Axitinib beyond first-line therapy of Metastatic Renal Cell Carcinoma: Real World Data from the STAR-TOR registry
Abstract
Objective:
To evaluate the effectiveness and safety profile of the tyrosine kinase inhibitor Axitinib for patients with advanced or metastatic renal cell carcinoma (a/mRCC) in a real-world setting.
Methods:
Adult patients from the German non-interventional post-approval multicenter STAR-TOR registry with a/mRCC (NCT00700258) were included if treated with Axitinib in second line or beyond. Overall survival (OS), progression-free survival (PFS) and adverse events were evaluated across subgroups using descriptive statistics and survival analyses.
Results:
Between November 2012 and December 2020, 75 study sites recruited 210 patients treated with Axitinib (69,6% male; median age 69 years; median Karnofsky Index 80%). Clear cell RCC was the most frequent histological subtype (81.0%). Axitinib was administered as second-line in 51.4%, third-line in 24.8%, and fourth-line treatment and beyond in 23.8% of the patients, respectively. MSKCC score was 15.0% favorable, 33.6% intermediate, and 51.3% poor risk. Median PFS was 5.6 months, and median OS 18.3 months. Patients with lactate dehydrogenase (LDH) levels > 300U/l had a nominally significantly shorter OS than patients with LDH≤300U/l (8.2 vs. 19.0 months, p = 0.008).
Drug related adverse and serious adverse events were reported in 56.7% and 17.6% of the patients, respectively (most common adverse event: gastrointestinal disorders; 37.6%).
Conclusions:
This real-world study confirms the clinical relevance of Axitinib in the second-line and beyond setting for a/mRCC with OS and PFS reported in concordance with pivotal trials, while demonstrating a favorable safety profile. A high LDH serum level could be a negative predictive marker for Axitinib effectiveness, which can aid in clinical decision making.
1INTRODUCTION
Treatment of advanced or metastatic renal cell carcinoma (a/mRCC) is continuously evolving, with checkpoint inhibitors (CPIs) being now part of all first line treatment regimens for suitable patients [1–3]. To simultaneously target various tumor pathways, CPIs are preferably administered as combination therapy [1–3]. The approval of combined Pembrolizumab or Avelumab and Axitinib for all a/mRCC risk groups in 2019 has lately highlighted the clinical relevance of the anti-VEGF tyrosine kinase inhibitor (TKI) Axitinib [4, 5]. While now considered a first-line treatment in combination with checkpoint inhibitors, Axitinib monotherapy had originally been approved in 2012 as second-line treatment after Sunitinib or cytokines according to the pivotal AXIS trial data [6]. The first phase III trial in which Axitinib was evaluated as monotherapy in second-line after cytokine or Sunitinib in mRCC patients demonstrated a longer median progression-free survival (PFS) of 6.7 months for Axitinib versus 4.7 months for Sorafenib (p < 0.0001) [6].
Although combinations of CPI and targeted therapy have demonstrated superior outcomes to previous regimens, few of the targeted therapies have been comprehensively examined for their efficacy and adverse effect profile in a single-agent setting [7–9]. This complicates the assessment of the contribution to efficacy and toxicity of each individual agent in immunotherapy combinations.
In addition, Axitinib is one of the standard second and third line treatment options after CPI-treatment failure [10]. As demonstrated by a large-scale national study in Germany, 56% of fist-line patients progress to second-line, 29% to third-line, and up to 13% to subsequent lines [11]. This context highlights the clinical relevance of Axitinib monotherapy, even in the CPI era. A recent meta-analysis by Wenzel et al. suggests a better second-line median PFS for Pazopanib after IO-failure, when compared to Axitinib [12]. Yet, the authors themself admit their analyses to be based on retrospective studies with small sample size and heterogeneous patient populations. Nevertheless, the study demonstrates that Axitinib plays an important role as treatment option for second and later lines.
Still, real-world data from large a/mRCC patient cohorts treated with Axitinib is limited [13–18].
Therefore, this study aims to evaluate Axitinib as a single-agent in the real-world setting. The study is one of the largest patient cohorts with clinical routine data that has been published so far. The here presented details bear several yet unknown but important details that can aid in understanding the effects seen in combination therapies.
2MATERIAL AND METHODS
This study evaluates data originating from the prospective STAR-TOR registry maintained by Pfizer Pharma GmbH, Berlin, Germany, and was conducted in accordance with the Declaration of Helsinki in its most recent version. The STAR-TOR registry received prior approval by the ethics committee of Münster University Hospital in 2007 (Nr.2007-484-f-S) and was registered in the US library of medicine database (NCT00700258). The registry is intended to evaluate the effectiveness, tolerability, and safety of patients with advanced RCC, recurrent or refractory mantle cell lymphoma and gastrointestinal stromal tumors treated with a) Axitinib, b) Sunitinib, or c) Temsirolimus in a routine clinical setting. Additional details regarding the STAR-TOR registry have been previously published [19–23].
2.1Study cohort
For the here presented analyses, inclusion criteria were histologically confirmed a/mRCC treated with Axitinib as single-agent drug at the STAR-TOR study centers between November 2012 and May 2020. The STAR-TOR registry enrolled only adult patients after giving written informed consent. All study centers were located in Germany.
