REhabilitation Approaches in CHildren with cerebellar mutism syndrome (REACH): An international cross-disciplinary survey study
Abstract
OBJECTIVE:
Pediatric cerebellar mutism syndrome (pCMS) can occur following resection of a posterior fossa tumor and, although some symptoms are transient, many result in long-lasting neurological deficits. A multi-disciplinary rehabilitation approach is often used in cases of pCMS; however, there have been no clinical trials to determine gold standards in rehabilitation practice in this population, which remains a research priority. The purpose of this study was to identify and compare intervention practices used in pCMS throughout the disciplines of occupational and physical therapy, speech-language pathology, and neuropsychology across geographic regions.
METHODS:
A 55-question e-survey was created by an international multidisciplinary research group made up of members of the Posterior Fossa Society and sent to rehabilitation professionals in pediatric neuro-oncology centers in the US, Canada, and Europe.
RESULTS:
Although some differences in the type of intervention used in pCMS were identified within each discipline, many of the targeted interventions including dose, frequency, and intensity were similar within disciplines across geographic regions. In addition, there were common themes identified across disciplines regarding challenges in the rehabilitation of this population.
CONCLUSION:
These results provide a foundation of current practices on which to build future intervention-based clinical trials.
1Introduction
Pediatric cerebellar mutism syndrome (pCMS) occurs in 8–31% of children who undergo surgical resection of a tumor in the posterior fossa [1]. Results from a Delphi analysis were interpreted during a consensus meeting among an international group of professionals representing the disciplines of neurology, oncology, neuroradiology, neurosurgery, psychiatry, physiatry, neurolinguistics, neuropsychology (NP), speech-language pathology (SLP) and physiotherapy leading to the current definition of pCMS [2]:
Postoperative pediatric CMS is characterized by delayed onset of mutism/reduced speech and emotional lability after cerebellar or 4th ventricle tumor surgery in children. Additional common features include hypotonia and oropharyngeal dysfunction / dysphagia. It may frequently be accompanied by cerebellar motor syndrome, cerebellar cognitive affective syndrome and brainstem dysfunction including long tract signs and cranial neuropathies. The mutism is always transient. But recovery from CMS may be prolonged. Speech and language may not return to normal; and other deficits of cognitive, affective and motor function often persist.
Despite the extent of rehabilitation involvement in pCMS, there is a gap in the literature regarding the efficacy of therapeutic approaches used with this population [3] as well as rehabilitation in general pediatric neuro-oncology [4]. In order to lay the foundation for future interventions studies, current rehabilitation practices in pCMS must be identified. The purpose of this study was to establish a clearer understanding of current rehabilitation approaches and dose of intervention being used in children with CMS across rehabilitation disciplines as well as challenges to rehabilitation and resources needed for more efficacious treatment.
2Materials and methods
2.1Participants and recruitment
A database was created using contacts from the Posterior Fossa Society and an internet search of neuro-oncology and pediatric rehabilitation centers in the United States, Canada, and Europe, including the United Kingdom. A total of 484 emails and phone calls were placed to reach rehabilitation professionals in the fields of including occupational therapy (OT), NP, physical therapy (PT), and SLP. The survey link was distributed via email. Inclusion criteria for the participants were 1) experience in providing service to children with posterior fossa tumor resection with and/or without pCMS and 2) licensure and/or certification in a specific discipline. If neither of these criteria were met, the participants were excluded from the survey. If deemed eligible to participate, the survey automatically took respondents to the e-consent page of the survey.
2.2Survey design
A multidisciplinary research group of members of the Posterior Fossa Society created a 55-question e-survey in REDCap for rehabilitation professionals in the fields of SLP, OT, PT, and NP. The previous ASPECT study survey was used as a model [15]. It consisted of four parts: 1) demographic information including geographic region, primary clinical setting, gender, years of experience, and experience working with children with posterior fossa tumors; 2) aspects of intervention for children who had undergone posterior fossa tumor resection; 3) intensity, frequency and duration of treatment; and 4) goal-writing and discharge procedures. Survey questions included multiple choice, select all that apply, ranking, and rubric response options.
