MET Inhibitors for Papillary Renal Cell Carcinoma
Abstract
BACKGROUND:
Papillary renal cell carcinoma (PRCC) has a relatively poor prognosis in the metastatic setting. In contrast to clear cell kidney cancer, there are limited treatment options specifically tested in PRCC. Alterations in the MET pathway are common in PRCC and may play a pivotal role in promoting tumor growth and the development of resistance to systemic therapy.
OBJECTIVE:
Current data on the efficacy of MET inhibitors over standard of care in PRCC is immature and evolving. The purpose of this systematic review is to assess and summarize the results and limitations of landmark trials of MET inhibitors for PRCC as well as to discuss barriers faced by trials of these drugs.
METHODS:
Manuscripts and abstracts were collected from PubMed, the American Society of Clinical Oncology (ASCO) historical abstracts and European Society for Medical Oncology (ESMO) historical abstracts. Included studies must have been either a clinical trial, systematic review or narrative review and included PRCC patients. Patients must have been treated with a selective or non-selective MET inhibitor. After the final application of criteria, 30 studies were included.
RESULTS/CONCLUSIONS:
Cabozantinib has the best evidence for use showing improved outcomes in PRCC. Other MET inhibitors, including savolitinib, crizotinib, and foretinib have shown possible benefit in patients with MET-positive disease, but the inconsistent definition of MET status and a low patient accrual rate prevented further extrapolation of the individual trial results. Future trials of single agent savolitinib, as well as combination MET inhibitor/ immuno-oncology (IO) therapies, have the potential to change the therapeutic landscape of using MET inhibitors for PRCC.
INTRODUCTION
Papillary renal cell carcinomas (RCCs) account for approximately 75% of non-clear cell RCCs [1]. The World Health Organization (WHO) pathology criteria previously subcategorized PRCC into type 1 and type 2, though the updated version no longer supports this approach to subtyping [2]. PRCC is a genomically heterogenous group of cancers with MET alterations being one of the more common genomic drivers [3]. Papillary renal cell carcinoma (PRCC) has a relatively poor prognosis in the metastatic setting [4]. Unlike the clear cell counterpart, there are limited studied treatment options for PRCC. Per National Comprehensive Cancer Network (NCCN) guidelines, the preferred regimen includes enrollment in a clinical trial when available, cabozantinib (a broad range tyrosine kinase inhibitor), and sunitinib. Once the first line therapy fails, there is no strong recommendation regarding further treatment options based on the available evidence [5]. Hence there exists an unmet need to identify newer treatment options to improve the clinical outcomes of the patients suffering from metastatic PRCC.
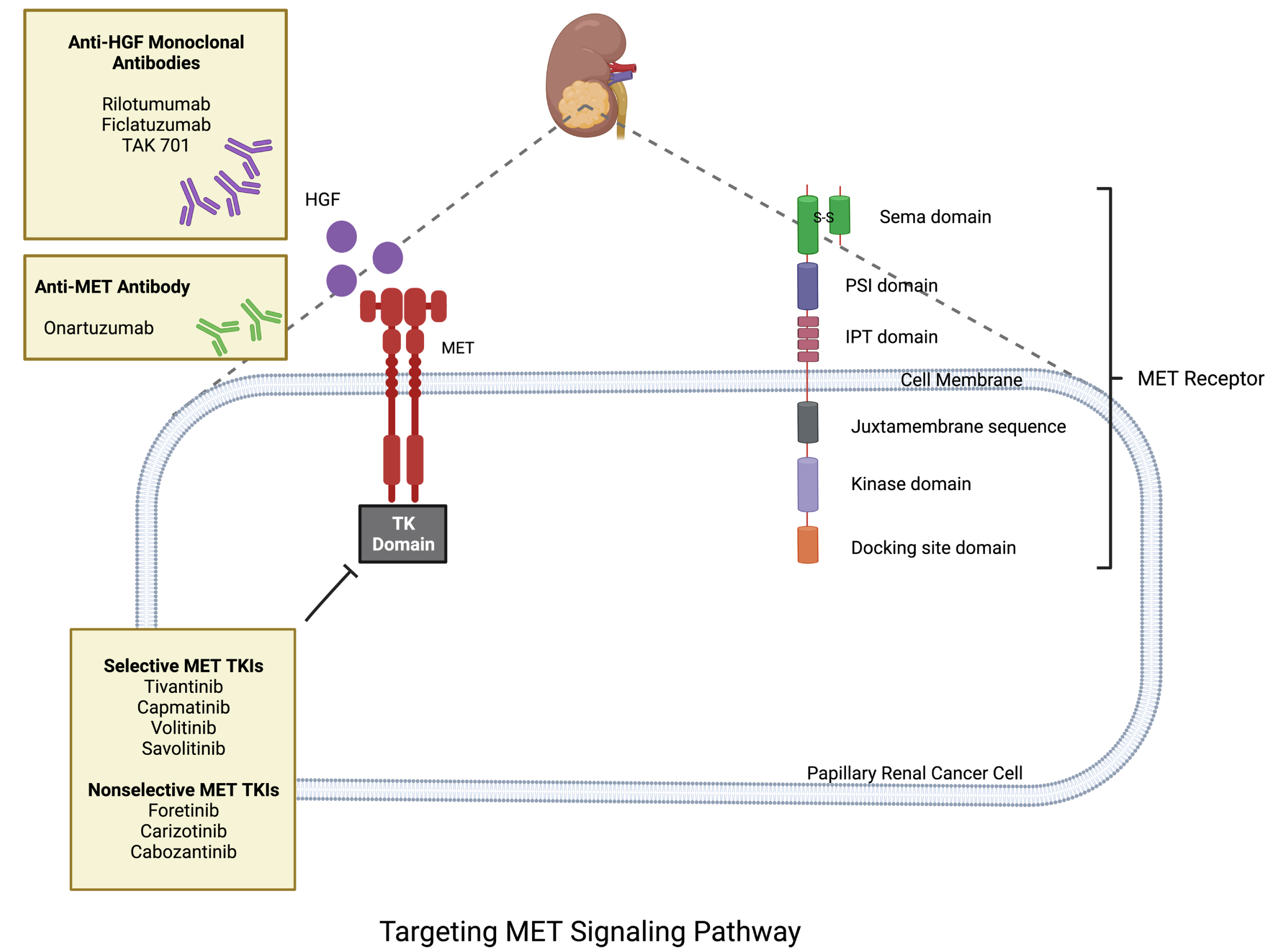
Recently, the MET pathway has been found to be dysregulated in a number of different cancers leading to excessive proliferation of tumor cells and metastasis. MET alterations are relatively common in PRCC, with studies reporting frequencies ranging from approximately 10% to 20% [6]. Studies have reported a higher frequency of MET alterations in type 1 PRCC as compared to type 2 PRCC [6]. It is extremely important to note that the frequency of MET alterations depends on the specific type of alteration being studied (for instance, MET gene amplification, overexpression, mutations or fusions) [6]. These alterations have also been recognized to play a pivotal role in promoting papillary tumor growth and the development of resistance to the first line agents [7]. The MET oncogene encodes a transmembrane receptor tyrosine kinase which binds with its ligand hepatocyte growth factor (HGF) that leads to activation of downstream signals promoting cancer cell growth, migration, and metastasis. MET/HGF pathway deregulation occurs by c-MET overexpression, mutation, amplification and HGF over secretion. Targeting the MET pathway, and downstream signals provides an alternative option in the treatment landscape of PRCC (Fig. 1). Hence, MET inhibitors are a group of drugs that could provide a viable option in patients with PRCC harboring MET alterations [8].
Fig. 1
Biology of MET pathway in PRCC.
Current data on the efficacy of MET inhibitors over standard of care in PRCC is evolving. The purpose of this systematic review is to narratively assess and summarize the results and limitations of landmark trials on MET inhibitors for PRCC as well as to discuss barriers facing trials of these drugs.
