First-Line Immune Checkpoint Inhibitor-Based Therapy for Metastatic Renal Cell Carcinoma: A Systematic Review
Abstract
Background:
Immune checkpoint inhibitors (CPIs) have come to the forefront as a major component of the management of metastatic renal cell carcinoma ( mRCC). Over a short period of time, several studies have shown benefit in using these agents in the first-line setting.
Objective:
In this systematic review, the available evidence regarding the use of CPI-based regimens in previously untreated mRCC was reviewed.
Methods:
A systematic search for phase II and III studies was conducted of the PubMed and Embase databases as per the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement. The search retrieved abstracts to February 1, 2020. Data was compiled and summarized in narrative and tabular formats.
Results:
Fifty-five abstracts from 11 clinical trials were included, including four phase III clinical trials and seven phase II trials. The most recent phase III data demonstrates overall survival (OS) benefit for ipilimumab plus nivolumab (for intermediate and poor risk patients) and pembrolizumab plus axitinib combination regimens over sunitinib. Two other regimens (avelumab plus axitinib and atezolizumab plus bevacizumab) have shown benefits in progression free survival, but not in OS to date. Toxicity data shows varying patterns of adverse events between the four treatments. Phase II data indicate CPI has activity as a single agent, and in patients with non-clear cell subtypes of RCC.
Conclusions:
CPI-based regimens improve outcomes in virtually all subgroups of mRCC patients when used as front-line therapy. This is certain to change the landscape of mRCC treatment.
INTRODUCTION
The management of advanced or metastatic renal cell carcinoma ( mRCC) has greatly relied on therapies that target its susceptibility to angiogenic and immune mediated pathways. More recently, the development of immune checkpoint inhibitors (CPIs) has changed the management of patients with mRCC, with multiple practice-changing trials demonstrating an improvement in several clinical outcomes with CPI-based therapy. The CPIs currently in clinical use target either the programmed cell death-1 (PD-1)/programmed death-ligand 1 (PD-L1) pathway, or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4).
With such promising results, the use of CPIs in the first-line setting is of great interest. The standard of care is rapidly shifting towards upfront use of CPI-based regimens, as evidenced by recently updated clinical practice guidelines [1, 2]. However, as the number of options increases, questions regarding how to select a first-line regimen, logistics, and the most appropriate means of monitoring for and managing toxicities remain. Understanding the currently available data, along with its limitations, is crucial for clinicians to implement these therapies into their practice.
We performed a systematic review of the available evidence from phase II and III clinical trials regarding the use of CPI therapies in previously untreated mRCC patients. Data regarding efficacy outcomes, toxicities, and quality of life were considered. Results are presented in narrative and tabular form. In addition to providing a summary of the data, we will discuss some of the pertinent issues relevant to delivering these therapies in the real-world setting.
METHODS
Search strategy
This systematic review was performed according to the PRISMA standards. A systematic search using pre-defined search terms was performed of the PubMed and Embase databases on August 25, 2019. The search was updated on February 1, 2020 using the same search terms to retrieve additional abstracts. The search terms are outlined in the Supplement. In addition, information regarding ongoing phase III studies was retrieved from ClinicalTrials.gov, also using specified search terms (see Supplementary Material). A hand search was also performed of abstracts from recent oncology meetings as well as citations from retrieved full text articles.
Study criteria
The intended study population was adult patients (≥ age 18) with a diagnosis of metastatic or advanced RCC who had undergone no prior systemic therapy for mRCC (prior adjuvant therapy was permitted). Both clear cell (ccRCC) and non-clear cell (nccRCC) subtypes were permitted. Studies were limited to phase II or III clinical trials (combined phase I/II studies were allowed if outcomes of the phase II study population of treatment-naive mRCC patients were reported separately), with at least one arm of the trial including a CPI. Single-arm studies were permitted. Abstracts from meetings without associated peer-reviewed publications were included. Retrospective studies, review articles, case reports and non-English texts were excluded.
Outcome measures
Outcome measures of interest included overall survival (OS), progression free survival (PFS), overall response rate (ORR), complete response (CR) rate, toxicities, patient-reported outcomes (PROs), and health-related quality of life (HRQoL). Efficacy data were gathered based on the patient population the primary outcome was tested in, or in the overall population if the outcome of interest was not a primary endpoint. Overall survival and PFS were reported as hazard ratios (HR) where applicable. ORR and CR rate was reported as percentages, with 95% confidence intervals (CI), if reported. Risk of bias was assessed using the Cochrane handbook for systematic reviews of interventions for phase III trials only [3].
Data collection
Retrieved abstracts were initially screened based on title and abstract content. Eligible articles were then reviewed in detail to insure eligibility. In instances where multiple abstracts presented on the same outcome measures for the same clinical trial, the most recent abstract was considered, with preference to peer-reviewed publications. The following details were extracted for each study: design, patient population, number of patients, treatment arms, outcomes, rate of grade 3 or greater toxicity, rate of treatment discontinuation due to toxicity, treatment related deaths, and PRO data.
Data synthesis
Due to heterogeneity of trials (patient population, primary outcomes, treatment strategies), outcome measures were not combined. Collected data was collated and summarized.
RESULTS
Search results are summarized in Fig. 1. Database search yielded 3478 citations, with an additional 9 abstracts added after hand search. After elimination of duplicates, 3138 abstracts were screened. Of these, 79 were subjected to full-text review. Fifty-five abstracts from eleven clinical trials were selected for this review. This included four phase III trials, one randomized phase II trial, and six single-arm phase II trials (see Table 1). The efficacy outcomes of the four phase III trials are summarized in Table 2, while toxicity data are summarized in Table 3. The assessment of risk of bias for the phase III trials is summarized in Table 4.
Fig. 1
Flow diagram of abstract selection and data acquisition.
