Adjuvant Therapy in Renal Cell Carcinoma: Are We Ready for Prime Time?
Abstract
The standard of care for localized renal cell carcinoma (RCC) is radical or partial nephrectomy. Despite complete resection, a subset of patients will develop locoregional recurrence or metastatic disease. Adjuvant immunotherapy has been studied since the 1980 s as the primary method to mitigate tumor recurrence after definitive surgery. We herein discuss published and ongoing clinical trials investigating adjuvant therapy in localized or locoregional RCC.
INTRODUCTION
Renal cell carcinoma (RCC) is common among men and women worldwide [1]. Due to widespread use of computed tomography and renal ultrasound, the incidence of RCC has been increasing over the past three decades [1, 2]. While there has been a significant increase in the incidence of RCC, survival for patients with local, regional, and distant disease has only modestly improved over the past several decades. From 2000 to 2011, 5-year cancer-specific survival for RCC was 94% for localized disease, 71% for regional disease, and 12% for distant disease [3]. In the metastatic setting, most recent data from contemporary phase 3 studies demonstrate a 5-year overall survival of 48% for patients with intermediate and poor risk RCC being treated with ipilimumab and nivolumab and 3-year overall survival of 63% for patients with RCC receiving pembrolizumab and axitinib [4]. For patient with localized disease, the standard of care continues to be radical or partial nephrectomy. Despite definitive surgical resection, a subset of patients with localized disease go on to develop distant metastases and lethal RCC.
Outcomes for patients with localized RCC are heterogeneous. According to one systematic review of contemporary data, 5-year recurrence free survival varied from 42% to 98% [5]. In this review, we highlight scoring models and algorithms to aid in risk stratification for patients with localized RCC. There have been a multitude of agents that have been tested in the adjuvant setting including cytokine based treatments, targeted therapies, and most recently checkpoint inhibitors. Despite the volume of agents tested, there still remains a very limited number of treatments which have demonstrated clinical efficacy in the adjuvant setting. In this review, we discuss the role of adjuvant immunotherapy in RCC.
RISK STRATIFICATION
Given the heterogeneous outcomes in terms of cancer recurrence and metastasis after nephrectomy for localized RCC, risk stratification tools have been developed to identify patients at increased likelihood of adverse outcomes. The TMN staging system, which takes into account tumor size and disease extension as estimated by T stage, is an important prognostic tool in RCC. This staging system has also been incorporated with a number of prognostic factors into nomograms (Table 1). The local and distant recurrence rates at a mean follow-up of 56 months was 0% and 4.4% for T1; 2.0% and 5.3% for T2, 8.2% and 11.5% for T3a, 10.6% and 14.9% for T3b, respectively [6]. Possibly due to relatively low frequency of lymph node dissection in RCC, the frequency of node positive disease is low but when present is associated with poor prognosis [7]. In addition to TNM staging, tumor grade has been historically shown to be associated with tumor recurrence independent of tumor stage [8]. For example, for T1 tumors, 5-year cancer-specific survival for grade 1, 2, 3, and 4 histology was 91%, 83%, 60%, and 0% [8]. The TNM staging system has been incorporated with several other factors potentially associated with recurrence such as histologic features, performance status, Fuhrman grade, tumor size, tumor necrosis, presence of symptoms, and margin status. These risk factors are reflected in several nomograms developed for disease prognostication in the setting of localized disease (Table 1). These nomograms vary either in their input clinical or pathologic characteristics or their computed outcomes, for example disease-free survival, cancer specific survival, or overall survival.
Table 1
Models for prognostication in localized renal cell carcinoma
| Model | Parameters | Outcome | Type |
| UISS | TNM, grade, ECOG PS | OS | KM analysis |
| Leibovich | TNM, pN+, tumor size, grade, tumor necrosis | MFS | Algorithm |
| SSIGN | TNM, pN+, pM+, tumor size, grade, tumor necrosis | CSS | Algorithm |
| MSKCC | TNM, tumor size, grade, tumor necrosis, symptoms | RFS | Nomogram |
| Kattan | TNM, tumor size, histology, symptoms | RFS | Nomogram |
| Yaycioglu | Tumor size, symptoms | RFS | Formula |
| Karakiewicz | TNM, age, sex, + margin, tumor size, symptoms | CSS | Nomogram |
| Cindolo | Tumor size, symptoms | RFS | Formula |
UISS = University of California Los Angles Integrated Staging System, SSIGN = Stage, Size, Grade and Necrosis Score for Renal Cell Carcinoma; MSKCC = Memorial Sloan Kettering Cancer Center Nomogram, ECOG = Eastern Cooperative Oncology Group, PS = Performance Status, pN+ = pathologically confirmed nodal metastasis; pM+ = pathologically confirmed distant metastasis, + margin = positive margin, OS = overall survival, MFS = metastasis-free survival, CSS = cancer-specific survival, RFS = recurrence-free survival, KM = Kaplan-Meier.
In addition to patient clinical and pathologic characteristics, several studies have investigated biomarkers for predicting post-surgical disease recurrence [9, 10]. Several molecular assays have been developed. ClearCode34, a 34-gene classifier was developed to sub-stratify clear cell RCC to estimate recurrence-free survival and overall survival [11]. In contrast, the cell cycle proliferation (CCP) score is an RNA assay characterizing expression of cell cycle proliferation genes [12]. On multivariable regression analyses, CCP was significantly associated with recurrence and disease-specific mortality after radical nephrectomy in localized clear cell, papillary, and chromophobe RCC [12]. Moreover, long non-coding RNA signature has also been shown to exhibit potential utility in disease prognostication in RCC [13]. There is a great deal of ongoing interest in utilization of molecular markers for risk stratification, disease prognostication, and potentially guiding neoadjuvant or adjuvant systemic therapy in advanced RCC. Currently, however, there exists no validated criteria beyond clinical information and histopathology for risk assessment.
