Geographical Differences in Kidney Cancer Outcomes of Patients Treated with Immunotherapy: A Systematic Review
Abstract
BACKGROUND:
Immune checkpoint inhibitors (ICI) have shown clinical benefit among patients with advanced kidney cancer. Their cost burden hardens its access, especially in low- and middle-income countries. To set solutions, the impact of geographical and socioeconomic differences in the clinical outcomes and survival of renal cell carcinoma (RCC) patients needs to be explored.
OBJECTIVE:
This review aimed to understand if geographical differences affected the clinical outcomes of RCC patients receiving immunotherapy.
METHODS:
This study reviewed 45 studies that examined the OS and PFS of RCC patients undergoing ICI (2010–2020) selected from a 3028-study database search conducted on PubMed and grey literature. The selected studies were divided into groups: Asia, multicentric studies, Europe and Anglo-America. The lethality and income of the geographical locations were measured and discussed.
RESULTS:
Weighted average (WAVG) of mPFS and mOS were 8,47 months, and 40,6 months in Asia. The WAVG of mOS were 12.2 months, and 20.22 months in the Anglo-American population (15 studies; 943 patients). In multicentric studies (4 studies; 1834 patients) the WAVG mPFS was 10,06. European group (13 studies; 3143 patients) had 6.1 and 20.24 months mPFS and mOS, respectively. The exploratory analysis on income and RCC lethality has shown an absolute decline of 8.7% (CI 10.1 to 7.3% - p < 0.05) in RCC lethality, when income is raised by 100%.
CONCLUSION:
Clinical benefit from ICI varies across the globe. A wide access to ICI, and evaluation of biological aspects of the disease will allow a better understanding of the impact of geographic regions in the clinical outcome of patients receiving ICI and the etiology of potential differences.
INTRODUCTION
Renal Cell Carcinoma (RCC) accounts for approximately 73,750 new cases and 14,830 cancer deaths yearly in the US, representing more than 400,000 new cases, and 175,000 deaths worldwide as reported by GLOBOCAN [1, 2]. In 2017, it represented an estimated 3,200,000 Disability-Adjusted Life Years (DALYs) globally, which indicates its overall importance and Global Burden of Disease (GBD) [3]. Although incidence rates for many cancer types are 2 to 3-fold higher in countries with transitioned economies when compared to the ones transitioning, the mortality rates are not so different. This is in part explained due to a higher case fatality in countries with a lower Human Development Index (HDI) [2].
According to 2017 data on RCC incidence, Southern Latin America (11.6), along with high-income North America (12.1) and Eastern Europe (10.0), had the highest age-standardized incidence rates, while South Asia (1.9), Eastern Sub-Saharan Africa (2.5), and Central Sub-Saharan Africa (2.7) had the lowest rates. As to mortality, Southern Latin America (4.3), Central Europe (3.8), and Eastern Europe (3.8) had the highest age-standardized death rates, and the regions with the lowest rates were South Asia (0.62), Eastern Sub-Saharan Africa (0.77) and Central Sub-Saharan Africa (0.85) [3].
In the last decades, a better understanding of RCC pathogenesis has led to the development of new targeted agents, such as tyrosine kinase inhibitors targeting the vascular endothelial growth factor (VEGF) pathway, and immune checkpoint inhibitors (ICI) that have dramatically changed the clinical outcome of patients with advanced disease [4, 5]. ICI have proved to be an essential part of mRCC systemic therapy, with the use of antibodies against programmed cell death receptor 1 (PD-1), programmed cell death ligand receptor 1 (PDL-1) either alone or in combination with other ICI, such as cytotoxic T-lymphocyte antigen 4 (CTLA-4), or VEGF-targeted agents [6]. Immunotherapy and targeted agents have changed the cancer prognosis, by showing longer overall survival (OS) and better quality of life among oncological patients. However, the cost burden associated to these medications created an urgent need to find solutions that enable better access to these drugs, without harming the health systems worldwide.
