New-onset hydrocephalus in an adult with cerebral palsy: A case report and review of the literature
Abstract
Hydrocephalus is a common comorbidity associated with brain injuries, including cerebral palsy (CP). In CP, hydrocephalus typically presents in infancy or early childhood. This report describes a patient in their mid 20 s with mixed dyskinetic-spastic CP with adult-onset hydrocephalus of unknown cause initially presenting with new-onset bilateral lower extremity spasms. Multiple interventions were trialed, including ischial bursal steroid injections, botulinum toxin injections, trigger point injections, multiple oral medications, and physical and massage therapies without benefit. Given lack of treatment response, imaging of the neuraxis was obtained. Magnetic resonance imaging (MRI) of the brain demonstrated new diffuse moderate ventriculomegaly compared to prior MRI. Ophthalmologic evaluation demonstrated papilledema, and opening pressure on lumbar puncture was elevated to 44 mmHg H2O. The patient underwent ventriculoperitoneal shunt placement with rapid and near-resolution of their spasms and pain. This patient represents a unique case of new-onset hydrocephalus in an adult with CP. To ensure appropriate and timely diagnosis and treatment, individuals with neurologic conditions such as CP should have ongoing surveillance and comprehensive evaluation for any neurologic or functional changes, including changes in baseline tone. Future research is needed to better understand if adults with CP are at higher risk for the development of hydrocephalus in adulthood.
1Case report
Many individuals with cerebral palsy (CP) have concomitant hydrocephalus, though this often develops in infancy or early childhood [1]. Acquired hydrocephalus is a known neurologic issue in adults with multiple identified causes including trauma and infection [2], though little is known about newly acquired hydrocephalus in adults with CP without these other risk factors. This report describes an adult with CP who presented with worsening hypertonia ultimately identified as being due to hydrocephalus.
A 27-year-old woman with mixed dyskinetic (athetoid, dystonic) spastic CP due to birth-related hypoxic ischemic injury presented with new-onset bilateral lower extremity spasms, causing severe pain and distress. Associated symptoms included decreased oral intake, generalized weakness, and headaches, which were attributed to her overall discomfort from the hypertonia. At baseline, she utilized a power wheelchair for mobility, was fully dependent for activities of daily living, and was nonverbal, communicating with use of an augmentative and alternative communication (AAC) device. Her medical history also included bilateral hip dysplasia status-post bilateral varus derotational osteotomies at six years old, neuromuscular scoliosis, and cervical myelopathy status-post C1-2 fusion. This presented as worsening left upper limb weakness and atrophy nine years prior to current presentation, with partial though incomplete improvement after fusion.
Ischial bursitis was initially suspected, as her pain and spasms developed in the setting of a leak found in her custom wheelchair cushion. However, despite replacement of the wheelchair cushion, symptoms persisted. Multiple interventions were trialed over an eight-month period, including ischial bursal steroid injections, botulinum toxin injections, trigger point injections, and multiple oral and topical pain and tone management medications without significant or lasting benefit. Additionally, she continued receiving physical and massage therapies with trials of various modalities throughout this time with minimal improvement in symptoms.
