Activity of Immunotherapy Regimens on Primary Renal Tumours: A Systematic Review
Abstract
BACKGROUND:
Immune checkpoint inhibitors (ICPIs) are widely used in treating metastatic renal cell carcinoma (RCC). Cytoreductive nephrectomy (CN) forms part of multimodality treatment in advanced disease, however there is no prospective evidence for its use in the ICPI era. Trials of neoadjuvant ICPIs in RCC are underway; understanding the anticipated effect of ICPIs on the primary tumour may help clinical decision making in both localised and advanced settings.
METHODS:
A systematic search (PubMed, Web of Science, clinicaltrials.gov) of English literature from 2012 to 2022 was performed according to PRISMA guidelines. 2,398 records were identified, 54 were included in the analysis.
RESULTS:
In the metastatic setting, response in the primary tumour (≥30% reduction in size) is seen in 33–56% of patients treated with dual ICPI or ICPI + VEGFR-TKI. Pathological complete response rates were 14% for patients undergoing CN after a period of ICPI therapy. In the neoadjuvant setting there is a single published trial of VEGFR-TKI + ICPI, 30% of patients had a≥30% reduction in size of the primary. This appears superior to single agent ICPI. Grade 3 adverse event rates are comparable to the metastatic setting.
CONCLUSIONS:
A period of ICPI combination therapy followed by nephrectomy may be considered for selected patients as a strategy to manage metastatic disease. In the neoadjuvant setting, it is not clear whether ICPI + VEGFR-TKI is superior to VEGFR-TKI alone. There is minimal data on whether either CN after ICPI in metastatic patients, or neoadjuvant ICPI therapy for localised disease, improves long term survival.
INTRODUCTION
Immunotherapy is now firmly established in the treatment of renal cell cancer (RCC). Phase 3 clinical trial data supports its use in dual immune checkpoint inhibitor (ICPI) combinations [1] or in combination with small molecule vascular endothelial growth factor receptor tyrosine kinase inhibitors (VEGFR-TKIs) [2–5]. It is approved for both first line and subsequent lines of treatment in the metastatic setting [1, 2, 6, 7], and as adjuvant therapy following curative surgery [8, 9].
The clinical benefit of removing the primary tumour for patients with metastatic disease, termed cytoreductive nephrectomy (CN), remains uncertain. In the era of cytokine-based treatment, trial data showed that adding CN to interferon alpha therapy improved survival, and this was considered standard of care [10]. Additionally, there may be palliative benefit in removing the primary to reduce pain, bleeding, or paraneoplastic phenomena such as hypercalcaemia [11]. Following the success of VEGFR-TKIs for treatment of metastatic RCC, the CARMENA and SURTIME trials examined whether the addition of CN to sunitinib improved outcomes, however the results indicated that up-front CN confers no benefit for these patients, and this is no longer considered standard of care for patients needing systemic treatment [12, 13].
The treatment landscape for RCC has moved on considerably since the results of CARMENA and SURTIME with the advent of ICPIs [1, 2, 7] and second generation VEGFR TKIs [14–16]. This has revived the questions surrounding the role of CN: do patients treated with immunotherapy benefit from CN, and if so, in what sequence should it be performed relative to starting ICPI treatment? Recent ASCO guidelines suggest that CN may be offered to selected patients based on International Metastatic RCC Database Consortium (IMDC) risk scoring and clinician assessment [17]. ESMO guidelines also recommend CN for good performance status (PS) patients who do not need immediate systemic therapy [18]. There is retrospective data indicating a benefit for CN in patients not needing immediate TKI therapy, a group not included in CARMENA [19]. However, practice varies considerably between centres and countries and these recommendations are based on evidence from VEGFR-TKI treated patients. Phase III trials of CN with immunotherapy are underway, however they require large numbers of patients and are some time from reporting results. At present, a proportion of patients are pragmatically managed with up-front or delayed CN in combination with immunotherapy.
For clinicians assessing patients for suitability for CN in the era of ICPI therapy, a key consideration is the anticipated effect of ICPI on the primary tumour. This is particularly relevant in clinical situations when patients may be suitable for a ‘delayed CN’, after a period of ICPI therapy. Examples are where immediate control of metastatic disease is needed, so up-front surgery would be detrimental to the patient, and where the primary tumour is initially inoperable but may be down-staged by ICPI. In the absence of prospective trial data for management of these cases, useful information might be gained from existing publications regarding the effect of ICPI on primary renal tumours.
The question of the effect of ICPI on the primary tumour is becoming increasingly relevant to patients without metastatic disease. Promising data have been reported in for Pembrolizumab in reducing the risk of recurrence for high-risk patients [8, 9]. This has led to change in practice with international approval of Pembrolizumab monotherapy in the adjuvant setting. However, Immotion 010 (Atezolizumab), Checkmate 914 (Ipilimumab + Nivolumab) and PROSPER (neoadjuvant and adjuvant Nivolumab) have all recently reported negative results, which makes the utility of adjuvant ICPI less certain [20–22]. The RAMPART Trial (adjuvant Durvalumab + Tremelimumab) is still ongoing [23].
Neoadjuvant treatments (systemic therapy given before surgery to potentially operable patients) may offer advantages over adjuvant therapy: in addition to long term survival benefits there may be improvements in surgical outcomes and perioperative morbidity [24]. Demonstrating an overall survival benefit can be challenging due to the numbers needed for recruitment or the influence of treatment on progression, so neoadjuvant trials often use surrogate end points such as primary tumour response [25–27]. A potential downside to the neoadjuvant approach is treatment related toxicity that may complicate the surgical management. Based on initial results, combination neoadjuvant treatment with VEGFR-TKI and ICPI appears safe and effective [27], but full results are pending, and many other trials are active in this area. In the meantime, understanding the anticipated effects of ICPI on the primary tumour from existing data may aid clinical decision making and neoadjuvant trial designs.
We performed a systematic literature review to assess the current evidence relating to the effect of immunotherapy on the primary tumour in these clinical settings.
Aims of the review:
(1) Assess the activity of immunotherapy on the primary tumour for patients with metastatic disease.
(2) Assess the activity of immunotherapy on the primary tumour for patients receiving treatment in the neoadjuvant setting
(3) Review toxicities of treatment in the pre-operative setting
(4) Summarise relevant active clinical trials in the pre-operative setting
METHODS
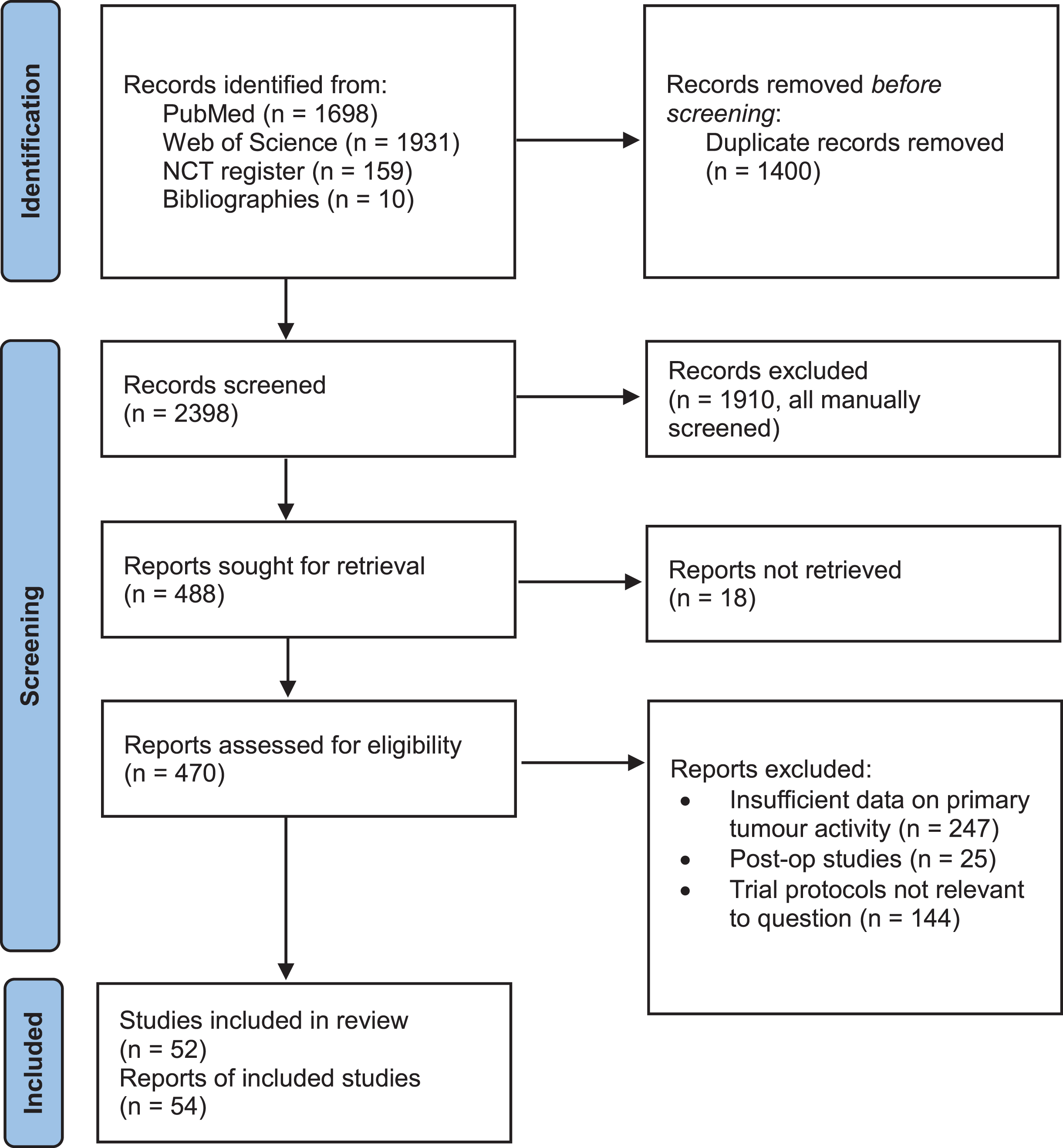
A systematic literature review was conducted. The review process is summarised in a PRISMA diagram (Fig. 1) [28].