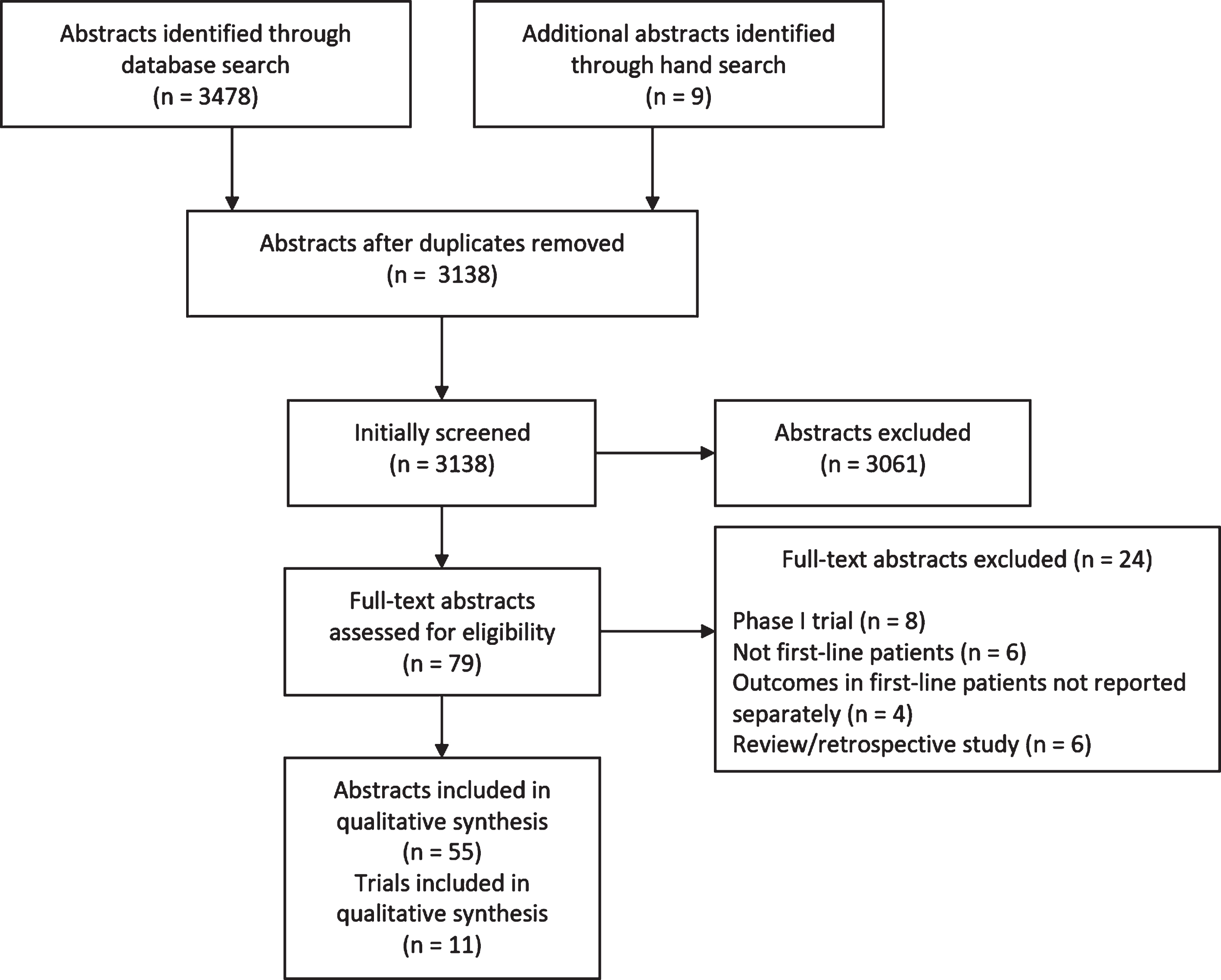
Table 1
Characteristics and design of retrieved clinical trials
| Clinical Trial | Design | Patient population enrolled | N | Treatment arms | Primary outcome(s) |
| Phase III Trials | |||||
| NCT02231749 (CheckMate 214) | Open-label, randomized phase III | Previously untreated mccRCC | 1096 | 1. Ipilimumab plus nivolumab2. Sunitinib | OS, PFS, ORR (IMDC int/poor risk only) |
| NCT02420821 (IMmotion151) | Open-label, randomized phase III | Previously untreated mccRCC | 915 | 1. Atezolizumab plus bevacizumab2. Sunitinib | PFS in PD-L1 + b; OS in ITT |
| NCT02684006 (JAVELIN Renal 101) | Open-label, randomized phase III | Previously untreated mccRCC | 886 | 1. Avelumab plus axitinib2. Sunitinib | OS and PFS in PD-L1 + a |
| NCT02853331 (KEYNOTE-426) | Open-label,randomized phase III | Previously untreated mccRCC | 861 | 1. Pembrolizumab plus axitinib2. Sunitinib | OS and PFS |
| Phase II Trials | |||||
| NCT01984242(IMmotion150) | Open-label, randomized phase II | Previously untreated mccRCC | 305 | 1. Atezolizumab2. Atezolizumab plus bevacizumab3. Sunitinib | PFS in PD-L1 + b and ITT |
| NCT02348008 (BTCRC-GU14-003) | Open-label,single-armphase Ib/II | Previously untreated mccRCC (in phase II component) | 48 | Pembrolizumab plus bevacizumab | ORR |
| NCT02446860 (ADAPTeR) | Open-label,single-armphase II | Previously untreated mccRCC planned for CN | 15 | Nivolumab (before and after CN) | Safety |
| NCT027224878 | Open-label,single-armphase II | Previously treatedand untreated mnccRCCand/or ≥20% sarcomatoiddifferentiation | 39 (in previously untreated cohort) | Atezolizumab plus bevacizumab | ORR |
| NCT02819596 (CALYPSO) | Open-label,single-armphase I/II | Previously treatedand untreated papillary mRCC | 28 (in previously untreated cohort) | Pembrolizumab plus savolitinib | ORR |
| NCT02853344 (KEYNOTE-427) | Open-label,single-arm,dual cohortphase II | Previously untreated mccRCC (Cohort A) or mnccRCC (Cohort B) | Cohort A: 107 Cohort B: 165 | Pembrolizumab | ORR |
| NCT03136627 (TiNivo) | Open-label,single-armphase Ib/II | Previously treatedand untreated mccRCC | 12 (in previously untreated cohort) | Tivozanib plus nivolumab | Safety |
a≥1% PD-L1 expression of tumor-infiltrating immune cells assessed using the Ventana SP263 assay. b≥1% PD-L1 expression of tumor-infiltrating immune cells assessed using the Ventana SP142 assay. N = number of subjects; OS = overall survival; PFS = progression free survival; ORR = overall response rate; IMDC = International Metastatic Renal Cell Carcinoma Database Consortium criteria; int = intermediate; PD-L1+ = programmed death-ligand 1 positive; ITT = intention-to-treat; mccRCC = clear cell metastatic renal cell carcinoma; CN = cytoreductive nephrectomy; mnccRCC = non-clear cell metastatic renal cell carcinoma; mRCC = metastatic renal cell carcinoma
Table 2
Summary of efficacy outcomes in phase III trials of immune checkpoint inhibitors in previously untreated metastatic renal cell carcinoma
| Trial [reference] | Treatment arm | Median follow up - mo | Median PFS (95% CI) - mo | PFS HR | ORR (95% CI) | CR rate | Median OS (95% CI) - mo | OS HR |
| CheckMate 214 [6] | Ipilimumab plus nivolumab | 32 | 8.2a (6.9–10.0) | 0.77a (p = 0.001) | 42% a (37–47) | 11% a | NEa (35.6-NE) | 0.66a (p < 0.0001) |
| Sunitinib | 8.3a (7.0–8.8) | 29% a (25–34) | 1% a | 26.6a (22.1–33.4) | ||||
| KEYNOTE-426 [13] | Pembrolizumab plus axitinib | 13 | 15.1 (12.6–17.7) | 0.69 (p < 0.001) | 59.3% (54.5–63.9) | 5.8% | NE | 0.53 (p < 0.0001) |
| Sunitinib | 11.1 (8.7–12.5) | 35.7% (31.1–40.4) | 1.9% | NE | ||||
| JAVELIN Renal 101 [10] | Avelumab plus axitinib | 12 | 13.8b (11.1-NE) | 0.61b (p < 0.001) | 55.2% b (49.0–61.2) | 4.4% b | NRb | 0.82b (p = 0.38) |
| Sunitinib | 7.2b (5.7–9.7) | 25.5% b (20.6–30.9) | 2.1% b | NRb | ||||
| IMmotion151 [9] | Atezolizumab plus bevacizumab | 15 | 11.2b (8.9–15.0) | 0.74b (p = 0.022) | 37% (32–41) | 5% | 33.6 (29.0-NE) | 0.93 (p = 0.475) |
| Sunitinib | 7.7b (6.8–9.7) | 33% (29–38) | 2% | 34.9 (28.8-NE) |
