Seventy-nine (94%) patients had histologic subtype of clear-cell RCC without sarcomatoid features; five (6%) patients were not classifiable. The lungs were the most common sites of metastases (74%) and two patients had brain metastases treated with stereotactic radiosurgery. Fifteen (18%) patients did not have previous nephrectomy. All patients received tyrosine kinase inhibitors (TKIs) as first-line therapy (46 [55%] sunitinib, 25 [30% ] pazopanib, and 13 [15% ] other TKIs). Patient prognosis at nivolumab therapy start was classified using the IMDC risk model into intermediate (n = 69, 82%) and poor (n = 15, 18%). Mean fibrinogen levels were 6.4 g/L and 2.9 g/L in cohorts H and L, respectively.
Fig. 1
Diagram of patient flow.
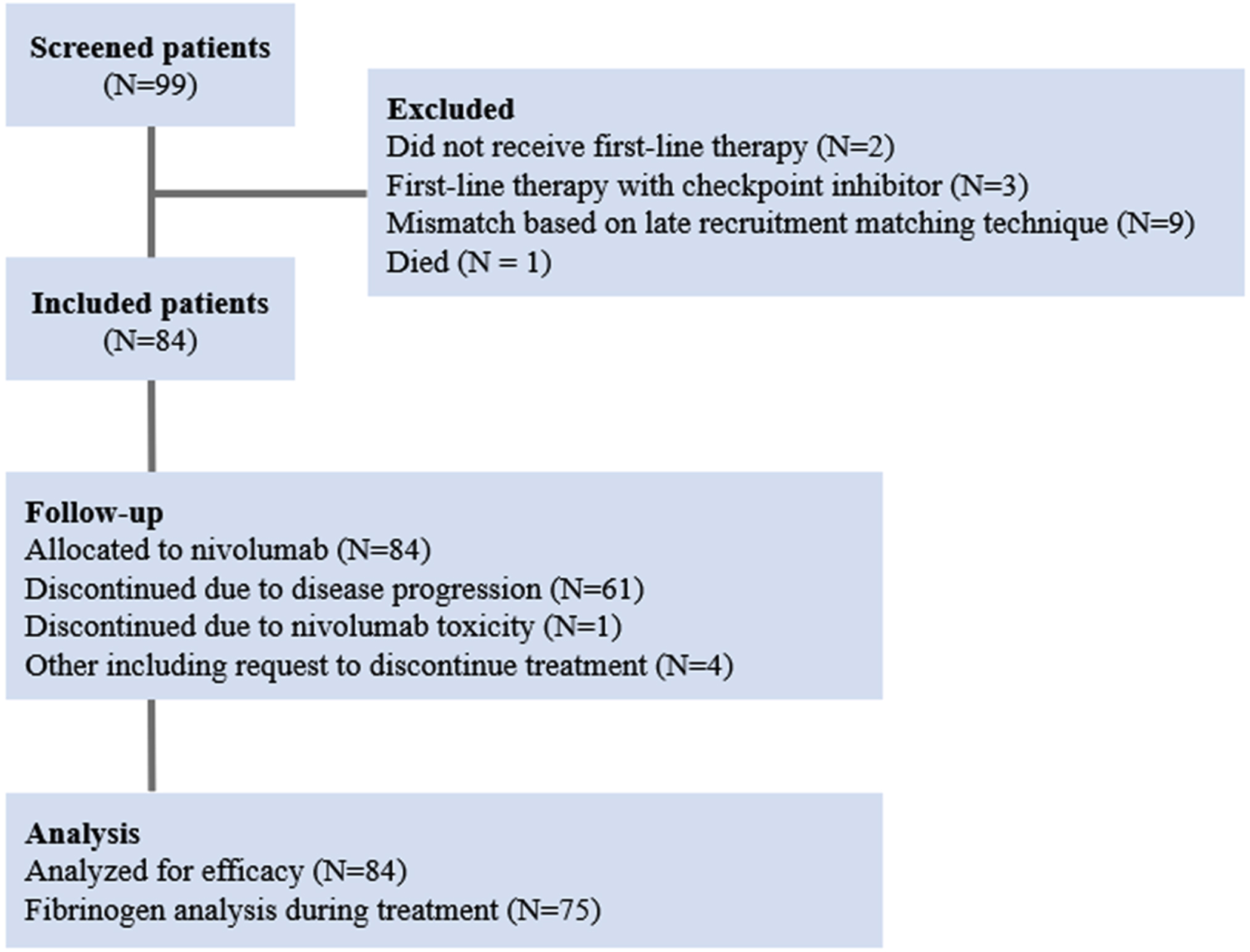
Table 1
Patient characteristics in 2 cohorts (H –high fibrinogen level as ≥5 g/L; N –normal fibrinogen level <5 g/L) of patients with mRCC receiving second-line therapy with nivolumab
| H cohort, | N cohort, | |
| N = 42 | N = 42 | |
| Age (years), mean (range) | 58.4 (39–65) | 62.1 (44–65) |
| IMDC risk factors, N (%) | ||
| 2 | 34 (81) | 35 (83) |
| 3 | 8 (19) | 7 (17) |
| ECOG PS, N (%) | ||
| 0-1 | 30 (71) | 30 (71) |
| 2 | 12 (29) | 12 (29) |
| Site of metastases, N (%) | ||
| Lung | 29 (69) | 33 (79) |
| Liver | 8 (19) | 6 (14) |
| Bone | 10 (23) | 9 (21) |
| Other (lymph nodes, adrenal, soft tissues, brain) | 15 (36) | 18 (43) |
| History of nephrectomy, N (%) | ||
| Yes | 35 (83) | 34 (81) |
| No | 7 (17) | 8 (19) |
| First-line TKIs, N (%) | ||
| Sunitinib | 25 (59.5) | 21 (50) |
