Real-World Results from One Year of Therapy with Tivozanib
Abstract
We report our one-year experience on the use of Tivozanib in metastatic Renal Cell Carcinoma (RCC) in n=23 patients treated within a year after approval. Tumor response according to RECIST criteria was PR in 39.1%, SD in 52.2% and PD in 8.7% of the patients. Median progression free survival (PFS) was 14.9 months (95% CI 5.1-24.8).
Abstract
Introduction:
Tivozanib was approved in Europe in November 2017. We report on our initial experience of the use of Tivozanib in metastatic Renal Cell Carcinoma (RCC).
Material and Methods:
N = 23 patients undergoing Tivozanib therapy were included in this retrospective analysis from a prospective database at the Multidisciplinary Center on Renal Tumors, Department of Urology, University of Munich between Nov. 2017 and Oct. 2018.
Results:
Median age was 69.1 years (range 42.7–83.8) n = 8 patients were started on tivozanib in first line (34.8%) and n = 15 (75.2% in later line therapy (2nd– 6th line). Tumor response according to RECIST criteria was PR in 39.1%, SD in 52.2% and PD in 8.7% of the patients. Median progression free survival (PFS) was 14.9 months (95% CI 5.1–24.8). Median overall survival (OS) has not been reached. Although not statistically significant the difference in median PFS for first line patients was 30.3 months versus later line patients of 8.6 months (CI 5.1–12.2) (p = 0.291). The most commonly reported side-effects were diarrhea, hypertension, fatigue and hoarseness. 34.8% of the patients had grade 2 side effects and 21.7% had grade 3 adverse events, leading to treatment discontinuation in 3 patients (13%).
Conclusion:
Tivozanib is a highly active tyrosine kinase inhibitor even in later lines of therapy. The side effects are well tolerated, and no new safety signals were detected.
CLINICAL PRACTICE POINTS
What is already know about this subject?
Tivozanib is a multi-kinase inhibitor approved for first-line therapy in patients with advanced renal cell carcinoma (RCC) in Europe. The approval was based on findings from a phase-III trial showing a prolonged PFS of 11.9 months versus sorafenib (9.1 months). Tivozanib has also shown a superior side effect profile over sorafenib, rendering it one of the best tolerated oral anti-angiogenic drugs for the treatment of renal cell carcinoma.
What are the new findings?
We specifically can show that patients in later lines of treatment also benefit from tivozanib with low levels of adverse events. In this small mixed population of all-line therapies, we could see a progression free survival of 14.9 months, exceeding the pivotal data.
How might it impact clinical practice in the foreseeable future?
The field of kidney cancer is rapidly changing, as immune-modulating drugs such as, nivolumab, ipilimumab and pembrolizumab have replaced previous front-line treatments. As patients are living longer, the need for later line options is growing. Data on patient experiences in third- and fourth-line therapy are limited, however as patients progress through treatment lines, there is a natural shift toward tolerability rather than efficacy. Given Tivozanib’s impressive PFS in these patients as well as its superior tolerability profile, it’s an appealing option for heavily pre-treated patients and should also be considered in front-line patients where immune modulation is not an option.
INTRODUCTION
Renal cell carcinoma (RCC) is the seventh most common cancer in the European Union. Most recent estimates predict approximately 100,000 new cases of RCC each year and over 40,000 deaths from the disease [1]. In Europe, incidence of kidney cancer increased for nearly two decades but have since plateaued. At diagnosis, 30 percent of patients will present with metastatic disease, where survival rates remain dismally low [2]. Over the last two decades, treatment of metastatic renal cell carcinoma (mRCC) has improved significantly with the advent of targeted therapies inhibiting vascular endothelial growth factor receptors (VEGFR) and most recently the introduction of newer immunotherapy agents [3].
Tivozanib is a multi-kinase or tyrosine-kinase inhibitor (TKI) that inhibits selectively VEGFR 1,2 and 3 and was approved by the European Medicines Agency in 08/2017 for the first line treatment of adult patients with advanced renal cell carcinoma (RCC) and for adult patients who are VEGFR and mTOR pathway inhibitor-naïve following disease progression after one prior treatment with cytokine therapy for advanced RCC [4].