aIn IMDC intermediate/poor risk patient population only. bIn PD-L1 + patient population only. PFS = progression free survival; CI = confidence interval; mo = months; HR = hazard ratio; ORR = overall response rate; CR = complete response; OS = overall survival; min = minimum; NE = not estimable; NR = not reported.
Table 3
Summary of adverse events in phase III trials of immune checkpoint inhibitors in previously untreated metastatic renal cell carcinoma
| Trial | Treatment | Treatment related AE ≥grade 3 | Any treatment stopped for AE | All treatment stopped for AE | High-dose glucocorticoid usea | Treatment related deaths |
| CheckMate 214 [4] | Ipilimumab plus nivolumab | 46% | 22% | 22% | 35% | 1.6% |
| IMmotion151 [9] | Atezolizumab plus bevacizumab | 40% | 7% | 5% | 9% | 1.1% |
| JAVELIN Renal 101 [10] | Avelumab plus axitinib | 71.2% | NR | 7.6% | 11% | 0.7% |
| KEYNOTE-426 [13] | Pembrolizumab plus axitinib | 62.9% | 30.5% | 10.7% | NR | 0.9% |
| (Range from above trials) | Sunitinib | 54–71.5% | NA | 8–13.9% | NA | 0.2–1.6% |
aHigh-dose glucocorticoid use indicates treatment with≥40 mg of prednisone a day, or equivalent. AE = adverse effect; NR = not reported; NA = not applicable.
Table 4
Assessment of risk of bias in reviewed phase III clinical trials
| Study | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) |
| CheckMate 214 | Low | Low | Unclear | Unclear | Low | Low |
| IMmotion151 | Low | Low | High | High | Low | Low |
| (for PFS endpoint) | (for PFS endpoint) | |||||
| JAVELIN Renal 101 | Low | Low | Low | Low | Low | Low |
| KEYNOTE-426 | Low | Low | Low | Low | Low | Low |
PFS = progression free survival.
CPI plus CPI combination therapies in metastatic clear cell RCC
The landmark CheckMate 214 phase III trial is the only included study investigating treatment with combinations of CPIs [4]. In this large, open-label randomized trial, 1096 treatment-naïve patients were assigned to standard therapy with sunitinib or to four cycles of combination ipilimumab plus nivolumab, followed by nivolumab monotherapy. While the trial included patients of any risk group, the coprimary endpoints of OS, PFS, and ORR were assessed only in the patients with intermediate or poor risk disease as per the International Metastatic Kidney Cancer Database Consortium (IMDC) criteria. The same endpoints were assessed in the intention-to-treat (ITT) population as secondary endpoints, as well as in the limited number of favorable risk patients as an exploratory analysis.
At a median follow up of 25 months, ipilimumab plus nivolumab showed a significant improvement in OS over sunitinib in the IMDC intermediate/poor risk subgroup, with a HR of 0.63 (p < 0.001). Median OS was not reached in the combination CPI arm and 26.0 months in the sunitinib arm. The ORR also favored the combination group over sunitinib monotherapy (42% versus 27%). The PFS coprimary endpoint did not meet statistical significance. These results were updated with a minimum of 30 months of follow up, confirming the OS and ORR benefit in the intermediate and poor risk patients [5], and are summarized in Table 2.
Of note, analysis of OS also seemed to favor combination ipilimumab plus nivolumab over sunitinib in the ITT population (HR 0.68, p < 0.001), although ORR and PFS did not meet thresholds for significance. When the patients with favorable risk disease (comprising 23% of the total trial population) were analyzed, ipilimumab plus nivolumab appeared to be inferior to sunitinib in terms of OS (HR 1.45), ORR (29% for ipilimumab plus nivolumab versus 52% for sunitinib), and PFS (median PFS of 15.3 months compared to 25.1 months, favoring sunitinib). While the trend towards worse OS in favorable risk patients with ipilimumab and nivolumab persisted with longer follow up, there appeared to be less of a difference in the two arms based on the HR of 1.22 [5]. Longer follow up is necessary to determine if this difference will continue to converge over time, particularly given the long survival of favorable risk patients.