The impact of geographical differences and socioeconomic disparities in the clinical outcomes and survival of mRCC patients was not well explored. In the VEGF-targeted therapy era, geographical differences did not significantly change the outcomes of advanced-stage patients. Thus, access to the new agents is necessary to provide similar results across the globe. There are no studies that systematically explored the results from various trials to check if health determinants according to the geographic region impacted the outcomes of patients with mRCC treated with ICI. An increased knowledge on population diversities and health determinants have provided a unique opportunity to improve cancer care across geographic regions and countries. In this study, we aimed to understand if geographical differences affected the clinical outcomes of RCC patients receiving immunotherapy.
In this study we sought to understand the differences in clinical outcome in patients treated with immunotherapy across different geographic regions, and to describe potential factors, including its socio-economic disparities, that impact the clinical outcomes.
MATERIALS AND METHODS
We identified studies examining the outcome of RCC in patients undergoing ICI therapy by searching the electronic database MEDLINE (PubMed), and grey literature (American Society of Clinical Oncology (ASCO), the European Society of Medical Oncology (ESMO), DART, OpenGrey, Proquest, and Ethos). The word sequence was based on Bittern Systematic Review Protocol: Specific for Immunotherapy in RCC [7].
The search was designed to identify articles reporting the OS and progression-free survival (PFS) in mRCC patients undergoing ICI therapy (Supplementary Material 1). Search were carried out on November 10th, 2020. We applied the filter “year of publication” (from 2010 to 2020). No accessibility or language filters were applied. After the search, non-English articles were excluded. Programs from the annual meetings were searched manually. Only posters from ASCO and ESMO were included. In cases of duplicate publications, the most complete and contemporaneous report of the study was included. The authors reviewed all screened titles and abstracts for relevance and the full text of relevant articles was retrieved to assess eligibility. The complete sequence used in PUBMED can be found in the supplementary material.
Eligibility criteria
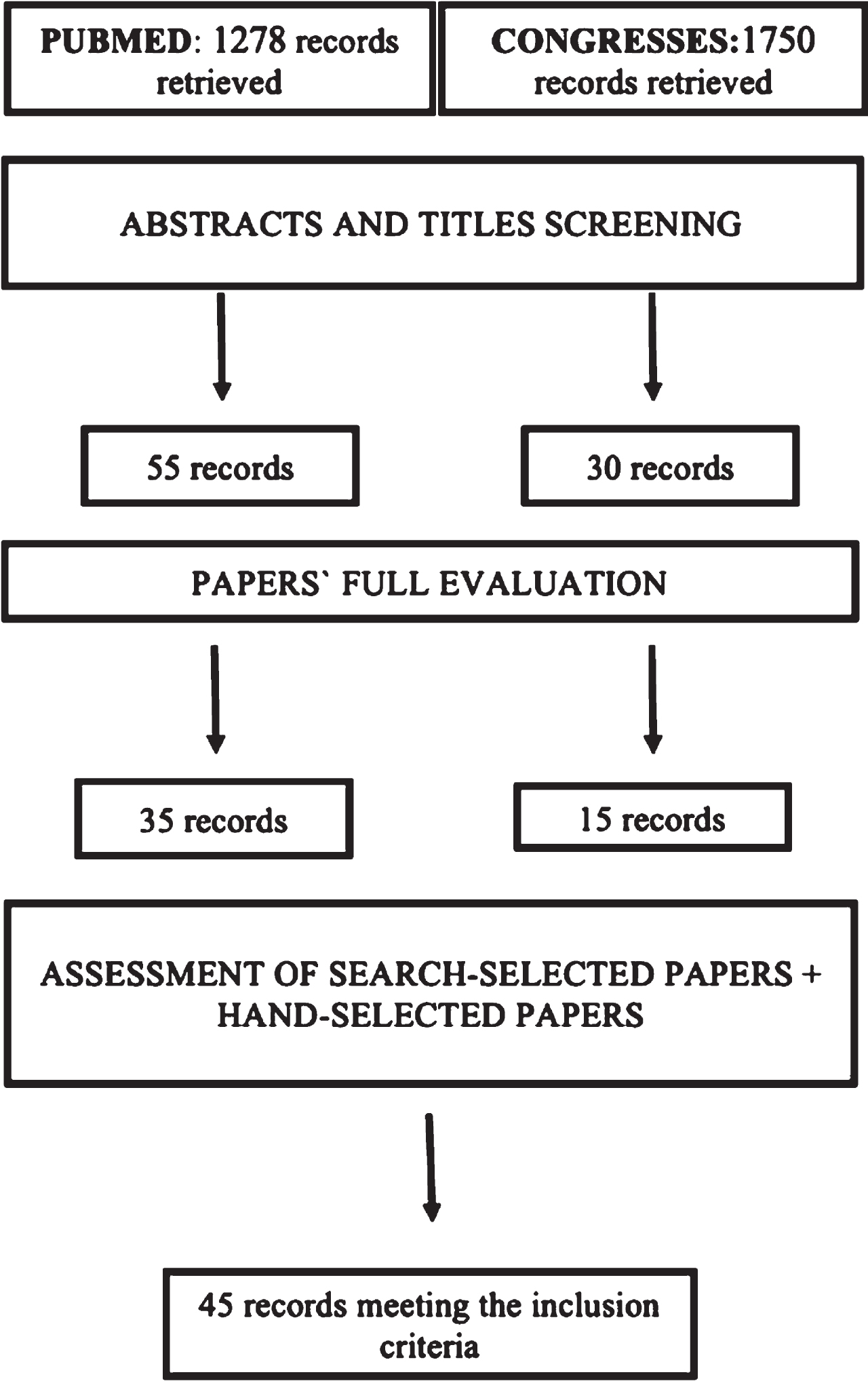
