Influences of Age and Comorbidities on Indication for Partial Nephrectomy: A Systematic Review
Abstract
BACKGROUND:
The influence of age and comorbidities during decision-making for patients with renal cell carcinoma remains controversial.
OBJECTIVE:
To comprehensively review the available evidence regarding the impacts of age and comorbidities on the decision to perform partial nephrectomy (PN).
EVIDENCE ACQUISITION:
A systematic review was conducted in accordance with PRISMA and registered with PROSPERO (CRD42022344759). Only randomized control trials, prospective cohort studies, registry-based studies, or single/multi-institutional retrospective cohort studies comparing PN to other therapeutic options for cT1N0M0 renal masses were considered. The primary outcome was to assess differences in patients’ baseline characteristics between different treatments in order to investigate how those aspects have influenced clinical decision-making. Finally, perioperative outcomes were compared across the different options.
EVIDENCE SYNTHESIS:
Overall, patients who underwent PN were 3 to 11 years younger than those who underwent other treatments. Baseline renal function was slightly better in patients who underwent PN than in those who underwent radical nephrectomy (RN), active surveillance (AS), or tumor ablation. Patients undergoing PN had an average pre-treatment eGFR 4 to 6 points (mL/min/1.73 m2) higher than patients undergoing RN or tumor ablation. Likewise, the proportion of baseline chronic kidney disease (CKD) before treatment was higher in patients undergoing other treatments, with a rate of CKD between 6% and 56% higher compared with that for PN. A slightly higher proportion of baseline diabetes mellitus (DM) and cardiovascular comorbidities (CVD) were found in patients who underwent PN than in those who underwent RN (20% vs. 21% for DM and 37% vs. 41% for CVD). On average, patients who underwent AS and tumor ablation had more comorbidities, in terms of Charlson comorbidity index (CCI), DM, and CVD (50% vs. 38% for CCI ≥2; 25% vs. 20% for DM; and 43% vs. 37% for CVD). In terms of Eastern Cooperative Oncology Group (ECOG) Performance Status and American Society of Anesthesiologists (ASA) classification, no major differences were found between PN and other treatments, but a trend emerged whereby more fit patients underwent PN compared with RN (16% of ECOG >1 for PN vs. 18% for RN and 15% of ASA grade ≥3 for PN vs. 26% for RN). Again, tumor ablation was preferred for less fit patients (31% of ASA grade ≥3). No study included in our systematic review reported the baseline frailty status of patients treated for cT1 renal masses. The rates of perioperative complications and length of hospital stay (LOS) were similar between different techniques.
CONCLUSIONS:
Patients who underwent PN tended to be younger and fitter than those who underwent other available treatments for cT1 renal masses. Since this technique aims at reducing renal function impairment after surgery, a greater effort should be made to optimize patient selection to include more comorbid patients for whom PN might be useful.
INTRODUCTION
For many decades, radical nephrectomy (RN) was the backbone of therapy for all renal masses. Although cancer-specific survival associated with RN is excellent, its negative impact on renal function and the idea of an overtreatment, especially in case of cT1 renal masses, led to expand indication for partial nephrectomy (PN).
Nowadays, PN is considered the gold standard treatment for cT1 renal masses [1, 2], and its use is becoming more common owing to the increasing number of incidentally diagnosed cT1 renal masses. Furthermore, PN has been demonstrated to improve renal function and to reduce cardiovascular events [3–5], with non-inferior oncologic outcome for c/p T1 renal masses compared to RN [1, 2]. PN is also being used increasingly in cases of complex renal masses, with acceptable rates of perioperative complications and mid-term oncological and functional outcomes [6–8].
Nevertheless, the clinical decision on whether to perform PN or RN is more complex. Clinicians should take into account different aspects, both surgical (surgeon expertise, hospital volume, and surgical technique availability) and host factors (tumor and patient characteristics).
The recent advancements of surgical techniques and the adoption of a minimally invasive approach, both laparoscopic PN (LPN) and robotic PN (RAPN), that have been demonstrated to have equivalent oncologic outcomes relative to open approaches [9, 10], have taken an important step forward in optimizing perioperative outcomes and preserving renal function after PN [11].
So, in the era of minimally invasive surgery characterized by continuous technical refinements, the focus is also shifting on several host factors that can affect the pre-operative probability of PN success [12]. Patient age and baseline comorbidities are key factors in surgical success and should be considered by surgeons before performing PN, leading also to the evaluation of other possible therapeutic approaches [13].
Although PN remain the gold standard treatment for renal masses, international guidelines recommend that also non-surgical treatment (active surveillance (AS) and tumor ablation (TA)) should be considered for frail and/or comorbid patients with T1 renal masses, especially in case of non-eligibility for surgery [1, 2].
AS is defined as the monitoring over time of tumor size by serial abdominal imaging with delayed intervention reserved for tumors showing clinical progression during follow-up [1, 2]. A growing number of retrospective studies and meta-analyses evaluate the safety of AS and compare it to surgical treatments, showing that in selected patients, with advanced age and/or comorbidities, AS could be considered appropriate as first approach to cT1 renal masses [14–17].
Regarding TA, different techniques to treat small renal masses have been described over years, all with the objective of developing a less invasive approach able to preserve renal function, improve patient procedural tolerance and reduce the risk of complications related to surgery. In a recent systematic review, TA for cT1 renal masses was found to be safe in terms of complications and adverse events, but its long-term oncological effectiveness compared with surgery remained unclear [18].
Despite literature, everyday life clinical decision on how to approach a renal mass is more complex. There is still no clear consensus on which factors should guide the choice of treatment and especially on how patient’s baseline features can affect surgical outcomes. Therefore, this systematic review aims to comprehensively investigate the available evidence on the influence of age and comorbidities on the choice to perform PN for cT1 renal masses.
MATERIALS AND METHODS
This systematic review was performed according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [19] and is registered in the International Prospective Registry of Systematic Reviews (PROSPERO; CRD42022344759).
Search strategy
A systematic review of the literature was conducted using PubMed/MEDLINE, Cochrane Library Central, EMBASE, and Scopus databases. A literature search of original English language articles was performed. Search terms are listed in the supplementary material and include combinations of the following: partial nephrectomy, radical nephrectomy, comorbidity, Eastern Cooperative Oncology Group (ECOG) Performance Status, American Society of Anesthesiologists (ASA) classify, frailty, performance status, Charlson comorbidity index, ablation, thermoablation, cryoablation, and active surveillance. The titles and abstracts of the manuscripts obtained from the search were used to screen for initial study inclusion. A full-text review was performed when the abstract was insufficient to determine study inclusion. The reference lists of the included studies were manually searched for completeness, and if a study was suitable for inclusion, it was included in our systematic review. Four authors (C.R., F.C., G.M., and D.C.) independently completed the study selection. Potential disagreements were resolved by consensus among all the co-authors.
Inclusion criteria
Only randomized control trials, prospective cohort studies, registry-based studies, and single/multi-institutional retrospective cohort studies were considered. No time restrictions were applied during the search period. Mandatory inclusion criteria for the qualitative synthesis were as follows: (1) only full-text English original articles comparing PN (open, laparoscopic, or robotic) to other types of treatments for kidney tumors [RN (open, laparoscopic, or robotic), tumor ablation (cryoablation, radiofrequency ablation, stereotactic ablative radiotherapy, or other ablative techniques) or active surveillance]; (2) only adult patients (age >18 years); (3) only cT1 (cT1a or cT1b) cN0 cM0 renal masses (any histology was included); and (4) data available for patients’ baseline characteristics before surgery, including one or more of the following: age, BMI, Estimated Glomerular Filtration Rate (eGFR), and comorbidities as scored with the Charlson comorbidity index (CCI), Eastern Cooperative Oncology Group (ECOG) Performance Status, American Society of Anesthesiologists (ASA) classification, and/or frailty index.
Exclusion criteria
Previous reviews or meta-analyses, commentaries, editorials, letters, abstracts, and brief communications were excluded from the search. Non-English reports were excluded from this study. An additional exclusion criterion was the non-availability of full-text articles after contacting the corresponding authors. Reports were considered relevant and included if they provided extractable data on the baseline characteristics of patients before cT1 renal mass treatment.
