Renal Function: Implications on the Surgical Treatment of RCC
Abstract
The good oncologic results after partial nephrectomy for stage 1 RCC show that radical nephrectomy is an overtreatment in most cases, and that many healthy nephrons are removed unnecessarily. However, partial nephrectomy is a difficult operation, with increased blood loss and a higher risk of complications. Therefore, the advantage of preserved function has to be weighed up against the increased trauma of surgery in each individual patient, and the assessment of preoperative function may influence this decision, among other factors such as comorbidities and age.
In most studies, renal function has been assessed by means of estimated glomerular filtration rate, and this parameter is very helpful for long-term studies in large populations. However, more precise measurement based on clearance studies are sometimes required for more sophisticated investigations.
The technique of partial nephrectomy has evolved substantially in recent years, resulting in the preservation of more nephrons, less damage to the remaining parenchyma, less blood loss, and a decreased risk of complications. The introduction of minimally invasive surgery for this purpose has also decreased the overall morbidity of surgery.
In the long-term, chronic kidney disease may result in increased cardiac mortality. There is ongoing discussion on this problem, however, this potential negative influence on overall survival is not only influenced by the rate of renal insufficiency, but also to a great extent by other comorbidities such as hypertension and diabetes. Therefore, in addition to providing the best surgery for any given patient, we have to make sure that the treatment of the comorbidities will also be part of our patient management, since the risk of cardiac failure may be greater than the risk of poor oncologic outcome.
ABBREVIATIONS AND ACRONYMS
AKD | Acute kidney disease |
CKD | Chronic kidney disease |
DSS | Disease-specific survival |
eGFR | Estimated glomerular filtration rate |
ESKD | End-stage kidney disease |
GFR | Glomerular filtration rate |
MAG3 | Technetium-99m mercaptoacetyltriglycine |
OS | Overall survival |
RCC | Renal cell cancer |
PN | Partial nephrectomy |
RN | Radical nephrectomy |
INTRODUCTION
In recent years, several modalities have been developed to effectively treat renal cell cancer (RCC). However, despite these efforts, surgery remains the only possibility to cure this disease. The technique of state-of-the-art radical nephrectomy (RN) for RCC was first described by Robson in 1969 [1]. The next most important step in the evolution of RN was the introduction of laparoscopy to perform this procedure [2]. Initially, partial nephrectomy (PN) was mainly performed as an alternative to RN for an imperative indication in patients with compromised renal function. With growing experience, it was realized that RN was an overtreatment when removing small tumors, since the oncologic results were comparable with PN, which was therefore increasingly performed in patients with a normal contralateral kidney [3]. As a next step, larger tumors up to 7cm were also considered suitable for nephron-sparing surgery [4]. Without doubt, preserving renal tissue by means of PN is associated with a reduced risk of chronic kidney disease (CKD) compared to RN. The patient will probably profit from PN as long as they are not confronted with major surgical complications and the oncologic outcome is not compromised. In this context, it is important to precisely evaluate the risk of a resulting CKD after surgery for RCC, and to understand the influence of CKD on associated disorders such as chronic heart disease and hypertension, which will ultimately influence overall survival.
RENAL FUNCTION –ETIOLOGY OF IMPAIRMENT
The quality and impact of clinical studies investigating renal function after surgery for RCC ess-entially depend on the methods used. The situation after RN is relatively simple, with only one kidney remaining. Indeed, it is more demanding to precisely assess the function of the ipsilateral kidney before and after PN, because its function is overlapped by that of the contralateral one. An exact and sophisticated preoperative investigation of the ipsilateral kidney, as well as the global function, is essential if we want to precisely determine and quantify the renal impairment that will be induced by PN. More global factors of kidney function such as serum creatinine and the glomerular filtration rate (GFR) are useful and sufficient for the investigation of the degree of CKD and its long-term influence of CKD on other medical conditions such as chronic heart failure and hypertension. However, these simple investigations alone are not adequate to fully understand the etiology of CKD.
Chronic kidney disease from medical causes is present in 25% to 30% of patients before surgery for renal cancer [5]. Initially impaired global renal function may be the result of medical conditions such as diabetes and hypertension, and it will deteriorate further after surgery in the short-term, but probably also long-term. The function of the tumor-bearing kidney deteriorates after surgery for several reasons; a certain amount of healthy renal parenchyma will be excised together with the tumor, whereas the tumor tissue does not add to function so that its removal will not result in any change. Further damage and loss of tissue occurs due to the repair with parenc-hymal sutures. The remaining tissue, however, is not affected by these measures and is therefore fully fun-ctional and healthy. Warm ischemia, a technique fre-quently used with laparoscopic/robotic PN, results in a different type of damage and reduction of fun-ction. There is no loss of volume, but a potentially chronic and maybe even continuously increasing damage of all of the remaining parenchyma. This type of CKD (normal number of nephrons with red-uced function) will possibly result in different and more pronounced long-term sequelae than CKD as a result of the reduced volume of unimpaired, nor-mally-functioning parenchyma, even if GFR is comparable.
