Meta analysis of the second course of radiotherapy for recurrent esophageal cancer1
Abstract
BACKGROUND:
How to improve efficacy and reduce side effects in treating recurrent esophageal cancer by applying the second course of radiotherapy alone and its combination with chemotherapy has been attracting broad research interest.
OBJECTIVE:
This review paper aims to systematically evaluate efficacy and side effects of applying the second course of anterograde radiotherapy alone and its combination with chemotherapy in treating recurrent esophageal cancer.
METHODS:
First, the relevant research papers are retrieved from PubMed, CNKI and Wanfang databases. Next, Redman 5.3 software is used to calculate the relative risk and 95% confidence interval to evaluate the efficacy and adverse reactions of applying the single-stage radiotherapy with and without combining single/multi dose chemotherapy to treat recurrent esophageal cancer. Then, a meta data analysis is applied to examine the effectiveness and side effects of radiation alone and re-course radiotherapy plus chemotherapy in treating esophageal cancer recurrence after the first radiotherapy.
RESULTS:
Fifteen papers are retrieved, which included 956 patients. Among them, 476 patients received radiotherapy combined with single drug/multi drug chemotherapy (observation) and others received only radiotherapy (control). Data analysis results show that the incidence of radiation induced lung injury and bone marrow suppression is high in the observation group. Subgroup analysis also shows the higher effective rate or one-year overall survival rate of patients treated with the second course radiotherapy combined with single drug chemotherapy.
CONCLUSION:
The meta-analysis result demonstrates that combining the second course of radiotherapy with single-drug chemotherapy has advantages in treating recurrent esophageal cancer with the manageable side effects. However, due to insufficient data, it is not possible to conduct the further subgroup analysis comparing the side effects of restorative radiation with the combined chemotherapy using between a single drug and multiple drugs.
1Introduction
Esophageal cancer refers to malignant tumor of esophageal mucosal epithelial cells from hypopharynx to junction of esophagus and stomach, which mainly includes two histological types: squamous cell carcinoma (SCC) and adenocarcinoma (AC) [1–3]. The length of esophagus is generally 25∼30 cm. In 2002, union for international cancer control (UICC) divided esophagus into cervical segment, upper thoracic segment, middle thoracic segment, and lower thoracic segment according to anatomical structure of esophagus. The wall thickness of esophageal tube is about 0.3∼0.5 cm [4, 5]. Because the esophagus itself has no serosa layer, esophageal cancer is easy to invade and infiltrate outside tube wall, resulting in perforation, esophageal trachea, and other complications. Every year, there are about 450000 new cases of esophageal cancer worldwide, with 400000 deaths, half of which are Chinese patients.
There are 277000 new cases and 206000 deaths in China every year, which has caused serious harm to health of our people [6]. Patients with advanced esophageal cancer account for more than 70% in China, and most of them are treated with definitive chemoradiotherapy (DCRT). Due to complexity of surrounding anatomical structure, high difficulty of surgery and more postoperative complications, DCRT is also often selected for cervical and upper thoracic esophageal cancer [7]. With continuous emergence and progress of new technologies such as intensity modulated radiation therapy (IMRT), 5-year survival rate of esophageal cancer patients treated with radical radiotherapy in China has increased from 5% in era of two-dimensional conventional radiation therapy (2DCRT) to 15% –20% now [8].
With huge number of patients with radical radiotherapy and continuous improvement of survival rate, more and more patients have local recurrence, it is reported that 65% of esophageal cancer recurred in irradiation field after radiotherapy [9–11]. The recurrence site is mostly in the primary lesion area. The recurrence may be caused by necrosis and hypoxia of central tissue of tumor, resulting in decline of radiosensitivity. Once recurrence occurs, 5-year survival rate of esophageal cancer patients will drop to 0% –11%, and most patients with recurrent esophageal cancer who have not been systematically treated will die within one year. Salvage surgery is often recommended for patients with recurrence after radiotherapy, but only 4–29% of patients can really be treated by surgery. Radiotherapy is an important local treatment for advanced esophageal cancer. The development of radiation technology has improved consistency and uniformity of radiation field, adjusted dose according to shape of tumor, reduced radiation dose of normal tissue, and adverse reactions are mild. Radiation esophagitis is most common adverse reaction of radiotherapy in process of esophageal radiotherapy, but general symptoms are light, no special treatment is required, and more serious complications, such as bleeding, perforation, radiation spinal cord injury and so on, are rare. Chemotherapy is a systemic treatment and can also be used as a radiosensitizer [12–14]. It can kill subclinical lesions and systemic micro metastasis while improving local tumor control.
Before starting to study related issues, this paper collected a lot of information about esophageal cancer and chemotherapy related knowledge to solve how to improve the side effects of esophageal cancer after chemotherapy. This paper systematically evaluates efficacy and side effects of the second course radiotherapy alone and the second course of radiotherapy combined with chemotherapy in treatment of recurrence of esophageal cancer after radiotherapy, to provide evidence, support for clinical practice of the second course of radiotherapy of esophageal cancer.
Additionally, to find a solution to the issue of how to mitigate the unfavorable effects of chemotherapy on patients afflicted with esophageal cancer, the researchers behind this study amassed a substantial quantity of information relating to both that disease and to chemotherapy. The purpose of this paper is to conduct a comprehensive analysis of the efficacy and side effects of radiotherapy alone as well as re-course radiotherapy combined with chemotherapy in the treatment of recurrence of esophageal cancer following initial re-course radiotherapy. The goal of this analysis is to provide evidence support for the clinical practice of re-course radiotherapy of esophageal cancer.
This study gathered a lot of information on esophageal cancer and chemotherapy to discover a way to reduce its side effects. This research examines the effectiveness and side effects of radiation alone and re-course radiotherapy plus chemotherapy in treating esophageal cancer recurrence after first radiotherapy. This study supports esophageal cancer re-course radiation.
This meta-analysis reveals that repeated radiation with single-drug chemotherapy may cure recurrent esophageal cancer after radiotherapy with acceptable adverse effects. Data limits prevent subgroup analysis of restorative radiation with single-drug chemotherapy and recourse radiotherapy with multi-drug chemotherapy adverse effects. Carefully analyze and validate this study’s results. Finally, radiation and chemotherapy work for recurrent esophageal cancer.
2Literature review
It has been determined that the systemic character of the illness at the time of presentation is to blame for the ineffectiveness of surgery alone [26, 27]. In addition to surgical resection, early and highly effective systemic chemotherapy as well as local radiation that is focused on micro-metastases might contribute to enhanced survival rates. There have been a lot of clinical investigations that have assessed the role of adjuvant treatment, both preoperatively and postoperatively, and the findings have been inconsistent. Chemotherapy and radiation therapy are the standard treatments for patients diagnosed with cervical esophageal cancer. This is done either before or after surgery, with the goal of preserving the larynx and avoiding the need for a laryngo-esophagectomy. Although most of the research has been conducted on squamous cell carcinomas, some of the studies have also included adenocarcinomas. However, this guideline report does not differentiate between the two histological subtypes because previous research has not consistently found that they react differently to chemotherapy or radiation [28–36]. This study gathered a lot of information on esophageal cancer and chemotherapy to discover a way to reduce its side effects. This research examines the effectiveness and side effects of radiation alone and re-course radiotherapy plus chemotherapy in treating esophageal cancer recurrence after first radiotherapy. This study supports esophageal cancer re-course radiation.