Variable collection and outcome measures
Variables were collected according to a proforma and categorized as follows:
• Age (<70 vs. >70 years, when possible)
• BMI (normal <25 vs. abnormal ≥25, when possible),
• Glomerular filtration rate (GFR) [chronic kidney disease (CKD) (GFR <90 mL/min/1.73 m2) vs. non-CKD patients (GFR ≥90 mL/min/1.73 m2), when possible]
• Cardiovascular comorbidities (CVD), including hypertension, myocardial infarction, vascular problems, valve problems, and other cardiovascular diseases (yes vs. no)
• Diabetes mellitus (DM), type I or II (yes vs. no)
• CCI (mild risk, CCI ≤2 vs. moderate-severe risk, CCI >2 when possible) [20]
• ECOG (ECOG ≤1 vs. ECOG >1, when possible) [21],
• ASA classification (ASA ≤2 vs. ASA ≥3 when possible) [22, 23]
• Frailty status (cut-off for frail and non-frail patients might vary across studies; therefore, frailty status might be presented with different indexes).
The primary outcome of this systematic review was to show differences in patients’ baseline characteristics between those who underwent PN and those who underwent other treatments for cT1 renal masses in order to investigate how patient baseline characteristics affected the choice of performing PN. In addition, perioperative outcomes of different techniques were analyzed, considering intra- and post-operative complication rates, classified according to the Clavien–Dindo classification, and the length of hospital stay.
RESULTS
Evidence selection
Figure 1 details the full studies selection, as for PRISMA guidelines [19]. Out of 526 studies initially found through our search, 47 reports complying with our inclusion/exclusion criteria were included in the final systematic review, enrolling a total of 143,732 patients. All the reports included in the qualitative synthesis and the baseline characteristics of the patients are summarized in Table 1. Of 47, 20 studies compared PN to RN for a total of 46,705 vs. 48,858 patients; 24 studies compared PN to tumor ablation for a total of 23,365 vs. 7,335 patients, respectively; 2 compared PN, RN, and tumor ablation for a total of 5,377 vs. 11,031 vs. 899 patients, respectively; and 1 compared PN, RN, tumor ablation, and AS for a total of 65 vs. 15 vs. 14 vs. 68 patients, respectively. Overall, of the 143,732 patients included in the qualitative synthesis, 75,512 underwent PN (OPN, LPN or RAPN), 59,904 RN (ORN, LRN or RARN), 8248 underwent tumor ablation (including percutaneous ablation, cryoablation, thermal ablation, radiofrequency ablation, local tumor ablation and laparoscopic radiofrequency ablation assisted tumor enucleation) and 68 underwent AS.
Fig. 1
PRISMA 2022 flow diagram for new systematic reviews – Study selection with inclusion and exclusion criteria of the reviewed studies.
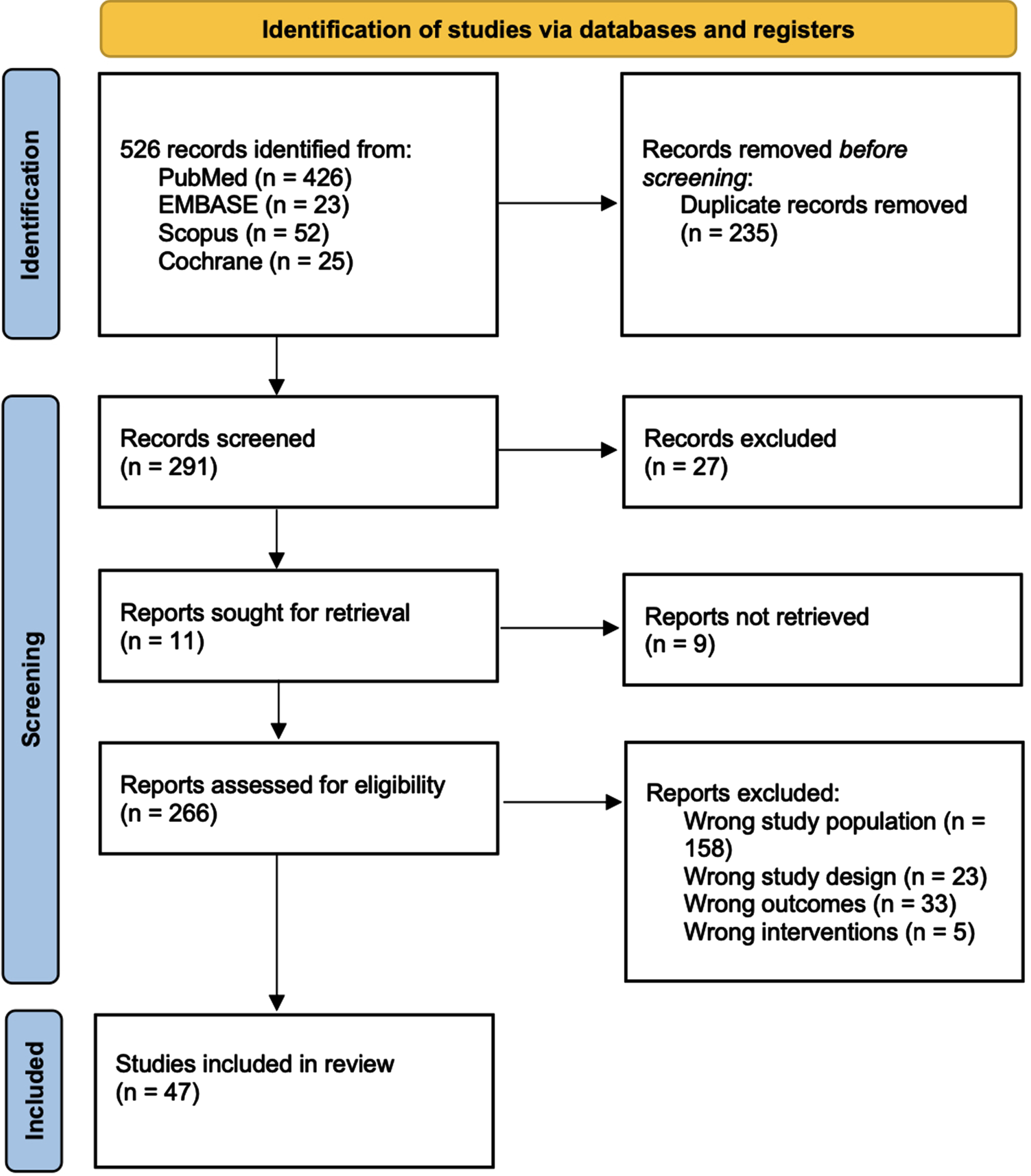
Table 1
Overview of the studies included in this review and patients’ baseline characteristics before partial nephrectomy or other types of treatment
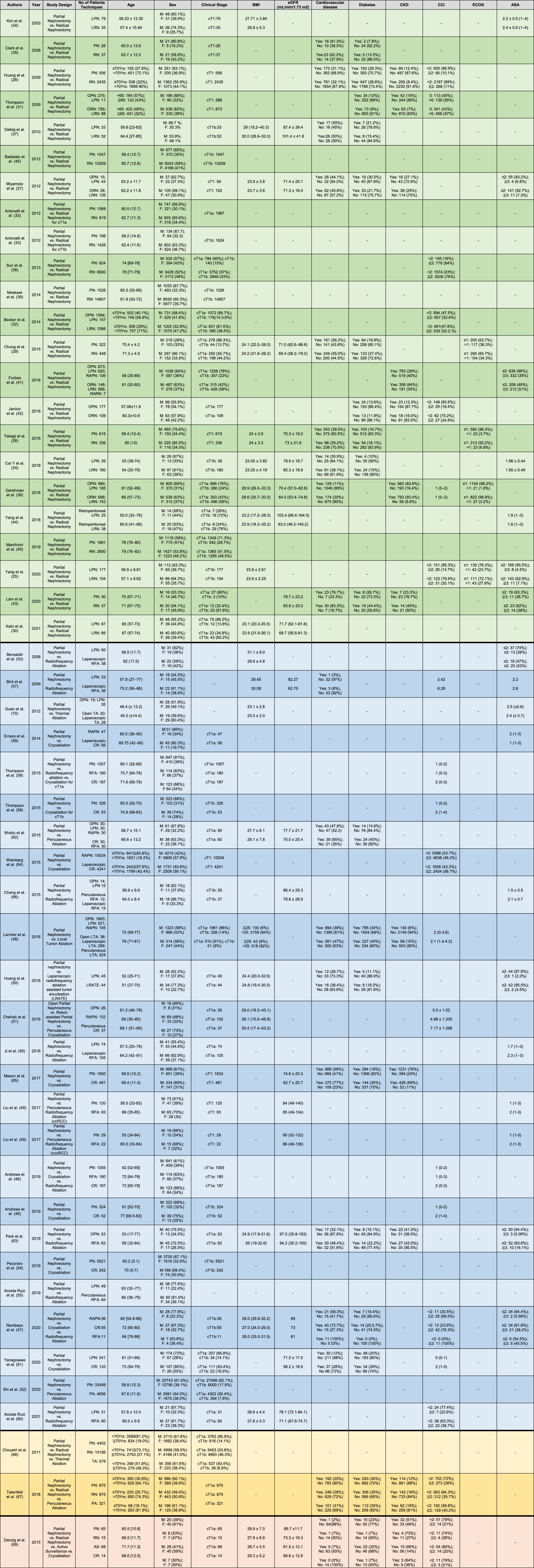
Overview of the studies included in the systematic review and patients’ baseline characteristics before partial nephrectomy or other types of treatment, including: studies comparing Partial Nephrectomy to Radical Nephrectomy (background color: green), studies comparing Partial Nephrectomy to Tumor Ablation (background color: blue), studies comparing Partial Nephrectomy to Radical Nephrectomy and Tumor Ablation (background color: yellow) and studies comparing Partial Nephrectomy to Radical Nephrectomy, Tumor Ablation and Active Surveillance (background color: orange). BMI: body mass index; eGFR: estimated glomerular filtration rate pre-treatment; CKD: chronic kidney disease; CCI: Charlson comorbidity index; ECOG: Eastern Cooperative Oncology Group performance status scale; ASA: American Society of Anesthesiologists physical status classification system; PN: partial nephrectomy; RN: radical nephrectomy; OPN: open partial nephrectomy; ORN: open radical nephrectomy; LPN: laparoscopic partial nephrectomy; LRN: laparoscopic radical nephrectomy; RAPN: robot-assisted partial nephrectomy; RARN: robot-assisted radical nephrectomy; AS: active surveillance; PA: percutaneous ablation; CR: cryoablation; TA: thermal ablation; RFA: radiofrequency ablation; LTA: local tumor ablation; LRATE: laparoscopic radiofrequency ablation assisted tumor enucleation; ccRCC: clear cell renal cell carcinoma; nccRCC: non clear cell renal cell carcinoma.
Age
