Collecting Duct Carcinoma of the Kidney: Analysis of Our Experience at the SPANISH ‘Grupo Centro’ of Genitourinary Tumors
Abstract
Introduction:
Collecting duct carcinomas (CDC), also known as Bellini’s tumors, are a rare and aggressive subtype of renal cell carcinoma. Therefore, there are very few data about their management, and there is no standard therapy for this malignancy. We report the outcome of CDC patients treated on institutions belonging to the ‘Grupo Centro’ of Genitourinary Tumors, a novel networking cooperative group in Spain.
Material and Methods:
Patients with CDC diagnosed between 1995 and 2015 were included. They had to have an appropriate follow-up, as well as available tissue for further correlative studies. Demographic baseline features and therapy outcomes were collected in a retrospective fashion. Approval for this data collection was obtained from a central ethical committee.
Results:
A total of 43 patients were analysed, with a median overall survival (OS) of 14 months (95% CI: 9.2–18.8 months). 29 of them (67.4%) were diagnosed as localized disease, and 14 (32.6%) as metastatic disease. For the subgroup of patients diagnosed without metastases, median relapse-free survival (RFS) is 22 months (95% CI: 12.4–35.6 months), and median OS, 53 months (95% CI: 35.5–84.3 months). For the subgroup of patients with metastatic disease, median OS is 6 months (95% CI: 4.1–7.8 months). 16 patients (55.2%) with stage IV disease received systemic therapy, mainly platinum-based chemotherapy, with a response rate of 12.5% and a median progression-free survival (PFS) of 2 months.
Conclusions:
CDC of the kidney is a malignancy with poor prognosis and few responses to therapy. Median OS of our group in the metastatic setting is similar to what has been observed in previous series. There is a clear need to improve the armamentarium we have for the systemic approach of patients with advanced CDC.
INTRODUCTION
Collecting duct carcinoma (CDC) of the kidney, also known as Bellini tumor, is a rare kidney neoplasm, accounting for about 1–2% of the total of epithelial kidney tumors. It is known to originate in the distal part of the collecting duct (1), and has a very dismal prognosis, with short survival and poor response to therapies (2). Data regarding its clinical course are scarce and are mainly restricted to case reports and short series of patients.
The classic clinical presentation of CDC is usually in an advanced metastatic stage, due to its aggressiveness (3); more than 60% of patients are symptomatic when diagnosed, and the most common sites of dissemination are lungs, lymph nodes and bone. This disease has been usually treated as an urothelial tumor, with chemotherapy combination regimens, usually a platinum (cisplatin or carboplatin) plus gemcitabine (4, 5). Results with these therapy schedules are poor, with survival being usually less than 12 months (6). There is less experience with other approaches, such as targeted therapy and immunotherapy, but results do not seem to be improved with these drugs.
The ‘Grupo Centro’ of Genitourinary Tumors is a networking cooperative group that comprises a total of 28 hospitals in the centre part of Spain, mainly Madrid and surrounding areas. Through a collaborative effort, we aimed to review our experience with CDCs of the kidney.
MATERIAL AND METHODS
We retrospectively reviewed the archives of the 28 hospitals that belong to the ‘Grupo Centro’, searching for CDCs of the kidney, including localized tumors as well as metastatic disease at diagnosis. The study was approved by a central Ethical Committee. Patients had to have an appropriate follow-up, and mixed histologies were excluded. Patients were diagnosed between 1995 and 2015, and had to have tissue available for further studies.
For each patient included, data were collected regarding age, gender, stage at diagnosis, symptoms at diagnosis, previous nephrectomy, site of metastases, systemic therapies, response to treatment, and outcome (relapse, survival). All data were analysed by descriptive statistics. Survival measurements were estimated using the Kaplan-Meier method using SPSS v21.
RESULTS
43 patients with CDC were identified; 29 of them (67.4%) were diagnosed as localized disease, and 14 (32.6%) as metastatic disease. Median age at presentation is 68 years (range 37–85). 27 patients are male (62.8%), and 16 (37.2%) are female. Patients’ clinical characteristics are summarized in Table 1.