The influence of patient comorbidities such as hy-pertension and diabetes on CKD should not be underestimated. On the one hand, comorbidity may be the reason for preexisting renal failure, and on the other, it may initially substantially aggravate surgically induced CKD, also in the longer term. The conflicting data reported in the literature on the long-term sequelae of CKD after RN and PN may be explained to some extent by comorbidities not realized as bias.
RENAL FUNCTION –ASSESSMENT
Plasma creatinine
A product of the metabolism of creatine and phosphocreatine in skeletal muscle –is the basic parameter used to measure renal function. Serum creatinine is elevated when there is a significant reduction in GFR, however, about 50% of kidney function must be lost before a rise in serum creatinine can be detected. Therefore, the creatinine level alone is not useful to monitor the effect of PN in a patient with a normal contralateral kidney.
Glomerular filtration rate (GFR)
Measuring the creatinine clearance using the serum creatinine level and a timed urine collection gives a good estimate of glomerular filtration rate: creatinine clearance= (urine creatinine x volume)/serum creatinine. Measured GFR provides solid data for investigations after renal surgery. However, urine collection is cumbersome and sometimes inaccurate. Measurement of split renal function requires the placement of a catheter in the kidney, which is rarely feasible.
Estimated GFR (eGFR)
Is not as reliable as measured GFR, but is widely used in clinical practice. Several formulas for the calculation of eGFR have been published (Cockroft/Gault equation, 6-variable MDRD, 4-variable MDRD). In addition to the serum creatinine levels, parameters such as age, sex and weight are used for calculation (6. Many publications provide eGFR data, and eGFR is very useful for long-term follow-up and investigation in large cohorts, however, when analyzing and comparing such data one should be aware which formula has been used for calculation.)
GFR measurement using radionuclides
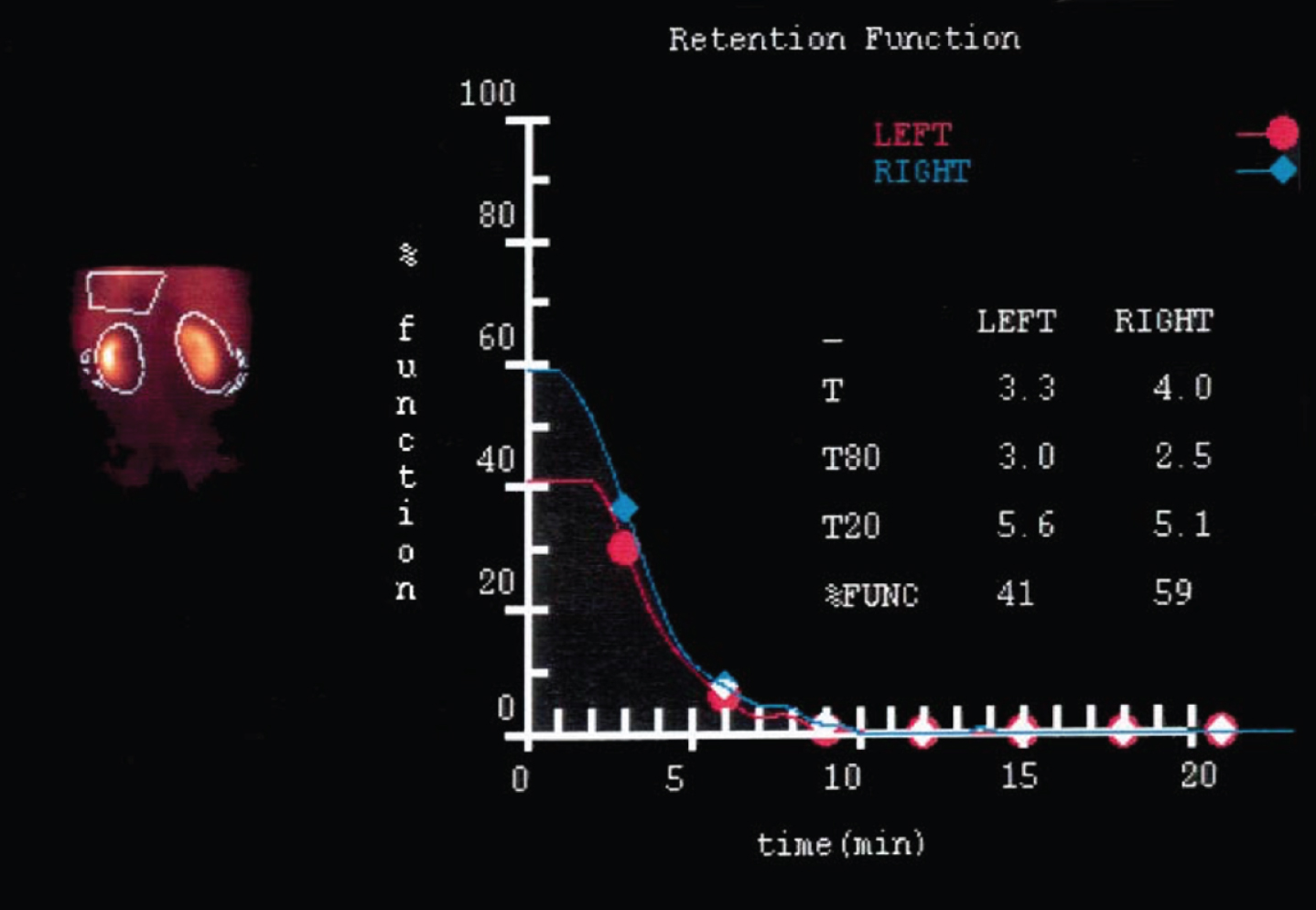
Correlates closely with inulin clearance. Tech-netium-99m mercaptoacetyltriglycine (MAG3) has proved to be very useful for this purpose and provides the most reliable data for scientific studies [7]. It also allows for the precise determination of split renal function [8] (Table 1). Deconvolution analysis of the peak concentration time documented in MAG3 studies enables the differentiation of whether a loss of function is due to reperfusion injury after ischemia (unchanged number of nephrons) or to loss of tissue, and the operated and non-operated kidney can also be directly compared in this respect [8] (Fig. 1).
Table 1