Of the 20 studies included in the qualitative synthesis comparing PN to RN, the mean (or median) age of patients who underwent PN ranged between 53 and 78 years, whereas that of those who underwent RN ranged between 54 and 79 years [24–45]. Thompson et al. [31] reported that the proportion of patients aged >65 years undergoing PN was 43%, compared with 52% undergoing RN (p = 0.08). Furthermore, the proportions of patients aged >70 years reported by Becker et al. [32] and Huang et al. [26] were 59.9% and 72.1% for patients undergoing PN compared with 71% and 78% of patients undergoing RN, respectively (p < 0.001).
Comparing PN and tumor ablation (cryoablation, radiofrequency ablation, stereotactic ablative radiotherapy, or other ablative techniques), the mean (or median) age of patients who underwent PN ranged between 46.4 and 72 years, while that of those undergoing tumor ablation techniques ranged between 45.5 and 84 years [46–66]. Weinberg et al. [64] reported a rate of 16.2% for patients >70 years undergoing PN, compared with 42.4% for those undergoing tumor ablation (p < 0.001).
In addition, considering the two studies comparing PN to RN and tumor ablation to AS, patients who underwent PN were the youngest, followed by those who underwent RN and tumor ablation, with those who underwent AS the oldest [67–69].
Body mass index
Of the 47 studies included in our systematic review, 21 reported patients’ BMI during treatment. Considering the studies that compared PN to RN, the mean (or median) BMI of patients treated with PN ranged between 23.1 and 29.0 kg/m2 while that of those treated with RN ranged between 23.3 and 30.0 kg/m2 [24, 25, 27–30, 34, 36, 37, 44].
Considering the studies that compared PN vs. tumor ablation, the mean (or median) BMI of patients undergoing PN ranged between 23.1 and 31.1 kg/m2 while that of those undergoing tumor ablation techniques ranged between 23.5 and 30.4 kg/m2 [47, 50, 51, 53, 57, 60, 62, 63, 70].
The median BMI for patients elected for AS was 28.7±5.5 kg/m2 [69].
Renal function
Of the 47 studies included in the qualitative synthesis, 19 reported pre-treatment renal function expressed as estimated glomerular filtration rate (eGFR, mL/min/1.73 m2).
Considering the studies that compared PN to RN, the mean (or median) eGFR of patients submitted to PN ranged between 70.3 and 103.4 mL/min/1.73 m2 while that of those submitted to RN ranged between 64.0 and 101.4 mL/min/1.73 m2 [24, 27–30, 36, 37, 43, 44, 69]. The median eGFR values reported by Gershman et al. [36] and Chung et al. [29] were 70.4 (57.0–82.6) and 71.0 (62.6–86.6) for patients undergoing PN compared with 64.0 (53.4–74.8) and 69.4 (58.2–79.2) of patients undergoing RN, respectively (p < 0.001 and p = 0.004).
Considering the studies that compared PN to tumor ablation techniques, the eGFR of patients submitted to tumor ablation ranged between 58.2 and 94.2 mL/min/1.73 m2 while that of those submitted to PN ranged between 71.2 and 97.5 mL/min/1.73 m2 [47, 49, 57, 60–63, 65, 66]. A statistical difference in pre-treatment eGFR was reported by Rembeyo et al. [47] (85 vs. 67, p = 0.001), Acosta Ruiz et al. [60] (78.1 vs. 71.1, p = 0.03), Yanagisawa et al. [61] (71.2 vs. 58.2, p < 0.001), and Bird et al. [57] (82.3 vs. 62.8, p < 0.05).
Furthermore, considering active surveillance, patients had worse renal function than patients undergoing PN (81.5±12.1 vs. 89.7±11.7, p < 0.001) [69].
The incidence of pre-operative CKD (GFR <90 mL/min/1.73 m2) among patients undergoing PN ranged between 15.0% and 27.1%, while that of those who underwent RN ranged between 7.0% and 64.0% (36,41–43,67). Gershman et al. [36] reported a lower baseline proportion of moderate to severe CKD in a cohort of patients submitted to PN than in patients who underwent RN (29.0% vs. 40.0%, p < 0.001). However, Thompson et al. [31] reported the opposite, with a higher rate of pre-operative kidney failure in the PN population than in the RN population (15.0% vs. 7.0%, p < 0.001).
Comparing PN to tumor ablation, the proportion of patients with baseline CKD ranged between 6.0% and 76.0% for patients undergoing PN and between 10.0% and 89.0% for patients who underwent tumor ablation [48, 63, 65, 67]. Mason et al. [65] reported a proportion of CKD between patients undergoing PN of 76%, compared with 89% of patients undergoing tumor ablation (p < 0.001).
Danzig et al. [69], comparing PN to RN, AS, and cryoablation, reported a lower rate of CKD in patients undergoing PN compared with the other treatments (51.0% vs. 73.0% vs. 88.0% vs. 64.0%, respectively, p < 0.001).
