A Systematic Review of Systemic Treatment Options for Advanced Non-Clear Cell Renal Cell Carcinoma
Abstract
Introduction:
There have been a number of recent advances in the management of advanced clear cell renal cell carcinoma (ccRCC). However, the majority of these studies excluded patients with non-clear cell RCC (nccRCC), and optimal management of nccRCC remains unknown.
Materials and Methods:
A systematic review of the literature was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to evaluate systemic treatment options in locally advanced or metastatic nccRCC between 2000-2019. Randomized controlled trials, single-arm phase II–IV trials, and prospective analyses of medication access programs were included. The primary outcome measures were progression free survival (PFS), overall survival (OS), and objective response rate (ORR).
Results:
A total of 31 studies were included in the final analysis. There was the highest level of evidence to support first-line treatment of nccRCC with sunitinib. Additional single-arm trials support the use of other vascular endothelial growth factor (VEGF) inhibitors with axitinib and pazopanib, as well as mammalian target of rapamycin (mTOR) inhibition with temsirolimus or everolimus +/–bevacizumab. Immune checkpoint inhibition has an emerging role in nccRCC, but optimal sequencing of available options is not clear. Prospective data to support the use of newer immunotherapy combinations are lacking. Treatment for collecting duct carcinoma remains platinum-based chemotherapy.
Conclusions:
The availability of randomized trials in nccRCC is limited, and most studies include outcomes for nccRCC as a group, making conclusions about efficacy by subtype difficult. This systematic review supports consensus guidelines recommending sunitinib or clinical trial enrollment as preferred first-line treatment options for nccRCC, but also suggests a more nuanced approach to management and new options for therapy such as immune checkpoint inhibition.
INTRODUCTION
Cancers of the kidney and renal pelvis account for about 4% of all new cancer diagnoses per year in the US with an estimated 73,820 new diagnoses in 2019 [1]. The vast majority of these are renal cell carcinomas (RCC) with clear cell renal cell carcinoma (ccRCC) as the most common subtype, comprising 75–80% of all RCC cases [2]. The remainder of cases are classified as non-clear cell renal cell carcinoma (nccRCC), which are then divided into multiple distinct subtypes based on histological and molecular characteristics. Subtypes of nccRCC include papillary, chromophobe, collecting duct, renal medullary, and translocation RCC, which represent 10–15%, 5–7%, 1–2%, <1%, and < 1% of all RCCs, respectively [3]. Unclassifiable cases of RCC are also typically included under the nccRCC umbrella, and both ccRCC and nccRCC can have sarcomatoid differentiation.
Median survival of patients with localized nccRCC varies with histology, with more favorable outcomes in patients with papillary and chromophobe RCC and less favorable outcomes in patients with renal medullary and translocation RCC [4]. In the metastatic setting, however, survival in all subtypes of nccRCC is uniformly worse compared to ccRCC [5], due to the inherent aggressiveness of these cancers, and a lack of effective systemic treatment options. Median survival following a diagnosis of metastatic nccRCC remains poor with 5 year overall survival rates of 7–12% [6].
Recently, there have been a number of promising advances in the treatment of metastatic ccRCC, particularly with immune checkpoint inhibitors (ICIs) and novel tyrosine kinase inhibitors (TKIs) [7–10]. These clinical trials have generally excluded patients with nccRCC and so data to support the use of these newer agents in the nccRCC population are lacking. To date, there are only 3 randomized controlled trials (RCTs) that exclusively enrolled nccRCC patients and another 2 RCTs that stratified results by histology [11–15]. However, there are a number of single-arm trials and prospective analyses of expanded access programs that evaluate additional therapeutic options for nccRCC patients and can provide valuable information for this under-represented cohort.
The goal of this systematic review was to evaluate the existing prospective literature regarding systemic treatment of advanced or metastatic nccRCC. In particular, we sought to highlight new agents and combinations that show potential, and to compile the existing evidence base for treatment stratified by nccRCC histologic subtype.
MATERIALS AND METHODS
Search strategy
A systematic review of the literature was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocol [16] to identify studies evaluating systemic treatment options in locally advanced or metastatic nccRCC. Study selection was performed in duplicate by C.O. and T.R. The PubMed-Medline and Embase databases were searched for studies published between January, 2000 and June, 2019 using one or a combination of the following search terms: renal cell carcinoma (RCC), advanced, metastatic, non-clear cell renal cell carcinoma, papillary RCC, chromophobe RCC, collecting duct RCC, translocation RCC, medullary RCC, systemic treatment, and clinical trial. Abstracts from the 2019 American Society of Clinical Oncology (ASCO) Annual Meeting and Genitourinary Cancer Symposium, and references found in relevant publications were also evaluated for inclusion. Results were restricted to English language only.
Study title and abstract were screened to determine initial relevance. Eligible articles then underwent full text evaluation for final inclusion in this review. Studies included were RCTs, single-arm phase II–IV trials, and prospective analyses of expanded access programs, while phase I trials, retrospective analyses, case series, case reports, meta-analyses, and reviews were excluded. If there were multiple publications reporting on the same cohort, only the most recent publication was included to avoid over-representation. Studies that did not report results for nccRCC patients alone, included less than 10 nccRCC patients, or evaluated surgical or radiation therapy were excluded.
Data extraction
A data extraction form was generated and included study design, baseline patient characteristics including histology, intervention(s), and outcome measures. Data extraction was performed independently by C.O.
Outcome measures
The primary outcome measures were progression free survival (PFS), overall survival (OS), and objective response rate (ORR). Due to the heterogeneous populations and methodologies of the included studies, data were not pooled for meta-analysis.
Risk of bias assessment
The Cochrane Collaboration tool was used to assess risk of bias in RCTs [17].
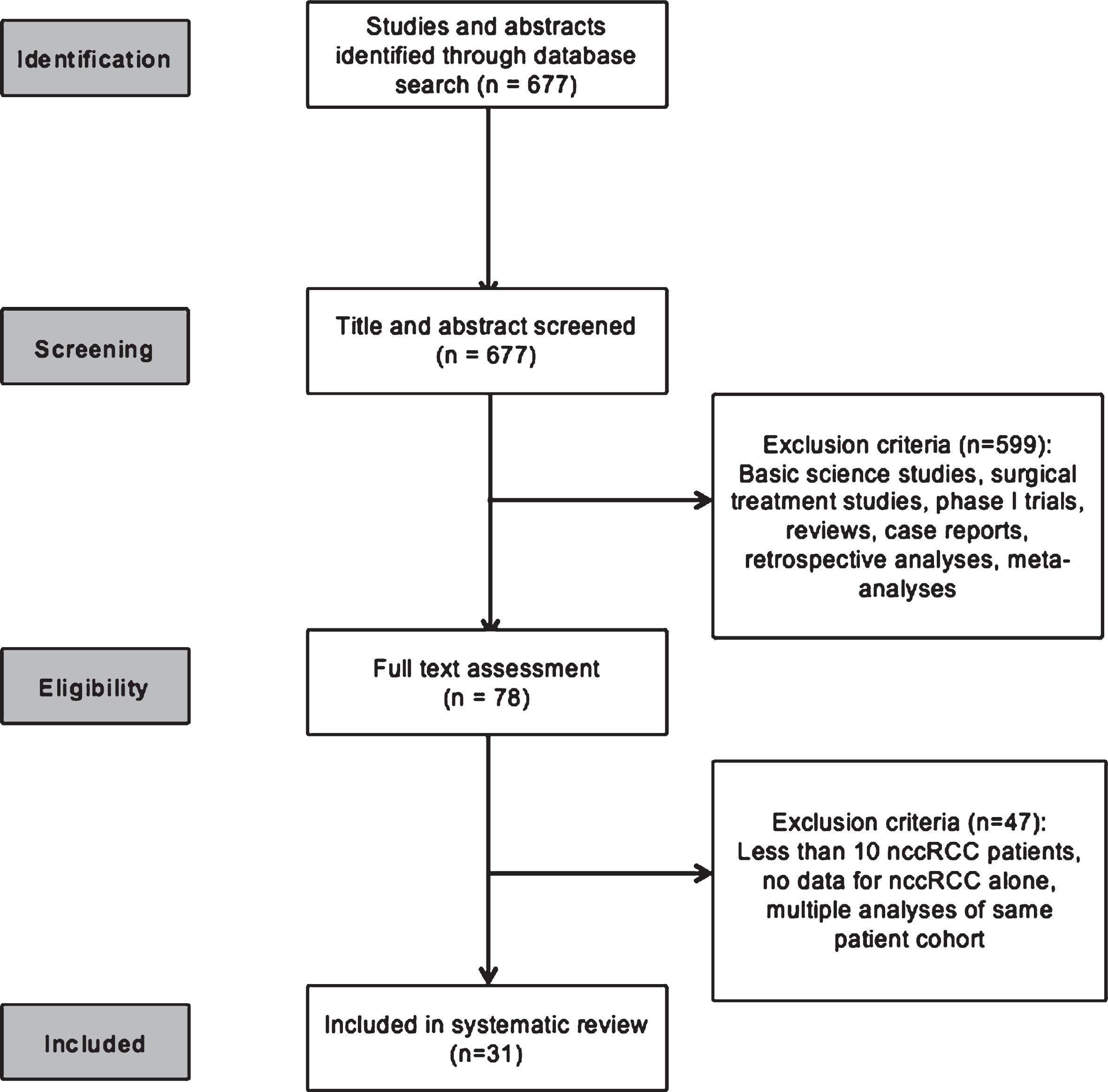
RESULTS
The systematic search strategy identified 677 publications for screening. Of these, 78 studies underwent full text assessment and a total of 31 were included in the final systematic review (Fig. 1).