A problematic clinical situation is one in which the cancer of the oesophagus returns after receiving aggressive treatment. Re-irradiation is one of the effective salvage therapy alternatives that are needed since there is a need for these choices. A meta-analysis of recurring re-course radiation after radical radiotherapy for the treatment of esophageal cancer may give insights into the efficacy and safety of this strategy in comparison to alternative choices for salvage treatment.
The patient population, the initial radiotherapy treatment modality and dose, the timing of recurrence, the re-irradiation dose and fractionation, concurrent chemotherapy use, and the outcomes of interest such as survival, local control, and toxicity are some of the relevant factors that could be included in such a meta-analysis. Other relevant factors that could be included are toxicity, survival, and local control of cancer.
According to the most recent research in the field, re-irradiation after radical radiotherapy for recurring esophageal cancer has the potential to produce satisfactory results while maintaining an acceptable level of risk. However, the selection of patients and the planning of treatments continue to be essential components.
In general, a meta-analysis on this subject might give useful information that could be used to influence clinical decision-making, treatment recommendations, and indicate areas that need more investigation.
3Data and methods
3.1Literature search and screening
Search PubMed, CNKI and Wanfang databases, and search time is from establishment of database to July 1, 2021. The subject words (mesh) and free words were used for joint search. The English search words were: “esophageal neoplasts” “re irradiation”, and Chinese search words were: “esophageal cancer”, “reradiotherapy”, “secondary radiotherapy”. With reference to inclusion criteria and exclusion criteria, first screen according to topic and abstract, and then read full text further to determine whether it is included. Those who are not clearly described or cannot be determined are excluded.
3.2Document acceptance and exclusion criteria
Inclusion criteria: research type is clinical research on recurrence of esophageal cancer after radiotherapy with three-dimensional conformal radiation therapy (3D-CRT) and three-dimensional intensity modulated radiation therapy (IMRT); The subjects were patients diagnosed with local recurrence after radical radiotherapy of esophageal cancer; The intervention factors are simple re course radiotherapy and re course radiotherapy combined with single drug or multi drug chemotherapy, and these two methods are required to be carried out in same study; The outcome measures were effective rate, 1,2,3-year overall survival rate, and incidence of adverse reactions such as radiation-induced lung injury, radiation esophagitis, and bone marrow suppression.
Exclusion criteria: including surgery, targeted and traditional Chinese medicine and other treatment methods; Study with sample size <10 cases; There is no outcome index needed in this study; There is no detailed summary and full text cannot be obtained.
3.3Literature extraction and quality evaluation
The author, year of publication, sample size, number of patients in observation (including re course radiotherapy combined with single drug chemotherapy and re course radiotherapy combined with multi drug chemotherapy) and control (re course radiotherapy alone, treatment), gender, radiotherapy technology and dose, chemotherapy scheme and dose of observation, efficacy outcome indicators and adverse reaction incidence were extracted from literature.
The methodological quality of the extracted papers was then evaluated according to bias risk evaluation criteria of randomized controlled trials in Cochrane manual.
3.4Case inclusion and exclusion criteria
Inclusion criteria:
1) At initial diagnosis, there was a clear pathology, and it was determined that patient was esophageal squamous cell carcinoma or adenocarcinoma.
2) Those who were initially diagnosed with radical radiotherapy did not undergo surgical treatment, and chemotherapy was not limited.
3) Local recurrence was confirmed by gastroscopic pathology or esophageal feeding angiography or CT.
4) After local recurrence, patients were treated with re IMRT without salvage surgery.
Exclusion criteria:
1) People with other serious cardiopulmonary basic diseases.
2) Patients with other malignant tumors within 5 years.
3) Immunotherapy or molecular targeted therapy after local recurrence.
4) Important case data are incomplete.
3.5Treatment process and method
At present, there is no unified standard for radiotherapy after local recurrence treated by re IMRT. The following is a unified radiotherapy scheme adopted by our hospital.
1) Radiotherapy equipment:
Elekta precision linear accelerator, Ge light speed NCT positioning CT, XIO 4.8 treatment planning system, etc.
2) Radiotherapy positioning:
After fully informing the patient of his condition and obtaining consent of patient and his family, radiotherapy positioning was performed [15–17]. Take supine position, with both arms extended to side of body or elbows alternately placed in front of forehead. Vacuum pad fixation for patients with cervical and upper chest segments, and fixation of body membrane for patients with middle and lower chest segments. Improve positioning CT scanning, with a layer thickness of 0.5 cm. It is feasible to Enhance CT positioning according to needs of disease. After uploading positioning images to treatment planning system (TPS), feasible target area is outlined.
3) Target area delineation:
The gross tumor volume (GTV) is determined by combining length and invasion range of primary tumor shown by esophagography and CT imaging: clinical target volume (CTV) is 0.5∼0.8 cm left and right, and 2 cm up and down on basis of GTV, and target range is adjusted according to anatomical structure after external radiation; The plan target volume (PTV) is extended by 0.5 cm based on CTV, and PTV range can be appropriately extended according to positioning error (as shown in Fig. 1). In Fig. 1, combined with esophagography and CT imaging, three shaded areas of different sizes are the length and extent of invasion of the primary tumor, respectively. The small shade represents the small length and narrow penetration of the primary tumor, and the medium shade represents the size of the primary tumor. The length and extent of invasion of the primary tumor are adequately adapted, and the largest shading represents an area with large length and extent of invasion of the primary tumor.
Fig. 1
Schematic diagram of standardized radiotherapy for esophageal cancer.
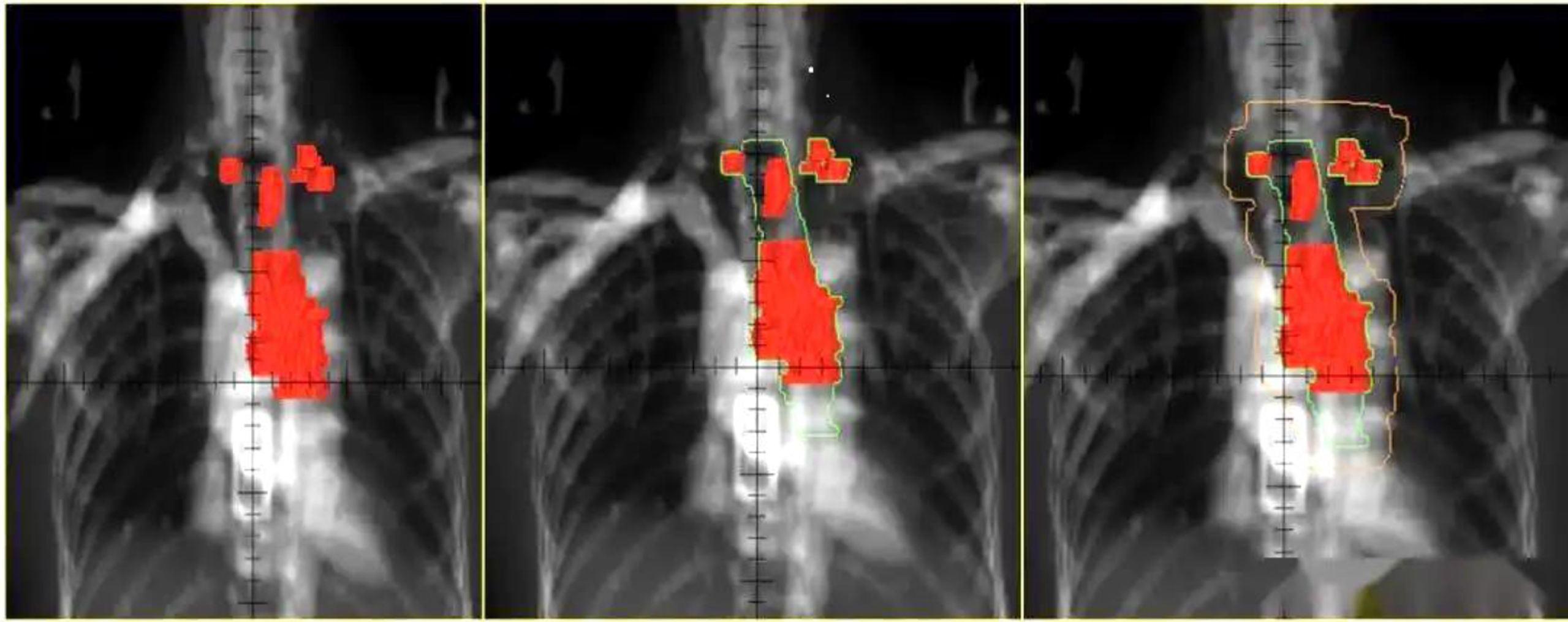
4) Prescription dose:
95% PTV 40∼60 Gy/2 Gy/20∼30 f.