2.3Procedure
The research team sent an introductory email that described the nature of the study and a direct link to the survey to individuals in the database. The Institutional Review Board (IRB) deemed the study exempt from IRB approval due to the design. The survey was constructed in REDCap which also included an e-consent process. Survey completion was estimated to take between 25–30 minutes.
2.4Data analysis
There was a total of 55 questions asked in this survey and responses from 19 were used in this analysis (Table 1). Although not all respondents completed the entire survey, all responses for questions were utilized in this analysis. As such, all responses were recorded and analyzed regardless of completion. The questions that required a selection of a response from a given list were analyzed by simply calculating the percentage for each discipline. To better understand the relationships between scaled variables and demographic (ordinal) variables, Spearman’s rank correlation analysis was conducted and, where appropriate, factorial analysis of variance was used to determine main effect and interaction. The questions requiring a written narrative response were transcribed into an Excel database and reviewed by the research team to create themes or categories of responses.
Table 1
Survey questions chosen for this analysis
| Survey question | Disciplines | |||
| NP | OT | PT | SLP | |
| Which type of therapy intervention do you use to treat children with posterior fossa tumors? Choose all that apply from a list. | X | X | X | X |
| Which of the therapy interventions do you use most often in children with posterior fossa tumors? Choose one from a list. | X | X | X | X |
| Rank the top three interventions you think are most effective for this population. | X | X | X | X |
| What adjuncts to therapy intervention do you use in children with posterior fossa tumors? Choose all that apply from a list. | X | X | X | |
| Which adjuncts do you use most often? Choose one from a list. | X | X | X | |
| Do you base your intervention on any clinical guidelines specifically for the treatment of children with posterior fossa tumors? Yes/No If yes, specify in textbox. | X | X | X | X |
| Do you base your intervention on any research evidence? Yes/No If yes, specify in textbox. | X | X | X | X |
| How often do you typically treat these children in an inpatient setting? Choice of frequency. | X | X | X | X |
| How often do you typically treat these children in the outpatient/community/school setting? Choice of frequency. | X | X | X | X |
| What is the length of a typical intervention session? Choice of session length. | X | X | X | X |
| What is the mode of delivery used for intervention? | X | X | X | |
| Do you feel therapy sessions are optimally timed with regard to frequency, session length, and duration? Yes/No If no, describe barriers. | X | X | X | |
| Are there any frequent problems/challenges you encounter when treating children following surgical resection of posterior fossa tumor? Yes/No If yes, describe. | X | X | X | X |
| Please list any common problems you have identified in relation to therapy management with the transition from inpatient to outpatient setting. | X | X | X | X |
| Please list any common problems you have identified in relation to therapy management regarding reintegration into the school setting. | X | X | X | X |
| How beneficial do you believe your intervention to be in this population? If no, what is needed to improve the benefit? | X | X | X | X |
| Do you administer neuropsychological assessments to children with posterior fossa tumors? Yes/No If yes, choose all that apply from a list. | X | |||
| Which area of functioning do you assess in children with posterior fossa tumors? Choose all that apply from a list. | X | |||
| Do you use a specific model or approach to intervention for children with posterior fossa tumors? Yes/No If yes, specify. | X | |||
3Results
There were a total of 134 respondents across all disciplines, 84 (63%) of whom fully completed the survey and 49 (36%) of whom completed a portion. Sample sizes per discipline can be found in Table 2. Total years of experience and years of experience providing service to children with brain tumors are reported in Table 3, and geographic distribution can be found in Table 4. The majority of NP respondents were evenly divided between inpatient and outpatient facilities at 38% each. All OT respondents reported working in an inpatient facility. Of the 75 PT respondents, 89% worked in an inpatient setting, 64% an outpatient setting, 64% in clinics, 6% in community, and 10% in schools. Some respondents reported working across more than one setting. Fifty-seven percent of SLP respondents reported their primary setting to be inpatient with 26% reporting an outpatient setting and one reporting working in a clinic setting. None of the SLP respondents worked in community or school settings.
