The Value of Multiparametric MRI for Assessment of Inferior Vena Cava Wall Invasion by Renal Cell Carcinoma Thrombus: A Prospective Feasibility Study
Abstract
Background:
Renal cell carcinoma (RCC) thrombus invasion in the inferior vena cava (IVC) wall needs adequate resection to improve survival. Surgical planning to avoid positive vascular surgical margins and recurrent disease can be facilitated by imaging capable of diagnosing this invasion and the extent of the thrombus.
Objective:
To evaluate the diagnostic performance of multiparametric MR imaging (mpMRI) for detecting inferior vena cava (IVC) wall invasion and extent of renal cell carcinoma (RCC) thrombus.
Methods:
In this prospective study, twenty consecutive patients underwent mpMRI before open radical nephrectomy with IVC thrombectomy was performed in a single high-volume institution by one urologist (PM) blinded for mpMRI findings. Primary endpoint was mpMRI negative- and positive predictive value (NPV/PPV) for IVC wall invasion as encountered during surgery. IVC wall invasion was assessed on mpMRI by a radiologist prior to the surgery based on qualitative imaging parameters. Also, NPV and PPV were calculated with incremental use of quantitative parameters. Therefore, the association between IVC wall invasion and the maximum IVC and renal vein orifice diameter, and the cranial extent of the thrombus was evaluated by logistic regression. After ROC analysis an optimal cut-off point was selected for significantly associated quantitative parameters. NPV and PPV were calculated using this optimal cut-off point.
Results:
mpMRI NPV and PPV for IVC wall invasion based on qualitative parameters was 82% and 100%, respectively. An increase in IVC diameter was significantly associated with IVC wall invasion (p < 0.05). With incremental use of an IVC diameter cut-off value of 27 mm NPV and PPV for IVC wall invasion were 100% and 89%, respectively.
Conclusion:
mpMRI is able to reliably diagnose IVC wall invasion of RCC thrombus and can optimize radical surgical resection.
INTRODUCTION
Renal cell carcinoma (RCC) is the most common renal cortical malignancy, with an annual incidence of 65.000 and 84.000 in the United States and the European Union, respectively [1, 2]. Of all malignancies that may present with a thrombus in the inferior vena cava (IVC), this condition is most frequently found in case of RCC [3]. In up to 10% of all RCC cases, a thrombus is found in the renal vein or IVC [4].
Next to the cranial extent of the thrombus, invasion of the IVC wall is a predictive factor for radical surgery [5]. IVC wall invasion has been described in up to 23% of in cases with tumoral venous extension. It is a known negative prognostic factor, upgrading RCC to a stage T3c according to the American Joint Committee on Cancer (AJCC) classification, independent of the cranial extent of the thrombus [6]. This subgroup harbors a 10-year cancer specific survival rate of 25% for which microscopic IVC wall invasion is an important predictive factor [6, 7]. Positive vascular wall margins following surgery result in significantly shorter recurrence free intervals (22 versus 70 months) [8]. Therefore, radical surgery, sometimes even with partial vena cava wall resection, is warranted to ensure optimal oncological outcomes.
The cranial extent of the thrombus, categorized by the Neves classification as level 1–4, usually determines the type of surgery [9, 10]. In level 1-2 thrombi, extending into the IVC but not cranially of the hepatic veins, partially clamping of the IVC using a Satinsky clamp followed by cavotomy with removal of the thrombus, and subsequent en-block removal of the kidney may be sufficient. Total clamping of the IVC and mobilization of the liver with Pringle clamping or even thoracotomy with cardiac arrest may be necessary to radically remove a thrombus with a more cranial extent [11, 12]. Total clamping of the IVC may be prevented when a thrombus can be retracted manually to a more caudal level. However, in case of unrecognized IVC wall invasion thrombus retraction can cause a positive vascular margin and may hamper radical surgery.