The approval was based on data from a global, open-label, randomized, multi-center Phase 3 trial (TIVO-1). The efficacy and tolerability of tivozanib was compared to sorafenib in 517 patients with advanced RCC. Patients treated with tivozanib experienced superior PFS (11.9 vs. 9.1 months in the overall population [HR, 0.797; 95% CI, 0.639 to 0.993; P = .042] and 12.7 vs. 9.1 months in treatment naïve patients [HR, 0.756; 95% CI, 0.580 to 0.985; P = 0.037]) versus sorafenib. There was also an improved side effect profile with tivozanib, with only 14% (versus 43% with sorafenib) requiring a dose reduction due to adverse events (AEs). In addition, fewer patients on tivozanib experienced clinically relevant side effects, such as diarrhea (23% of patients vs 33%) and hand-foot syndrome (14% of patients vs 54%) [5]. Real life evidence is rare as tivozanib is only available in Europe and reimbursement is mainly ensured in Germany.
We present our clinical experience one year after the drug was available in Europe.
MATERIAL & METHODS
This single center study analyzed patients with mRCC on systemic therapy with tivozanib between 11/2017 and 11/2018. Patients were treated with tivozanib until progression or intolerable toxicity. Patients were identified in a prospective institutional database after institutional review board approval. Adverse effects were prospectively graded according to the Common Terminology Criteria for Adverse Events CTCAE Ver. 5.0. Imaging of patients on systemic therapy was performed using computed tomography (CT) of chest, abdomen, and pelvis every 3 months, and brain CT once a year if no brain metastases were known. Response evaluation of systemic therapy was done according to the RECIST criteria [6] and categorized at each surveillance scan as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Patients were treated until PD or intolerable toxicity and were subsequently switched to another therapy. Time from initiation of tivozanib therapy until disease progression either on imaging or clinical deterioration was recorded as progression free survival. Overall survival was calculated from start of tivozanib therapy.
Calculations were performed using IBM SPSS Statistics, version 25 (IBM Corp., Armonk, NY, USA). Kaplan Meier analysis was used to estimate PFS and OS and log-rank calculations with a one-sided p-value.
RESULTS
We included 23 patients at a median age of 69.1 years (range 42.7–83.8) with 15 being male and male to female ratio of 0.65. N = 8 patients were started on tivozanib in first line (34.8%) and n = 15 (75.2%) in later line therapy (2nd– 6th line). 17.4% of the patients were classified as low risk according to the IMDC risk groups, 65.2% intermediate and 17.4% high risk [7]. Median follow-up was 16.9 months (range6.4–32.3) (see Table 1).
Table 1
Patient and outcome characteristics
| Male | n = 15 (65.2%) |
| Median age (years) | 69.9 (range 42.7–83.8) |
| IMDC risk group | |
| low | n = 5 (21.7%) |
| intermediate | n = 15 (65.2%) |
| high | n = 3 (13.0%) |
| ECOG Performance status | |
| 0 | n = 17 (73.9%) |
| 1 | n = 5 (21.7 %) |
| 2 | n = 1 (4.3%) |
| Median Duration of therapy (months) | 12.5 (range 2.3–30.6) |
| Treatment line | |
| 1st line | n = 7 (30.4%) |
| 2nd line | n = 4 (17.4 %) |
| Later line | n = 12 (52.2%) |
| Response | |
| Progression | n = 2 (8.7) |
| Partial response | n = 9 (39.1%) |
| Stable disease | n = 12 (52.2%) |
Tumor response according to RECIST criteria was PR in 39.1% (n = 9), SD in 52.2% (n = 12) and PD in 8.7% (n = 2) of the patients.