5) Normal tissue limit:
V20 in both lungs (the lung is irradiated with a dose of 20 Gy, accounting for volume of whole lung)<28%, V40 in heart (the heart is irradiated with a dose of 40 Gy, accounting for total volume of heart)<20%, and maximum dose of spinal cord <16 Gy.
6) Evaluate and determine radiotherapy plan:
Dose volume histogram (DVH) and isodose curve were used.
3.6Concurrent chemotherapy and symptomatic treatment
Concurrent chemoradiotherapy (CCRT), It can also be sequential chemoradiotherapy (Scrt), Chemotherapy drugs are adjusted according to first chemotherapy regimen, and specific course of treatment is determined according to patient’s condition. Commonly used drugs include cisplatin (DDP), 5-fluorouracil (5-FU), paclitaxel, irinotecan, docetaxel, gemcitabine, teggio, etc. The schemes used include pf scheme (ddp+5-fu), TP (paclitaxel +ddp), or single drug teggio chemotherapy, etc.
Additionally, nutritional support, antibiotics, hormones, and other symptomatic treatment can also be given during radiotherapy.
3.7Observation content
1) The distribution of gender, age and other general characteristics of patients with esophageal cancer undergoing IMRT.
2) Short term efficacy of IMRT patients after local recurrence.
3) Overall survival (OS) and 1 -, 2 -, and 3-year survival rates of patients with recurrent IRMT after local recurrence.
4) Adverse reactions of IMRT after recurrence.
5) The value of IMRT combined with chemotherapy.
6) Analysis of influencing factors of OS after relapse of intensity modulated radiotherapy.
3.8Statistical data analysis
Using software revman5.3 of Cochrane Collaboration Network and taking relative risk (RR) as effect index, Calculate 95% confidence intervals (CI). When heterogeneity between studies is obvious (heterogeneity test p < 0.1 or f > 50%), random effect model is used, on contrary, fixed effect model is used. When heterogeneity is obvious, sensitivity analysis and subgroup analysis are carried out to further analyze causes of heterogeneity. The publication bias of Naren literature was evaluated by funnel chart.
4Results
A total of 377 articles were obtained by searching database [18–20]. According to inclusion and exclusion criteria, 15 articles were finally included in this study, with a total of 956 patients, including 476 in observation and 480 in control.
The general situation of each research is basically matched and comparable [21–23]. According to Cochrane manual, risk of low bias, uncertainty of bias risk and high bias risk were used to determine. The results showed that bias risk of included studies was moderate, acceptable and no significant publication bias was found. The funnel chart of one-year overall survival rate is shown in Fig. 2. In the funnel chart of the overall survival rate in Fig. 2, we can see that there are many circles at the apex and few at the bottom, indicating that the fertility rate is declining. A total of 14 literatures were included in treatment effectiveness rate, and heterogeneity test result was p = 59%. Using random effect model, 95% CI was 1.03∼1.32, p = 0.002. Sensitivity analysis was used to exclude literature findings one by one. When study by Ding Lingli was excluded, P decreased to 42%, and heterogeneity test 1 of other literatures was >50%, considering that it may be source of heterogeneity [24].
Fig. 2
