The Current Status of Kidney Cancer Urine Markers – A Systematic Review
Abstract
BACKGROUND:
Renal cell carcinoma is the 9th most common malignant disease in the Western World. Typically, patients develop symptoms in a late stage of the disease and most of them are diagnosed by chance. Up to 30% of the patients at the time of diagnosis had metastatic disease. Therefore, highly specific and sensitive biomarkers for the detection and progression of kidney cancer are of great importance. Here, urine markers can be a major advantage and can have a huge clinical impact on the diagnosis, differentiation and prognosis of kidney cancer. At the moment there are several approaches to improve these conditions..
METHODS:
A systematic literature research was performed according to the PRISMA guidelines to identify studies reporting urine markers for kidney cancer between 2012 and 2021. A two-step process for the selection of the studies was initiated. In total 287 studies were considering for the final analysis. In total, 6 studies, which presented potential urinary biomarker were analyzed in depth.
RESULTS:
The major focus was on urinary markers for the detection, progression and differentiation of renal cell carcinoma. In total, a study population of 1099 patients were investigated in the different studies that were analyzed in depth. The median patient sample size of the different studies was 157 patients. The focus was based on the investigation of different microRNAs and proteins as urinary marker for kidney cancer detection.
CONCLUSION:
Overall, there are different approaches present for the detection, prognosis and differentiation of kidney cancer in urine but most of the studies are based on a small sample size and need to be validated in a greater collective. Furthermore, the standard should be improved to bring these biomarkers into routine clinical practice.
INTRODUCTION
Renal cell carcinoma (RCC) accounts 2% of global cancer diagnosis and is the 9th most common maligned disease in the Western World [1]. This group of malignant tumors comprises 80 to 90% of all malignant kidney tumors and 70% of all solid kidney tumors [2]. RCC is more frequent in men than in women and most of the cases are diagnosed between the 4th and 6th decade of life [3]. RCC present with a wide range of symptoms. This is a major challenge in diagnosing RCC. Most of the patients are asymptomatic and only become symptomatic in a late-stage of the disease. Asymptomatic patients generally only receive their diagnosis as an incidental finding [4, 5]. The classical symptoms like flank pain, palpable abdominal mass and hematuria develop at an advanced stage of the disease [6]. Up to 30% of the diagnosed patients already, have metastatic disease, especially in those patients with a tumor size larger than 4 cm [4, 5]. However, approximately, 20% of the large tumors (larger than 3 cm) are not malignant [5].
The development of highly sensitive and specific liquid biomarkers for the detection of renal cell carcinoma is of particular great interest, and some even allow differentiation between the different renal cancer subtypes. Blood and urine are especially useful sources for biomarkers detection, since the material is easily to get [6]. Biomarkers are defined as objective, quantifiable characteristics of biological processes that can measure a physiological state [7]. In the case of renal tumors, the analysis of liquid biomarkers from blood or urine seems to be a very interesting approach, since this kind of marker has the ability to improve diagnosis in the clinic [6]. The use of urine biomarkers is an easy approach to improve the diagnosis, prognosis or even the differentiation of kidney cancers. Therefore, a systematic review on the current status of potential urine biomarkers for the detection of kidney cancers was performed.
MATERIALS AND METHODS
Search strategy
A systematic literature analysis was performed according to the PRISMA guidelines [8]. The publications used in this systematic review were obtained from PubMed and published between 2012 and 2021. Here, a free hand search was performed by using different keywords and the following keywords were used in combination: kidney cancer biomarker and kidney cancer urine marker. The selection process was done by two stages. In the first stage non-English articles and non-original articles (including reviews, books, meta- analyses and clinical trials) and repeated publications were excluded. In the second stage, literature of irrelevant studies after full text analysis was excluded. Here, the full text accesses were due to open access publications and publications, which were readable due to university accesses. Furthermore, journals not listed in PubMed were manually screened to avoid any missing and eligible study. Also, literature was excluded for the detection of rare kidney tumors. This includes studies that do not analyze the potential marker within a patient cohort or studies which do not present any potential urine marker for kidney cancer.