METHODS
Search and Extraction
Manuscripts and abstracts were collected from PubMed, the American Society of Clinical Oncology (ASCO) historical abstracts and European Society for Medical Oncology (ESMO) historical abstracts. Search terms were created and agreed on by all the authors individually prior to performing any search. In each database, the following search terms were used: “papillary renal cell carcinoma,” “non-clear cell renal cell carcinoma,” “savolitinib,” “crizotinb,” “foretinib,” “amuvantinib,” “capmatinib,” “cabozantinib” and “tivantinib.” The drugs used in the search criteria do not reflect all MET inhibitors, but they did capture all MET inhibitors with development in PRCC. For information relating to other MET inhibitors that did not include PRCC, see a review by Smith et al. [9]. In PubMed, the results of the search were filtered to include only study designs accepted by criteria which are detailed below. Data were then extracted by PubMed’s CSV export tool. Abstracts from the ASCO and ESMO websites were extracted using a web scraping tool, Octoparse.
Inclusion and Exclusion Criteria
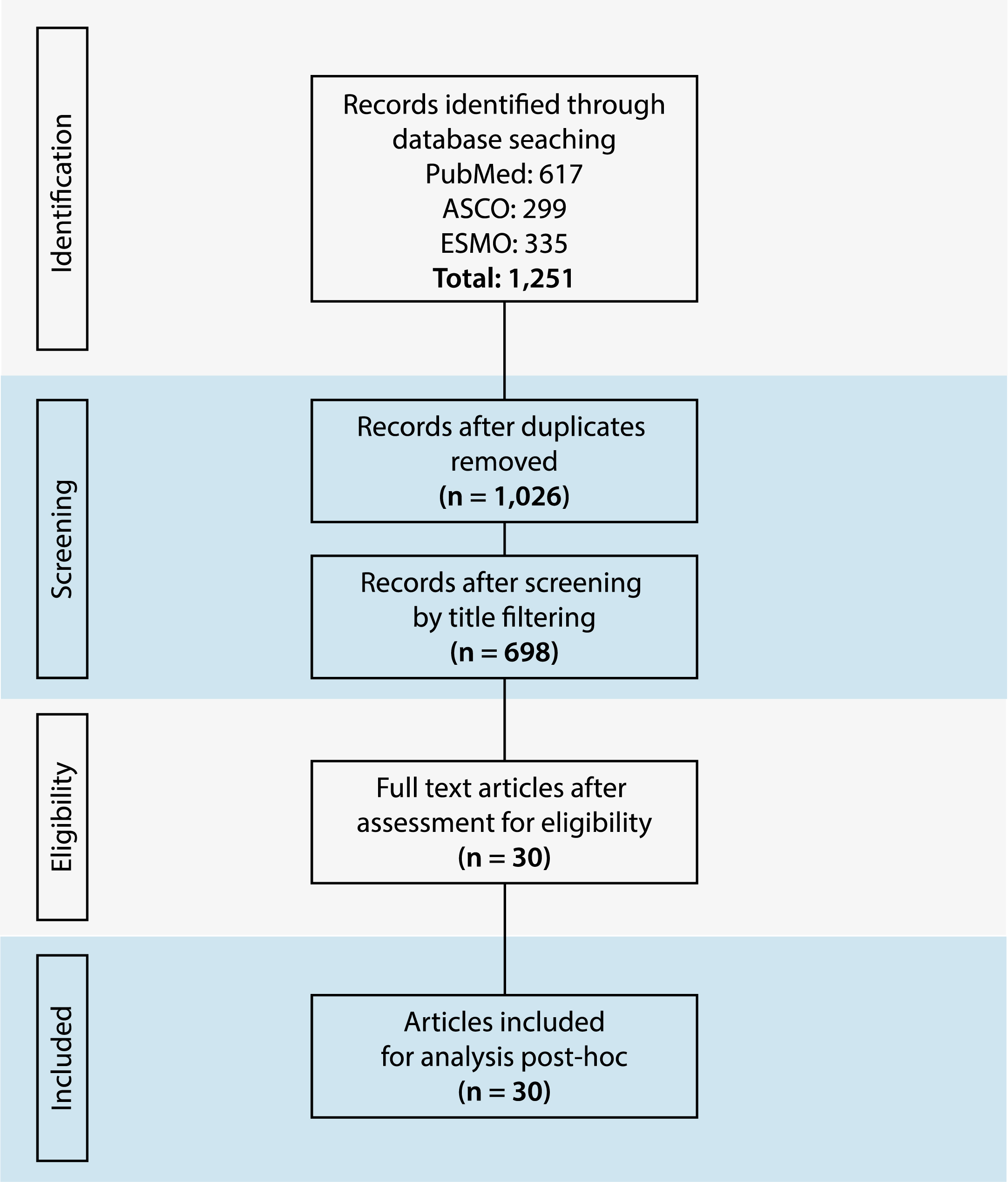
To be included in this systematic review, the papers or abstract were required to meet the following criteria. The study must be either a clinical trial, systematic review or narrative review. They must include information about PRCC specifically. Patients must have been treated with a MET inhibitor, however drugs with multiple targets that included the MET pathway were allowed. Two independent reviewers (JB and KS) assessed the criteria for each study found during the search. When the reviewers disagreed on a study, the third reviewer (BM) reviewed it again. There were no instances where after a second review, the reviewers had a disagreement on the criteria for a particular study. This process can be seen by the PRISMA diagram displayed in Fig. 2.
Fig. 2
The PRISMA flow diagram.
Summary of individual MEK inhibitors based on the trial data (Figure 3)
Foretinib
Foretinib is an inhibitor of MET, VEGF, TIE-2, RON, and AXL. The phase 1 study on the safety of foretinib was first published in 2010, that included patients with a variety of cancers, including four patients with PRCC [10, 11]. Of the three patients which showed partial response from all cancer subtypes, two had PRCC [10]. Following this, the MET111644 trial tested foretinib in 67 patients with histologically confirmed advanced PRCC with no more than one prior systemic therapy [12, 13]. The overall response rate (ORR) was 13.5% in the unselected population. However, the ORR was increased to 50% in patients with MET germline mutations (5 of 10) but not in other MET pathway alterations [12–14]. While this study did not meet its primary endpoint of ORR > 25%, it showed that foretinib is more effective in patients with MET germline mutations than other MET pathway alterations, and unselected patients. Clinical development of foretinib stopped based on these results.