Chart of total survival rate.
Re-radiotherapy combined with chemotherapy was divided into three subgroups: the second course of radiotherapy combined with single drug chemotherapy, the second course of radiotherapy combined with multi drug chemotherapy, and the second course of radiotherapy combined with uncertain chemotherapy. Only one study in of the second course of radiotherapy combined with uncertain chemotherapy was excluded, P was 39%, 60%, and 95% CI was 1.11∼1.76, 0.93∼1 25, P values were 0.004 and 0.30 respectively, considering that chemotherapy regimen is not source of heterogeneity. The effective rate of observation was higher (as shown in Fig. 3 and Table 1).
Fig. 3
Chart of treatment effectiveness.
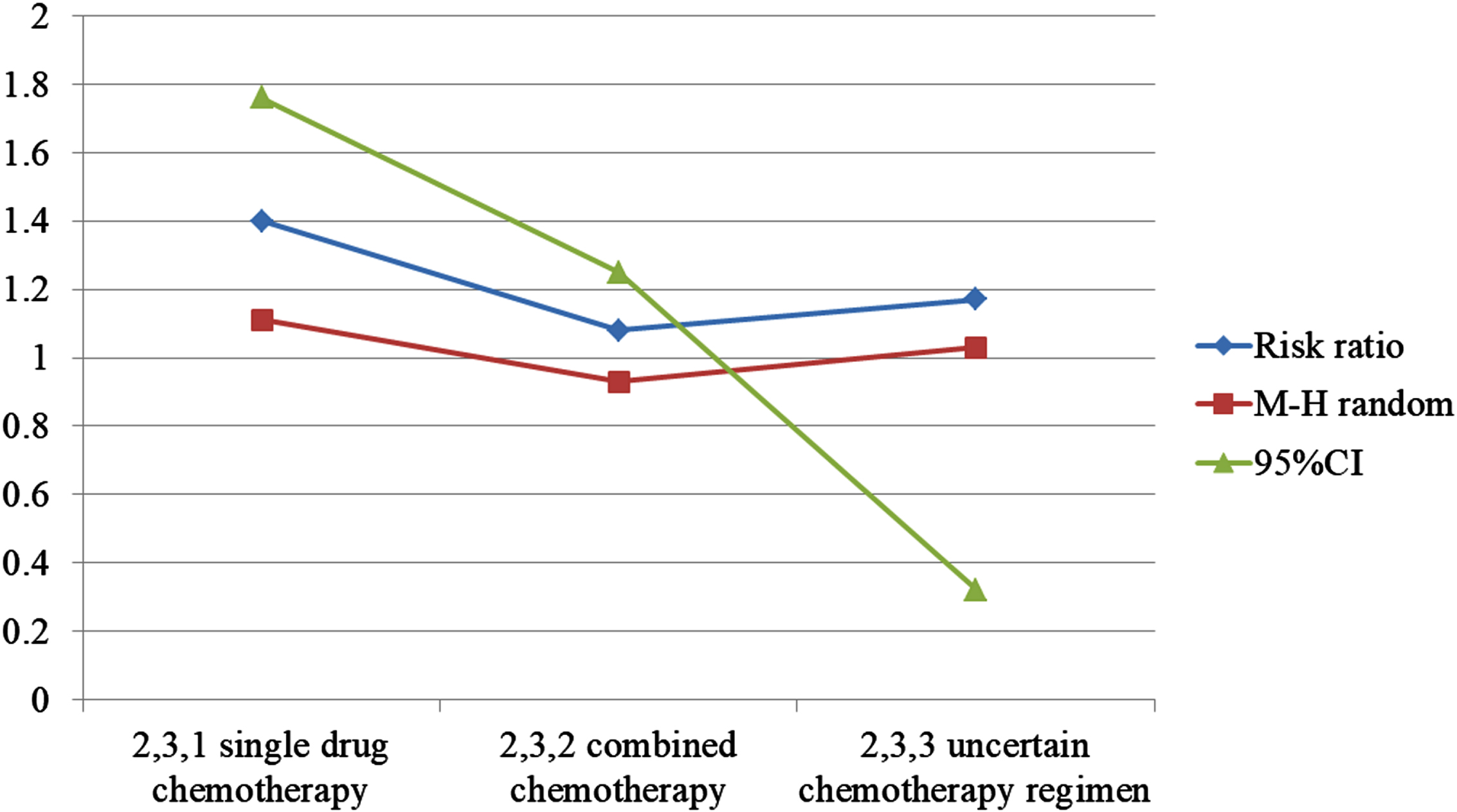
Table 1
Single drug chemotherapy research record
| Experimental | Control | Risk Ratio | ||||
| study or subgroup | Events | Total | Events | Total | Weight | M-H Random. 95% CI |
| 2.3.1 single drug chemotherapy | ||||||
| Zhou X. 2019 | 23 | 35 | 13 | 33 | 4.4% | 1.67[1.03,2.71] |
| Zhang Q. 2015 | 28 | 30 | 24 | 30 | 9.9% | 1.17[0.95,1.43] |
| Gu H. 2014 | 23 | 31 | 14 | 30 | 5.1% | 1.59[1.03,2.46] |
| Gu X. 2018 | 21 | 27 | 18 | 36 | 5.9% | 1.56[1.06,2.28] |
| Subtotal (95% Cl) | 123 | 129 | 25.2% | 1.40[1.11,1.76] | ||
| Total events | 95 | – | 69 | – | – | – |
| 2,3,2 combined chemotherapy | ||||||
| Ding L. 2014 | 32 | 43 | 39 | 43 | 9.9% | 0.82[0.67,1.00] |
| Liu G. 2018 | 38 | 40 | 31 | 40 | 10.4% | 1.23[1.02,1.47] |
| Peng J. 2013 | 34 | 44 | 37 | 46 | 9.6% | 0.96[0.78,1.19] |
| Wang J. 2017 | 24 | 32 | 22 | 30 | 7.7% | 1.02[0.76,1.37] |
| Luo H. 2013 | 19 | 23 | 20 | 25 | 8.2% | 1.03[0.79,1.35] |
| Min B. 2015 | 17 | 22 | 18 | 22 | 7.5% | 0.94[0.70,1.28] |
| Chen J. 2015 | 30 | 44 | 19 | 44 | 5.7% | 1.58[1.06,2.34] |
| Gao H. 2019 | 27 | 47 | 25 | 47 | 6.2% | 1.08[0.75,1.55] |
| Kaolin 2015 | 18 | 21 | 9 | 20 | 4.1% | 1.90[1.14,3.19] |
| Subtotal (95% Cl) | 239 | – | 220 | – | 69.3% | 1.08[0.93,1.25] |
| 2,3,3 uncertain chemotherapy regimen | ||||||
| Zhang X. 2015 | 20 | 28 | 14 | 24 | 5.4% | 1.22[0.81,1.85] |
| Subtotal (95% Cl) | – | 28 | – | 24 | 5.4% | 1.22[0.81,1.85] |
| Total events | 20 | – | – | – | – | – |
| Total (95% CI) | – | 467 | – | 470 | 100% | 1.17[1.03,1.32] |
| Total events | 354 | – | 303 | – | – | – |
Next, in analyzing data of one-year total survival outcome index presented in 14 literatures, the heterogeneity test result was p = 0%. Using the fixed effect model, 95% CI was 1.08∼1.36, p = 0.001. According to subgroup analysis, 95% CI of re course radiotherapy combined with single drug chemotherapy was 1.13∼1.85, p = 0.003, that is, one-year survival rate of re course radiotherapy combined with single drug chemotherapy was higher, See Fig. 4. The total 2-year survival rate was included in 11 literatures. The heterogeneity test result was f = 0%. Using the fixed effect model, 95% CI was 1.08∼1.69, P = 0.009, that is, 2-year survival rate of observation was higher. The 3-year overall survival rate was included in 10 literatures [25]. The heterogeneity test result was f = 0%. Using fixed effect model, 95% CI was 1.22∼2 60, p = 0.003, that is, 3-year survival rate of observation is higher (as shown in Table 2).
Fig. 4
Chart of annual total survival rate.
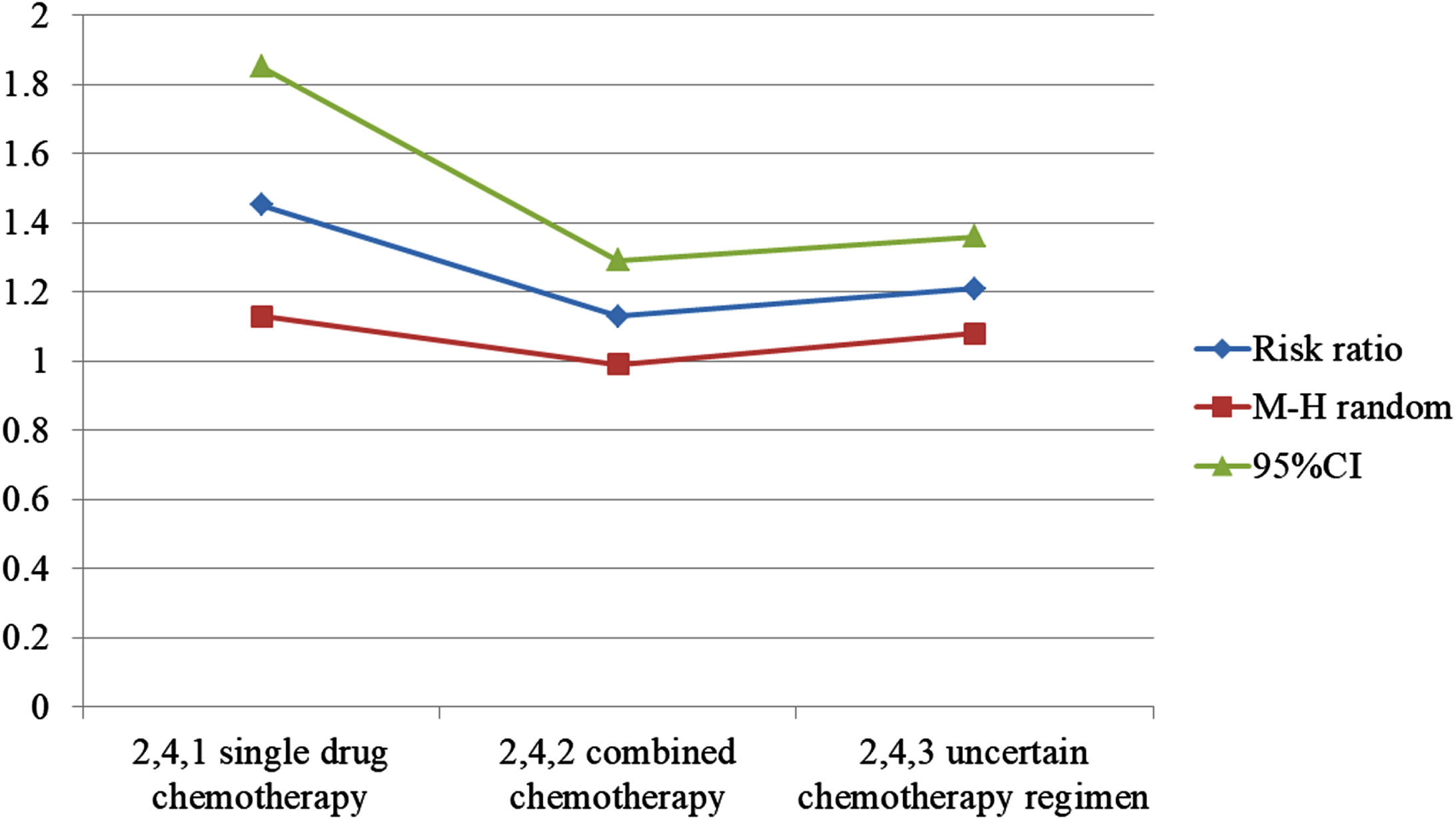
Table 2
Statistical table of survival rate difference