The study population included mRCC patients treated with ICI, and had the OS and/or PFS rates assessed. Studies used to do this analysis were clinical trials (pilots or not, randomized or not), case-control studies, retrospective analysis, or reviews of trials (Table 1) [8]. The trimming process was divided into three phases: abstracts and title screening, paper’s full evaluation, assessment of search-selected and hand-selected papers (Fig. 1). We read all the titles and abstracts and selected only the ones that gave OS or PFS of mRCC patients under ICI. After, we read all the selected articles and peaked the ones that analyzed the results based on the geographical region of the patients. Lastly, grey literature and database final articles were joined in a table, for results calculation. During this process, the last screening assessment was made, to ensure optimal results and data. Studies that analyzed only non-clear cell RCC were excluded.
Table 1
PICOS. Study methodology based in the PICOS system
| PICOS | Inclusion criteria | Exclusion criteria |
| Participants | Metastatic kidney cancer patients | Only non-clear cell histology mRCC |
| Interventions | Targeted immunotherapy | Interleukin and interferon therapies were not considered |
| Comparisons | World region (Anglo-America, Western Europe, Asia, and Other Countries) | When analysis did not include results by region |
| Outcomes | Overall survival, progression-free survival | Studies that did not report the outcomes of interest |
| Study design | Clinical trials (pilots, non-randomized and randomized), reviews, and retrospective analyses | Case series, case reports, case-control studies, books, and documents. |
| Publications | Published in English, full access online, or access to abstract if gray literature | Study protocols only |
Fig. 1
Fluxogram. Fluxogram of this study’s database search, divided in PUBMED and congresses findings.
We gathered and analyzed data, dividing each study into groups based on geographical location or study format (for multicenter trials) from where we could find relevant clinical data. Our groups were Asia, multicentric clinical trials, Europe and Anglo-America. In sequence, we proceeded to calculate the weighted average based on the number of participants for mPFS and mOS per region. After, we calculated a simple average of the weighted average, which was 9.235 and 27 for mPFS and mOS for the four groups, respectively (Table 4).
All multinational multicenter studies considered Asia as part of “Other Countries”. They also did not calculate the mPFS nor mOS per region, giving only the HR for the U.S, Europe, and “Other Countries” population. Articles involving Interleukin and Interferon were excluded from the analysis.
Data extraction
All of the authors assessed relevant articles for study eligibility and performed data extraction. The disagreement was resolved by consensus. Recorded information included: study title, authors, year of publication, location, period, evaluated therapy, line of treatment, PFS, OS, link to the article, control and comparison therapy, sample size, clinical outcomes, and pertinent statistical analyses.