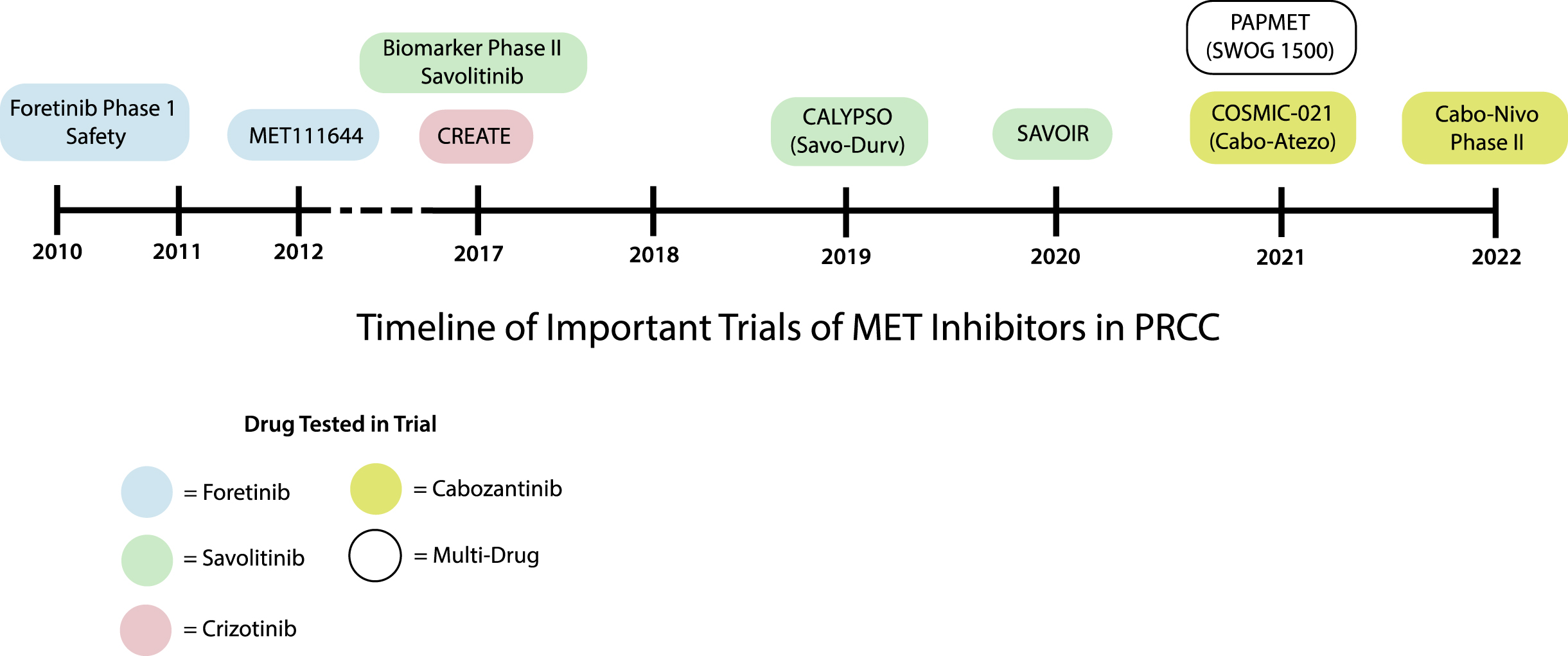
Fig. 3
Timeline of key clinical trials in PRCC.
Crizotinib
Crizotinib is an inhibitor of the MET, ALK and ROS1 pathways [15]. Safety and anti-tumor activity in PRCC was first reported in the CREATE trial. This phase 1 trial successfully accrued 27 patients with PRCC type 1 disease. These patients were further stratified to MET alteration positive versus MET alteration negative disease as defined by sequencing of axons 16–19 of the MET gene in tumor tissue [16, 17]. Only 4 of 27 patients exhibited MET altered disease. The CREATE trial achieved an ORR of 50% (2 out of 4 patients) in patients with MET alterations and 6.3% (1 out of 16 patients) in MET wild-type patients. The final dose escalation and safety data was published in 2020 but did not alter the initial findings [18]. Crizotinib was also tested in the PAPMET (SWOG 1500) trial. In this trial patients were randomized 1:1:1:1 to sunitinib, crizotinib, savolitinib or cabozantinib. Each experimental arm was individually compared to the defacto standard of care arm, sunitinib. This trial was not designed to compare the experimental arms to each other. A pre-planned futility analysis was performed. The crizotinib arm was discontinued early since it failed to demonstrate superior progression free survival compared to sunitinib [19, 20]. It is worth noting that the S1500 trial did not stratify patients by MET alteration status, but rather treated all patients with histologically known PRCC [19, 20]. Authors noted that while a trial stratified by MET alteration status would have been ideal in order to show efficacy of MET inhibitors such as crizotinib, the rarity of the disease posed a significant barrier in accrual of patients. Even with expanded inclusion criteria of including all PRCC patients, authors still reported a lower accrual rate to the study than originally anticipated. This further demonstrates the difficulty in accruing trials for PRCC, especially for biomarker selected treatment. No further studies on crizotinib in mPRCC have been published since the S1500 trial in 2021.
Savolitinib
Savolitinib (previously AZD6094, HMPL-504 and volitinib) is a selective MET inhibitor which showed potential for treatment of PRCC with MET alterations in its first in human studies [21, 22]. The only three patients which achieved partial response (PR) in these trials among various cancers were those with PRCC and MET alterations as defined by tumor tissue next generation sequencing (NGS) demonstrating MET copy number changes (focal amplification or chromosome 7 gains) [21].
The initial trials were followed by biomarker-selected trials of single agent savolitinib in patients with histologically proven advanced or metastatic PRCC [23]. The first was a single arm trial in which MET status was defined by tumor chromosome 7 copy gain, focal MET or HGF gene amplification, or MET kinase domain mutations [23]. Patients with or without MET alteration were enrolled, and a pre-planned comparison between MET altered versus wild-type was performed. The trial accrued 109 patients with 40% (n = 44) of them having MET altered PRCC and 42% (n = 46) having MET wild-type disease [23]. It demonstrated PR in 18% of patients with MET altered disease but no response in MET wild-type disease [23]. Additionally, 61% of patients with MET-altered disease experienced some degree of tumor shrinkage, while only 20% of MET wild-type disease had tumor shrinkage [23]. Positive results from this trial served as the foundation for the SAVOIR trial. The SAVOIR trial was a phase 3 randomized clinical trial that compared single-agent savolitinib versus standard-of-care sunitinib for locally advanced or metastatic PRCC in patients with MET altered disease. Like the preceding trial, MET status was defined by chromosome 7 gain, MET amplification, MET kinase domain variations or HGF amplification in tumor tissue. However, in this study, patients with Von Hippel-Lindau (VHL) and fumarate hydratase (FH) variations were excluded [24]. The results of the SAVOIR trial have limited interpretability due to the trial being closed early due to accrual challenges.
Of the patients with MET driven disease randomized and treated before closure, progression free survival (PFS) and overall survival (OS) were numerically but not statistically greater for savolitinib of versus sunitinib with fewer grade 3 or higher adverse events in the savolitinib arm [24]. PFS was 7 vs. 5.6 months and OS was not reached compared with 13.2 months for the savolitinib and sunitinib groups respectively [24]. The PFS in the sunitinib cohort was longer than anticipated by the initial power calculation. A new power calculation indicated the need for a larger study, and hence further recruitment of patients for SAVOIR was halted, limiting the interpretation of its results. In 2021, further evidence emerged regarding single agent savolitinib from the S1500 trial. This trial compared single agent savolitnib to sunitinib in patients with advanced or metastatic PRCC regardless of MET status [19]. The savolitinib arm was also closed early due to lack of improved PFS with savolitinib compared to sunitinib [19]. The results of S1500 for savolitinib were perhaps unsurprising given that all the previous evidence for savolitinib were in the setting of MET altered disease, albeit by slightly different definitions [19].
Additionally, savolitnib has been studied in combination with durvalumab; a PD-L1 inhibiting immune therapy. The CALYPSO trial was a single arm phase I/II trial for the use of combination savolitinib and durvalumab in patients with metastatic PRCC at any line of treatment. This trial did not select patients based on MET status. Initial results showed an ORR of 27% (11/41) with a median PFS of 3.3 months [25]. Surprisingly, a follow up analysis of the CALYPSO trial by MET status did not show improved benefit of the drug in biomarker positive disease [26]. No further trials on the combination use of savolitinib and durvalumab have been conducted.
Cabozantinib
Cabozantinib is a MET, RET and AXL inhibitor approved for the treatment of medullary thyroid cancer and advanced ccRCC [27]. Of all the MET inhibitors studied in PRCC, cabozantinib has been tested the most extensively. Evidence for Cabozantinib accumulated without stratifying by MET pathway alterations. This enabled more data to be gathered through retrospective studies which enrolled all PRCC patients irrespective of the specific mutation status [28–31]. This review will focus first on single agent cabozantinib followed by the various combinations.