| Experimental | Control | Risk Ratio | ||||
| study or subgroup | Events | Total | Events | Total | Weight | M-H Random. 95% CI |
| 2.4.1 single drug chemotherapy | ||||||
| Zhou X. 2019 | 15 | 35 | 6 | 33 | 2.7% | 2.36[1.04,5.34] |
| Zhang Q. 2015 | 20 | 28 | 15 | 28 | 6.7% | 1.29[0.84,1.96] |
| Gu H. 2014 | 17 | 31 | 13 | 30 | 5.8% | 1.27[0.75,2.13] |
| Gu X. 2018 | 20 | 27 | 19 | 36 | 7.1% | 1.40[0.96,2.05] |
| Subtotal (95% Cl) | – | 127 | – | 127 | 22.3% | 1.45[1.13,1.85] |
| Total events | 72 | – | 53 | – | – | – |
| 2.4.2 combined chemotherapy | ||||||
| Ding L. 2014 | 23 | 43 | 22 | 43 | 9.6% | 1.05[0.70,1.57] |
| Liu G. 2018 | 24 | 40 | 17 | 40 | 7.4% | 1.41[0.91,2.19] |
| Peng J. 2013 | 25 | 44 | 24 | 46 | 10.3% | 1.09[0.75,1.59] |
| Wang J. 2017 | 16 | 32 | 16 | 30 | 7.2% | 0.88[0.53,1.45] |
| Luo H. 2013 | 13 | 23 | 13 | 25 | 5.5% | 1.09[0.65,1.83] |
| Luo H. 2019 | 21 | 40 | 20 | 40 | 8.8% | 1.05[0.68,1.61] |
| Min B. 2015 | 14 | 22 | 12 | 22 | 5.3% | 1.17[0.71,1.91] |
| Chen J. 2015 | 43 | 44 | 35 | 44 | 15.3% | 1.23[1.05,1.44] |
| Kaolin 2015 | 11 | 21 | 9 | 20 | 4.0% | 1.16[0.62,2.19] |
| Subtotal (95% CI) | – | 309 | – | 310 | 73.4% | 1.13[0.99,1.29] |
| Total events | 189 | – | 168 | – | – | – |
| 2.4.3 uncertain chemotherapy regimen | ||||||
| Zhang X. 2015 | 14 | 28 | 9 | 24 | 4.2% | 1.33[0.71,2.52] |
| Subtotal (95% Cl) | – | 28 | – | 24 | 4.2% | 1.33[0.71,2.52] |
| Total events | 14 | – | 9 | – | – | – |
| Total (95% Cl) | 459 | – | 461 | 100% | 1.21[1.08,1.36] | |
| Total events | 275 | – | 230 | – | – | – |
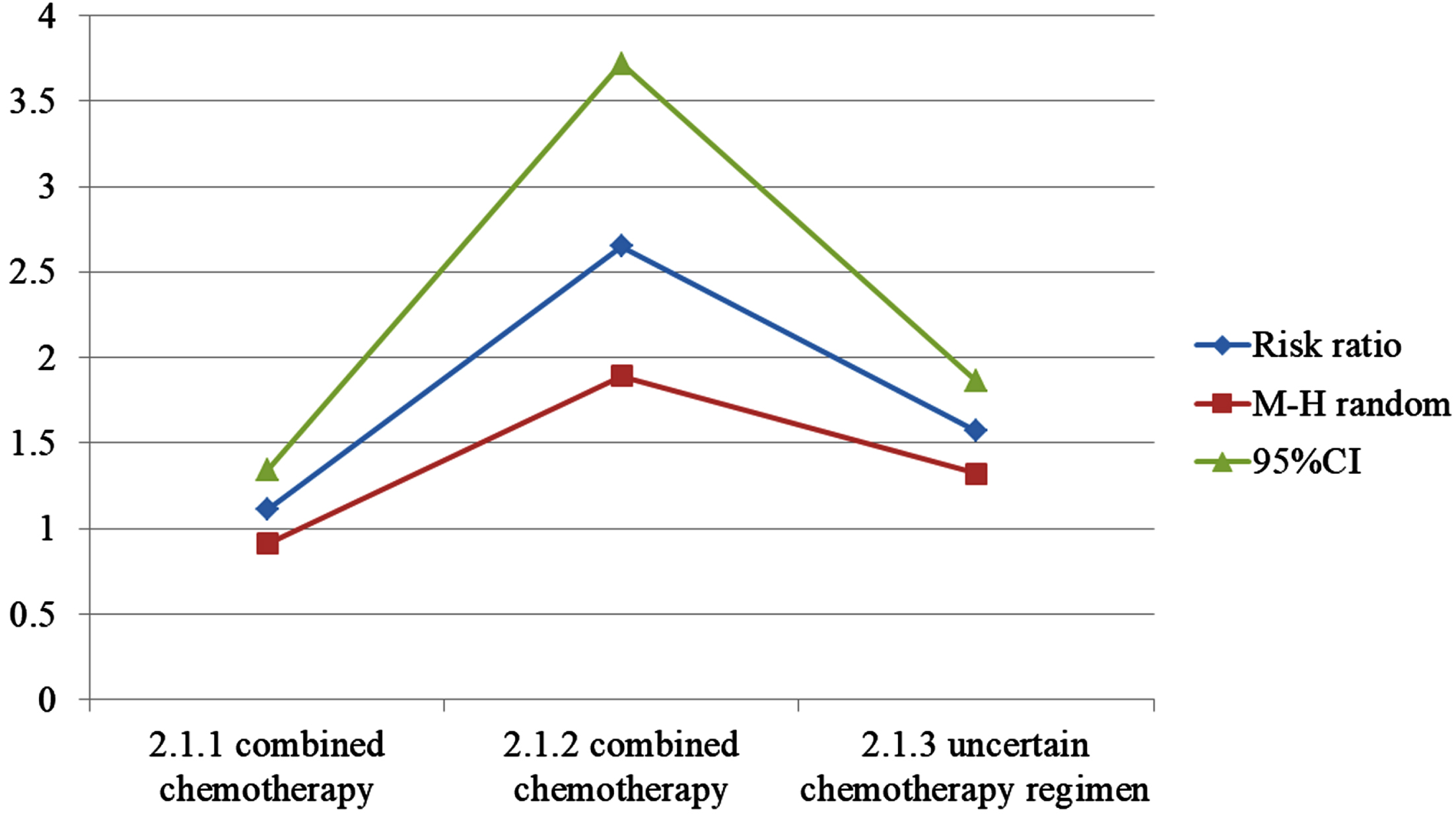
A total of 9 literatures on radiation-induced lung injury were included, and heterogeneity test result was f = 26%. The fixed effect model was used, 95% CI was 1.34∼2.60, P = 0.0002, indicating that incidence of radiation-induced lung injury in observation was higher. A total of 11 literatures on bone marrow suppression were included. The heterogeneity test results were p = 79%. The random effect model was used, and 95% CI was 1.73∼2.58, p0.0005; Sensitivity analysis showed that f was >50% after excluding literature one by one; Subgroup analysis; The 1 of the second course of radiotherapy combined with single drug chemotherapy and the second course of radiotherapy combined with multi drug chemotherapy were 0%, 95% CI were 1.01 1.59 and 2.05∼3.97 respectively, P values were 0.04 and <0.00001 respectively, indicating that the second course of radiotherapy combined with single drug chemotherapy The incidence of bone marrow suppression in re course radiotherapy combined with multi drug chemotherapy was higher than that in control, and chemotherapy regimen may be reason for heterogeneity. A total of 11 literatures were included in radiation esophagitis. The heterogeneity test result was p = 69%. Using random effect model, 95% CI was 1.32∼1.86, p0.003; Sensitivity analysis showed that f was >50% after excluding literature one by one; Subgroup analysis, the second course of radiotherapy combined with single drug chemotherapy The P of re course radiotherapy combined with multidrug chemotherapy was 0%, 34%, while incidence of radiation esophagitis in the second course of radiotherapy combined with multi drug chemotherapy is higher. Considering that chemotherapy regimen may be cause of heterogeneity (see Table 3 and Fig. 5).