ADJUVANT CYTOKINE THERAPY
Cytokine therapy was first among many classes of therapies to be investigated in the adjuvant setting after nephrectomy. Beginning in the 1980 s, seven key studies investigated the role of adjuvant cytokine-based treatment post nephrectomy. These were the first trials to test immunotherapy strategies in the adjuvant setting. One of the earliest trials by Trump et al. and Porzolt et al. utilized adjuvant lymphoblastoid interferon (IFN) and recombinant IFN-2a in RCC with perinephric fat, renal vein, or inferior vena cava involvement [14, 15]. These therapies did not improve disease-free survival. From the 1990 s to 2000 s, additional studies evaluated the efficacy of IFN-α2b (rIFNα2b) and IFN-NL; both these trials were negative [16, 17].
Interleukin 2 (IL-2) was evaluated in the 2000 s as a potential adjuvant therapy in RCC. In a randomized phase III clinical trial, Clark et al. evaluated high dose bolus IL-2 in patients with high-risk RCC post resection [18]. The primary endpoint of 30% improvement in 2-year disease-free survival was not met [18]. Additional trials investigated IFN in comparison to IL-2 and chemotherapy and all failed to improve outcomes for patients and were associated with increased toxicity [19, 20]. The adjuvant cytokine trials are summarized in Table 2. Currently, there is no role for adjuvant cytokine-based treatments in RCC.
Table 2
Adjuvant cytokine therapy trials in renal cell carcinoma
| Trials | Population | Arms | N | Primary | Hazard Ratio Confidence Interval |
| Trump et al. (1987) | pT3-4aN0 or pTxN1-3 | L-IFN vs. Observation | 294 | Recurrence | No Difference. Hazard Ratio NA |
| Porzsolt et al. (1988) | pT3-4N0 or pTxN1-3 | IFN-α vs. Observation | 270 | TTF/Survival | No Difference. Hazard Ratio NA |
| Clark et al. (1990) | pT3b-4Nx or pTxN1-3 | IL-2 vs. Observation | 138 | 2-year DFS | No Difference. Hazard Ratio NA |
| Pizzocaro et al. (2001) | pT3-4aN0 or pTxN1-3 | IFN-a vs. Observation | 247 | 5-year OS | 1.040 (95% CI, 0.671–1.613) p = 0.861 |
| Messing et al. (2003) | pT3-4aN0 or pTxN1-3 | IFN-α vs. Observation | 283 | 5-year OS | 1.35 (95% CI 0.98–1.36) p = 0.09 |
| Atzpodien et al. (2005) | pT3b-4Nx or pTxN1-3 | IL-2/IFN-a/5-FU vs. Observation | 203 | 2-year DFS | p = 0.2398. Hazard Ratio NA |
| Aitchison et al. (2014) | pT3b-4Nx or pTxNa-2 or +margins/vascular invasion | IL-2/IFN-a/5-FU vs. Observation | 309 | 3-year DFS | 0.87 (95% CI 0.61–1.23) p = 0.428 |
+ margin = positive margin, NA = not available, IFN-α= interferon alpha, L-IFN = lymphoblastoid interferon, IL-2 = interleukin 2, 5-FU = 5 fluorouracil, TTF = time to treatment failure, DFS = disease-free survival, OS = overall survival, vs = versus.
ADJUVANT TARGETED THERAPY
After these negative cytokine trials, there was a hiatus in exploring adjuvant therapy in localized RCC. During this time, tyrosine kinase inhibitors and mammalian target of rapamycin (mTOR) inhibitors began to show efficacy for metastatic RCC. Thus, the next rationale was to test these agents in the adjuvant setting. The adjuvant targeted therapy studies, summarized in Table 3, have produced mixed results. These trials tested the efficacy of adjuvant sunitinib, sorafenib, pazopanib, axinitib and everolimus with the primary endpoint of disease-free survival [21–27]. Of the trials discussed in this domain, the ASSURE and S-TRAC trials are most frequently highlighted in the literature [21, 23–27]. The ASSURE trial was a phase III randomized, double-blind, placebo-controlled study evaluating sunitinib, sorafeninb, and placebo in patients with non-metastatic RCC (including non-clear cell histologies) after complete resection [26]. It was the largest of the adjuvant studies to date. The primary endpoint, disease-free survival, was not met among the treatment arms [26]. Furthermore, subgroup analyses in patients with only clear cell histology or those with pT3 or pN1 disease showed no benefit to treatment [22].
Table 3
Adjuvant tyrosine inhibitor trials in renal cell carcinoma
| Trial | Arms | Years | N | Primary Endpoint | Clear Cell Only | Eligibility | Hazard Ratio Confidence Interval |
| ASSURE | Sunitinib vs. Sorafenib vs. Placebo | 1 | 1943 | DFS | No | pT1bG3-4N0, pT2-4GxN0, TxGxN + | Sunitinib –1.02 (97.5% CI 0.85–1.23), p = 0.8038 Sorafenib –0.97 (97.5% CI 0.80 –1.17), p = 0.7184 |
| S-TRAC | Sunitinib vs. Placebo | 1 | 615 | DFS | Yes | pT3-4GxN0-x, TxGxN1-2 | 0.76 (95% CI 0.59–0.98), p = 0.03 |
| PROTECT | Pazopanib vs. Placebo | 1 | 1538 | DFS | Yes | pT2G3-4N0, pT3-4N0, pTxN1 | 0.86 (95% CI 0.70–1.06), p = 0.165 |
| ATLAS | Axinitib vs. Placebo | 1–3 | 724 | DFS | Yes | pT2-GxN0, pTxN1 | 0.870 (95% CI 0.66–1.147), p = 0.3211 |
| SORCE | Sorafenib vs. Placebo | 1–3 | 1711 | DFS | No | Leibovich scores 3–11 | 1.01 (95% CI 0.83–1.23), p = 0.95 |
| EVEREST | Everolimus vs. Placebo | 1 | 1545 | RFS | No | pT1bG3-4N0 to pT3a G1-2N0, pT3aG2-4 to pT4 G1-4 or N1 | 0.85 (95% CI, 0.72 –1.00); 1-sided p = 0.0246, not significant because p greater than one-sided significance level of 0.022. |
DFS = disease-free survival, RFS = recurrence-free survival, G1-4 = grade 1 to 4, CI = confidence interval, vs = versus.