Fig. 1
CONSORT diagram outlining the study evaluation and selection process.
Characteristics of included studies
The included studies were comprised of 5 RCTs, 1 single-arm phase IIIB/IV trial, 21 single-arm phase II trials, and 4 prospective analyses of expanded access medication programs. A total of 22 different systemic treatments for locally advanced or metastatic nccRCC were evaluated across a combined total of 2,134 nccRCC patients. Study characteristics and outcomes for all included studies are detailed in Tables 1–4 and supplementary Table 1.
Table 1
Study characteristics and summary of outcomes of the included randomized controlled trials
| Study | Comparator | Line | Total n | NCC | mOS, months (95% CI) | OS HR (95% CI) | mPFS, months (95% CI) | PFS HR (95% CI) | ORR (%) |
| ASPEN | Everolimus | First | 57 | 57 (100) | 13.2 (9.7 – 37.9) | 1.12 (0.7 – 2.1) | 5.6 (80% CI 5.5–6.0) | 1.41 (80% CI 1.03–1.92) | 9% |
| Sunitinib | First | 51 | 51 (100) | 31.5 (14.8 – NR) | 8.3 (80% CI 5.8–11.4) | 18% | |||
| RECORD-3 | Everolimus | First | 238 | 31 (13) | – | – | 5.1 (2.6–7.9) | 1.5 (0.9–2.8) | – |
| Sunitinib | First | 233 | 35 (15) | – | 7.2 (5.4–13.8) | – | |||
| ESPN | Everolimus | First | 35 | 35 (100) | 14.9 (8.0 – 23.4) | – | 4.1 (2.7–10.5) | – | 3% |
| Sunitinib | First | 33 | 33 (100) | 16.2 (14.2 – NR) | 6.1 (4.2–9.4) | 9% | |||
| ARCC | Interferon-α | First | 207 | 36 (17) | 4.3 (3.2 – 7.3) | 1.8 (1.6–2.1) | 8% | ||
| Temsirolimus | First | 209 | 37 (18) | 11.6 (8.9–14.5) | 0.49 (0.29 – 0.85) | 7.0 (3.9–8.9) | 0.38 (0.23–0.62) | 5% | |
| SWOG 1107 | Tivantinib | First Second | 25 | 25 (100) | 10.3 (7.3 – 15.7) | – | 2.0 (1.8–3.0) | – | 0% |
| Tivantinib + Erlotinib | First Second | 25 | 25 (100) | 11.3 (6.7–21.9) | 3.9 (1.8 – 7.3) | 0% |
Legend: (-) = Data not reported; CR = complete response; HR = hazard ratio; NCC = non-clear cell renal cell carcinoma; NR = not reached; mOS = median overall survival; mPFS = median progression-free survival; ORR = objective response rate.
Table 2
Study characteristics and summary of outcomes of trials in patients with papillary RCC. For trials that include patients with other histologies, only outcomes for the papillary patients are reported
| Author | Year | Treatment | Line | Total n | Papillary n (%) | mOS, months (95% CI) | mPFS, months (95% CI) | ORR (%) |
| VEGF TKIs | ||||||||
| Park et al. | 2018 | Axitinib | Second or later | 040 | 26 (65) | 8.3 (4.1–12.5) | 3.5 (0–10.9) | 38% |
| Jung et al. | 2018 | Pazopanib | Any | 029 | 19 (66) | NR | 17.3 (14.8–19.8) | 39% |
| Stadler et al. | 2010 | Sorafenib | Any | 2504 | 107 (4) | – | – | 3% |
| Armstrong et al. | 2016 | Sunitinib | First | 051 | 33 (65) | – | 8.1 (80% CI 5.8–11) | 24% |
| Lee et al. | 2012 | Sunitinib | Any | 031 | 22 (71) | – | – | 36% |
| Molina et al. | 2012 | Sunitinib | Any | 023 | 08 (35) | – | 5.6 (1.4–7.1) | – |
| Procopio et al. | 2008 | Sorafenib | Second or later | 136 | 15 (11) | – | – | 7% |
| Ravaud et al. | 2015 | Sunitinib | First | 061 | 61 (100) | Type 1:17.8 (5.7–26.1) | Type 1:6.6 (2.8–14.8) | Type 1:13% |
| Type 2:12.4 (8.2–16) | Type 2:5.5 (3.8–7.1) | Type 2:11% | ||||||
| Tannir et al. | 2012 | Sunitinib | First Second Third | 057 | 27 (47) | 12.6 (7.3–36.9) | 1.6 (1.4–5.4) | 0% |
| Tannir et al. | 2016 | Sunitinib | First | 033 | 14 (42) | 14.9 (7.1 –22.7) | 5.7 (1.4–19.8) | – |
| Twardowski et al. | 2017 | Tivantinib | First Second | 025 | 25 (100) | 10.3 (7.3 –15.7) | 2.0 (1.8–3.0) | 0% |
| Immune Checkpoint Inhibitors | ||||||||
| Suarez et al. | 2019 | Pembrolizumab | First | 165 | 118 (71) | – | – | 28% |
| MET inhibitors | ||||||||
| Schoffski et al. | 2017 | Crizotinib | Any | 023 | 23 (100) | 30.5 (12.3 –NR) | 5.8 (2.6–30.5) | 17% |
| Choueiri et al. | 2013 | Foretinib | First Second | 074 | 74 (100) | NR | 9.3 (6.9–12.9) | 14% |
| Choueiri et al. | 2017 | Savolitinib | Any | 109 | 109 (100) | – | – | 7% |
| mTOR inhibitors | ||||||||
| Armstrong et al. | 2016 | Everolimus | First | 057 | 37 (65) | – | 5.5 (80% CI 4.4–5.6) | 5% |
| Escudier et al. | 2016 | Everolimus | First | 088 | 88 (100) | 21.4 (15.4–28.4) | 4.1 (3.6–5.5) | 1% |
| Koh et al. | 2012 | Everolimus | Any | 049 | 29 (60) | 10.9 | 3.4 | 7% |
| Tannir et al. | 2016 | Everolimus | First | 035 | 13 (37) | 16.6 (5.9–NR) | 4.1 (1.5–7.4) | – |
| Dutcher et al. | 2009 | Temsirolimus | First | 209 | 25 (12) | 10.9 (7.8–15.1) | 5.9 (3.7–9.0) | – |
| Chemotherapy | ||||||||
| Bylow et al. | 2009 | Carboplatin+Paclitaxel | First | 017 | 16 (94) | – | – | 0% |
| Other/Combination Therapies | ||||||||
| McKay et al. | 2019 | Atezolizumab+Bevacizumab | Any | 065 | 12 (18) | – | – | 25% |
| Powles et al. | 2019 | Durvalumab+Savolitinib | Any | 041 | 41 (100) | NR | 5.3 (1.5–12.0) | 27% |
| Voss et al. | 2016 | Everolimus+Bevacizumab | First | 035 | 04 (11) | – | 13.8 (1.4–NR) | 25% |
| Dutcher et al. | 2009 | Interferon-α | First | 207 | 30 (14) | 5.7 (4.3–7.8) | 2.1 (1.8–4.3) | – |
| Twardowski et al. | 2017 | Tivantinib+Erlotinib | First Second | 025 | 25 (100) | 11.3 (6.7–21.9) | 3.9 (1.8 –7.3) | 0% |
Legend: (-) = Data not reported; CR = complete response; HR = hazard ratio; NE = not evaluable; NR = not reached; mOS = median overall survival; mPFS = median progression-free survival; ORR = objective response rate.