Because radical surgical resection is the only curative option for localized RCC with an IVC thrombus, pre-operative imaging plays a crucial role in surgical planning [10]. Unfortunately, IVC wall invasion is most often encountered during surgery due to the inability to assess it on pre-operative imaging [13]. By informing the surgeon on both the extent of the tumor thrombus and the probability of IVC wall invasion, mpMRI has a potential benefit to tailor the surgical strategy during radical nephrectomy and IVC thrombectomy to ensure radical removal.
The objective of this study was to evaluate the diagnostic performance of mpMRI for IVC wall invasion caused by RCC thrombus. Secondary endpoints were mpMRI performance for diagnosing thrombus extent and presence of tumor cells in the thrombus.
PATIENTS AND METHODS
Study population
A subset of twenty patients with RCC and a caval thrombus were prospectively and consecutively selected from the included patients in the study registered under NCT02325921 on clinicaltrials.gov. Institutional Review Board (IRB) approval was obtained for this study, and informed consent was waived. Inclusion criteria for this study were a renal lesion suspicious for RCC with an IVC thrombus for which radical nephrectomy with cavotomy and thrombectomy was planned. All included patients with metastasis underwent surgery before systemic treatment was initiated. Exclusion criteria were contraindications for MR imaging (non MR compatible metal device/foreign bodies, claustrophobia).
Imaging protocol
MR scans were acquired using a 3T MRI system (Magnetom Trio, Siemens, Erlangen, Germany) at a median of 8.5 days (range 1–25) prior to surgery. Patient position was feet-first supine. A combination of a 32-channel receiver coil and phased array body surface coil was used. The scanning protocol consisted of anatomical T1-weighted VIBE (volumetric interpolated breath-hold examination), with and without fat suppression, and T2-weighted fat suppressed HASTE (Half-Fourier-Acquired Single-shot Turbo spin Echo) sequences in axial and coronal directions using breath hold. Water diffusion was measured in three directions using a respiratory triggered coronal single-shot-echo-planar imaging (SS-EPI) sequence with b-values of 50, 800 and 1400 s/mm2. Subsequent dynamic contrast enhanced (DCE) fat saturated T1-weighted VIBE 3D imaging was acquired in the corticomedullary, nephrographic, and excretory phase. The extended imaging parameters are listed in Supplemental Table 1.
Surgery
Nephrectomy was planned based on cranial extent of the thrombus on pre-operative CT scan and performed by one experienced urologist (PM) blinded for mpMRI findings. During surgery a transperitoneal abdominal approach was used. Dissection of the kidney included ligation of the gonadal vein, the ureter, and the renal artery. Subsequently control was gained over the contralateral renal vein and the vena cava caudally of the renal veins and cranially of the thrombus extent. In case of level I/II thrombi the thrombus was manually massaged caudally whenever possible in order to avoid total IVC clamping and enable clamping with a Satinsky around the renal vein orifice. If Satinsky clamping was not possible total IVC clamping was performed. When the thrombus extended cranially of the hepatic veins a surgeon specialized in hepatic surgery was consulted and the liver was laterally mobilized. In case of thrombus extension into the right atrium a cardiothoracic surgeon was consulted and a thoracotomy was performed, and when necessary, hypothermia with cardiac arrest was done prior to opening of the right atrium for thrombus removal via cavotomy. In that case trans-esophageal cardiac ultrasound was performed for thrombus extension monitoring. After clamping (partially or total) of the vena cava the renal vein orifice was incised circumferentially and cavotomy was performed followed by en-block removal of the tumor and thrombus. If the thrombus could easily be removed no IVC wall invasion was reported. When adherence to the IVC wall hampering manual removal of the thrombus was encountered IVC wall invasion was reported, and when possible partial removal of the caval wall was performed. The site at which the adherence was found was documented. Without IVC wall invasion cavorrhaphy was done after removal of the thrombus using continuous prolene suturing.
Pathology report
Histological RCC subtype was defined according to the 2016 World Health Organization classification and International Society of Urological Pathology nuclear grading was reported [14, 15]. Presence of tumor cells in the IVC thrombi was evaluated and reported. Also, tumor ingrowth in the caval wall was evaluated.