Cardiovascular diseases
Only 19 studies included in the qualitative synthesis reported the baseline cardiovascular comorbidities (CVD) in the cohorts included in the analyses.
Considering the studies that compared PN and RN, the proportion of patients with CVD among patients undergoing PN ranged between 11.0% and 76.7%, while that for those who underwent RN ranged between 20.0% and 83.3% [24, 26–29, 35–37, 43]. Gershman et al. [36] reported a proportion of CVD between patients undergoing PN of 11%, compared with 28% of patients undergoing RN (p < 0.001).
Furthermore, considering the studies that compared PN and tumor ablation, the proportion of patients with baseline cardiovascular comorbidities among patients undergoing PN ranged between 3.0% and 59.0%, while those who underwent tumor ablation ranged between 8.0% and 100.0% [47, 48, 50, 57, 61–63, 65]. There were no studies in which patients who underwent PN had a higher proportion of baseline CVD than those who underwent tumor ablation.
In their study comparing PN to RN and percutaneous ablation, Talenfeld et al. [67] reported a significantly lower proportion of patients presenting baseline cardiovascular comorbidity in those submitted to PN than in those submitted to RN or percutaneous ablation (20% vs. 28% vs. 31%, respectively, p = 0.03). Furthermore, Danzig et al. reported a higher proportion of patients with baseline cardiovascular comorbidity for those who underwent AS than for those who underwent PN (7.0% vs. 2.0%, p = 0.3) [69].
Diabetes mellitus
The proportion of patients with baseline type I or II DM before surgery ranged between 7.8% and 30.5% among patients undergoing PN, while that of those who underwent RN ranged from 9.0% to 44.4% [24, 26–29, 31, 35, 37, 42, 43]. Chung et al. [29] reported a proportion of DM between patients undergoing PN of 20% compared with 27% of patients undergoing RN (p = 0.03).
In all the included studies comparing PN to tumor ablation, the proportion of patients with baseline DM was lower for patients who underwent PN than for those who underwent tumor ablation [47, 48, 50, 61–63, 65], ranging between 11.1% and 34.0% for PN and between 18.2% and 40.0% for tumor ablation [48, 62, 65]. A statistical difference in pre-treatment DM incidence was reported by Woldu et al. [62] (16% vs. 40%, p = 0.001), Mason et al. [65] (18% vs. 30%, p < 0.001), and Larcher et al. [48] (34% vs. 40%, p = 0.01).
Talenfeld et al. reported a significantly lower proportion of baseline DM in patients undergoing PN than in patients undergoing RN or PA (30% vs. 35% vs. 35%, respectively, p = 0.01) [67].
Similarly, Danzig et al. [69] reported baseline DM in 2% of patients who underwent PN and 7% of those who underwent AS (p = 0.06).
Charlson comorbidity index
Eighteen studies reported data on CCI at intervention. No differences were found between the patients who underwent PN and those who underwent RN. The proportion of patients with a moderate-to-severe grade of comorbidities (CCI ≥2) ranged between 10.1% and 84.0% for patients undergoing PN and between 11.0% and 76.0% for those undergoing RN [25, 26, 31, 32, 36, 38, 42].
In contrast, all studies comparing PN to tumor ablation reported a better CCI for patients undergoing PN [46–48, 51, 57, 58, 60, 61, 64]. Rembeyo et al. [47] and Weinberg et al. [64] reported proportions of CCI ≥2 between patients undergoing PN of 70% and 46% compared with 88% and 57% for patients undergoing RN, respectively (p < 0.001).
Talenfeld et al. compared PN to RN and PA and reported that patients undergoing PA had a worse baseline CCI than patients undergoing RN or PN, with those who underwent PN being healthier [67] (PA: 40% of CCI ≥2, RN: 36% and PN 28%, p = 0.04). No differences were found by Danzig et al. between cohorts of patients who underwent PN or AS [69].
ECOG performance status
Overall, only four studies comparing PN to RN reported patients’ ECOG performance status before surgery. Gershman et al. [36] reported an higher proportion of patients with a worse performance status (ECOG grade >1) in the RN group compared with patients undergoing PN (3% vs. 2%, p < 0.001). Two other studies reported similar results [25, 28]. Only one study reported a worse performance status in patients undergoing PN, but this was not statistically significant [29].
American Society of Anesthesiologists classification
From the studies comparing PN to RN, no major differences in ASA score were found between the two cohorts, even if a non-significant trend toward a lower ASA can be seen for patients who underwent PN [24, 25, 27, 34, 41, 43, 44].
Considering patients who underwent PN vs. tumor ablation, a higher proportion of patients with worse ASA scores (ASA ≥2) was observed in those undergoing tumor ablation [47, 49, 50, 53, 56, 57, 59, 63, 66, 70].
Frailty index
None of the studies included in our systematic review reported the baseline frailty index of patients treated for cT1 renal masses.
Complication rate and length of hospital stay
The intra-operative and post-operative complication rates, complication grade, and length of hospital stay (LOS) values are shown in Table 2.
Table 2
Brief report on patients’ outcomes after treatment for renal masses (PN vs. other treatments) of the included studies
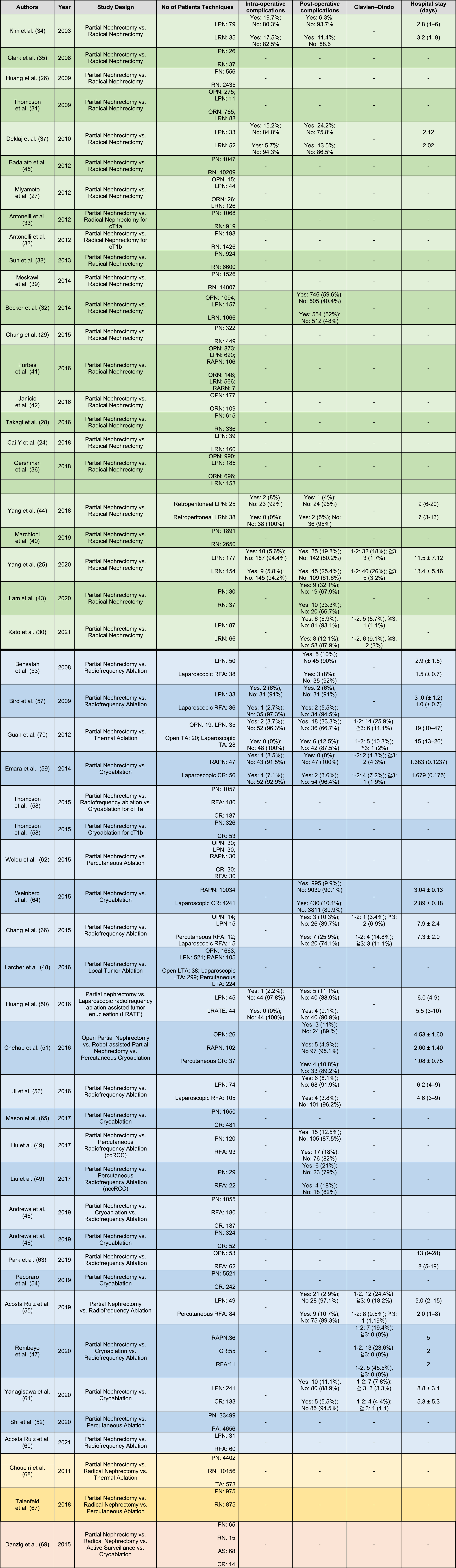
Brief report on patients’ outcomes after treatment for renal masses (PN vs. other treatments) of the included studies: studies comparing Partial Nephrectomy to Radical Nephrectomy (background color: green), studies comparing Partial Nephrectomy to Tumor Ablation (background color: blue), studies comparing Partial Nephrectomy to Radical Nephrectomy and Tumor Ablation (background color: yellow) and studies comparing Partial Nephrectomy to Radical Nephrectomy, Tumor Ablation and Active Surveillance (background color: orange). PN: partial nephrectomy; RN: radical nephrectomy; OPN: open partial nephrectomy; ORN: open radical nephrectomy; LPN: laparoscopic partial nephrectomy; LRN: laparoscopic radical nephrectomy; RAPN: robot-assisted partial nephrectomy; RARN: robot-assisted radical nephrectomy; AS: active surveillance; PA: percutaneous ablation; CR: cryoablation; TA: thermal ablation; RFA: radiofrequency ablation; LTA: local tumor ablation; LRATE: laparoscopic radiofrequency ablation assisted tumor enucleation; ccRCC: clear cell renal cell carcinoma; nccRCC: non clear cell renal cell carcinoma.