Fig. 1
PRISMA flow diagram illustrating the systematic review process. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71.
The National Library of Medicine (PubMed) and Web of Science databases were searched for the following terms:
(“kidney cancer” OR “renal cell carcinoma” OR “renal cell cancer”) AND (metastatic OR advanced) AND (immunotherapy)
(“kidney cancer” OR “renal cell carcinoma” OR “renal cell cancer”) AND (neoadjuvant OR perioperative) AND (immunotherapy)
The criteria for including studies were interventional clinical studies of adult patients with kidney cancer, reporting data on the effects of immunotherapy on the primary tumour. Both clear cell RCC (ccRCC) and non-clear cell RCC (non-ccRCC) were included.
A date range of January 2012 –May 2022 was used for screening. The rationale for the start date of the window was that the key phase 1 trials showing clinical effect of PD-1 and PD-L1 directed therapies were published in 2012 [29, 30], thus defining the era of combination immunotherapy, and also to screen at least the last 10 years’ worth of publications. Both full articles and published abstracts were included in the screen.
Records were first screened to remove duplicates. This list was then screened to identify primary clinical studies of immunotherapy in adult renal cancer for further review. Reasons for excluding records at the screening stage included: review articles/opinion pieces, educational presentations, pre-clinical studies, studies not relating to RCC, paediatric studies, and observational studies not reporting the effect of immunotherapy. Non-English language reports were also not included.
The remaining studies were then reviewed in depth for data on the primary tumour, with those that did not report adequate data on the primary tumour excluded at this stage. 18 records could not be accessed for in depth review.
For eligible studies, data on number of evaluable participants, IMDC risk, intervention, primary tumour response by radiological and pathological assessment, systemic tumour radiological response, time on treatment before surgery, and grade 3+ adverse event rate were collected.
Additionally, we screened the clinical trials.gov database for active interventional trials in adult patients in the pre-operative setting. (search terms: “Kidney cancer AND Immunotherapy”, (128 records) “Kidney cancer AND Neoadjuvant” (31 records); filters applied: Recruiting, Not yet recruiting, Active, Not recruiting, Enrolling by invitation, Unknown status, Interventional Studies, Adult, Older Adult). Both metastatic and neoadjuvant trials were included. The main reasons for excluding trials were as follows: Non-interventional studies, trials not assessing immunotherapy, and trials not assessing effects on the primary. For these protocols we collected the intervention and primary outcome measure.
Studies were grouped into completed studies with palliative treatment intent, completed studies neoadjuvant treatment intent, and ongoing clinical studies for the analysis.
RESULTS
Studies identified by systematic review process
The review process and outputs are summarised in a PRISMA diagram (Fig. 1) [28]. 2236 discrete records were identified following removal of duplicates. An additional 9 publications were identified from citations within papers during the review process. From these studies, 335 primary research publications on adult renal cancer were identified. Of these, 38 studies reported sufficient data on effects on the primary tumour to be included in the review. Our search of the clinical trials.gov database identified 152 discrete records, from which we identified 15 relevant trials. A further trial was also identified from the literature search and included in the review. In total, 54 reports or trial registrations were identified, relating to 52 distinct studies which were included in the synthesis of evidence.
Effect of immunotherapy on the primary in the metastatic setting
We identified 14 studies that have addressed the effect of immunotherapy on the primary tumour in patients with metastatic RCC, where treatment was given with palliative intent [31–44]. The studies included a variety of treatment combinations: single agent immunotherapy [2], dual immunotherapy [6], and immunotherapy in combination with VEGFR-TKIs [2]. Five of the studies reported data from pooled groups without specific data on each treatment combination. Data were available for both clear cell RCC (ccRCC) and non-clear cell RCC (non-ccRCC) subtypes. The trials included mainly IMDC poor risk (range 26–69%) and intermediate risk (range 31–64%) patients, with fewer favourable risk patients (range 0–10%). These studies are summarised in Table S1.
Radiological Response Assessments
Nine studies reported data on radiological response in the primary tumour [32, 33, 36, 39–44]. All defined a partial response in the primary tumour (PR-primary) as≥30% reduction in size from baseline. Seven used RECIST version 1.1 to assess this, one used the maximal tumour dimension [36], and one used a volumetric method [40]; we felt that the methods were broadly comparable. The results are summarised in Fig. 2.
Fig. 2
Response rates are summarised in the primary tumour and metastatic disease. Numbers of patients and % clear cell RCC subtype are indicated, where the data was available.
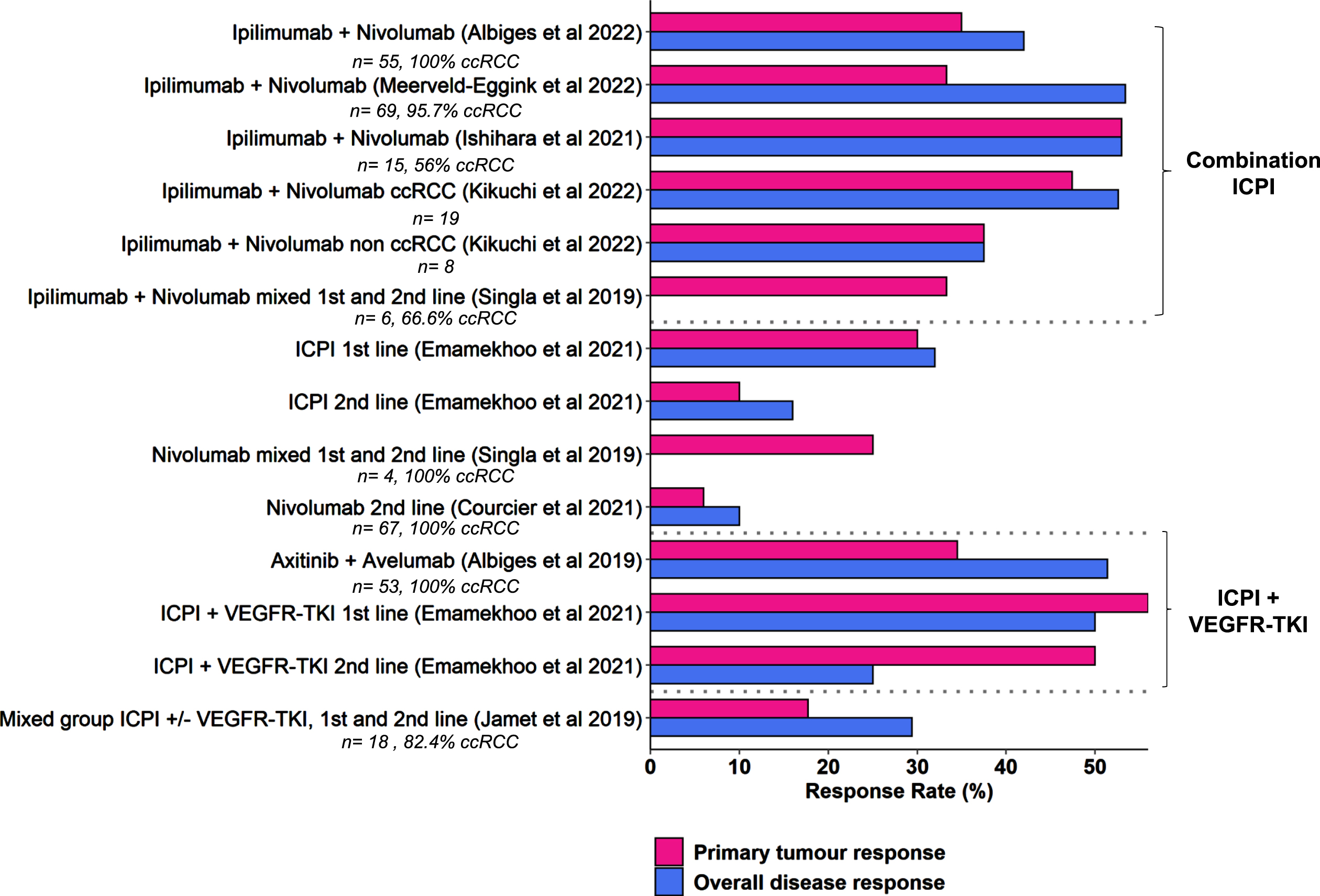
One study provided specific data on the use of single agent nivolumab in four patients (one first line, three second line or greater), one of whom had a response in the primary tumour (25% PR-primary rate) [35]. A further study reported the use of single agent nivolumab in the second line setting, with a 6% PR-primary rate, compared to 10% systemic objective response rate (ORR) [41]. Five studies reported specific data for the combination of Ipilimumab and Nivolumab, with 33.3–53% PR-primary rate [32, 36, 39, 42, 44]. The ORR for systemic disease was approximately 50% with this combination. One smaller study reported data for both clear cell and non-clear cell RCC, which had similar PR-primary rates [36]. Where reported, the median time to response in the primary ranged from 2.8 –4.8 months [32, 39, 42]. Data specific to IO-TKI combinations were reported by two studies, with PR-primary rates of 34.5% and 56% in the first line setting [40, 43]. The median time to response was reported by one study as 4.4 months [43]. A final study reported a mixed group of patients treated with ICPI or ICPI + VEGFR-TKI in the first- or second-line setting, PR-primary rates were accordingly lower (17.7%) [33]. Several studies commented on the good correlation between primary and secondary tumour responses [32, 41, 43], however this was not universal, with some patients exhibiting differing response in the primary and metastatic sites [33].
