Targeted Therapies Following First-Line Immune Checkpoint Inhibitor Combination in Metastatic Renal Cell Carcinoma: A Single Center Experience
Abstract
Background:
Both late and early phase immune checkpoint inhibitor (CPI) combination trials indicate an impending role of combinations in the first-line treatment of metastatic renal cell carcinoma (mRCC). Sequencing the options following failure of CPI combinations is an emerging conundrum.
Objective:
To present our single-center clinical experience with targeted therapies (TT) following first-line CPI combinations.
Methods:
mRCC patients who received TT following failure of a combination regimen with CPI were identified from an institutional database. Clinical information including tumor characteristics, survival outcomes, and adverse events was retrieved from medical records. Descriptive statistics and Kaplan-Meier survival functions were performed.
Results:
Of 11 patients identified, median age was 63 (31–79) and 8 (73%) patients were male. First-line treatment was a CPI and TT combination in 7 (64%) patients while the rest received combination of two CPIs. The majority of patients (82%) were intermediate risk category at the initiation of targeted therapies. TTs utilized included cabozantinib (46%), lenvatinib and everolimus (27%), sunitinib (18%), and temsirolimus (9%). Best response was stable disease for 10 (91%) and partial response for 1 (9%) patient. In a median follow up of 9.1 months (range, 4.9–34.1), median progression free survival was 7.7 (95% CI 4.6–10.8) months. Progression has occurred in 7 patients, and 3 patients remain on treatment. One patient discontinued treatment due to toxicity.
Conclusions:
In our report, TTs demonstrate effective disease control and safety. Further exploration in prospective setting is warranted.
INTRODUCTION
Renal cell carcinoma is the seventh most common solid cancer type worldwide. Approximately 15000 cancer related deaths occur due to renal cell carcinoma each year [1]. Within the last 15 years, dynamic advances in cancer research led to an expansion of the treatment armamentarium for metastatic renal cell carcinoma (mRCC) [2]. Previously, the only evidence based treatment approach was conventional immunotherapy that included interleukin-2 and interferon alfa. Immune activation was effective in a small subset of patients [3]. Targeted therapies (TT) acting via vascular endothelial growth factor (VEGF) or mammalian target of rapamycin (mTOR) pathway blockade were first introduced, with VEGF-inhibitors doubling the progression free survival (PFS) over existing standards [4].
Recently, immunotherapy has been established as a standard in this disease [2]. Nivolumab, a programmed death-1 (PD-1) inhibitor, was the first CPI with activity in second-line treatment following TTs [5]. Perhaps the most groundbreaking advance was the results of the CheckMate214 study where the efficacy of nivolumab and ipilimumab was compared to sunitinib in first-line treatment of intermediate and poor risk patients. The combination demonstrated an absolute overall survival (OS) benefit over sunitinib [6]. Furthermore, patient reported outcomes were recently published confirming the better tolerability of the combination CPI over sunitinib [7].
Efficacy of the two different strategies led to the investigation of combined approaches in order to foster efficacy and tolerability. To date, two phase 3 studies were published comparing CPI and TT combination with sunitinib in first-line setting. Firstly, the Immotion151 study met the primary endpoint of PFS with combination of bevacizumab, a VEGF receptor inhibitor and atezolizumab, a programmed cell death-ligand 1 (PD-L1) directed checkpoint inhibitor (CPI), over sunitinib [8]. Subsequently, in the Javelin Renal 101 study, axitinib, a VEGF-TKI and avelumab, a PD-L1 directed CPI combination demonstrated PFS benefit over sunitinib across patients from all risk categories [9]. More recently, the results of Keynote-426 trial were positive, with the PD-1 inhibitor pembrolizumab and axitinib in combination showing a superior PFS, OS and response rate compared to sunitinib [10]. Several other early phase studies employing the combined CPI and TT approach have shown promise [11–13].