Considering PN vs. RN [25, 30, 32, 34, 37, 43, 44], the rate of intra-operative complications among patients undergoing PN ranged from 5.6% to 19.7%. Similarly, that of patients who underwent RN ranged between 5.7% and 17.5%. Furthermore, the post-operative complication rate for PN ranged from 4.0% to 59.6%, whereas that after RN was between 5.0% and 52.0%. In addition, considering high-grade complications (CD ≥3), the rates were similar between PN and RN [25, 30]. The mean (or median) LOS of patients who underwent PN ranged between 2.1 and 11.5 days, and that for patients who underwent RN was comparable, ranging between 2.0 and 13.4 days [25, 34, 37, 44].
Moreover, when comparing PN to tumor ablation [47, 49–51, 53, 55–57, 59, 61, 63, 64, 66, 70], the rate of intra-operative complications among patients undergoing PN ranged between 2.2% and 8.5%, while that of patients who underwent tumor ablation ranged between 0.0% and 7.1%. Furthermore, the post-operative complication rate for PN ranged from 0.0% to 33.3% and between 3.6% and 25.9% for tumor ablation. Additionally, the rate of complications classified as Clavien–Dindo ≥3 ranged between 0.0% and 18.2% for PN and between 0.0% and 11.1% for tumor ablation. The mean (or median) LOS for patients who underwent PN ranged from 1.3 to 19.0 days, while that of patients who underwent tumor ablation ranged from 1.1 to 15.0 days.
DISCUSSION
This systematic review examined 47 papers reporting baseline characteristics of patients undergoing PN compared with those of patients undergoing other treatments (RN, AS, and tumor ablation) for cT1 renal masses, with the aim of describing how host factors influenced the decision to perform PN and their impact on perioperative outcomes.
Overall, patients who underwent PN were on average three years younger than those who underwent RN, regardless of the surgical approach (open, laparoscopic, or robotic), 8 years younger than those who underwent tumor ablation, and 11 years younger than those who were candidates for AS. In addition, in most of the included studies, baseline renal function was slightly better in patients who underwent PN than in those who underwent RN, AS, or tumor ablation, although statistical significance was not reached in all the included studies. Patients undergoing PN had an average pre-treatment eGFR 4 points higher than patients undergoing RN. Furthermore, eGFR was on average 6 points higher in patients undergoing PN than in patients undergoing tumor ablation. Likewise, the proportion of baseline CKD before treatment was higher in patients undergoing other treatments than in those who underwent PN. The rate of CKD among patients undergoing RN was 6% greater than that for patients undergoing PN. This percentage increased considering tumor ablation or AS, being 13% and 56% greater than PN, respectively. There were few differences in baseline overall comorbidities (CCI) between PN and RN. However, slightly higher proportions of baseline DM and CVD were found in patients who underwent PN than in those who underwent RN (20% vs. 21% for DM and 37% vs. 41% for CVD). On average, patients who underwent AS and tumor ablation had more comorbidities in terms of CCI, DM, and CVD (50% vs. 38% of CCI ≥2; 25% vs. 20% for DM; and 43% vs. 37% for CVD). Regarding ECOG and ASA grade, no major differences were found between PN and other treatments, but a trend toward more fit patients emerged for those who underwent PN compared with RN, although this trend was not statistically significant in most cases (16% of ECOG >1 for PN vs. 18% for RN and 15% of ASA grade ≥3 for PN vs. 26% for RN). Again, tumor ablation was preferably chosen for less fit patients (31% of ASA grade ≥3). Concerning frailty index, no study included in our systematic review reported the baseline frailty status of patients treated for cT1 renal masses.
Given these differences in baseline characteristics, we also assessed perioperative outcomes, such as intra- and post-operative complications and LOS. Despite PN being a more complex procedure than RN and more invasive than AS or tumor ablation, the rates of perioperative complications and LOS were similar.
PN plays a key role in the treatment of cT1 renal masses [1, 71]. Several retrospective studies have suggested that nephron-sparing surgery could result in better preserved kidney function, decreased cardiovascular-specific mortality, and improved overall survival compared with RN [26, 72–76]. Several studies have reported a lower other cause of mortality (OCM) in elderly patients subjected to PN than in other treatments [3, 40, 77]. Moreover, PN resulted in lower cancer-specific mortality (CSM) in patients ≥75 years. Marchioni et al. [40] suggested that PN should also be considered in elderly patients with comorbidities. However, our systematic review reported that PN has been performed over the past few years, mostly in younger men. Given the feasibility of this surgery in older and comorbid men, greater effort should be dedicated to providing PN to this subgroup of patients.
Additionally, baseline renal function is one of the most important parameters that surgeons consider in decision-making regarding the treatment of cT1 renal masses. Several studies have suggested that PN guarantees better kidney function preservation than RN after surgery [3, 4]. Furthermore, the majority of patients undergoing PN, even if they might experience an acute post-operative decrease in renal function, usually recover within a few months of surgery [78, 79] and generally have stable long-term renal function [4]. Huang et al. [80] found that 26% of patients with newly diagnosed RCC had a baseline eGFR <60 mL/min. Moreover, they demonstrated that RN, compared with PN, increased the risk of developing chronic kidney disease in the long term. In our systematic review, no particular differences were found in terms of eGFR between PN and other treatments. Therefore, more effort should be made to expand indications for PN to patients with lower eGFR, with the aim of improving post-operative outcomes.
This is also supported by the fact that intra- and perioperative morbidity and complication rates, oncological outcomes, and quality of life after surgery are similar between PN and RN for cT1 renal masses [3, 72, 81–83].
The general population is aging, and the rate of comorbidities is increasing [84]. In this context, proper patient selection for decision-making in the treatment of cT1 renal masses is of primary importance. In some studies, the benefits of PN seemed to only apply to younger and fitter patients with fewer baseline comorbidities. Indeed, Sun et al. [38, 85] found a reduction in CSM and OCM among patients diagnosed with localized RCC who were treated with surgery compared with non-surgical treatments. Nevertheless, no benefits were observed among patients aged ≥75 years or those with multiple comorbidities. Furthermore, Rosiello et al. [86], in their comprehensive assessment of frailty status on surgical, functional, and oncologic outcomes in patients treated with PN, found that the risk of OCM significantly overcame the risk of dying due to RCC in frail patients. Moreover, he found that frail patients experienced a permanent decrease in renal function over time, even after adjusting for pre-operative eGFR, BMI, WIR, or EBL, without any renal function plateau or improvement during follow-up.
These findings suggest that the most important risk factors for unfavorable outcomes after PN remain unclear, and that the choice of performing PN in elderly and comorbid patients should be weighed against the risk of suboptimal surgical outcomes. There is a void in the guidelines on renal cancer regarding the recommendations for PN based on patient characteristics. There are no available randomized trials on this issue, and the majority of available studies are retrospective and have a high risk of bias.
Despite its strengths, our systematic review was not devoid of limitations. First, our findings are limited by the heterogeneity of the published data in terms of the study population, exposure, and outcome definition. Second, because of the purpose of the study, we took into account only patients’ baseline features (age and comorbidities), however performing or not PN for small renal masses is a more complex decision that should be driven also by surgical (surgeon expertise, hospital volume, and surgical technique availability) and tumor factors (e.g. nephrometry scores: RENAL or PADUA). Third, while evidence is more solid for PN compared with RN, there are fewer data comparing PN to other treatment options. In addition, given the above-mentioned limitations, the high risk of bias, and the heterogeneity of the included studies, we were not able to perform any summary statistics apart from reporting the ranges of the mean/median values in each study.