Table 2
Trimming methods. Number of articles found in each base and the total number of articles selected during the article filter and selection
| Trimming methods | PUBMED | ASCO | ESMO |
| Abstracts and titles screening | 1278 | 1472 | 278 |
| Papers‘ full evaluation | 55 | 23 | 07 |
| Assessment of search-selected papers + hand-selected papers | 35 | 14 | 1 |
| Total final selection | 45 |
Risk of bias in individual studies and across them
This analysis was retrospective study, so some biases need to be considered. The studies presented slightly different interventions, such as dosage forms, combinations, and schedules. The evaluated immunotherapies might also have different efficacy when compared to each other. Since we analyzed a heterogeneous group of patients, throughout studies, the efficacy of immunotherapy might differ between those populations.
Evidence synthesis
The literature search strategy yielded 3028 unique articles. This includes the hand search which identified 1750 conference abstracts. 85 articles met the criteria for full-text review (Fig. 1). A total of 45 articles were selected for data extraction and subsequent review (Supplementary Material 1 and 2).
RCC lethality (MIR) and income (GDP per capita)
As a measure of lethality, the mortality-to-incidence ratio (MIR) is a well-known proxy in cancer patients [9]. It is calculated by the division of the number of deaths by the incidence of a specific cancer type during a certain period. This ratio shows the percentage of patients that will die from cancer (lethality) while the remaining share represents all the patients that will be cured with treatment (survival ratio = 1 –MIR). It must be noted that both early diagnosis and better treatment have an impact in this ratio, reducing the number of deaths in relation to the total number of cases. To generate the income per capita of each country we used the gross domestic product per capita (GDPpc), since they are equivalent [9–12].
In order to create this regression, we used data from 2 different sources. The cancer incidence and mortality data for each country from the International Agency for Research on Cancer, an institution of the World Health Organization (WHO). The Gross Domestic Product per capita was acquired from the World Bank database. The regression was performed with an Ordinary Least Square (OLS) methodology. Because of the exponential distribution of GDPpc across the world, we transformed the data to the logarithmic form [9–12].
RESULTS
We have analyzed a total 45 studies, including 6.619 patients. Thirteen studies were Asian, from which twelve were Japanese and one was Indian, with a total of 699 subjects. Tables 3 and 4 show the total number of patients and the average mPFS and mOS for each group. Our weighted average mPFS and mOS were 8,47 months (9% lower than the average) and 40,6 months (50% higher than the average).
Table 3
Result without multicentric studies included in average. Results without multicentric studies included in the average. This table has mPFS and mOS weighted average and the total number of studies and patients in each regional group
| Weighted average mPFS | Weighted average mOS | Studies | Patients (n) | |
| Asia | 8.47 | 40.6 | 13 | 699 |
| Anglo-America | 12.2 | 20.22 | 15 | 943 |
| Europe | 6.1 | 20.24 | 13 | 3143 |
| Multicentric studies | 10.7 | – | 4 | 1834 |
| Average | 8.95 | 27.04 | – | 4785* |
*: total; n: number; mPFS: median Progression-Free Survival; mOS: median Overall Survival.
Table 4
Results with multicentric studies included in average
| Weighted average mPFS | Weighted average mOS | Studies | Patients (n) | |
| Asia | 8.47 | 40.6 | 13 | 699 |
| Anglo-America | 12.2 | 20.22 | 15 | 943 |
| Europe | 6.1 | 20.24 | 13 | 3143 |
| Multicentric studies | 10.7 | – | 4 | 1834 |
| Average | 9.235 | 27.04 | – | 6619* |
*: total; n: number; mPFS: median Progression-Free Survival; mOS: median Overall Survival.
For the Anglo-American population, fifteen studies were included. Those studies contained 943 patients from American and Canadian trials. The weighted average mPFS and mOS for this category were 12.2 months (32% higher than the average) and 20.22 months (26% lower than the average).
Only four trials were categorized as multicentric, containing 1834 patients. The weighted average mPFS was 10,06 (8% higher than the average). There was not enough data to determine the average mOS for this group, as most of the trials had not reached mOS specially in this specific population.
3143 subjects were included within thirteen studies in the European group. This category had 6.1 and 20.24 months of mPFS (34% lower than the average) and mOS (26% lower than the average), respectively.