Despite the above, ipilimumab plus nivolumab induced a higher rate of complete responses compared to sunitinib, regardless of risk group. In the intermediate/poor risk patients, CR was attained by 11% in the combination group, versus 1% in the sunitinib group [6]. In the favorable risk group, 8% had a CR with CPI therapy compared to just 4% with sunitinib.
Grade 3 or 4 toxicities occurred in 46% of patients in the ipilimumab plus nivolumab arm, while 63% occurred in the sunitinib group. However, more patients discontinued CPI therapy due to toxicities than sunitinib (22% versus 12%), and 35% of patients in the experimental arm required high-dose glucocorticoids for toxicity management. Of note, in cases of significant immune related adverse events, the trial mandated complete discontinuation of both ipilimumab and nivolumab without subsequent administration of nivolumab maintenance therapy. More treatment-related deaths occurred in the combination CPI arm (8 patients total, compared to 4 for sunitinib). Patient-reported outcome data indicate that patients receiving ipilimumab plus nivolumab experienced better HRQoL and longer time to deterioration during the initial 145 weeks of treatment [7].
CPI plus anti-VEGF combination therapies in metastatic clear cell RCC
Both phase II and III trials investigating the benefit of combining CPIs with therapies targeting the VEGF pathway in treatment naïve mRCC patients were identified.
IMmotion150 was a randomized phase II clinical trial of 305 previously untreated patients with mRCC, involving three arms: monotherapy with atezolizumab, combination therapy with atezolizumab plus the anti-VEGF monoclonal antibody bevacizumab, and standard therapy with sunitinib [8]. The coprimary endpoints were PFS in the ITT and PD-L1+ (defined as≥1% expression on tumor-infiltrating immune cells) patient populations. The results demonstrated that PFS was comparable between the atezolizumab monotherapy arm and sunitinib arm for both the ITT population (HR 1.19; 95% CI 0.82–1.71) and the PD-L1+ subgroup (HR 1.03; 95% CI 0.63–1.67). For the atezolizumab plus bevacizumab arm, PFS appeared to be improved amongst the PD-L1+ patients compared to sunitinib (median PFS 14.7 months versus 7.8 months, HR 0.64; 95% CI 0.38–1.08). This benefit was attenuated in the ITT population (HR 1.00; 95% CI 0.69–1.45).
Based on these data, the IMmotion151 clinical trial was undertaken. This open-label phase III study enrolled 915 patients, who were randomized to first-line therapy with either atezolizumab plus bevacizumab or to sunitinib monotherapy [9]. Based on IMmotion150, the coprimary endpoints selected were PFS in the PD-L1+ subpopulation (representing 40% of the study population) and OS in the ITT population. After a minimum follow up of 12 months, the co-primary endpoint of PFS in the PD-L1+ group was met, with a median of 11.2 months for combination therapy versus 7.7 months for sunitinib (HR 0.74; 95% CI 0.57–0.96). However, the other co-primary endpoint for OS was not met in the ITT group at last analysis, with a HR of 0.93 (95% CI 0.76–1.14). There was no significant prolongation of OS in the PD-L1+ subgroup either (HR 0.84; 95% CI 0.62–1.15). The ORR for the atezolizumab plus bevacizumab arm was 37% in the ITT group and 43% for the PD-L1+ subgroup. This included a CR rate of 5% and 9%, respectively. In comparison, patients treated with sunitinib had an ORR of 33% (including 2% CR) in the ITT population, and 38% (with 4% CR) in the PD-L1+ subgroup. Safety data appeared to favor the combination regimen, with fewer grade 3 or 4 toxicities (40% versus 54%) and treatment discontinuation due to toxicity (5% versus 8%), although more treatment-related deaths were observed (5 total, compared to one in the sunitinib group). PROs were also in favor of combination therapy, indicating these patients had improved HRQoL compared to sunitinib [10].
Two phase III clinical trials have utilized combining CPIs with the oral VEGF-targeting TKI axitinib. In JAVELIN Renal 101, an open-label randomized trial, 886 patients received either the combination regimen of avelumab with axitinib or standard therapy with sunitinib [11]. Although enrollment was not restricted based on PD-L1 status, the primary endpoints of PFS and OS were only assessed in PD-L1+ patients (defined as≥1% expression on tumor-infiltrating immune cells). PD-L1 patients comprised 63.2% of the patient population. At the first interim analysis, the primary endpoint of PFS in PD-L1+ patients was positive, with a median PFS of 13.8 months in the avelumab plus axitinib group, compared to 7.2 months in the sunitinib group (HR 0.61; 95% CI 0.47–0.79). Avelumab plus axitinib was also found to prolonged PFS in the ITT population (HR 0.69; 95% CI 0.56–0.84). Statistical significance was not yet met for the OS endpoint in both the PD-L1+ subgroup (HR 0.82; 95% CI 0.53–1.28) and the ITT population (HR 0.78; 95% CI 0.55–1.08), with a relatively short median follow-up of 12 months. The ORR was 55.2% (with 4.4% CR) amongst PD-L1+ patients with combination therapy, compared to 25.5% (2.1% CR) for sunitinib. The ORR was similar in the overall population. Importantly, the PFS and ORR benefit from avelumab plus axitinib was observed in all subgroups, including IMDC risk groups and various PD-L1 expression levels [12]. Grade 3 or greater toxicity occurred in 71.2% of patients in the experimental arm, versus 71.5% in the sunitinib arm. Treatment was discontinued due to adverse events in 7.6% of patients receiving avelumab plus bevacizumab, compared to 13.4% of those in the sunitinib arm. Three treatment-related deaths occurred in the combination arm compared to one receiving sunitinib.
The second phase III trial involving axitinib was the KEYNOTE-426 trial of pembrolizumab plus axitinib [13]. This open-label, randomized trial enrolled 861 patients and assigned them either to treatment with the combination regimen or sunitinib monotherapy. The primary endpoints were OS and PFS in the overall population. At the first interim analysis, this study met both endpoints with a median follow-up of just over a year. The HR for OS was 0.53 (95% CI 0.38–0.74; p < 0.0001), with an estimated 12-month survival of 89.9% for the pembrolizumab plus axitinib arm versus 78.3% for sunitinib. Pembrolizumab plus axitinib also significantly prolonged PFS, with a median of 15.1 months (95% CI 12.6–17.7) versus 11.1 months (95% CI 8.7–12.5), and a HR of 0.69 (95% CI 0.57–0.84). The prolongation of survival and PFS was seen in all subgroups, irrespective of IMDC risk category and tumor PD-L1 expression. The ORR also significantly favored pembrolizumab plus axitinib over sunitinib (59.3% versus 35.7%; p < 0.001). In the combination arm, 5.8% attained a CR, compared to 1.9% with sunitinib. Adverse events of grade 3 or higher occurred in 75.8% of patients receiving the combination therapy, compared to 70.6% in the control arm. Discontinuation of both pembrolizumab and axitinib due to toxicity was observed in 10.7% (30.5% required discontinuation of either medication), compared to 13.9% of patients who needed to discontinue sunitinib. Four patients died from treatment-related adverse events in the combination arm, compared to 7 patients in the sunitinib arm.