The S-TRAC trial was the only positive trial of the targeted therapy studies. It was a phase III randomized, double-blind, placebo-controlled study evaluating sunitinib in patients with locoregional high-risk clear cell RCC [23]. The trial was carefully designed and more specific in its patient selection. Unlike the ASSURE trial, which included patients with lower risk tumors, the S-TRAC trial selected for patients with only clear cell histology and high-risk locoregional disease (tumor stage 3 or higher, regional lymph-node metastasis, or both) [23, 26]. The primary endpoint of disease-free survival was longer in the sunitinib treatment arm (median 6.8 vs 5.6 years, Hazard Ratio [HR] 0.76, 95% Confidence Interval [CI] 0.59–0.98; p = 0.03) [23]. However, sunitinib did not result in improvement in overall survival and was associated with increased incidence of grade 3 and 4 adverse events and decreased quality of life [23]. Ultimately, sunitinib was approved by the Food and Drug Administration in the United States [28]. The European Medicines Agency, however, did not approve adjuvant sunitinib. In clinical practice, adjuvant sunitinib is not routinely administered and requires careful shared decision making with patients regarding the risks and benefits of treatment.
Several explanations have been proposed to examine the reasons behind the failures of the adjuvant TKI trials. One, these negative trials were potentially weakened by inclusion of lower risk patients. For example, 34–35% of patients in the treatment arms of ASSURE were AJCC stage I or II [26]. Two, toxicity of TKI required several trials to lower the starting dose of TKI, potentially decreasing efficacy. In SOURCE, only 13% of patients received the full starting dose [21]. Three, it is possible that from a mechanistic standpoint, TKIs alone lack the capability to eradicate micrometastatic disease given the lack of overall survival benefit in these trials. This finding is mirrored in the metastatic setting; for example in poor risk metastatic RCC treated with sunitinib compared to IFN-α, median PFS and OS did not significantly differ between the arms HR = 0.660 (95% CI, 0.360 to 1.207) [29].
ADJUVANT IMMUNO-ONCOLOGY (IO) THERAPY
At present there is a renaissance of immunotherapy for advanced RCC in the form of immune checkpoint inhibitors. Multiple studies have demonstrated the efficacy of immune checkpoint inhibitors either as monotherapy or in combination with vascular endothelial growth factor (VEGF) pathway targeting agents [30–36]. This reinvigorated enthusiasm about testing the role of immunotherapy in the adjuvant setting and led to a series of clinical trials.
The first phase III checkpoint inhibitor trial to be reported was Keynote 564 [37]. In this randomized, double-blind, placebo-controlled trial, enrolled patients had intermediate-high risk (pT2N0M0 grade 4 or sarcomatoid, or pT3N0M0 any grade) or high-risk (pT4N0M0 any grade, or pTxN1M0 any grade) after definitive radical or partial nephrectomy [37]. Additionally, patients with M1 disease resected to no evidence of disease (NED)≤1 year from nephrectomy were also included [37]. These patients were randomized to pembrolizumab 200 mg every 3 weeks for approximately 12 months versus placebo [37]. The primary endpoint was disease-free survival [37]. At a median follow-up of 24.1 months, adjuvant pembrolizumab resulted in improved disease-free survival compared to placebo (77.3% vs 68.1%, HR 0.68, 95% CI 0.53–0.87; p = 0.0010) [37]. Updated analysis after a median follow-up of 30.1 months demonstrated persistent disease-free survival benefit of pembrolizumab (HR 0.63, 95% CI 0.50–0.80) [38]. While overall survival data is still immature given limited events, at the first interim analysis the HR for death was 0.54 (95% CI 0.30–0.96), which has persisted at the 30.1 month follow up at 0.52 (95% CI 0.31–0.86) [37, 38]. Although pembrolizumab was associated with increased rate of grade 3 or 4 adverse events, the investigators noted that the rate of grade 3 to 4 immune-mediated adverse events were low and comparable with previous trials involving pembrolizumab [33, 36, 37]. These results constitute the basis for FDA approval in 2021 of pembrolizumab for adjuvant therapy in RCC at intermediate-high or high risk of recurrence after nephrectomy or with M1 NED post resection [39]. While there has been some adoption of adjuvant pembrolizumab in the clinic, many questions remain including optimizing patient selection for treatment given risk of overtreatment of those at lower risk of recurrence and potentially under treatment of those at highest risk who would otherwise receive combination immunotherapy for metastatic disease. Additionally, additional follow up will be necessary to assess the impact of therapy on overall survival.
Most recently, a number of parallel studies investigating perioperative/adjuvant immune checkpoint blockage were conducted. These trials are summarized in Fig. 1 and Table 4. Currently, the results of these trials are pending or negative [40–42]. IMmotion010 evaluated the role of adjuvant atezolimumab versus placebo in patients with surgically resected pT2 grade 4, pT3a grade 3–4, pT3b-c, pT4a grade 1–4, pTxN1 grade 1–4, pTxpNxpM1 rendered NED by surgery [40]. Unlike pembrolizumab, atezolimumab is a programmed death ligand 1 (PD-L1) monoclonal antibody, and does not have approval in the metastatic setting. Hence the negative results may be partly explained by differing mechanism of action of atezolizumab compared to other PD-1 targeting monoclonal antibodies. CheckMate 914 was a randomized placebo-controlled trial that investigated adjuvant nivolumab as well as adjuvant nivolumab and ipilimumab in the non-metastatic setting in patients with surgically resected pT2a grade 3-4 N0M0, pT2b grade 1–4 N0M0, pT3 grade 1–4 N0M0, pT4 grade 1–4 N0M0, pTxpN1M0 [41]. Patients in this trial were randomized to receive nivolumab 240 mg intravenous every 2 weeks for 12 doses and ipilimumab 1 mg/kg every 6 weeks for four doses (6 months of therapy) [41]. Unlike Keynote 564 and IMmotion 010, patients with M1 NED were not eligible [41]. Over a median follow-up of 37.0 months, the primary efficacy endpoint of disease-free survival by independent review was not met (HR 0.92, 95% CI 0.71–1.19; p = 0.5347) [41]. Median DFS was not reached among patients who received nivolumab and ipilimumab and was 50.7 months among those who received placebo [41]. Landmark 2-year DFS was 76.4% and 74.0%, respectively [41]. Adverse events were also higher in the treatment arm with serious grade≥3 or higher treatment-related adverse events occurring in 28.5% and 2.0% of patients in the treatment and control arm, respectively [41]. There are multiple possible explanations for these study results including selection of patients at lower risk of recurrence, duration of therapy of 6 months alone, and toxicity that may have limited therapy exposure (despite delayed ipilimumab dosing schedule) [41, 43]. Notably, 43% of patients discontinued therapy for all causes and 33% discontinued therapy due to drug toxicity, and this could additionally exert the effect of biasing the results towards the null [43]. This is higher than Keynote 564, which reported 38.9% all-cause discontinuation, 21.3% due to adverse event, and may partly explain the null outcomes in CheckMate 914 [37, 43].