CONCLUSIONS
Patients who underwent PN are younger and healthier than those who underwent other available treatments for T1 renal masses. Since this technique is aimed at reducing renal function impairment after surgery, a greater effort should be made to optimizing patient selection and including more comorbid patients for whom PN might be clinically meaningful.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
Conception: Cignoli, Fallara, Montorsi, Capitanio.
Performance of data: Cignoli, Re, Musso, Cei.
Interpretation of data: Cignoli, Fallara.
Writing the article: Cignoli, Fallara.
Supervision: Montorsi, Larcher, Capitanio.
All the authors had complete access to the data.
CONFLICT OF INTEREST
Cignoli, Re, Fallara, Cei, Musso, Basile, Rosiello, Larcher, Montorsi and Capitanio have no conflict of interest to report.
REFERENCES
[1] | Ljungberg B , Albiges L , Bedke J , Bex A , Capitanio U , Giles RH , Hora M , Klatte T , Marconi L , Powles TAV . EAU Guidelines on renal cell carcinoma presented at the EAU Annual Congress Milano 2023. Eur Urol. 2023. |
[2] | Campbell SC , Clark PE , Chang SS , Karam JA , Souter L , Uzzo RG . Renal mass and localized renal cancer: Evaluation, management, and follow-up: AUA guideline: Part I. J Urol. (2021) ;206: (2):199–208. |
[3] | Van Poppel H , Da Pozzo L , Albrecht W , Matveev V , Bono A , Borkowski A , et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. (2011) ;59: (4):543–552. |
[4] | Scosyrev E , Messing EM , Sylvester R , Campbell S , Van Poppel H . Renal function after nephron-sparing surgery versus radical nephrectomy: Results from EORTC randomized trial 4. Eur Urol. (2014) ;65: (2):372–377. |
[5] | Capitanio U , Larcher A , Cianflone F , Trevisani F , Nini A , Mottrie A , et al. Hypertension and cardiovascular morbidity following surgery for kidney cancer. Eur Urol Oncol. (2020) ;3: (2):209–215. |
[6] | Juvet TS , Thompson RH , Potretzke AM . Robot-assisted partial nephrectomy is safe and effective for complex renal masses when performed by experienced surgeons. Translational andrology and urology. (2020) ;9: :2474–2478. |
[7] | Veccia A , Dell’oglio P , Antonelli A , Minervini A , Simone G , Challacombe B , et al. Robotic partial nephrectomy versus radical nephrectomy in elderly patients with large renal masses. Minerva Urol Nefrol. (2020) ;72: (1):99–108. |
[8] | Mari A , Tellini R , Porpiglia F , Antonelli A , Schiavina R , Amparore D , et al. Perioperative and mid-term oncological and functional outcomes after partial nephrectomy for complex (PADUA Score ≥10) renal tumors: A prospective multicenter observational study (the RECORD2 Project). Eur Urol Focus. (2021) ;7: (6):1371–1379. |
[9] | Lane BR , Campbell SC , Gill IS . 10-year oncologic outcomes after laparoscopic and open partial nephrectomy. J Urol. (2013) ;190: (1):44–49. |
[10] | Fallara G , Larcher A , Dabestani S , Fossati N , Järvinen P , Nisen H , et al. Recurrence pattern in localized RCC: Results from a European multicenter database (RECUR). Urol Oncol. (2022) ;40: (11):494.e11–494.e17. |
[11] | Cignoli D , Fallara G , Larcher A , Rosiello G , Montorsi F , Capitanio U . How to improve outcome in nephron-sparing surgery: The impact of new techniques. Curr Opin Urol. (2021) ;31: (3):255–261. |
[12] | Cacciamani GE , Gill T , Medina L , Ashrafi A , Winter M , Sotelo R , et al. Impact of host factors on robotic partial nephrectomy outcomes: Comprehensive systematic review and meta-analysis. J Urol. (2018) ;200: (4):716–730. |
[13] | Chandrasekar T , Boorjian SA , Capitanio U , Gershman B , Mir MC , Kutikov A . Collaborative review: Factors influencing treatment decisions for patients with a localized solid renal mass. Eur Urol. (2021) ;80: (5):575–588. |
[14] | Abouassaly R , Lane BR , Novick AC . Active surveillance of renal masses in elderly patients. J Urol. (2008) ;180: (2):505–509. |
[15] | Abou Youssif T , Kassouf W , Steinberg J , Aprikian AG , Laplante MP , Tanguay S . Active surveillance for selected patients with renal masses: Updated results with long-term follow-up. Cancer. (2007) ;110: (5):1010–1014. |
[16] | Crispen PL , Viterbo R , Boorjian SA , Greenberg RE , Chen DYT , Uzzo RG . Natural history, growth kinetics, and outcomes of untreated clinically localized renal tumors under active surveillance. Cancer. (2009) ;115: (13):2844–2852. |
[17] | Rosales JC , Haramis G , Moreno J , Badani K , Benson MC , McKiernan J , et al. Active surveillance for renal cortical neoplasms. J Urol. (2010) ;183: (5):1698–1702. |
[18] | Abu-Ghanem Y , Fernández-Pello S , Bex A , Ljungberg B , Albiges L , Dabestani S , et al. Limitations of available studies prevent reliable comparison between tumour ablation and partial nephrectomy for patients with localised renal masses: A systematic review from the european association of urology renal cell cancer guideline panel. Eur Urol Oncol. (2020) ;3: (4):433–452. |
[19] | Moher D , Shamseer L , Clarke M , Ghersi D , Liberati A , Petticrew M , et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) statement. Syst Rev. (2015) ;4: (1):1. |
[20] | Charlson ME , Pompei P , Ales KL , MacKenzie CR . A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. (1987) ;40: (5):373–383. |
[21] | Oken MM , Creech RH , Tormey DC , Horton J , Davis TE , McFadden ET , et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group Am J Clin Oncol. (1982) ;5: (6):649–655. |
[22] | Saklad M . Grading of patients for surgical procedures. Anesthesiology [Internet]. (1941) May 1;2: (3):281–284. Available from: https://doi.org/10.1097/00000542-194105000-00004 |
[23] | Dripps RD , Lamont A , Eckenhoff JE . The role of anesthesia in surgical mortality. JAMA. (1961) ;178: :261–266. |
[24] | Cai Y , Li H-Z , Zhang Y-S . Comparison of partial and radical laparascopic nephrectomy: Long-term outcomes for clinical T1b renal cell carcinoma. Urol J. (2018) ;15: (2):16–20. |
[25] | Yang F , Zhou Q , Xing N . Comparison of survival and renal function between partial and radical laparoscopic nephrectomy for T1b renal cell carcinoma. J Cancer Res Clin Oncol. (2020) ;146: (1):261–272. |
[26] | Huang WC , Elkin EB , Levey AS , Jang TL , Russo P . Partial nephrectomy versus radical nephrectomy in patients with small renal tumors–is there a difference in mortality and cardiovascular outcomes? J Urol. (2009) ;181: (1):52–55. |
[27] | Miyamoto K , Inoue S , Kajiwara M , Teishima J , Matsubara A . Comparison of renal function after partial nephrectomy and radical nephrectomy for renal cell carcinoma. Urol Int. (2012) ;89: (2):227–232. |
[28] | Takagi T , Kondo T , Iizuka J , Omae K , Kobayashi H , Yoshida K , et al. Comparison of survival rates in stage 1 renal cell carcinoma between partial nephrectomy and radical nephrectomy patients according to age distribution: A propensity score matching study. BJU Int. (2016) ;117: (6B):E52–E59. |
[29] | Chung JS , Son NH , Lee SE , Hong SK , Lee SC , Kwak C , et al. Overall survival and renal function after partial and radical nephrectomy among older patients with localised renal cell carcinoma: A propensity-matched multicentre study. Eur J Cancer. (2015) ;51: (4):489–497. |