2.2Axitinib administration
The starting dose of Axitinib was 5 mg orally twice per day (bis in die; b.i.d.). The dosage could be changed according to tolerability or effectiveness from 2 mg to 10 mg b.i.d.. It is the character of a non-interventional trial that no exact provisions for documentation could be made, and so patients from first- to fourth-line and beyond were recruited. In this analysis, however, only pretreated patients from second line and beyond are evaluated.
2.3Variables
Up to six lines of systemic therapy were documented.
The MSKCC score for pretreated patients was calculated according to the algorithm published by Motzer et al. [24]. Relevant factors were Karnofsky performance status < 80%, low serum hemoglobin (≤13 vs > 13 mg/dL for males and≤11.5 vs > 11.5 mg/dL for females), and high corrected calcium (<10 vs≥10 mg/dL). Documentation of laboratory values was voluntary in this non-interventional study, so not for all patients the MSKCC score could be calculated.
Serum lactate dehydrogenase (LDH) levels were assessed during the course of the therapy by local laboratories. High LDH was defined as LDH level > 300 U/L. This cutoff was chosen because the MSKCC score for treatment-naïve patients rates a LDH concentration of 1.5 times the upper level of the normal as risk factor [24].
2.4Outcomes
A total of three primary outcome measures were evaluated in this study: overall survival (OS), progression-free survival (PFS), and adverse events (AE). Follow-up visits were timed corresponding to the clinical routine at the participating sites and occurred in intervals of 8 to 12 weeks. At each follow-up visit, the sites documented treatment status and response, tumor staging, laboratory values, and AEs. Participating study centers were randomly monitored to ensure correct patient assessment and data entry.
OS was defined as time from the baseline visit at a participating STAR-TOR center until death from any cause. In case no death was documented, the subject was censored with the latest available contact date. PFS was defined as the time from the baseline visit to the date of the progression event or death. If no progression was documented, the patient was censored at the day of the last visit. In case these rules led to a missing duration or a negative duration, PFS and OS duration were set to “1 day”. If data were missing, appropriate provisions were made in the statistical analysis plan.
Response rate to treatment was evaluated according to RECIST 1.1 [25]. Best response was determined by the physician’s assessment at final visit. If “best response” was missing or inconclusive, it was derived from the best response documented during previous follow-up visits. If response evaluation at the study sites was not routinely performed according to RECIST standards, the physician’s assessment was accepted instead.
Study sites reported any AE according to NCI-CTCAE criteria [26]. In the analyses used for this publication, AEs were summed up in groups according to NCI-CTCAE standards.
2.5Statistical analyses
Continuous data were reported as median and range. Categorial data were described using absolute and relative frequencies (percent).
PFS and OS were visualized with Kaplan Meyer plots and compared between subgroups using log rank tests. Exploratory subgroup analyses for the primary outcomes were performed for Axitinib treatment line, patient age (≤65 vs.>65 years), MSKCC prognosis group, histological subtype, and LDH levels.
All analyses are univariate as multivariate models would have demanded imputation of missing values or exclusion of multiple patients with incomplete data jeopardizing the informative value of our results.
All statistical analyses were conducted using SAS software (version 9.2). All provided tests are two-sided and have to be interpreted purely exploratively.
The statistical analyses were performed by Dr. Thomas Fischer (Winicker-Norimed GmbH, Nuremberg, Germany).
3RESULTS
3.1Study cohort
A total of 210 patients from 75 study centers were included in this study, with a median age of 69 years. Most patients were male (69.6% of 207) and presented with a clear cell RCC (81% of 210), a high-risk MSKCC score (51.3% of 113) and Karnofsky performance status≤80% (60.8% of 204). Radical nephrectomy was the predominant treatment modality (82.4% of 210), achieving a complete resection in 75.1% of n = 185 cases (R0 status).
With respect to metastatic sites upon inclusion in the study, pulmonary metastases were most frequent (52.4% of 210) followed by lymph node (31.4%) and bone metastases (28.6%). All relevant baseline characteristics are shown in Table 1.
Table 1
Patient demographics, tumor variables and treatment characteristics
| Patient number, total n = 210 | Median (range) % | |
| Age, median, years | 210 | 69 (28-84) |
| Gender, male/female | 207 | 69.6 / 30.4 |
| Risk assessment according to MSKCC * | 113 | |
| favorable risk | 17 | 15.0 |
| intermediate risk | 38 | 33.6 |
| high risk | 58 | 51.3 |
| Karnofsky performance status | 204 | 80 (50-100) |
| T stage | 210 | |
| T1 | 48 | 22.9 |
| T2 | 38 | 18.1 |
| T3 | 95 | 45.2 |
| T4 | 10 | 4.8 |
| TX or missing | 19 | 9.0 |
| N stage | 210 | |
| N0 | 100 | 47.6 |
| N1 | 19 | 9.0 |
| N2 | 18 | 8.6 |
| NX or missing | 73 | 34.8 |
| M stage | 210 | |
| M0 | 79 | 37.6 |
| M1 | 77 | 36.7 |
| MX or missing | 54 | 25.7 |
| Histological subtype † | 210 | |
| Clear cell | 170 | 81.0 |
| Papillary | 27 | 12.9 |
| Chromophobe | 4 | 1.9 |
| Other | 8 | 3.8 |
| Unclassified | 5 | 2.4 |
| Nephrectomy | 210 | |
| total nephrectomy | 173 | 82.4 |
| partial nephrectomy | 16 | 7.6 |
| no nephrectomy | 21 | 10.0 |
| Primary tumor resection | 185 | |
| R0 | 139 | 75.1 |
| R+ | 46 | 24.9 |
| Additional radiation therapy | 142 | |
| Yes | 9 | 6.3 |
| No | 133 | 93.7 |
| Location of metastases at diagnosis ‡ | 210 | |
| Lung | 110 | 52.4 |
| Lymph nodes | 66 | 31.4 |
| Bone | 60 | 28.6 |
| Liver | 52 | 24.8 |
| Adrenal gland | 21 | 10.0 |
| Contralateral kidney | 10 | 4.8 |
| Central nervous system | 8 | 3.8 |
| Other | 46 | 21.9 |
| Number of metastatic sites | 210 | 2 (0-6) |
| LDH level in serum | 163 | |
| ≤300 U/l | 131 | 80.4 |
| >300 U/l | 32 | 19.6 |
* the MSKCC score for pretreated patients was calculated. † multiple types possible. ‡multiple entries allowed.