Table 3
Record of multi drug chemotherapy research
| Experimental | Control | Risk Ratio | ||||
| study or subgroup | Events | Total | Events | Total | Weight | M-H Random. 95% CI |
| 2.1.1 combined chemotherapy | ||||||
| Zhou X. 2019 | 15 | 35 | 14 | 33 | 12.6% | 1.01[0.58,1.75] |
| Zhang Q. 2015 | 21 | 30 | 20 | 30 | 17.5% | 1.05[0.74,1.48] |
| Gu H. 2014 | 14 | 31 | 11 | 30 | 9.8% | 1.23[0.67,2.27] |
| Gu X. 2018 | 25 | 27 | 29 | 36 | 21.8% | 1.15[0.95,1.39] |
| Subtotal (95% Cl) | 123 | 129 | 61.7% | 1.11[0.91,1.34] | ||
| Total events | 76 | 74 | ||||
| 2.1.2 combined chemotherapy | ||||||
| Ding L. 2014 | 20 | 43 | 7 | 43 | 6.1% | 2.86[1.35,6.05] |
| Peng Y. 2013 | 19 | 44 | 6 | 46 | 5.1% | 3.31[1.46,7.51] |
| Wang J. 2017 | 13 | 32 | 4 | 30 | 3.6% | 3.05[1.12,8.31] |
| Luo H. 2013 | 11 | 23 | 4 | 25 | 3.4% | 2.99[1.11,8.08] |
| Luo H. 2019 | 16 | 40 | 4 | 40 | 3.5% | 4.00[1.47,10.92] |
| Kaolin 2015 | 11 | 21 | 9 | 20 | 8.1% | 1.16[0.62,2.19] |
| Subtotal (95% Cl) | 203 | 204 | 29.8% | 2.65[1.89,3.72] | ||
| Total events | 90 | 34 | ||||
| 2.1.3 uncertain chemotherapy regimen | ||||||
| Zhang X. 2015 | 12 | 28 | 9 | 24 | 8.5% | 1.14[0.58,2.23] |
| Subtotal (95% Cl) | 28 | 24 | 8.5% | 1.14[0.58,2.23] | ||
| Total events | 12 | 9 | ||||
| Total (95% Cl) | 354 | 357 | 100% | 1.57[1.32,1.86] | ||
| Total events | 177 | 177 | ||||
Fig. 5
Chart of incidence of radiation esophagitis.
5Discussion
According to the findings of the search, there are several research that concentrate on forecasting outcomes and identifying the most effective therapies for patients with esophageal cancer who are undergoing radiation therapy. In these types of investigations, machine learning models and molecular assays are often used to analyze the impact of radiation treatment and other factors on patient outcomes.
Both radiation and neoadjuvant chemotherapy, in their respective forms, were evaluated using the samples to see how effective they were. The hyperparameters of the machine learning models that were trained and evaluated using these datasets were fine-tuned with the assistance of cross-validation, which was employed in the process.
Although the experimental conditions differed from one research to the next, they often included patient factors including age, gender, and comorbidities in addition to radiation dosage and frequency, chemotherapeutic regimens, and the frequency and amount of radiation exposure. Patients with esophageal cancer who were participating in the studies were given radiation therapy with the intention of determining which treatment strategies proved to be the most successful overall. It was hoped that by doing this, patients would get better outcomes from their radiation therapy and have fewer of the negative side effects that are often linked with the treatment.
The results of the research show that machine learning models and molecular assays have the potential to be valuable tools for establishing optimal treatment decisions and predicting outcomes for patients with esophageal cancer who are receiving radiation therapy. This was the conclusion drawn from the findings of the study. To improve these technologies and figure out the most efficient ways to employ them in clinical settings, further research will need to be conducted.
The prognosis of recurrent esophageal cancer after the second course of radiotherapy may be related to many factors. We conducted a univariate analysis of various clinical factors to explore which factors will affect patients’ OS [12]. Showed that gender, tumor location, lesion length and survival time were not statistically related. In this study, patient’s gender (x = 0.906, p = 0.341), lesion segmentation (x = 1.778, p = 0.620), lesion length (x0.316, P 0.574) and prognosis were not statistically significant. Age is a factor affecting prognosis of esophageal cancer after the second course of radiotherapy (x = 6.335, p = –0.012). The 1, 2 and 3-year survival rates of patients aged <66 years old are higher than those aged >66 years old, which indicates that younger patient is, more obvious survival benefit of the second course of radiotherapy is [15]. It is considered that recurrence interval is a factor affecting prognosis [18]. It was found that patients with an interval of more than 2 years between the second course of radiotherapy had a longer survival time [21]. Pointed out that one-year and 2-year survival rates of patients with recurrence interval >1 year were higher than those with recurrence interval <1 year [23]. 260 patients with esophageal cancer after radical radiotherapy were followed up, of which 75 cases recurred. The recurrence interval ranged from 2 to 84 months, with an average interval of 19.7 months. Among 65 patients in this study, recurrence interval was 6–204 months, and median recurrence time was 41 months [24].
The development time of a cancer recurrence in our study is prolonged, which may be due to development and optimization of the radiotherapy technology in recent years. Thus, curative effect is better than before. As a result, the disease-free survival state of patients after first treatment lasts for a long time. The recurrence interval was further divided into three groups. Compared of patients with <36 months, 36–60 months and >60 months, it shows that patients with long recurrence interval have not improved survival after the second course of radiotherapy. Whether KPS score is related to prognosis is still controversial, believes that it is not related to short-term efficacy. [16–19] shows that a high KPS score means a good prognosis. Our data showed that there was no difference between KPS score and prognosis (x = 0.887, p = 0.642). The reason may be that doctors have some subjectivity in evaluating patients’ KPS score, and KPS score can only reflect the general situation of patients at this stage before treatment, which is time-consuming.