Table 1
Patients’ characteristics
| Number of patients (%) | |
| Median age at diagnosis | 68 years (range 37 – 78) |
| Gender | |
| Male | 27 (62.8%) |
| Feale | 16 (37.2%) |
| Radical nephrectomy | |
| Yes | 42 (97.7%) |
| No | 1 (2.3%) |
| Stage at diagnosis | |
| Localized | 29 (67.4%) |
| Metastatic | 14 (32.6%) |
| MSKCC prognostic score (only for patients with metastasis; | |
| n = 29) | |
| Good | 1 (3.4%) |
| Intermediate | 12 (41.4%) |
| Poor | 13 (44.8%) |
| Unknown | 3 (10.4%) |
| Heng prognostic score (only for patients with metastasis; n = 29) | |
| Good | 1 (3.4%) |
| Intermediate | 11 (37.9%) |
| Poor | 14 (48.3%) |
| Unknown | 3 (10.4%) |
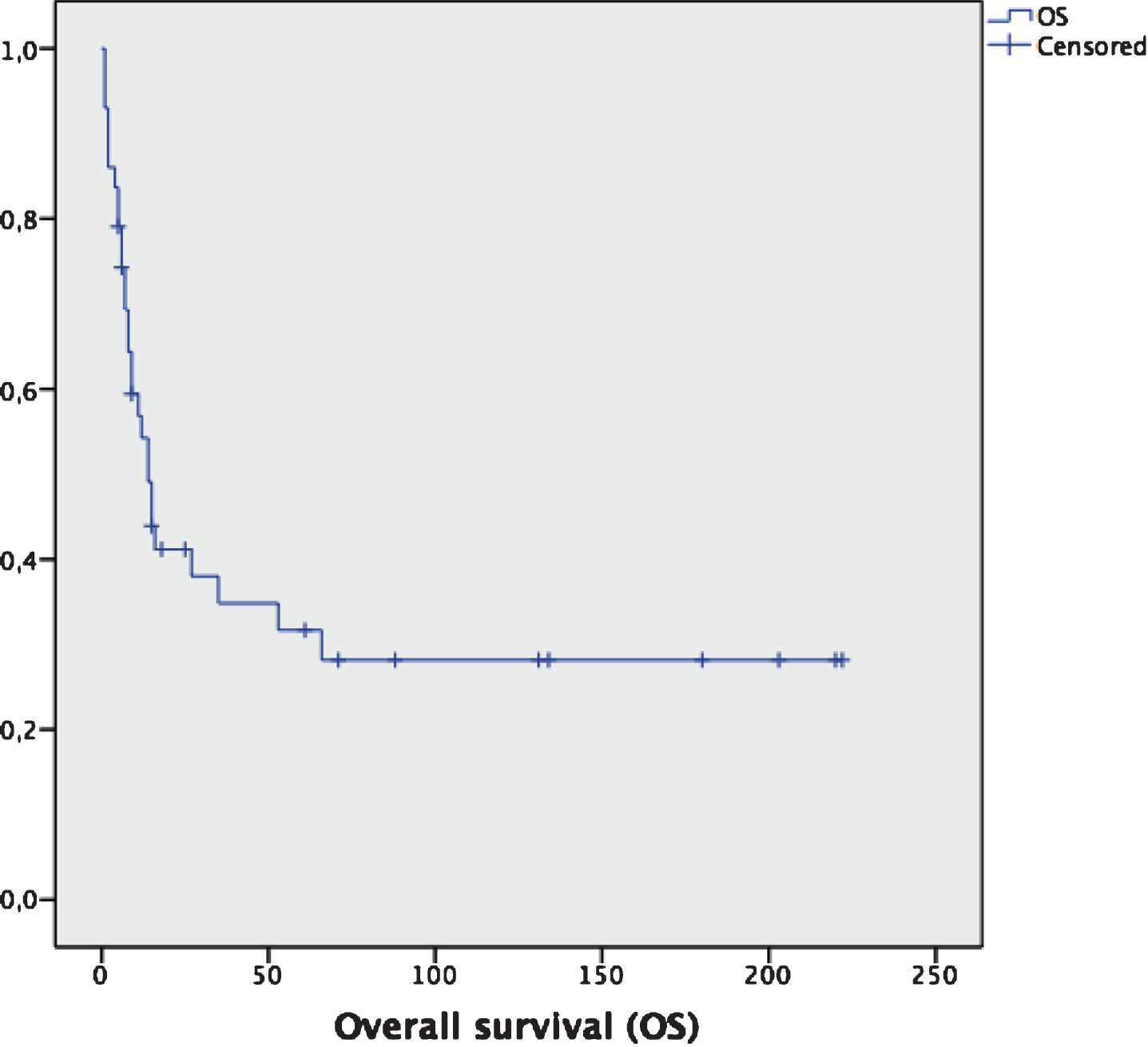
For the whole cohort of patients (n = 43), median OS is 14 months (95% CI: 9.2–18.8 months) (Fig. 1). For the subgroup of patients diagnosed without metastases (n = 29), 15 of them (51.7%) relapsed, with a median relapse-free survival (RFS) of 22 months (95% CI: 12.4–35.6 months). None of them received any adjuvant therapy after surgery. Median overall survival (OS) for this subgroup is 53 months (95% CI: 35.5–84.3 months).