| Pazopanib | 10 (23.8) | 15 (36) |
| Others | 7 (16.7) | 6 (14) |
| Duration of the first-line therapy, median, months | 9.9 | 11.3 |
| Fibrinogen (baseline), g/L, mean (range) | 6.4 (5.1–8.4) | 2.9 (1.8–3.7) |
3.2Overall survival
After a median follow-up of 35.7 months, the median OS was 20.9 months (95% CI, 18.1 to 23.7) in cohort H and 31.5 months (95% CI, 27.9 to 35.1) in cohort N (Fig. 2). Death occurred in 29 of the 42 patients (69%) in cohort H and in 23 of the 42 patients (55%) in cohort N. The hazard ratio (HR) for death (from any cause) in cohort N versus cohort H was 0.39 (98.5% CI, 0.21 to 0.7; P = 0.002). Higher OS in cohort N was observed across subgroups, including sex, ECOG PS, prior nephrectomy, prior targeted agent, and individual IMDC risk factors. The heterogeneity of the treatment effect within each subgroup was tested with the use of an interaction test in a Cox proportional-hazards model. None of the interaction terms were significant at the 0.05 level. We also explored the correlation between fibrinogen level and OS. In cohort H, a below-median fibrinogen level (<6.4 g/L) was associated with longer observed OS (median, 23.7 vs. 19.4 months; P = 0.03). Table 2 showed the results of multivariate analysis in ITT population.
Fig. 2
Kaplan–Meier curve for overall survival (OS).
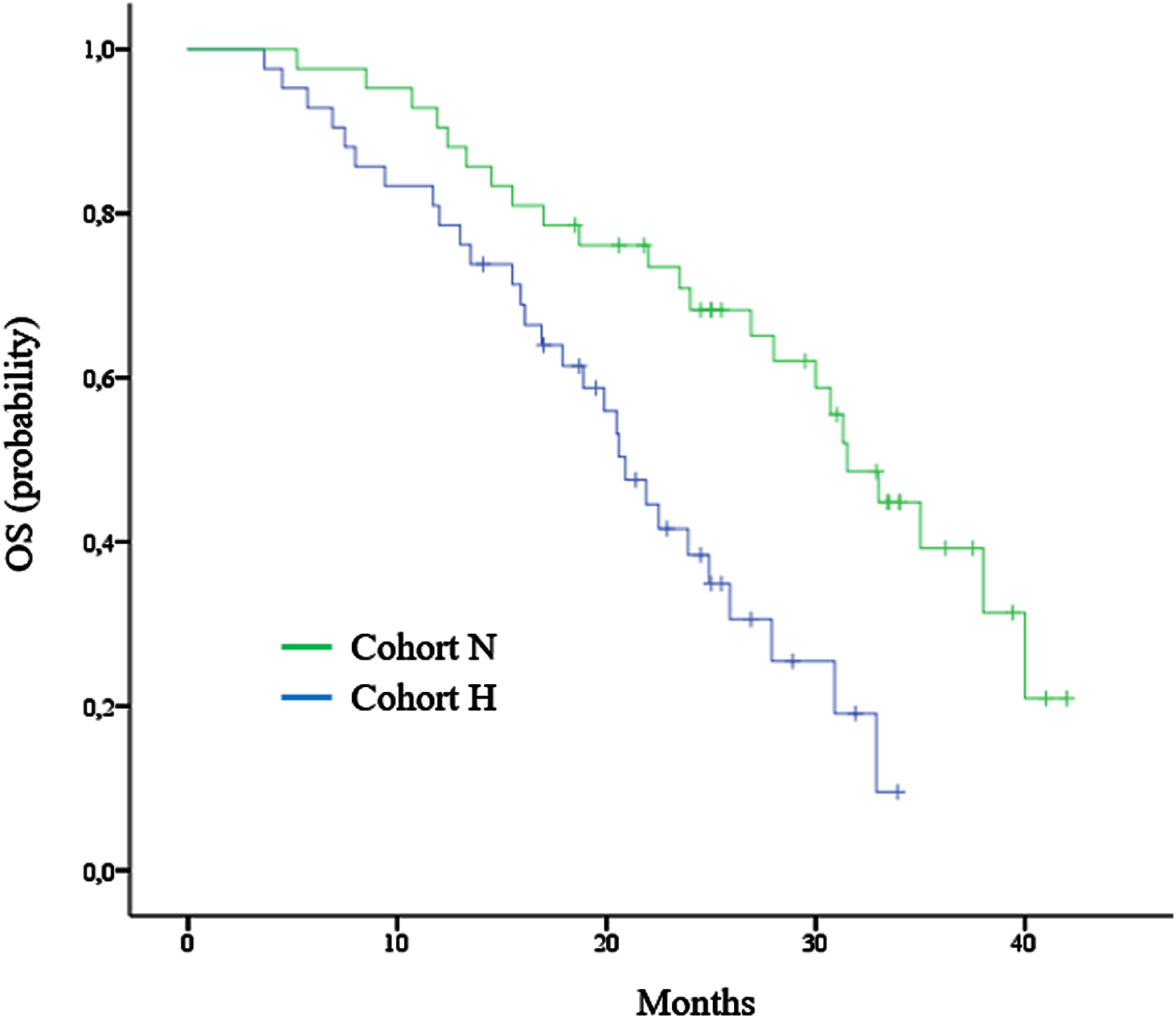
Table 2
Results of multivariate analysis in ITT population (N = 84)
| Parameter | Parameter Estimate±SE | Hazard Ratio | 95% CI | P |
| Fibrinogen level > median | 0.85±0.12 | 2.31 | 1.69 to 3.80 | <0.0001 |