3.2Axitinib treatment
The majority of the patients received Axitinib as second-line treatment (51.4%) over a median duration of 4.9 months, with Sunitinib being the most common preceding drug (75.2%).
For 175 patients, data regarding changes of dosage were reported. Between first and last dose there was no change of dosage in 111 (63.4%) patients. In 52 (29.7%) patients the starting dose was reduced from the first to the last application, in 12 (6.9%) patients the dose was increased. A total of 67 patients (31.9% of 210) permanently discontinued Axitinib, whereas 38 patients (18.8% .) temporarily interrupted their treatment. Neither the length of the treatment pause, nor the reason for the discontinuation were documented in the STAR-TOR register. Further details on Axitinib treatment are provided in Table 2.
Table 2
Details of Axitinib treatment
| Patient number, total n = 210 | Median (range) % | |
| Axitinib was used in | 210 | |
| Second-line | 108 | 51.4 |
| Third-line | 52 | 24.8 |
| Fourth-line | 22 | 10.5 |
| ≥Fifth-line and beyond | 28 | 13.3 |
| Number of previous systemic therapies | 210 | 1 (1-5) |
| Previous systemic therapies‡ | ||
| Sunitinib | 158 | 75.2 |
| Pazopanib | 68 | 32.4 |
| Everolimus | 54 | 25.7 |
| Sorafenib | 39 | 18.6 |
| Interferon | 20 | 9.5 |
| Nivolumab | 15 | 7.1 |
| Bevacizumab | 12 | 5.7 |
| Cabozantinib | 7 | 3.3 |
| Interleukin | 5 | 2.4 |
| 5-Fluorouracil | 3 | 1.4 |
| Ipilimumab | 1 | 0.5 |
| Lenvatinib | 1 | 0.5 |
| Mitomycin-C | 1 | 0.5 |
| Vinblastine | 1 | 0.5 |
| Investigational Drug | 1 | 0.5 |
| Treatment duration * | 186 | 4.9 (0-59.6) |
| Duration of survival follow-up | 119 | 10.9 (0-55.1) |
| Therapy discontinuation | 210 | |
| Temporary | 38 | 18.1 |
| Permanent | 67 | 31.9 |
‡ multiple entries allowed. * only available for pts with final examination.
3.3Axitinib treatment response
207 patients were analyzed for best overall response. The clinical benefit rate of Axitinib was 50.7% : 3 patients (1.4%) experienced a complete response (CR), 35 patients (16.9%) had partial remission (PR), and 67 patients (32.4%) experienced a stable disease (SD). Progressive disease (PD) was observed in 56 patients (27.1%). 46 patients were not assessable. For 3 patients, response data were missing. Table A in the supplementary material shows the response rates by therapeutic line. We did not detect marked differences even though the results have to be interpreted cautiously as patient numbers decreased in later lines.
The overall median PFS was 5.6 months and median OS was 18.3 months. Median PFS and OS for 108 second-line patients were 4.8 and 16.1 months. Patients in later lines (n = 102) demonstrated numerically longer PFS (6.3 months) and OS (18.4 months), respectively (p = 0.68 and 0.75).
For patients older than 65 years compared to patients≤65 years of age, PFS was noticeably longer with 6.5 vs. 4.3 months (p = 0.005), whereas the OS difference was only numerically longer with 18.4 vs. 15.6 months (p = 0.31). Detailed survival data are shown in Table 3.
Table 3
Progression-free and overall survival according to patient subgroups
| Patient number n | Progression-free survival Months | Overall survival Months | |
| All patients | 210 | 5.6 | 18.3 |
| Second line patients | 108 | 4.8 | 16.1 |
| ≥Third line patients | 102 | 6.3 | 18.4 |
| Favourable risk patients *† | 17 | 10.5 | 37.6 |
| Intermediate risk patients *† | 38 | 5.7 | 25.8 |
| Poor risk patients *† | 58 | 3.5 | 9.3 |
| Patients with LDH≤300 U/l † | 131 | 6.2 | 19.0 |
| Patients with LDH > 300 U/l † | 32 | 5.1 | 8.2 |
| Patients with clear cell histology | 170 | 5.6 | 18.3 |
| Patients with other histologies | 40 | 5.8 | 22.2 |
| Patients with bone metastases | 60 | 5.2 | 12.7 |
* MSKCC score for pretreated patients. † exploratorative analysis proved statistical significance with p < 0.05.
As depicted in Fig. 1, survival also differed relevantly by MSKCC prognosis group: median PFS for patients with favorable, intermediate, and poor risk was 10.5, 5.7, and 3.5 months (p = 0.01). For the respective prognosis groups, OS also differed with a median of 37.6, 25.8 and 9.3 months (p = 0.004).