Data extraction
The data were extracted independently by two authors. Afterwards a double-check was performed. In this study the following data were extracted: number of patients, outcome of the study. All extracted data were double checked to exclude any double data.
RESULTS
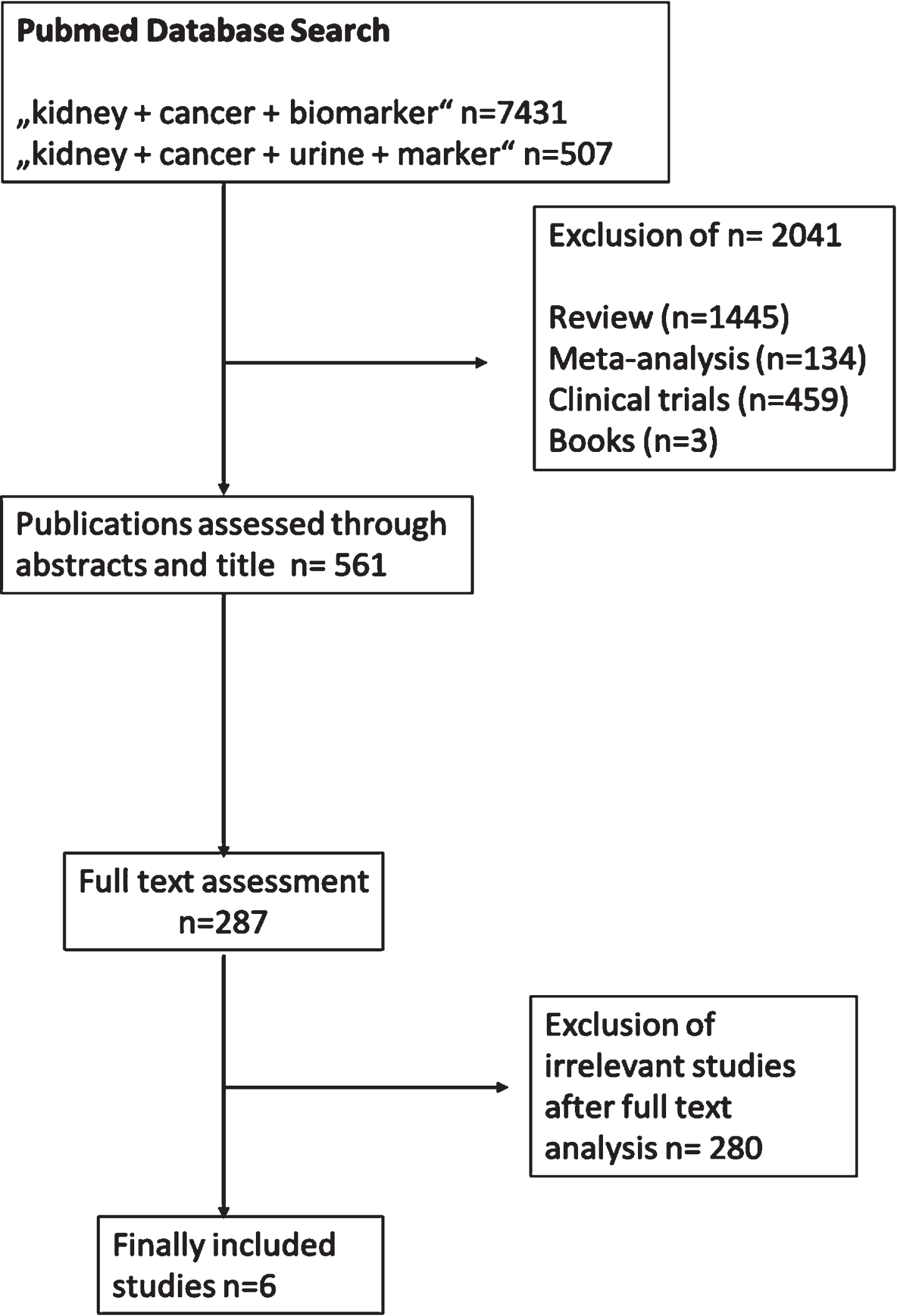
The selection process is presented by a CONSORT diagram (Fig. 1). The initial online search for the different keywords presented 7938 publications. In total, 2321 of the publications were excluded based of a two-step selection process. In the first selection step 2041 publications were excluded. Overall, 287 reached the full text accesses. Full text accesses were due to open access publications and university accesses. After a second screening of the publications in total, 280 non-relevant studies were excluded. Finally, 6 different studies were included in the analysis. These include two different kinds of potential urinary biomarker (microRNAs and proteins) for kidney cancer. Furthermore, all these studies include the analysis of patient material. The main focus of the different studies were the diagnosis and differentiation of the clear renal cell carcinoma.