| ECOG PS≥2 | 0.42±0.19 | 1.39 | 1.19 to 1.73 | 0.019 |
| Time from diagnosis to treatment <1 year | 0.46±0.11 | 1.47 | 1.24 to 1.79 | 0.012 |
| Hemoglobin < LLN | 0.62±0.19 | 1.83 | 1.37 to 2.06 | <0.0001 |
| Calcium > ULN | 0.48±0.18 | 1.51 | 1.21 to 1.92 | 0.0097 |
| Neutrophil count > ULN | 0.76±0.20 | 1.95 | 1.55 to 2.66 | <0.0001 |
SE, standard error; ECOG PS, Eastern Cooperative Oncology Group Performance Status; LLN, lower limit of normal; ULN, upper limit of normal; CI, Confidential interval.
Progression-free survival and objective response rate
The median PFS was 9.4 months (95% CI, 5.5 to 14.1) in cohort N and 4.0 months (95% CI, 2.9 to 5.1) in cohort H (HR, 0.65; 95% CI, 0.51 to 0.72; P < 0.001).
The ORR was higher in patients with a normal level of fibrinogen (33% vs. 17% ; P = 0.012). Partial responses were observed in 13 (31%) patients in cohort N and in 7 (17%) patients in cohort H. Complete response was observed in 1 (2%) patient in cohort N; there were no complete responses in cohort H. The median time to response and the median duration of response were 3.2 months (range, 3.0 to 18.6) and 15.4 months (range, 4.5 to 42.0) in cohort N and 4.1 months (range, 3.1 to 19.5) and 11.8 months (range, 3.7 to 33.9) in cohort H, respectively Stable disease was observed in 17 (40.5%) and 28 (67%) patients in cohorts N and H, respectively. Among subjects with objective response and stable disease, 11 patients (35%) with a normal fibrinogen level and 6 (17%) patients with hyperfibrinogenemia had a durable disease control, lasting through the end of the study.