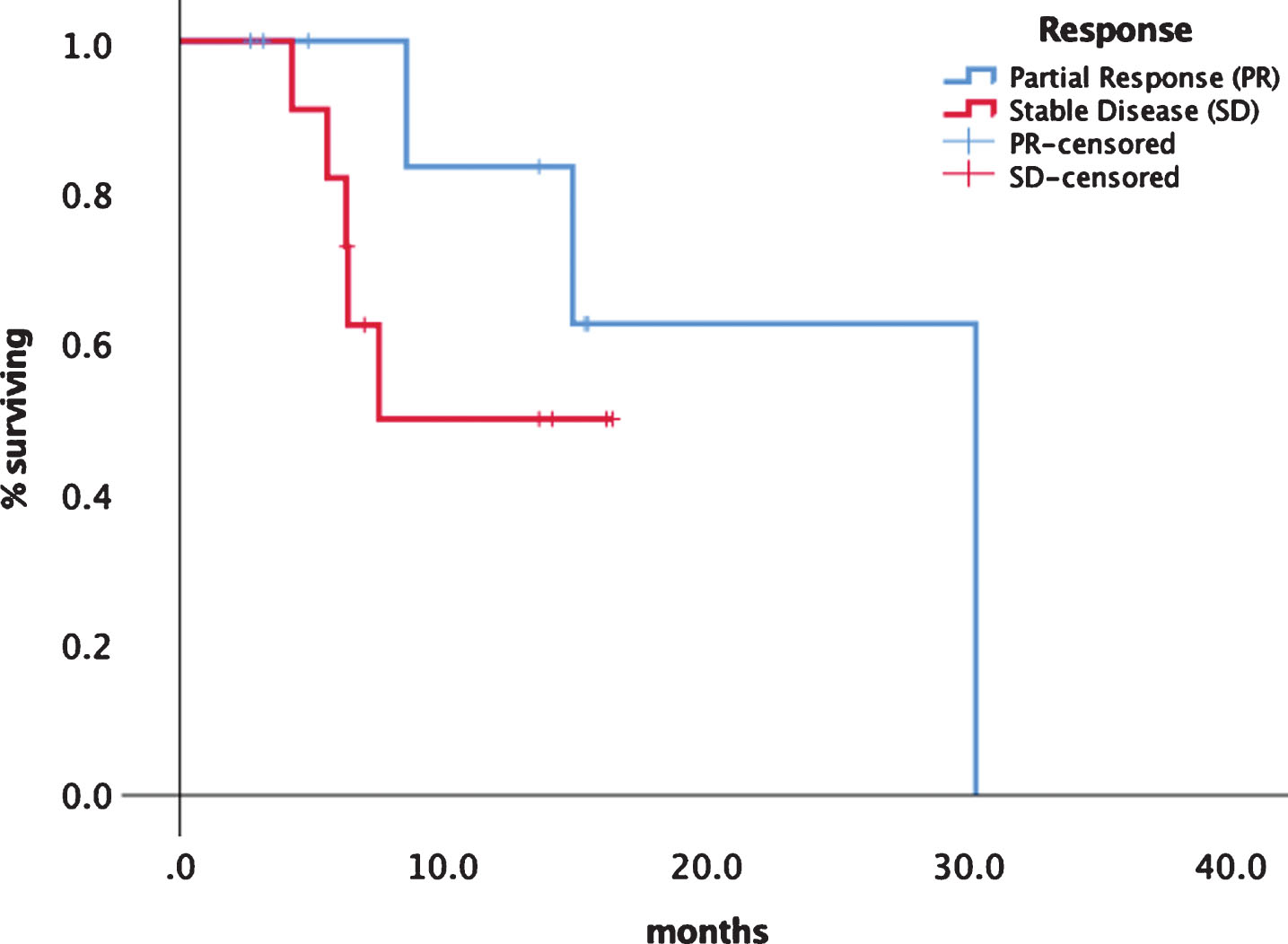
Median progression free survival (PFS) was 14.9 months (95% CI 5.1–24.8) (see Fig. 1). Median overall survival has not been reached so far. Although statistically not significant there was a difference in median PFS for first line patients with 30.3 (95% CI 13.3–33.1) months versus later line patients of 8.6 months ()5% CI 5.1–12.2) as shown in Fig. 2. Patients experiencing a PR had a median PFS of 30.2 months (95% CI 14.2–32.8) versus patients with SD had a median PFS of 7.5 months (95% CI 7.3–13.9) (Fig. 3). No significant PFS difference was seen between IMDC risk groups. (data not shown).
Fig.1
Progression free survival of tivozanib in all patients.