Fig.1
Overall survival for the entire cohort (n = 43). Median OS: 14 months (95% CI: 9.2–18.8 months).
For the subgroup of patients with metastatic disease (n = 29), taking together patients with first diagnosis as a stage IV disease and patients that relapsed after previous surgery, median OS is 6 months (95% CI: 4.1–7.8 months), with a 2-year OS of 10.3% (3 out of 29 patients) (Fig. 2). Only 16 out of these 29 patients (55.2%) received any systemic therapy, mainly platinum-based chemotherapy, with a response rate of 12.5%, and a median duration of response of 6 months. Median OS for patients who began any systemic therapy for advanced disease were 6 months from the beginning of therapy, compared to 3 months for patients with advanced disease that received no systemic therapy; this difference was not statistically significant (p = 0.32). The type of systemic therapy and its outcomes are summarized in Table 2.
Fig.2
Overall survival (OS) of localized vs metastatic patients at diagnosis. Median OS: 53 months (localized) vs 6 months (metastatic).
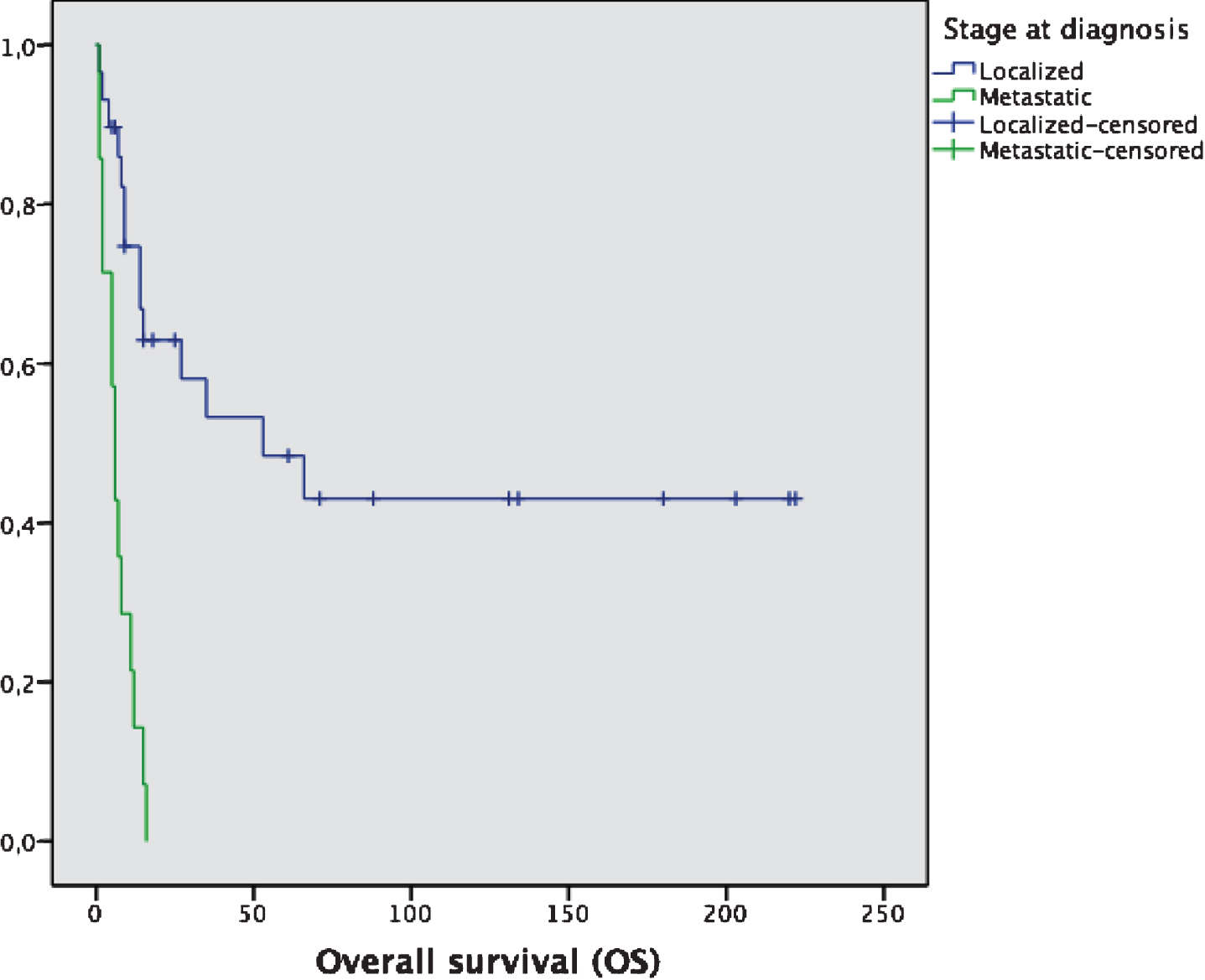
Table 2
Systemic therapy for CDC
| Line of treatment | Number of patients | Response rate | Median PFS |
| First-line therapy | 16/29 (55.2%) | 2 months | |
| Platinum-based chemotherapy | 13/16 (81.2%) | 12.5% | |
| Tyrosine-kinase inhibitors | 2/16 (12.5%) | 0% | |
| Cytokines | 1/16 (6.3%) | 0% | |
| Second-line therapy | 6/29 (20.7%) | 2 months | |
| Chemotherapy | 6/16 (37.5%) | 0% |
PFS: progression-free survival.
DISCUSSION
CDC is a very aggressive subtype of renal cell carcinoma, that is usually hard to identify; the main differential diagnosis are renal medullary carcinoma, fumarate-hydratase deficient renal cell carcinoma, urothelial malignancies of the upper urinary tract, type 2 papillary renal cancer, or even metastatic carcinoma with spread to the kidney (7, 8). There is an increasing effort in understanding the underlying molecular biology of CDC. One of the most relevant attempts to address this issue was published in 2016 (9); this study analysed 17 cases of CDC by comprehensive genomic profiling. The most common genomic alterations were found in NF2 (29% of cases), SETD2 (24%), SMARCB1 (18%) and CDKN2A (17%). Of 9 cases assessed for fumarate hydratase genomic alterations, 2 cases had fumarate hydratase homozygous loss. These findings could suggest a possible benefit from targeted therapy, particularly with mTOR inhibitors in patients with NF2 alterations. A similar study with 7 cases of CDC performed whole exome sequencing (WES) and transcriptome sequencing (RNAseq) (10), and confirmed a frequent loss (62.5%) of CDKN2A expression. In addition, SLC7A11 (a cisplatin resistance associated gene) was found to be overexpressed in 4 out of 5 cases (80%) of CDC, as compared to matched non-tumor tissue.
Finally, another study compared the transcriptomic profile of CDC with upper tract urothelial carcinoma (UTUC) and renal cell carcinoma (RCC) (11). It showed that CDC displays a unique transcriptomic profile, closer to that of other RCC subtypes than to UTUC. It also provides the first evidence that CDC is a metabolic disease, with a profound impairment in oxidoreductase activity, that leads to increased aerobic glycolysis and decreased AMPK gene expression. Another finding that could be of importance is that CDC expressed a highly enriched immune signature, with a high percentage of T-cell infiltrates that is even higher in metastatic tumors compared with non-metastatic tumors.
In our series, in contrast with what has been previously reported (12), a high proportion of patients (67.4%) were diagnosed as localized disease without symptoms; this may reflect the broader use of radiologic techniques and the incidental finding of renal masses that, in some cases, are ultimately resected and diagnosed as CDC. Our survival data are similar to the largest series published, which is the one by the National Cancer Institute (NCI), comparing the outcome of CDC with clear cell renal cell carcinoma (CCRCC) (13). In this database analysis, including 577 patients with CDC and 201.686 CCRCC from 2004 to 2013, the median OS of patients with CDC was 13.2 months (14 months in our group). OS for the metastatic cohort was 6.4 months (6 months in our group). Similar to our results, few patients received any systemic therapy in the metastatic setting (76/184, 41.3%; 55.2% in our group).