A fifth clinical trial has also reported results of CPI plus VEGF targeted therapy in the first-line setting for mccRCC. The single-arm BTCRC-GU14-003 phase Ib/II clinical trial utilized the combination of pembrolizumab plus bevacizumab [14]. The phase II component of the trial included 48 treatment-naïve patients. The primary outcome of interest was ORR, which was reported to be 60.9% (95% CI 45.4–74.9), although no CRs were reported. The median PFS was 17.0 months (95% CI 11.3–24.8), while median OS had not been reached. While this regimen demonstrated activity in this trial, the small number of patients and lack of a control arm preclude use of pembrolizumab plus bevacizumab outside of a clinical trial.
Preliminary results are available from an additional single-arm phase Ib/II trial of 25 mRCC patients treated with the anti-VEGF TKI tivozanib combined with nivolumab [15]. For the 12 treatment-naïve patients in this trial, the median PFS was 18.9 m. For the entire cohort, including previously treated patients, 56% experienced a grade 3 or higher toxicity. Further data on outcomes for the previously untreated patient cohort were not yet reported.
CPI monotherapy in metastatic clear cell RCC
KEYNOTE-427 assessed first-line CPI monotherapy in the treatment of mccRCC [16]. This single-arm, phase II trial enrolled treatment-naïve patients to receive single-agent pembrolizumab in two cohorts –“cohort A” included only ccRCC patients (n = 110). The most recent report from this cohort described an ORR (the primary outcome) of 36.4%, with 2.7% CRs. Median PFS was 7.1 months, with median OS not yet reached. Another single-arm phase II trial of nivolumab monotherapy given pre- and post-cytoreductive nephrectomy in 15 mRCC patients, reported an ORR of 37% [17]. The aforementioned IMmotion150 phase II trial also included an arm of atezolizumab monotherapy; results are summarized above, but atezolizumab did not demonstrate an advantage over sunitinib. These data indicate CPI monotherapy has activity in the first-line setting, however comparison to combination therapy in a randomized trial is necessary.
CPI-based regimens in metastatic renal cell carcinoma with sarcomatoid differentiation
Several of the above-mentioned trials have reported subgroup analyses for patients with advanced RCC and sarcomatoid differentiation, a pathological feature associated with poor response to therapy and worse prognosis [18]. In CheckMate 214, 112 intermediate or poor risk patients with sarcomatoid RCC treated with ipilimumab plus nivolumab had a significant improvement in several efficacy outcomes compared to sunitinib, demonstrated in an exploratory subgroup analysis [19]. Overall survival was improved, with a median OS of 31.2 m versus 13.6 m (HR 0.55; 95% CI 0.33–0.90), as was PFS (median 8.4 m versus 4.9 m), ORR (56.7% compared to 19.2%), and CR rate (18.3% versus 0%). In a prespecified analysis of IMmotion151, among the 142 patients with any sarcomatoid component the combination of atezolizumab plus bevacizumab yielded improved OS (median not reached versus 15 months), PFS (median 8.3 m versus 5.3 m), ORR (49% versus 14%), and CR rate (10% versus 3%) compared to sunitinib [20]. The activity of CPI-axitinib combinations was demonstrated in exploratory subgroup analyses of KEYNOTE-426 and JAVELIN Renal 101. For the 105 patients with sarcomatoid differentiation, combination therapy with pembrolizumab and axitinib demonstrated benefit over sunitinib for OS (median not reached for either arm; HR 0.58, 95% CI 0.21–1.59), PFS (median not reached versus 8.4 m), ORR (58.8% versus 31.5%), and CR rate (11.8% versus 0%) [21]. For the combination of avelumab plus axitinib, PFS (median 7.0 m versus 4.0 m) and ORR (47% versus 21%) appeared to be improved compared to sunitinib in the 108 patients with sarcomatoid differentiation [22]. Even CPI monotherapy appears to be active; in KEYNOTE-427, patients with sarcomatoid differentiation treated with pembrolizumab had an ORR of 63.6% for ccRCC (11 patients), and 42.1% for nccRCC (38 patients) [16, 23]. Thus, CPI-based therapies appear to be of particular benefit over TKI monotherapy for this subgroup of historically difficult-to-treat patients.
CPI-based regimens in non-clear cell metastatic renal cell carcinoma
No phase III trials including patients with nccRCC have reported results to date. The single-arm phase II KEYNOTE-427 trial of pembrolizumab monotherapy, described above, included a cohort of patients with nccRCC (“cohort B”) [23]. Of the 165 enrolled patients, the majority (72%) were of the papillary subtype, with a further 13% chromophobe and 16% unclassified. Preliminary results with a median of 15 months of follow up reported an ORR of 26.1% (95% CI 19.5–33.5) including 6% CRs. The ORR by subtype was 28% for papillary, 9.5% for chromophobe, and 30.8% for unclassified. The ORR was more pronounced in those with tumors that had a PD-L1 combined positive score (CPS) of≥1% (35.3%) compared to a CPS < 1% (10.3%). A second single-arm phase I/II trial enrolled both treatment-naïve and previously treated patients with papillary mRCC to receive combination therapy with savolitinib (a MET-targeting TKI) and durvalumab [24]. In the 28 treatment-naïve patients, the ORR was 29% and median PFS was 12 months (95% CI 1.5-NR). Finally, a third single-arm phase II trial investigated the combination of atezolizumab and bevacizumab in both previously untreated and treated patients with variant histology RCC (nccRCC and/or the presence of ≥20% sarcomatoid component) [25]. Only the ORR (the primary outcome) was reported separately for the 39 treatment-naïve patients, at 31%. These trials lend support to the notion that CPIs, both as monotherapy and in combination with targeted therapy, have activity in nccRCC.