Pathological response assessment
Eight studies reported histopathological results for patients that had CN after a period of ICPI therapy [31, 34–39, 44]. These results are summarised in Fig. 3. The duration of preoperative ICPI therapy varied, with medians quoted between 3 and 13 months. Four studies provided specific data for Ipilimumab + Nivolumab: the pathological complete response (pCR) rates ranged from 0.0 –50% [35, 36, 38, 39]. Three of the studies reported data for both patients that did and did not undergo CN, showing that a relatively small proportion of patients who start ICPI go on to have CN (16–26%) [36, 38, 39].
Fig. 3
Pathological response rates are summarised for patients who underwent nephrectomy after a period of ICPI treatment. The number of patients who were on treatment but did not undergo surgery are included where the data was available. Numbers of patients in the study and % clear cell RCC subtype are also indicated where available.
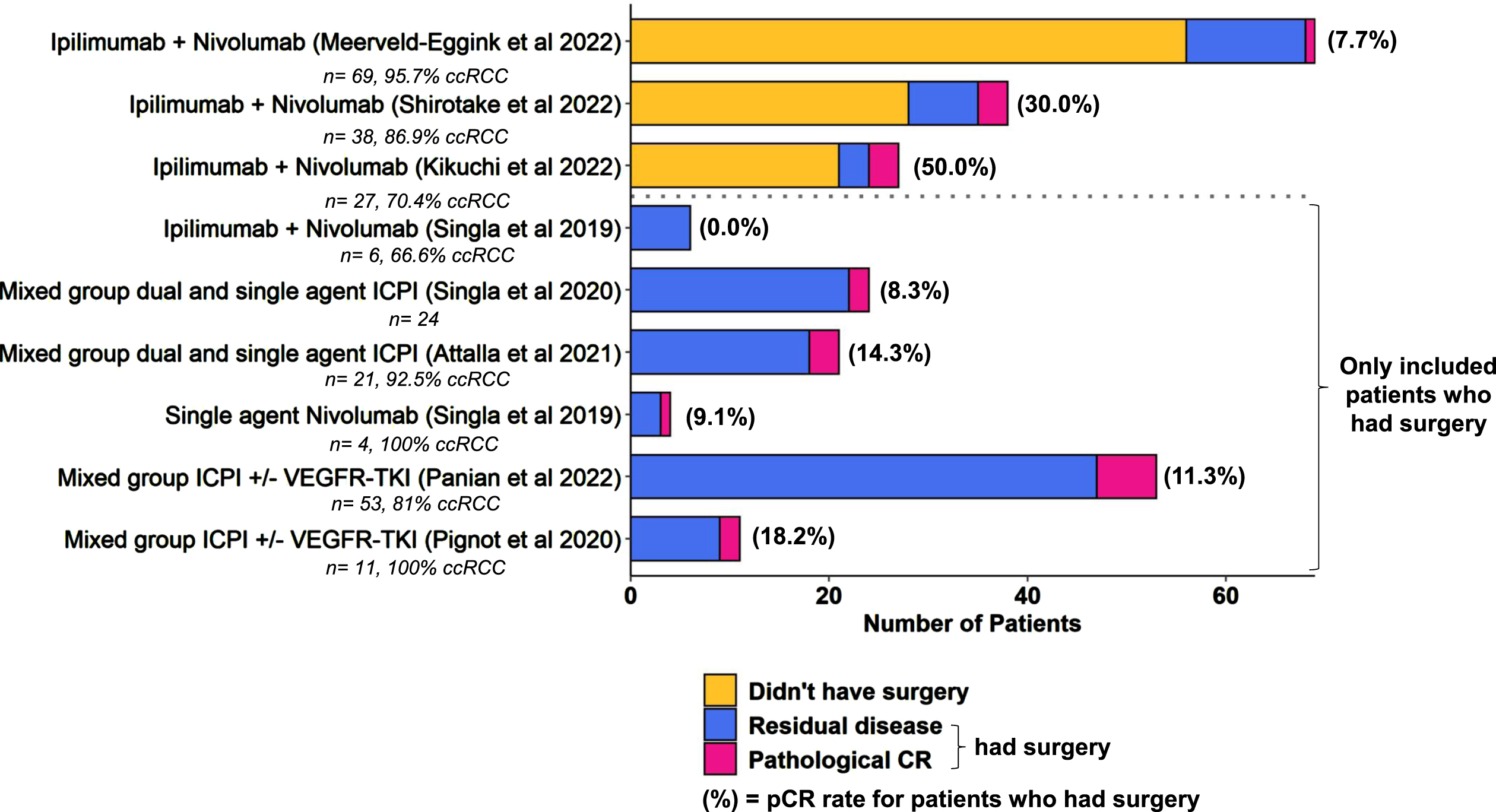
The remaining five studies only selected patients that had surgery [31, 34, 35, 37, 44]. These included a mix of treatments in the first- and second-line settings, pCR rates ranged from 8.3 –18.2%. If we pool all the patients who had surgery across studies, 21 of 149 (14.1%) had a pCR.
Two studies commented on downstaging of the cT (clinical tumour) stage compared to pT (pathological tumour) stage at nephrectomy [35, 37], though this is difficult to interpret as this downstaging was also seen in patients that had up-front CN without systemic therapy. During our review we also identified 18 case reports (Table S2) which comment on downstaging of the primary tumour with immunotherapy [45–62]. Of these, 14 commented on evidence of treatment effect, either the presence of pathological complete responses, or the infiltration of immune cells into the residual tumour bed indicating activation of an anti-tumour immune response.
Adverse event rates
For combination Ipilimumab + Nivolumab therapy CTCAE grade 3+ adverse event (AE) rates were 31–59% [32, 38, 39, 42]. Two studies of mixed treatment groups reported grade 3+ AE rates of 27.3% and 23.8% [31, 34].
Effect of immunotherapy on the primary tumour in the neoadjuvant setting
Four neoadjuvant studies were identified by our literature search (Table 1). Two studies assessed the use of neoadjuvant nivolumab given every 2 weeks, for either six or eight weeks [25, 26]. No radiological PR-primary was seen in either of these studies. There was limited pathological evidence of tumour immune response and regression in the post operative specimens: Carlo et al. provided specific data on complete pathological response, which was not seen in any patients, though 10 of 14 patients showed at least 5% tumour regression. Gorin et al. commented on the presence of ‘immune pathological response’ which was only seen in one patient, and also assessed for changes in tumour infiltrating lymphocytes (TILs) after treatment, though no significant differences were found. Carlo et al reported grade 3+ immunotherapy related AE rates of 11.1%, two patients needed to stop nivolumab early (due to arthralgia and transaminitis). Two patients had Clavien-Dindo Grade 3b surgical complications. Gorin et al. reported grade 3+ AEs in two patients (11.8%), one of which was attributed to nivolumab (lymphopenia). There were no Clavien-Dindo grade 3 or above complications. All patients completed surgery without delay in both studies.
Table 1
Neoadjuvant studies
| Title | Study Type | Patients | Risk Scoring | Treatment | Primary tumour radiological response | Primary tumour pathologic response | Grade 3+ AEs |
| Efficacy, safety, and biomarker analysis of neoadjuvant avelumab/axitinib in patients (pts) with localized renal cell carcinoma (RCC) who are at high risk of relapse after nephrectomy (NeoAvAx). Bex et al. Journal of Clinical Oncology 2022 | Single arm phase II | 40 (100% ccRCC) | High risk non metastatic patients (cT1b-4c, N0-1, M0, Grades 3-4) | Axitinib + Avelumab, 12 weeks | 30% PR (RECIST) | Upregulation of PD-L1 and CD8 infiltration when pre vst post treatment samples were compared. pCR not stated. | Hypertension (2.5%) Infusion reaction (2.5%) Fatigue (2.5%) Nausea (2.5%) Hand-foot syndrome (2.5%) Raised LFTs (2.5%) |
| Phase II Study of Neoadjuvant Nivolumab in Patients with Locally Advanced Clear Cell Renal Cell Carcinoma Undergoing Nephrectomy. Carlo et al. European Urology 2022 | Single arm phase II | 18 (100% ccRCC) | 12 year probability of metastases of≥20% | Nivolumab, 8 weeks | 0% PR, 100% SD (RECIST) | 0% pCR. At least 5% tumour regression was seen in 10/14 cases, there were no significant changes in TILs. | 11.1% |
| Neoadjuvant Nivolumab in Patients with High-risk Nonmetastatic Renal Cell Carcinoma. Gorin et al. European Urology Oncology 2022 | Single arm phase II | 15 (100% ccRCC) | High risk non metastatic patients (T2a-T4 Nany M0 or Tany N1 M0) | Nivolumab, 6 weeks | 0% PR 100% SD (RECIST) | One patient described to have ‘irPR’: evidence of regression with immune infiltrate. pCR rate not stated. | 11.8% |
| Neoadjuvant combination of pazopanib or axitinib and programmed cell death protein-1-activated dendritic cell-cytokine-induced killer cells immunotherapy may facilitate surgery in patients with renal cell carcinoma. Zhang et al. Translational Andrology and Urology 2021 | Retrospective case series | 16 (87.5% ccRCC) | Both locally advanced and metastatic patients included | Pazopanib + PD-1/CD-CIK cells (9) Axitinib + PD-1/CD-CIK cells (7) | 18.6% PR by RECIST (61.54% PR by volumetric assessment) | Not specified. Six patients declined surgery. | 55.6% (Pazopanib + PD-1/CD-CIK cells) 0% (Axitinib + PD-1/CD-CIK cells) |
Regarding neoadjuvant ICPI + VEGFR-TKI combinations, results of the NeoAvAx study were presented at GU ASCO in early 2022 [27]. This was a phase II neoadjuvant study assessing the effect of 12 weeks of pre-operative Axitinib + Avelumab. 40 patients were enrolled. PR-primary was seen in the in 30% of patients. Grade 3 systemic treatment related AEs occurred in a small number of patients; the pattern was similar to those expected for Axitinib + Avelumab in the metastatic setting (one grade 3 occurrence each of: hypertension, infusion reaction, fatigue, nausea, hand-foot syndrome, raised liver function tests) [2]. All patients had surgery, though one operation was delayed due to drug toxicity (immunotherapy related hypothyroidism). Five patients had Clavien-Dindo grade 3 or greater surgical complications. Of particular interest, the investigators had access to pre-treatment biopsies which were compared to the resection specimens. Significant increases were seen in CD8+ T cell infiltration and PD-L1 staining after treatment which may reflect an increase in immune activation against the tumour.