Fibrinogen levels during therapy
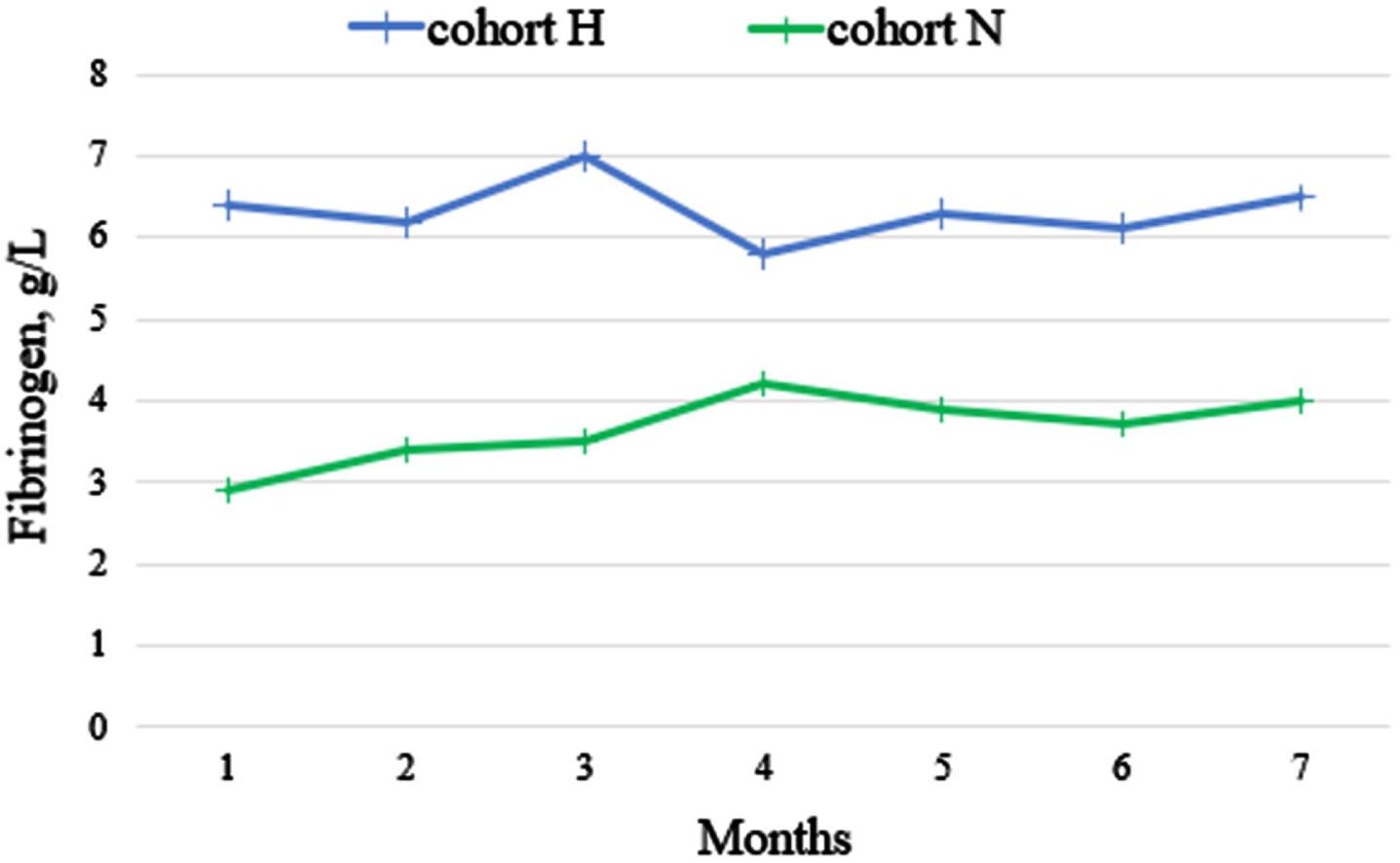
We measured fibrinogen levels in 462 plasma samples in a single-blind central laboratory (INVITRO) using a standardized commercial kit (normal range of fibrinogen level, 2–4 g/L). Twelve (<3%) samples were excluded from analysis due to technical issues. Figure 3 shows the change in a median fibrinogen concentration during nivolumab therapy. Despite a numerical increase in fibrinogen concentration during nivolumab therapy, especially in cohort N, no statistically significant changes were found (all P > 0.05).