Single agent cabozantinib
Data on single agent cabozantinib for PRCC has come primarily through retrospective studies. In 2017, there were presentations demonstrating clinical benefit for patients with “variant histology RCC.” In Campbell et al., in which 11/19 patients had PRCC, there were no statistically significant differences in PFS or OS detected between PRCC and non-PRCC [29]. Prisciandoro et al. enrolled 18 patients, out of which 12 patients had histologically confirmed PRCC and showed a median PFS of 7.83 months. This PFS duration is comparable to the cabozantinib arm in the phase III METEOR clinical trial in patients with metastatic ccRCC which was also 7.4 months [32]. Results from Prisciandoro et al. were not subdivided by histological type of PRCC, nor were specific prior lines of therapy disclosed, though it is unlikely these patients received previous MET inhibitors given the timing of the trial [32]. Similarly, Gan et. al. demonstrated in a retrospective analysis of 413 patients, of which 72 had nccRCC, that the ORR and time to treatment failure (TTF) was comparable with single agent cabozantinib throughout lines 1–4 of therapy. The ORR remained between 25–32% and the TTF remained between 7 and 8.3 months across all lines of therapy. This suggests that single agent cabozantinib remains effective regardless of the prior therapies due to minimal to no cross-resistance with other treatments currently in use for kidney cancer. In 2022, Graham et al. showed that in 260 patients with mRCC, of which 22 had PRCC, cabozantinib dose reduction was associated with improved OS (15.28 vs. 29.6 months) and TTF (6.44 vs 12.75 months), in mRCC [30]. Results were not provided specifically for patients with PRCC. Results from SWOG 1500, which enrolled 41 patients in the cabozantinib arm, showed that single-agent cabozantinib was the only of three MET inhibitors (savolitinib and crizotinib) to outperform standard-of-care sunitinib in patients with PRCC regardless of MET status who had not received prior MET or VEGF targeting agents [20]. It demonstrated a PFS of 9.0 months vs. 5.6 months, with two patients in the cabozantinib arm achieving complete response [20]. Based on the results of S1500, cabozantinib is the recommended first-line treatment for patients with metastatic PRCC.
Cabozantinib combinations
Cabozantinib has also been studied in combination with other agents that have shown independent benefits in patients with ccRCC and nccRCC. One phase 1 trial tested a combination of a molecule, CB-839 (a glutaminase inhibitor), and cabozantinib in 13 patients with ccRCC [11] and PRCC [2] who had received multiple other lines of therapy, though the number of PRCC patients is not specified in the abstract. The combination regimen demonstrated an ORR of 50%, but results were not subspecified for PRCC patients [33]. Subsequently the CANTATA trial was conducted, which enrolled 444 ccRCC patients. Unfortunately, this study did not achieve its primary endpoint of improved PFS, demonstrating a PFS of 9.2 vs 9.3 months (p = 0.65) comparing combination cabozantinib + CB-839 to cabozantinib, making it unlikely that this combination will be pursued further in PRCC [34]. Cabozantinib has also been tested in combination with atezolizumab, a PD-L1 inhibitor in the COSMIC-021 trial. In this phase 1b trial, 102 patients with both ccRCC and nccRCC were enrolled, of which 15 patients had PRCC [35]. The study was divided into 3 arms, cabozantinib 40 mg + atezolizumab in ccRCC, cabozantinib 60 mg + atezolizumab in ccRCC and cabozantinib 40 mg + atezolizumab for nccRCC. In the non-clear cell RCC group, an ORR of 31% was achieved with a median PFS of 9.5 months [35]. These encouraging results led to the CONTACT-03 trial, which is ongoing currently, evaluating combination of cabozantinib, and atezolizumab for ccRCC and nccRCC in patients who have previously been treated with a PD-1/PD-L1 antagonist. Finally, cabozantinib has also been tested in combination with nivolumab, another PD-L1 inhibitor in non-clear cell RCC. This trial is ongoing at the time of writing with an estimated completion date in August 2023. Interim results have been published at the annual ASCO meeting in 2022. The trial enrolled patients into two cohorts. Cohort 1 included patients with metastatic PRCC (N = 32). Cohort 2 included patients with metastatic chromophobe (N = 6). Each cohort was designed with an independent Simon two-stage stopping rule applied independently for each cohort. Enrollment would proceed as long as the ORR was at least 25% in each cohort. For cohort one the stopping rule was not met allowing for complete enrollment. The stopping rule was met for cohort two so further enrollment was halted. The ORR for cohort 1 was 48% with a median PFS of 12.5 months [36]. Additionally, genetic and genomic testing showed associations with Neurofibromatosis type 2 (NF2) and FH mutations and response to treatment. MET alterations were not investigated [36].
DISCUSSION/FUTURE DIRECTIONS
Cabozantinib is recommended as the first-line treatment for patients with metastatic PRCC based on the current literature. It is the only MET inhibitor that has outperformed sunitinib regardless of MET status (S1500) [19]. There is also evidence suggesting clinical benefit of single-agent cabozantinib in patients with PRCC at later lines of therapy [31]. Other MET inhibitors, including savolitnib, crizotinib, and foretinib showed initial promising results in MET-positive PRCC, but ultimately were not found superior to the standard of care [10, 12–14, 16, 17, 19, 24]. However, these trials faced accrual challenges and inconsistency in the definition of MET status, which makes it difficult to rule out potential benefits. In particular, the SAVOIR trial results remain promising specifically in patients with MET altered disease but further clinical trials are needed to confirm the preliminary findings [24]. This has led to the activation of an international phase III clinical trial, SAMETA [37].
Clinical trials of MET inhibitors in biomarker selected (i.e. MET altered) disease are biologically sound but have faced significant barriers in clinical trial execution
One issue faced by biomarker selected trials is the variable and inconsistent definition of MET alteration. Early work in foretinib showed benefit only in patients with MET germline mutations but not those with somatic alterations, while work with later agents defined MET status only by mutations in tumor tissue [12, 13]. Even among trials which utilized tumor tissue, definition of MET driven disease was variable. Trials of crizotinib leading to and including the CREATE trial defined MET altered status as any mutation to exons 16–19 of the MET gene, excluding other possible mechanisms of MET-driven disease [16, 17, 20]. In contrast, trials of savolitnib initially defined MET altered disease as MET copy number changes with focal amplification or chromosome 7 gains. In a later trial this was expanded to include tumor chromosome 7 copy gain, focal MET or HGF gene amplification, or MET kinase domain mutations. Finally, the SAVOIR trial used the same definition but excluded patients with VHL and FH mutations [23, 24]. The variability in definition of MET altered disease makes comparison of each of these relatively small trials challenging. Clarifying the molecular biology may help in the designing future clinical trials that clearly identify patients with potential benefit from selective MET inhibitors, such as savolitinib, as opposed to multi-targeted drugs such as cabozantinib.
The rarity of MET altered PRCC has also made accrual for trials of MET inhibitors in PRCC a challenge. All of the biomarker selected trials experienced slow accrual rates [12, 13, 16, 17, 24]. PRCC is a rare cancer so enrolling a biomarker selected population of a rare cancer leads to especially slow accrual. For example, the post-trial analysis of the SAVOIR trial suggests that there may be a benefit of savolitnib over sunitinib, but better than expected performance of sunitinib prompted closure and redesign of the trial [24] because longer follow up and more patients would be needed for full enrollment. This issue of slow accrual is particularly important since regulatory bodies such as the EMA and FDA continue to approve treatments regardless of histologic type resulting in physicians frequently treating all kidney cancers with the same treatment strategy as ccRCC. Because these trials take so long to accrue, the results are based on treatment strategies that are frequently no longer used. For instance, in ccRCC immune therapy-based combinations are most frequently used now as first-line treatment but S1500 only tested single-agent TKIs which was the standard of care for ccRCC at the time of study initiation. Trials of cabozantinib have proceeded much more easily in part because all PRCC patients are included. The CALYPSO trial, testing MET-selective savolitinib, avoided the issue of slow accrual by enrolling all PRCC patients but doing a pre-planned secondary PFS analysis stratified by MET status. [25]. Of note, this study showed no effect of MET status on disease response [25].