Table 3
Study characteristics and summary of outcomes of trials in patients with chromophobe histology. For trials that include patients with other histologies, only outcomes for the chromophobe patients are reported
| Author | Year | Intervention | Line | Total | Chromophobe | mOS, | mPFS, | ORR |
| n | n (%) | months | (95% CI) | (%) | ||||
| (95% CI) | ||||||||
| VEGF TKIs | ||||||||
| Park, I | 2018 | Axitinib | Second or later | 040 | 4 (10) | 22.2 (-) | 11.0 (-) | 25% |
| Jung, K | 2018 | Pazopanib | Any | 029 | 3 (10) | 18.9 (-) | 18.3 (11.9–24.7) | 33% |
| Procopio, G | 2008 | Sorafenib | Second or later | 136 | 3 (2) | – | – | 0% |
| Stadler, W | 2010 | Sorafenib | Any | 2504 | 20 (1) | – | – | 5% |
| Armstrong, A | 2016 | Sunitinib | First | 051 | 10 (19.6) | – | 5.5 (80% CI 3.2–19.7) | 10% |
| Lee, J | 2012 | Sunitinib | Any | 031 | 3 (10) | – | – | 33% |
| Tannir, N | 2012 | Sunitinib | First Second Third | 057 | 5 (9) | – | 12.7 (8.5–NR) | 40% |
| Tannir, N | 2016 | Sunitinib | First | 033 | 6 (18) | 31.6 (14.2–NR) | 8.9 (2.9–20.1) | – |
| Immune Checkpoint Inhibitors | ||||||||
| Suarez, C | 2019 | Pembrolizumab | First | 165 | 21 (13) | – | – | 10% |
| mTOR inhibitors | ||||||||
| Armstrong, A | 2016 | Everolimus | First | 057 | 6 (10.5) | – | 11.4 (80% CI 5.7–19.4) | 33% |
| Koh, Y | 2012 | Everolimus | Any | 049 | 8 (16) | 21.6 | 13.1 | 29% |
| Tannir, N | 2016 | Everolimus | First | 035 | 6 (17) | 25.1 (4.7–NR) | NR | – |
| Other/Combination Therapies | ||||||||
| McKay, R | 2019 | Atezolizumab+Bevacizumab | Any | 065 | 10 (15) | – | – | 10% |
| Voss, M | 2016 | Everolimus+Bevacizumab | First | 035 | 5 (14) | – | NR (1.9–NR) | 40% |
Legend: (-) = Data not reported; CR = complete response; HR = hazard ratio; NR = not reached; mOS = median overall survival; mPFS = median progression-free survival; ORR = objective response rate.
Table 4
Study characteristics and summary of outcomes of trials in patients with collecting duct histology
| Author | Year | Intervention | Line | Total n | CD n (%) | OS, months (95% CI) | PFS, months (95% CI) | ORR (%) |
| Oudard, S | 2007 | Gemcitabine + Cisplatin or Carboplatin | First | 23 | 23 (100) | 10.5 (3.8–17.1) | 7.1 (3.0–11.3) | 26% |
| Sheng, X | 2018 | Gemcitabine + Cisplatin + Sorafenib | Any | 26 | 26 (100) | 12.5 (9.6–15.4) | 8.8 (6.7–10.9) | 31% |
| Tannir, N | 2012 | Sunitinib | First Second Third | 57 | 6 (11) | – | 3.1 (1.4–NR) | 0% |
Legend: (-) = Data not reported; CD = collecting duct; CR = complete response; HR = hazard ratio; NR = not reached; mOS = overall survival; mPFS = progression-free survival; ORR = objective response rate.
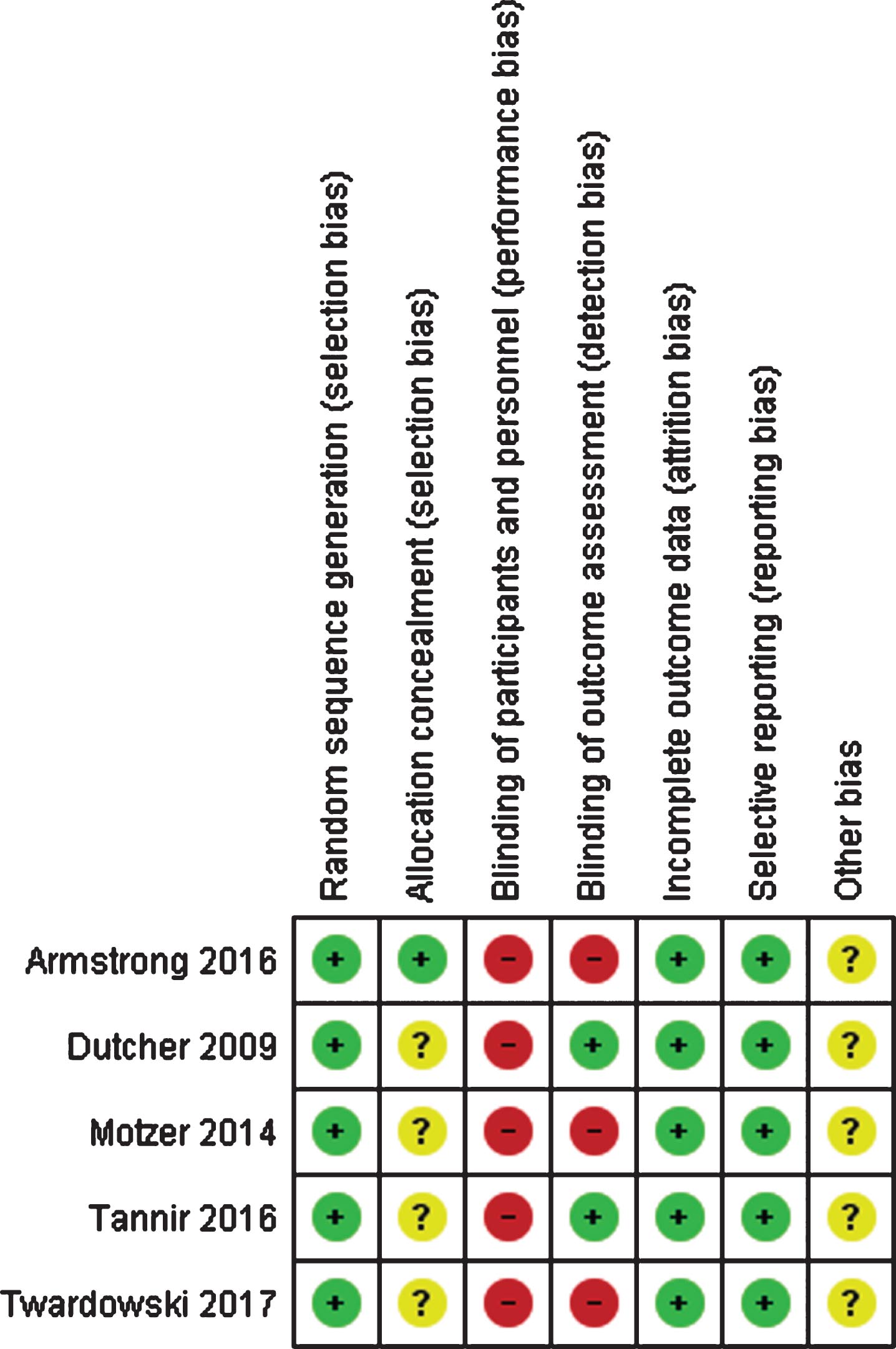
Risk of bias assessment of included studies
All 5 RCTs had a low overall risk of bias, although all of them were open-label and only 2 of the 5 trials included blinded independent review for outcome assessment (Fig. 2). The remaining single arm studies and expanded access programs had at least a moderate risk of bias, however they were still included in this systematic review as they represent much of the best available evidence for treatment in this patient population. Based on the inclusion of multiple negative studies within this review, we do not suspect that publication bias had a significant impact on our results or conclusions.