Fig. 3
The change in median fibrinogen concentration during nivolumab therapy.
DISCUSSION
In this study, we explored the association of fibrinogen with efficacy of nivolumab in mRCC. Collectively, our results indicate that plasma fibrinogen levels correlated with survival, progression-free interval, and response to treatment with nivolumab. The coagulation assessment is a simple and inexpensive test that can be carried out before treatment and can help to individualize therapy based on the risk factor assessment.
At the screening stage, we found that almost half of patients with clear-cell mRCC, who had previously received targeted therapy, had elevated fibrinogen levels, averaging 6 g/L with a normal value of up to 4 g/L. From a biological point of view, it is well known that malignancy affects the hemostasis system. Cancer patients have a number of blood clotting disorders that increase the likelihood of both thrombosis and hemorrhage [13]. As it was shown earlier, about 40% of mRCC patients have an abnormal coagulation [14]. The precise mechanism inducing elevation of fibrinogen level as well as an increased number of platelets in association with mRCC is uncertain. Potential factors include overproduction of factor×activators and cytokines/growth factors stimulating the tissue factor pathway and megakaryocytes in case of thrombocytosis [15, 16]. Tissue factor is a glycoprotein in charge of initiating extrinsic pathway of coagulation and contributes to a variety of pathologic processes, such as thrombosis, metastasis, tumor growth, and tumor angiogenesis [17]. Immunohistochemical studies showed that kidney cancer cells express tissue factor on their cell surfaces [18]. Moreover, tissue factor has also been detected in endothelial cells in RCC [19]. In preclinical studies tissue factor was assessed as a potential target for CAR-NK cell immunotherapy of triple-negative breast cancer [20]. At the same time, in vitro studies showed that interleukins (IL), such as IL-6 and IL-1 could impact coagulation and plasma levels of fibrinogen [21, 22]. More than a half of patients with mRCC have increased levels of circulating IL-6, which also correlates with increased C-reactive protein levels [23]. In a study by Walther et al. [24], IL-6 was found in 19 of 21 (90%) renal cancer cell lines obtained from 20 patients with mRCC and was also detected in the serum in 33 of 59 (56%) patients with mRCC. Elevation of the cytokines was correlated with paraneoplastic manifestations including coagulation disorders.
More importantly, fibrinogen, which is synthesized by cancer cells, promotes the proliferation of fibroblast growth factor-2 (FGF2) [25]. In turn, activation of the FGF-FGFR pathway stimulates the pathogenesis of RCC and is a possible mechanism of resistance to immunotherapy [26–29]. The studies of fibrinogen-deficient mice suggest that fibrinogen plays an important role in spontaneous metastasis, facilitating the stable adhesion and/or survival of metastatic emboli after tumor cell intravasation [30]. Fibrin degradation products have been reported to have angiogenic, chemoattractant, and anti-inflammatory activities and these proteolytic derivatives of fibrin might also be of biologic relevance to tumor progression.
In our study, the two cohorts were well balanced for known predictive factors, including the IMDC model. Two cohorts differed only by the levels of fibrinogen, which varied on average by 3 times. We excluded patients with high platelet counts, as well as individuals with cardiovascular diseases and those receiving anticoagulants to avoid potential strong confounding factors.
In the cohort of patients with high fibrinogen levels, the median OS was lower than previously described in the CheckMate 025 study (20.9 vs. 25.8 months) [12]. It should be noted that in our study, patients received nivolumab only as a second-line therapy, therefore, this patient population should have performed better than the population of patients enrolled on CheckMate 025 trial. Conversely, in the cohort of patients with normal fibrinogen levels, the median OS exceeded 31 months. Of interest, subjects in cohort H with fibrinogen values closer to normal had better survival than subjects with more pronounced hyperfibrinogenemia. The secondary endpoints –PFS and ORR –were also significantly better in cohort N. The results of our study do not support that fibrinogen can be used as a liquid biomarker to monitor and predict response to nivolumab therapy in patients with mRCC. Fibrinogen levels did not change significantly in patients with response or disease progression and were largely stable over the assessment period of 6 months. Nivolumab did not cause hyperfibrinogenemia in any subjects.