Zhang et al. reported a series of patients treated in the neoadjuvant setting with Cytokine Induced Killer Cells: ex-vivo generated cells stimulated by mature dendritic cells which can be used for adoptive cell therapy (referred to as DC-CIK cells) [63]. Treatment was given in combination with VEGFR-TKIs, resulting in PR-primary rates of 18.6% by RECIST criteria, and 61.5% when a volumetric assessment method was used. 31 % of patients experienced grade 3+ AEs related to treatment. 10/16 patients underwent surgery, with the remainder declining an operation, there was one Clavien-Dindo grade 4 complication. No data was provided on pathological response.
Ongoing pre-operative immunotherapy studies
We identified 16 active studies investigating the role of pre-operative immunotherapy in RCC. These are summarised in Table 2. There are two phase III trials actively addressing the role of CN in patients treated with immunotherapy in the metastatic setting. The PROBE trial (NCT04510597) involves an induction phase of 9–12 weeks of therapy with FDA approved immunotherapy combinations. Response is assessed at this point, and patients who are benefiting from treatment are randomised to either have CN and then restart ICPI; or continue ICPI directly. The target enrolment is 364 patients. The primary outcome is OS in the intention to treat (ITT) population, and secondary outcomes include response in the primary tumour. In the NORDIC-SUN trial (NCT03977571), patients receive three months of pre-operative Ipilimumab + Nivolumab or an ICPI+ VEGFR-TKI combination therapy. Patients with fewer than three IMDC risk factors and a resectable tumour may be randomised to CN at this point. Patients that are not operable or have≥3 IMDC risk factors (defined as ‘high risk’ for the purposes of the trial) continue systemic treatment for a further three months and are re-assessed at this point, at which time if they are operable they undergo randomisation. The trial is aiming to recruit 400 patients, primary outcome is OS, with secondary outcomes including primary tumour response, immune infiltration, and translational genetic and microbiome assessments. These trials should hopefully provide critical evidence in answering the question of benefit of CN in the combination IO era.
Table 2
Active trials
| Trial Name | Phase | Pre-surgical intervention | Primary Outcome Measures |
| Comparing the Outcome of Immunotherapy-Based Drug Combination Therapy With or Without Surgery to Remove the Kidney in Metastatic Kidney Cancer, the PROBE Trial (PROBE) NCT04510597 | III | 12 weeks initial immunotherapy (Nivolumab, Pembrolizumab + Axitinib or Avelumab + Axitinib) followed by randomisation to CN or continuation of systemic treatment | Overal survival |
| Deferred Cytoreductive Nephrectomy in Synchronous Metastatic Renal Cell Carcinoma: The NORDIC-SUN-Trial (NORDIC-SUN) NCT03977571 | III | Surgery after induction therapy with IO/IO or a TKI/IO-combination, followed by maintenance therapy with nivolumab or a TKI/IO-combination.Vs. Induction therapy wih IO/IO or a TKI/IO-combination, followed by maintenance therapy with nivolumab or a TKI/IO-combination. | Overal survival |
| Neoadjuvant Study With Combination Immuno-oncology for Primary Clear Cell Renal Cell Cancer (NESCIO) NCT05148546 | II | 2 cycles of nivolumab 360 mg every 3 weeks (arm A) 2 cycles of ipilimumab 1 mg/kg + nivolumab 3 mg/kg every 3 weeks (arm B) 2 cycles of relatlimab 360 mg + nivolumab 360 mg every 3 weeks (arm C) | Pathological response rate (complete or partial) |
| Pembrolizumab With or Without Axitinib for Treatment of Locally Advanced or Metastatic Clear Cell Kidney Cancer in Patients Undergoing Surgery NCT04370509 | II | 3 cycles of pembrolizumab every 3 weeks followed by surgery. 1-2 years adjuvant pembrolizumab depending on resection status (Cohort A) 3 cycles of pembrolizumab + axitinib every 3 weeks followed by surgery. 1-2 years adjuvant pembrolizumab + axitinib depending on resection status (Cohort B) | Proportion of patients with≥2 fold increase in number of TILs |
| NeoAdjuvant Pembrolizumab and STEreotactic Radiotherapy Prior to Nephrectomy for Renal Cell Carcinoma (NAPSTER) NCT05024318 | II | Stereotactic Ablative Radiotherapy (SABR) 42 Gy in 3 fractions, followed by surgery within 9–12 weeks Or: SABR followed by surgery as above plus 3 cycles neoadjuvant pembrolizumab every 3 weeks | mPR rate (<10% residual tumour) CD8+ Tissue resident memory cell counts TCF-1+ TIL counts |
| WIRE - Novel Treatments in Renal Cell Cancer (WIRE) NCT03741426 | II | Arm 1: Cediranib (mininum 2 weeks), Arm 2: Olaparib (mininum 2 weeks) Arm 3: Olaparib + Cediranib (mininum 2 weeks), Arm 4: Durvalumab (maximum 4 weeks), Arm 5: Olaparib (minimum 2 weeks) + Durvalumab (maximum 4 weeks) | Cediranib arms and olaparib single agent arm: 30% ktrans change pre-treatment vs post treatment DCE-MRI Durvalumab arms: 30% change in CD8 T cell density |
| Toripalimab Combined With Axitinib as Neoadjuvant Therapy for Advanced/Metastatic Non-clear Cell Renal Cell Carcinoma NCT04385654 | II | 6 weeks of toripalimab plus axitinib | mPR rate (<10% residual tumour) pCR rate (0% residual tumour) pNR rate (>90% residual tumour) |
| CYTO Reductive Surgery in Kidney Cancer Plus Immunotherapy and Targeted Kinase Inhibition (Cyto-KIK) NCT04322955 | II | 3 months cabozantanib + nivolumab | % patients with CR |
| Lenvatinib and Pembrolizumab Before Surgery for the Treatment of Locally Advanced Non-Metastatic Kidney Cancer NCT04393350 | II | 4 cycles lenvantinib + pembrolizumab every 3 weeks | Objective response rate |
| Neoadjuvant Lenvatinib and Pembrolizumab for IVC Tumor Thrombus NCT05319015 | II | 4 cycles of lenvatinib and pembrolizumab every 3 weeks prior to surgery, followed by up to 13 cycles adjuvant pembrolizumab | Disease control rate Local and metastatic progression rate 90 day post operative complications |
| Pembrolizumab and Axitinib as Neoadjuvant Therapy for Locally Advanced Non-metastatic Clear Cell Renal Cell Carcinoma (PANDORA) NCT04995016 | II | 4 cycles pembrolizumab and axitinib every 3 weeks | mPR rate (<10% residual tumour) |
| Toripalimab Combined With Axitinib as Neoadjuvant Therapy in Patients With Non-metastatic Locally Advanced Nonmetastatic Clear Cell Renal Cell Carcinoma NCT04118855 | II | 4 cycles of toripalimab and axitinib every 3 weeks | Objective response rate |
| A Study on the Safety and Effectiveness of Tislelizumab Combined With Axitinib for Neoadjuvant Treatment of ccRCC NCT05172440 | II | 4 cycles of tiselizumab and axitinib every 3 weeks | Objective response rate |
| Neoadjuvant Sitravatinib in Combination With Nivolumab in Patients With Clear Cell Renal Cell Carcinoma NCT03680521 | II | Sitravatinib for 2 weeks, then in combination with nivolumab every 2 weeks, for 6–8 weeks. | Objective response rate |
| Nivolumab With or Without Bevacizumab or Ipilimumab Before Surgery in Treating Patients With Metastatic Kidney Cancer That Can Be Removed by Surgery NCT02210117 | I | 3 cycles of nivolumab every 2 weeks (arm A) 3 cycles of nivolumab + bevacizumab every 2 weeks (arm B) 2 cycles of nivolumab + ipilimumab every 3 weeks (arm C) | Incidence of therapy related G3+ AEs |
| A Study of Combination Spartalizumab and Canakinumab in Patients With Localized Clear Cell Renal Cell Carcinoma (SPARC-1) NCT04028245 | I | 2 cycles of canakinumab and spartalizumab every 4 weeks | % patients that proceed to radical nephrectomy |
We found fourteen ongoing trials investigating the role of neoadjuvant therapy for patients with localised disease (Table 2). It is notable that all but one of these trials involve some form of combination therapy, generally with a VEGFR-TKI. This is in keeping with the observations in the palliative and neoadjuvant studies summarised above that higher response rates are seen with dual treatments. Between 6 and 12 weeks of therapy are used, with most trials using 12 weeks therapy prior to surgery. There is variation in the primary outcomes, though most use an immediate measure of tumour response: six studies specify radiological objective response rates and four use a pathological response assessment of the nephrectomy specimen. Three use changes in tumour infiltrating lymphocytes as a biological assessment of response, which may correlate with the degree of immune activation achieved. Two of the studies include the use of adjuvant pembrolizumab in the protocol [8, 9].