Fig. 1
Phase 3 adjuvant immune checkpoint inhibitor trials for localized or locally advanced renal cell carcinoma.
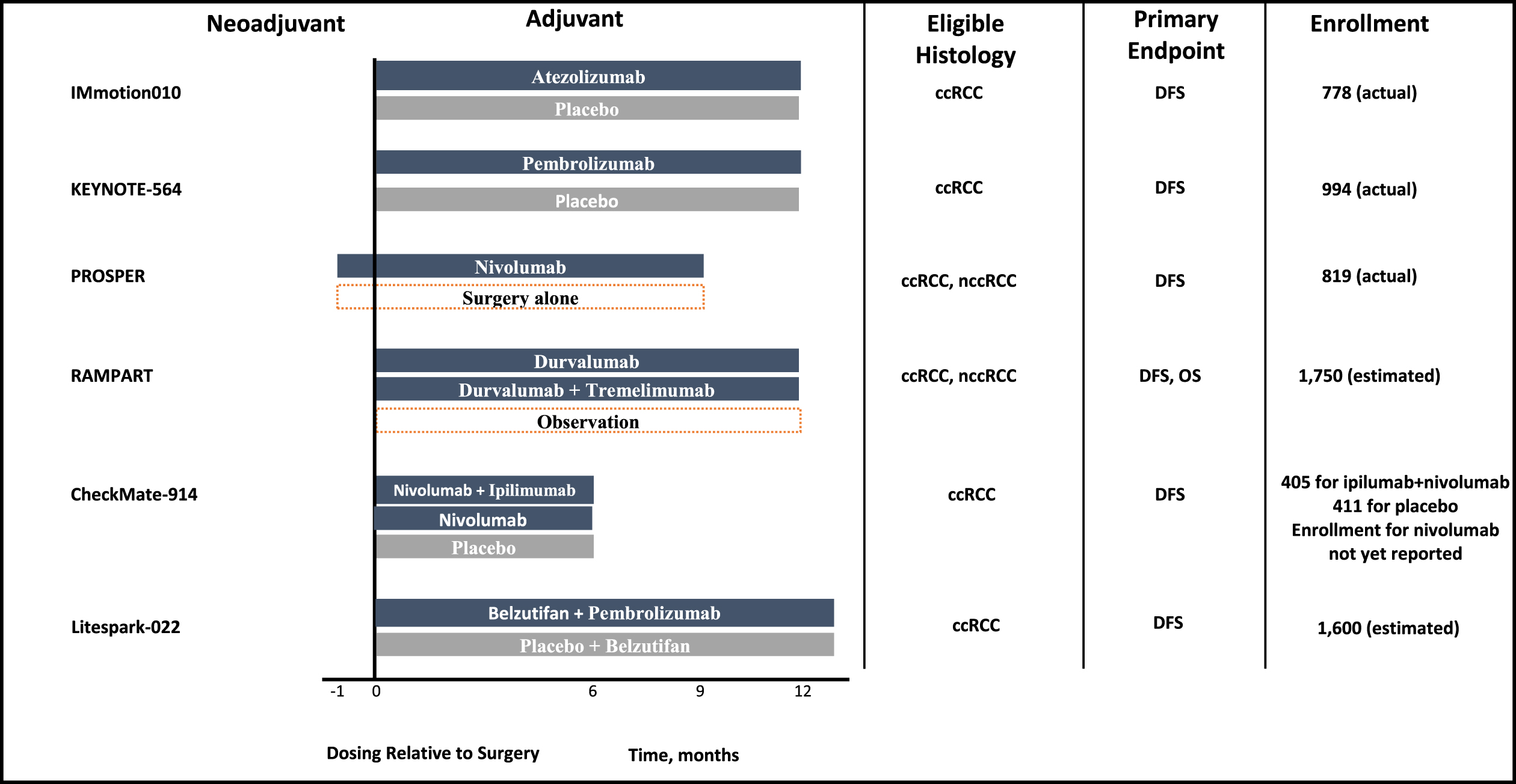
Table 4
Adjuvant immune checkpoint trials for localized or locally advanced renal cell carcinoma
| Trial | Arms | Blinded | Median treatment duration | Median Followup (months) | Primary Endpoint | Clear Cell Only | Surgical Management | Eligibility | Hazard Ratio Confidence Interval |
| IMmotion010 | Atezolimumab vs placebo | Yes | Not specified Planned duration: 16 cycles or 1 year | 44.7 | DFS | No | Not specified | pT2 G4, pT3a G3-4, pT3b-c, pT4a G1-4, pTxN1 G1-4, pTxpNxpM1 NED | 0.93 (95% CI 0.75 –1.15) |
| CheckMate 914 | Nivolumab + Ipilumumab vs Nivolumab vs. Placebo | Yes | 5.1 months | 37.0 | DFS | Yes | Radical or partial nephrectomy with negative margins | pT2a G3-4, pT2b G1-4, pT3 G1-4, pT4 G1-4, pTxpN1M0 | 0.92 (95% CI 0.71–1.19) |
| PROSPER RCC | Neoadjuvant Nivolumab + Adjuvant Nivolumab vs. Surgery + surveillance | No | Planned duration: 1 dose preop + 9 adjuvant doses | N/A | RFS | Yes | Partial or radical nephrectomy | cT2 or higher, cTxcN1. pM1 NED within 12 weeks of surgery | 0.97 (95% CI 0.74–1.28) |
| Keynote 564 | Pembrolizumab vs. Placebo | Yes | Planned duration: 17 cycles or 1 year Median duration: 17 cycles (11.1 months) | 24.1 | DFS | Yes | Nephrectomy | pT2 G4, pT3 G1-4, pT4 G1-4, pTxpN1M0, pM1 NED | 0.68(95% CI 0.53–0.87) |
| RAMPART | Durvalumab vs Durvalumab + tremelimumab vs observation | No | Planned duration (durvalumab): 13 cycles or 1 year Planned duration (tremelimumab): 2 cycles | N/A | DFS | No | Nephrectomy | Leibovich scores 3–11 | Pending |
| Litespark-022 | Belzutifan + pembrolizumab vs placebo + pembrolizumab | Yes | Belzutifan 54 weeks, pembrolizumab 9 cycles | N/A | DFS | Yes | Partial or radical nephrectomy | pT2G4/ sarcomatoid, pT3G1-4, pT4G1-4, pTxpN1M0, pTxpNxpM1 NED | Pending |
vs = versus, DFS = disease-free survival, RFS = recurrence-free survival, NED = no evidence of disease, G1-4 = grade 1 to 4, CI = confidence interval.