Fig. 1
Consort diagram, which present the selection process of the included studies.
In total 1099 patients were analyzed in the different studies, which includes healthy controls, different types of kidney tumors and other urological malignancies (like prostate cancer and urothelial carcinoma). The other urological malignancies were used to proof and validate the potential urinary marker. The median number of included patients was 157. The biggest study was performed by von Brandenstein et al., and Outeriro-Pinho et al., [9, 10]. Here, a study of approximately 350 patients was analyzed, including healthy controls. In the study from von Brandenstein et al., two different proteins called Vimentin3 (Vim3) and Mxi-2 were investigated as markers for the differentiation between Oncocytoma (benign kidney tumor) and renal cell carcinoma (RCC) [9]. These proteins are truncated versions of their full length versions which differ in their biological function [11]. The two different entities of kidney tumors present different expression patterns. Significantly high Vim3 levels were detectable in urine samples from patients with Oncocytoma, whereas significant high levels of Mxi-2 were found in urine samples from patients with RCC [9]. The second biggest study was performed by Outeriro-Pinho et al., [10]. Here, a downregulation of the miR-30a-5p is associated with clear cell renal cell carcinoma (ccRCC) [10]. This downregulation seems to be a general mechanism only found in RCC. Armed with the understanding of this mechanism Outeriro-Pinho et al., tried to identify patients with an increased risk of RCC progression [10]. These two major studies describe the two potential sources of urinary markers for RCC.
One focus of new potential urine markers was the analysis of microRNAs (miRs), which are small non-coding RNAs. The main function of miRs is to bind to the 3’ UTR of the mRNA resulting in the downregulation of this mRNA [12]. In 50% of the 6 final studies, the expression of different miRs were analyzed. The other 50% of the evaluated studies predominantly focus on proteins as a source for urine markers. In the study from Song et al., [13] different candidates were first identified by Next Generation Sequencing. Here the exosomal expression of the miR-30c-5p in urine samples from RCC patients was analyzed in depth. A significant downregulation of the miR-30c-5p was identified in urine samples from RCC patients, compared with healthy controls. Furthermore, it was also possible for the authors to show that the overexpression of the miR-30c-5p is associated with a decrease of progression in RCC [13]. The analysis of components of exosomes can also be used as urinary marker. Exosomes are cell derived vesicles and can be secreted by almost all type of cells, including tumor cells [14, 15]. These membranes bound particles contain various biomolecules, including proteins, mRNAs and miRs from the secreted cells [14]. A third miR, which was described as a novel kidney cancer urine marker is the miR-210 [16]. Li et al., report that the overexpression of the miR-210 was only found in urine samples from RCC patients. Furthermore, they separate the RCC patients into three different stages, although differentiation between the stages was not possible [16]. Therefore, it could be assumed that the urinary marker could be used for the diagnosis of RCC.
The second type of urinary marker for kidney cancer detection is based on protein levels. In addition to the analysis of Vim3 and Mxi-2 for the differentiation between Oncocytoma and clear cell RCC, a third protein was described as marker for the detection of renal cell carcinomas, named kidney injury protein-1 (KIM-1) [17, 18]. KIM-1 was investigated by different groups including those of Zhang et al., and Bialek et al., in 2021. In the study by Zhang et al., [17] the expression of the protein was found in renal cell carcinoma and it was possible to show that the levels of KIM-1 were downregulated after surgery. In the study by Bialek et. al., [18] the expression of KIM-1 was compared between urine samples from RCC patients with urine samples from urothelial carcinoma patients. Here, the authors identify that the KIM-1 urine expression is higher in urine samples from urothelial carcinoma patients than in urine samples from RCC patients [18].