Future phase III trials with CPI
A search of registered clinical trials on ClinicalTrials.gov found eight phase III trials that are in progress. These studies are summarized in Table 5.
Table 5
Ongoing phase III clinical trials with immune checkpoint inhibitors in treatment-naïve metastatic renal cell carcinoma (trial enrollment status as of December 31, 2019)
| ClinicalTrials.gov identifier | Patient enrollment | Experimental arm(s) | Control arm | Active enrollment | Primary outcome(s) |
| NCT02811861 | 1069 | 1. Pembrolizumab plus lenvatinib | Sunitinib | No | PFS |
| 2. Everolimus plus lenvatinib | |||||
| NCT03141177 | 638 | 1. Nivolumab plus cabozantinib | Sunitinib | No | PFS |
| 2. Ipilimumab plus nivolumab plus cabozantinib | |||||
| NCT03260894 | 129 | Pembrolizumab plus epacadostat | Sunitinib or pazopanib | No | ORR |
| NCT03729245 | 600 (IMDC int/poor) | NKTR-214 plus nivolumab | Sunitinib or cabozantinib | Yes | ORR and OS |
| NCT03793166 | 1046 (IMDC int/poor) | Ipilimumab plus nivolumab followed by nivolumab plus cabozantinib | Ipilimumab plus nivolumab, followed by nivolumab monotherapy | Yes | OS |
| NCT03873402 | 418 (IMDC int/poor) | Nivolumab | Ipilimumab plus nivolumab, followed by nivolumab monotherapy | Yes | PFS and ORR |
| NCT03937219 | 676 (IMDC int/poor) | Ipilimumab plus nivolumab plus cabozantinib followed by nivolumab plus cabozantinib | Ipilimumab plus nivolumab, followed by nivolumab monotherapy | Yes | PFS |
| NCT03977571 | 400 (IMDC int/poor) | Ipilimumab plus nivolumab followed by cytoreductive nephrectomy, followed by nivolumab monotherapy | Ipilimumab plus nivolumab, followed by nivolumab monotherapy | Yes | OS |
PFS = progression free survival; ORR = overall response rate; IMDC = International Metastatic Renal Cell Carcinoma Database Consortium criteria; int = intermediate; OS = overall survival.
DISCUSSION
This systematic review of first-line immune CPI-based therapies yielded data from four phase III and seven phase II clinical trials. All demonstrate activity in mRCC of various CPI regimens, including CPI monotherapy, combined PD-1 and CTLA-4 blockade, and CPIs combined with targeted therapy.
The strengths of this analysis are incorporation of the most recent data, the inclusion of phase II studies, and of nccRCC trials. Although the majority of phase II trials in this review were not randomized, they illustrate the potential benefits of novel CPI treatment strategies (such as single-agent pembrolizumab) and in variant RCC subtypes. A weakness of this systematic review is that we did not combine efficacy measures across the phase III trials. Due to the differences in treatment, trial design (primary outcomes and patient populations), and immaturity of OS data in at least two of the phase III studies, it was not felt to yield meaningful conclusions. However, in sum these data not only support the concept that RCC is a disease susceptible to immune system modulation, but also that immunotherapy can alter the natural course of advanced disease.
Clinical trials represent valuable, scientifically rigorous tools to inform clinicians and patients regarding optimal treatment strategies. This is especially true of the management of treatment-naïve patients, where selecting the “best” upfront therapy is likely to be the most impactful on survival. Within a short timeframe, four large phase III trials have reported results as described above. Additionally, several ongoing phase III trials are studying different CPI combinations (Table 5). This markedly increases the number of potential options for first-line treatment. However, many unanswered questions remain, including which treatment option to utilize and, logistically, how one delivers CPI treatments to the entire mRCC population to maximize both efficacy and safety. In practice, implementing clinical trial treatments and obtaining similar results can be less than straightforward.
In terms of choosing a specific treatment in the first-line setting, none of the four regimens studied in the current phase III trials have been directly compared to one another, and to date no clinically relevant predictive biomarkers have been identified to aid in selection. Thus, treatment selection requires comparison across trials despite the potentially confounding differences in patient population, baseline characteristics, primary endpoints, and durations of follow up. Most notably, only two of the phase III trial combinations, ipilimumab plus nivolumab and pembrolizumab plus axitinib, show a significant OS advantage at this point, and therefore would seem to be the top two choices for intermediate and poor risk patients. Pembrolizumab plus axitinib is seemingly the preference for favorable risk patients if the OS endpoint is considered the most important factor. Conversely, atezolizumab plus bevacizumab would not be considered an optimal treatment option due to the lack of benefit seen in the OS endpoint.
Clinicians and patients will need to consider other factors when making treatment decisions given the lack of direct comparisons. Examples of such factors include complete response rates and toxicities. The increase in CRs seen in with CPI therapy in all these clinical trials raises the question of whether attaining a CR should be a goal of therapy, and thus an endpoint worth pursuing in future clinical trials. While a CR is not necessarily synonymous with long-term control, retrospective evidence from the targeted therapy era demonstrates long periods of disease control amongst patients who attained a CR with VEGF-targeted therapy [26]. There are indications that CRs with CPI therapies yield durable disease control; 30 month follow-up data from the CheckMate 214 trial showed that 88% of those in the ITT population attaining a CR with ipilimumab plus nivolumab had an ongoing CR [5]. The issue of relying on CR rates to select therapy is highlighted by the CheckMate 214 data in favorable risk patients. In this subgroup, the CR rate was higher with ipilimumab plus nivolumab than with sunitinib (8% versus 4%) despite the OS, PFS, and ORR endpoints favoring sunitinib [6]. In situations such as these, where there is a discordance between the OS benefit and the CR rate, it is not clear that prioritizing CR as a goal is a valid strategy unless it can be shown that a CR truly represents long-term disease control, or an OS benefit is demonstrated with prolonged follow-up. Longer follow-up of complete responders, and outcomes of patients with a CR on CPI plus anti-VEGF combinations, will be critical. Despite this, CR rates will remain an important consideration when selecting first-line therapies.