Considering the efficacy of combined approaches, another shift in treatment algorithm for mRCC is anticipated. However, the sequencing strategies after failure of first-line combinations have not been examined prospectively. Therefore, the question regarding how to juxtapose several available treatment options has been emerging in clinical practice. We conducted a retrospective analysis to examine outcomes with second-line targeted therapies following treatment failure with a combination of two CPIs or a CPI and TT in patients with mRCC.
MATERIALS AND METHODS
Patient selection and data collection
Patients who received first-line combination treatment with immune checkpoint inhibitor for treatment of metastatic renal cell carcinoma at the City of Hope Comprehensive Cancer Center Medical Oncology department between April 2014 and January 2019 were retrospectively identified using an institutional database. Those who were treated with a second-line TT following the CPI combination were included in the final analysis. Patient characteristics in first and second-line treatment initiation, response to treatment, survival and adverse event information were retrieved from the medical records.
This retrospective study was approved by the Institutional Review Board of the City of Hope Comprehensive Cancer Center. Confidentiality of study subjects was protected in compliance with the regulations of Health Insurance Portability and Accountability Act.
Statistical analyses
The International mRCC Database Consortium (IMDC) model was utilized for first and second-line prognostic risk calculation and categorization of patients. Response to treatment was determined according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
Median, range and standard deviation were calculated using descriptive statistics. PFS and OS with 95% confidence interval were evaluated by Kaplan-Meier survival function. All analyses were performed using SPSS software 23 (IBM SPSS Statistics for Mac, version 23.0, Armonk, NY: IBM Corp).
RESULTS
Patient characteristics in first-line
In total, 11 patients received second-line TT following first-line CPI combination. Median age at the time of the first-line treatment initiation was 63 (range, 31–79), and 73% of participants were male. The majority of patients, 7 (64%), were in an intermediate prognostic risk category while there were two patients in favorable and poor prognostic groups each. First-line treatment was CPI and TT combination in 7 patients; CPI and CPI combination in four patients. The entire cohort received first-line CPI combination by means of clinical trial enrollment. First-line CPI resulted in disease control in 6 (54%) patients in contrast to the 5 (46%) patients whose best response was progressive disease. Median duration of CPI combination was 2.76 months (range, 5.0–49.9). Two patients discontinued first-line treatment due to immune related toxicities (nephropathy and myasthenia gravis, respectively). The remaining 9 patients had progression of their disease that resulted in transition to second-line targeted treatment (Table 1).
Table 1
Patient and tumor characteristics during first-line treatment with checkpoint inhibitor combination
| N = 11 (%) | |
| Age, median (range) | 63 (31–79) |
| Gender | |
| Female | 3 (27%) |
| Male | 8 (73%) |
| Stage at the time of diagnosis | |
| Stage I–III | 4 (36%) |
| Stage IV | 7 (64%) |
| Nephrectomy | |
| Yes | 9 (82%) |
| First-line prognostic group (IMDC) | |
| Favorable | 2 (18%) |
| Intermediate | 7 (64%) |
| Poor | 2 (18%) |
| First-line treatment | |
| CPI + TT | 7 (64%) |
| CPI + CPI | 4 (36%) |
| Best response to first-line treatment | |
| Partial response | 2 (18%) |
| Stable disease | 4 (36%) |
| Progressive disease | 5 (46%) |
| Duration of treatment with first-line CPI treatment | |
| Median (range) (in months) | 2.8 (1.3–25.9) |
| Reason to stop first-line treatment | |
| Progression | 9 (82%) |
| Toxicity | 2 (18%) |
| CPI: Checkpoint inhibitor, TT: Targeted therapy |
Patient characteristics in second-line
At the time of second-line targeted treatment initiation, 9 (82%) patients were in an intermediate risk category and there was one patient in favorable and poor risk categories each. The median interval between first-line treatment discontinuation and second-line initiation was 23 days (range, 0–80 days). Five patients received cabozantinib, 3 patients received lenvatinib and everolimus combination, 2 patients received sunitinib and 1 patient received temsirolimus. Most frequent metastatic sites at the second-line treatment initiation were lung, lymph node, bone and soft tissue, present in 9 (82%), 6 (55%), 3 (27%) and 3 (27%) patients, respectively (Table 2).