Kidney cancer lethality (MIR) and income (GDP per capita)
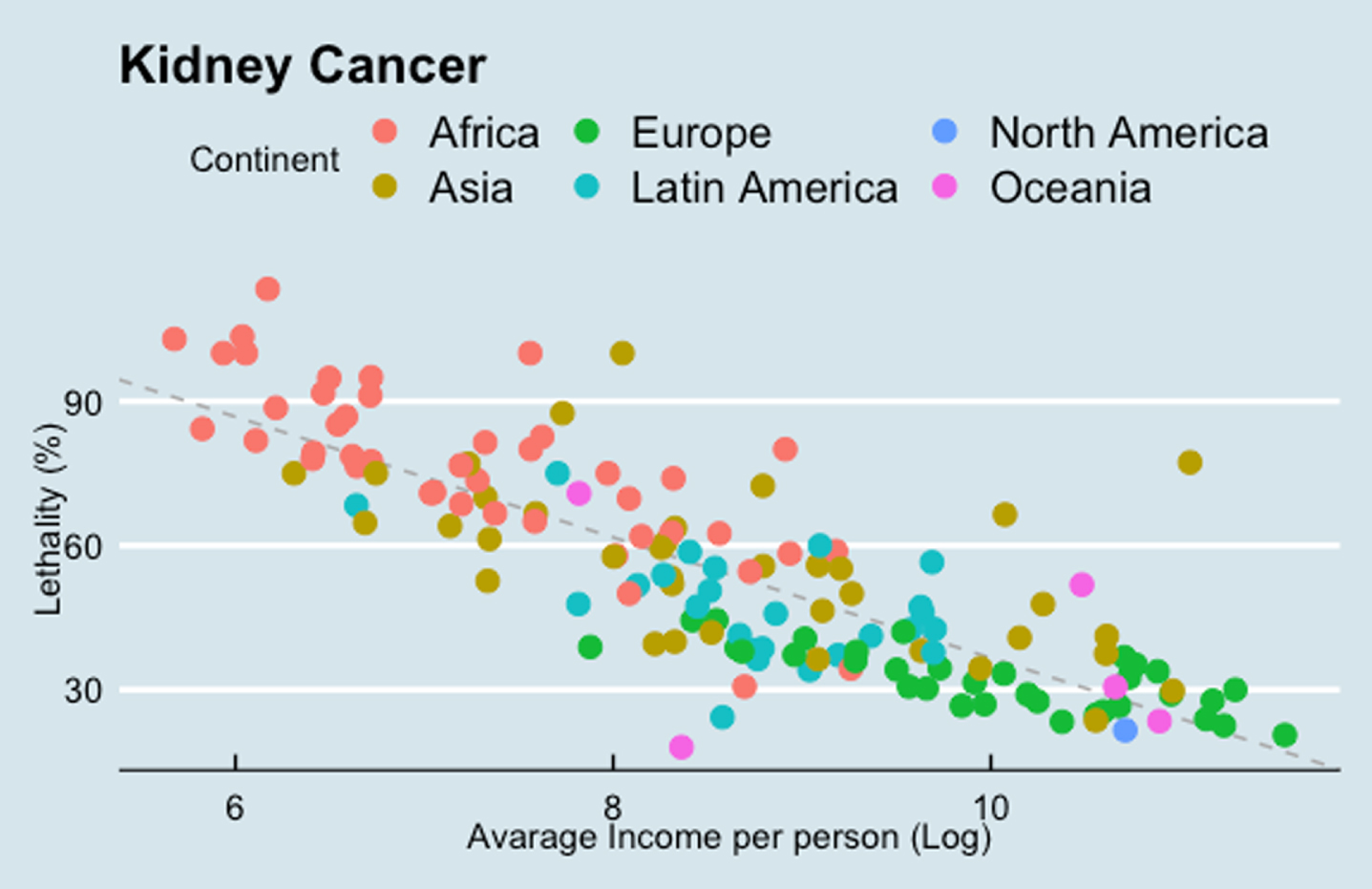
The exploratory analysis on income and RCC lethality has shown that when a country‘s income is raised in 100%, its RCC lethality has an absolute decline of 8.7% (CI 10.1 to 7.3% - p < 0.05). There is also a clear difference in the results between continents (Fig. 2)
Fig. 2
Results of kidney cancer lethality (MIR) and income (GDP per capita). Each point indicates one single country. The gray bar indicates the correlation between these two variables.