[30] | Kato D , Nakane K , Enomoto T , Tomioka M , Nakai C , Takai M , et al. The utility of laparoscopic partial nephrectomy with renal function preservation, regardless of warm ischemia time, compared with laparoscopic radical nephrectomy. Asian J Endosc Surg. (2021) ;14: (3):386–393. |
[31] | Thompson RH , Siddiqui S , Lohse CM , Leibovich BC , Russo P , Blute ML . Partial versus radical nephrectomy for 4 to 7cm renal cortical tumors. J Urol. (2009) ;182: (6):2601–2606. |
[32] | Becker A , Ravi P , Roghmann F , Trinh Q-D , Tian Z , Larouche A , et al. Laparoscopic radical nephrectomy vs laparoscopic or open partial nephrectomy for T1 renal cell carcinoma: Comparison of complication rates in elderly patients during the initial phase of adoption. Urology. (2014) ;83: (6):1285–1291. |
[33] | Antonelli A , Ficarra V , Bertini R , Carini M , Carmignani G , Corti S , et al. Elective partial nephrectomy is equivalent to radical nephrectomy in patients with clinical T1 renal cell carcinoma: Results of a retrospective, comparative, multi-institutional study. BJU Int. (2012) ;109: (7):1013–1018. |
[34] | Kim FJ , Rha KH , Hernandez F , Jarrett TW , Pinto PA , Kavoussi LR . Laparoscopic radical versus partial nephrectomy: Assessment of complications. J Urol. (2003) ;170: (2 Pt 1):408–411. |
[35] | Clark ATD , Breau RH , Morash C , Fergusson D , Doucette S , Cagiannos I . Preservation of renal function following partial or radical nephrectomy using 24-hour creatinine clearance. Eur Urol. (2008) ;54: (1):143–149. |
[36] | Gershman B , Thompson RH , Boorjian SA , Lohse CM , Costello BA , Cheville JC , et al. Radical versus partial nephrectomy for cT1 renal cell carcinoma. Eur Urol. (2018) ;74: (6):825–832. |
[37] | Deklaj T , Lifshitz DA , Shikanov SA , Katz MH , Zorn KC , Shalhav AL . Laparoscopic radical versus laparoscopic partial nephrectomy for clinical T1bN0M0 renal tumors: Comparison of perioperative, pathological, and functional outcomes. J Endourol. (2010) ;24: (10):1603–1607. |
[38] | Sun M , Bianchi M , Trinh Q-D , Hansen J , Abdollah F , Hanna N , et al. Comparison of partial vs radical nephrectomy with regard to other-cause mortality in T1 renal cell carcinoma among patients aged ≥75 years with multiple comorbidities. BJU Int. (2013) ;111: (1):67–73. |
[39] | Meskawi M , Becker A , Bianchi M , Trinh Q-D , Roghmann F , Tian Z , et al. Partial and radical nephrectomy provide comparable long-term cancer control for T1b renal cell carcinoma. Int J Urol Off J Japanese Urol Assoc. (2014) ;21: (2):122–128. |
[40] | Marchioni M , Preisser F , Bandini M , Nazzani S , Tian Z , Kapoor A , et al. Comparison of partial versus radical nephrectomy effect on other-cause mortality, cancer-specific mortality, and 30-day mortality in patients older than 75 years. Eur Urol Focus. (2019) ;5: (3):467–473. |
[41] | Forbes CM , Rendon RA , Finelli A , Kapoor A , Moore RB , Breau RH , et al. Disease progression and kidney function after partial vs. radical nephrectomy for T1 renal cancer. Urol Oncol. (2016) ;34: (11):486.e17–486.e23. |
[42] | Janicic A , Bumbasirevic U , Pekomezovic T , Cekerevac M , Acimovic M , Dzamic Z , et al. Partial versus radical nephrectomy for pT1a renal cancer in Serbia. J BUON. (2016) ;21: (6):1449–1453. |
[43] | Lam JKJ , Tan SY , Chong KT . Is partial nephrectomy worth performing compared to radical nephrectomy for small, localised renal cortical tumours in geriatric patients? Singapore Med J. (2020) ;61: (4):190–193. |
[44] | Yang C , Wang Z , Huang S , Xue L , Fu D , Chong T . Retroperitoneal laparoscopic partial nephrectomy versus radical nephrectomy for clinical T1 renal hilar tumor: Comparison of perioperative characteristics and short-term functional and oncologic outcomes. J Laparoendosc Adv Surg Tech A. (2018) ;28: (10):1183–1187. |
[45] | Badalato GM , Kates M , Wisnivesky JP , Choudhury AR , McKiernan JM . Survival after partial and radical nephrectomy for the treatment of stage T1bN0M0 renal cell carcinoma (RCC) in the USA: A propensity scoring approach. BJU Int. (2012) ;109: (10):1457–1462. |
[46] | Andrews JR , Atwell T , Schmit G , Lohse CM , Kurup AN , Weisbrod A , et al. Oncologic outcomes following partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol. (2019) ;76: (2):244–251. |
[47] | Rembeyo G , Correas J-M , Jantzen R , Audenet F , Dariane C , Delavaud C , et al. Percutaneous ablation versus robotic partial nephrectomy in the treatment of cT1b renal tumors: Oncologic and functional outcomes of a propensity score-weighted analysis. Clin Genitourin Cancer. (2020) ;18: (2):138–147. |
[48] | Larcher A , Meskawi M , Valdivieso R , Boehm K , Trudeau V , Tian Z , et al. Comparison of renal function detriments after local tumor ablation or partial nephrectomy for renal cell carcinoma. World J Urol. (2016) ;34: (3):383–389. |
[49] | Liu N , Huang D , Cheng X , Chong Y , Wang W , Gan W , et al. Percutaneous radiofrequency ablation for renal cell carcinoma vs. partial nephrectomy: Comparison of long-term oncologic outcomes in both clear cell and non-clear cell of the most common subtype. Urol Oncol. (2017) ;35: (8):530.e1–530.e6. |
[50] | Huang J , Zhang J , Wang Y , Kong W , Xue W , Liu D , et al. Comparing zero ischemia laparoscopic radio frequency ablation assisted tumor enucleation and laparoscopic partial nephrectomy for clinical T1a renal tumor: A randomized clinical trial. J Urol. (2016) ;195: (6):1677–1683. |
[51] | Chehab M , Friedlander JA , Handel J , Vartanian S , Krishnan A , Wong C-YO , et al. Percutaneous cryoablation vs partial nephrectomy: cost comparison of T1a tumors. J Endourol. (2016) ;30: (2):170–176. |
[52] | Shi L , He Y , Liu C , Qian X , Wang Z . Local ablation vs partial nephrectomy in T1N0M0 renal cell carcinoma: An inverse probability of treatment weighting analysis. Cancer Med. (2020) ;9: (21):7988–8003. |
[53] | Bensalah K , Zeltser I , Tuncel A , Cadeddu J , Lotan Y . Evaluation of costs and morbidity associated with laparoscopic radiofrequency ablation and laparoscopic partial nephrectomy for treating small renal tumours. BJU Int. (2008) ;101: (4):467–471. |
[54] | Pecoraro A , Palumbo C , Knipper S , Mistretta FA , Tian Z , Shariat SF , et al. Cryoablation predisposes to higher cancer specific mortality relative to partial nephrectomy in patients with nonmetastatic pT1b kidney cancer. J Urol. (2019) ;202: (6):1120–1126. |
[55] | Acosta Ruiz V , Ladjevardi S , Brekkan E , Häggman M , Lönnemark M , Wernroth L , et al. Periprocedural outcome after laparoscopic partial nephrectomy versus radiofrequency ablation for T1 renal tumors: A modified R. E.N.A.L nephrometry score adjusted comparison. Acta Radiol. (2019) ;60: (2):260–268. |
[56] | Ji C , Zhao X , Zhang S , Liu G , Li X , Zhang G , et al. Laparoscopic radiofrequency ablation versus partial nephrectomy for cT1a renal tumors: Long-term outcome of 179 patients. Urol Int. (2016) ;96: (3):345–353. |
[57] | Bird VG , Carey RI , Ayyathurai R , Bird VY . Management of renal masses with laparoscopic-guided radiofrequency ablation versus laparoscopic partial nephrectomy. J Endourol. (2009) ;23: (1):81–88. |