Some developing targeted therapies were validated in tumors found to have a specific mutation rather than for a specific tumor tissue. While some of the initial phase 1 trials MET inhibitors were performed in studies with multiple primary tumors, the general approach for further validation has been tissue rather than mutation specific. For other primary tumors, such as from the lung and papillary thyroid, MET inhibitors are more effective. The more limited efficacy in PRCC may be related to the increased heterogeneity of the TME or driver mutations. PRCC is well known for being highly heterogenous. Additionally, there is some data indicating that responsiveness to MET inhibitors vary by type of MET alteration. For example, Kou et. al. demonstrated that MET TKIs but not anti-MET antibodies inhibit tumor cell growth with MET amplified tissue [38]. However, both MET TKIs and antibodies were able to inhibit HGF-autocrine tumor growth [38]. Further characterization of the mechanism of MET inhibitors may aid in the design of trials that highlight populations that will most benefit from selective MET inhibitors.
The difficulty in determining MET status reflects a common problem in RCC, which is that typical biomarkers of response to therapy, even those closely related to proposed drug MOA, have been difficult to establish. As a different example, biomarkers of response for immunotherapy, such as PDL1 status, have not proven effective biomarkers in RCC despite efficacy of these drugs [39]. These findings have been especially surprising for PDL-1 inhibitors, where the biomarker is thought to be closely related to the mechanism of action of the drug. However, emerging data suggests that immune therapies may act on tumors via additional mechanisms. For example, a group of studies found that the gut microbiome of responders to immune therapy has the potential to restore drug effectiveness in some patients who previously failed PDL-1 treatment [40, 41]. A prospective trial has now demonstrated the efficacy of a standardized microbiome supplement which improved responsiveness to immune therapy [41]. Together, these studies suggest that mechanisms of immune therapy may be more complex than initially thought and not just restricted to PD-1/PD-L1 status. It may be that these additional mechanisms play a key role in responsiveness to immune therapy in RCC. As our understanding of these mechanisms develops, new potential biomarkers may emerge. Similarly, as the understanding of the complete MOA for MET inhibitors improves, it is possible that accurate biomarkers may emerge.
As these biomarkers develop, it will be important to consider lessons learned from biomarker development in RCC. Other biomarkers of response to therapy as well as general prognostic biomarkers are at various stages of development. Broadly, these markers can be divided into imaging based (MRI, CT, molecular imaging), peripheral tissue based (serum and urine markers, ctDNA) or tumor tissue based (histology, NGS) [42]. These divisions largely represent differences in invasiveness and cost. Similarly, the biomarkers can also be thought of as single factor (IL-6 level, PDL-1 status), composite easily interpretable scores (Heng score, MSKCC score, Motzer score) and composite difficult to interpret scores (NGS biomarkers derived from PCA or machine learning) [42–49]. As these biomarkers develop, it will be important to consider both the ease with which they can be collected as well as their known relationship to drug or tumorigenesis/invasive pathways.
Recent guideline updates have eliminated the clinical distinction between what was previously type 1 and type 2 PRCC. It was previously thought that type 1 PRCC patients may be good candidates for MET inhibition because approximately 85% of type 1 PRCC tumors contained MET alterations. When tumor genetic testing was less available, stratifying clinical trials by histology represented a good way to target MET altered tumors. Practically, only one MET targeted trial stratified by histology classifications with most opting instead to rely on tumor genetic analysis to determine MET status directly. As sequencing becomes increasingly available, the distinction between type 1 and 2 PRCC becomes less important for the progress of MET targeted therapies.
Part of the initial excitement around MET inhibitors was the opportunity to provide targeted therapies for PRCC towards the presumed biologic driver of this disease. However, it is possible that less targeted approaches may work better because MET may not be the sole driver or even the main biologic driver of this disease. It is possible that non-selective agents such as cabozantinib may be more active because multiple key pathways are co-occuring such as VEGFR [37]. For example, in ccRCC, the combination lenvatinib/pembrolizumab showed improved OS, PFS and ORR compared to sunitinib prompting investigators to test this same combination in nccRCC [50]. Lenvatinib is a multitarget TKI, antagonizing the VEGF, FGF, PDGFB KIT and RET pathways, but not the MET pathway [51]. KEYNOTE-B61 is a single arm clinical trial testing pembrolizumab and lenvatinib, in nccRCC [52]. In total 147 patients with nccRCC were enrolled, of which 87 were classified as histologic PRCC. The ORR was 47.6% in the nccRCC population [52]. Results have not been published for patients with PRCC. This ORR is higher than previously published with any prior clinical trial evaluating monotherapy treatment. Likely the high response rate is related to the combination approach taken. It is important to observe that the response rate is higher than that observed with pembrolizumab monotherapy in the PRCC population in the KEYNOTE-427 clinical trial. The ASPEN and ESPN trials also highlight this concept. The MTOR inhibitor everolimus was tested against sunitinib in both trials. MTOR therapy was inferior to sunitinib especially when evaluating the subgroup of patients specifically with PRCC. Treatment with a single mechanism appear to perform less well in PRCC possibly due to the biologic diversity of this histologic diagnosis [53, 54]. Alternative pathways beyond MET also seem to play a crucial role in the biology of this disease [55]. Together, these data support the use of multitarget therapies, such as cabozantinib or suntinib, in the treatment of PRCC.
Table 1
Prospective clinical trials in PRCC
| Study Title | Study design | Treatment | Control Arm | MET alteration type | Number of Patients | ORR, % | PFS | OS |
| TRIALS ON SINGLE-AGENT TKI | ||||||||
| Crizotinib achieves long-lasting disease control in advanced papillary renal-cell carcinoma type 1 patients with MET mutations or amplification: EORTC 90101 CREATE trial (Schoffski et al.) [16, 17] | Phase II (NCT01524926) | Crizotinib | None | MET mutation exons (16–19)/MET amp | 41 (23 eligible with PRCC) | 50.0% | 1-year PFS 75.0%; 2-year PFS 75.0% | 1 yr OS: 75% |
| Sunitinib Versus Cabozantinib, Crizotinib or Savolitinib in Metastatic Papillary Renal Cell Carcinoma (pRCC): Results from the Randomized Phase II SWOG 1500 Study (SWOG 1500 study) (Pal et al.) [19] | Phase III (NCT02761057) | Cabozantinib 60 mg orally daily, crizotinib 250 mg orally daily, savolitinib 600 mg orally daily. | sunitinib 50 mg orally daily (with 4 weeks on and 2 weeks off) | Not specified | 147 PRCC patients | ORR (%): 22.72 (cabozantinib) vs. 0 (crizotinib) vs. 3.44 (savolitinib) vs. 4.34 (sunitinib) | PFS (median): 9 (cabozantinib) vs. 2.8 (crizotinib) vs. 3 (savolitinib) vs. 5.6 (sunitinib) months | OS (median): 20 (cabozantinib) vs. 19.9 (crizotinib) vs. 11.7 (savolitinib) vs. 16.4 (sunitinib) months |
| A Phase II and Biomarker Study of the Dual MET/VEGFR2 Inhibitor Foretinib in Patients With Papillary Renal Cell Carcinoma (Choueiri et al.) [13] | Phase II NCT 00726323 | Foretinib | None | Germline MET mutation (n = 11); somatic mutation (n = 5); gain of chromosome 7 = (n = 18); MET amp (n = 2) | 74 PRCC patients | ORR: 13.5% (10/74) | Median PFS: 9.3 months | Median OS: 12.8 months |
| TRIALS ON COMBINATION THERAPY OF TKI AND ICI | ||||||||
| Cabozantinib in Combination With Atezolizumab for Advanced Renal Cell Carcinoma: Results From the COSMIC-021 Study (Pal et al.) [35] | Phase I/II NCT03170960 | Cabozantinib and Atezolizumab | None | Not specified | 32 nccRCC patients | ORR: 31% | Median PFS: 9.5 months | Median OS: not reached |
| Phase II Trial of Cabozantinib Plus Nivolumab in Patients With Non-Clear-Cell Renal Cell Carcinoma and Genomic Correlates. (Lee et al.) [36] | Phase II (NCT03635892) | Cabozantinib and Nivolumab | None | Not specified | 40 patients (papillary, unclassified, or translocation-associated RCC) | ORR: 47.5% | Median PFS: 12.5 months | Median OS: 28 months |
| Clinical activity of durvalumab and savolitinib in MET-driven, metastatic papillary renal cancer. (Powles et al.) [25] | Phase I/II (NCT02819596) | Durvalumab and savolitinib | None | MET DNA alterations (central analysis: chromosome 7 gain/MET or HGF amplification/MET kinase domain mutations) | 41 PRCC patients | confirmed response rate: 29% | Median PFF: 4.9 months | Median OS in MET-driven patients: 14.1 months |
New research studies are quickly incorporating immune checkpoint inhibitors for the treatment of PRCC with initial promising results. To date there have not been any randomized studies completed for this population that included immune checkpoint inhibitors. S2200 (PAPMET2) is cooperative group trial comparing cabozantinib versus cabozantinib/atezolizumab in PRCC regardless of MET biomarker status. As mentioned previously, SAMETA is another ongoing study in MET-biomarker selected patients randomizing patients 1:1:1 to savolitinib/durvalumab, sunitinib or durvalumab monotherapy. The value of MET status and combination versus monotherapy will likely be clarified based on the results of these two clinical trials.