MAG3 clearance before and after partial nephrectomy in cold ischemia. The decrease\\ in mean split function from 105ml/min to 73ml/min was exclusively due to loss of\\ volume and not to parenchymal damage (see Fig. 1)(ref.8)
| pre-op n=14 | post-op n=20 | p-value | |
| MAG3 clearance ml/min | 219 (193–243) | 186 (133–230) | 0.008 |
| split renal function % operated kidney | 47.9% (35–52) | 38.2% (35–60) | |
| split MAG3 clearance ml/min operated kidney | 105 (79–122) | 73 (42–108) | 0.012 |
Fig. 1
Deconvolution analysis of peak concentration time (MAG 3 renal scan): After partial nephrectomy in cold ischemia, the function of the operated left kidney is decreased due to loss of volume. The function of the remaining parenchyma, however, is not impaired (ref.8).
Proteinuria
An important parameter for the nephrologists, is largely neglected in the investigation of CKD after surgery for RCC. However, preoperative proteinuria is a significant predictor of overall sur-vival in patients who undergo nephrectomy. Classification according to preoperative glomerular filtration rate and proteinuria more accurately predicts survival than using the glomerular filtration rate alone after accounting for cancer stage. This information supports routine evaluation of proteinuria in patients with kidney cancer [9].
RENAL FUNCTION –INFLUENCE OF SURGICAL TECHNIQUE
Loss of healthy nephrons - resection
Enucleation of the tumor seems to be the techni-que which best preserves renal function. There re-mains some concern with regard to the oncologic out-come, however. In a recent large prospective, but not randomized multi-center study (16 referral centers) including 507 patients with T1a (66.6%), T1b (37,8%) and T2 (3.0%) RCC, the tumors were either removed by means of enucleation (52%), enucleo-resection (30%), or resection (18%) [10]. Operative time, WIT, estimated blood loss and duration of hospitalization were comparable among all patients. Clavien-Dindo grade 2 complications were more frequently recorded after enucleo-resection (10.7%) than after enucleation (4.2%) and resection (3.3%). The proportion of patients experiencing acute kidney disease (AKD) was significantly higher after enucleo-resection than after enucleation (32.0% vs 20.7%, p=0.01), however, it did not differ between the enucleo-resection and resection cohorts. Also on multivariable analysis, the resection technique (enucleo-resection vs enucleation OR 1.67, 95% CI 1.01–2.76, p=0.046) was significantly associated with postoperative AKD. Interestingly, the incidence of positive surgical margins was also significantly higher after enucleo-resection compared to after enucleation (10.0% vs 4.9%, p=0.046) and resection (10.0% vs 2.2%, p=0.02). Unfortunately, there are no data on direct comparison of enucleation versus resection. In another study, volume loss, not ischemia time, was the primary determinant of ultimate renal function after PN [11].