The study has a number of limitations. The number of enrolled subjects was small, although powered to detect the difference in OS between two cohorts of subjects. Fibrinogen levels could vary over time before treatment initiation, thus making subject allocation to 2 cohorts erroneous. Third, we cannot rule out that hyperfibrinogenemia was a side-effect of previous targeted therapies. Due to the fact that our trial was not designed as a biomarker study and, given the above limitations, it seems premature to state that fibrinogen is a strong predictor of the effectiveness of immunotherapy. Therefore, a larger study is required, preferably in patients receiving first-line immunotherapy. It would be important to evaluate the integration of the hyperfibrinogenemia as a risk factor in the IMDC model. Clinical trials assessing additional methods that affect hypercoagulability, such as addition of low molecular weight heparins to checkpoint inhibitors in patients with abnormal coagulation, should be considered.
In conclusion, our study demonstrated a worse survival in patients with mRCC and high levels of fibrinogen treated with second-line nivolumab. Upon next clinical validation, fibrinogen could be used as a simple biomarker of response to immunotherapy in patients with mRCC.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
Conception: IT.
Performance of work: IT, GM, IU, IG, AO, SM, and NO.
Interpretation of data: IT, KZ, MI, and TM.
Writing the article: All authors.
CONFLICT OF INTEREST
IT is an Editorial Board member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
GM, IU, KZ, IG, AO, SM, NO, MV and TM have no conflict of interest to report.
DATA AVAILABILITY
Data sharing is not applicable to this article as no datasets were generated or analyzed during the course of this study.
REFERENCES
[1] | Fotia G , Stellato M , Guadalupi V , et al.Current status of predictive biomarker development in metastatic renal cell carcinoma. Curr Oncol Rep. (2023) ;25: (6):671–677. |
[2] | Tenold M , Ravi P , Kumar M , et al. Current approaches to the treatment of advanced or metastatic renal cell carcinoma. Am Soc Clin Oncol Educ Book. (2020) ;40: :1–10. |
[3] | Tsimafeyeu I . Nivolumab: 5 years sinceFDAapproval of the first checkpoint inhibitor for renal cell carcinoma. Kidney Cancer. 2021:63-71. |
[4] | Machlus KR , Cardenas JC , Church FC , Wolberg AS . Causal relationship between hyperfibrinogenemia, thrombosis, and resistance to thrombolysis in mice. Blood. (2011) ;117: (18):4953–4963. |
[5] | Toss H , Lindahl B , Siegbahn A , Wallentin L . Prognostic influence of increased fibrinogen and C-reactive protein levels in unstable coronary artery disease: FRISC Study Group. Circulation. (1997) ;96: (12):4204–4210. |
[6] | Calvet L , Thouy F , Mascle O , et al. Hypercoagulability in critically ill patients with COVID 19, an observational prospective study. PLoS One. (2022) ;17: (11):e0277544. |
[7] | Zhu S , Yu Y , Qu M , et al. Neutrophil extracellular traps contribute to immunothrombosis formation via the STING pathway in sepsis-associated lung injury. Cell Death Discov. (2023) ;9: (1):315. |
[8] | Barbhaiya M , Zuily S , Naden R , et al. 2023 ACR/EULAR antiphospholipid syndrome classification criteria. Ann Rheum Dis. 2023:ard-2023-224609. |
[9] | Tian Y , Hong M , Jing S , et al. Clinical and prognostic effect of plasma fibrinogen in renal cell carcinoma: A meta-analysis. Biomed Res Int. (2017) ;2017: :9591506. |
[10] | Qian X , Cai J , Qi Q , et al. Preoperative fibrinogen is associated with the clinical survival of primary liver cancer patients and promotes hepatoma metastasis via the PTEN/AKT/mTOR pathway. Heliyon. (2023) ;9: (6):e16696. |
[11] | Tian J , Tian H , Yang M , Yang L , Liu D . Prognostic value of pretreatment serum fibrinogen in young patients with small cell lung cancer: A cross-sectional study. Health Sci Rep. (2023) ;6: (8):1507. |