Another retrospective review from Korea included 35 patients (14), including all stages of the disease (localized as well as metastatic). Median OS for metastatic patients was 8.6 months, with improved results for patients that received any systemic therapy compared to those with no treatment at all (median OS: 18.4 vs 4.5 months). Several other retrospective studies have shown similar results, reflecting the aggressive course of this disease, with few responses to systemic therapy and short survival (15, 16). Nevertheless, in our group of patients we did not show such an improvement for patients who received systemic therapy, with poor outcomes regardless of having received it or not.
Given the infrequency of CDC, the optimal management of this malignancy remains unclear. There have been previous reports of platinum-based chemotherapy for CDC, with poor results. The phase II published in 2007 by Oudard and cols. (17) treated 23 patients with metastatic CDC with gemcitabine and a platinum salt (either cisplatin o carboplatin), for a response rate of 26% (1 complete response and 5 partial responses), median PFS of 7.1 months, and median OS of 10.2 months. This is to our knowledge the only prospective trial focused specifically on CDC testing the efficacy of systemic therapy.
A retrospective review from several Italian hospitals, published in 2014, showed similar outcomes when evaluating targeted therapies (18). With 13 patients included, median OS was 4 months, with a response rate of 23% (1 response with Temsirolimus, 1 with Sorafenib and 1 with Sunitinib). Another recent study published in 2019 (19) tested the efficacy of Cabozantinib in metastatic nonn-clear-cell renal cell carcinoma; amongst the 112 patients included, there were 4 cases of CDC, with a 50% response rate to Cabozantinib.
A phase II multicentre, single arm trial, also published in 2018, tested the combination of chemotherapy (Cisplatin plus Gemcitabine) with Sorafenib (20). A total of 26 patients were enrolled in the trial, showing an objective response rate of 30.8%, a median PFS of 8.8 months, and a median OS of 12.5 months. Therefore, even though the most common approach for systemic therapy is platinum-based chemotherapy, there may also be a role for targeted therapies in this setting. Nevertheless, even though there are some other case reports that could suggest some activity with targeted therapies (21–25), there are also other studies with no response to these agents (26–28).
Regarding immunotherapy, the expression of PD-L1 in CDC is not rare. A study published in 2014 showed PD-L1 expression in 20% of CDC tumor cells, raising to 100% PD-L1 expression in tumor-infiltrating mononuclear cells (TIMC). PD-L1 positivity in tumor cells was associated with higher tumor grade and stage, as well as shorter OS. PD-L1 in TIM showed only a trend to worse OS (29). These results could give a rational to explore immunotherapy in CDC, and there are some case reports with promising activity (30, 31).
There is an ongoing phase II clinical trial testing the combination of gemcitabine, a platinum salt and Bevacizumab for metastatic collecting duct carcinomas (GETUG-AFU 24, NCT02363751), and another one exploring the role of Cabozantinib as first line treatment (caBozantinib in cOllectiNg ductS Renal Cell cArcInoma – the BONSAI trial, NCT03354884). If these trials can recruit appropriately, we may have prospective data about the role of targeted therapies focused on antiangiogenesis in the upcoming years. There are no immunotherapy trials ongoing specifically for CDC; nevertheless, there is an ongoing study with Nivolumab for rare tumors in France, with a cohort for non-clear cell RCC that includes CDC (Secured Access to Nivolumab for Adult Patients With Selected Rare Cancer Types (AcSé), NCT03012581). There is also a phase IIIb study with Atezolizumab (NCT02928406) focused in urotehlial neoplasms that allows the inclusion of CDCs.
Clinical and molecular findings could suggest that, although chemotherapy is usually the preferred option for first-line therapy of advanced CDC, outcomes are poor. Genomic abnormalities found to be common in CDC frequently cause platinum-resistance, and lead to a profile more similar to RCC than UTUC. Therefore, higher interest in targeted therapies and immunotherapy for this neoplasm, and a thorough examination of underlying mechanisms of oncogenesis and disease progression, could be the way to improve the outcomes of patients with CDC.
CONCLUSIONS
CDC is a rare and aggressive subtype of RCC. It usually presents with symptoms and has a poor prognosis irrespective of the systemic therapies administered. Platinum-based chemotherapy has been the most common systemic treatment used in our series, with a response rate of 12.5% and median OS in the metastatic setting of 6 months. Due to these poor results, research about biology and new therapeutic options for this disease is necessary.