DISCUSSION
In this study we evaluate the differences in survival outcome across different geographic regions. We also explored the impact of income per capita income and RCC lethality as a potential explanation for outcome disparities. The main objective was to describe and to understand the use of ICI therapy in a population from developing countries in whom socioeconomical disparities could reflect different outcomes and also different access patterns. Interestingly, pivotal studies have failed to include a large number of patients representing all regions of the globe. This may impact an adequate analysis regarding the use of ICI therapy in developing countries such as Latin America.
In this analysis, we found a numerically lower PFS in Europe and higher in Anglo-America (6.1 vs. 12.2 months, respectively). However, a similar OS was observed in these regions (20.24 vs. 20.22 months). Interestingly, a numerically higher OS was observed in the Asian population (40,6 months - 50% higher than the average). In this context, Peng and colleagues had published a meta-analysis of trials with various types of cancer treated with ICI that demonstrated higher OS in Asian versus non-Asian patients (HR 0.84; 95% CI, 0.75–0.94) [13]. However, this data should be interpreted very cautiously considering we are exploring different studies which have included different patient population. However, it may raise an hypothesis that ethnic, socioeconomical disparities and/or genomic profiling may impact clinical outcomes or may predict prognosis. In the VEGF-targeted therapies era, Guo and colleagues have shown that Asians, when compared with non-Asians, may have a distinct toxicity profile when treated with pazopanib or sunitinib, suggesting that ethnic differences or other environmental factors may influence treatment tolerance [14]. However, no difference in survival outcome was observed between Asian vs. non-Asian patients with mRCC treated with VEGF-targeted therapy [15].
Cancer care varies significantly across the globe. In contrast to high-income countries, an adequate and quality cancer care in low- and middle-income countries is a big problem. System resources and allocation of resources across levels of care, quality of care, income, and socio-cultural disparities may influence clinical outcome of cancer patients [16]. Tartari and colleagues, in a study that evaluated Nivolumab in mRCC patients, have shown that the one-year per-patient cost for treating mRCC with nivolumab was estimated to be $32,130 to achieve a median PFS of 4.6 months, at a worldwide cost of $2.7 billion [17]. The lack of access to these high-cost agents has become a worldwide concern, once it doubts the sustainability of the health system. In the last years, the use of clinical trials as an alternative to enable patients access to these drugs became a quick worldwide solution [18]. These approaches to improve accessibility are hardened in low to middle-income countries where the number of trials conducted is small because of regulatory issues and a lack of infrastructure [19]. Data from the European Federation of Pharmaceutical Industries Association indicate that among the new drugs released into the market in the last 5 years, approximately 90% are used predominantly in three regions: the United States (64.7%), five countries in Western Europe (17.5%), and Japan (7.3%). This leaves about 10% of the consumption for the rest of the world [20].Given the small number of clinical trials performed in LMICs, together with the small number of patients recruited relative to the population at risk of disease, conducting clinical trials does not substantially improve access. Socioeconomic, cultural, and economic characteristics of each region also influence the participation in the studies [20].
The exploratory analysis to evaluate the impact of country income and RCC lethality has shown a statistically significant decline in the RCC lethality according to an increase in country‘s income (Fig. 2). This may reflect treatment access, and therefore explain distinct clinical outcomes in mRCC patient across the globe in the immunotherapy era.
This analysis has many limitations. First, many informations that may impact clinical outcome such as clinicopathological data, tumor characteristics, biological insights, lines of treatment and type of treatment were not available and not reported in the clinical studies for the different subgroups. It may result in imbalances that may explain differences in clinical outcome. Individual information may be needed to better explore these questions. In addition, IMDC classification is lacking across the different geographic regions and may also influence the hypothesis we have raised. Similarly, differences in health care systems have not been considered in this analysis.
CONCLUSIONS
The use of ICI has significantly improved the clinical outcome of patents with mRCC across the globe. However, this clinical benefit varies across geographic regions and not all regions are well represented in clinical studies. Clinical outcome to ICI may be different according to ethnic differences or socioeconomical disparities. A worldwide access to ICI should be stimulated to allow a better understanding on the impact of geographic regions in the clinical outcome of patients receiving this class of agents and the etiology of potential differences that may appear.