PROSPER RCC was a distinct clinical trial from the ones described above given that it included a neoadjuvant component in addition to an adjuvant component [42]. It was an open-label randomized trial that investigated neoadjuvant nivolumab followed by adjuvant nivolumab after surgery compared to surgery alone in patients with clinical stage cT2 or higher, clinically node positive disease, or oligometastatic disease rendered NED within 12 weeks of surgery [42]. In addition to the non-blinded design, patients were enrolled based on clinical rather than pathologic stage, including lower risk cT2 tumors [42]. Furthermore, the trial allowed non-clear cell histologies [42]. This was a negative study demonstrating no benefit in event-free survival with perioperative nivolumab compared to observation (HR 0.97, 95% CI 0.74–1.28; p = 0.43) [42]. There are a number of factors that may have influenced the results of this trial, including: (1) the study including patients based on clinical stage as opposed to pathologic stage, (2) the study being open label, (3) no blinded radiology review, (4) imbalance in delays during the biopsy portion of the trial, (5) toxicity in response to starting nivolumab preoperatively, (6) not all patients undergoing surgery, (7) surgical complications and other potential issues with restarting nivolumab after surgery, (8) possibility of inadequate duration of neoadjuvant nivolumab (1 cycle), (9) inclusion of non-clear cell histology (13% of cohort), and (10) relatively short median followup of 16 months [43]. While nivolumab has a role as monotherapy or in combination with either nivolumab or cabozantinib for patients with advanced disease, the results of the Checkmate 914 and Prosper trials highlight that there is no current role for nivolumab with or without ipilimumab in the adjuvant setting.
Hence the encouraging results from Keynote 564 are juxtaposed by a sea of negative parallel trials. These negative trials also raise the question of whether adjuvant immunotherapy was ineffective in these trials due to study design or due to factors intrinsic to the biology of adjuvant immunotherapy in RCC. The negative results from IMotion 010, the only one among these that used an antibody against PD-L1 raises the possibility that inhibitors to PD-L1 are potentially less effective in RCC. This is buttressed by results of Keynote 564 for pembrolizumab, an antibody to PD-1, and subanalysis of CheckMate 914, which showed activity of nivolumab, an antibody against PD-1, in the sarcomatoid subgroup 0.29 (95% CI 0.09–0.91) [43].
In addition to the aforementioned technicalities in study design that may have biased results towards the null hypothesis, an additional aspect worth mentioning is the inclusion of patients with pTxpNxpM1 rendered NED by surgery. One concern this raises is the possibility that RCC that develop metastasis are biologically more similar to metastatic RCC, and that this difference remains even after surgical consolidation of all metastatic sites. One might find some support for this hypothesis based on the updated 30-month follow-up data for Keynote 564, which showed that the greatest benefit in disease-free survival was seen in the pTxpNxpM1cohort rendered NED by surgery (HR 0.28, 95% CI 0.12–0.66) [38]. A corollary to this hypothesis is the possibility of undertreatment of this pTxpNxpM1cohort. For overt metastatic disease, the standard of care is dual immunotherapy-based treatment. While these are the patients that seem to derive benefit from adjuvant pembrolizumab monotherapy, given high risk of recurrence, there is concern for potential undertreatment as these patients, if overtly metastatic, would receive therapy escalation.
While the positive results from Keynote 564 would suggest that the results may be related to trial design, we cannot neglect the fact that we do not fully understand the biology of tumor recurrence in RCC and the role adjuvant immunotherapy in intercepting this process. One hypothesis that arises from considering these negative trials is that presence of tumor in situ exerts a priming effect on immunotherapy and thus renders them more biologically efficacious. Under this hypothesis, complete resection of all tumor requisite in these adjuvant trials and in clinical practice results in an inhibitory effect on the collective efficacy of adjuvant immunotherapy. Another hypothesis is that there are ever-present factors fueling cancer recurrence in high-risk RCC after definitive surgery such that adjuvant therapy needs to be given for an extended period in order to render a meaningful therapeutic response.
These aforementioned negative studies also raise the possibility that the null hypothesis is in fact valid –that adjuvant immunotherapy does not exert a significant impact in the non-metastatic setting. The overall survival data is still immature for Keynote 564. The corollary is that the presence of visible tumor in situ is a prerequisite for immune checkpoint inhibitors to exert their effects, and that circulating microscopic disease after nephrectomy is insufficient to illicit an immune response towards existing circulating microscopic disease. This hypothesis could suggest that neoadjuvant therapy may generate a stronger tumor response compared to adjuvant therapy. This is supported by recent published results of SWOG S1801, where we see significantly improved event-free survival in patients with advanced melanoma randomized to neoadjuvant pembrolizumab compared to adjuvant pembrolizumab HR 0.59 (95% CI 0.40–0.86), p = 0.0015 [44].