Fig. 2
Risk of bias assessment of the randomized controlled trials included in the systematic review. Green (+): low risk of bias; yellow (?): unclear risk of bias; red (-): high risk of bias.
RCTs in nccRCC
Everolimus versus sunitinib
There were 3 RCTs comparing the mammalian target of rapamycin (mTOR) inhibitor everolimus to the vascular endothelial growth factor (VEGF) TKI sunitinib in first line treatment of metastatic nccRCC. The ASPEN and ESPN trials enrolled only nccRCC, and the RECORD-3 trial enrolled patients with any RCC histology but reported PFS results for nccRCC alone [11, 12, 15]. Median overall survival was numerically greater in the sunitinib group compared to the everolimus group in both ASPEN (31.5 months vs. 13.2 months; HR 1.12 (95% CI 0.7–2.1)) and ESPN (16.2 months vs. 14.9 months; stratified log-rank p = 0.18), however this failed to reach statistical significance in either trial. The median PFS was numerically longer with first line sunitinib compared to everolimus in all 3 trials, but was only statistically significant in the ASPEN (8.3 months vs. 5.6 months; HR 1.41 (80% CI 1.03–1.92)) and RECORD-3 (7.2 months vs. 5.1 months; HR 1.5 (95% CI 0.9–2.8)) trials. Response rates were reported in ASPEN and ESPN with higher ORR seen for the sunitinib group in both trials (18% vs. 9% and 9% vs. 3%, respectively).
Interferon-alpha (IFNα) versus temsirolimus
The phase 3 Advanced Renal Cell Carcinoma (ARCC) trial randomized patients with poor risk RCC of any histology to treatment with the mTOR inhibitor temsirolimus or interferon-α (IFNα). The study subsequently performed an exploratory subgroup analysis of outcomes for nccRCC patients alone [14]. In nccRCC patients, median OS and PFS were significantly longer in the temsirolimus group compared to the IFNα group (11.6 months vs. 4.3 months; HR 0.49 (95% CI 0.29–0.85) and 7.0 months vs. 1.8 months; HR 0.38 (95% CI 0.23–0.62)), respectively. Response rates were not different between groups. Clinical benefit, defined as complete response (CR) plus partial response (PR) plus stable disease (SD), was reported in 15/37 (41%) temsirolimus patients and 3/36 (8%) IFNα patients (p = 0.002).
Tivantinib versus tivantinib plus erlotinib
The Southwest Oncology Group (SWOG) 1107 trial compared the VEGF TKI tivantinib with or without the epidermal growth factor receptor (EGFR) TKI erlotinib in the first or second line setting for papillary RCC [13]. Unfortunately, the ORR was 0% in both arms and median OS and PFS were not different between the two arms.
SINGLE-ARM TRIALS AND PROSPECTIVE ANALYSES OF EXPANDED ACCESS PROGRAMS IN nccRCC
Anti-angiogenesis agents/Tyrosine kinase inhibitors
The majority of the single arm studies involving nccRCC patients evaluated TKIs targeting the VEGF pathway, including sunitinib [18–21], sorafenib [22–24], axitinib [25], and pazopanib [26].
Sunitinib
Three single-arm studies of sunitinib enrolled only nccRCC patients with a total accrual of 111 patients, and a global expanded access program of sunitinib analyzed an additional 532 nccRCC patients. All four studies reported ORR (range 4.5–35.5%) and median PFS (range 2.7–6.4 months). Median OS was reported in two studies and ranged from 12.2–16.8 months.
Sorafenib
Two single-arm studies and 1 expanded access program reported response rates to sorafenib for nccRCC patients. Khaled et al evaluated sorafenib in the first line setting and found a disease control rate (CR + PR + SD) of 81.8% for nccRCC patients, but ORR was not reported. In the second line setting, Procopio et al reported 1 papillary RCC patient with a partial response to sorafenib out of 18 total nccRCC patients (PR rate 5.6%). The expanded access trial by Stadler et al reported 4 partial responses out of 127 papillary or chromophobe RCC patients treated with sorafenib (ORR rate 3.1%). None of these studies reported OS or PFS results for nccRCC patients alone.
Axitinib
One single-arm trial investigated axitinib in 40 nccRCC patients who had failed prior treatment with temsirolimus. The median OS, PFS, and ORR of the entire cohort were 12.1 months (95% CI 6.4–17.7), 7.4 months (95% CI 5.2–9.5), and 37.5%, respectively. Results were also reported by histology, with a median OS of 8.3 months (95% CI 4.17–12.5) and PFS of 3.5 months (95% CI 0–10.9) for papillary RCC, 22.2 months and 11.0 months for chromophobe RCC, and 16.9 months and 11.1 months (95% CI 7.6–14.6) for MiT family translocation RCC.
Pazopanib
Pazopanib was evaluated in 29 nccRCC patients, primarily with papillary histology (65.5%). The ORR was 28% with a median PFS of 16.5 months (95% CI 10.9–22.1). Median OS was not reached, but the 1 year overall survival rate was 69%.
mTOR inhibitors
In addition to the previously mentioned RCTs, there was one phase II trial of everolimus in nccRCC patients [27]. In this trial, the median OS of the entire cohort of 49 patients was 14.0 months with a PFS of 5.2 months and ORR of 10%. There was a trend toward increased PFS in patients with chromophobe RCC compared to papillary RCC (13.1 months vs. 3.4 months, p = 0.08), but no significant difference in OS (21.6 months vs. 10.9 months, p = 0.39)(27).
mTOR inhibitors + bevacizumab
Two phase II trials evaluated the use of the angiogenesis inhibitor bevacizumab in combination with mTOR inhibition. A first-line trial of everolimus plus bevacizumab in nccRCC demonstrated a promising ORR of 26% [28]. In this trial, there were significant differences in outcomes based on histology, with the presence of papillary features associated with improved response. Compared to tumors without papillary features, those with papillary features had an increased ORR (43% versus 11%), PFS (12.9 months vs. 1.9 months), and OS (28.2 months vs. 9.3 months, p < 0.001). Furthermore, tumor genetic testing found mutations in ARID1A in 5 of 14 patients with a major papillary component but in none of the other histologic variants, and all 5 of these patients had a PFS > 6 months.
A trial of 40 RCC patients, including 13 with nccRCC, evaluated the combination of temsirolimus plus bevacizumab in patients that had disease progression or intolerable toxicity with a VEGF TKI [29]. Among patients with nccRCC, the ORR was 8%, although an additional 77% of patients had stable disease. Median OS was 13.1 months (95% CI 5.0–24.6) and median PFS was 5.6 months (95% CI 3.4–13.7).