Image analysis
All imaging was evaluated prior to the nephrectomy on a standard PACS (IMPAX, Agfa Healthcare, Mortsel, Belgium) work station by a radiologist (JF) with 15-years of experience in urogenital MR-imaging.
No wall invasion was suspected when the entire thrombus could be delineated from the vena cava wall and the vessel wall had a homogeneous signal intensity on T2-weighted or contrast enhanced imaging at any level. In case of no clear delineation of the thrombus from the vessel wall or an altered signal within the vessel wall, a change in wall thickness or a clear breach of the vessel wall (defined as discontinuity of the wall), vein wall invasion was suspected. The imaging findings were correlated with the intra-operative findings.
The association between IVC wall invasion and maximum IVC and renal vein orifice diameter measured in anterior-posterior direction and thrombus level on MRI was evaluated. For the significantly associated quantitative parameters an optimal cut-off point that maximized diagnostic accuracy was determined.
Secondary endpoints were MRI NPV and PPV for diagnosing thrombus level and presence of tumor cells in the thrombus. For the latter thrombi were categorized as a tumor-, bland- (tumor cell free) or mixed thrombus based on imaging. Presence of tumor cells within the thrombus was suspected in case of contrast enhancement with equal intensity compared to the primary tumor or in case of diffusion restriction within the thrombus comparable with solid tumor tissue. Thrombus level was correlated with intra-operative findings. Imaging findings for presence of tumor cells in the thrombi were correlated with final histopathology.
Statistical analysis
For statistical analysis SPSS (version 22.0; IBM; Amonk; New York) and MATLAB R2014b (MathWorks; Natick; MA, USA) were used. Continuous variables are expressed as median (range) or mean (±standard deviation). NPV and PPV were calculated based on the reported qualitative MRI findings. Odds ratios were calculated to quantify the association between IVC wall invasion and maximum IVC and renal vein orifice diameter, and thrombus level using univariate logistic regression analysis. ROC analysis was performed for significantly associated quantitative parameters, and the optimal cut-off point was selected using the Youden method. NPV and PPV were calculated using this cut-off point in combination with the qualitative parameters. Differences between means of groups were tested using a Mann-Whitney test. For all tests, a p-value <0.05 was considered significant.
RESULTS
Of the 20 included patients, 19 were available for final analysis. In one patient with two known peritoneal metastatic depositions a cytoreductive nephrectomy was planned. During surgery extensive depositions in the mesentery of the colon were detected. Therefore, the operation was terminated before the nephrectomy was performed. Demographics and tumor characteristics of the 19 patients used for final analysis are listed Table 1.
Table 1
Demographics and tumor characteristics based on surgical findings and histopathology
| Median (range) or n (%) | |
| Age | 68 (48–79) |
| Female | 5 (26%) |
| Left sided tumor | 5 (26%) |
| Tumor diameter | 106 mm (58–230) |
| Synchronous metastasis | 3 (16%) |
| IVC thrombus level | |
| I | 3 (16%) |
| II | 10 (53%) |
| III | 3 (16%) |
| IV | 3 (16%) |
| RCC subtype | |
| Clear cell | 18 (95%) |
| Papillary type 2 | 1 (5%) |
| ISUP nuclear grading | |
| 1 | 1 (5%) |
| 2 | 5 (26%) |
| 3 | 6 (32%) |
| 4 | 7 (37%) |
IVC Inferior vena cava; RCC renal cell carcinoma; ISUP International Society of Urological Pathology.
MRI NPV and PPV for IVC wall invasion based on qualitative parameters were 82% and 100%, respectively. IVC wall invasion was present in 10 (53%) cases. (Fig. 1). In total two false negative scans were scored. In one case a level IV thrombus was intra-operatively found to be adherent to the IVC wall at the level of the diaphragm. In the second case adherence was encountered during surgery and after thrombectomy an additional biopsy of the caval wall was obtained. Histopathology did not confirm IVC wall invasion at the site of the thrombus IVC wall adherence.