Additionally, it is well known that cytotoxic T lymphocytes require priming by tumor associated antigens on antigen presenting cells [45]. However, most tumor associated antigens are intracellular, rendering them difficult to target [45]. Thus the exposure of the immune system to these tumor associated antigens seems to be a key event in stimulating a humoral response to tumor in vivo. This line of reasoning is one of the key drivers behind (1) the development of cancer vaccines, whereby antigens are introduced from an external source, and (2) utilizing radiation to release tumor antigens to parallel the effects of a vaccine [45, 46]. Under this hypothesis, the absence of visible tumor in these adjuvant RCC trials would significantly attenuate antigen presentation and thus may be postulated to shed light on biologic mechanisms underlying the relative inefficacy of these adjuvant trials.
Biomarkers to better select patients for adjuvant therapy remain elusive. Several are currently under investigation to inform prognosis and potentially also predict response to therapy and include tissue, blood and urine based tests. These include molecular DNA markers and RNA signatures from tissue and tissue pathologic assessment through artificial intelligence/machine learning algorithms. Blood based tests including circulating tumor DNA (ctDNA) and circulating tumor cell (CTC) have been developed for other malignancies and have potential application to RCC. Additionally exploratory urine DNA assays are being developed to understand recurrence risk in RCC.
There is now significant interest in molecular characterization of RCC tumors. Perhaps one of the most important updates in this domain by Motzer et al. highlighted the potential of transcriptomic analysis of RCC tumors into 7 subtypes, whereby tumors with angiogenesis RNA profile showed response to angiogenesis inhibitors and those with angiogenesis-poor and immune-rich profiles exhibited poor response to angiogenesis inhibitors but significant response to immune checkpoint inhibition [47]. Additionally, there is emerging interest in utilization of circulating tumor DNA (ctDNA) in RCC. This is based on promising results from its utilization in urothelial carcinoma, which showed that patients with positive ctDNA after cystectomy had improved disease-free survival and overall survival in response to adjuvant atezolizumab compared to observation [48]. In addition to circulating tumor DNA, identification of circulating tumor cells could potentially be utilized in a similar manner for risk stratification of high risk RCC after nephrectomy. Most recently, it has been shown in a longitudinal study of metastatic RCC on systemic therapy, decreased quantity of detectable circulating tumor cells correlated with radiographic tumor response [49]. Translational correlates such as these have potential to play a significant role in future adjuvant therapy trials to facilitate tumor-specific therapy selection.
Based on the results of existing adjuvant therapy trials, the ideal adjuvant therapy remains elusive at this time. Such therapy would not only delay but prevent recurrences, would be easy to administer, would be associated with limited toxicity, with maintenance of quality of life. Currently, pembrolizumab is being investigated in combination with belzutifan, a hypoxia-inducible factor 2 alpha inhibitor as adjuvant therapy in a randomized, double-blind, placebo-controlled trial of patients with clear cell RCC after nephrectomy [50]. This trial includes patients with pT2 grade 4 or sarcomatoid, pT3 grade 1–4, pT4 grade 1–4, pTxpN1M0, and pTxpNxpM1 rendered NED by surgery [50]. Additionally, durvalumab, a novel antibody against PD-L1, in combination with tremelimumab, a novel antibody against cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), are being investigated in a phase III randomized multi-arm multi-stage controlled trial (RAMPART) against active monitoring and durvalumab monotherapy in clear cell and non-clear cell RCC with Leibovich score 3–11 after surgery [51]. These trials are currently enrolling in centers around the world.
CONCLUSION
We have been investigating potential adjuvant therapies for high risk local or locally advanced RCC for the past 40 years. This sustained effort has yielded two positive trials that were associated with improved disease-free survival. While adjuvant sunitinib was not associated with improved overall survival, the data on overall survival are still maturing for adjuvant pembrolizumab. Should adjuvant pembrolizumab lead to improved overall survival, this would likely exert a transformational effect on the way we manage localized high-risk, locally advanced, and oligometastatic RCC, perhaps augment overall patient survival for this disease. In light of Keynote 564, the recently released results from the adjuvant trials highlight our inadequate understanding of how exactly kidney cancer recurs on a biologic level, and perhaps the need for future trials to be more stringent in selection of truly high risk RCC tumors for therapy.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
Study conception and design: Luke Wang, Rana McKay.
Data collection: Luke Wang, Rana McKay.
Analysis and interpretation: All authors.
Draft manuscript preparation: All authors.
All authors reviewed the results and approved the final version of the manuscript.
CONFLICT OF INTEREST
Luke Wang, Ava Saidian, Elizabeth Pan, and Justine Panian have no conflicts of interest to report.
Rana McKay has the following conflicts of interest:
Advisory board/consultant: Aveo, AstraZeneca, Bayer, Bristol Myers Squibb, Calithera, Caris, Dendreon, Exelixis, Janssen, Lilly, Merck, Myovant, Novartis, Pfizer, Sanofi, Sorrento Therapeutics, Tempus, Telix. Research support from Astrazenca, Bayer, Tempus, Oncternal.