No relevant conflict of interest to disclose by any of the authors.
The study was approved by a Central Ethics Committee for Research.
CONFLICT OF INTEREST
The authors report no conflict of interest.
REFERENCES
[1] | Seo AN , Yoon G , Ro JY . Clinicopathologic and molecular pathology of collecting duct carcinoma and related renal cell carcinomas. Adv Anat Pathol. (2017) ;24: :65–77. |
[2] | May M , Ficarra V , Shariat SF , Zigeuner R , Chromecki T , Cindolo L , et al. Impact of clinical and histopathological parameters on disease specific survival in patients with collecting duct renal cell carcinoma: development of a disease specific risk model. J Urol. (2013) ;190: :458–63. |
[3] | Kanayama HO , Fukumori T , Fujimoto H , Nakanishi H , Ohyama C , Suzuki K , et al. Clinicopathological characteristics and oncological outcomes in patients with renal cell carcinoma registered in 2007: The first large-scale multicentre study from the Cancer Registration Committee of the Japanese Urological Association. Int J Urol. (2015) ;22: :S1–7. |
[4] | Diamond E , Molina AM , Carbonaro M , Akhtar NH , Giannakakou P , Tagawa ST , et al. Cytotoxic chemotherapy in the treatment of advanced renal cell carcinoma in the era of targeted therapy. Crit Rev Oncol Hematol. (2015) ;96: :518–26. |
[5] | David KA , Milowsky MI , Nanus DM . Chemotherapy for non-clear-cell renal cell carcinoma. Clin Genitourin Cancer. (2006) ;4: :263–8. |
[6] | Dason S , Allard C , Sheridan-Jonah A , Gill J , Jamshaid H , Aziz T , et al. Management of renal collecting duct carcinoma: a systematic review and the McMaster experience. Curr Oncol. (2013) ;20: :223–32. |
[7] | Ohe C , Smith SC , Sirohi D , Divatia M , de Peralta-Venturina M , Paner GP , et al. Reappraisal of morphologic differences between renal medullary carcinoma, collecting duct carcinoma, and fumarate hydratase-deficient renal cell carcinoma. Am J Surg Pathol. (2018) ;42: :279–92. |
[8] | Amin MB , Smith SC , Agaimy A , Argani P , Comperat EM , Delahunt B , et al. Collecting duct carcinoma versus renal medullary carcinoma: an appeal for nosologic and biological clarity. Am J Surg Pathol. (2014) ;38: :871–4. |
[9] | Pal SK , Choueiri TK , Wang K , Khaira D , Karam JA , Van Allen E , et al. Characterization of clinical cases of collecting duct carcinoma of the kidney assessed by comprehensive genomic profiling. Eur Urol. (2016) ;70: :516–21. |
[10] | Wang J , Papanicolau-Sengos A , Chintala S , Wei L , Liu B , Hu Q , et al. Collecting duct carcinoma of the kidney is associated with CDKN2A deletion and SLC family gene up-regulation. Oncotarget. (2016) ;7: :29901–15. |
[11] | Malouf GG , Comperat E , Yao H , Mouawad R , Lindner V , Rioux-Leclerq N , et al. Unique transcriptomic profile of collecting duct carcinomas relative to upper tract urothelial carcinomas and other kidney carcinomas. Sci Rep. (2016) ;6: :30988. |
[12] | Pepek JM , Johnstone PAS , Jani AB . Influence of demographic factors on outcome of collecting duct carcinoma: a Surveillance, Epidemiology, and End Results (SEER) database analysis. Clin Genitourin Cancer. (2009) ;7: :E24–E27. |
[13] | Sui W , Matulay JT , Robins DJ , James MB , Onyeji IC , Choudhury AR , et al. Collecting duct carcinoma of the kidney: disease characteristics and treatment outcomes from the National Cancer Database. Urol Oncol. (2017) ;35: :540.e13–540.e18. |
[14] | Kwon KA , Oh SY , Kim HY , Kim HS , Lee HY , Kim TM , et al. Clinical features and treatment of collecting duct carcinoma of the kidney from the Korean Cancer Study Group Genitourinary and Gynecologic Cancer Committee. Cancer Res Treat. (2014) ;46: :141–7. |