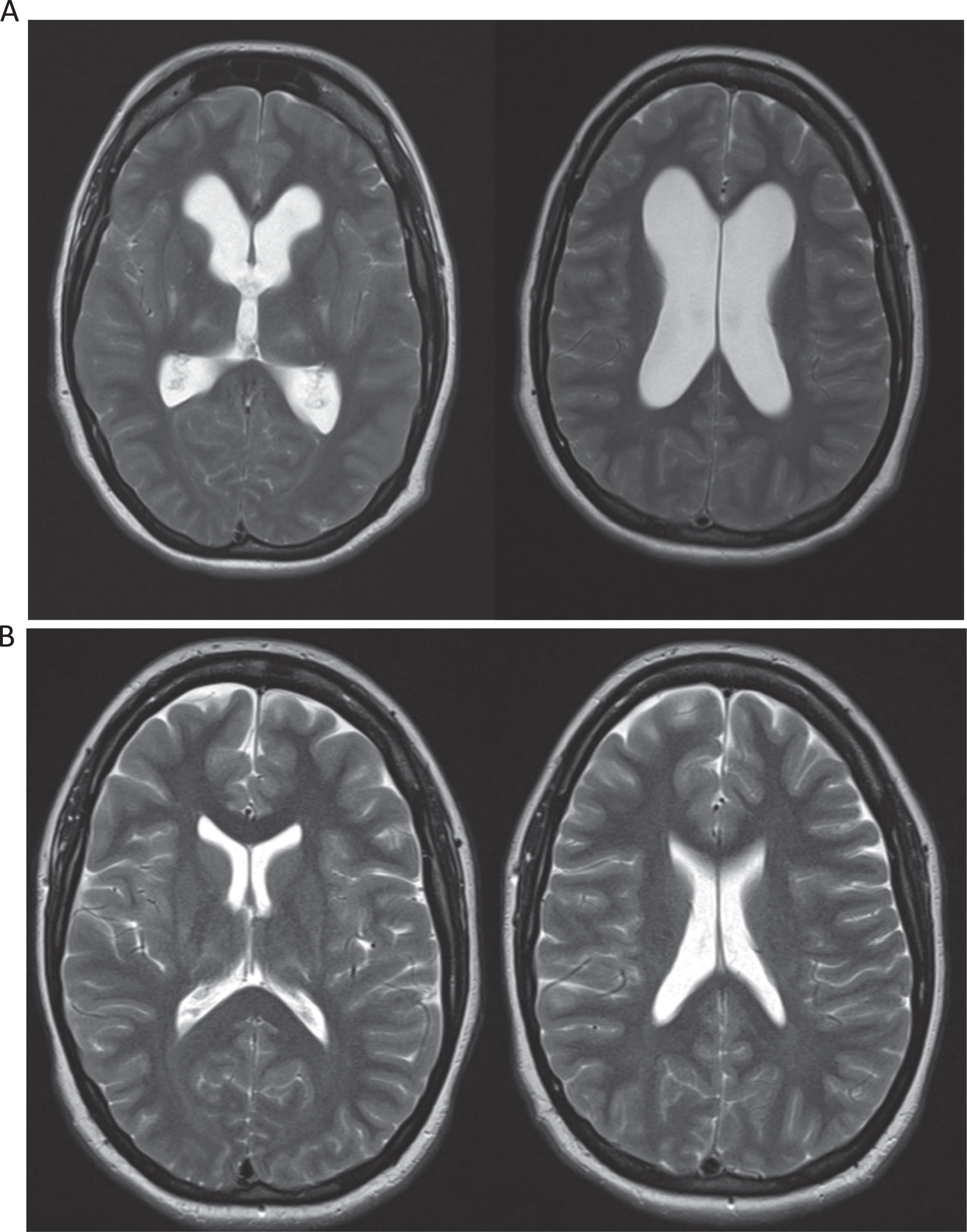
Given persistence of symptoms despite multimodal treatment, additional imaging of the neuraxis was obtained. The patient had a reassuring lumbar spine MRI without neuroforaminal or central stenosis and stable cervical spine MRI with post-surgical C1-C2 changes, chronic myelomalacia at C2, and mild degenerative findings at C6-7. However, an MRI of the brain demonstrated new diffuse moderate ventriculomegaly without transependymal edema compared to a prior MRI nine years earlier (Fig. 1A and B), compatible with non-obstructive hydrocephalus. There was associated papilledema (grade 2 on right, grade 3 on left) on ophthalmologic exam and elevated opening pressure of 44 cm H2O on lumbar puncture (LP). Cerebrospinal fluid (CSF) protein and glucose were both normal. While generalized hypertonia may have partly contributed to the elevated opening pressure, the MRI brain findings and papilledema were concerning for increased intracranial pressure. She therefore underwent CSF diversion via ventriculoperitoneal shunt replacement with rapid and near-resolution of her symptoms.
Fig. 1
T2 magnetic resonance imaging (MRI) studies of the brain of this patient during presentation (1A) and nine years prior (1B). MRI of the brain demonstrated new diffuse moderate ventriculomegaly without transependymal edema compared to prior MRI nine years earlier (Fig. 1A and B), compatible with non-obstructive hydrocephalus.
2Discussion
This is a unique and complex case describing new-onset of non-obstructive hydrocephalus in an adult with CP without other identified risk factors for hydrocephalus. Diagnostic evaluation made obstructive, hypersecretory, and normal pressure hydrocephalus unlikely. Imaging findings did not reveal any lesions blocking CSF flow leading to obstructive hydrocephalus. No tumors that may cause overproduction of CSF such as plexus papilloma or carcinoma were identified [1]. The elevated pressure on lumbar puncture ruled out normal pressure hydrocephalus. Though there was no transependymal edema and white matter was normal for age, papilledema and elevated opening pressure on LP along with the patient’s neurologic changes that improved with CSF diversion also made hydrocephalus ex-vacuo unlikely [3]. One manuscript by Albright et al. describes occult ventriculomegaly (without hydrocephalus) in childhood CP due to intraventricular hemorrhage or periventricular leukomalacia [4]. However, this patient’s cause of CP was in the setting of hypoxic ischemic injury. Additionally, she had multiple prior neuroimaging studies without hydrocephalus; therefore, this was not congenital hydrocephalus. Despite the clinical course and evaluation consistent with non-obstructive hydrocephalus, no causative preceding neurologic process such as acute hemorrhage or infection was identified. The patient did have a history of cervical myelopathy, and there are case reports describing obstructive hydrocephalus after a cervical spinal fracture and dislocation due to penetrating trauma (which this patient did not experience) [5, 6]. However, the bony compression and instability was previously repaired nine years prior, and neuroimaging did not demonstrate signs of obstruction. Additional diagnostic considerations included genetic testing [7] for assessment of risk or imaging studies of CSF flow to assess for abnormal flow patterns [8].