Loss of healthy nephrons - repair
Deep parenchymal sutures are frequently used to approximate the cut edges after tumor excision and to provide hemostasis, and this open surgical technique could successfully be duplicated by means of laparoscopy [12]. These sutures are effective, but also quite traumatic. An important improvement of the technique was the introduction of a first-layer running suture through the interstitial tissue at the bottom of the tumor bed. This suture provides both closure of the possibly-opened collecting system as well as hemostasis. Now, the hilum is unclamped prior to the parenchymal sutures as second layer, allowing for re-perfusion of the kidney. Any remaining bleeding is readily detected and precisely secured using additional sutures [13]. The final parenchymal sutures should not be sited too deep and need not be put under tension, since they are no longer required for hemostasis but only for re-approximation of the cut edges. This technique has two advantages with regard to tissue preservation; ischemia time is substantially reduced, and the parenchymal sutures are less trau-matic since they are no longer required for hemostasis. They can sometimes even be omitted completely, especially with a shallow tumor bed.
It is difficult to assess how many nephrons are lost due either to resection or to subsequent repair. Dec-line in function after partial nephrectomy averages approximately 20% in the operated kidney as asses-sed in a metanalysis [14]. These data, however, are based on eGFR only and not on measured split renal function. We studied renal function after PN in a small series using pre- and postoperative MAG3 scans providing precise data on the operated kidney, and the mean decrease in function was 31% (Table 1, Fig. 1) [8].
Damage to healthy nephrons –ischemia
In nephron-sparing surgery, vascular control is often necessary during the period of tumor excision and parenchymal repair to minimize blood loss as well as increase visibility. So-called warm ischemia is achieved by temporary occlusion of the renal artery alone or the artery and vein together. There is, however, abundant literature describing the deteriorating effect of warm ischemia on renal function. This effect is best studied in patients with a solitary kidney. In a multi-institutional study, 537 patients with a solitary kidney were investigated following open nephron-sparing surgery; 85 did not require clamping of the artery, and 174 and 278 were performed in warm and cold ischemia, respectively. Preoperative creatinine was similar in the three groups (1.4, 1.3 and 1.4mg/dl). Warm and cold ischemia was associated with a significantly increased risk of acute (P<0.001) and chronic (P<0.027) renal failure and temporary dialysis (P<0.028) compared with patients with no ischemia. Warm ischemia for longer than 20 minutes and cold ischemia for longer than 35 minutes were both associated with a higher incidence of acute renal failure (P=0.002 and 0.003, respectively). Additionally, warm ischemia for more than 20 minutes was associated with an increased risk of chronic renal insufficiency (41 vs. 19%, P=0.008), increase in creatinine greater than 0.5 (42 vs. 15%, P<0.001) and permanent dialysis (10 vs. 4%, P=0.145) [15]. There is now general consensus that a warm ischemia time of up to 20 minutes is safe in a kidney without preexisting damage, and warm ischemia times of more than 30 minutes should be omitted [16]. Ischemic damage can be reduced if only the segmental artery feeding the tumor is clamped. An exact preoperative evaluation of the vascular anatomy in correlation to the tumor site is an absolute precondition. If the tumor is supplied by a clearly identifiable artery, selective clamping minimizes the intra-operative warm ischemic injury and improves early post-operative renal function compared to main renal artery clamping [17].
Ischemic damage of nephrons –protection by hypothermia
Slush ice surface cooling during ischemia protects the kidney, offers the surgeon extra time, and since it is easy and quick to apply it has become standard in open surgery. To duplicate the results of hypothermia, which has proved so useful in open surgery, several techniques have been developed to integrate cold ischemia into laparoscopy as well. The feasibility of laparoscopic renal surface hypothermia by applying ice slush, and renal hypothermia by retrograde ureteral cold saline perfusion respectively, has been reported [18, 19]. We developed a technique of renal cooling by means of arterial perfusion [20]. The functional results were excellent. The excision of the tumor and the repair of the parenchyma resulted in a mean GFR decrease (MAG3 measured) of 31% (operated side) and 15% (global function) respectively (Table 1). However, the function of the remaining tissue on the operated side was not compromised despite an ischemia time of 36 (25–86) minutes (Fig. 1) [8]. Obviously the protective effect of cold arterial perfusion is not only due to hypothermia, but is also due to the continuous washing out of toxic radicals which are accumulated during arrest of circulation, and are responsible for ischemia-reperfusion injury [21].
Ischemic damage of nephrons –increase of ischemic tolerance by renoprotective drugs
The goal is the preoperative or intra-operative application of a drug which prevents or decreases renal ischemia-reperfusion injury by increasing the ischemic tolerance of the kidney. Thirty years ago, Wickham used inosin for this purpose during difficult renal stone surgery, but the efficacy of inosin for this purpose was never clearly proved and interest waned [22]. Now, with PN, a new indication has come to light. Mannitol is widely used to decrease ischemic damage [23], but without clear evidence, and several studies have failed to prove a positive effect [24].