ABBREVIATIONS LIST
GBD: Global Burden of Disease
DALYs: Disability-Adjusted Life Years
HDI: Human Development Index
KC: Kidney Cancer
mRCC: Metastatic Renal Cell Carcinoma
RCC: Renal Cell Carcinoma
OS: Overall Survival
WAVG: Weighted average
IMDC: International Metastatic Renal Cell Carcinoma
MSKCC: Memorial Sloan-Kettering Cancer Center (MSKCC/Motzer) Score
LARCG: Latin American Renal Cell Group
VEGF: Vascular Endothelial Growth Factor
BMI: Body Mass Index
ECOG: Eastern Cooperative Oncology Group
ASA: American Society of Anesthesiologists
KPS: Karnofsky Performance
LDH: High Lactate Dehydrogenase
CSS: Cancer-specific Survival
mPFS: median Progression-Free Survival
mOS: median Overall Survival
ICI: immune checkpoint inhibitors
IFNa: Interferon-alpha
ILK-2: Interleukin-2
PD-1: Programmed Cell Death Receptor
PDL-1: Programmed Cell Death Ligand Receptor 1
CTLA-4: Cytotoxic T-lymphocyte antigen 4
ASCO: American Society of Clinical Oncology
ESMO: European Society of Medical Oncology
LMICs: Low and middle-income countries
HICs: High-income countries
MIR: Mortality-to-incidence ratio
GDPpc: Gross domestic product per capita
WHO: World Health Organization
OLS: Ordinary Least Square
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
•Conception or design of the study
- Vinicius Goncalves, Andre Fay
•Acquisition, analysis, or interpretation of data
- All authors
•Writing (original draft or revision for important intellectual content)
- All authors
•Supervision and/or final approval of the version to be submitted.
- Andre Fay
CONFLICT OF INTEREST
Vinicius Knackfuss Gonçalves, Fernando Sabino Marques Monteiro, Antonia Angeli Gazola, Felipe Pizzolo, Júlia Elisa Hübner, Rodrigo Pellegrini, Alessandra Borba and André P. Fay have no conflicts of interest to report.
SUPPLEMENTARY MATERIAL
[1] The supplementary materials can be found in the following links below:
1: https://drive.google.com/file/d/1Eip6hxeKwyy3gyZGkOVQhxe2mewvP9uo/view?usp = sharing
2: https://drive.google.com/file/d/1Y6GWpKiWKwLDCEVtujNwArK93SfHPRQ/view?usp = sharing
REFERENCES
[1] | Siegel RL , Miller KD , Jemal A . Cancer statistics, 2020. CA Cancer J Clin [Internet]. (2020) ;70: (1):7–30. Available from: https://onlinelibrary.wiley.com/doi/abs/10.3322/caac.21590 |
[2] | Bray F , Ferlay J , Soerjomataram I , Siegel RL , Torre LA , Jemal A . Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) ;68: (6):394–424. |
[3] | Safiri S , Kolahi A-A , Mansournia MA , Almasi-Hashiani A , Ashrafi-Asgarabad A , Sullman MJM , et al. The burden of kidney cancer and its attributable risk factors in 195 countries and territories, 1990–2017. Sci Rep [Internet]. (2020) ;10: (1):13862. Available from: http://www.nature.com/articles/s41598-020-70840-2 |
[4] | Linehan WM , Schmidt LS , Crooks DR , Wei D , Srinivasan R , Lang M , et al. The Metabolic Basis of Kidney Cancer. Cancer Discov [Internet]. (2019) ;9: (8):1006–21. Available from: http://cancerdiscovery.aacrjournals.org/lookup/doi/10.1158/2159-8290.CD-18-1354 |
[5] | Hirsch BR , Burke JM , Agrawal M , Hauke RJ , Hutson TE , Doshi G , et al. Sequential Therapy in Metastatic Renal Cell Carcinoma. J Kidney Cancer VHL [Internet]. (2016) ;3: (1):23–35. Available from: https://jkcvhl.com/index.php/jkcvhl/article/view/46 |
[6] | Lalani A-KA , McGregor BA , Albiges L , Choueiri TK , Motzer R , Powles T , et al. Systemic Treatment of Metastatic Clear Cell Renal Cell Carcinoma in 2018: Current Paradigms, Use of Immunotherapy, and Future Directions. Eur Urol [Internet]. (2019) ;75: (1):100–10. Available from: https://linkinghub.elsevier.com/retrieve/pii/S03022_83818307516 |