CONCLUSION
In summary, cabozantinib has the best evidence for use as a first-line treatment based on improved PFS when directly compared with sunitinib regardless of MET status. Other MET inhibitors including savolitinib, crizotinib, and foretinib have shown possible benefit in patients with MET-altered disease, but the inconsistent definition of MET status and early trial termination prevents further extrapolation of the data. Of these drugs, savolitinib has the most promising preliminary data in MET altered disease. Future trials of MET selective therapies, as well as combinations including immune checkpoint inhibitors have the potential to further improve the prognosis for patients with metastatic PRCC.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
Conception or design of the work: Kamal Kant Sahu, James Brundage, Benjamin L. Maughan.
Data collection: Kamal Kant Sahu, James Brundage, Benjamin L. Maughan.
Data analysis and interpretation: Kamal Kant Sahu, James Brundage, Benjamin L. Maughan.
Drafting the article: Kamal Kant Sahu, James Brundage, Benjamin L. Maughan.
Critical revision of the article: Kamal Kant Sahu, James Brundage, Benjamin L. Maughan.
Final approval of the version to be published: Kamal Kant Sahu, James Brundage, Benjamin L. Maughan.
CONFLICT OF INTEREST
Dr. Benjamin Maughan: Roche/Genentech, Pfizer, AVEO Oncology, Janssen Oncology, Astellas, Bristol-Myers Squibb, Clovis, Tempus, Merck, Exelixis, Bayer Oncology, Peloton Therapeutics (C/A), Exelixis, Bavarian-Nordic, Clovis, Genentech, Bristol-Myers Squibb (FR–institutional).
James Nicholas Brundage and Kamal Kant Sahu have no conflict of interest to report.
REFERENCES
[1] | Hsieh JJ , Purdue MP , Signoretti S , Swanton C , Albiges L , Schmidinger M , et al. Renal cell carcinoma. Nat Rev Dis Primers [Internet] (2017) [cited 2023 Jan 17];3: :17009. Available from: /pmc/articles/PMC5936048/ |
[2] | Moch H , Amin MB , Berney DM , Compérat EM , Gill AJ , Hartmann A , et al. The World Health Organization Classification of Tumours of the Urinary System and Male Genital Organs—Part A: Renal, Penile, and Testicular Tumours. Eur Urol. (2022) ;82: (5):458–68. |
[3] | Compérat E , Amin MB , Epstein JI , Hansel DE , Paner G , Al-Ahmadie H , et al. The Genitourinary Pathology Society Update on Classification of Variant Histologies, T1 Substaging, Molecular Taxonomy, and Immunotherapy and PD-L1 Testing Implications of Urothelial Cancers. Adv Anat Pathol [Internet]. (2021) [cited 2023 Mar 4];28: (4):196–208. Available from: https://pubmed.ncbi.nlm.nih.gov/34128484/ |
[4] | Deng J , Li L , Xia H , Guo J , Wu X , Yang X , et al. A comparison of the prognosis of papillary and clear cell renal cell carcinoma: Evidence from a meta-analysis. Medicine [Internet]. (2019) [cited 2023 Jan 17];98: (27). Available from: /pmc/articles/PMC6635153/ |
[5] | Powles T . Recent eUpdate to the ESMO Clinical Practice Guidelines on renal cell carcinoma on cabozantinib and nivolumab for first-line clear cell renal cancer: Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up 1. Annals of Oncology [Internet]. (2021) [cited 2023 Jan 17];32: (3):422–3. Available from: http://www.annalsofoncology.org/article/S0923753420431710/fulltext. |
[6] | Linehan WM , Spellman PT , Ricketts CJ , Creighton CJ , Fei SS , Davis C , et al. Comprehensive Molecular Characterization of Papillary Renal Cell Carcinoma. N Engl J Med [Internet]. (2016) [cited 2023 Mar 4];374: (2):135. Available from: /pmc/articles/PMC4775252/ |
[7] | Nandagopal L , Sonpavde GP , Agarwal N . Investigational MET inhibitors to treat Renal cell carcinoma. https://doi.org/101080/1354378420191673366 [Internet]. 2019 [cited 2023 Jan 17];28: (10):851–60. Available from: https://www.tandfonline.com/doi/abs/10.1080/13543784.2019.1673366 |
[8] | Jardim DLF , De Melo Gagliato D , Falchook G , Zinner R , Wheler JJ , Janku F , et al. MET abnormalities in patients with genitourinary malignancies and outcomes with c-MET inhibitors. Clin Genitourin Cancer [Internet]. (2015) [cited 2023 Jan 17];13: (1):e19. Available from: /pmc/articles/PMC5144738/ |
[9] | Rhoades Smith KE , Bilen MA . A Review of Papillary Renal Cell Carcinoma and MET Inhibitors. Kidney Cancer [Internet]. (2019) [cited 2023 Jan 17];3: (3):151. Available from: /pmc/articles/PMC6918905/ |
[10] | Eder JP , Shapiro GI , Appleman LJ , Zhu AX , Miles D , Keer H , et al. A phase I study of foretinib, a multi-targeted inhibitor of c-Met and vascular endothelial growth factor receptor 2. Clin Cancer Res [Internet]. (2010) [cited 2023 Jan 15];16: (13):3507–16. Available from: https://pubmed.ncbi.nlm.nih.gov/20472683/. |
[11] | Qian F , Engst S , Yamaguchi K , Peiwen Y , Won KA , Mock L , et al. Inhibition of tumor cell growth, invasion, and metastasis by EXEL-2880 (XL880, GSK1363089), a novel inhibitor of HGF and VEGF receptor tyrosine kinases. Cancer Res [Internet]. (2009) [cited 2023 Mar 4];69: (20):8009–16. Available from: https://pubmed.ncbi.nlm.nih.gov/19808973/. |
[12] | Choueiri TK , Vaishampayan UN , Rosenberg JE , Logan T , Harzstark AL , Rini BI , et al. A phase II and biomarker study (MET111644) of the dual Met/VEGFR-2 inhibitor foretinib in patients with sporadic and hereditary papillary renal cell carcinoma: Final efficacy, safety, and PD results. https://doi.org/101200/jco2012305 suppl355. (2012) ;30: (5 suppl):355–355. |
[13] | Choueiri TK , Vaishampayan U , Rosenberg JE , Logan TF , Harzstark AL , Bukowski RM , et al. Phase II and biomarker study of the dual MET/VEGFR2 inhibitor foretinib in patients with papillary renal cell carcinoma. J Clin Oncol [Internet]. (2013) [cited 2022 Nov 27];31: (2):181–6. Available from: https://pubmed.ncbi.nlm.nih.gov/23213094/. |
[14] | Srinivasan R , Bottaro DP , Choueiri TK , Vaishampayan UN , Rosenberg JE , Logan T , et al. Correlation of germline MET mutation with response to the dual Met/VEGFR-2 inhibitor foretinib in patients with sporadic and hereditary papillary renal cell carcinoma: Results from a multicenter phase II study (MET111644). https://doi.org/101200/jco2012305 suppl372. (2012) ;30: (5 suppl):372–372. |
[15] | Rikova K , Guo A , Zeng Q , Possemato A , Yu J , Haack H , et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell [Internet]. (2007) [cited 2023 Mar 4];131: (6):1190–203. Available from: https://pubmed.ncbi.nlm.nih.gov/18083107/. |