[58] | Thompson RH , Atwell T , Schmit G , Lohse CM , Kurup AN , Weisbrod A , et al. Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol. (2015) ;67: (2):252–259. |
[59] | Emara AM , Kommu SS , Hindley RG , Barber NJ . Robot-assisted partial nephrectomy vs laparoscopic cryoablation for the small renal mass: Redefining the minimally invasive “gold standard”. BJU Int. (2014) ;113: (1):92–99. |
[60] | Acosta Ruiz V , Båtelsson S , Onkamo E , Wernroth L , Nilsson T , Lönnemark M , et al. Split renal function after treatment of small renal masses: Comparison between radiofrequency ablation and laparoscopic partial nephrectomy. Acta Radiol. (2021) ;62: (9):1248–1256. |
[61] | Yanagisawa T , Miki J , Shimizu K , Fukuokaya W , Urabe F , Mori K , et al. Functional and oncological outcome of percutaneous cryoablation versus laparoscopic partial nephrectomy for clinical T1 renal tumors: A propensity score-matched analysis. Urol Oncol. (2020) ;38: (12):938.e1–938.e7. |
[62] | Woldu SL , Thoreson GR , Okhunov Z , Ghandour R , Rothberg MB , RoyChoudhury A , et al. Comparison of renal parenchymal volume preservation between partial nephrectomy, cryoablation, and radiofrequency ablation using 3D volume measurements. J Endourol. (2015) ;29: (8):948–955. |
[63] | Park JM , Yang SW , Shin JH , Na YG , Song KH , Lim JS . Oncological and functional outcomes of laparoscopic radiofrequency ablation and partial nephrectomy for T1a renal masses: A retrospective single-center 60 month follow-up cohort study. Urol J. (2019) ;16: (1):44–49. |
[64] | Weinberg AC , Woldu SL , Wen T , Deibert CM , Korets R , Badani KK . Utilization and perioperative complications of laparoscopic cryoablation vs. robotic partial nephrectomy for localized renal tumors. Int Braz J Urol. (2015) ;41: (3):473–485. |
[65] | Mason RJ , Atwell TD , Lohse C , Bhindi B , Weisbrod A , Boorjian SA , et al. Renal functional outcomes in patients undergoing percutaneous cryoablation or partial nephrectomy for a solitary renal mass. BJU Int. (2017) ;120: (4):544–549. |
[66] | Chang X , Zhang F , Liu T , Ji C , Zhao X , Yang R , et al. Radio frequency ablation versus partial nephrectomy for clinical T1b renal cell carcinoma: Long-term clinical and oncologic outcomes. J Urol. (2015) ;193: (2):430–435. |
[67] | Talenfeld AD , Gennarelli RL , Elkin EB , Atoria CL , Durack JC , Huang WC , et al. Percutaneous ablation versus partial and radical nephrectomy for T1a renal cancer: A population-based analysis. Ann Intern Med. (2018) ;169: (2):69–77. |
[68] | Choueiri TK , Schutz FAB , Hevelone ND , Nguyen PL , Lipsitz SR , Williams SB , et al. Thermal ablation vs surgery for localized kidney cancer: A Surveillance, Epidemiology, and End Results (SEER) database analysis. Urology. (2011) ;78: (1):93–98. |
[69] | Danzig MR , Ghandour RA , Chang P , Wagner AA , Pierorazio PM , Allaf ME , et al. Active surveillance is superior to radical nephrectomy and equivalent to partial nephrectomy for preserving renal function in patients with small renal masses: Results from the DISSRM registry. J Urol. (2015) ;194: (4):903–909. |
[70] | Guan W , Bai J , Liu J , Wang S , Zhuang Q , Ye Z , et al. Microwave ablation versus partial nephrectomy for small renal tumors: Intermediate-term results. J Surg Oncol. (2012) ;106: (3):316–321. |
[71] | Van Poppel H , Da Pozzo L , Albrecht W , Matveev V , Bono A , Borkowski A , et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol [Internet]. (2011) ;59: (4):543–552. Available from: https://doi.org/10.1016/j.eururo.2010.12.013 |
[72] | MacLennan S , Imamura M , Lapitan MC , Omar MI , Lam TBL , Hilvano-Cabungcal AM , et al. Systematic review of perioperative and quality-of-life outcomes following surgical management of localised renal cancer. Eur Urol. (2012) ;62: (6):1097–1117. |
[73] | Capitanio U , Terrone C , Antonelli A , Minervini A , Volpe A , Furlan M , et al. Nephron-sparing techniques independently decrease the risk of cardiovascular events relative to radical nephrectomy in patients with a T1a-T1b renal mass and normal preoperative renal function. Eur Urol. (2015) ;67: (4):683–689. |
[74] | Thompson RH , Boorjian SA , Lohse CM , Leibovich BC , Kwon ED , Cheville JC , et al. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol. (2008) ;179: (2):463–468. |
[75] | Kates M , Badalato GM , Pitman M , McKiernan JM . Increased risk of overall and cardiovascular mortality after radical nephrectomy for renal cell carcinoma 2cm or less. J Urol. (2011) ;186: (4):1247–1253. |
[76] | Sun M , Trinh Q-D , Bianchi M , Hansen J , Hanna N , Abdollah F , et al. A non-cancer-related survival benefit is associated with partial nephrectomy. Eur Urol. (2012) ;61: (4):725–731. |
[77] | Shuch B , Hanley J , Lai J , Vourganti S , Kim SP , Setodji CM , et al. Overall survival advantage with partial nephrectomy: A bias of observational data? Cancer. (2013) ;119: (16):2981–2989. |
[78] | Mir MC , Ercole C , Takagi T , Zhang Z , Velet L , Remer EM , et al. Decline in renal function after partial nephrectomy: Etiology and prevention. J Urol. (2015) ;193: (6):1889–1898. |
[79] | Porpiglia F , Fiori C , Bertolo R , Morra I , Russo R , Piccoli G , et al. Long-term functional evaluation of the treated kidney in a prospective series of patients who underwent laparoscopic partial nephrectomy for small renal tumors. Eur Urol. (2012) ;62: (1):130–135. |
[80] | Huang WC , Levey AS , Serio AM , Snyder M , Vickers AJ , Raj G V , et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: A retrospective cohort study. Lancet Oncol. (2006) ;7: (9):735–740. |
[81] | Poulakis V , Witzsch U , de Vries R , Moeckel M , Becht E . Quality of life after surgery for localized renal cell carcinoma: Comparison between radical nephrectomy and nephron-sparing surgery. Urology. (2003) ;62: (5):814–820. |
[82] | Kunath F , Schmidt S , Krabbe L-M , Miernik A , Dahm P , Cleves A , et al. Partial nephrectomy versus radical nephrectomy for clinical localised renal masses. Cochrane database Syst Rev. (2017) ;5: (5):CD012045. |
[83] | Van Poppel H , Da Pozzo L , Albrecht W , Matveev V , Bono A , Borkowski A , et al. A prospective randomized EORTC intergroup phase 3 study comparing the complications of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. (2007) ;51: (6):1606–1615. |
[84] | Kinsella K , He W . U.S. Census Bureau, International Population Reports,P95/09-1,An Aging World: 2008. 2009;(June). Available from: http://www.census.gov/content/dam/Census/library/publications/2009/demo/p95-09-1.pdf |
[85] | Sun M , Becker A , Tian Z , Roghmann F , Abdollah F , Larouche A , et al. Management of localized kidney cancer: Calculating cancer-specific mortality and competing risks of death for surgery and nonsurgical management. Eur Urol. (2014) ;65: (1):235–241. |
[86] | Rosiello G , Larcher A , Fallara G , Cignoli D , Re C , Martini A , et al. A comprehensive assessment of frailty status on surgical, functional and oncologic outcomes in patients treated with partial nephrectomy–A large, retrospective, single-center study. Urol Oncol. 2022. |