DISCUSSION
Cytoreductive Nephrectomy for Patients with Metastatic Disease
In the metastatic setting, data from post-hoc analyses of phase II and III trials, and retrospective case series, provides support for the use of up-front immunotherapy in patients with ccRCC to reduce the size of the primary tumour prior to CN. The best first-line data is available for the combination of Ipilimumab and Nivolumab, with PR-primary reported in one third to one half of patients, with median time to response from 2.8–4.4 months [32, 36, 39, 42, 44]. One study also provided direct support for this combination in non-ccRCC [36]. Similar PR-primary response rates were seen with ICPI + VEGFR-TKI combination treatment [40, 43]. There was no data specific to the use of single agent ICPI in the first line setting, but it is inferred from the data on mixed treatment groups that response rates are lower [40, 44]. This reflects the existing trial data for systemic treatments, where higher quality data is available for first line combinations than single agent treatments [1, 7]. Several studies commented that whilst the response in the primary and metastases were generally similar, differing responses did occur [32, 33, 41, 43]. This may be due to different subclones, molecular profiles or microenvironments found in metastatic sites, resulting in divergent treatment sensitivity [64–66]. Lower PR-primary rates were seen with second line ICPI, in the range of 10% [41]. Combination treatment comes at the cost of increased side effects, though for patients with metastatic disease the clinical decision on dual versus single agent therapy is based on factors other than the plan for nephrectomy, such as fitness, IMDC risk score and co-morbidities [17, 18]. The adverse event rates observed were comparable to those seen in larger phase III trials of these agents [1, 2].
Considering pathological measures of response, we estimated the pCR rate as 14% for patients with metastatic disease who underwent nephrectomy after a period of ICPI treatment. The range seen was wide, from 0–50% [31, 34–39, 44]. This figure includes both dual and single agent therapy with ICPI or in combination with VEGFR-TKIs. It is important to note that good response to initial ICPI treatment was a factor in selecting patients for nephrectomy [39], so the pCR is likely an overestimate of the true rate. The duration of preoperative treatment was also variable, with median times on treatment ranging from 3 to 13 months.
A key unanswered question which is outside the scope of our review is whether CN improves outcomes in the long term for patients with metastatic disease. This will be addressed by the results of the PROBE and NORDIC-SUN trials. Both studies however are investigating at delayed CN, whereas looking at existing practice data, many CN surgeries are done immediately [35], before systemic treatment. It is also not clear how current risk scoring systems, such as IMDC, factor into decision making regarding either immediate or deferred CN. NORDIC-SUN does consider IMDC risk, but at different thresholds to those used in selection of systemic treatments and in the ASCO guidelines for CN with VEGFR-TKI therapy [17]. The optimal duration of treatment is not known; in the current trials reviewed the range is wide (typically three to 12 months) and led by individual patient factors. The design of NORDIC-SUN may address this to an extent. A final concern is that given the rapid progress in development of immunotherapies, the standard of care may have moved on by the time these trials report, as has now happened for CARMENA and SURTIME [12, 13].
The observation of pCRs in the primary after ICPI treatment also raises interesting questions about the biological rationale for CN in the metastatic setting. A high proportion of patients had residual disease, and so may benefit from removal of these malignant cells. However, one could speculate that in the patients with pCR, the ICPI treatment may actually have achieved long term control of the disease, and so CN was not needed. The ‘ideal’ delayed CN patient may therefore be one who has radiological CR following ICPI in their metastatic disease, but a residual primary tumour on imaging. CN may benefit these patients by directly achieving long term disease control, or as an opportunity to stop systemic therapy and provide the patient with a duration of time off treatment. Looking at future technologies, patients may be assigned to initial systemic ICPI or VEGFR-TKI by molecular profiling, as has been done in the BIONIKK trial [67]. Following a period of systemic treatment, patients with complete ‘molecular response’ could then be identified (for example through circulating nucleic acids) to inform the decision on further surgical treatment [68].
Neoadjuvant ICPI treatment
We identified three prospective trials that have reported data in the neoadjuvant setting. Poor response rates were seen in two trials with neoadjuvant nivolumab [25, 26]. Combined axitinib and avelumab treatment resulted in a PR-primary rate of 30% [27]. Taken together with the observations on PR-primary in the metastatic setting, if the intention of treatment is downstaging within a clinical meaningful timeframe, combination treatment with IO-VEGFR-TKI seems to be the best option, however this is based on only one trial and so the results of further prospective studies are awaited It is noteworthy that most ongoing studies use combination treatment (Table 2).
Something that must be considered however is the potential to use single agent VEGFR-TKI. Several studies have assessed this (summarised in Table S3M): Wood et al. used 8 weeks of pazopanib treatment, response rates were 38% [69]. All patients went to surgery. Karam et al assessed 12 weeks of axitinib, 45.8% had a partial response by RECIST [70]. One patient required early surgery. The NAXIVA trial assessed 8 weeks of axitinib for patients with venous tumour thrombus (VTT): PR-primary rates were 16.7%, and response rates in the VTT were 35% [71]. Four patients did not undergo surgery, although they comprised a very high-risk patient group. These studies suggest that comparable PR-primary rates can be achieved with single agent TKI.
Toxicity is another factor to consider when comparing ICPI + VEGFR-TKI to VEGFR-TKI alone in the neoadjuvant setting. NeoAvAx has not reported the overall grade 3 toxicity rate, though from data presented at GU ASCO in 2022 it can be estimated that grade 3 AEs occurred in no more than 15% of patients [27]. This is lower than that for single agent axitinib in comparable patients (40–52%) [70, 71], but this comes from a small number of phase II studies. Looking at toxicity rates in the Javelin Renal 101 trial, approximately 70% of patients experienced grade 3+ toxicity in both the axitinib + avelumab and sunitinib arms [2], but the duration of treatment was longer. It is therefore difficult to comment on whether adding ICPI to VEGFR-TKI increases the grade 3+ toxicity rate in the neoadjuvant setting. A further question is whether there is a qualitative difference in the toxicity experienced. Although the grade 3+ rates are high in the neoadjuvant VEGFR-TKI trials, the predominant toxicities are hypertension, mucositis, hand-foot syndrome and fatigue, all of which are manageable with supportive therapy and reversible on withdrawal of the drug [70, 71]. Immunotherapy can cause autoimmune phenomena (‘Immune related adverse effects’, IRAEs) which are more unpredictable, can cause significant morbidity, may require systemic steroids or immunosuppressants, and in some cases are irreversible [72, 73]. The neoadjuvant immunotherapy trials reported some instances of IRAEs: grade 2 hypothyroidism which delayed surgery, grade three arthralgia, transaminitis and lymphopenia and a grade 3 post-operative immunotherapy induced colitis [25–27]. Whilst all patients in these trials went to surgery, a concern is that with wider use, more IRAEs will occur, as are seen in the metastatic treatment setting, which might delay or preclude surgery.
Future pre-operative studies
Most of the ongoing neoadjuvant trials use either primary tumour dimension or a pathological assessment, including TIL density, to assess primary treatment outcomes. Imaging based assessments, such as MRI, can also be used to assess functional response [74]. However, it is not yet clear how these correspond to long term outcomes such as survival benefit. Given the apparent similarity of efficacy with single agent TKI and combination ICPI plus VEGFR-TKI based on primary tumour response alone, the long-term survival data will be key in deciding whether this approach is worthwhile when balanced against potential increases in toxicity.