Table 1
Evaluated studies, by comparing the study size, the aim of the test and the method used method for the detection of the urine biomarker. In the last column, the results of the study are summarized
| Author | Year | Publication title | Study size | Aim of the biomarker | Technique | Results |
| Zhang et al., [17] | 2014 | Urine kidney injury molecule 1: a potential non-invasive biomarker for patients with renal cell carcinoma | Kidney tumor = 19 | Detection of renal cell carcinoma | TMA, ELISA | Downregulation of KIM-1 positive patients (evaluated via TMA) after surgery –KIM-1 positivity was found in malignant and not in benign kidney tumors |
| Li et al., [16] | 2017 | Detection of urinary cell-free miR-210 as a potential tool of liquid biopsy for clear cell renal carcinoma | Control n = 45 RCC n = 75 | Detection of renal cell carcinoma | qRT-PCR | Overexpression of cell-free miR-210 was detected in RCC patients –a downregulation of the miR was found in patients after surgery |
| Song et al., [13] | 2019 | Urinary exosome miR-30c-5p as a biomarker for clear renal cell carcinoma that inhibits progression by targeting HSPA5 | Control n = 30 Bladder cancer n = 30 Prostate cancer n = 30 RCC n = 70 | Detection of renal clear cell carcinoma | Next-generation Sequencing, qRT-PCR | Analysis of candidates was performed by Next-Generation Sequencing- Identification of the exosomal miR-30c-5p as a potential marker for the diagnosis of RCC |
| Outeiro-Pinho et al., [10] | 2020 | MicroRNA-30a-5pme: a novel diagnostic and prognostic biomarker for clear renal cell carcinoma in tissue and urine samples | RCC n = 224 Control n = 142 | Detection and prognosis of renal clear cell carcinoma | qRT-PCR | Downregulation of the miR 30a-5p seems a common mechanism in RCC and can be used for diagnosis and prognostic purposes |
| Von Brandenstein et al., [9] | 2021 | Non-invasive urine marker for the differentiation between RCCs and oncocytoma | Control n = 40 Oncocytoma n = 20 Chromophobe RCC n = 50 Papillary RCC n = 40 RCC n = 200 | Differentiation between renal cell carcinoma and oncocytoma | ELISA | Vim3 and Mxi-2 are highly specific markers, which allow the differentiation between RCC and benign Oncocytoma –here also a pre-surgical differentiation was possible |
| Bialek et al., [18] | 2021 | Human kidney injury molecule-1 as a urine biomarker for differentiating urothelial and renal cell carcinoma | Kidney cancer n = 30 Urothelial carcinoma n = 27 | Differentiation between urothelial and renal cell carcinoma | ELISA | Expression of KIM-1 allows the differentiation between RCC and urothelial carcinoma –can maybe support pre-surgical decisions |
DISCUSSION
The aim of the systematic review was the comparison of kidney cancer urine markers and to provide a comprehensive overview of the studies performed. To reduce biases the studies were carefully identified (by two independent persons) and included in this overview of the current state of urine marker for kidney cancer. One major challenge in the diagnosis of kidney cancer is the heterogenicity of the disease. Renal masses can be divided into either begin, clinically indolent or up to very aggressive [7]. Currently, the most important factor for the prognosis of kidney cancer is the pathological classification [5]. Therefore, the development of biomarkers for the early detection, differentiation or diagnosis would have a major clinical impact. That’s a reason why the focus should be on the development of these kind of biomarkers. Another factor, why the development of such biomarkers is of great important is the fact that small renal masses are often indolent. This can lead to overdiagnosis and overtreatment [19]. Therefore, the development of such markers would be a major benefit to reduce overdiagnosis and overtreatment and reduce costs.