[12] | Motzer RJ , Escudier B , George S , et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: Updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer. (2020) ;126: (18):4156–4167. |
[13] | Falanga A , Marchetti M , Vignoli A . Coagulation and cancer: Biological and clinical aspects. J Thromb Haemost. (2013) ;11: (2):223–233. |
[14] | Tsimafeyeu IV , Demidov LV , Madzhuga AV , Somonova OV , Yelizarova AL . Hypercoagulability as a prognostic factor for survival in patients with metastatic renal cell carcinoma. J Exp Clin Cancer Res. (2009) ;28: (1):30. |
[15] | Gordon SG , Mielicki WP . Cancer procoagulant: A factor X activator, tumor marker and growth factor from malignant tissue. Blood Coagul Fibrinolysis. (1997) ;8: (2):73–86. |
[16] | Motzer RJ , Escudier B , Bukowski R , et al. Prognostic factors for survival in patients treated with sunitinib for metastatic renal cell carcinoma. Br J Cancer. (2013) ;108: (12):2470–2477. |
[17] | Kasthuri RS , Taubman MB , Mackman N . Role of tissue factor in cancer. JCO. (2009) ;27: :4834–4838. |
[18] | Wojtukiewicz MZ , Zacharski LR , Memoli VA , Kisiel W , Kudryk BJ , Rousseau SM , Stump DC: Fibrinogen-fibrin transformation in situ in renal cell carcinoma. Anticancer Res. (1990) ;10: (3):579–582. |
[19] | Aird WC . Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. (2007) ;100: (2):174–190. |
[20] | Hu Z , Tissue factor as a new target for CAR-NK cell immunotherapy of triple-negative breast cancer. Sci Rep. (2020) ;10: :2815. |
[21] | Wong LY , Leung RY , Ong KL , Cheung BM . Plasma levels of fibrinogen and C-reactive protein are related to interleukin-6 gene -572C>G polymorphism in subjects with and without hypertension. J Hum Hypertens. (2007) ;21: (11):875–882. |
[22] | Luc G , Bard JM , Juhan-Vague I , et al. C-reactive protein, interleukin-6, and fibrinogen as predictors of coronary heart disease: The PRIME Study. Arterioscler Thromb Vasc Biol. (2003) ;23: (7):1255–1261. |
[23] | Gudbrandsdottir G , Aarstad HH , Bostad L , et al. Serum levels of the IL-6 family of cytokines predict prognosis in renal cell carcinoma (RCC). Cancer Immunol Immunother. (2021) ;70: (1):19–30. |
[24] | Walther MM , Johnson B , Culley D , et al. Serum interleukin-6 levels in metastatic renal cell carcinoma before treatment with interleukin-2 correlates with paraneoplastic syndromes but not patient survival. J Urol. (1998) ;159: (3):718–722. |
[25] | Sahni A , Francis C . Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood. (2000) ;96: :3772–3778. |
[26] | Tsimafeyeu I , Volkova M , Olshanskaia A , et al. Expression of receptor tyrosine kinases on peripheral blood mononuclear cells and tumor-infiltrating lymphocytes in patients with renal cell carcinoma and healthy donors. Oncology. (2020) ;98: (4):252–258. |
[27] | Volkova M , Tsimafeyeu I , Olshanskaya A , et al. Immunochemical expression of fibroblast growth factor and its receptors in primary tumor cells of renal cell carcinoma. Am J Clin Exp Urol. (2021) ;9: (1):65–72. |
[28] | Tsimafeyeu I , Statsenko G , Vladimirova L , et al. A phase 1b study of the allosteric extracellular FGFR2 inhibitor alofanib in patients with pretreated advanced gastric cancer. Invest New Drugs. (2023) ;41: (2):324–332. |
[29] | Tsimafeyeu I , Smith J , Yin W , et al. 165P - Neutralizing anti-FGFR1 antibody as a combined partner of anti-PD-1 antibodies in tumor models. Annals of Oncology. (1016) ;33: (suppl_7):S758–S771.10.1016/annonc/annonc1078 |
[30] | Palumbo JS , Potter JM , Kaplan LS , Talmage K , Jackson DG , Degen JL . Spontaneous hematogenous and lymphatic metastasis, but not primary tumor growth or angiogenesis, is diminished in fibrinogen-deficient mice. Cancer Res. (2002) ;62: (23):6966–6972. |