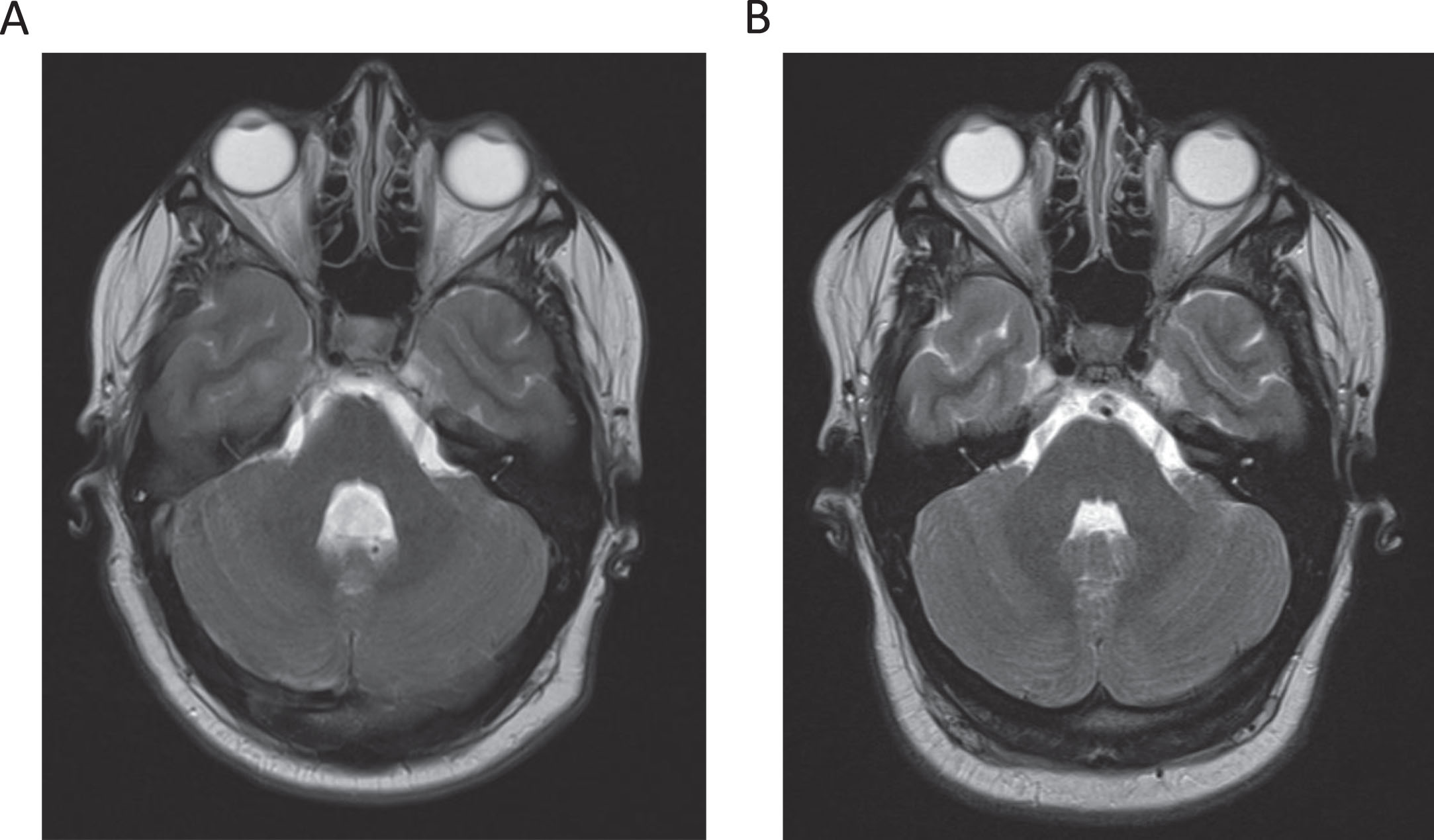
Fig. 2
T2 magnetic resonance imaging (MRI) studies of the brain of this patient during presentation (2A) and nine years prior (2B). MRI of the brain demonstrated new fourth ventricle ventriculomegaly compared to prior MRI nine years earlier.