In the field of markers for kidney tumors, there are a lot of different approaches. Most of the studies were based on the diagnosis of RCC [10, 13, 16, 17]. Based on the fact that kidney cancer is typically diagnosed in a late stage, this could have a huge clinical impact. Another use for urinary markers is the differentiation between urothelial and renal cell carcinomas [18] and one candidate allows differentiation between Oncocytoma and RCC [9]. The differentiation between different entities has also a high clinical impact, especially when tumors belong to a very heterogenies group. A further major impact of this study is that these potential non-invasive biomarkers also allow the detection of small renal tumors [9]. Concluding, these different approaches indicate the importance of the identification of urinary markers for the detection of renal cell carcinoma. Studies can be divided in two different usable biomarkers detection methods, either the identification of miRs or the expression of proteins. The first study of urine miR as a RCC biomarker was described by Brandenstein et al., in 2012. Since that timepoint the number of publications on miRs in RCC has increased [3, 20]. These research groups also identified a second miR, which allows the detection of renal Oncocytoma [21]. The analysis of miRs as a urinary biomarker for the detection of RCC could be a promising tool [3]. Nevertheless, the analysis of miRs as a biomarker is relatively time consuming and expensive. Furthermore, the results are frequently dependent on the housekeeping gene for neutralization as well as the qRT-PCR reader [22]. In addition, to standardize the analysis of miRs as a urinary biomarker for the detection of RCC is also a challenge, which should be solved before adoption in routine clinical practice [3]. Consequently, the analysis of proteins as biomarkers is a promising alternative, since it is less time consuming, inexpensive and the determination of protein levels is quite stable. One example for a rapid generation of urine-based results was developed by von Brandenstein et al. in form of a lateral flow assay [9].
One difference in the studies was the sensitivity and specificity of the presented urine protein-based markers. Here, KIM-1 shows a specificity of 73,3% and sensitivity of 62.6% whereas Mxi-2 has a specificity of 82,4% and a sensitivity of 90,2%. [9, 18]. These two different markers had a specificity over 70% but only Mxi-2 had a sensitivity higher than 70% (slightly over 90%). The low sensitivity of the KIM-1 analysis in RCC urine can be could be a result of the small sample size. In this study only 57 patients were analyzed. For all miR-based urinary markers the sensitivity and specificity look quite similar compared to the protein-based markers. The three miR-based markers all reached a specificity of over 80%, and the analysis of the exosomal miR-30c-5p even reached a specificity of 100% [13]. For the sensitivity the values look quite different. Nevertheless, only the analysis of the miR-30a-5p reached a sensitivity over 80% [10]. The two other studies only reached a sensitivity between 57- 68%. This could be further increased by an increase in the general sample size of the study, since in this study the used collective and the study sizes were very small.
A major limitation of the 6 different analyzed studies was the small collective. The median of all studies was 157 participants but this number was only reached by two studies which tested around 350 participants. All the other studies featured a much smaller collective. This small number of participants has a high impact on the statistical power of the analysis and causes an increase of bias within the study [23]. To identify a solid and usable urinary marker studies should increase the sample size.
One finding of the systematic review was that the evaluation of potential urinary markers for the diagnosis, prognosis and differentiation of kidney cancer is part of the current research but not the main focus. At the moment the number of described blood-based markers for kidney tumors is much higher [7]. However, while liquid biomarker analysis is of great importance, we also need tissue-based markers [7].
One major advantage of the development of urine biomarkers is that the analysis of these markers is minimally invasive, safer and easy to evaluate. In addition, the analysis is easily repeated, allowing a continuous monitoring in the progression of the disease. This narrow control also allows a rapid switch in the case for therapy by any changes [19].
CONCLUSION
To summarize the data from this systematic review, it can be said that there is a focus on the development on markers for the detection of malignant kidney tumors but most of them were performed in small patient collectives resulting in statistical problems. At the moment there is no commercially available biomarker for the detection or for the progression of kidney cancer. Therefore, the focus should be still on the development of urine markers for the detection, differentiation and prognosis of kidney cancer to allow their routine use in the clinic.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTION
BK and MVB: conception, design, interpretation of the results, manuscript writing. AH supported the interpretation of the results and performed manuscript review.
CONFLICT OF INTEREST
BK reports no relevant conflicts of interest.
AH reports no relevant conflicts of interest.
MVB reports no relevant conflicts of interest.