Fig.1
Progression-Free and Overall Survival according to LDH level and MSKCC risk score.
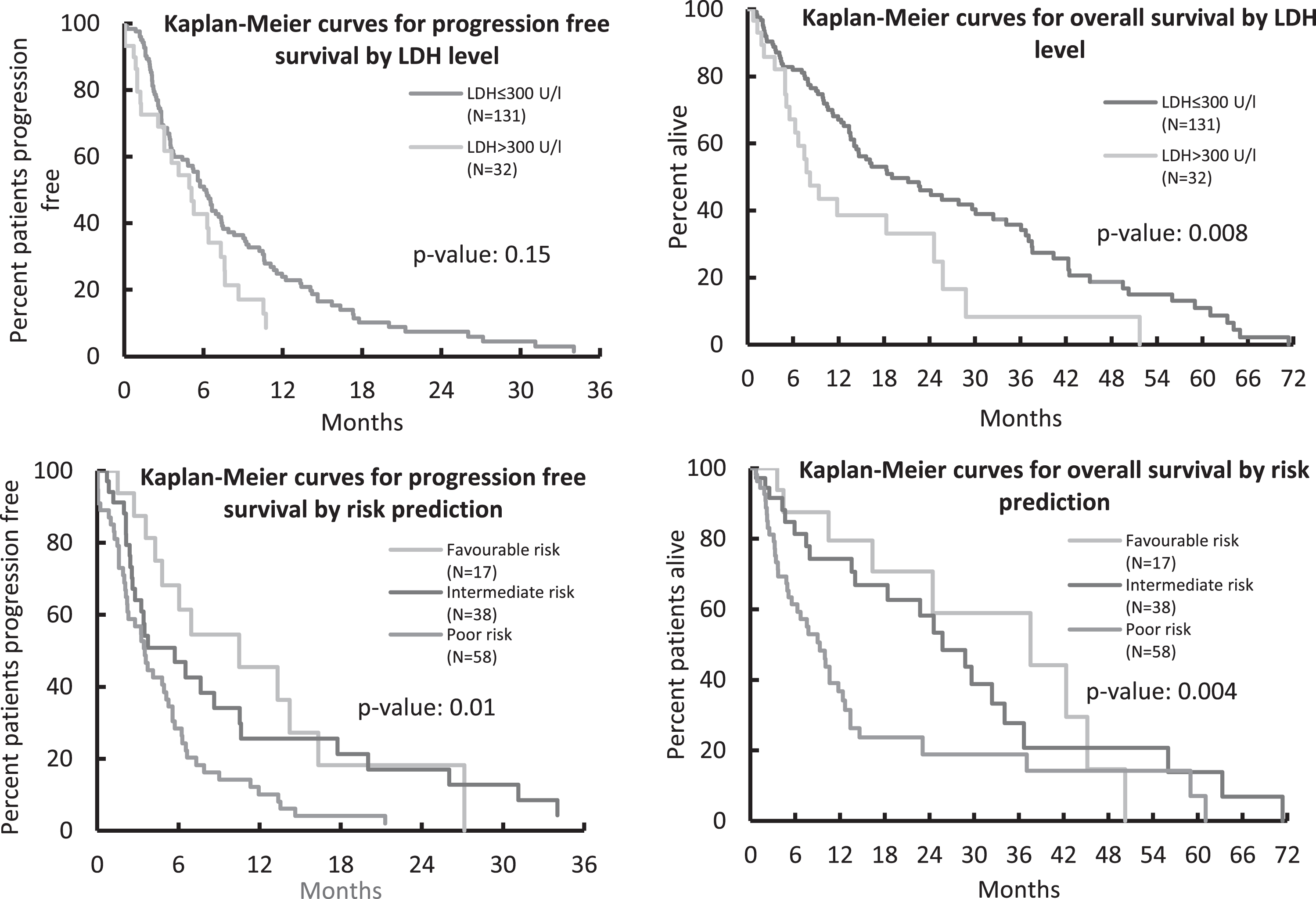
Clear cell RCC patients and those with non-clear cell histologies had similar outcomes in terms of PFS (5.6 and 5.8 months, p = 0.19) and OS (18.3 and 22.2 months, p = 0.28). Due to small numbers, non-clear cell histologies were not differentiated further.
Patients with high baseline LDH levels showed a worse OS and PFS than patients with LDH levels≤300 U/l (OS: 8.2 vs. 19.0 months, p = 0.008; PFS: 5.1 vs 6.2 months, p = 0.15). The corresponding Kaplan-Meier plots are also shown in Fig. 1.
3.4Adverse events
The 75 participating centers reported at least one treatment-related AE for a total of 119 patients (56.7%). CTCAE grade 3 and 4 events were reported in 37 patients (17.6%). The most frequently reported AEs were gastrointestinal disorders (37.6%), general disorders (21.0%), and skin and subcutaneous tissue disorders (16.2%). Vascular disorders (primarily hypertension of any grade) were reported in 7.6% of the patients. Table 4 shows all AEs which were reported with a frequency of≥2%, according to the Medical Dictionary for Regulatory Activities MedDRA [27].