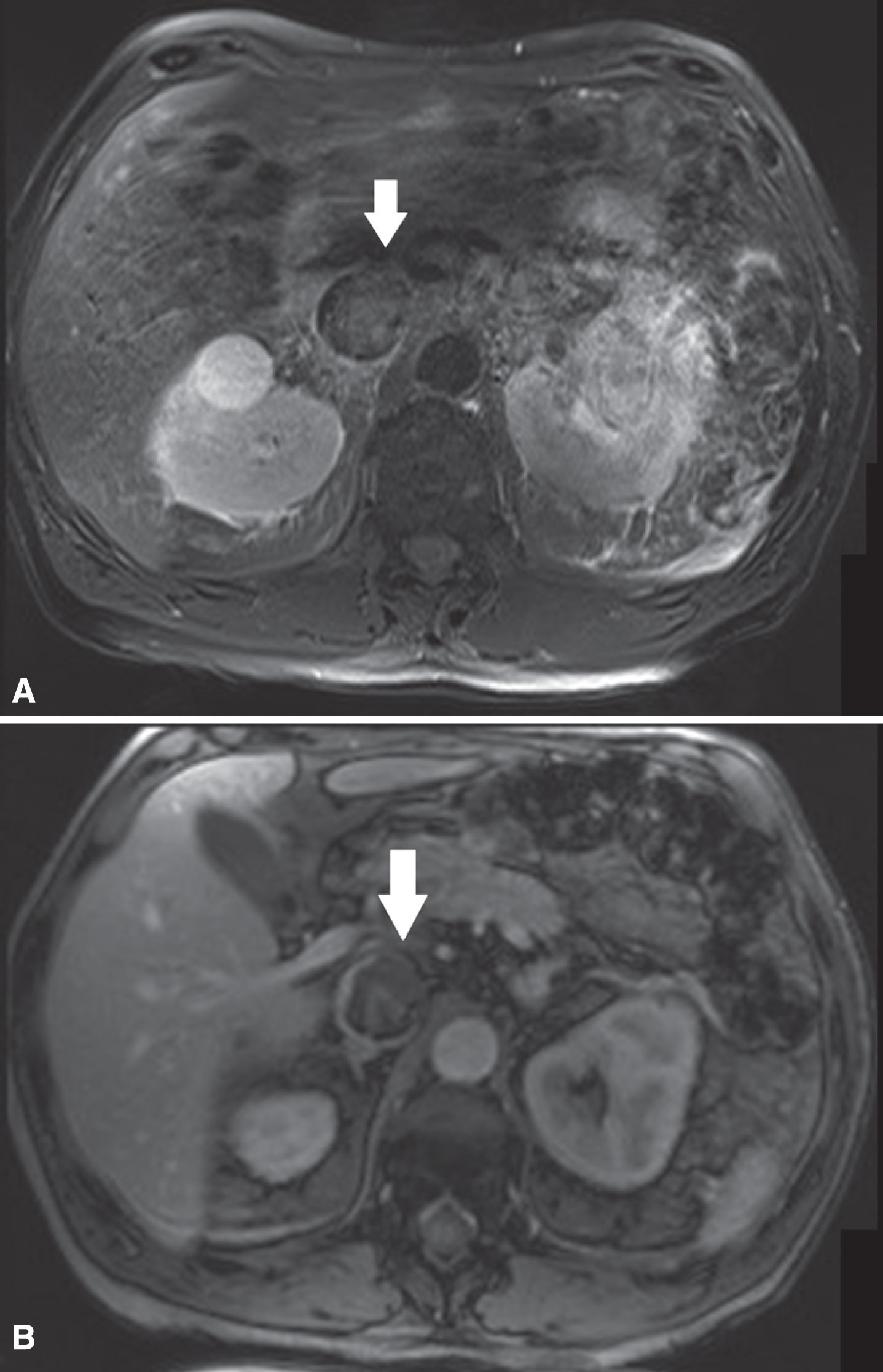
Fig.1
(A) In this patient with a left sided tumor and level 2 thrombus, the T2-weighted MR images showed loss of high signal intensity at the medial/ventral side of the vena cava wall (white arrow) suspicious for IVC wall invasion. (B) The corresponding DCE sequence showed evident loss of vein wall signal (white arrow) supporting the suggestion of IVC wall invasion. Surgical findings confirmed medial/ventral IVC wall invasion.