This case highlights the critical need for ongoing neurologic surveillance in adults with CP [9] who are also at eight-times greater risk of myelopathy and two-times greater risk of stroke compared to the general population. They therefore require comprehensive evaluation for neurologic causes if presenting with changes in or development of new symptoms, including changes in spasticity, dystonia, or other patterns of hypertonia. Having a baseline MRI of the brain was valuable for comparison and interpretation of current imaging. It may be useful for patients with CP to have baseline imaging. Comprehensive evaluation was better able to be performed given her use of augmentative communication, emphasizing its importance not just for daily life but for medical needs as well. Seizures were also considered for this patient, though hydrocephalus was discovered prior to evaluation for seizures. Earlier identification in the patient may have led to earlier shunting, prevention of excessive procedures, and symptomatic improvement. As she wrote with her AAC device, “The spasms were really debilitating…I was up all night screaming in horrific pain, couldn’t eat, exercise, or enjoy life. I was so depressed – I thought I was dying.” Definitive treatment resulted in a profound improvement in comfort and function: “I can now do everything again.”
As far as the authors are aware, there are currently no studies describing new-onset hydrocephalus in adults with CP. Consequently, future research is needed to better understand if adults with CP are at higher risk for the development of hydrocephalus in adulthood. Furthermore, with a diagnosis as heterogeneous as CP, this risk may vary depending on type, distribution, and/or etiology of CP. As such, systematic study of the risk of and unique risk factors for hydrocephalus in adults with CP is needed. Overall, the growing population of those with CP warrants increased attention and specialized rehabilitation care to better understand their unique health risks.
Acknowledgments
The authors have no acknowledgments.
Conflicts of interest
No conflicts exist for authors. No funding was provided from any source and there were no financial benefits to the authors.
Contributions
Dr. Sarmiento was involved with the contribution to patient care, procedures involved, and creation of this manuscript. Drs. Ratnasingam and Roberts were involved with creation of this manuscript and literature review.
Ethical considerations/informed consent
Written consent and approval obtained from patient for this case report.
References
[1] | Koleva M , De Jesus O . Hydrocephalus, In: StatPearls [Internet].Treasure Island (FL): StatPearls Publishing; (2022) [cited 2023 Feb 6]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK560875/. |
[2] | Isaacs AM , Riva-Cambrin J , Yavin D , et al. Age-specific global epidemiology of hydrocephalus: Systematic review, metanalysis and global birth surveillance. PLoS ONE. (2018) ;13: (10):e0204926. doi: 10.1371/journal.pone.0204296. |
[3] | Adult-onset Hydrocephalus – Symptoms, Diagnosis and Treatments [Internet]. AANS Neurosurgical Members; 2024 [updated 2024 Jul 15; cited 2024 Jul 25]. Available from: https://www.aans.org/patients/conditions-treatments/adult-onset-hydrocephalus/. |
[4] | Albright AL , Ferson S , Carlos S . Occult hydrocephalus in children with cerebral palsy. Neurosurgery. (2005) ;56: (1):93. doi: 10.1227/01.neu.0000144779.32401.a2. |
[5] | Chung YY , Ju CI , Kim SW , Kim DM . Acute hydrocephalus as a complication of cervical spine fracture and dislocation: A case report. Korean J Spine. (2014) ;11: (2):74–6. doi: 10.14245/kjs.2014.11.2.74. |
[6] | Joseph G , Johnston RA , Fraser MH , McLean AN . Delayed hydrocephalus as an unusual complication of a stab injury to the spine. Spinal Cord. (2005) ;43: (1):56–8. doi: 10.1038/sj.sc.3101655. |
[7] | Garcia-Bonilla M , McAllister JP , Limbrick DD . Genetics and molecular pathogenesis of human hydrocephalus. Neurol India. (2021) ;69: (Supplement):S268–74. doi: 10.4103/0028-3886.332249. |
[8] | Korbecki A , Zimny A , Podgórski P , Sąsiadek M , Bladowska J . Imaging of cerebrospinal fluid flow: Fundamentals, techniques, and clinical applications of phase-contrast magnetic resonance imaging. Pol J Radiol. (2019) ;84: :e240–50. doi: 10.5114/pjr.2019.86881. |
[9] | Smith SE , Gannotti M , Hurvitz EA , et al. Adults with cerebral palsy require ongoing neurologic care: A systematic review, Ann Neurol. (2021) ;89: (5):860–71. doi: 10.1002/ana.26040. |