Immune checkpoint inhibitors
More recently, the safety and efficacy of immune checkpoint inhibitors (ICIs) has been explored in nccRCC through the KEYNOTE-427 study of pembrolizumab, a subgroup analyses of the CheckMate 374 study of nivolumab, and an expanded access program for nivolumab [30–32]. Additionally, a phase II trial of atezolizumab and bevacizumab included patients with nccRCC and clear cell renal cell carcinoma with sarcomatoid differentiation (sccRCC) [33].
Cohort B of the KEYNOTE-427 study [30] was the largest of the ICI studies and included 165 patients with nccRCC, the majority of which had papillary RCC (71.5%). One year PFS and OS rates were 24.7% and 73.7%, respectively. The ORR in the entire nccRCC cohort was 26.1%, including 6.1% of patients achieving a CR. ORR varied by histology, with an ORR of 28.0% for papillary RCC, 9.5% for chromophobe RCC, and 30.8% for unclassified RCC.
Subgroup analyses of CheckMate 374 [31] and an expanded access program of nivolumab [32] both showed activity in nccRCC, with an ORR of 13% and 19%, respectively. CheckMate 374 also reported a median PFS of 2.2 months (95% CI 1.8–5.4) and OS of 16.3 months (95% CI 9.2-NR). Additionally, a trial of atezolizumab plus bevacizumab had an ORR of 26% in patients with nccRCC, with survival data not yet mature [33].
Three of the four studies of ICIs included subgroup analysis of patients by PD-L1 status, all showing a numerically increased response rate in PD-L1 positive patients compared to PD-L1 negative patients, although the studies were not powered to detect a significant difference between these groups.
Chemotherapy
Aside from trials limited to patients with collecting duct histology, there were 2 studies of traditional chemotherapy: one with carboplatin and paclitaxel and the second using capecitabine [34, 35]. Of the 16 patients who received carboplatin and paclitaxel, there was only 1 documented response to treatment, which was a CR in the patient with collecting duct histology. The trial of capecitabine in 51 patients with nccRCC had an ORR of 26%, including 2 patients with CR. Median PFS was 10.1 months (95% CI 8.7–11.5) and median OS was 18.3 months (95% CI 15.5–21.1) with a 1 year overall survival rate of 71%.
TREATMENT OF nccRCC SUBTYPES
Papillary nccRCC
A total of 7 studies, including 1 RCT and 6 single-arm phase II trials, included only patients with papillary histology, and an additional 15 studies of mixed histology reported results for papillary patients alone. The majority of the papillary-specific studies investigated the use of c-MET inhibition, due to the increased incidence of alterations in the MET proto-oncogene in these tumors [36]. Agents investigated included single agent savolitinib [37], foretinib [38], tivantinib [13], and crizotinib [39], as well as combination therapy with savolitinib plus durvalumab [40] and tivantinib plus erlotinib [13]. Tumor responses were mixed, ranging from an ORR of 0% for both tivantinib alone and tivantinib plus erlotinib [13], to an ORR of 27% for durvalumab plus savolitinib [40].
Three of these trials also included response rates stratified by the presence or absence of an alteration in the MET gene. Although the definition of “MET-altered” varied across trials, all found an increased ORR in patients with MET alterations compared to those without. In patients treated with savolitinib, all of the observed responses were in patients with MET-driven tumors with an ORR of 18% in this subgroup [37], while patients with a germline mutation in MET also had an improved response to foretinib compared to those without a mutation (ORR 50% vs. 9%) [38]. Additionally, in a trial of crizotinib, MET-altered patients had an ORR of 50% and 2 year OS rate of 75%, compared to an ORR of 6% and 2 year OS rate of 36.9% for wild-type patients [39].
As previously described, the ASPEN and ESPN trials each compared everolimus versus sunitinib in the first line setting [11, 12]. The overall trial results favoring sunitinib remained consistent for patients with papillary histology, with an ORR of 24% for sunitinib and 5% for everolimus in the ASPEN trial in this subset [12]. Use of sunitinib was also associated with longer PFS and OS compared with everolimus, when reported.
The RAPTOR and SUPAP trials evaluated everolimus and sunitinib respectively, in single arm trials of patients with type 1 and type 2 papillary RCC [41, 42]. Both trials showed modest activity in this subset [42]. Full results are summarized in Table 2.
Chromophobe nccRCC
There were no studies that exclusively enrolled patients with chromophobe histology, however the ASPEN and ESPN trials included results for the subgroup of chromophobe patients. Contrary to the overall results, the median PFS was longer in the everolimus group than the sunitinib group in both trials, with a median PFS of 11.4 months for everolimus and 5.5 months for sunitinib in the ASPEN trial, and not reached for everolimus and 8.9 months for sunitinib in the ESPN trial (both non-significant). Two trials involving ICIs reported response rates for chromophobe patients alone. The ORR of pembrolizumab was 9.5% in chromophobe patients in Keynote-427 [30]; and the ORR of atezolizumab plus bevacizumab was 10% [33].
In studies of targeted therapies, chromophobe patients had comparable responses compared to all nccRCC patients with everolimus (ORR 29% vs. 10%; PFS 13.1 months vs. 5.2 months; OS 21.6 months vs. 14.0 months) [27], everolimus plus bevacizumab (ORR 40% vs. 29%) [28], axitinib (ORR 25% vs. 38%; PFS 11.0 months vs. 7.4 months; OS 22.2 months vs. 12.1 months) [25], and pazopanib (ORR 33% vs. 28%; PFS 18.3 months vs. 16.5 months; OS 18.9 months vs. NR) [26].
Collecting duct nccRCC
Two single-arm phase II trials enrolled only patients with collecting duct histology. One study of gemcitabine plus cisplatin or carboplatin had a median PFS of 7.1 months (95% CI 3–11.3) and median OS of 10.5 months (95% CI 3.8–17.1) with an ORR of 26%, including 1 patient with a CR [43]. A similar trial of the combination of gemcitabine, cisplatin, and sorafenib reported a median PFS of 8.8 months (95% CI 6.7–10.9) and median OS of 12.5 months (95% CI 9.6 –15.4) with an ORR of 30.8% [44]. Additionally, one trial of sunitinib reported results for collecting duct patients alone, with an ORR of 0% and median PFS of 3.1 months (95% CI 1.4 –NR) [21].
DISCUSSION
The total evidence base to guide treatment for patients with locally advanced or metastatic nccRCC remains limited and many questions regarding the optimal therapeutic strategy in this population are still unanswered. To our knowledge, there is only one prior systematic review and meta-analysis comparing the effectiveness and toxicities of systemic therapies for nccRCC [45] and a second review and meta-analysis comparing the efficacy of targeted therapies between ccRCC and nccRCC [46]. Given this limited evidence base, current clinical practice RCC guidelines from the European Association of Urology (EAU) and National Comprehensive Cancer Network (NCCN) recommend treatment based on limited data, and randomized studies using newer agents are desperately needed for this patient population.
Recently, the EAU RCC Guideline Panel decided to recommend sunitinib over everolimus and temsirolimus for first-line treatment of nccRCC based on a meta-analysis trend toward increased PFS favoring sunitinib over everolimus, although this did not reach statistical significance [45]. NCCN guidelines similarly categorize sunitinib as a “preferred regimen” for nccRCC, while everolimus is an “other recommended regimen,” and temsirolimus is a category 1 recommendation for patients in the poor-prognosis risk group but category 2A for other risk groups [47]. Our results support these general guidelines but also highlight differences in therapeutic strategies and treatment response across histologic subtypes of nccRCC [11, 12, 19, 21, 23–28, 33]. Trials in nccRCC continue to lump this diverse subgroup of cancers together, when the underlying biology and treatment efficacy clearly differs by subtype.