Table 2
Patient and tumor characteristics during second-line targeted therapy
| N = 11 (%) | |
| Second-line prognostic group (IMDC) | |
| Favorable | 1 (9%) |
| Intermediate | 9 (82%) |
| Poor | 1 (9%) |
| Second-line treatment | |
| Cabozantinib | 5 (46%) |
| Lenvatinib + everolimus | 3 (27%) |
| Sunitinib | 2 (18%) |
| Temsirolimus | 1 (9%) |
| Site of metastasis, second-line | |
| Lung | 9 (82%) |
| Lymph node | 6 (55%) |
| Bone | 3 (27%) |
| Soft tissue | 3 (27%) |
| Liver | 1 (9%) |
| Pleura | 1 (9%) |
| Pancreas | 1 (9%) |
| Peritoneum | 1 (9%) |
| Skin | 1 (9%) |
| Adrenal | 1 (9%) |
Clinical outcome
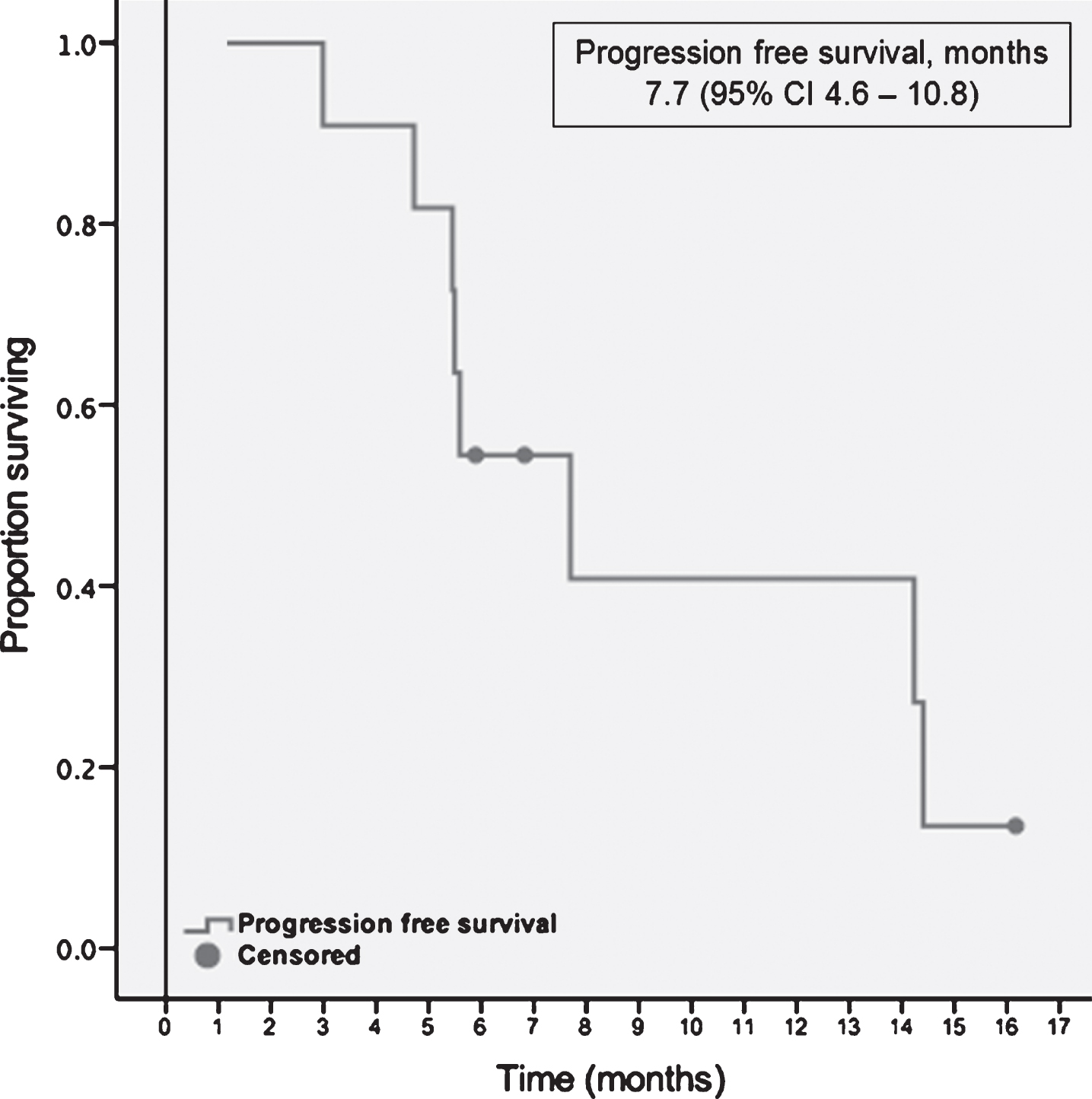
Starting with the initiation of the first-line TT, median follow-up was 9.1 months (range, 4.9–34.1). At the time point of the data cut off, each patient had at least one response evaluation. Median time to treatment response was 2.6 months, ranging between 1.2 and 4.2 months. The majority of the population, 10 (91%) had stable disease as their best response to treatment. One patient demonstrated partial response 7 weeks after initiation of