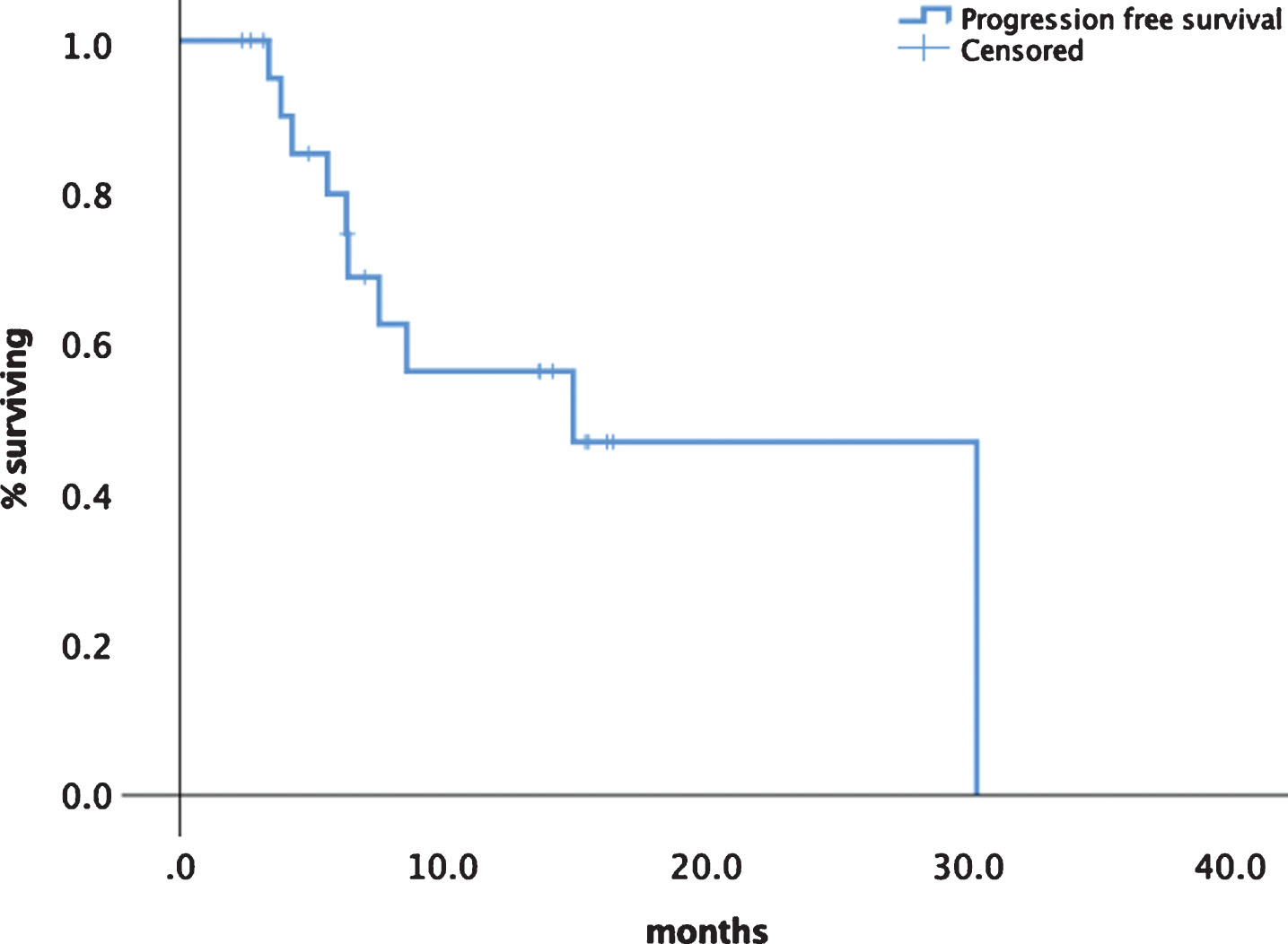
Fig.2
Comparison of treatment lines first vs. later line of tivozanib.