Additionally, newer strategies show promise in the upfront management of nccRCC, but comparative studies are lacking. Most notably, immune checkpoint inhibitors, alone or in combination, appear to have activity in papillary and unclassified RCC. With the need for additional high-level evidence to support treatment decisions, enrollment in clinical trials should be considered a preferred option for management of all patients with nccRCC. There are a number of ongoing trials in this setting, including a study of nivolumab plus cabozantinib (NCT03635892) and a study of lenvatinib plus everolimus in nccRCC (NCT02915783). These trials, among others, will hopefully provide further insight regarding optimal nccRCC management in the near future. Treatments with documented activity in larger promising phase 2 trials, such as pembrolizumab and the combination of atezolizumab plus bevacizumab, should be incorporated into guidelines to guide treatment choices given the lack of other effective agents and randomized trials. Additionally, combination regimens such as pembrolizumab plus axitinib have distinct rationale for use in nccRCC as well, given the modest activity of both checkpoint inhibitors and VEGF TKIs as monotherapy, although prospective data to support use of combination therapy is lacking.
Papillary
Papillary RCC (pRCC) is the most common subtype of nccRCC and it is therefore possible to draw some conclusions from subgroup analyses and subtype-specific trials in pRCC. The highest level of evidence for treatment comes from the ASPEN and ESPN trials, both of which found that sunitinib is the preferred first-line treatment over everolimus based on a numerically superior OS and PFS [11, 12].
Recently, there has been an increased focus on genetic and molecular drivers of pRCC. Two such drivers are alterations in MET, which are found in 17–33% of type 1 papillary and 7% of type 2 pRCCs [48], and mutations in the gene for fumurate hydratase, which result in the familial syndrome of Hereditary Leiomyomatosis and Renal Cell Cancer (HLRCC) that is associated with an aggressive variant of type 2 pRCC. Our review found the results of trials using MET inhibitors to be somewhat underwhelming for unselected patients with pRCC, but ORR for patients harboring MET mutations are as high as 50% and further study of biomarker-selected patients is needed [37–40]. Cabozantinib, an inhibitor of multiple tyrosine kinases including c-MET and VEGFR2, has demonstrated efficacy in metastatic ccRCC [49, 50], but as of yet there are no published prospective studies evaluating its efficacy in nccRCC. However, retrospective studies suggest that it also has activity in nccRCC, with observed ORRs ranging from 27% [51] to 35% [52], including 1 patient with papillary RCC that achieved a CR [51]. The PAPMET trial (NCT02761057) comparing cabozantinib, crizotinib, savolitinib, or sunitinib in patients with metastatic papillary RCC is nearing completion of accrual and analysis of this study will hopefully provide additional evidence regarding the use of MET inhibitors in this population.
An additional study that did not meet criteria for inclusion in this review utilized bevacizumab plus erlotinib in patients with either HLRCC or sporadic pRCC. Patients with HLRCC had particularly robust response to this regimen with an ORR of 60% and PFS of 24.2 months, compared with an ORR of 29% and PFS of 7.4 months in patients with sporadic pRCC [53]. These targeted therapies appear promising within a select population, but genetic and molecular sequencing will need to be more widely used in order to appropriately identify patients that may benefit.
Finally, ICIs with or without TKIs are now standard of care for metastatic ccRCC, and our results suggest that this therapeutic approach has activity in papillary RCC as well, with ORRs of 28%, 25%, and 27% for pembrolizumab, atezolizumab plus bevacizumab, and durvalumab plus savolitinib, respectively [30, 33, 40]. However, survival data from these studies are not yet mature and it remains unknown if there is any benefit to combination therapies over single agent ICI.
Chromophobe
Chromophobe RCC is typically a more indolent subtype of RCC with a lower risk of tumor progression or metastasis and longer cancer-specific survival. However, patients who do progress with locally advanced or metastatic disease have poor outcomes [54]. Our results suggest that there is at least modest efficacy in chromophobe RCC with VEGF TKIs, including sunitinib, axitinib, and pazopanib, and mTOR inhibition with everolimus or everolimus plus bevacizumab, and therefore these represent reasonable first-line treatment options. Since the ASPEN and ESPN trials both suggested a numerically longer median PFS with everolimus compared with sunitinib, this could be considered a standard at this point. Few chromophobe patients have been included in trials of ICIs thus far, but based on the two trials reported in this review, immune checkpoint inhibition may have limited efficacy in this subgroup [30, 33].
Rare subtypes of nccRCC
Collecting duct carcinoma remains a rare but aggressive variant of nccRCC. A commonly utilized treatment for treatment is platinum-based chemotherapy, such as gemcitabine plus cisplatin or carboplatin. There are a handful of case reports describing patients with collecting duct carcinoma who responded to either cabozantinib [55], sunitinib [56], or sorafenib [57], however there are no prospective studies supporting the use of these therapies outside of a clinical trial setting. Our results support first-line use of chemotherapy and confirm the limited efficacy of TKIs in patients with collecting duct carcinoma. There were no studies of renal medullary carcinoma or translocation RCC that met criteria for inclusion in this review.
In the future, additional prospective studies enrolling nccRCC patients are required to further elucidate optimal treatment strategies and sequencing. Given the small number of patients with this disease, collaborative multi-institutional efforts are needed to provide the statistical power necessary to perform subgroup analyses based on patient and tumor factors. In particular, this review highlights a number of differences in treatment response between nccRCC histologies. Additional investigation will be required to determine whether these apparent differences may be related to differing efficacy of the treatment, inherent differences in tumor behavior, or differences in other patient-level characteristics. As our understanding of the molecular and genetic basis of nccRCC continues to improve, more studies will be needed to develop consensus definitions of clinically relevant mutations and to assess the prognostic and predictive value of existing and novel biomarkers.
One strength of this study is our review of the data stratified by histologic subtype. As previously mentioned, there can be significant variability in response between tumor histology and description of these differences is important. This study also includes review of 4 new trials utilizing ICIs, which is an area of growing interest and potential promise. A limitation of our study is the inability to perform a meta-analysis. As a systematic review, we are limited to population level rather than patient level data, and the significant heterogeneity of this population precluded pooling of results. Additionally, the majority of the studies included were single-arm phase II trials and expanded access programs, which are a less rigorous source of evidence than RCTs. This review focused on the efficacy of different therapies for nccRCC and as such does not include data regarding toxicity or quality of life for patients undergoing these treatments. However, as has been previously reported [45], the toxicities experienced by nccRCC patients are typically not different from those experienced by ccRCC patients receiving the same medications and are generally well-recognized class effects of each therapy. Despite these limitations, this study provides a valuable synthesis of the existing literature and highlights the need for ongoing efforts in this disease.
CONCLUSIONS
This systematic review supports current consensus guidelines recommending sunitinib or enrollment in a clinical trial as first-line treatment options for nccRCC, but also suggests a more nuanced approach to management and new options for therapy such as immune checkpoint inhibition. All patients with locally advanced or metastatic nccRCC should have genetic and molecular sequencing to identify those that may benefit from targeted therapies.
FUNDING
This research was partially supported by a National Research Service Award/Post-Doctoral Traineeship from the Agency for Healthcare Research and Quality sponsored by The Cecil G. Sheps Center for Health Services Research, The University of North Carolina at Chapel Hill, Grant No. T32-HS000032.
AUTHOR CONTRIBUTIONS
All authors contributed to the study conception, performance and interpretation of data.
CONFLICT OF INTEREST
TLR receives research funding from Merck, GeneCentric Therapeutics, Bristol-Myers Squibb, and X4 Pharmaceuticals. CKO has no conflict of interest to report.
SUPPLEMENTARY MATERIAL
[1] One additional table and the completed PRISMA checklist are included within the supplementary material. Supplemental Table 1 describes the study characteristics and outcomes of all included trials in patients with any non-clear cell renal cell carcinoma histology.