NPV was 100% and PPV dropped to 89% for IVC wall invasion when a cut-off value of 27 mm was used for maximum IVC diameter combined with the qualitative parameters. Mean IVC diameter was significantly higher (p < 0.01) for the group with wall invasion (35.9 mm±8.1) versus no wall invasion (23.4 mm±3.3). Univariate logistic regression analysis showed that an increase in IVC diameter was associated with a higher probability of IVC wall invasion (Odds Ratio 1.78; 95% CI 1.08–2.94; p < 0.05). The optimal cut-off selected was 27 mm (Youden index = 0.7889). In contrast, IVC wall invasion was not significantly associated with RV orifice diameter and thrombus level (Table 2).
Table 2
Quantitative parameters for assessment of IVC wall invasion
| No invasion (n = 9) | Invasion (n = 10) | OR for IVC wall invasion1 | 95% CI | p-value1 | |
| Maximum IVC diameter* | 23.4 (±3.3) | 35.9 (±8.1) | 1.78 | 1.08–2.94 | 0.03 |
| Renal vein orifice diameter* | 23.5 (±4.5) | 26.5 (±5.4) | 1.14 | 0.93–1.41 | 0.22 |
| Thrombus level | Level 1 33% (n = 3) | Level 1 none | 6.53 | 0.94–45.39 | 0.06 |
| Level 2 56% (n = 5) | Level 2 50% (n = 5) | ||||
| Level 3 11 % (n = 1) | Level 3 20% (n = 2) | ||||
| Level 4 none | Level 4 30% (n = 3) |
*Measured in anterior posterior direction. 1Calculated using univariate logistic regression. OR odds ratio, IVC infrarenal vena cava; CI confidence interval.
MRI NPV and PPV for diagnosing thrombus level were both 100%. In all cases tumor cells were detected in the thrombus on histopathology, therefore only the PPV for presence of tumor cells in the thrombus could reliably be calculated and was 100%. Remarkably, in two (11%) cases no contrast enhancement of the thrombus was seen. However, clear diffusion restriction was visualized and both thrombi were therefore marked as tumor thrombi (Fig. 2).
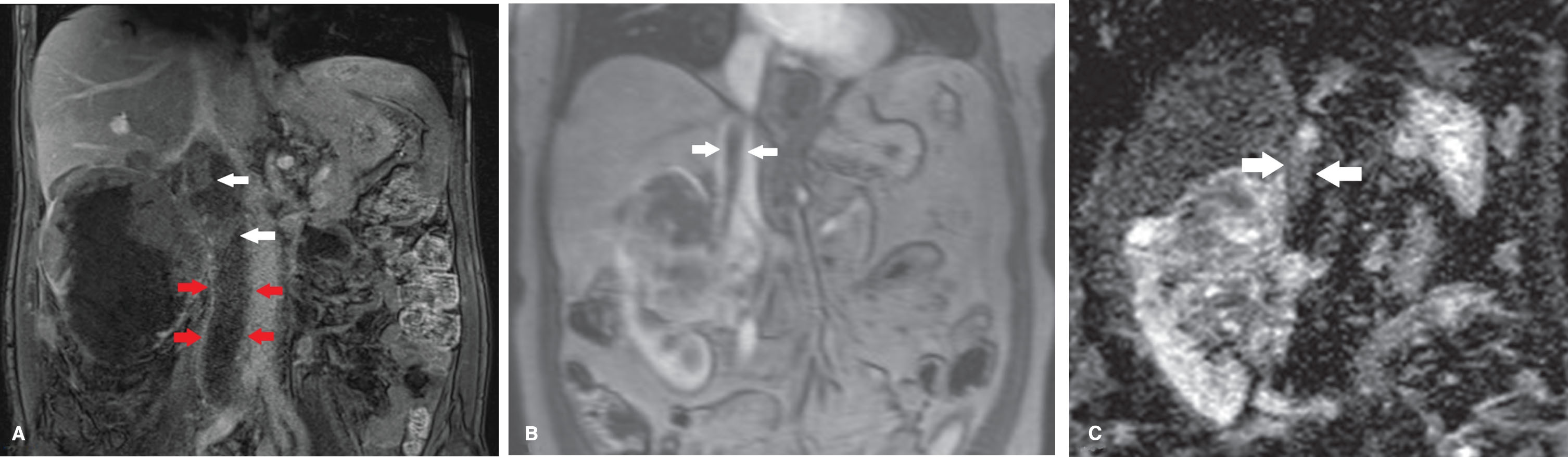
Fig.2
(A) In a patient with a right sided tumor with a level 2 thrombus (including occlusion of the iliac veins) the DCE sequence showed no enhancement of the thrombus caudal of the renal veins (between 4 most caudal arrows). Clear heterogeneous enhancement of the tumor thrombus is seen at more cranial level (white arrows) suggestive for vital tumor. (B) In contrast, in this patient with a level 3 thrombus and right sided tumor, no contrast enhancement of the thrombus is seen (between white arrows). (C) On the DW-MRI sequence clear diffusion restriction of the thrombus is seen suggestive for vital tumor (between white arrows). Histopathology confirmed the finding of vital tumor in the thrombus.
DISCUSSION