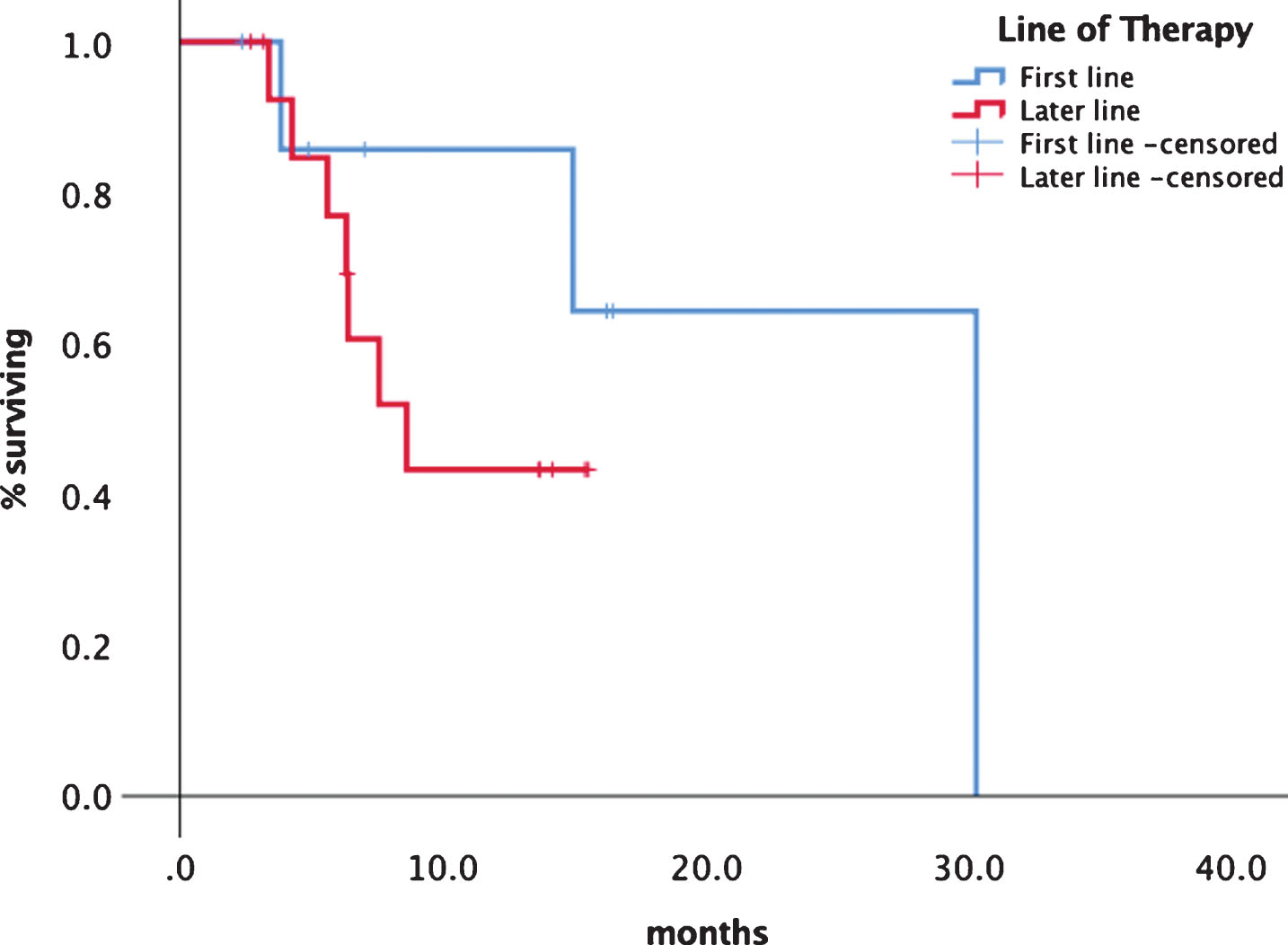
Fig.3
Progression free survival depending on response category PR vs. SD.
Frequent side effects consisted of diarrhea, hypertension, fatigue and hoarseness. 34.8% of the patients had grade II side effects, namely diarrhea and fatigue and 21.7% had grade 3 adverse events, mostly diarrhea, leading to treatment discontinuation in 3 patients (13%). Hoarseness, although not graded high, was especially bothering n = 3 patients, as it limits their ability of communication (Table 2).