REFERENCES
[1] | Padala SA , et al. Epidemiology of Renal Cell Carcinoma. World J Oncol. (2020) ;11: (3):79–87. |
[2] | Hancock SB , Georgiades CS . Kidney Cancer. Cancer J. (2016) ;22: (6):387–92. |
[3] | Oto J , et al. Urinary microRNAs: Looking for a New Tool in Diagnosis, Prognosis, and Monitoring of Renal Cancer. Curr Urol Rep. (2020) ;21: (2):11. |
[4] | Iafolla MAJ , et al. Systematic Review and STARD Scoring of Renal Cell Carcinoma Circulating Diagnostic Biomarker Manuscripts. JNCI Cancer Spectr. (2020) ;4: (5):pkaa050. |
[5] | Gray RE , Harris GT . Renal Cell Carcinoma: Diagnosis and Management. Am Fam Physician. (2019) ;99: (3):179–84. |
[6] | Pastore AL , et al. Serum and urine biomarkers for human renal cell carcinoma. Dis Markers. (2015) ;2015: :251403. |
[7] | Farber NJ , et al. Renal cell carcinoma: the search for a reliable biomarker. Transl Cancer Res. (2017) ;6: (3):620–32. |
[8] | Liberati A , et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) ;339: :b2700. |
[9] | von Brandenstein M , et al. Non-invasive urine markers for thedifferentiation between RCCs and oncocytoma. J Clin Lab Anal. (2021) ;35: (5):e23762. |
[10] | Outeiro-Pinho G , et al. MicroRNA-30a-5p(me): a novel diagnostic and prognostic biomarker for clear cell renal cell carcinoma in tissue and urine samples. J Exp Clin Cancer Res. (2020) ;39: (1):98. |
[11] | von Brandenstein M , et al. Beyond the 3’UTR binding-microRNA-induced protein truncation via DNA binding. Oncotarget. (2018) ;9: (67):32855–67. |
[12] | von Brandenstein M , Richter C , Fries JW . MicroRNAs: Small but amazing, and their association with endothelin. Life Sci. (2012) ;91: (13-14):475–89. |
[13] | Song S , et al. Urinary exosome miR-30c-5p as a biomarker of clear cell renal cell carcinoma that inhibits progression by targeting HSPA5. J Cell Mol Med. (2019) ;23: (10):6755–65. |
[14] | Huda MN , et al. Potential Use of Exosomes as Diagnostic Biomarkers and in Targeted Drug Delivery: Progress in Clinical and Preclinical Applications. ACS Biomater Sci Eng. (2021) ;7: (6):2106–49. |
[15] | Wong CH , Chen YC . Clinical significance of exosomes as potential biomarkers in cancer. World J Clin Cases. (2019) ;7: (2):171–90. |
[16] | Li G , et al. Detection of urinary cell-free miR-210 as a potential tool of liquid biopsy for clear cell renal cell carcinoma. Urol Oncol. (2017) ;35: (5):294–9. |
[17] | Zhang PL , et al. Urine kidney injury molecule- a potential non-invasive biomarker for patients with renal cell carcinoma. Int Urol Nephrol. (2014) ;46: (2):379–88. |
[18] | Bialek L , et al. Human kidney injury molecule-1 as a urine biomarker differentiating urothelial and renal cell carcinoma. Cent European J Urol. (2021) ;74: (3):295–9. |
[19] | Marchioni M , et al. Biomarkers for Renal Cell Carcinoma Recurrence: State of the Art. Curr Urol Rep. (2021) ;22: (6):31. |
[20] | von Brandenstein M , et al. MicroRNA 15a, inversely correlated to PKCalpha, is a potential marker to differentiate between benign and malignant renal tumors in biopsy and urine samples. Am J Pathol. (2012) ;180: (5):1787–97. |
[21] | von Brandenstein M , et al. MicroRNAs as Urinary Biomarker for Oncocytoma. Dis Markers. (2018) ;2018: :6979073. |
[22] | Koshiol J , et al. Strengths and limitations of laboratory procedures for microRNA detection. Cancer Epidemiol Biomarkers Prev. (2010) ;19: (4):907–11. |
[23] | Button KS , et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. (2013) ;14: (5):365–76. |