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/KCA-190078.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
REFERENCES
[1] | National Cancer Institute. SEER Cancer Stat Facts: Kidney and Renal Pelvis Cancer. [Internet]. [cited 2019 Jul 8]. Available from: http://seer.cancer.gov/statfacts/html/kidrp.html |
[2] | Linehan WM , Vasselli J , Srinivasan R , Walther MM , Merino M , Choyke P , et al. Genetic basis of cancer of the kidney: Disease-specific approaches to therapy. In: Clinical Cancer Research. 2004. |
[3] | Inamura K . Renal cell tumors: Understanding their molecular pathological epidemiology and the 2016 WHO classification. Vol. 18, International Journal of Molecular Sciences. 2017. |
[4] | Kim SH , Yang HK , Moon KC , Lee ES . Localized non-conventional renal cell carcinoma: Prediction of clinical outcome according to histology. Int J Urol. (2014) ;21: (4):359–64. |
[5] | de Velasco G , McKay RR , Lin X , Moreira RB , Simantov R , Choueiri TK . Comprehensive Analysis of Survival Outcomes in Non–Clear Cell Renal Cell Carcinoma Patients Treated in Clinical Trials. Clin Genitourin Cancer. (2017) ;15: (6):652–660.e1. |
[6] | Siegel RL , Miller KD , Jemal A . Cancer statistics, 2016. - PubMed - NCBI. CA Cancer J Clin [Internet]. (2016) [cited 2019 Aug 25];66: (1):7–30. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26742998 |
[7] | Rini BI , Plimack ER , Stus V , Gafanov R , Hawkins R , Nosov D , et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med [Internet]. (2019) [cited 2019 Aug 7];380: (12):1116–27. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30779529 |
[8] | Motzer RJ . Nivolumab plus Ipilimumab Versus Sunitinib in Advanced Renal-cell Carcinoma. N Engl J Med. 2018; |
[9] | Motzer RJ , Penkov K , Haanen J , Rini B , Albiges L , Campbell MT , et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. (2019) ;380: (12):1103–15. |
[10] | Rini BI , Powles T , Atkins MB , Escudier B , McDermott DF , Suarez C , et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open-label, phase 3, randomised controlled trial. Lancet [Internet]. (2019) [cited 2019 Jul 15];393: (10189):2404–15. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31079938 |
[11] | Tannir NM , Jonasch E , Albiges L , Altinmakas E , Ng CS , Matin SF , et al. Everolimus Versus Sunitinib Prospective Evaluation in Metastatic Non-Clear Cell Renal Cell Carcinoma (ESPN): A Randomized Multicenter Phase 2 Trial. Eur Urol. (2016) ;69: (5):866–74. |
[12] | Armstrong AJ , Halabi S , Eisen T , Broderick S , Stadler WM , Jones RJ , et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): A multicentre, open-label, randomised phase 2 trial. Lancet Oncol [Internet]. (2016) Mar [cited 2019 Jul 18];17: (3):378–88. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26794930 |
[13] | Twardowski PW , Tangen CM , Wu X , Plets MR , Plimack ER , Agarwal N , et al. Parallel (Randomized) Phase II Evaluation ofTivantinib (ARQ197) and Tivantinib in Combination with Erlotinib in Papillary Renal Cell Carcinoma: SWOGS1107. Kidney Cancer. (2017) ;1: (2):123–32. |
[14] | Dutcher JP , De Souza P , McDermott D , Figlin RA , Berkenblit A , Thiele A , et al. Effect of temsirolimus versus interferon-α on outcome of patients with advanced renal cell carcinoma of different tumor histologies. Med Oncol. (2009) ;26: (2):202–9. |
[15] | Motzer RJ , Barrios CH , Kim TM , Falcon S , Cosgriff T , Harker WG , et al. Phase II randomized trial comparing sequential first-line everolimus and second-line sunitinib versus first-line sunitinib and second-line everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol. (2014) ;32: (25):2765–72. |
[16] | Liberati A , Altman DG , Tetzlaff J , Mulrow C , Gøtzsche PC , Ioannidis JPA , et al. Guidelines and Guidance The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. |
[17] | Higgins JPT , Altman DG , Gøtzsche PC , Jüni P , Moher D , Oxman AD , et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011; |
[18] | Gore ME , Szczylik C , Porta C , Bracarda S , Bjarnason GA , Oudard S , et al. Final results from the large sunitinib global expanded-access trial in metastatic renal cell carcinoma. Br J Cancer [Internet]. (2015) ;113: (1):12–9. Available from: www.bjcancer.com |
[19] | Lee JL , Ahn JH , Lim HY , Park SH , Lee SH , Kim TM , et al. Multicenter phase II study of sunitinib in patients with non-clear cell renal cell carcinoma. Ann Oncol. (2012) ;23: (8):2108–14. |
[20] | Molina AM , Feldman DR , Ginsberg MS , Kroog G , Tickoo SK , Jia X , et al. Phase II trial of sunitinib in patients with metastatic non-clear cell renal cell carcinoma. Invest New Drugs. (2012) ;30: (1):335–40. |
[21] | Tannir NM , Plimack E , Ng C , Tamboli P , Bekele NB , Xiao L , et al. A phase 2 trial of sunitinib in patients with advanced non-clear cell renal cell carcinoma. Eur Urol. (2012) ;62: (6):1013–9. |
[22] | Khaled H , Azim HA , Barsoum E , Chahine G , Shamseddine A , Metal GA , et al. A multicenter, phase II study of the RAF-kinase inhibitor sorafenib in patients with advanced renal cell carcinoma. Mol Clin Oncol. (2015) ;3: (5):1099–102. |
[23] | Procopio G , Verzoni E , Gevorgyan A , Mancin M , Pusceddu S , Catena L , et al. Safety and activity of sorafenib in different histotypes of advanced renal cell carcinoma. Oncology. (2008) ;73: (3-4):204–9. |
[24] | Stadler WM , Figlin RA , McDermott DF , Dutcher JP , Knox JJ , Miller WH , et al. Safety and efficacy results of the advanced renal cell carcinoma sorafenib expanded access program in North America. Cancer. (2010) ;116: (5):1272–80. |
[25] | Park I , Lee SH , Lee JL . A Multicenter Phase II Trial of Axitinib in Patients With Recurrent or Metastatic Non–clear-cell Renal Cell Carcinoma Who Had Failed Prior Treatment With Temsirolimus. Clin Genitourin Cancer. (2018) ;16: (5):e997–1002. |
[26] | Jung KS , Lee SJ , Park SH , Lee JL , Lee SH , Lim JY , et al. Pazopanib for the treatment of non-clear cell renal cell carcinoma: A single-arm, open-label, multicenter, phase II study. Cancer Res Treat. (2018) ;50: (2):488–94. |
[27] | Koh Y , Lim HY , Ahn JH , Lee JL , Rha SY , Kim YJ , et al. Phase II trial of everolimus for the treatment of nonclear-cell renal cell carcinoma. Ann Oncol. (2013) ;24: (4):1026–31. |
[28] | Voss MH , Molina AM , Chen YB , Woo KM , Chaim JL , Coskey DT , et al. Phase II trial and correlative genomic analysis of everolimus plus bevacizumab in advanced non-clear cell renal cell carcinoma. J Clin Oncol. (2016) ;34: (32):3846–53. |
[29] | Mahoney KM , Jacobus S , Bhatt RS , Song J , Carvo I , Cheng SC , et al. Phase 2 Study of Bevacizumab and Temsirolimus After VEGFR TKI in Metastatic Renal Cell Carcinoma. Clin Genitourin Cancer. (2016) ;14: (4):304–313.e6. |
[30] | Suárez C , Lee J-L , Ziobro M , Gafanov RA , Matveev VB , Donskov F , et al. First-line pembrolizumab (pembro) monotherapy for advanced non-clear cell renal cell carcinoma (nccRCC): Updated follow-up for KEYNOTE-427 cohort B. Ann Oncol [Internet]. 2019 Oct 1 [cited 2019 Nov 19];30(Supplement 5). Available from: https://academic.oup.com/annonc/article/doi/10.1093/annonc/mdz249.044/5576679 |