mpMRI can accurately detect IVC wall invasion based on qualitative imaging parameters. Incremental use of the maximum IVC diameter increases the NPV for IVC wall invasion. These findings support that mpMRI is of added value for surgical planning for nephrectomy with IVC thrombectomy, and can aid in reducing positive vascular wall margins.
Several studies have evaluated prognostic factors for survival of RCC patients with an IVC thrombus. In spite of the debate, the cranial extent of the thrombus has not been established as prognostic marker [16]. In contrast, non-radical surgery is inversely correlated with survival [6]. This emphasizes the importance of surgical planning in order to achieve radical removal, which is of paramount importance in the setting of non-metastatic disease as surgery is the only potentially curative option [10].
Two previous studies with the objective to predict IVC wall invasion on MRI have been conducted. A study by Sohaib et al. found a sensitivity and specificity of 100%, and 89%, respectively, for IVC wall invasion in 12 patients on MRI [17]. In 48 patients, Adams et al. found a sensitivity and specificity of 92%, and 86%, respectively [18]. Comparable to our study, both studies evaluated qualitative MRI features. In our study one out of two false negative MRI scan was scored at the level of the diaphragm in a level 4 thrombus. This specific area is susceptible to breathing artifacts, which may lead to false negative scans [17]. Previously, false positive scans due to small areas of adherence (<1 cm) and compression of the caval wall by tumor mass were described [17, 18]. We aimed to increase the detection of tumor thrombi and concomitant adherence and small areas of adherence using a multiparameteric scanning protocol.
We included DW-MRI in the scanning protocol. Qualitative analysis of DWI-MR images increased the detection of tumor containing thrombi, showing two tumor thrombi not characterized as such by DCE-MRI. This stresses the importance of a multiparametric scanning protocol. The rationale for evaluating presence of vital tumor in thrombi is that the neovascularisation bed of the tumor thrombus causes the vein wall invasion, warranting a change in surgical approach to avoid positive vascular margins [19]. In case of absence of both thrombus contrast enhancement and diffusion restriction IVC wall invasion is unlikely. Unfortunately, due to the absence of pure bland thrombi in our study no association between the presence of tumor thrombi and IVC wall invasion probability could be reliably calculated.