[16] | Schoffski P , Wozniak A , Escudier B , Rutkowski P , Anthoney A , Bauer S , et al. Effect of crizotinib on disease control in patient with advanced papillary renal cell carcinoma type 1 with MET mutations or amplification: Final results of EORTC 90101 CREATE. https://doi.org/101200/JCO2018366suppl580. (2018) ;36: (6 suppl):580–580. |
[17] | Schöffski P , Wozniak A , Escudier B , Rutkowski P , Anthoney A , Bauer S , et al. Crizotinib achieves long-lasting disease control in advanced papillary renal-cell carcinoma type 1 patients with MET mutations or amplification. EORTC 1 CREATE trial. Eur J Cancer. (2017) ;87: , 147–63. |
[18] | Clark JW , Ross Camidge D , Kwak EL , Maki RG , Shapiro GI , Chen I , et al. Dose-escalation trial of the ALK, MET & ROS1 inhibitor, crizotinib, in patients with advanced cancer. Future Oncol [Internet]. (2020) [cited 2023 Jan 15];16: (1):4289–301. Available from: https://pubmed.ncbi.nlm.nih.gov/31778074/. |
[19] | Pal SK , Tangen C , Thompson IM , Haas NB , George DJ , Heng DYC , et al. Sunitinib versus cabozantinib, crizotinib or savolitinib in metastatic papillary renal cell carcinoma (pRCC): Results from the randomized phase II SWOG 1500 study. Journal of Clinical Oncology [Internet]. (2021) [cited 2023 Jan 15];39: (6_suppl):270–270. https://ascopubs.org/doi/10.1200/JCO.2021.39.6_suppl.270 |
[20] | Pal SK , Tangen C , Thompson IM , Balzer-Haas N , George DJ , Heng DYC , et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: a randomised, open-label, phase 2 trial. Lancet [Internet]. (2021) [cited 2022 Nov 27];397: (10275):695. Available from: /pmc/articles/PMC8687736/ |
[21] | Gan HK , Millward M , Hua Y , Qi C , Sai Y , Su W , et al. First-in-Human Phase I Study of the Selective MET Inhibitor, Savolitinib, in Patients with Advanced Solid Tumors: Safety, Pharmacokinetics, and Antitumor Activity. Clin Cancer Res [Internet]. (2019) [cited 2022 Nov 27];25: (16):4924–32. Available from: https://pubmed.ncbi.nlm.nih.gov/30952639/. |
[22] | Gan HK , Lickliter J , Millward M , Gu Y , Weiguo S , Qi C , et al. cMet: Results in papillary renal cell carcinoma of a phase I study of AZD6094/volitinib leading to a phase 2 clinical trial with AZD6094/volitinib in patients with advanced papillary renal cell cancer (PRCC). https://doi.org/101200/jco2015337 suppl487. (2015) ;33: (7 suppl):487–487. |
[23] | Choueiri TK , Plimack E , Arkenau HT , Jonasch E , Heng DYC , Powles T , et al. Biomarker-Based Phase II Trial of Savolitinib in Patients With Advanced Papillary Renal Cell Cancer. J Clin Oncol [Internet]. (2017) [cited 2022 Nov 27];35: (26):2993–3001. Available from: https://pubmed.ncbi.nlm.nih.gov/28644771/. |
[24] | Choueiri TK , Heng DYC , Lee JL , Cancel M , Verheijen RB , Mellemgaard A , et al. Efficacy of Savolitinib vs Sunitinib in Patients With MET-Driven Papillary Renal Cell Carcinoma: The SAVOIR Phase 3 Randomized Clinical Trial. JAMA Oncol [Internet]. (2020) [cited 2022 Nov 27];6: (8):1. Available from: /pmc/articles/PMC7260692/ |
[25] | Powles T , Larkin JMG , Patel P , Pérez-Valderrama B , Rodriguez-Vida A , Glen H , et al. A phase II study investigating the safety and efficacy of savolitinib and durvalumab in metastatic papillary renal cancer (CALYPSO). https://doi.org/101200/JCO2019377suppl545. (2019) ;37: (7 suppl):545–545. |
[26] | Rodriguez C . Overall survival results for durvalumab and savolitinib in metastatic papillary renal cancer. J Clin Oncol. 2020; |
[27] | Yakes FM , Chen J , Tan J , Yamaguchi K , Shi Y , Yu P , et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther [Internet]. (2011) [cited 2023 Mar 4];10: (12):2298–308. Available from: https://pubmed-ncbi-nlm-nih-gov.ezproxy.lib.utah.edu/21926191/. |
[28] | Gomez de Liano Lista A . Cabozantinib in metastatic renal cell carcinoma (mRCC): data from UK expanded access program (EAP). In 2018. |
[29] | Campbell MT , Bilen MA , Duran C , Altinmakas E , Lim ZD , Jonasch E , et al. Cabozantinib for the treatment of patients with metastatic variant histology renal cell carcinoma (vhRCC): A retrospective study. Journal of Clinical Oncology [Internet]. (2017) Feb 20 [cited 2023 Jan 15];35: (6 suppl):478–478. Available from: https://ascopubs.org/doi/10.1200/JCO.2017.35.6suppl.478 |
[30] | Graham J , Basappa NS , Ghosh S , Zhang H , Hansen AR , Lalani AKA , et al. Association of cabozantinib dose reductions for toxicity with clinical effectiveness in metastatic renal cell carcinoma (mRCC): Results from the Canadian Kidney Cancer Information System (CKCis). Journal of Clinical Oncology [Internet]. (2022) [cited 2023 Jan 15];40: (6 suppl):316–316. Available from: https://ascopubs.org/doi/10.1200/JCO.2022.40.6suppl.316 |
[31] | Gan C . Cabozantinib real-world effectiveness in the first through fourth-line settings for the treatment of metastatic renal cell carcinoma (mRCC): Results from the International mRCC Database Consortium (IMDC). J Clin Oncol. 2020; |
[32] | Prisciandaro M , Ratta R , Massari F , Fornarini G , Caponnetto S , Iacovelli R , et al. Safety and Efficacy of Cabozantinib for Metastatic Nonclear Renal Cell Carcinoma: Real-world Data From an Italian Managed Access Program. Am J Clin Oncol [Internet]. (2019) [cited 2023 Jan 15];42: (1):42–5. Available from: https://pubmed.ncbi.nlm.nih.gov/30204614/. |
[33] | Meric-Bernstam F , Lee RJ , Carthon BC , Iliopoulos O , Mier JW , Patel MR , et al. CB-839, a glutaminase inhibitor, in combination with cabozantinib in patients with clear cell and papillary metastatic renal cell cancer (mRCC): Results of a phase I study. Journal of Clinical Oncology [Internet]. (2019) [cited 2023 Jan 15];37: (7 suppl):549–549. Available from: https://ascopubs.org/doi/10.1200/JCO.2019.37.7suppl.549 |
[34] | Tannir NM , Agarwal N , Porta C , Lawrence NJ , Motzer R , McGregor B , et al. Efficacy and Safety of Telaglenastat Plus Cabozantinib vs Placebo Plus Cabozantinib in Patients With Advanced Renal Cell Carcinoma: The CANTATA Randomized Clinical Trial. JAMA Oncol [Internet]. (2022) [cited 2023 Jan 22];8: (10):1411–8. Available from: https://jamanetwork.com/journals/jamaoncology/fullarticle/2795979. |
[35] | Pal SK , McGregor B , Suárez C , Tsao CK , Kelly W , Vaishampayan U , et al. Cabozantinib in Combination With Atezolizumab for Advanced Renal Cell Carcinoma: Results From the COSMIC-021 Study. Journal of Clinical Oncology [Internet]. (2021) [cited 2022Nov 27];39: (33):3725. Available from: /pmc/articles/PMC8601305/ |