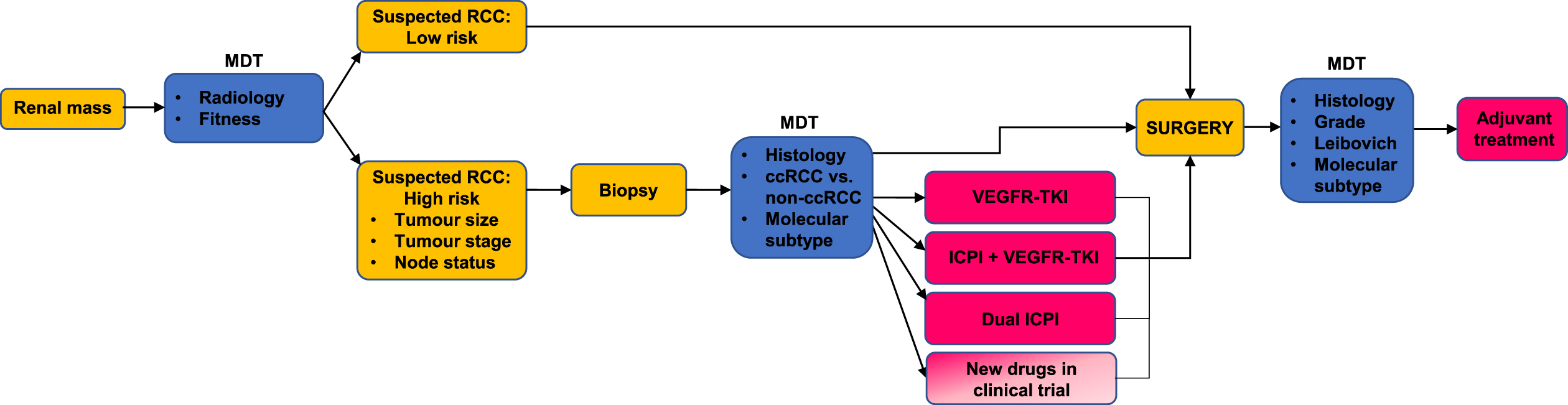
One future approach may be to risk stratify patients for neoadjuvant therapy. Those with high-risk disease, venous tumour thrombus, or those that are initially inoperable are the main groups we foresee benefiting from neoadjuvant approaches. These definitions would need formalising; at present trials have used tumour size, nodal status and grade to assess risk (Table 1) [25–27]. A pre-operative biopsy would seem to be a requirement, as we would not want to inadvertently expose patients with less common histological types or benign lesions to several months of oncological treatment. A biopsy would also provide the opportunity for molecular risk scoring, for example identifying patients with VEGFR-TKI sensitive disease who only need single agent therapy or predicting treatment resistant patients who should proceed directly to surgery [65, 67]. The duration of treatment should also be considered: most trials use 6–12 weeks of neoadjuvant therapy, which has basis in practice in advanced disease: for example, the median time to response with axitinib + avelumab in the metastatic setting was approximately 11 weeks [2]. Neoadjuvant trials generally include a mid-point assessment, which can be used as an ‘exit route’ for patients with progressive disease who need to go to surgery. We have summarised a proposed pathway for neoadjuvant treatment in Fig. 4 illustrating the factors that need to be considered and that might be investigated in future studies.
Fig. 4
A patient with a new renal mass is referred to the multidisciplinary team MDT. Patients with a suspected low risk RCC proceed straight to surgery. Patients assessed to have high risk disease undergo a biopsy. They are then assigned to neoadjuvant treatment taking into account histological information and molecular subtype. They may also take part in a clinical trial of neoadjuvant treatment. The postoperative findings are reviewed at the MDT, and patients continue on adjuvant treatment where appropriate.
A further complication is the adjuvant immunotherapy trial data [8, 9, 20–22]. These trials have had mixed success, however results for pembrolizumab are positive and have changed practice. Interpreting the ongoing neoadjuvant trials may be challenging with adjuvant immunotherapy as standard of care, and only two of the trials include this in their designs. From a biological perspective, it is not clear if the presence of the primary tumour helps or hinders the generation of the immune response after ICPI treatment: the bulk of the tumour may provide a greater supply of antigens against which a response can be generated but may also be accompanied by immune-suppressive cells recruited to the tumour microenvironment which blunt the immune response [75–77]. Translational research efforts in parallel to these active trials will hopefully provide more information on the effects of the microenvironment of treatment sequencing.
Summary
We have conducted a comprehensive literature review on the effects of ICPI on primary renal tumours, identifying numerous studies addressing this question. Most trials defined their primary tumour response in similar ways (≥30% reduction in tumour size from baseline), and so a valid comparison could be made between these trials. We identified a small number of neoadjuvant trials that have reported data, and ongoing neoadjuvant trials that will provide further information.
A key limitation is that despite the large number of immunotherapy trials we identified in our search, only a small number report the treatment effect on the primary tumour. There are likely to be large numbers of archived scans from these trials which could be analysed to provide more robust data on this question. Much of the evidence we identified comes from single arm phase II trials and retrospective studies, some of which had low numbers of patients. Only two phase III studies were included, and these were both post-hoc subgroup analyses. Concerning toxicity in the neoadjuvant setting, although most patients underwent surgery, a relatively small number of patients have been assessed across the reported trials, and so further IRAEs complicating surgery may yet emerge. The toxicity data quoted have a wide variation, and so rates must still be inferred from the metastatic setting, in which the treatments are given for a longer period in less fit patients.
The role of pre-operative immunotherapy for RCC patients remains an open question in both the metastatic and neoadjuvant setting. In the metastatic setting, there is evidence that first line combination treatment results in clinically meaningful reductions in the primary tumour, which may facilitate cytoreductive nephrectomy. Whether this procedure benefits patients in terms of survival outcome is unanswered in the ICPI era but may be addressed by active trials.
Early data from the neoadjuvant setting favours the use of VEGFR-TKIs alone or in combination with ICPIs. Results from single agent neoadjuvant ICPI studies have been disappointing. Toxicity is a key consideration, although from initial data neoadjuvant approaches appear to be safe and do not compromise patients’ chances of having surgery. A personalised medicine approach may be needed in selecting patients for neoadjuvant therapy, based on tumour risk assessment, co-morbidities, and molecular profiling. Long term survival data and the results of ongoing neoadjuvant trials will be key in determining whether neoadjuvant treatment is widely adopted.
ACKNOWLEDGMENTS
The authors would like to thank the Editors for the invitation to write this review.
FUNDING
JOJ is funded by an NIHR Academic Clinical Lectureship
AUTHOR CONTRIBUTIONS
JOJ, WHJI, SJW and GDS developed the review questions and approach. JOJ and WHJI conducted the systematic review of records. JOJ wrote the tables and figures. JOJ, WHJI, GDS and SJW wrote the manuscript.
CONFLICT OF INTEREST
JOJ, WHJI and SJW have no conflict of interest to declare. GDS—educational grants from Pfizer, AstraZeneca and Intuitive Surgical; consultancy fees from Pfizer, Merck, EUSA Pharma and CMR Surgical; Travel expenses from Pfizer and Speaker fees from Pfizer.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/KCA-220012.
REFERENCES
[1] | Motzer RJ , Tannir NM , McDermott DF , Arén Frontera O , Melichar B , Choueiri TK , et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. New England Journal of Medicine. 2018. |
[2] | Motzer RJ , Penkov K , Haanen J , Rini B , Albiges L , Campbell MT , et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. New England Journal of Medicine. 2019; |
[3] | Choueiri TK , Powles T , Burotto M , Escudier B , Bourlon MT , Zurawski B , et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. New England Journal of Medicine. (2021) ;384: (9). |
[4] | Motzer R , Alekseev B , Rha SY , Porta C , Eto M , Powles T , et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. New England Journal of Medicine. (2021) ;384: (14). |
[5] | Rini BI , Plimack ER , Stus V , Gafanov R , Hawkins R , Nosov D , et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. New England Journal of Medicine. 2019; |
[6] | Motzer RJ , Escudier B , George S , Hammers HJ , Srinivas S , Tykodi SS , et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: Updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer. (2020) ;126: (18). |
[7] | Motzer RJ , Rini BI , McDermott DF , Redman BG , Kuzel TM , Harrison MR , et al. Nivolumab for metastatic renal cell carcinoma: Results of a randomized phase II trial. Journal of Clinical Oncology. (2015) ;33: (13). |
[8] | Choueiri TK , Tomczak P , Park SH , Venugopal B , Ferguson T , Chang YH , et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. New England Journal of Medicine. (2021) ;385: (8). |
[9] | Choueiri TK , Tomczak P , Park SH , Venugopal B , Ferguson T , Symeonides SN , et al. Pembrolizumab as post nephrectomy adjuvant therapy for patients with renal cell carcinoma: Results from 30-month follow-up of KEYNOTE-564. Journal of Clinical Oncology. (2022) ;40: (6_suppl). |
[10] | Flanigan RC , Salmon SE , Blumenstein BA , Bearman SI , Roy V , McGrath PC , et al. Nephrectomy Followed by Interferon Alfa-2b Compared with Interferon Alfa-2b Alone for Metastatic Renal-Cell Cancer. New England Journal of Medicine. (2001) ;345: (23). |
[11] | Umbreit E , McIntosh A , Suk-Ouichai C , Karam J , Wood C . The current role of cytoreductive nephrectomy for metastatic renal cell carcinoma. Vol. 37, Indian Journal of Urology. 2021. |