REFERENCES
[1] | Key Statistics About Kidney Cancer. American Cancer Society. https://www.cancer.org/cancer/kidney-cancer/about/key-statistics.html. Published 2022. Accessed September 12, 2022. |
[2] | Kane CJ , Mallin K , Ritchey J , Cooperberg MR , Carroll PR . Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer. (2008) ;113: (1):78–83. |
[3] | Haifler M , Neheman A , Zisman A . Has Stage Migration in Renal Cancer Run Its Course? A SEER Database Analysis. Clin Genitourin Cancer. (2020) ;18: (4):e368–e373. |
[4] | Klaassen Z . ASCO 2021: Pembrolizumab plus Axitinib Versus Sunitinib as First-Line Therapy for Advanced Clear Cell Renal Cell Carcinoma: Results from 42-Month Followup ofKEYNOTE-426ASCO2021 Kidney CancerWeb site. https://www.urotoday.com/conference-highlights/asco-2021/asco-2021-kidney-cancer/130133-asco-2021-pembrolizumab-plus-axitinib-versus-sunitinib-as-first-line-therapy-for-advanced-clear-cell-renal-cell-carcinoma-results-from-42-month-follow-up-of-keynote-426.html |
[5] | Speed JM , Trinh QD , Choueiri TK , Sun M . Recurrence in Localized Renal Cell Carcinoma: a Systematic Review of Contemporary Data. Curr Urol Rep. (2017) ;18: (2):15. |
[6] | Hafez KS , Novick AC , Campbell SC . Patterns of tumor recurrence and guidelines for followup after nephron sparing surgery for sporadic renal cell carcinoma. J Urol. (1997) ;157: (6):2067–70. |
[7] | Terrone C , Cracco C , Porpiglia F , et al. Reassessing the current TNM lymph node staging for renal cell carcinoma. Eur Urol. (2006) ;49: (2):324–31. |
[8] | Tsui KH , Shvarts O , Smith RB , Figlin RA , deKernion JB , Belldegrun A . Prognostic indicators for renal cell carcinoma: a multivariate analysis of 643 patients using the revised 1997 TNM staging criteria. J Urol. (2000) ;163: (4):1090–5; quiz 1295. |
[9] | Kattan MW , Sternberg CN , Mehmud F , Bhatt K , McCann L , Motzer RJ . Development and Validation of a Prognostic Nomogram for Progression-Free Survival in Patients with Advanced Renal Cell Carcinoma Treated with Pazopanib. Oncology. (2015) ;89: (4):235–41. |
[10] | Leibovich BC , Blute ML , Cheville JC , et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. (2003) ;97: (7):1663–71. |
[11] | Brooks SA , Brannon AR , Parker JS , et al. ClearCode A prognostic risk predictor for localized clear cell renal cell carcinoma. Eur Urol. (2014) ;66: (1):77–84. |
[12] | Morgan TM , Mehra R , Tiemeny P , et al. A Multigene Signature Based on Cell Cycle Proliferation Improves Prediction of Mortality Within 5 Yr of Radical Nephrectomy for Renal Cell Carcinoma. Eur Urol. (2018) ;73: (5):763–9. |
[13] | Qu L , Wang ZL , Chen Q , et al. Prognostic Value of a Long Non-coding RNA Signature in Localized Clear Cell Renal Cell Carcinoma. Eur Urol. (2018) ;74: (6):756–63. |
[14] | Porzsolt F , Messerer D , Hautmann R , et al. Treatment of advanced renal cell cancer with recombinant interferon alpha as a single agent and in combination with medroxyprogesterone acetate. A randomized multicenter trial. J Cancer Res Clin Oncol. (1988) ;114: (1):95–100. |
[15] | Trump DL , Elson PJ , Borden EC , et al. High-dose lymphoblastoid interferon in advanced renal cell carcinoma: an Eastern Cooperative Oncology Group Study. Cancer Treat Rep. (1987) ;71: (2):165–9. |
[16] | Pizzocaro G , Piva L , Colavita M , et al. Interferon adjuvant to radical nephrectomy in Robson stages II and III renal cell carcinoma: a multicentric randomized study. J Clin Oncol. (2001) ;19: (2):425–31. |
[17] | Messing EM , Manola J , Wilding G , et al. Phase III study of interferon alfa-NL as adjuvant treatment for resectable renal cell carcinoma: an Eastern Cooperative Oncology Group/Intergroup trial. J Clin Oncol. (2003) ;21: (7):1214–22. |
[18] | Clark JW , Smith JW , 2nd, Steis RG , et al. Interleukin 2 and lymphokine-activated killer cell therapy: analysis of a bolus interleukin 2 and a continuous infusion interleukin 2 regimen. Cancer Res. (1990) ;50: (22):7343–50. |
[19] | Atzpodien J , Schmitt E , Gertenbach U , et al. Adjuvant treatment with interleukin-2- and interferon-alpha2a-based chemoimmunotherapy in renal cell carcinoma post tumour nephrectomy: results of a prospectively randomised trial of the German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN). Br J Cancer. (2005) ;92: (5):843–6. |
[20] | Aitchison M , Bray CA , Van Poppel H , et al. Adjuvant 5-flurouracil, alpha-interferon and interleukin-2 versus observation in patients at high risk of recurrence after nephrectomy for renal cell carcinoma: results of a phase III randomised European Organisation for Research and Treatment of Cancer (Genito-Urinary Cancers Group)/National Cancer Research Institute trial. Eur J Cancer. (2014) ;50: (1):70–7. |
[21] | Eisen T , Frangou E , Oza B , et al. Adjuvant Sorafenib for Renal Cell Carcinoma at Intermediate or High Risk of Relapse: Results From the SORCE Randomized Phase III Intergroup Trial. J Clin Oncol. (2020) ;38: (34):4064–75. |
[22] | Haas NB , Manola J , Dutcher JP , et al. Adjuvant Treatment for High-Risk Clear Cell Renal Cancer: Updated Results of a High-Risk Subset of the ASSURE Randomized Trial. JAMA Oncol. (2017) ;3: (9):1249–52. |
[23] | Ravaud A , Motzer RJ , Pandha HS , et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. N Engl J Med. (2016) ;375: (23):2246–54. |
[24] | Motzer RJ , Haas NB , Donskov F , et al. Randomized Phase III Trial of Adjuvant Pazopanib Versus Placebo After Nephrectomy in Patients With Localized or Locally Advanced Renal Cell Carcinoma. J Clin Oncol. (2017) ;35: (35):3916–23. |
[25] | Gross-Goupil M , Kwon TG , Eto M , et al. Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: results from the phase III, randomized ATLAS trial. Ann Oncol. (2018) ;29: (12):2371–78. |