Table 4
Axitinib-associated adverse events according to the Medical Dictionary for Regulatory Activities MedDRA [27]
| 2cn=210 | ||
| total (%) | Grade 3/4 (%) | |
| Total no. of patients with AE | 56.7 | 17.6 |
| Gastrointestinal disorders | 37.6 | 8.1 |
| General disorders | 21.0 | 4.3 |
| Skin and subcutaneous tissue disorders | 16.2 | 1.0 |
| Investigations* | 10.5 | 1.0 |
| Metabolism and nutrition disorders | 11.4 | 3.3 |
| Nervous system disorders | 9.5 | 3.3 |
| Respiratory, thoracic and mediastinal disorders | 9.5 | 1.9 |
| Vascular disorders | 7.6 | 2.4 |
| Endocrine disorders | 5.7 | 0.0 |
| Infections and infestations | 4.3 | 1.9 |
| Musculoskeletal and connective tissue disorders | 3.8 | 0.5 |
| Cardiac disorders | 2.4 | 1.4 |
| Blood and lymphatic system disorders | 2.9 | 0.0 |
| Neoplasms, benign, malignant and unspecified | 2.4 | 0.0 |
*Investigations: collective term for abnormal laboratory values, and examination results such as ECG- and echocardiography results.
4DISCUSSION
In the ever-evolving landscape of systemic treatment for a/mRCC, current guidelines advocate for combined targeting of various tumor pathways, including CPIs and TKIs [1–3]. Still, the real-world evidence on the effectiveness and adverse event profile on the TKI Axitinib as a single-agent is limited, in particular in a prospective multicenter setting in a European-based patient cohort.
Here, the results of the multicenter STAR-TOR Axitinib cohort are reported, which is one of the largest real-world Axitinib datasets. In this analysis, Axitinib yielded a median PFS of 5.6 months and OS of 18.3 months in a/mRCC patients in second-line treatment and beyond.
High serum LDH levels were identified as a potential prognostic marker of unfavorable outcome: LDH levels > 300U/l were associated with a clinically relevant shorter OS than LDH levels < 300U/l (8.2 vs. 19.0 months, p = 0.008).
The STAR-TOR real-world data compares well to the phase III AXIS trial evaluating Axitinib versus Sorafenib as second-line treatment in mRCC [6, 28]. The AXIS trial reported a median PFS of 4.8 months for patients pretreated with Sunitinib and median OS of 15.2 months, including 33% of MSKCC poor-risk patients.
To provide a comprehensive overview of the literature, Table 5 summarizes outcomes from studies evaluating Axitinib for a/mRCC. Compared to other studies, the STAR-TOR cohort included the highest proportion of MSKCC poor-risk patients (51.3% vs.15.5% and 37% in the studies by Facchini et al. and Rini et al.) [6, 9, 13–17].
Table 5
Selection of Axitinib real-world data (empty table element = no data available)
| n | Risk stratification, % | Overall response rate, % | PFS | OS | |||||||
| good | int. | poor | CR | PR | SD | PD | months | months | |||
| Rini et al., 2011 [6]; | 361 | 28 * | 37 * | 33 * | 0 | 19 | 50 | 22 | 6.7 | 20.1 | |
| Motzer et al., 2013 [28] | |||||||||||
| MacLean, 2016 [13] | 659 † | NA | NA | NA | NA | NA | NA | NA | 3.8 † | NA | |
| Hutson et al., 2017 [14] | 135 | NA | NA | NA | NA | NA | NA | NA | 4.6 Δ | NA | |
| Matias et al., 2017 [15] | 106 | 13 ‡ | 54 ‡ | 32 ‡ | 0 | 32 | 40 | 27 | 8.3 | 16.4 | |
| Miyake et al., 2017 [16] | 124 | 6.5 † | 67.7 † | 25.8 † | 0 | 16.9 | 70.2 | 9.7 | 9.3 | 27.0 | |
| Facchini et al., 2019 [17] | 148 | 24.3 ‡ | 60.1‡ | 15.5 ‡ | 0.6 | 16.0 | 54.0 | 29.4 | 7.1 | 15.5 | |
| Osawa et al., 2020 [18] | 485 | 12.2 ‡ | 64.3 ‡ | 19.6 ‡ | 2.3 | 19.8 | 56.1 | 20.6 | 13 | 34 | |
| Uhlig et al., this publication | 210 | 15.0 * | 33.6 * | 51.3 * | 1.5 | 15.7 | 34.0 | 26.4 | 5.6 | 18.3 | |
* MSKCC score for pretreated patients. † only second line subset (data for the total population not available), no PFS but duration of treatment given. ‡ IMDC risk score. Δ no PFS but duration of treatment given. Abbreviations: CR: complete remission, NA: Not Available; PR: partial remission, SD: stable disease, PD: progressive disease, PFS: progression-free survival, OS: overall survival.
Interestingly, despite the inclusion of a remarkable number of poor-risk patients, PFS and OS rates in the STAR-TOR registry are comparable to those reported in other studies. For example, Rini et al. described a median PFS of 6.7 months and OS of 20.1 months for their total study population and 4.8 and 15.2 months for the patients pretreated with Sunitinib, respectively. This compares well to 5.6 and 18.3 months in the STAR-TOR cohort, respectively [6, 28]. These observations could lead to the interpretation that even in poor-risk a/mRCC patients Axitinib yields a high effectiveness. Still, longer PFS and OS rates than reported in Caucasian patients were described in Japanese populations by Miyake et al. and Osawa et al., although the retrospective design and exclusion of roughly 10% of patients with treatment duration of less than 1 month in the latter trial may have distorted the results [16, 18].