A 3-year survival rate may be used in cancer prognostication since they have the potential to provide essential insights on the efficacy of therapies and the possibility of a disease recurrence. In addition, survival rates calculated over a period of three years may be a better metric for shorter-term follow-up research, while survival rates calculated over a period of five years are often utilized for longer-term investigations. It is also crucial to note that survival rates may vary greatly based on several circumstances, including the kind and stage of cancer, the patient’s general health, as well as the availability of therapies and how successful those treatments are.
The search results reference a meta-analysis that sheds light on the use of concurrent chemoradiotherapy and radiation alone in the treatment of locally advanced esophageal cancer. However, the topic of repeat radiation courses after the first radical treatment for esophageal cancer is also up for debate. This strategy, which involves re-irradiation in addition to chemo-immunotherapy, has also been studied for recurring lung cancer. Some studies have assessed the appropriate re-irradiation dosage for locally recurrent esophageal cancer, with conflicting findings. If esophageal cancer returns after radiotherapy, other treatments, such as surgery, re-radiation, chemotherapy, or brachytherapy, may be necessary [37, 38]. Chronic sinusitis is one late consequence of radiation therapy for head and neck cancer that may be difficult for patients to manage. Depending on molecular markers and other criteria, the care of locoregional breast cancer recurrence may comprise a mix of surgery, radiation therapy, and systemic therapies.
Based on research results of [21], CCRT is standard treatment for inoperable esophageal cancer. At present, there is still no consensus on whether retraining radiotherapy should be combined with chemotherapy [14]. The clinical efficacy and adverse reactions of 41 patients with recurrent esophageal cancer were analyzed. The effective rates of radiotherapy alone and radiotherapy and chemotherapy were 45.00% and 85.71% respectively; However, there was no difference in survival rate and toxic and side effects. Therefore, it is considered that combination of the second course of radiotherapy and chemotherapy is worth expanding clinical research [17]. 48 patients with recurrent esophageal and tubular carcinoma after radiotherapy were divided into CCRT and radiotherapy only. The results showed that local control rate was different (82.6% vs 80.0%), and 1 -, 2 -, and 3-year survival rates were different (56.5%, 47.8%, 21.7%, and 52.0%, 28.0%, 8.0%), On whole, curative effects of two regimens are similar, but toxic and side effects of CCRT are more obvious; patients with kps270 and no chemotherapy contraindications can use CCRT. If the above conditions are not met, they should be carefully selected [14]. It was confirmed that MST, 1, 2 and 3-year survival rate of patients after the second course of radiotherapy and chemotherapy were higher than those who only received radiotherapy. Radiotherapy locally irradiates tumor tissue, while chemotherapy is systemic. Theoretically, combination of two has a synergistic effect, which can enhance original efficiency and kill more tumor cells. However, when two drugs are used together, toxic and side effects also increase exponentially. This reminds us that we should choose scheme flexibly, and we must weigh benefit and risk, to decide whether to combine chemotherapy [21]. Shows that whether 3DCRT or IMRT is used has no effect on long-term survival of patients.
Our results are consistent with this (x2 = 0.023, p = 0.879). After the first course of radiotherapy, short-term efficacy is an important factor affecting prognosis. Those with short-term efficacy of Cr are better than those with PR or NR in terms of survival rate and local control rate. In this study, long-term survival of patients with the second course of radiotherapy was correlated with remission rate (x2 = 51.747, p < 0.001). Patients with good short-term efficacy tended to have better prognosis and survival, which was consistent with conclusion of [9]. It is suggested that this index can be used in clinical work to predict long-term survival of patients. In addition, we found that weight loss, nature of recurrence and occurrence of various adverse reactions were not related to prognosis. As one of second-line treatment schemes after local recurrence of esophageal cancer, primary purpose of the second course of radiotherapy is to alleviate discomfort symptoms of patients, improve quality of life, and then improve prognosis and prolong survival time. At present, most scholars believe that short-term and long-term efficacy of the second course of radiotherapy for esophageal cancer is significant, and its clinical application value is worthy of affirmation, which can be further promoted and applied in clinic.
6Conclusion
The meta-analysis results of this study show that recurrent radiotherapy combined with single drug chemotherapy may be an effective treatment for patients with recurrent esophageal cancer after radiotherapy, and the adverse reactions can be tolerated. This study has some limitations. The quality and quantity of some accepted documents are low. Some results are still heterogeneous and may have publication bias. For subgroup analysis, due to data limitations, the adverse reactions of remedial radiotherapy combined with single drug chemotherapy and re course radiotherapy combined with multi drug chemotherapy are not comprehensive. Considering the above reasons, the results of this study should be treated objectively and cautiously, and more high-quality studies are needed to further confirm the conclusions. To sum up, patients with recurrent esophageal cancer after radiotherapy, especially radiotherapy plus chemotherapy, are effective means to treat recurrent esophageal cancer.
The goal of this research was to find ways to lessen the unpleasant effects of chemotherapy for esophageal cancer. This study compares the efficacy and safety of second-round radiotherapy with chemotherapy for the treatment of esophageal cancer that has returned after first irradiation. Re-irradiation for esophageal cancer is supported by these findings. This meta-analysis shows that combining further radiation with single-drug chemotherapy may successfully treat recurrent esophageal cancer with manageable side effects. Due to insufficient data, it is not possible to conduct a subgroup study comparing the side effects of restorative radiation with those of single-drug chemotherapy and of recourse radiation with those of many drugs. Thus, researchers need to analyze and verify the findings of this investigation thoroughly. Finally, recurrent esophageal cancer may be treated with radiation and chemotherapy.
Data availability
The experimental data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of interest
The authors declared that they have no conflicts of interest regarding this work.
Funding statement
There is no specific funding to support this research.