[26] | Haas NB , Manola J , Uzzo RG , et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN Ea double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. (2016) ;387: (10032):2008–16. |
[27] | Ryan Cea . EVEREST: Everolimus for renal cancer ensuing surgical therapy—A phase III study (SWOG S0931, NCT01120249). 2022. https://ascopubs.org/doi/abs/10.1200/JCO.2022.40.17_suppl.LBA4500. Accessed September 20, 2022. |
[28] | Administration FaD. FDA approves sunitinib malate for adjuvant treatment of renal cell carcinoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-sunitinib-malate-adjuvant-treatment-renal-cell-carcinoma. Published 2017. Accessed September 17, 2022. |
[29] | Motzer RJ , Hutson TE , Tomczak P , et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. (2009) ;27: (22):3584–90. |
[30] | Motzer RJ , Escudier B , McDermott DF , et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. (2015) ;373: (19):1803–13. |
[31] | McDermott DF , Lee JL , Bjarnason GA , et al. Open-Label, Single-Arm Phase II Study of Pembrolizumab Monotherapy as First-Line Therapy in Patients With Advanced Clear Cell Renal Cell Carcinoma. J Clin Oncol. (2021) ;39: (9):1020–8. |
[32] | Motzer RJ , Choueiri TK , McDermott DF , et al. Biomarker analysis from CheckMate 214: nivolumab plus ipilimumab versus sunitinib in renal cell carcinoma. J Immunother Cancer. (2022) ;10: (3). |
[33] | Rini BI , Plimack ER , Stus V , et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. (2019) ;380: (12):1116–27. |
[34] | Motzer RJ , Penkov K , Haanen J , et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. (2019) ;380: (12):1103–15. |
[35] | Choueiri TK , Powles T , Burotto M , et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. (2021) ;384: (9):829–41. |
[36] | Motzer R , Alekseev B , Rha SY , et al. Lenvatinib plus Pembrolizumabor Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med. (2021) ;384: (14):1289–300. |
[37] | Choueiri TK , Tomczak P , Park SH , et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. N Engl J Med. (2021) ;385: (8):683–94. |
[38] | Powles T , Tomczak P , Park SH , et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2022) ;23: (9):1133–44. |
[39] | Administration FaD. FDA approves pembrolizumab for adjuvant treatment of renal cell carcinoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-adjuvant-treatment-renal-cell-carcinoma. Published 2021. Accessed September 17, 2021. |
[40] | al BAe. LBA66 - IMmotion010: Efficacy and safety from the phase III study of atezolizumab (atezo) vs placebo (pbo) as adjuvant therapy in patients with renal cell carcinoma (RCC) at increased risk of recurrence after resection. 2022. https://oncologypro.esmo.org/meeting-resources/esmo-congress/immotion010-efficacy-and-safety-from-the-phase-iii-study-of-atezolizumab-atezo-vs-placebo-pbo-as-adjuvant-therapy-in-patients-with-renal-cell. Published September 10, 2022. |
[41] | Motzer R , Russo P , Gruenwald V , et al. LBA4 - Adjuvant nivolumab plus ipilimumab (NIVO+IPI) vs placebo (PBO) for localized renal cell carcinoma (RCC) at high risk of relapse after nephrectomy: Results from the randomized, phase III CheckMate 914 trial. 2022. https://oncologypro.esmo.org/meeting-resources/esmo-congress/adjuvant-nivolumab-plus-ipilimumab-nivo-ipi-vs-placebo-pbo-for-localized-renal-cell-carcinoma-rcc-at-high-risk-of-relapse-after-nephrectomy. Published September 11, 2022. |
[42] | Allaf Mea. LBA67 - Phase III randomized study comparing perioperative nivolumab (nivo) versus observation in patients (Pts) with renal cell carcinoma (RCC) undergoing nephrectomy (PROSPER, ECOG-ACRIN EA8143), a National Clinical Trials Network trial. 2022. https://oncologypro.esmo.org/meeting-resources/esmo-congress/phase-iii-randomized-study-comparing-perioperative-nivolumab-nivo-versus-observation-in-patients-pts-with-renal-cell-carcinoma-rcc-undergoing. Published September 10, 2022. |
[43] | Bedke J , Albiges L , Capitanio U , et al. The 2022 Updated European Association of Urology Guidelines on the Use of Adjuvant Immune Checkpoint Inhibitor Therapy for Renal Cell Carcinoma. European Urology. 2022; In Press. |
[44] | Patel S , Othus M , Prieto V , et al. LBA6 - Neoadjvuant versus adjuvant pembrolizumab for resected stage III-IV melanoma (SWOG S1801). Annals of Oncology. (2022) ;33: :S808–S869. |
[45] | Lin MJ , Svensson-Arvelund J , Lubitz GS , et al. Cancer vaccines: the next immunotherapy frontier. Nat Cancer. (2022) ;3: (8):911–26. |
[46] | Kaur P , Asea A . Radiation-induced effects and the immune system in cancer. Front Oncol. (2012) ;2: :191. |
[47] | Motzer RJ , Banchereau R , Hamidi H , et al. Molecular Subsets in Renal Cancer Determine Outcome to Checkpoint and Angiogenesis Blockade. Cancer Cell. (2020) ;38: (6):803–817 e804. |
[48] | Powles T , Assaf ZJ , Davarpanah N , et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature. (2021) ;595: (7867):432–7. |
[49] | Bootsma M , McKay RR , Emamekhoo H , et al. Longitudinal Molecular Profiling of Circulating Tumor Cells in Metastatic Renal Cell Carcinoma. J Clin Oncol. (2022) ;40: (31):3633–41. |
[50] | LLC MSD. A Study of Belzutifan (MK-6482) Plus Pembrolizumab (MK-3475) Versus Placebo Plus Pembrolizumab in Participants With Clear Cell Renal Cell Carcinoma Post Nephrectomy (MK-6482-022). Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT05239728. Published 2022. Accessed September 20, 2022. |
[51] | Renal Adjuvant MultiPle Arm Randomised Trial (RAMPART). ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT03288532 Published 2022. Accessed December 4, 2022. |