cabozantinib. No complete responses were observed within the cohort. At the data cut off three patients were still on second-line TT. The remaining 8 (73%) patients discontinued the treatment; seven due to progression of the disease and one due to toxicity (Grade 3 mucositis and fatigue). Median PFS with the second-line targeted therapies was 7.7 months (95% CI, 4.6–10.8) (Table 3). A swimmer plot of the included patients and PFS function can be appreciated in Figs. 1 and 2 respectively.
Table 3
Response to second-line targeted therapy and survival
| N = 11 (%) | |
| Best response to second-line treatment | |
| Response evaluable | 11 (100%) |
| Stable disease | 10 (91%) |
| Partial response | 1 (9%) |
| Complete response | 0 |
| Progressive disease | 0 |
| Objective response rate | 9% |
| Disease control rate | 100% |
| Progression free survival, months | 7.7 (95% CI 4.6–10.8) |
| Second-line treatment stopped | 8 (73%) |
| Reason to stop second treatment | |
| Progression | 7 (88%) |
| Toxicity | 1 (12%) |
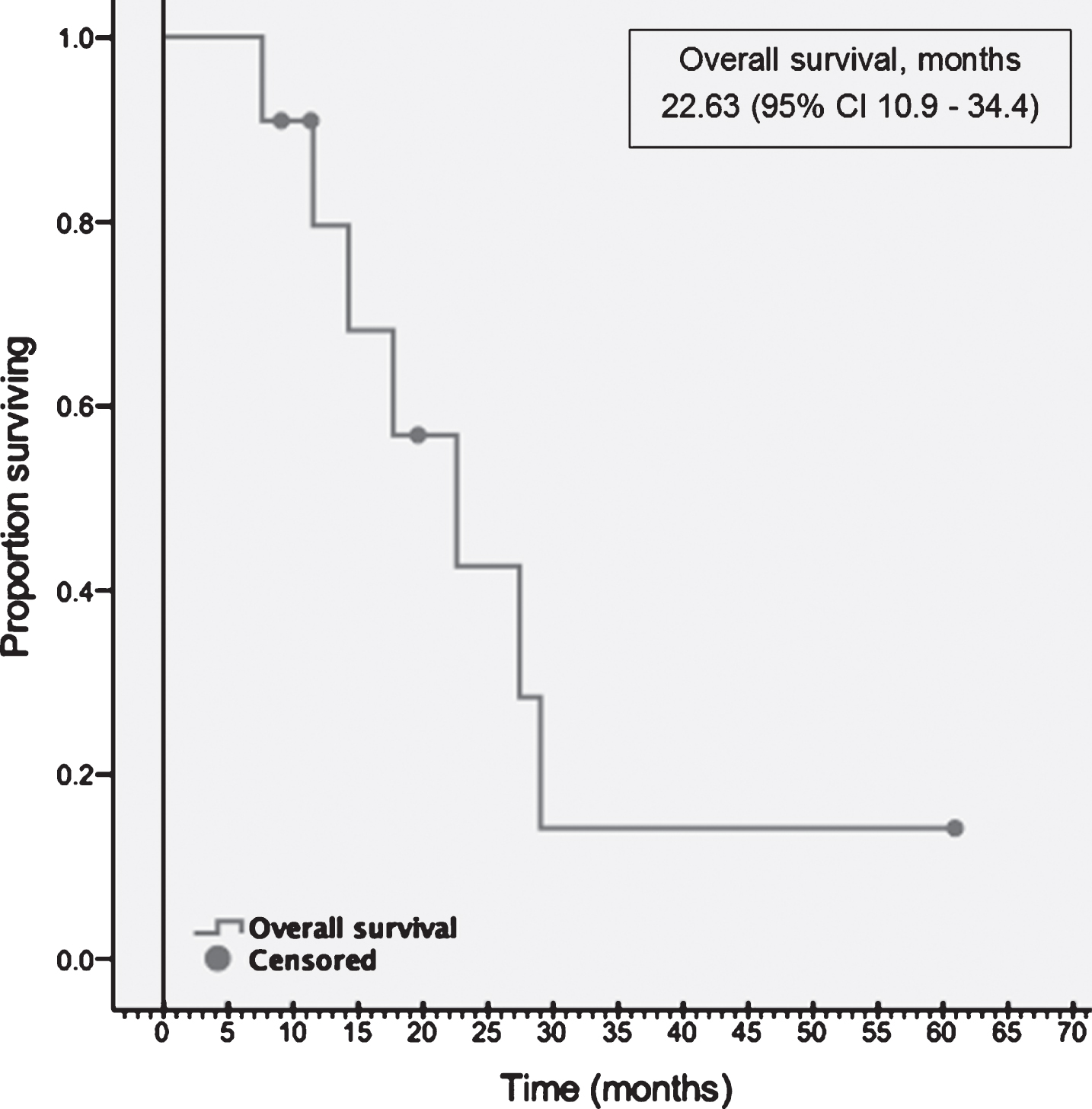
| Overall Survival, months | 22.7 (95% CI 10.9–34.3) |