[31] | Vogelzang NJ , McFarlane JJ , Kochenderfer MD , Molina AM , Arrowsmith E , Bauer TM , et al. Efficacy and safety of nivolumab in patients with non-clear cell renal cell car- cinoma (RCC): Results from the phase IIIb/IV CheckMate 374 study. J Clin Oncol. (2019) ;37: (7_suppl):562. |
[32] | De Giorgi U , Cartenì G , Giannarelli D , Basso U , Galli L , Cortesi E , et al. Safety and efficacy of nivolumab for metastatic renal cell carcinoma: Real-world results from an expanded access programme. BJU Int. (2019) ;123: (1):98–105. |
[33] | McKay RR , McGregor BA , Gray K , Steinharter JA , Walsh MK , Braun DA , et al. Results of a phase II study of atezolizumab and bevacizumab in non-clear cell renal cell carcinoma (nccRCC) and clear cell renal cell carcinoma with sarcomatoid differentiation (sccRCC). J Clin Oncol. (2019) ;37: (7_suppl):548. |
[34] | Bylow KA , Atkins MB , Posadas EM , Stadler WM , McDermott DF . Phase II trial of carboplatin and paclitaxel in papillary renal cell carcinoma. Clin Genitourin Cancer. (2009) ;7: (1):39–42. |
[35] | Tsimafeyeu I , Demidov L , Kharkevich G , Petenko N , Galchenko V , Sinelnikov I , et al. Phase II, multicenter, uncontrolled trial of single-agent capecitabine in patients with non-clear cell metastatic renal cell carcinoma. Am J Clin Oncol Cancer Clin Trials. (2012) ;35: (3):251–4. |
[36] | Linehan WM , Spellman PT , Ricketts CJ , Creighton CJ , Fei SS , Davis C , et al. Comprehensive Molecular Characterization of Papillary Renal-Cell Carcinoma. N Engl J Med. (2016) ;374: (2):135–45. |
[37] | Choueiri TK , Plimack E , Arkenau HT , Jonasch E , Heng DYC , Powles T , et al. Biomarker-based phase II trial of savolitinib in patients with advanced papillary renal cell cancer. In: Journal of Clinical Oncology. (2017) . pp. 2993–3001. |
[38] | Choueiri TK , Vaishampayan U , Rosenberg JE , Logan TF , Harzstark AL , Bukowski RM , et al. Phase II and biomarker study of the dual MET/VEGFR2 inhibitor foretinib in patients with papillary renal cell carcinoma. J Clin Oncol [Internet]. (2013) ;31: (2):181–6. Available from: www.jco.orgonwww.jco.org. |
[39] | Schöffski P , Wozniak A , Escudier B , Rutkowski P , Anthoney A , Bauer S , et al. Crizotinib achieves long-lasting disease control in advanced papillary renal-cell carcinoma type 1 patients with MET mutations or amplification. EORTC 90101 CREATE trial. Eur J Cancer [Internet]. (2017) [cited 2019 Jul 24];87: :147–63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29149761 |
[40] | Powles T , Larkin JMG , Patel P , Pérez-Valderrama B , Rodriguez-Vida A , Glen H , et al. A phase II study investigating the safety and efficacy of savolitinib and durvalumab in metastatic papillary renal cancer (CALYPSO). J Clin Oncol. (2019) ;37: (7_suppl):545. |
[41] | Escudier B , Molinie V , Bracarda S , Maroto P , Szczylik C , Nathan P , et al. Open-label phase 2 trial of first-line everolimus monotherapy in patients with papillary metastatic renal cell carcinoma: RAPTOR final analysis. Eur J Cancer. (2016) ;69: :226–35. |
[42] | Ravaud A , Oudard S , De Fromont M , Chevreau C , Gravis G , Zanetta S , et al. First-line treatment with sunitinib for type 1 and type 2 locally advanced or metastatic papillary renal cell carcinoma: A phase II study (SUPAP) by the French Genitourinary Group (GETUG). Ann Oncol. (2015) ;26: (6):1123–8. |
[43] | Oudard S , Banu E , Vieillefond A , Fournier L , Priou F , Medioni J , et al. Prospective Multicenter Phase II Study of Gemcitabine Plus Platinum Salt for Metastatic Collecting Duct Carcinoma: Results of a GETUG (Groupe d’Etudes des Tumeurs Uro-Génitales) Study. J Urol. (2007) ;177: (5):1698–702. |
[44] | Sheng X , Cao D , Yuan J , Zhou F , Wei Q , Xie X , et al. Sorafenib in combination with gemcitabine plus cisplatin chemotherapy in metastatic renal collecting duct carcinoma: A prospective, multicentre, single-arm, phase 2 study. Eur J Cancer. (2018) ;100: :1–7. |
[45] | Fernández-Pello S , Hofmann F , Tahbaz R , Marconi L , Lam TB , Albiges L , et al. A Systematic Review and Meta-analysis Comparing the Effectiveness and Adverse Effects of Different Systemic Treatments for Non-clear Cell Renal Cell Carcinoma [Internet]. Vol. 71, European Urology. (2017) [cited 2019 Aug 13]. pp. 426–36. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27939075 |
[46] | Vera-Badillo FE , Templeton AJ , Duran I , Ocana A , De Gouveia P , Aneja P , et al. Systemic therapy for non-clear cell renal cell carcinomas: A systematic review and meta-analysis. European Urology. 2015. |
[47] | National Comprehensive Cancer Network. Kidney Cancer (Version 2.2020) [Internet]. 2019 [cited 2019 Aug 7]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf |
[48] | D’Avella C , Abbosh P , Pal SK , Geynisman DM . Mutations in renal cell carcinoma. Urologic Oncology: Seminars and Original Investigations. 2018; |
[49] | Choueiri TK , Hessel C , Halabi S , Sanford B , Michaelson MD , Hahn O , et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression-free survival by independent review and overall survival update. Eur J Cancer. (2018) ;94: :115–25. |
[50] | Choueiri TK , Escudier B , Powles T , Tannir NM , Mainwaring PN , Rini BI , et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): Final results from a randomised, open-label, phase 3 trial. Lancet Oncol. (2016) ;17: (7):917–27. |
[51] | Martínez Chanzá N , Xie W , Asim Bilen M , Dzimitrowicz H , Burkart J , Geynisman DM , et al. Cabozantinib in advanced non-clear-cell renal cell carcinoma: A multicentre, retrospective, cohort study. Lancet Oncol [Internet]. (2019) ;20: (4):581–90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30827746 |
[52] | Prisciandaro M , Ratta R , Massari F , Fornarini G , Caponnetto S , Iacovelli R , et al. Safety and efficacy of cabozantinib for metastatic nonclear renal cell carcinoma: Real-world data from an Italian managed access program. Am J Clin Oncol Cancer Clin Trials. (2019) ;42: (1):42–5. |
[53] | Srinivasan R , Su D , Stamatakis L , Siddiqui MM , Singer E , Shuch B , et al. Mechanism based targeted therapy for hereditary leiomyomatosis and renal cell cancer (HLRCC) and sporadic papillary renal cell carcinoma: Interim results from a phase 2 study of bevacizumab and erlotinib. Eur J Cancer. (2014) ;50: :8. |
[54] | Volpe A , Novara G , Antonelli A , Bertini R , Billia M , Carmignani G , et al. Chromophobe renal cell carcinoma (RCC): Oncological outcomes and prognostic factors in a large multicentre series. BJU Int [Internet]. (2012) Jul [cited 2019 Aug 27];110: (1):76–83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22044519 |
[55] | Mennitto A , Verzoni E , Peverelli G , Alessi A , Procopio G . Management of Metastatic Collecting Duct Carcinoma: An Encouraging Result in a Patient Treated With Cabozantinib. Clin Genitourin Cancer. (2018) ;16: (3):e521–3. |
[56] | Miyake H , Haraguchi T , Takenaka A , Fujisawa M . Metastatic collecting duct carcinoma of the kidney responded to sunitinib. Int J Clin Oncol. (2011) ;16: (2):153–5. |
[57] | Ansari J , Fatima A , Chaudhri S , Bhatt RI , Wallace M , James ND . Sorafenib Induces Therapeutic Response in a Patient with Metastatic Collecting Duct Carcinoma of Kidney. Oncol Res Treat [Internet]. (2009) [cited 2019 Nov 20];32: (1-2):44–6. Available from: https://www.karger.com/Article/FullText/183736 |