Toxicity profiles will also be an important consideration when selecting first-line therapy. When comparing the rates of adverse events in the four phase III clinical trials (Table 3), there is again differing patterns between the various CPI strategies. While CPI-axitinib combinations were associated with a higher rate of grade 3 or greater toxicity than ipilimumab plus nivolumab, the rates of treatment discontinuation due to AEs (22% for ipilimumab plus nivolumab versus 7.6–10.7% for CPI-axitinib combinations) and rate of treatment-related deaths (1.6% versus <1%, respectfully) were higher with ipilimumab plus nivolumab. Additionally, the rate of high-dose glucocorticoid use was dramatically higher in trial patients receiving ipilimumab plus nivolumab (35%), compared to CPI-anti-VEGF combinations (9 to 11%; see Table 3). It should be noted that patients who discontinued ipilimumab plus nivolumab for AEs had similar efficacy outcomes compared to those who did not, and 42% of these patients had not required second line therapy at 24 months [27]. Whether a similar effect is seen in patients discontinuing treatment with the other CPI regimens remains to be seen. While PROs have only been reported for CheckMate 214 and IMmotion151 to date, both favored the CPI regimens over sunitinib, a notable finding given the rates of steroid use and discontinuation from toxicity in CheckMate 214.
As ICI therapies move to the forefront of treatment of most RCC patients, specific subgroups of patients will greatly benefit from their uptake. The above data show that the subgroup of ccRCC patients with sarcomatoid differentiation have improvements in OS, PFS, ORR, and CR rates with CPI regimens compared to classical anti-VEGF TKI therapy. Further, several phase II trials demonstrate activity of CPI regimens in non-clear cell RCC subtypes. This represents a group of RCC patients who have been shown to have only modest benefit from TKI monotherapy. Although these are subgroup analyses or relatively small, non-randomized trials, patients with nccRCC or sarcomatoid differentiation are underrepresented in phase III RCC trials in general and have few established systemic treatment options. In this context, these data support the use of CPI regimens in this group of patients in high need of effective treatment options, and these regimens should be preferentially selected for such patients.
Translating these phase III results to daily practice leads to unavoidable considerations regarding the costs of delivering these treatments. These costs extend to include financial expenditures and resources such as personnel and time. The CPI regimens described above are notable for their direct and indirect financial cost, and for many patients and health care systems access will be an issue. This will be a larger barrier in most developing countries. In addition, beyond monetary costs several other factors add to the use of health care resources. The unpredictable and insidious nature of immune-related adverse events from CPIs means patients (and their caregivers) must be well-informed, reliable to report symptoms, and well-supported. This requires additional time spent by patients’ oncologists, nurses, and pharmacists in education, advocacy, and ensuring safety. Further, as immune-related AEs can arise suddenly and require prompt management to avoid organ- or life-threatening complications, the patient’s oncology clinic must have the resources to accommodate unexpected visits, frequent reassessments, and potential inpatient management. This will require an expansion in both human resources and in the available physical space for outpatient and inpatient care. Outside of the oncology clinic, peripheral centers, community emergency departments, and primary care providers must also be educated and prepared to recognize and potentially manage immune-related AEs, particularly in more remote practices. While such an increase in resource utilization may be feasible in certain practice environments (especially urban, well-funded, centrally located ones), many practices worldwide may not have the flexibility to expand their limited resources. However, not doing so puts patients receiving CPI therapies at risk of developing serious morbidity as a result of toxicities.
Future trials, including some currently in progress (Table 5), may help determine if newer combinations or novel strategies improve on the efficacy, safety, and appropriate delivery of the current regimens. This includes trials randomizing patients to CPI-TKI combinations (such as NCT03937219) or CPI monotherapy (such as NCT03873402) using ipilimumab plus nivolumab as the comparator arm as opposed to TKI monotherapy, as has been used to date. This may help shed light on the important question of which patients could safely utilize CPI monotherapy or benefit from the addition of TKI therapy to CPIs.
The data from the reviewed trials indicate very promising activity for CPI-based therapies, superiority over standard anti-VEGF therapy in several patient populations, and better outcomes for many mRCC patients. We expect that CPIs will form the foundation of mRCC treatment in the first-line management of the vast majority of patients moving forward. Clinicians, patients, and health care systems must become resourceful and innovative to prepare for the ripple effects of the widespread incorporation of CPIs and the complexities of their administration.
FUNDING
The authors report no funding.
CONFLICT OF INTEREST
MT has no conflicts to declare. LW has served as an advisory board member (with no personal financial compensation) for Astellas, Merck, Ipsen, BMS and Pfizer; and has participated in clinical trials supported by Aragon, AstraZeneca, BMS, Exelixis, Merck, Pfizer, and Roche with financial support going to her institution.
SUPPLEMENTARY MATERIAL
[1] The Supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/KCA-190076.
ACKNOWLEDGMENTS
The authors have no acknowledgments.