Table 2
Adverse events
| °1 and °II | °III | |
| Fatigue | n = 4 (17.4%) | |
| Diarrhea | n = 3 (13.0%) | n = 2 (8.7%) |
| Hoarseness | n = 3 (13.0%) | |
| Hypertension | n = 3 (13.0%) |
DISCUSSION
In the last three years, front line treatment in Renal Cell Carcinoma has shifted dramatically, first with the approval of the combination of ipilimumab plus nivolumab in intermediate and high-risk patients and most recently new therapies combining TKI and PD-1/PD-L1 inhibition [8–10]. Trials have shown that these combinatory approach increase PFS and OS in mRCC patients as a front-line therapy. This has led to a shift away from front-line therapy using single agent targeted therapies.
Tivozanib has shown potential as an agent that is conducive to combination therapy. In 2013, Fishman et al. 2013 enrolled 27 patients in a trial combining temsirolimus with tivozanib. PR and SD were 23% and 68%, which is in the range of our results. Tolerability was good and thus this combination was thought to be feasible for further investigations [11].
Ongoing trials are evaluating tivozanib in the setting of refractory disease and the utility of tivozanib in combination with the checkpoint inhibitor nivolumab (clinicaltrials.gov NCT03136627).
Recent data from trials combining axitinib with either PD-L1 inhibitor avelumab and PD-1 inhibitor pembrolizumab indicate that the duration of tumor control (PFS) is most likely linked to the TKI efficacy, thus a TKI offering a longer PFS would seem to be promising. Tivozanib has one of the longest durations of response in a first line setting with over 12.7 months, compared to 8.4 and 9.5 months with pazopanib and sunitinib making it an excellent candidate for future combinations [5, 12].
The TIVO-3 trial (clinicaltrials.gov, NCT02627963) recently showed a 44% improvement in median PFS and 26% reduction in risk of progression or death (HR = 0.74, p = 0.02) for tivozanib compared to sorafenib after at least 2 lines of therapy [13]. Our data show a long PFS in a real-world setting, proving that tivozanib is active in subsequent therapeutic lines. This is of importance as the question of how to sequence therapies beyond first-line combinations is growing in significance.
Notably, our data showed impressive PFS rates for patients being treated from second to sixth line therapy, showing that tivozanib is effective regardless of tumor biology.
Besides overall survival data are pending, PFS stays a meaningful endpoint for the evaluation of treatment benefit to patients. OS continuously prolongs in the era of targeted therapy. Recent analysis from the SEER database has shown an increase to 23.4 months in 2012 from 16.7 months before 2003. 5-year survival rates have doubled in the last decade based on the availability of more surgical and systemic treatment options [14].
It has recently been shown that patients in the real-world setting have the same outcome as patients in trials if they fulfill the same criteria as asked for by study inclusion data [15]. Thus, our data can be compared with the results as all of the patients included in this analysis would have been eligible for a clinical trial as well. The indication for off-trial therapy mainly derived from the fact that at the moment of inclusion into this evaluation no trial was available. But given the excellent results we are convinced that the observed data can be achieved by other non-trial centers.
Besides these results there are conflicting data on real-world efficacy of TKI therapy. Although some data show equivalence others don’t and cannot reveal equal outcomes, especially on PFS and OS. Nazha et all showed that pazopanib has an adverse outcome of PFS and OS compared to sunitinib in Canadian multicenter analysis with patients being treated with pazopanib showng a shorter OS and PFS than in the pivotal trial or a European multicenter analysis, that proofed equivalence [16, 17]. Furthermore, these oncological differences have an impact on cost efficacy as well [10, 16, 18]. Our data are the first on tivozanib to show that efficacy and tolerability are comparable to the pivotal trial and therefore proof that the use of tivozanib is safe and oncological beneficial.
Nonetheless our data have to be interpreted with caution, given the small sample size and short follow-up which limit the results. Further pooled analyses and research especially towards overall survival are eagerly awaited. Pending data on later line therapy especially after immunotherapy are expected to be presented soon.