References
[1] | Zhu C. , Wang S. , You Y. , et al., Risk factors for esophageal fistula in esophageal cancer patients treated with radiotherapy: A systematic review and meta-analysis. Oncology Research and Treatment 43: (1-2) ((2020) ), 34–41. |
[2] | Wu J. , Ni M. , Zhu J. , et al., Clinical evaluation of javanica oil emulsion injection combined with the radiotherapy in the treatment of esophageal cancer: A systematic review and meta-analysis. The Journal of Alternative and Complementary Medicine 25: (5) ((2019) ), 542–551. |
[3] | Wu J. , Ni M. , Zhu J. , et al., Clinical evaluation of javanica oil emulsion injection combined with the radiotherapy in the treatment of esophageal cancer: A systematic review and meta-analysis. The Journal of Alternative and Complementary Medicine 25: (5) ((2019) ), 542–551. |
[4] | Sohda M. and Kuwano H. , Current status and future prospects for esophageal cancer treatment. Annals of Thoracic and Cardiovascular Surgery 23: (1) ((2017) ), 1–11. |
[5] | Jang R. , Darling G. and Wong R.K. , Multimodality approaches for the curative treatment of esophageal cancer. Journal of the National Comprehensive Cancer Network 13: (2) ((2015) ), 229–238. |
[6] | Xie X. , Pan X. , Zhang W. and An J. , A context hierarchical integrated network for medical image segmentation. Computers and Electrical Engineering 101: ((2022) ), 108029. |
[7] | Ikebe M. , Morita M. , Yamamoto M. and Toh Y. , Neoadjuvant therapy for advanced esophageal cancer: The impact on surgical management. General Thoracic and Cardiovascular Surgery 64: (7) ((2016) ), 386–394. |
[8] | Xie X. , Pan X. , Shao F. , et al., Mci-net: Multi-scale context integrated network for liver ct image segmentation. Computers and Electrical Engineering 101: ((2022) ), 108085. |
[9] | Cerrati E.W. , Nguyen S.A. , Farrar J.D. and Lentsch E.J. , The efficacy of photodynamic therapy in the treatment of oral squamous cell carcinoma: A meta-analysis. Ear, Nose & Throat Journal 94: (2) ((2015) ), 72–79. |
[10] | Deressa B.T. , Tigeneh W. , Bogale N. , et al., Short-course 2-dimensional radiation therapy in the palliative treatment of esophageal cancer in a developing country: A phase II study (Sharon Project). International Journal of Radiation Oncology* Biology* Physics 106: (1) ((2020) ), 67–72. |
[11] | Agas R.A.F. , Co L.B.A. , Jacinto J.C. , et al., Neoadjuvant radiotherapy versus no radiotherapy for stage iv rectal cancer: A systematic review and meta-analysis. Journal of Gastrointestinal Cancer 49: (4) ((2018) ), 389–401. |
[12] | Morimoto H. , Fujiwara Y. , Lee S. , et al., Treatment results of neoadjuvant chemoradiotherapy followed by radical esophagectomy in patients with initially inoperable thoracic esophageal cancer. Japanese Journal of Radiology 36: (1) ((2018) ), 23–29. |
[13] | Ashok A. , Niyogi D. , Ranganathan P. , et al., The enhanced recovery after surgery (ERAS) protocol to promote recovery following esophageal cancer resection. Surgery today 50: (4) ((2020) ), 323–334. |
[14] | Xie X. , Zhang W. and Wang H. , Dynamic adaptive residual network for liver CT image segmentation. Computers and Electrical Engineering 91: ((2021) ), 107024. |
[15] | Mariette C. , Gronnier C. , Duhamel A. , et al., Self-expanding covered metallic stent as a bridge to surgery in esophageal cancer: Impact on oncologic outcomes. Journal of the American College of Surgeons 220: (3) ((2015) ), 287–296. |
[16] | Nicolay N.H. , Rademacher J. , Oelmann-Avendano J. , et al., High dose-rate endoluminal brachytherapy for primary and recurrent esophageal cancer. Strahlentherapie und Onkologie 192: (7) ((2016) ), 458–466. |
[17] | Van Der Wilk B.J. , Eyck B.M. , Spaander M.C. , et al., Towards an organ-sparing approach for locally advanced esophageal cancer. Digestive Surgery 36: (6) ((2019) ), 462–469. |
[18] | Li Y.C. , Zhao L. , Wu J.P. , et al., Cytokine-induced killer cell infusion combined with conventional treatments produced better prognosis for hepatocellular carcinoma patients with barcelona clinic liver cancer B or earlier stage: A systematic review and meta-analysis. Cytotherapy 18: (12) ((2016) ), 1525–1531. |
[19] | Cellini F. , Manfrida S. , Casà C.V. , et al., Modern management of esophageal cancer: Radio-oncology in neoadjuvancy, adjuvancy and palliation. Cancers 14: (2) ((2022) ), 431. |
[20] | Unterrainer M. , Eze C. , Ilhan H. , et al., Recent advances of PET imaging in clinical radiation oncology. Radiation Oncology 15: (1) ((2020) ), 1–15. |
[21] | Hashimoto J. , Kato K. , Ito Y. , et al., Phase II feasibility study of preoperative concurrent chemoradiotherapy with cisplatin plus 5-fluorouracil and elective lymph node irradiation for clinical stage II/III esophageal squamous cell carcinoma. International Journal of Clinical Oncology 24: (1) ((2019) ), 60–67. |
[22] | Verma V. , Simone C.B. and Mishra M.V. , Quality of life and patient-reported outcomes following proton radiation therapy: A systematic review. JNCI: Journal of the National Cancer Institute 110: (4) ((2018) ), 341–353. |
[23] | Schlampp I. , Rieber J. , Adeberg S. , et al., Re-irradiation in locally recurrent lung cancer patients. Strahlentherapie und Onkologie 195: (8) ((2019) ), 725–733. |
[24] | Linde P. , Baues C. , Wegen S. , et al., Pentixa for PET/CT for imaging of chemokine receptor 4 expression in esophageal cancer–a first clinical approach. Cancer Imaging 21: (1) ((2021) ), 1–11. |
[25] | Wu F. , Hong J. , Du N. , et al., Long-term outcomes of neoadjuvant chemotherapy in locally advanced gastric cancer/esophagogastric junction cancer: A systematic review and meta-analysis. Anti-Cancer Agents in Medicinal Chemistry 22: (1) ((2022) ), 143–151. |
[26] | Anderson L.L. and Lad T.E. , Autopsy findings in squamous cell carcinoma of the esophagus. Cancer 50: ((1982) ), 1587–1590. |
[27] | Chan K.J.W. , Chan E.Y. and Chan C.W. , Carcinoma of the esophagus: An autopsy study of 231 cases. Pathology 18: ((1996) ), 400–405. |
[28] | Forastiere A.A. , Orringer M.B. , Perez-Tamayo C. , et al., Concurrent chemotherapy and radiation therapy followed by transhiatal esophagectomy for local-regional cancer of the esophagus. J Clin Oncol 8: ((1990) ), 119–127. |
[29] | Coia L.R. , Engstrom P.F. , Paul A.R. , et al., Long-term results of infusional 5-FU, mitomycin-C and radiation as primary management of esophageal carcinoma. Int J Radiat Oncol Biol Phys 20: ((1999) ), 29–36. |
[30] | Forastiere A.A. , Treatment of locoregional esophageal cancer. Semin Oncol 19: ((1992) ), 57–63. |
[31] | Gill P.G. , Denham J.W. , Jamieson G.G. , et al., Patterns of treatment failure and prognostic factors associated with the treatment of esophageal carcinoma with chemotherapy and radiotherapy either as sole treatment or followed by surgery. J Clin Oncol 10: , 1037–1043. |
[32] | Naunheim K.S. , Petruska P. , Roy T.S. , et al., Preoperative chemotherapy and radiotherapy for esophageal carcinoma. J Thorac Cardiovasc Surg 103: ((1992) ), 887–893. |
[33] | Jones D.R. , Detterbeck F.C. , Egan T.M. , et al., Induction chemoradiotherapy followed by esophagectomy in patients with carcinoma of the esophagus. Ann Thorac Surg 64: ((1997) ), 185–191. |
[34] | Ilson D.H. , Ajani J. , Bhalla K. , et al., Phase II trial of paclitaxel, fluorouracil, and cisplatin in patients with advanced carcinoma of the esophagus. J Clin Oncol 16: ((1998) ), 1826–1834. |
[35] | Orringer M.B. , Marshall B. and Iannettoni M.D. , Transhiatal esophagectomy: Clinical experience and refinements. Ann Surg 230: ((1992) ), 392–400. |
[36] | Altorki N.K. , Three-field lymphadenectomy for esophageal cancer. Chest Surg Clin N Am 10: ((2000) ), 553–560. |
[37] | Zhu L. , Yuan L. , Wang H. , et al., A meta-analysis of concurrent chemoradiotherapy for advanced esophageal cancer. PLOS ONE 10: (6) ((2015) ), e012816. |
[38] | Zhu C. , Wang S. , You Y. , et al., Risk factors for esophageal fistula in esophageal cancer patients treated with radiotherapy: A systematic review and meta-analysis. Oncol Res Treat 43: (1-2) ((2020) ), 34–41. |