IVC and renal vein (orifice) diameter have previously been suggested to be a predictive factor for IVC wall and renal vein orifice wall invasion [18, 20, 21]. We confirmed a significant correlation between IVC wall invasion and maximum IVC diameter. Previously reported mean IVC diameter in case of wall invasion varies between 28 mm (±10) and 43 mm (±9), compared to 35.9 mm (±8.1) in our study [18, 20]. Psutka et al. evaluated predictors for IVC resection during nephrectomy for RCC with caval thrombus. Many factors, including IVC and renal vein orifice diameter, were found to be of predictive value [21]. A secondary analysis to assess the ability to predict histological IVC wall invasion showed an AP diameter≥24 mm of the IVC at the renal vein orifice to be of value. The data in our study, which are in line with previous findings, confirms that IVC diameter can be of added value to predict IVC wall invasion next to qualitative imaging features. However, because the main goal of the imaging should be to facilitate surgical planning, IVC diameter alone cannot be used as a surrogate marker for IVC wall invasion. Qualitative parameters are of importance to determine the anatomical location of where invasion can beexpected.
This study has several limitations. The most prominent limitation is the limited sample size. Although this may limit extensive statistical analysis, the sample size was considered adequate to meet the study endpoints. Moreover, our hospital is a tertiary referral center for stage T3b-c RCC and the number of included patients is in line with previous single institution studies evaluating MRI for evaluation of T3 RCC [17–19, 22–24].
We conclude that mpMRI is able to reliably diagnose IVC wall invasion of RCC thrombus and helps adequate resection of tumor trombus.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/KCA-190071.
REFERENCES
[1] | Siegel RL , Miller KD , Jemal A . Cancer Statistics, 2018. Ca-Cancer J Clin, (2018) ;68: (1):7–30. |
[2] | Ferlay J , Steliarova-Foucher E , Lortet-Tieulent J , Rosso S , Coebergh JWW , Comber H , et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer. (2013) ;49: (6):1374–403. |
[3] | Kraft C , Schuettfort G , Weil Y , Tirneci V , Kasper A , Haberichter B , et al. Thrombosis of the inferior vena cava and malignant disease, Thromb Res. (2014) ;134: (3):668–73. |
[4] | Marshall VF , Middleton RG , Holswade GR , Goldsmith EI . Surgery for renal cell carcinoma in the vena cava, J Urol (1970) ;103: (4):414–20. |
[5] | Hatcher PA , Anderson EE , Paulson DF , Carson CC , Robertson JE . Surgical-Management and Prognosis of Renal-Cell Carcinoma Invading the Vena-Cava, J Urology. (1991) ;145: (1):20–4. |
[6] | Rodriguez Faba O , Linares E , Tilki D , Capitanio U , Evans CP , Montorsi F , et al. Impact of Microscopic Wall Invasion of the Renal Vein or Inferior Vena Cava on Cancer-specific Survival in Patients with Renal Cell Carcinoma and Tumor Thrombus: A Multi-institutional Analysis from the International Renal Cell Carcinoma-Venous Thrombus Consortium, Eur Urol Focus. (2018) ;4: (3):435–41. |
[7] | Kim SP , Alt AL , Weight CJ , Costello BA , Cheville JC , Lohse C , et al. Independent validation of the 2010 American Joint Committee on Cancer TNM classification for renal cell carcinoma: Results from a large, single institution cohort, J Urol. (2011) ;185: (6):2035–9. |