Theoretically there are two concepts to avoid isc-hemia-reperfusion injury. First, increase of resistance of the renal parenchyma in order to ensure that no, or only minor, damage occurs. N-acetylcysteine, a potent antioxidant, seems to be well suited for this purpose. This drug which is frequently used in pulmonology with minimal side effects; it is also used clinically to prevent renal failure after the application of reno-toxic contrast agents, and the effect is well documented [25, 26]. In an animal study, N-ace-tylcysteine was shown to ameliorate ischemic renal failure [27]. These data make it an ideal candidate for further investigation. Once the ischemic reperfusion injury has occurred, repair mechanisms can induce further damage, which may be avoided or reduced by the application of cortisone, immune-suppressant or similar drugs. Transplant surgeons and nephrologists have performed intensive research in this respect [28, 29].
Non-ischemic partial nephrectomy
The first published series on PT was performed without control of the vascular pedicle, and the tumors were directly excised, mainly by using bipolar coagulation for hemostasis [30]. This technique was, however, restricted to small tumors in a favorable location. Recently, a very similar technique was reported where the bipolar coagulation was replaced by a surgical laser, which was also used for cutting [32]. Not surprisingly, very similar limitations were encountered.
The novel concept of zero-ischemia anatomical robotic and laparoscopic PN represents a substantial improvement on non-ischemic PN. Instead of clamping the main or a segmental artery, pre-emptive control of tumor specific, tertiary or higher-order renal arterial branches is achieved using neurosurgical aneurysm micro-bulldog clamps [32]. With this technique, ischemia is restricted to the tumor, and the uninvolved renal tissue is not compromised. Because of the precise dissection and continuous hemostasis, excision of healthy tissue at the tumor border is restricted to a minimum. Also, deep hemostatic sutures to the healthy parenchyma are omitted, again saving healthy nephrons, so there is a threefold beneficial effect on renal function. This technique is especially helpful for PN in hilar tumors. In a recent systematic review, short- and long-term renal functional outcomes of PN were investigated, and they appeared superior in the off-clamp and super selective clamp groups compared with the on-clamp PN cohort. However, the authors comment that higher quality data are necessary for definitive conclusions in this regard [33].
RADICAL NEPHRECTOMY VERSUS PARTIAL NEPHRECTOMY: IMPACT ON SURVIVAL
There is no doubt that PN results in the retention of a higher number of nephrons as compared with RN; therefore the risk of CKD is reduced. However, the number of nephrons is not the only essential criterion, it is also important that these nephrons are fully functional (preoperatively-postoperatively after ischemia). More than 25% of patients with localized renal cancer have preexisting CKD and will benefit from optimized function after PN to minimize the risk of progression to renal failure, and RN is a significant risk factor for the development of chronic kidney disease [34].
In a large community-based population including 1,120,295 adults, an independent, graded association was observed between a reduced estimated GFR and the risk of death and cardiovascular events [35]. In a series of 499 patients with benign tumors on final pathology, PN was unexpectedly associated with better OS when compared to RN in patients with unanticipated benign tumors. This observed survival advantage appears partly to be the result of better preservation of eGFR [36]. Other factors, however, have not been investigated.
Another important observation has recently been reported. In a cohort of 1,783 patients without CKD, ten-year other cause mortality-free survival rates were 90% and 88% after nephron-sparing surgery and radical nephrectomy, respectively. However, radical nephrectomy increased the risk of other cause mortality according to the increasing baseline Charlson comorbidity index (interaction test p=0.0008). It is the patients who are more ill with relevant comorbidities (stratified by the Charlson comorbidity index) who benefit most from nephron-sparing surgery in terms of other-cause mortality [37].
Roughly 2% of the patients with normal estimated GFR before kidney surgery will develop end-stage renal disease (ESRD) in the first 10 years of follow-up. In addition to the already known protective benefits in terms of cardiovascular events and renal function preservation, PN seems to be associated with a lower risk of ESRD relative to RN. Nonetheless, individual risk factors inherent at baseline (especially age, diabetes, and uncontrolled hypertension) appear to be crucial predictors of ESRD regardless of the treatment delivered [38].