Fig.1
Swimmers plot for the response evaluable patients.
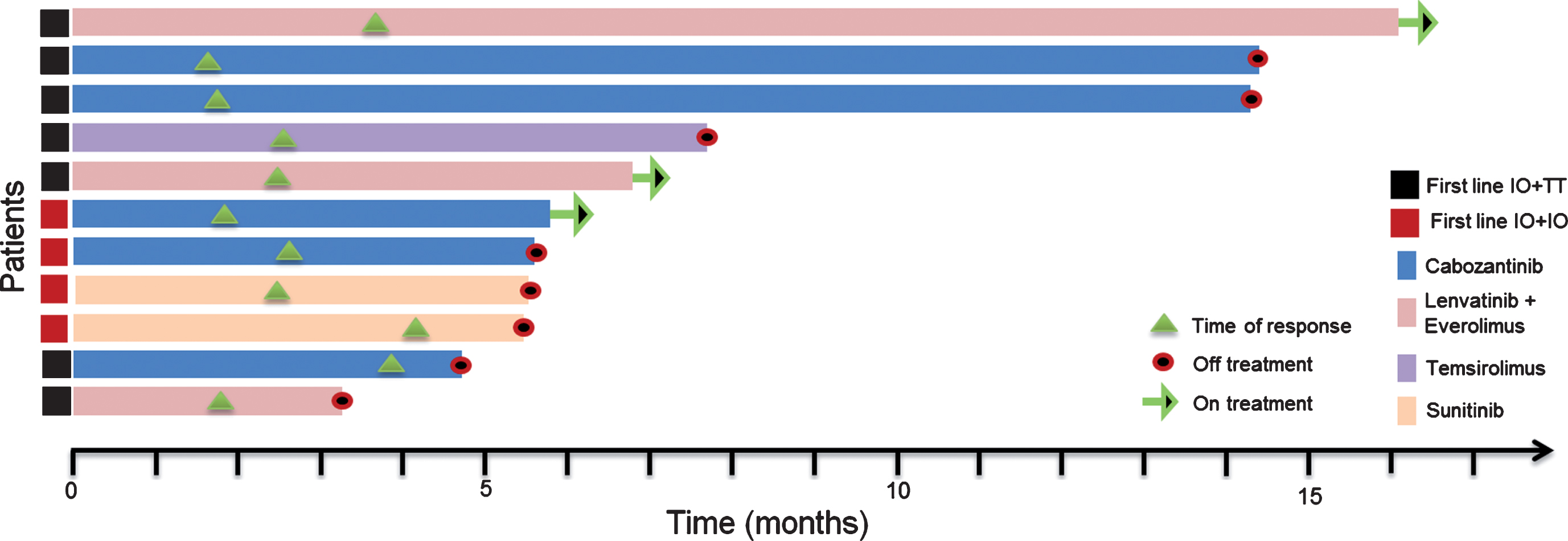
Fig.2
Progression free survival with second-line targeted therapies.
Following second-line TT, 4 patients received one more line of subsequent TT. One patient received one line of TT and one line of CPI subsequently. At the data cut off, 4 (36%) patients were alive, and OS was 22.6 months (10.9–34.4) (Fig. 3).
Fig.3
Overall survival.
Adverse events
During the second-line treatment with targeted therapies, 6 (54%) of patients experienced adverse events. The most commonly observed adverse events were fatigue, stomatitis, hand food syndrome, and diarrhea. Three (27%) patients had grade 3 adverse events. Grade 3 adverse events in two patients were managed with dose reduction but not resulted in treatment discontinuation. One patient experienced grade 3 mucositis and fatigue with the lenvatinib and everolimus combination that resulted in treatment discontinuation (Table 4).
Table 4
Side effect profile of targeted therapies in second-line setting
| Total N (%) | Grade 1-2 N (%) | Grade 3-4 N (%) | |
| All | 6 (54%) | 5 (45%) | 3 (27%) |
| Fatigue | 5 (45%) | 5 (45%) | 1 (9%) |
| Stomatitis | 3 (27%) | 2 (18%) | 1 (9%) |
| Hand foot syndrome | 2 (18%) | 1 (9%) | 1 (9%) |
| Diarrhea | 2 (18%) | 2 (18%) | – |
| Nausea - vomiting | 1 (9%) | 1 (9%) | – |
| Cytopenia | 1 (9%) | – | 1 (9%) |
| Hypertension | 1 (9%) | – | 1 (9%) |
DISCUSSION
Our results demonstrate that mRCC patients benefited from TT at the second-line setting after exposure to a CPI in combination with a CPI or a TT; specifically, disease control was achieved in the entire cohort. However, the overall response rate was 9%. Despite the high disease control rate, responses were not durable and disease progression had eventually occurred resulting in a PFS of 7.7 months this appears similar to the PFS in second-line historical trials of TTs used in our dataset [14–16]. The safety profile was overall similar to previous studies with targeted therapies. Unexpected toxicities were not encountered. The majority of the adverse events were manageable and only one patient required treatment discontinuation due to toxicity.