[12] | Méjean A , Ravaud A , Thezenas S , Colas S , Beauval JB , Bensalah K , et al. Sunitinib Alone or after Nephrectomy in Metastatic Renal-Cell Carcinoma. New England Journal of Medicine. (2018) ;379: (5). |
[13] | Bex A , Mulders P , Jewett M , Wagstaff J , van Thienen J v , Blank CU , et al. Comparison of Immediate vs Deferred Cytoreductive Nephrectomy in Patients with Synchronous Metastatic Renal Cell Carcinoma Receiving Sunitinib: The SURTIME Randomized ClinicalTrial. In: JAMAOncology. 2019. |
[14] | Motzer RJ , Hutson TE , Cella D , Reeves J , Hawkins R , Guo J , et al. Pazopanib versus Sunitinib in Metastatic Renal-Cell Carcinoma. New England Journal of Medicine. 2013; |
[15] | Motzer RJ , Nosov D , Eisen T , Bondarenko I , Lesovoy V , Lipatov O , et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: Results from a phase III trial. Journal of Clinical Oncology. (2013) ;31: (30). |
[16] | Choueiri TK , Hessel C , Halabi S , Sanford B , Michaelson MD , Hahn O , et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A03 CABOSUN randomised trial): Progression-free survival by independent review and overall survival update. Eur J Cancer. (2018) ;94: . |
[17] | Kimryn Rathmell W , Bryan; R , Peter; , van Veldhuizen J , Al-Ahmadie H , Hamid Emamekhoo; , et al. Management of Metastatic Clear Cell Renal Cell Carcinoma: ASCO Guideline [Internet]. Vol. 40, J Clin Oncol. 2022. Available from: www.asco.org/genitourinary-cancer-guidelines |
[18] | Escudier B , Porta C , Schmidinger M , Rioux-Leclercq N , Bex A , Khoo V , et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. (2019) ;30: (5):706–20. |
[19] | Roussel E , Verbiest A , Milenkovic U , van Cleynenbreugel B , van Poppel H , Joniau S , et al. Too good for CARMENA: criteria associated with long systemic therapy free intervals post cytoreductive nephrectomy for metastatic clear cell renal cell carcinoma. Scand J Urol. (2020) ;54: (6). |
[20] | Bex A , Uzzo R , Karam JA , Master VA , Donskov F , Suárez C , et al. LBA66 IMmotion Efficacy and safety from the phase III study ofatezolizumab (atezo) vs placebo (pbo) as adjuvant therapy inpatients with renal cell carcinoma (RCC) at increased risk ofrecurrence after resection. Annals of Oncology. (2022) ;33: :S1431–2. |
[21] | Motzer RJ , Russo P , Gruenwald V , Tomita Y , Zurawski B , Parikh OA , et al. LBA4 Adjuvant nivolumab plus ipilimumab (NIVO+IPI) vs placebo (PBO) for localized renal cell carcinoma (RCC) at high risk of relapse after nephrectomy: Results from the randomized, phase III CheckMate 914 trial. Annals of Oncology. (2022) ;33: :S1430. |
[22] | Allaf M , Kim SE , Harshman LC , McDermott DF , Master VA , Signoretti S , et al. LBA67 Phase III randomized study comparing perioperative nivolumab (nivo) versus observation in patients (Pts) with renal cell carcinoma (RCC) undergoing nephrectomy (PROSPER, ECOG-ACRIN EA8143), a National Clinical Trials Network trial. Annals of Oncology. (2022) ;33: :S1432–3. |
[23] | Oza B , Frangou E , Smith B , Bryant H , Kaplan R , Choodari-Oskooei B , et al. RAMPART: A phase III multi-arm multi-stage trial of adjuvant checkpoint inhibitors in patients with resected primary renal cell carcinoma (RCC) at high or intermediate risk of relapse. Contemp Clin Trials. (2021) ;108: . |
[24] | Stewart GD , Klatte T , Cosmai L , Bex A , Lamb BW , Moch H , et al. The multispeciality approach to the management of localised kidney cancer. Lancet. (2022) ;400: (10351):523–34. |
[25] | Gorin MA , Patel HD , Rowe SP , Hahn NM , Hammers HJ , Pons A , et al. Neoadjuvant Nivolumab in Patients with High-risk Nonmetastatic Renal Cell Carcinoma. Eur Urol Oncol. (2022) ;5: (1). |
[26] | Carlo MI , Attalla K , Mazaheri Y , Gupta S , Yildirim O , Murray SJ , et al. Phase II Study of Neoadjuvant Nivolumab in Patients with Locally Advanced Clear Cell Renal Cell Carcinoma Undergoing Nephrectomy. Eur Urol. (2022) ;81: (6). |
[27] | Bex A , Abu-Ghanem Y , van Thienen Jv , Graafland N , Lagerveld B , Zondervan P , et al. Efficacy, safety, and biomarker analysis of neoadjuvant avelumab/axitinib in patients (pts) with localized renal cell carcinoma (RCC) who are at high risk of relapse after nephrectomy (NeoAvAx). Journal of Clinical Oncology. (2022) ;40: (6_suppl). |
[28] | Page MJ , McKenzie JE , Bossuyt PM , Boutron I , Hoffmann TC , Mulrow CD , et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Vol. 372, The BMJ. 2021. |
[29] | Topalian SL , Hodi FS , Brahmer JR , Gettinger SN , Smith DC , McDermott DF , et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012; |
[30] | Brahmer JR , Tykodi SS , Chow LQM , Hwu WJ , Topalian SL , Hwu P , et al. Safety and Activity of Anti–PD-L1 Antibody in Patients with Advanced Cancer. New England Journal of Medicine. (2012) ;366: (26). |
[31] | Pignot G , Thiery-Vuillemin A , Walz J , Lang H , Bigot P , Werle P , et al. Nephrectomy After Complete Response to Immune Checkpoint Inhibitors for Metastatic Renal Cell Carcinoma: A New Surgical Challenge? European Urology. 2020. |
[32] | Ishihara H , Takagi T , Yoshida K , Hashimoto Y , Kondo T , Tanabe K . Tumor response in primary kidney lesions and metastatic lesions in nivolumab plus ipilimumab therapy for advanced renal cell carcinoma without prior nephrectomy: Preliminary results of a multi-institutional study. International Journal of Urology. (2021) ;28: (10). |
[33] | Jamet A , Caramella C , Guida A , Arfi-Rouche J , Merabet Z , Colomba E , et al. Effect of immunotherapy (IO) on primary renal tumor in metastatic renal cell cancer (mRCC). Journal of Clinical Oncology. (2019) ;37: (7_suppl). |
[34] | Attalla K , Carlo M , Gupta S , Patil S , Coskey D , Murray S , et al. PD40-05 NEOADJUVANT IMMUNOTHERAPY IN PATIENTS WITH RENAL CELL CARCINOMA UNDERGOING NEPHRECTOMY. Journal of Urology. (2021) ;206: (Supplement 3). |
[35] | Singla N , Hutchinson RC , Ghandour RA , Freifeld Y , Fang D , Sagalowsky AI , et al. Improved survival after cytoreductive nephrectomy for metastatic renal cell carcinoma in the contemporary immunotherapy era: An analysis of the National Cancer Database. Urologic Oncology: Seminars and Original Investigations. (2020) ;38: (6). |
[36] | Kikuchi H , Osawa T , Matsumoto R , Abe T , Maruyama S , Harabayashi T , et al. Efficacy of nivolumab plus ipilimumab as first-line therapy for primary tumors in patients with renal cell carcinoma. Urologic Oncology: Seminars and Original Investigations. (2022) ;40: (1). |
[37] | Panian J , Saidian A , Hakimi K , Ajmera A , Barata PC , Berg SA , et al. Pathologic outcomes at cytoreductive nephrectomy (CN) following immunotherapy (IO) for patients with advanced renal cell carcinoma (RCC). Journal of Clinical Oncology. (2022) ;40: (6_suppl). |
[38] | Shirotake S , Miyama YU , Baba Y , Tajima H , Okada Y , Nakazawa K , et al. Impact of Cytoreductive Nephrectomy Following Nivolumab Plus Ipilimumab Therapy for Patients With Advanced Renal Cell Carcinoma. Anticancer Res. (2022) ;42: (5):2727–35. |
[39] | Meerveld-Eggink A , Graafland N , Wilgenhof S , van Thienen J v , Lalezari F , Grant M , et al. Primary Renal Tumour Response in Patients Treated with Nivolumab and Ipilimumab for Metastatic Renal Cell Carcinoma: Real-world Data Assessment. Vol. 35, European Urology Open Science. 2022. |
[40] | Emamekhoo H , Hester D , Abbasi S , Eickhoff J , Bice T , Archaya L , et al. 294 Evaluation of radiographic response in the intact renal mass (intact-Rmass) to immune checkpoint inhibitor (ICI) combination regimens in patients with metastatic renal cell carcinoma (mRCC). J Immunother Cancer. (2021) ;9: (Suppl 2). |
[41] | Courcier J , Dalban H , Laguerre C , Ladoire S , Barthélémy P , Oudard S , et al. Primary Renal Tumour Response in Patients Treatedwith Nivolumab for Metastatic Renal Cell Carcinoma: Results from theGETUG-AFU 26 NIVOREN Trial. Eur Urol. 2021: ;80: (3). |
[42] | Albiges L , Tannir NM , Burotto M , McDermott D , Plimack ER , Barthélémy P , et al. First-line Nivolumab plus Ipilimumab Versus Sunitinib in Patients Without Nephrectomy and With an Evaluable Primary Renal Tumor in the Check Mate 214 Trial. Eur Urol. (2022) ;81: (3). |
[43] | Albiges L , Rini BI , Haanen JBAG , Motzer RJ , Kollmannsberger CK , Negrier S , et al. Primary renal tumour shrinkage in patients (pts) who did not undergo upfront cytoreductive nephrectomy (uCN): Subgroup analysis from the phase III JAVELIN Renal 101 trial of first-line avelumab + axitinib (A + Ax) vs sunitinib (S) for advanced renal cell carcinoma (aRCC). Annals of Oncology. (2019) ;30: . |