While the overall benefit rate of Axitinib in the STAR-TOR registry with 50.7% was lower than that reported in other studies, these differences might partly result from missing data in almost 23% of the patients owing to the STAR-TOR registry's design. This assumption is further supported by a disease progression rate of 30.5%, which is comparable to the most recent Axitinib studies by Facchini et al. and Osawa et al. [17, 18]. We did not observe marked differences in response rates when comparing the therapeutic lines. Similarly, Osawa et al. did not identify treatment line as a significant predictor of OS [18]. Yet, Matias et al. reported significantly longer PFS and OS for patients in second-line treatment versus third or later line [15] A publication by Graham et al. investigated PFS and ORR for patients treated with VEGFR-TKIs (Axitinib, Sunitinib, Cabozantinib, Pazopanib, Bevacizumab, and Sorafenib). The authors reported for second, third, fourth and later lines increasing PFS with 3.8, 5.7, and 6.1 months but decreasing ORR of 23%, 22%, and 10% [29]. We suppose that a strong selection bias may lead to the observed effects.
Regarding Axitinib-related AEs, there is heterogeneous data from the STAR-TOR cohort. On one hand, the rate of gastrointestinal AEs of 37.6% (including 29% diarrhea) is consistent with data obtained from other real-world datasets: Facchini et al. described gastrointestinal disorders in 36.5% of their Axitinib patients and Osawa et al. reported 22.5% of the patients from a Japanese to suffer from diarrhea [17, 18]. On the other hand, the rate of vascular disorders in the STAR-TOR registry (mainly arterial hypertension) with 7.6% was unexpectedly low: Miyake et al. reported hypertension in 58.9% of patients, while hypertension was evident in 40% of patients in the AXIS trial [6, 16]. One reason for these discrepancies might be a higher prevalence of pretreated and thus compensated hypertension among real-world patients enrolled in the STAR-TOR registry compared to the randomized controlled AXIS trial. Also, varying comedications and comorbidities might contribute to the observed differences. Finally, worsening, or new-onset hypertension might be clinically underreported in the real-world setting, as elevated blood pressure could be deemed clinically less relevant than other treatment-related AEs, such as gastrointestinal disorders.
Patients’ serum LDH levels were identified as a prognostic marker for survival in this study, where patients with lower LDH levels had a larger clinical benefit from Axitinib treatment in terms of longer OS than those with high LDH levels (median OS 19 vs. 8.2 months, p = 0.008). While the prognostic potential of LDH levels has so far been not described for Axitinib, other authors have reported similar results with favorable outcomes in mRCC patients with low LDH levels treated with Temsirolimus or interferon alpha [30]. Furthermore, a high serum LDH level is well known to be associated with poor survival in other solid tumors [31].
Interestingly, similar results were reported for patients with clear-cell and non-clear-cell RCC treated with Axitinib in this study. Still, the results need to be interpreted carefully given the small number of non-clear-cell RCC patients in our analysis. In particular, since the authors of other publications reported worse treatment outcomes for non-clear-cell histologies [32, 33].
The here presented study is not devoid of limitations, which are mainly rooted in its design: First, there were missing data across several variables, which is inherent in the nature of a non-interventional trial. While this data unavailability could have distorted our findings, the monitoring of participating STAR-TOR centers reduced the risk of relevant bias. Nevertheless, missing MSKCC score data in almost 50% of cases may have impacted the power of our statistical comparisons of patient survival by risk group.
The design as a register study may yield a certain degree of imprecision regarding the outcome measures. Although all STAR-TOR sites were exhorted to use the RECIST 1.1. criteria, this was not mandatory and radiological assessment of treatment response may lack precision. Likewise, monitoring of adverse events may lack precision as not all physicians used CTCAE.
Second, this study does not provide information about the treatment effectiveness of Axitinib with regard to the type of first-line treatment received since these data were not captured due to the design of the STAR-TOR registry. Furthermore, our study does not include the effect of Axitinib in immunotherapy combinations. Given the recent approval of Axitinib in combination with Pembrolizumab or Avelumab as first-line treatment for mRCC, these data have not been generated in clinical routine registers yet, as patient accrual must increase to provide adequately powered analyses.
Third, our results have to be compared carefully to studies using other methods of risk stratification, such as the IMDC score, even though we consider the MSKCC score used in this study for pretreated patients as an adequate proxy.
Finally, one cannot discount some reporting bias in a phase IV observational, multicenter design like the STAR-TOR registry even though participating centers were randomly monitored. This restriction, however, applies for any data acquisition in the everyday clinical routine setting.
Nevertheless, our study provides important evidence contributing to the existing knowledge concerning Axitinib beyond first line. Notwithstanding the many publications involving treatment strategies with Axitinib, data on real-world experience is sparse:
Our literature search yielded only five other real-world studies [13, 15–18]. Yet, the patient cohorts of Matias et al., Miyake et al., and Facchini et al. were of only about half the size than our study cohort with 210 individuals [15–17]. In addition, the largest publication by Mac Lean et al. with 659 patients did not report on adverse events [13]. Therapeutic lines later than second line were analyzed only by three studies with the publication by Osawa et al. presenting no data beyond third line [13, 15, 18]. Our study presents multicenter data what also holds true only for three of the other the publications [13, 17, 18]. As the studies by Miyake et al. and Osawa et al. report exclusively data on Japanese patients there seems to be limited comparable evidence for European patient populations [16, 18]. Another important point is that except our study only the publication by Matias et al. reports on prospectively collected data [15].