REFERENCES
[1] | Albiges L , Powles T , Staehler M , Bensalah K , Giles RH , Hora M , et al. Updated European Association of Urology Guidelines on Renal Cell Carcinoma: Immune Checkpoint Inhibition Is the New Backbone in First-line Treatment of Metastatic Clear-cell Renal Cell Carcinoma. Eur Urol. (2019) ;76: :151–6. |
[2] | National Comprehensive Cancer Network [Internet]. Plymouth Meeting, PA: the Network; c2019. Kidney Cancer (Version 2.2020). [Updated 2019 August 5, cited 2019 August 27]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/kidney_blocks.pdf |
[3] | Higgins J , Green S . Cochrane Handbook for Systematic Reviews of Interventions [Internet]. Version 5.1.0. London:The Cochrane Collaboration;2011 [cited 2019 August 30]. Available at www.handbook.cochrane.org. |
[4] | Motzer RJ , Tannir NM , McDermott DF , Arén Frontera O , Melichar B , Choueiri TK , et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. (2018) ;378: :1277–90. |
[5] | Motzer RJ , Rini BI , McDermott DF , Arén Frontera O , Hammers HJ , Carducci MA , et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. (2019) ;20: :1370–85. |
[6] | Tannir NM , Frontera OA , Hammers HJ , Carducci MA , McDermott DF , Salman P , et al. Thirty-month follow-up of the phase III CheckMate 214 trial of first-line nivolumab+ipilimumab (N+I) or sunitinib (S) in patients (pts) with advanced renal cell carcinoma (aRCC). J Clin Oncol. (2019) ;37: :547. |
[7] | Cella D , Escudier B , Ivanescu C , Mauer M , Lord-Bessen J , Gooden K . Quality of life in previously untreated patients with advanced renal cell carcinoma (aRCC) in CheckMate Updated results. Ann Oncol. (2019) ;30: :v383–4. |
[8] | McDermott DF , Huseni MA , Atkins MB , Motzer RJ , Rini BI , Escudier B , et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. (2018) ;24: :749–57. |
[9] | Rini BI , Powles T , Atkins MB , Escudier B , McDermott DF , Suarez C , et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. (2019) ;393: :2404–15. |
[10] | Escudier B , Motzer RJ , Rini BI , Powles T , McDermott DF , Suarez C , et al. Patient-reported outcomes (PROs) in IMmotion Atezolizumab (atezo)+bevacizumab (bev) vs sunitinib (sun) in treatment (tx) naive metastatic renal cell carcinoma ( mRCC). J Clin Oncol. (2018) ;36: :4511. |
[11] | Motzer RJ , Penkov K , Haanen J , Rini B , Albiges L , Campbell MT , et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. (2019) ;380: :1103–15. |
[12] | Choueiri TK , Motzer RJ , Campbell MT , Alekseev BY , Uemura M , Kollmannsberger CK , et al. Subgroup analysis from JAVELIN Renal Outcomes for avelumab plus axitinib (A+Ax) versus sunitinib (S) in advanced renal cell carcinoma (aRCC). J Clin Oncol. (2019) ;37: :544. |
[13] | Rini BI , Plimack ER , Stus V , Gafanov R , Hawkins R , Nosov D , et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. (2019) ;380: :1116–27. |
[14] | Dudek AZ , Liu LC , Alva AS , Stein M , Gupta S , Albany C , et al. Phase ib and phase II studies of pembrolizumab (P) with bevacizumab (B) for the treatment of metastatic renal cell carcinoma (RCC): BTCRC-GU14-003. J Clin Oncol. (2018) ;36: :4558. |
[15] | Barthelemy P , Escudier B , Negrier S , Ravaud A , Needle MN , Albiges L . TiNivo: Tivozanib combined with nivolumab results in prolonged progression free survival in patients with metastatic renal cell carcinoma ( mRCC): Final results. Ann Oncol. (2019) ;30: :v380–1. |
[16] | Larkin JMG , Tykodi SS , Donskov F , Lee J-L , Szczylik C , Malik J , et al. First-line pembrolizumab (pembro) monotherapy in advanced clear cell renal cell carcinoma (ccRCC): Updated follow-up for KEYNOTE-427 cohort A. Ann Oncol. (2019) ;30: :v381–2. |
[17] | Au L , Litchfield K , Rowan A , Horswell S , Byrne F , Nicol D , et al. ADAPTeR: A phase II study of anti-PD1 (nivolumab) therapy as pre- and post-operative therapy in metastatic renal cell carcinoma. Ann Oncol. (2019) ;30: :v359. |
[18] | El Mouallem N , Smith SC , Paul AK . Sarcomatoid renal cell carcinoma: Biology and treatment advances. Urol Oncol Semin Orig Investig. (2018) ;36: :265–71. |
[19] | McDermott DF , Choueiri TK , Motzer RJ , Aren OR , George S , Powles T , et al. CheckMate 214 post-hoc analyses of nivolumab plus ipilimumab or sunitinib in IMDC intermediate/poor-risk patients with previously untreated advanced renal cell carcinoma with sarcomatoid features. J Clin Oncol. (2019) ;37: :4513. |
[20] | Rini BI , Motzer RJ , Powles T , McDermott DF , Escudier B , Donskov F , et al. Atezolizumab (atezo)+bevacizumab (bev) versus sunitinib (sun) in pts with untreated metastatic renal cell carcinoma ( mRCC) and sarcomatoid (sarc) histology: IMmotion151 subgroup analysis. J Clin Oncol. (2019) ;37: :4512. |
[21] | Rini BI , Plimack ER , Stus V , Gafanov R , Hawkins R , Nosov D , et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for metastatic renal cell carcinoma ( mRCC): Outcomes in the combined IMDC intermediate/poor risk and sarcomatoid subgroups of the phase 3 KEYNOTE-426 study. J Clin Oncol. (2019) ;37: :4500. |
[22] | Choueiri TK , Larkin JMG , Pal SK , Motzer RJ , Venugopal B , Alekseev BY , et al. Efficacy and biomarker analysis of patients (pts) with advanced renal cell carcinoma (aRCC) with sarcomatoid histology (sRCC): Subgroup analysis from the phase III JAVELIN renal 101 trial of first-line avelumab plus axitinib (A+Ax) vs sunitinib (S). Ann Oncol. (2019) ;30: :v361. |
[23] | Suárez C , Lee J-L , Ziobro M , Gafanov RA , Matveev VB , Donskov F , et al. First-line pembrolizumab (pembro) monotherapy for advanced non-clear cell renal cell carcinoma (nccRCC): Updated follow-up for KEYNOTE-427 cohort B. Ann Oncol. (2019) ;30: :v381. |
[24] | Powles T , Larkin JMG , Patel P , Pérez-Valderrama B , Rodriguez-Vida A , Glen H , et al. A phase II study investigating the safety and efficacy of savolitinib and durvalumab in metastatic papillary renal cancer (CALYPSO). J Clin Oncol. (2019) ;37: :545. |
[25] | McGregor BA , McKay RR , Braun DA , Werner L , Gray K , Flaifel A , et al. Results of a Multicenter Phase II Study of Atezolizumab and Bevacizumab for Patients With Metastatic Renal Cell Carcinoma With Variant Histology and/or Sarcomatoid Features. J Clin Oncol. (2020) ;38: :63–70. |
[26] | Buchler T , Bortlicek Z , Poprach A , Pavlik T , Veskrnova V , Honzirkova M , et al. Outcomes for Patients with Metastatic Renal Cell Carcinoma Achieving a Complete Response on Targeted Therapy: A Registry-based Analysis. Eur Urol. (2016) ;70: :469–75. |
[27] | Tannir NM , Motzer RJ , Plimack ER , McDermott DF , Barthelemy P , Porta C , et al. Outcomes in patients (pts) with advanced renal cell carcinoma (aRCC) who discontinued (DC) first-line nivolumab+ipilimumab (N+I) or sunitinib (S) due to treatment-related adverse events (TRAEs) in CheckMate 214. J Clin Oncol. (2019) ;37: :581. |