[44] | Singla N , Elias R , Ghandour RA , Freifeld Y , Bowman IA , Rapoport L , et al. Pathologic response and surgical outcomes in patients undergoing nephrectomy following receipt of immune checkpoint inhibitors for renal cell carcinoma. Urologic Oncology: Seminars and Original Investigations. 2019; |
[45] | Woldu SL , Brugarolas J , Kapur P , Margulis V . What is the role of nephrectomy following complete response to checkpoint inhibitors? Urol Case Rep. (2018) ;18: . |
[46] | Watson DD , Farha NM , Kallail KJ , Dakhil S , Farha AJ . From Radical to Partial Nephrectomy in the Setting of Solitary Functioning Kidney: Neoadjuvant Treatment of Renal Cell Carcinoma. Rev Urol. (2020) ;22: (3):126–9. |
[47] | Suzuki I , Kijima T , Takada-Owada A , Nakamura G , Uematsu T , Sakamoto K , et al. A case of clear cell renal cell carcinoma with vena cava thrombus responding to presurgical avelumab, and axitinib. IJU Case Rep. (2021) ;4: (6). |
[48] | Studentova H , Zemankova A , Spisarova M , Skanderova D , Tudos Z , Melichar B , et al. A Pathological Complete Response to the Combination of Ipilimumab and Nivolumab in a Patient with Metastatic Renal Cell Carcinoma. Medicina (Lithuania). (2022) ;58: (3). |
[49] | Tucker MD , Beckermann KE , Gordetsky JB , Giannico GA , Davis NB , Rini BI . Complete Pathologic Responses With Immunotherapy in Metastatic Renal Cell Carcinoma: Case Reports. Front Oncol. (2020) ;10: . |
[50] | Reimers MA , Figenshau RS , Kim EH , Tucker J , Kasten N , Khan AS , et al. Elective Cytoreductive Nephrectomy After Checkpoint Inhibitor Immunotherapy in Patients With Initially Unresectable Metastatic Clear Cell Renal Cell Carcinoma. Clin Genitourin Cancer. (2020) ;18: (5). |
[51] | Pandey Y , Matin A , Broadfoot B , Kunthur A . Clinical and histological response to combination nivolumab and ipilimumab in metastatic renal cell carcinoma. Baylor University Medical Center Proceedings. (2020) ;33: (2). |
[52] | Nishimura K , Miura N , Sugihara N , Funaki K , Koyama K , Sawada Y , et al. Sequential immune-targeted surgical therapy resulted in disease-free survival in a case with advanced renal cell carcinoma. BMC Urol. (2021) ;21: (1). |
[53] | Marmarelis ME , Davis MR , Sethi NS , Krajewksi KM , McKay RR , Choueiri TK , et al. Tumor control with PD-1 inhibition in a patient with concurrent metastatic melanoma and renal cell carcinoma. J Immunother Cancer. (2016) ;4: (1). |
[54] | Mahmoud F , Abdallah AO , Arnaoutakis K , Makhoul I . Metastatic Renal Cell Carcinoma Presenting as Painful Chewing Successfully Treated with Combined Nivolumab and Sunitinib. Perm J. (2016) ;20: (3). |
[55] | Levitin M , Ofori J , Shin WJ , Huang J , Daly M , Cao D , et al. Radiation and Checkpoint Inhibitor Immunotherapy Lead to Long Term Disease Control in a Metastatic RCC patient With Brain Metastases. Front Oncol. (2020) ;10: . |
[56] | Labbate C , Hatogai K , Werntz R , Stadler WM , Steinberg GD , Eggener S , et al. Complete response of renal cell carcinoma vena cava tumor thrombus to neoadjuvant immunotherapy. J Immunother Cancer. (2019) ;7: (1). |
[57] | Kambe T , Yamasaki T , Mine Y , Hagimoto H , Kokubun H , Kubota M , et al. Complete remission of renal cell carcinoma with lung carcinomatous lymphangiosis after primary therapy with immune checkpoint inhibitors followed by partial nephrectomy for surgical consolidation. IJU Case Rep. (2022) ;5: (3). |
[58] | Ikarashi D , Kato Y , Katagiri H , Takahara T , Uesugi N , Shiomi E , et al. Case of complete response to neoadjuvant therapy using nivolumab in a patient with metastatic renal cell carcinoma. International Journal of Urology. (2018) ;25: (6). |
[59] | Furubayashi N , Taguchi K , Negishi T , Miura A , Sato Y , Miyoshi M , et al. Cytoreductive Nephrectomy after Combination of Nivolumab plus Ipilimumab for Mucinous Tubular and Spindle Cell Carcinoma of the Kidney with Bone Metastases: A Case Report. In Vivo (Brooklyn). (2022) ;36: (1). |
[60] | Dawsey SJ , Campbell SC , Ornstein MC . Cytoreductive nephrectomy following immunotherapy-base treatment in metastatic renal cell carcinoma: A case series and review of current literature. Current Oncology. (2021) ;28: (3). |
[61] | Berends J , Gourley E , Kaushik D . Robust response to nivolumab in patient with renal cell carcinoma inferior vena cava tumour thrombus. BMJ Case Rep. (2019) ;12: (4). |
[62] | Shimizu K , Tamada S , Matsuoka Y , Go I , Okumura S , Ogawa M , et al. Pathologic complete response with pembrolizumab plus axitinib in metastatic renal cell carcinoma. Int Cancer Conf J. (2022) ;11: (3):205–9. |
[63] | Zhang Z , Xiong L , Wu Z , Liu H , Ning K , Peng Y , et al. Neoadjuvant combination of pazopanib or axitinib and programmed cell death protein-1-activated dendritic cell-cytokine-induced killer cells immunotherapy may facilitate surgery in patients with renal cell carcinoma. Transl Androl Urol. (2021) ;10: (5). |
[64] | Turajlic S , Xu H , Litchfield K , Rowan A , Chambers T , Lopez JI , et al. Tracking Cancer Evolution Reveals Constrained Routes to Metastases: TRACERx Renal. Cell. 2018; |
[65] | Motzer RJ , Banchereau R , Hamidi H , Schiff C , Huseni MA , Rini B , et al. Article Molecular Subsets in Renal Cancer Determine Outcome to Checkpoint and Angiogenesis Blockade Article Molecular Subsets in Renal Cancer Determine Outcome to Checkpoint and Angiogenesis Blockade. Cancer Cell [Internet]. 2020;1-15. Available from: https://doi.org/10.1016/j.ccell.2020.10.011 |
[66] | McKay RR , Barata PC , Elliott A , Bilen MA , Burgess EF , Darabi S , et al. Molecular alterations across sites of metastasis in patients with renal cell carcinoma (RCC). Journal of Clinical Oncology. (2022) ;40: (6_suppl). |
[67] | Vano YA , Elaidi R , Bennamoun M , Chevreau C , Borchiellini D , Pannier D , et al. Nivolumab, nivolumab-ipilimumab, and VEGFR-tyrosine kinase inhibitors as first-line treatment for metastatic clear-cell renal cell carcinoma (BIONIKK): a biomarker-driven, open-label, non-comparative, randomised, phase 2 trial. Lancet Oncol [Internet]. 2022;1-13. Available from: http://www.ncbi.nlm.nih.gov/pubmed/35390339 |
[68] | Geertsen L , Koldby KM , Thomassen M , Kruse T , Lund L . Circulating Tumor DNA in Patients with Renal Cell Carcinoma. A Systematic Review of the Literature. Vol. 37, European Urology Open Science. 2022. |
[69] | Wood CG , Ferguson JE , Parker JS , Moore DT , Whisenant JG , Maygarden SJ , et al. Neoadjuvant pazopanib and molecular analysis of tissue response in renal cell carcinoma. JCI Insight. (2020) ;5: (22). |
[70] | Karam JA , Devine CE , Urbauer DL , Lozano M , Maity T , Ahrar K , et al. Phase 2 trial of neoadjuvant axitinib in patients with locally advanced nonmetastatic clear cell renal cell carcinoma. Eur Urol. (2014) ;66: (5). |
[71] | Stewart GD , Welsh SJ , Ursprung S , Gallagher FA , Jones JO , Shields J , et al. A Phase II study of neoadjuvant axitinib for reducing the extent of venous tumour thrombus in clear cell renal cell cancer with venous invasion (NAXIVA). Br J Cancer. 2022 Jun 23; |
[72] | Brahmer JR , Lacchetti C , Schneider BJ , Atkins MB , Brassil KJ , Caterino JM , et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. Journal of Clinical Oncology. (2018) ;36: (17):1714–68. |
[73] | Haanen JBAG , Carbonnel F , Robert C , Kerr KM , Peters S , Larkin J , et al. Management of toxicities from immunotherapy: ESMO ClinicalPractice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology [Internet]. (2018) ;29: (Supplement_4):iv264–6. Available from: https://academic.oucom/annonc/article/29/Supplement_4/iv264/5039693 |
[74] | Ursprung S , Mossop H , Gallagher FA , Sala E , Skells R , Sipple JAN , et al. The WIRE study a phase II, multi-arm, multi-centre, non-randomised window-of-opportunity clinical trial plat form using a Bayesian adaptive design for proof-of-mechanism of novel treatment strategies in operable renal cell cancer –a study protocol. BMC Cancer. (2021) ;21: (1). |
[75] | Blank CU , Rozeman EA , Fanchi LF , Sikorska K , van de Wiel B , Kvistborg P , et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. (2018) ;24: (11). |
[76] | Munn DH , Bronte V . Immune suppressive mechanisms in the tumor microenvironment. Vol. 39, Current Opinion in Immunology. 2016. |
[77] | Drake CG , Stein MN . The immunobiology of kidney cancer. Vol. 36, Journal of Clinical Oncology. 2018. |