5CONCLUSIONS
This real-world study confirms the clinical relevance of Axitinib for a/mRCC treatment. Its effectiveness is not only of value in the immunotherapy combinations but also as single-agent in the second-line and beyond setting. Although a high proportion of patients with unfavorable MSKCC poor-risk group were included, OS and PFS were in concordance with pivotal trials, while demonstrating a favorable safety profile. A high LDH serum level seems to be a negative prognostic marker for Axitinib effectiveness, which can aid in clinical decision making.
ACKNOWLEDGMENTS
The STAR-TOR registry was funded by Pfizer Pharma GmbH, Berlin, Germany. Pfizer Pharma GmbH provided the data and the statistical analyses which were conducted by Thomas Fischer, Winicker-Norimed GmbH, Nuremberg, Germany.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
Conceptualization of the research project: AU, JU, MW, ML, AS
Design of the Methodology: AU, JU, MW, TF
Data collection; LT, LB, MB, PJG, MR, KS, ML, AS
Data curation: MW, TF
Contribution to data analysis: AU, JU, MW, TF
Visualization: TF
Writing of the manuscript: AU, JU, MW, ML, AS
Reviewing and editing: TF, LT, LB, MB, PJG, MR, KS
ETHICAL CONSIDERATIONS
The STAR-TOR registry received prior approval by the ethics committee of Münster University Hospital in 2007 (Nr.2007-484-f-S) and was registered in the US library of medicine database (NCT00700258).
CONFLICT OF INTEREST
Michael Woike is an employee of Pfizer Germany. Annemarie Uhlig, Johannes Uhlig, Thomas Fischer, Lutz Trojan, Lothar Bergmann, Martin Bögemann, Peter J. Goebell, Michael Rink, Katrin Schlack, Marianne Leitsmann, and Arne Strauß have no conflicts of interest to disclose.
REFERENCES
[1] | Ward RD , Tanaka H , Campbell SC , Remer EM AUA Renal Mass and Localized Renal Cancer Guidelines: Imaging Implications. Radiographics. (2018) ;38: (7):2021–2033. |
[2] | Bedke J , Albiges L , Capitanio U et al. Updated European Associationof Urology Guidelines on Renal Cell Carcinoma: Nivolumab plusCabozantinib Joins Immune Checkpoint Inhibition CombinationTherapies for Treatment-naïve Metastatic Clear-Cell Renal CellCarcinoma. Eur Urol. (2021) ;79: (3):339–342. |
[3] | Ljungberg B , Albiges L , Abu-Ghanem Y et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The Update. European Urology. (2019) ;75: (5):799–810. |
[4] | Rini BI , Plimack ER , Stus V et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. New England Journal of Medicine. (2019) ;380: (12):1116–1127. |
[5] | Motzer RJ , Penkov K , Haanen J et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. New England Journal of Medicine (2019) ;380: 12:1103–1115. |
[6] | Rini BI , Escudier B , Tomczak P et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. The Lancet. (2011) ;378: (9807):1931–1939. |
[7] | Mcdermott DF , Lee J-L , Bjarnason GA et al. Open-Label, Single-Arm Phase II Study of Pembrolizumab Monotherapy as First-Line Therapy in Patients With Advanced Clear Cell Renal Cell Carcinoma. Journal of Clinical Oncology. (2021) ;39: 9:1020–1028. |
[8] | Donskov F , Mcdermott DF , Lee JL et al. KEYNOTE-427 cohort A: Pembrolizumab monotherapy as first-line therapy in advanced clear cell renal cell carcinoma (ccRCC). Annals of Oncology. (2018) ;29: , viii307. |
[9] | Vaishampayan U , Schöffski P , Ravaud A et al. Avelumab monotherapy as first-line or second-line treatment in patients with metastatic renal cell carcinoma: phase Ib results from the JAVELIN Solid Tumor trial. J Immunother Cancer. (2019) ;7: (1):275. |
[10] | Graham J , Shah AY , Wells JC et al. Outcomes of Patients with Metastatic Renal Cell Carcinoma Treated with Targeted Therapy After Immuno-oncology Checkpoint Inhibitors. European Urology Oncology. (2021) ;4: (1):102–111. |
[11] | Goebell PJ , Staehler M , Müller L et al. Changes in Treatment Reality and Survival of Patients With Advanced Clear Cell Renal Cell Carcinoma 
nalyses From the German Clinical RCC-Registry. Clinical Genitourinary Cancer. (2018) ;16: (6):e1101–e1115. |
[12] | Wenzel M , Deuker M , Nocera L et al. Median time to progression with TKI-based therapy after failure of immuno-oncology therapy in metastatic kidney cancer: A systematic review and meta-analysis. Eur J Cancer. (2021) ;155: :245–255. |
[13] | Maclean E , Cisar L , Mehle K , Eremina D , Quigley JM Real-World Axitinib Use in the United States: A Retrospective Study Using Linked Datasets. J Manag Care Spec Pharm. (2016) ;22: (6):723–732u. |
[14] | Hutson TE , Al-Shukri S , Stus VP et al. Axitinib Versus Sorafenib in First-Line Metastatic Renal Cell Carcinoma: Overall Survival From a Randomized Phase III Trial. Clin Genitourin Cancer. (2017) ;15: (1):72–76. |