To date, a number of reports aiming to examine the effect of second-line targeted therapies after CPIs have been presented or published. Two included patients who received CPI and TT combination in first-line [17, 18]. Barata et al. reported outcomes of 41 patients who progressed on nivolumab/ipilimumab, axitinib/avelumab or bevacizumab/atezolizumab [17]. First subsequent TT was cabozantinib, axitinib, pazopanib or sunitinib. Objective response rate was 29%, and disease control rate was 83%. Median PFS was 6.4 months. No difference in PFS was seen in patients received first-line dual CPI versus VEGF-TKI plus CPI. Characteristics and survival outcomes were comparable to the present study.
A similar analysis was conducted at the MD Anderson Cancer Center. Shah et al. reported their experience with VEGF-TKIs following progression on nivolumab alone or in combination with ipilimumab or bevacizumab [18]. In total, 43 patients who were treated with pazopanib, axitinib or cabozantinib were included. Objective response rate was 43% with one complete response. The rest of the cohort responded to treatment with stable disease as their best response, resulting in a disease control rate of 100%. Median PFS was 10 months, which is the longest among other studies reported to date.
Outcomes with second-line VEGF-TKIs after nivolumab/ipilimumab failure in CheckMate214 was more recently published, using data from 12 participating institutions [19]. VEGF-TKIs used included sunitinib, axitinib, pazopanib or cabozantinib. Objective response rate was 36%, and stable disease was the best response in 39% of patients. Median PFS was 8 months. PFS did not differ for first generation versus second generation TKIs (namely, sunitinib and pazopanib versus axitinib and cabozantinib).
Few studies have investigated the role of individual targeted therapies in the post-CPI setting. Ornstein et al. prospectively studied axitinib in an individualized dose titration schema and reported favorable tolerability and anti-tumor activity with an ORR of 38.7% and median PFS of 9.2 months [20]. A retrospective analysis of 69 patients who received cabozantinib at second or further lines following progression on a CPI-containing regimen yielded evidence of the safety and activity of cabozantinib [21].
In comparison to the other cited experiences, our study has a lower response rate, but the clinical benefit rate remains at 100%. Differing outcomes from these studies could be secondary to biologic characteristics. Recent data indicates that patients bearing PBRM1 alteration, for instance, may have particular sensitivity to CPI [22]. In contrast, mutations in TSC1/2 and MTOR have been well documented to confer sensitivity to mTOR inhibitors [23]. More recently, molecular signatures have been identified in the Immotion150 trial, for instance, an angiogenic signature was deciphered that identified patients with selective benefit from angiogenesis inhibition as opposed to CPI therapy [24]. Although the effect of genomic characteristics on treatment response has been exclusively studied in the front line setting, a similar association can be anticipated throughout the disease course. In these relatively small cohorts, biologic differences could drive variations in clinical outcome.
Several limitations to this report should be acknowledged. The study was retrospective with a small sample size. Furthermore, the limited follow-up may challenge our estimates of PFS and potentially underestimate latent responses. Despite these limitations, the clinical relevance of the question encouraged us to present our experience.
In conclusion, our data indicates that second-line targeted therapies in patients who progressed on CPI combinations appear to be safe and efficacious. Varying response rates and PFS estimates between existing studies can also be attributed to heterogeneous treatment types used. In an effort to address these important questions, prospective studies with larger patient cohorts are needed.
DISCLOSURES
Authors have no conflict of interest to report.