[7] | Universal Scientific Education and Research Network. Bitern: systematic review protocol.: specific immunotherapy in Renal Cell Carcinoma. |
[8] | Moher D , Liberati A , Tetzlaff J , Altman DG . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med [Internet]. (2009) ;6: (7):e1000097. Available from: https://dx.plos.org/10.1371/journal.pmed.1000097 |
[9] | Organization. WH. World Health Organization [Internet]. Available from: https://www.who.int/ |
[10] | Choi E , Lee S , Nhung BC , Suh M , Park B , Jun JK , et al. Cancer mortality-to-incidence ratio as an indicator of cancer management outcomes in Organization for Economic Cooperation and Development countries. Epidemiol Health [Internet]. (2017) ;39: :e2017006. Available from: http://www.eepih.org/journal/view.php?doi=10.4178/epih.e2017006 |
[11] | Asadzadeh Vostakolaei F , Karim-Kos HE , Janssen-Heijnen MLG , Visser O , Verbeek ALM , Kiemeney LALM . The validity of the mortality to incidence ratio as a proxy for site-specific cancer survival. Eur J Public Health [Internet]. (2011) ;21: (5):573–7. Available from: https://academic.oup.com/eurpub/articlelookup/doi/10.1093/eurpub/ckq120 |
[12] | Grady D . Harnessing the Immune System to Fight Cancer. [Internet]. The New York Times. (2016) . Available from: https://www.nytimes.com/2016/07/31/health/harnessing-the-immune-system-to-fight-cancer.html |
[13] | Peng L , Qin B-D , Xiao K , Xu S , Yang J-S , Zang Y-S , et al. A meta-analysis comparing responses of Asian versus non-Asian cancer patients to PD-1 and PD-L1 inhibitor-based therapy. Oncoimmunology [Internet]. (2020) Jan 1;9: (1):1781333. Available from: https://www.tandfonline.com/doi/full/10.1080/2162402X.2020.1781333 |
[14] | Guo J , Jin J , Oya M , Uemura H , Takahashi S , Tatsugami K , et al. Safety of pazopanib and sunitinib in treatmentnaive patients with metastatic renal cell carcinoma: Asian versus non-Asian subgroup analysis of the COMPARZ trial. J Hematol Oncol. (2018) ;11: (1):1–10. |
[15] | Wang Y , Choueiri TK , Lee JL , Tan MH , Rha SY , North SA , et al. Anti-VEGF therapy in mRCC: Differences between Asian and non-Asian patients. Br J Cancer [Internet]. (2014) ;110: (6):1433–7. Available from: http://dx.doi.org/10.1038/bjc.2014.28 |
[16] | Goss PE , Lee BL , Badovinac-Crnjevic T , Strasser-Weippl K , Chavarri-Guerra Y , Louis JS , et al. Planning cancer control in Latin America and the Caribbean. Lancet Oncol [Internet]. (2013) ;14: (5):391–436. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1470204513700482 |
[17] | Tartari F , Santoni M , Burattini L , Mazzanti P , Onofri A , Berardi R . Economic sustainability of anti-PD- 1 agents nivolumab and pembrolizumab in cancer patients: Recent insights and future challenges. Cancer Treat Rev [Internet]. (2016) ;48: :20–4. Available from: http://linkinghub.elsevier.com/retrieve/pii/S030573721630041X |
[18] | Weigmann K . The ethics of global clinical trials. EMBO Rep [Internet]. (2015) ;16: (5):566–70. Available from: https://onlinelibrary.wiley.com/doi/10.15252/embr.201540398 |
[19] | Okpechi IG , Swanepoel CR , Venter F . Access to medications and conducting clinical trials in LMICs. Nat Rev Nephrol [Internet]. (2015) ;11: (3):189–94. Available from: http://www.nature.com/articles/nrneph.2015.6 |
[20] | Barrios CH , Reinert T , Werutsky G . Global Breast Cancer Research: Moving Forward. Am Soc Clin Oncol Educ B [Internet]. (2018) ;(38):441–50. Available from: https://ascopubs.org/doi/10.1200/EDBK_209183 |