[36] | Lee CH , Voss MH , Carlo MI , Chen YB , Zucker M , Knezevic A , et al. Phase II Trial of Cabozantinib Plus Nivolumab in Patients With Non-Clear-Cell Renal Cell Carcinoma and Genomic Correlates. J Clin Oncol [Internet]. (2022) [cited 2022 Nov 27];40: (21):2333–41. Available from: https://pubmed.ncbi.nlm.nih.gov/35298296/. |
[37] | Savolitinib Plus Durvalumab Versus Sunitinib and Durvalumab Monotherapy in MET-Driven, Unresectable and Locally Advanced or Metastatic PRCC - Full Text View - ClinicalTrials.gov [Internet]. [cited 2023 Mar 4]. Available from: https://clinicaltrials.gov/ct2/show/NCT05043090?term=savolitinib+durvalumab&cond=Papillary+Renal+Cell+Carcinoma&draw=2&rank=1 |
[38] | Kou J , Musich PR , Staal B , Kang L , Qin Y , Yao ZQ , et al. Differential responses of MET activations to MET kinase inhibitor and neutralizing antibody. J Transl Med [Internet]. (2018) [cited 2023 May 7];16: (1):253. Available from: /pmc/articles/PMC6134500/ |
[39] | Carretero–gonzález A , Lora D , Sobrino IM , Sanz IS , Bourlon MT , Herranz UA , et al. The Value of PD-L1 Expression as Predictive Biomarker in Metastatic Renal Cell Carcinoma Patients: A Meta-Analysis of Randomized Clinical Trials. Cancers (Basel) [Internet]. (2020) [cited 2023 May 8];12: (7):1–16. Available from: /pmc/articles/PMC7409133/ |
[40] | Derosa L , Routy B , Fidelle M , Iebba V , Alla L , Pasolli E , et al. Gut Bacteria Composition Drives Primary Resistance to Cancer Immunotherapy in Renal Cell Carcinoma Patients. Eur Urol. (2020) ;78: (2):195–206. |
[41] | Dizman N , Hsu JA , Bergerot PG , Gillece JD , Folkerts M , Reining L , et al. Randomized trial assessing impact of probiotic supplementation on gut microbiome and clinical outcome from targeted therapy in metastatic renal cell carcinoma. Cancer Med [Internet]. (2021) [cited 2023 Mar 6];10: (1):79. Available from: /pmc/articles/PMC7826461/ |
[42] | Farber NJ , Kim CJ , Modi PK , Hon JD , Sadimin ET , Singer EA . Renal cell carcinoma: the search for a reliable biomarker. Transl Cancer Res [Internet]. (2017) [cited 2023 May 8];6: (3):620–32. Available from: https://tcr.amegroups.com/article/view/13604/html. |
[43] | Tian X , Xu WH , Xu FJ , Li H , Anwaier A , Wang HK , et al. Identification of prognostic biomarkers in papillary renal cell carcinoma and PTTG1 may serve as a biomarker for predicting immunotherapy response. Ann Med [Internet]. (2022) [cited 2023 May 8];54: (1):211. Available from: /pmc/articles/PMC8765283/ |
[44] | Wang Y , Tian X , Zhu SX , Xu WH , Anwaier A , Su JQ , et al. Identification of prognostic and therapeutic biomarkers in type 2 papillary renal cell carcinoma. World J Surg Oncol [Internet]. (2023) [cited 2023 May 8];21: (1):98. Available from: https://wjso.biomedcentral.com/articles/10.1186/s12957-022-02836-3. |
[45] | Xu Y , Kong D , Li Z , Qian L , Li J , Zou C . Screening and identification of key biomarkers of papillary renal cell carcinoma by bioinformatic analysis. PLoS One [Internet]. (2021) [cited 2023 May 8];16: (8):e0254868. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0254868. |
[46] | Koh MY , Sayegh N , Agarwal N . Seeing the forest for the trees—single-cell atlases link CD8+ T cells and macrophages to disease progression and treatment response in kidney cancer. Cancer Cell. (2021) ;39: (5):594–6. |
[47] | Simonaggio A , Epaillard N , Pobel C , Moreira M , Oudard S , Vano YA . Tumor Microenvironment Features as Predictive Biomarkers of Response to Immune Checkpoint Inhibitors (ICI) in Metastatic Clear Cell Renal Cell Carcinoma (mccRCC). Cancers (Basel). (2021) ;13: (2):1–22. |
[48] | Brannon AR , Reddy A , Seiler M , Arreola A , Moore DT , Pruthi RS , et al. Molecular Stratification of Clear Cell Renal Cell Carcinoma by Consensus Clustering Reveals Distinct Subtypes and Survival Patterns. Genes Cancer [Internet]. (2010) [cited 2022 Aug 2];1: (2):152–63. Available from: https://pubmed.ncbi.nlm.nih.gov/20871783/. |
[49] | Pourmir I , Noel J , Simonaggio A , Oudard S , Vano YA . Update on the most promising biomarkers of response to immune checkpoint inhibitors in clear cell renal cell carcinoma. World J Urol [Internet]. (2021) [cited 2022 Aug 2];39: (5):1377–85. Available from: https://pubmed.ncbi.nlm.nih.gov/33386948/. |
[50] | Motzer R , Alekseev B , Rha SY , Porta C , Eto M , Powles T , et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. New England Journal of Medicine [Internet]. (2021) [cited 2023 Mar 5];384: (14):1289–300. Available from: https://www.nejm.org/doi/10.1056/NEJMoa2035716 |
[51] | Matsui J , Yamamoto Y , Funahashi Y , Tsuruoka A , Watanabe T , Wakabayashi T , et al. Ea novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer [Internet]. (2008) [cited 2023 Mar 5];122: (3):664–71. Available from: https://pubmed.ncbi.nlm.nih.gov/17943726/. |
[52] | Phase II KEYNOTE-B61 study of pembrolizumab (Pembro) + lenvatinib (Lenva) as first-line treatment for non-clear cell renal cell carcinoma 952 (nccRCC) | OncologyPRO [Internet]. [cited 2023 Feb 23]. Available from: https://oncologypro.esmo.org/meeting-resources/esmo-congress/phase-ii-keynote-b61-study-of-pembrolizumab-pembro-lenvatinib-lenva-as-first-line-treatment-for-non-clear-cell-renal-cell-carcinoma-nccrcc |
[53] | Tannir NM , Jonasch E , Albiges L , Altinmakas E , Ng CS , Matin SF , et al. Everolimus Versus Sunitinib Prospective Evaluation in Metastatic Non-Clear Cell Renal Cell Carcinoma (ESPN): A Randomized Multicenter Phase 2 Trial. Eur Urol [Internet]. (2016) [cited 2023 May 28];69: (5):866–74. Available from: https://pubmed.ncbi.nlm.nih.gov/26626617/. |
[54] | Armstrong AJ , Halabi S , Eisen T , Broderick S , Stadler WM , Jones RJ , et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial. Lancet Oncol [Internet]. (2016) [cited 2023 May 28];17: (3):378–88. Available from: https://pubmed.ncbi.nlm.nih.gov/26794930/. |
[55] | McDermott DF , Lee JL , Ziobro M , Suarez C , Langiewicz P , Matveev VB , et al. Open-Label, Single-Arm, Phase II Study of Pembrolizumab Monotherapy as First-Line Therapy in Patients With Advanced Non-Clear Cell Renal Cell Carcinoma. Journal of Clinical Oncology [Internet]. (2021) [cited 2023 Mar 16];39: (9):1029. Available from: /pmc/articles/PMC8078262/ |