ACKNOWLEDGMENTS
None
REFERENCES
[1] | Siegel RL , Miller KD , Jemal A . Cancer statistics, 2019. CA: A Cancer Journal for Clinicians. (2015) . |
[2] | Lalani AA , et al. Systemic treatment of metastatic clear cell renal cell carcinoma in 2018: Current paradigms, use of immunotherapy, and future directions. Eur Urol. (2019) ;75: (1):10010. |
[3] | Cohen HT , McGovern FJ . Renal-cell carcinoma. New England Journal of Medicine. (2005) ;353: (23):2477–90. |
[4] | Choueiri TK , Motzer RJ . Systemic therapy for metastatic renal-cell carcinoma. New England Journal of Medicine. (2017) ;376: (4):354–66. |
[5] | Motzer RJ , et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. New England Journal of Medicine. (2015) ;373: (19):1803–13. |
[6] | Motzer RJ , et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. New England Journal of Medicine. (2018) ;378: (14):1277–90. |
[7] | Cella D , et al. Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): A randomised, phase 3 trial. The Lancet Oncology.(2019) . |
[8] | Motzer RJ , et al. IMmotion A randomized phase III study of atezolizumab plus bevacizumab vs sunitinib in untreated metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology. (2018) ;36: (6_suppl):578–8. |
[9] | Motzer RJ , et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. New England Journal of Medicine. (2019) ;380: (12):1103–15. |
[10] | Rini BI , et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. New England Journal of Medicine. (2019) ;380: (12):1116–27. |
[11] | Escudier B , et al. Tivozanib combined with nivolumab: Phase Ib/II study in metastatic renal cell carcinoma (mRCC). Journal of Clinical Oncology. (2018) ;36: (6_suppl):618–8. |
[12] | Nadal RM , et al. Results of phase I plus expansion cohorts of cabozantinib (Cabo) plus nivolumab (Nivo) and CaboNivo plus ipilimumab (Ipi) in patients (pts) with with metastatic urothelial carcinoma (mUC) and other genitourinary (GU) malignancies. Journal of Clinical Oncology. (2018) ;36: (6_suppl):515–5. |
[13] | Lee C-H , et al. Lenvatinib+pembrolizumab in patients with renal cell carcinoma: Updated results. Journal of Clinical Oncology. (2018) ;36: (15_suppl):4560–60. |
[14] | Choueiri TK , et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. New England Journal of Medicine. (2015) ;373: (19):1814–23. |
[15] | Motzer RJ , et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open-label, multicentre trial. Lancet Oncol. (2015) ;16: (15):1473–82. |
[16] | Hutson TE , et al. Randomized phase III trial of temsirolimus versus sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology. (2014) ;32: (8):760–7. |
[17] | Barata PC , et al. Clinical outcome of patients (Pts) with metastatic renal cell carcinoma (mRCC) progressing on front-line immune-oncology based combination (IO-COMBO) regimens. Journal of Clinical Oncology. (2018) ;36: (6_suppl):613–613. |
[18] | Shah AY , et al. Outcomes of patients (pts) with metastatic clear-cell renal cell carcinoma (mCCRCC) treated with second-line (2L) vascular endothelial growth factor receptor tyrosine kinase inhibitors (VEGFR-TKI) after first-line (1L) immune checkpoint inhibitors (ICI). Journal of Clinical Oncology. (2018) ;36: (6_suppl):682–2. |
[19] | Auvray M , et al. Second-line targeted therapies after nivolumab-ipilimumab failure in metastatic renal cell carcinoma. European Journal of Cancer. (2019) ;108: :33–40. |
[20] | Ornstein MC , et al. Prospective phase II multi-center study of individualized axitinib (Axi) titration for metastatic renal cell carcinoma (mRCC) after treatment with PD-1 / PD-L1 inhibitors. Journal of Clinical Oncology. (2018) ;36: (15_suppl):4517–17. |
[21] | McGregor B , et al. 879P Activity of cabozantinib (cabo) after PD-1/PD-L1 immune checkpoint blockade (ICB) in metastatic clear cell renal cell carcinoma (mccRCC). Annals of Oncology. (2018) ;29: (suppl_8):mdy283. 088. |
[22] | Miao D , et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science. (2018) ;359: (6377):801–6. |
[23] | Kwiatkowski DJ , et al. Mutations in TSC1, TSC2, and MTOR are associated with response to rapalogs in patients with metastatic renal cell carcinoma. Clin Cancer Res. (2016) ;22: (10):2445–52. |
[24] | McDermott DF , et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nature Medicine. (2018) ;24: (6):749–57. |