The prognostic risk of chronic kidney disease in patients with kidney cancer is increased when the preoperative glomerular filtration rate is less than 60ml/minute/1.73m2 or the postoperative rate is less than 45ml/minute/1.73m2. Additional factors, including nonsurgical causes of chronic kidney dis-ease and the degree of albuminuria, can also dramatically alter the consequences of chronic kidney disease after kidney cancer surgery. [39]. According to these data, a patient with an eGFR >60ml/minute/1.73m2 after nephrectomy and no comorbidities has no risk of increased overall mortality. This is exactly the situation of patients undergoing living donor nephrectomy. A cohort of 96,217 kidney donors in the United States and a cohort of non-donors at equally low risk of renal disease and free of contraindications to live donation (20,024 participants of the Third National Health and Nutrition Examination Survey=NHANES III) were compared in relation to their risk of developing ESKD. Estimated lifetime risk of ESRD was 90 per 10,000 donors, 326 per 10,000 unscreened non-donors (general population), and 14 per 10,000 healthy non-donors. Compared to matched healthy non-donors, kidney donors had an increased risk of ESRD; however, the magnitude of the absolute risk increase was small [40].
Prospective randomized study
The EORTC-GU (European Organisation for Res-earch and Treatment of Cancer Genito-Urinary Group) designed the non-inferiority phase 3 trial 30904 comparing the outcome of RN and PN [41]. The primary end point was OS. Secondary end points were disease-specific survival (DSS), progression, and surgical side-effects. Evaluation of renal function and CKD was not within the scope of the study. In the intention to treat population, PN seemed to be significantly less effective than RN in terms of OS. However, in the targeted population of RCC patients, the trend in favor of RN is no longer significant. The small number of progressions and deaths from renal cancer cannot explain any possible OS differences between treatment types. This study has several serious drawbacks. Only 541 of the planned 1,300 patients could be recruited, 55 patients switched treatment, and the study was closed prematurely. Despite the small number of patients, 46 centers and at least 57 surgeons of unknown experience participated. To further investigate the unexpected finding of better OS in favor of RN, additional data on kidney function were collected and then published [42]. However, although it is presented as such, this follow-up study can no longer be considered a prospective randomized study, but rather it is in fact a retrospective cohort study. The randomization process of the initial study did not include the parameter renal function. Accordingly, robust data on pre-operative renal function are not available, and not even precise creatinine values exist, therefore the influence of surgery on post-op function cannot be assessed. The follow-up now provides eGFR which demonstrate a possible beneficial impact of PN on renal function which did not result in improved OS, however, the initial study was not designed to test the hypothesis of improved renal function and therefore reduced cardiovascular events with PN. This also means that additional important data on comorbidity (cardiovascular disease, hypertension, diabetes, smoking status) are missing, and a meaningful analysis has become impossible. It is surprising that there has been so much discussion of these studies, because they certainly do not provide the level 1 evidence data needed to better understand the influence of CKD on cardiovascular disease and the related mortality rate.
CONCLUSION
The quality of partial nephrectomy for RCC has continuously and substantially increased over the past years, and we have learned to increase the number of spared nephrons by reducing the volume of excised functional parenchyma as well as by careful repair. It has also been possible to reduce ischemia times to a minimum by sophisticated techniques so that the spared nephrons are not compromised by ischemia-reperfusion injury. By comparison, it can be seen that PN results in the preservation of functional nephrons which would be lost with RN, however, the impact and advantage of this difference is not completely understood. Healthy patients with normal pre-operative function are likely to achieve good post-operative function with eGFR >60ml/min/1.73m2 after RN, so that resulting morbidity will not occur. PN will not make much difference in this setting. However, when CKD is present pre-operatively, every measure should be taken to preserve as many nephrons as possible. If an eGFR of >45ml/min/1.73m2 can be maintained, the risk of increased mortality will also be quite low. Comorbidities such as hypertension and diabetes have the potential to further compromise renal function. CKD carries the risk of inducing or aggravating cardiovascular disease, which will decrease OS. And comorbidities not only negatively influence renal function, but can also have a direct synergetic negative effect on cardiovascular disease. So our task is not only to best preserve renal function, but also to effectively treat comorbidity.
FUNDING
The author reports no funding.
AUTHOR CONTRIBUTIONS
There is one author only who is responsible for all parts of this manuscript.
CONFLICT OF INTEREST
The author has no conflict of interest to report.
ACKNOWLEDGMENTS
The author has no acknowledgments.