[15] | Tokuda N , Naito S , Matsuzaki O , Nagashima Y , Ozono S , Igarashi T . Collecting duct (Bellini duct) renal cell carcinoma: a nationwide survey in Japan. J Urol. (2006) ;176: :40–3. |
[16] | Karakiewicz PI , Trinh QD , Rioux-Leclercq N , de la Taille A , Novara G , Tostain J , et al. Collecting duct renal cell carcinoma: a matched analysis of 41 cases. Eur Urol. (2007) ;52: :1140–5. |
[17] | Oudard S , Banu E , Vieillefind A , Fournier L , Priou F , Medioni J , et al. Prospective multicenter phase II study of gemcitabine plus platinum salt for metastatic collecting duct carcinoma: results of a GETUG study. J Urol. (2007) ;177: :1698–702. |
[18] | Procopio G , Testa I , Iacovelli R , Grassi P , Verzoni E , Garanzini E , et al. Treatment of collecting duct carcinoma: current status and future perspectives. Anticancer Res. (2014) ;34: :1027–30. |
[19] | Martínez-Chanzá N , Xie W , Bilen MA , Dzimitrowicz H , Burkart J , Geynisman DM , et al. Cabozantinib in advanced non-clear-cell renal cell carcinoma: a multicentre, retrospective, cohort study. Lancet Oncol. (2019) ;20: :581–90. |
[20] | Sheng X , Cao D , Yuan J , Zhou F , Wei Q , Xie X , et al. Sorafenib in combination with gemcitabine plus cisplatin chemotherapy in metastatic renal collecting duct carcinoma: a prospective, multicentre, single-arm, phase 2 trial. Eur J Cancer. (2018) ;100: :1–7. |
[21] | Ansari J , Fatima A , Chaudhri S , Bahtt RI , Wallace M , James ND . Sorafenib induces therapeutic response in a patient with metastatic collecting duct carcinoma of the kidney. Onkologie. (2009) ;32: :44–6. |
[22] | Miyake H , Haraguchi T , Takenaka A , Fujisawa M . Metastatic collecting duct carcinoma of the kidney responded to sunitinib. Int J Clin Oncol. (2011) ;16: :153–5. |
[23] | Mennitto A , Verzoni E , Peverelli G , Alessi A , Procopio G . Management of metastatic collecting duct carcinoma: an encouraging result in a patient treated with Cabozantinib. Clin Genitourin Cancer. (2018) ;16: :e521–3. |
[24] | Yin M , Wang W , Rosenberg J , Kaag M , Joshi M , Holder S , et al. Targeted therapy in collecting duct carcinoma of the kidney: a case report and literature review. Clin Genitourin Cancer. (2016) ;14: :e203–6. |
[25] | Zhao RN , Lie NH , Gong R , Wang JZ , Wazir R , Liu LR , et al. Active targeted therapy for metastatic collecting duct carcinoma of the kidney: a case report and review of the literature. |
[26] | Soto-Delgado M , Pedrero-Márquez G , Arroyo-Maestre JM , Beardo-Villar P . Collecting duct carcinoma of the kidney: a contribution of 4 new cases. Arch Esp Urol. (2014) ;67: :14–7. |
[27] | Voss MH , Bastos DA , Karlo CA , Ajeti A , Hakimi AA , Feldman DR , et al. Treatment outcome for mTOR inhibitors for metastatic renal cell carcinoma with nonclear and sarcomatoid histologies. Ann Oncol. (2014) ;25: :663–8. |
[28] | Tannir NM , Plimack E , Ng C , Tamboli P , Bekele NB , Xiao L , et al. A phase 2 trial of sunitinib in patients with advanced non-clear-cell renal cell carcinoma. Eur Urol. (2012) ;62: :1013–9. |
[29] | Choueiri TK , Fay AP , Gray KP , Callea M , Ho TH , Albiges L , et al. PD-L1 expression in nonclear-cell renal cell carcinoma. Ann Oncol. (2014) ;25: :2178–84. |
[30] | Mizutani K , Horie K , Nagai S , Tsuchiya T , Saigo C , Kobayashi K , et al. Response to Nivolumab in metastatic collecting duct carcinoma expressing PD-L1: a case report. Mol Clin Oncol. (2017) ;9: :988–90. |
[31] | Rimar KJ , Meeks JJ , Kuzel TM . Anti-programmed death receptor 1 blockade induces clinical response in a patient with metastatic collecting duct carcinoma. Clin Genitourin Cancer. (2016) ;14: :e431–4. |