[8] | Abel EJ , Carrasco A , Karam J , Tamboli P , Delacroix S , Vaporciyan AA , et al. Positive vascular wall margins have minimal impact on cancer outcomes in patients with non-metastatic renal cell carcinoma (RCC) with tumour thrombus, Bju Int. (2014) ;114: (5):667–73. |
[9] | Neves RJ , Zincke H . Surgical-Treatment of Renal-Cancer with Vena-Cava Extension, Brit J Urol. (1987) ;59: (5):390–5. |
[10] | Lardas M , Stewart F , Scrimgeour D , Hofmann F , Marconi L , Dabestani S , et al. Systematic Review of Surgical Management of Nonmetastatic Renal Cell Carcinoma with Vena Caval Thrombus, Eur Urol. (2016) ;70: (2):265–80. |
[11] | Boorjian SA , Sengupta S , Blute ML . Renal cell carcinoma: Vena caval involvement, Bju Int. (2007) ;99: (5 Pt B):1239–44. |
[12] | Blute ML , Leibovich BC , Lohse CM , Cheville JC , Zincke H . The Mayo Clinic experience with surgical management, complications and outcome for patients with renal cell carcinoma and venous tumour thrombus, Bju Int. (2004) ;94: (1):33–41. |
[13] | Kandpal H , Sharma R , Gamangatti S , Srivastava DN , Vashisht S . Imaging the inferior vena cava: A road less traveled, Radiographics. (2008) ;28: (3):669–89. |
[14] | Delahunt B , Cheville JC , Martignoni G , Humphrey PA , Magi-Galluzzi C , McKenney J , et al. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters, Am J Surg Pathol. (2013) ;37: (10):1490–504. |
[15] | Moch H , Cubilla AL , Humphrey PA , Reuter VE , Ulbright TM . The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours, Eur Urol. (2016) ;70: (1):93–105. |
[16] | Klatte T , Pantuck AJ , Riggs SB , Kleid MD , Shuch B , Zomorodian N , et al. Prognostic factors for renal cell carcinoma with tumor thrombus extension, J Urol. (2007) ;178: (4 Pt 1):1189–95; discussion 95. |
[17] | Sohaib SAA , Teh J , Nargund VH , Lumley JSP , Hendry WF , Reznek RH . Assessment of tumor invasion of the vena caval wall in renal cell carcinoma cases by magnetic resonance imaging, J Urology. (2002) ;167: (3):1271–5. |
[18] | Adams LC , Ralla B , Bender YY , Bressem K , Hamm B , Busch J , et al. Renal cell carcinoma with venous extension: Prediction of inferior vena cava wall invasion by MRI, Cancer Imaging. (2018) ;18: (1):17. |
[19] | Oto A , Herts BR , Remer EM , Novick AC . Inferior vena cava tumor thrombus in renal cell carcinoma: Staging by MR imaging and impact on surgical treatment, Am J Roentgenol. (1998) ;171: (6):1619–24. |
[20] | Zini L , Destrieux-Garnier L , Leroy X , Villers A , Haulon S , Lemaitre L , et al. Renal vein ostium, wall invasion of renal cell carcinoma with an inferior vena cava tumor thrombus: Prediction by renal and vena caval vein diameters and prognostic significance, J Urology. (2008) ;179: (2):450–4. |
[21] | Psutka SP , Boorjian SA , Thompson RH , Schmit GD , Schmitz JJ , Bower TC , et al. Clinical and radiographic predictors of the need for inferior vena cava resection during nephrectomy for patients with renal cell carcinoma and caval tumour thrombus, Bju Int. (2015) ;116: (3):388–96. |
[22] | Myneni L , Hricak H , Carroll PR . Magnetic resonance imaging of renal carcinoma with extension into the vena cava: Staging accuracy and recent advances, Br J Urol. (1991) ;68: (6):571–8. |
[23] | Roubidoux MA , Dunnick NR , Sostman HD , Leder RA . Renal carcinoma: Detection of venous extension with gradient-echo MR imaging, Radiology. (1992) ;182: (1):269–72. |
[24] | Laissy JP , Menegazzo D , Debray MP , Toublanc M , Ravery V , Dumont E , et al. Renal carcinoma: Diagnosis of venous invasion with Gd-enhanced MR venography, Eur Radiol. (2000) ;10: (7):1138–43. |