REFERENCES
[1] | Robson CJ , Churchill BM , Anderson W . The results of radical nephrectomy for renal cell carcinoma. J Urol. (1969) ;101: (3):297–301. |
[2] | Clayman RV , Kavoussi LR , Soper NJ , Dierks SM , Meretyk S , Darcy MD et al Laparoscopic nephrectomy: initial case report. J Urol. (1991) ;146: (2):278–82. |
[3] | Herr HW . Partial nephrectomy for unilateral renal carcinoma and a normal contralateral kidney: 10-year follow-up. J Urol. (1999) ;161: (1):33–4. |
[4] | Leibovich BC , Blute M , Cheville JC , Lohse CM , Weaver AL , Zincke H . Nephron sparing surgery for appropriately selected renal cell carcinoma between 4 and 7 cm results in outcome similar to radical nephrectomy. J Urol. (2004) ;171: (3):1066–70. |
[5] | Lane BR , Campbell SC , Demirjian S , Fergany AF . Surgically induced chronic kidney disease may be associated with a lower risk of progression and mortality than medical chronic kidney disease. J Urol. (2013) ;189: (5):1649–55. |
[6] | Traynor J , Mactier R , Geddes CC , Fox JG . How to measure renal function in clinical practice BMJ. (2006) ;333: (7571):733–7. |
[7] | Itoh K . 99mTc-MAG review of pharmacinetics, clinical application to renal diseases and quantification of renal function. Ann Nucl Med. (2001) ;15: (3):179–90. |
[8] | Beri A , Lattouf JP , Deambros O , Grüll M , Gschwendtner M , Ziegerhofer J et al Partial nephrectomy using renal artery perfusion for cold ischemia: functional and oncologic outcomes. J Endourol. (2008) ;22: (6):1285–90. |
[9] | Tourojman M , Kirmiz S , Boelkins B , Noyes SL , Davis AT , O’Donnell K et al Impact of Reduced Glomerular Filtration Rate and Proteinuria on Overall Survival of Patients with Renal Cancer. J Urol. (2016) ;195: (3):588–93. |
[10] | Minervini A , Campi R , Lane BR , De Cobelli O , Sanguedolce F , Hatzichristodoulou G et al Impact of resection technique on perioperative outcomes and surgical margins after partial nephrectomy for localized renal masses: a prospective multicenter study. J Urol . (2020) ;203: (3):496–504. |
[11] | Simmons MN , Hillyer SP , Lee BH Fergany AF , Kaouk J , Campbell SC . Functional recovery after partial nephrectomy: effects of volume loss and ischemic injury J Urol. (2012) ;187: (5):1667–73. |
[12] | Gill IS , Desai MM , Kaouk JH , Meraney AM , Murphy DP , Sung GT , et al Laparoscopic partial nephrectomy for renal tumor: duplicating open surgical techniques. J Urol. (2002) ;167: (2Pt1):469–76. |
[13] | Baumert H , Ballaro A , Nimish Shah N , Mansouri D , Zafar N , Molinie V , et al Reducing warm ischaemia time during laparoscopic partial nephrectomy: a prospective comparison of two renal closure techniques. Eur Urol. (2007) ;52: (4):1164–9. |
[14] | Mir MC , Ercole C , Takagi T , Zhang Z , Velet L , Remer EM , et al Decline in renal function after partial nephrectomy: etiology and prevention. J Urol. (2015) ;193: (6):1889–98. |
[15] | Thompson RH , Frank I , Lohse CM , Saad IR , Fergany A , Zincke H , et al The impact of ischemia time during open nephron sparing surgery on solitary kidneys: a multiinstitutional study. J Urol. (2007) ;177: :471–6. |
[16] | Porpiglia F , Renard J , Billia M Musso F , Volpe A , Burruni R et al Is renal warm ischemia over 30 minutes during laparoscopic partial nephrectomy possible? One-year results of a prospective study. Eur Urol. (2007) ;52: (4):1170–8. |
[17] | Shao P , Qin C , Yin C , Meng X , Ju X , Li J et al Laparoscopic partial nephrectomy with segmental renal artery clamping: technique and clinical outcomes Eur Urol. (2011) ;59: (5):849–55. |
[18] | Gill IS , Abreu SC , Desai MM , Steinberg AP , Ramani AP , Ng C et al Laparoscopic ice slush renal hypothermia for partial nephrectomy: the initial experience. J Urol. (2003) ;170: (1):52–6. |
[19] | Landman J , Venkatesh R , Lee D , Vanlangendonck R , Morissey K , Andriole GL et al Renal Hypothermia achieved by retrograde endoscopic cold saline perfusion: technique and initial clinical application. Urology. (2003) ;61: (5):1023–5. |
[20] | Janetschek G , Abdelmaksoud A , Bagheri F , Al-Zahrani H , Leeb K , Gschwendtner M . Laparoscopic partial nephrectomy in cold ischemia: Renal artery perfusion. J Urol. (2004) ;171: (1):68–71. |
[21] | Woods RJ , Prueckner S , Safar P Radovsky A , Takasu A , Stezoski SW , et al Hypothermic aortic arch flush for preservation during exsanguination cardiac arrest of 15 minutes in dogs. J Trauma. (1999) ;47: (6):1028–36. |