CONCLUSION
In routine clinical practice tivozanib is an effective TKI with a long PFS even in later lines of therapy. Adverse effects are consistent with TKI therapy and no new safety signals are seen in a real-world setting. Due to its long PFS and preferable side effects further research should focus on combination therapies.
ACKNOWLEDGMENTS
This publication is supported by an unrestricted grant from EUSAPharma, Germany.
REFERENCES
[1] | Ferlay J , Steliarova-Foucher E , Lortet-Tieulent J , et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. European Journal of Cancer. (2013) ;49: :1374–403. |
[2] | Fischer CG , Waechter W , Kraus S , Fuentecilla Perez E , Weidner W , Dudeck J . Urologic tumors in the Federal Republic of Germany: Data on 56,013 cases from hospital cancer registries, Cancer. (1998) ;82: :775–83. |
[3] | Singer EA , Gupta GN , Marchalik D , Srinivasan R . Evolving therapeutic targets in renal cell carcinoma, Current Opinion in Oncology. (2013) ;25: :273–80. |
[4] | European Medicines Agency E. Summary of Opiinion Fotivda, EMA/CHMP/333095/2017 Vol 2019. https://www.ema.europa.eu/en/documents/smop-initial/chmp-summary-positive-opinion-fotivda_en.pdf 2017. |
[5] | Motzer RJ , Nosov D , Eisen T , et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: Results from a phase III trial, J Clin Oncol. (2013) ;31: :3791–9. |
[6] | Tsuchida Y , Therasse P . Response evaluation criteria in solid tumors (RECIST): New guidelines, Medical and Pediatric Oncology. (2001) ;37: :1–3. |
[7] | Motzer RJ , Bacik J , Mazumdar M . Prognostic factors for survival of patients with stage IV renal cell carcinoma: Memorial sloan-kettering cancer center experience, Clin Cancer Res. (2004) ;10: :6302S–3S. |
[8] | Motzer RJ , Tannir NM , McDermott DF , et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma, N Engl J Med. (2018) ;378: :1277–90. |
[9] | Rini BI , Plimack ER , Stus V , et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma, N Engl J Med. (2019) . |
[10] | Motzer RJ , Penkov K , Haanen J , et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma, N Engl J Med. (2019) . |
[11] | Fishman MN , Srinivas S , Hauke RJ , et al. Phase Ib study of tivozanib (AV-951) in combination with temsirolimus in patients with renal cell carcinoma, Eur J Cancer. (2013) ;49: :2841–50. |
[12] | Motzer RJ , Hutson TE , Cella D , et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma, N Engl J Med. (2013) ;369: :722–31. |
[13] | Rini B , Pal S , Escudier B , et al. TIVO-3: A phase III, randomized, controlled, multicenter, open-label study to compare tivozanib to sorafenib in subjects with refractory advanced renal cell carcinoma (RCC), Journal of Clinical Oncology. Journal of Clinical Oncology. (2019) ;3: :541–541. |
[14] | Pal SK , Ghate SR , Li N , et al. Real-world survival outcomes and prognostic factors among patients receiving first targeted therapy for advanced renal cell carcinoma: A SEER-medicare database analysis, Clin Genitourin Cancer. (2017) ;15: :e573–82. |
[15] | Marschner N , Staehler M , Muller L , et al. Survival of patients with advanced or metastatic renal cell carcinoma in routine practice differs from That in clinical trials-analyses from the german clinical RCC registry, Clin Genitourin Cancer. (2017) ;15: :e209–15. |
[16] | Nazha S , Tanguay S , Kapoor A , et al. Use of targeted therapy in patients with metastatic renal cell carcinoma: Clinical and economic impact in a Canadian real-life setting, Curr Oncol. (2018) ;25: :e576–84. |
[17] | Schmidinger M , Bamias A , Procopio G , et al. Prospective observational study of pazopanib in patients with advanced renal cell carcinoma (PRINCIPAL Study), Oncologist. (2019) ;24: :491–7. |
[18] | Nazha S , Tanguay S , Kapoor A , et al. Cost-utility of sunitinib versus pazopanib in metastatic renal cell carcinoma in canada using real-world evidence, Clin Drug Investig. (2018) ;38: :1155–65. |