[15] | Matias M , Le Teuff G , Albiges L et al. Real world prospective experience of axitinib in metastatic renal cell carcinoma in a large comprehensive cancer centre. Eur J Cancer. (2017) ;79: :185–192. |
[16] | Miyake H , Harada KI , Ozono S , Fujisawa M Assessment of Efficacy, Safety, and Quality of Life of 124 Patients Treated With Axitinib as Second-Line Therapy for Metastatic Renal-Cell Carcinoma: Experience in Real-World Clinical Practice in Japan. Clin Genitourin Cancer. (2017) ;15: (1):122–128. |
[17] | Facchini G , Rossetti S , Berretta M et al. Second line therapy with axitinib after only prior sunitinib in metastatic renal cell cancer: Italian multicenter real world SAX study final results. J Transl Med. (2019) ;17: (1):296. |
[18] | Osawa T , Kojima T , Hara T et al. Oncological outcomes of amulticenter cohort treated with axitinib for metastatic renal cellcarcinoma. Cancer Science. (2020) ;111: (7):2460–2471. |
[19] | Boegemann M , Hubbe M , Thomaidou D et al. Sunitinib Treatment Modification in First-Line Metastatic Renal Cell Carcinoma: Analysis of the STAR-TOR Registry. Anticancer Research. (2018) ;38: (11):6413–6422. |
[20] | Schrader AJ , Seseke S , Keil C et al. Temsirolimus in daily use: results of a prospective multicentre noninterventional study of patients with metastatic kidney cancer. Eur Urol. (2014) ;66: (2):275–281. |
[21] | Boegemann M , Goebell PJ , Woike M , Buncke J , Schlack K , Schrader AJ Assessment of prognosis by established prognosis scores and physicians’ judgement in mRCC patients: an analysis of the STAR-TOR registry. Translational Andrology and Urology. (2021) ;10: (10):4062–4074. |
[22] | Uhlig A , Uhlig J , Trojan L , Woike M , Leitsmann M , Strau\ensuremath ß A Toxicities of axitinib, sunitinib and temsirolimus: implications for progression-free and overall survival in metastatic renal cell cancer. Future Oncology. (2021) ;17: (1):45–56. |
[23] | Strauss A , Schmid M , Rink M et al. Real-world outcomes in patients with metastatic renal cell carcinoma according to risk factors: the STAR-TOR registry. Future Oncology. (2021) ;17: (18):2325–2338. |
[24] | Motzer RJ , Bacik J , Schwartz LH et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. (2004) ;22: (3):454–463. |
[25] | Eisenhauer EA , Therasse P , Bogaerts J et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1). Eur J Cancer. (2009) ;45: (2):228–247. |
[26] | Health UDO , Services H Common Terminology Criteria for Adverse Events. Version 5.0. Published November 27, 2017. ((2020) ). |
[27] | Brown EG , Wood L , Wood S The medical dictionary for regulatory activities (MedDRA). Drug Saf. (1999) ;20: (2):109–117. |
[28] | Motzer RJ , Escudier B , Tomczak P et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. (2013) ;14: (6):552–562. |
[29] | Graham J , Wells JC , Mckay R et al. Clinical outcomes of patients with metastatic renal cell carcinoma (mRCC) treated with vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitors (TKI) and mammalian target of rapamycin inhibitors (mTORI) after immuno-oncology (IO) checkpoint inhibitors. Annals of Oncology. (2018) ;29: :viii315. |
[30] | Armstrong AJ , George DJ , Halabi S Serum lactate dehydrogenase predicts for overall survival benefit in patients with metastatic renal cell carcinoma treated with inhibition of mammalian target of rapamycin. J Clin Oncol. (2012) ;30: (27):3402–3407. |
[31] | Petrelli F , Cabiddu M , Coinu A et al. Prognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studies. Acta Oncol. (2015) ;54: (7):961–970. |
[32] | Schwab M , Hofmann R , Heers H , Hegele A mRCC Outcome in the Treatment of Metastatic Renal Cell Carcinoma – A German Single-center Real-world Experience. In Vivo. (2018) ;32: (6):1617–1622. |
[33] | Costello BA , Bhavsar NA , Zakharia Y et al. A Prospective Multicenter Evaluation of Initial Treatment Choice in Metastatic Renal Cell Carcinoma Prior to the Immunotherapy Era: The MaRCC Registry Experience. Clinical Genitourinary Cancer. (2022) ;20: (1):1–10. |
Appendices
Appendix:
Table A
Response rates by therapeutic line
| Best response | ||||||
| CR | PR | SD | PD | NA | sum | |
| therapeutic line | ||||||
| 2nd | 3 (2.9) | 17 (16.2) | 31 (29.5) | 32 (30.5) | 22 (21.0) | 105 |
| 3rd | 0 (0.0) | 10 (18.5) | 19 (35.2) | 11 (20.4) | 14 (25.9) | 54 |
| 4th | 0 (0.0) | 5 (23.8) | 7 (33.3) | 6 (28.6) | 3 (14.3) | 21 |
| ≥5th | 0 (0.0) | 3 (11.1) | 10 (37.0) | 7 (25.9) | 7 (25.9) | 27 |
| Sum | 3 (1.4) | 35 (16.9) | 67 (32.4) | 56 (27.1) | 46 (22.2) | 207 |
Abbreviations: CR: complete remission, NA: Not Available; PR: partial remission, SD: stable disease, PD: progressive disease.