[22] | Fitzpatrick JM , Wallace DM , Whitfield HN Watkinson LE , Fernando AR , Wickham JE . Inosine in ischemic renal surgery: long-term follow-up. Br J Urol. (1981) ;53: (6):524–7. |
[23] | Consentino M , Breda A , Sanguedolce F , Landman J , Stolzenburg JU , Verze P et al The use of mannitol in partial and live donor nephrectomy: an international survey. World J Urol. (2013) ;31: (4):977–82. |
[24] | Spaliviero M , Power NE , Murray KS , Sjoberg DD , Benfante NE , Bernstein ML et al Intravenous mannitol versus placebo during partial nephrectomy in patients with normal kidney function: a double-blind, clinically-integrated, randomized trial Eur Urol. (2018) ;73: (1):53–59. |
[25] | Venkataraman R , Kellum JA . Prevention of acute renal failure. Chest. (2007) ;131: :300–8. |
[26] | Birck R , Krossok S , Markoetz F , Schnülle P , van der Woude FJ , Braun C Acetycysteine for prevention of contrast nephropathy: meta-analysis. Lancet. (2003) ;362: :598–603. |
[27] | DiMari J , Megyesi J , Udvarhelyi N , Price P , Davis R , Safirstein R . N-acetyl cysteine ameliorates ischemic renal failure. Am J Physiol. (1997) ;272: :292–8. |
[28] | van Alem CMA , Boonstra M , Prins J , Bezhaeva T , van Essen MF , Ruben J et al Local delivery of liposomal prednisolone leads to an anti-inflammatory profile in renal ischemia-reperfusion injury in the rat. Nephrol Dial Transplant. (2018) ;33: (1):44–53. |
[29] | Qiu L , Zhang ZJ . Therapeutic strategies of kidney transplant ischemia reperfusion injury: insight from mouse models. Biomed J Sci Tech Res. (2019) ;14: (5):002617. |
[30] | Janetschek G , Daffner P , Peschel R , Bartsch G . Laparoscopic nephron sparing surgery for small renal cell carcinoma. J Urol. (1998) ;159: (4):1152–5. |
[31] | Drerup M , Magdy A , Hager M , Colleselli D , Kunit T , Lusuardi L et al Non-ischemic laparoscopic partial nephrectomy using -nm diode laser for small exophytic renal tumors. BMC Urol. (2018) ;18: (1):99. |
[32] | Gill I , Patil MB , de Castro Abreu AL , Ng C , Cai J , Berger et al Zero ischemia anatomical partial nephrectomy: a novel approach. J Urol. (2012) ;187: (3):807–14. |
[33] | Cacciamani GE , Medina LG , Gill TS , Mendelsohn A , Husain F , Bhardwaj L , et al Impact of renal hilar control on outcomes of robotic partial nephrectomy: systematic review and cumulative meta-analysis. Eur Urol Focus. (2019) ;5: (4):619–35. |
[34] | Huang WC , Levey AS , Serio AM , Snyder M , Vickers AJ , Raj GV et al: Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. (2006) ;7: (9):735–40. |
[35] | Go AS , Chertow GM , Fan D , McCulloch CE , Hsu CY . Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. (2004) ;351: (13):1296–305. |
[36] | Weight CJ , Lieser G , Larson BT , Gao T , Lane BR , Campbell SC et al Partial nephrectomy is associated with improved overall survival compared to radical nephrectomy in patients with unanticipated benign renal tumours. Eur Urol. (2010) ;58: (2):293–8. |
[37] | Larcher A , Capitanio U , Terrone C , Volpe A , De Angelis P , Dehó F et al Elective nephron sparing surgery decreases other cause mortality relative to radical nephrectomy only in specific subgroups of patients with renal cell carcinoma. J Urol. (2016) ;196: (4):1008–13. |
[38] | Capitanio A , Larcher U , Terrone C , Antonelli A , Volpe A , Fiori C et al End-stage renal disease after renal surgery in patients with normal preoperative kidney function: balancing surgical strategy and individual disorders at baseline. Eur Urol. (2016) ;70: (4):558–61. |
[39] | Huang WC , Donin NM , Levey AS , Campbell SC . Chronic Kidney Disease and Kidney Cancer Surgery: New Perspectives. J Urol. (2020) ;203: (3):475–85. |
[40] | Muzaale AD , Massie AB , Wang MC , Montgomery RA , McBride MA , Wainright JL et al Risk of end-stage renal disease following live kidney donation. JAMA. (2014) ;311: (6):579–86. |
[41] | Van Poppel H , Da Pozzo L , Albrecht W , Matveev V , Bono A , Borkowski A et al A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. (2011) ;59: (4):543–52. |
[42] | Scosyrev E , Messing EM , Sylvester R , Campbell S , Van Poppel H . Renal function after nephron-sparing surgery versus radical nephrectomy: results from EORTC randomized trial 4. Eur Urol. (2014) ;65: (2):372–7. |