Table 2
Sample size distribution by discipline. Number of individuals who completed the survey across disciplines of neuropsychology (NP), occupational therapy (OT), physical therapy/physiotherapy (PT), and speech-language pathology (SLP)
| NP | OT | PT | SLP | TOTAL | |
| Total Respondents | 21 | 11 | 75 | 26 | 134 |
| Incomplete Survey | 5 | 2 | 33 | 9 | 119 |
| Complete Survey | 16 (76%) | 9 (81%) | 42 (56%) | 17 (65%) | 84 |
Table 3
Reported years of experience across disciplines
| Discipline | Total years of experience | Years of brain tumor care experience | ||||||
| 0–2 years | 3–5 years | 6–9 years | >10 years | 0–2 years | 3–5 years | 6–9 years | >10 years | |
| Neuropsychology | 0 | 14.3% | 28.6% | 57.1% | 4.8% | 33.3% | 19% | 42.9% |
| Occupational Therapy | 9.1% | 9.1% | 36.4% | 45.5% | 18.2% | 27.3% | 45.5% | 9.1% |
| Physical Therapy | 2.6% | 13.3% | 24% | 60% | 13.3% | 29.3% | 14.6% | 42.6% |
| Speech-Language Pathology | 0 | 19.2% | 23.1% | 53.8% | 23.1% | 26.9% | 11.5% | 34.6% |
Table 4
Geographic representation by discipline across neuropsychology (NP), occupational therapy (OT), physical therapy/physiotherapy (PT), and speech-language pathology (SLP)
| NP | OT | PT | SLP | |
| N = 21 | N = 11 | N = 75 | N = 26 | |
| Austria | 1 | 0 | 0 | 0 |
| Belgium | 4 | 0 | 2 | 1 |
| Denmark | 1 | 0 | 2 | 0 |
| England | 2 | 2 | 16 | 9 |
| France | 0 | 1 | 1 | 0 |
| Hungary | 0 | 0 | 1 | 0 |
| Ireland | 0 | 1 | 5 | 1 |
| Israel | 1 | 0 | 0 | 0 |
| Italy | 1 | 0 | 5 | 2 |
| Malta | 0 | 0 | 1 | 0 |
| Netherlands | 0 | 1 | 0 | 0 |
| Portugal | 1 | 0 | 0 | 0 |
| Russia | 2 | 0 | 0 | 0 |
| Scotland | 0 | 0 | 2 | 0 |
| Spain | 1 | 0 | 4 | 3 |
| United States | 7 | 6 | 36 | 10 |
3.1Types of interventions used across disciplines
3.1.1NP
Neuropsychologists responded to questions regarding types of assessments and interventions provided to children with CMS. NP respondents reported 1) types of assessment used to be neuropsychological tests (100%), interview (94%), and parent-reported questionnaires (94%) and 2) areas of assessment to be intelligence (94%), attention (94%), memory (94%), working memory (94%), executive functions (88%), visuomotor functioning (88%), emotional (88%), language (81%), visuospatial (81%), social (81%), motor functioning (69%), and academic (69%). When asked to identify one intervention they used most often, responses revealed psychoeducation (81%) followed by school interventions (69%), cognitive remediation (61%), cognitive behavioral therapy (CBT, 38%), supporting parent-child interaction (25%), and to a lesser extent, play therapy, problem-focused therapy, computerized cognitive training, group therapy, and psychodynamic or interpersonal therapy (less than 20% ; Fig. 1). None of the participating neuropsychologists reported using neurofeedback training. When asked to rank the top three interventions thought to be most effective in this population, pooled responses indicated school interventions, followed by CBT and psychoeducation (Table 5). The main goals of interventions were providing management strategies for parents (94%), supporting daily living (75%), training of neurocognitive functions (82%), supporting academic functioning and dealing with behavioral problems (69%), supporting emotional and social functioning (62%), and training of metacognitive skills (50%). Respondents reported the focus of neurocognitive training to be executive function (82%), attention (75%), and working memory (69%). With a small, unbalanced sample, comparisons in types of assessments and interventions used across geographic regions were unable to be conducted.
Fig. 1
Types of interventions used with children with CMS reported by A) neuropsychologists B) occupational therapists C) physical therapists/physiotherapists and D) speech-language pathologist across geographic regions. X axis represents the number of respondents; the y axis represents the types of interventions reported. In the occupational therapy data, there was also one response for each: yoga, virtual training and biofeedback. Education (Edu); Activities of Daily Living (ADLs); Neurodevelopmental Training (NDT); Range of Motion (ROM); Augmentative and Alternative Communication (AAC).
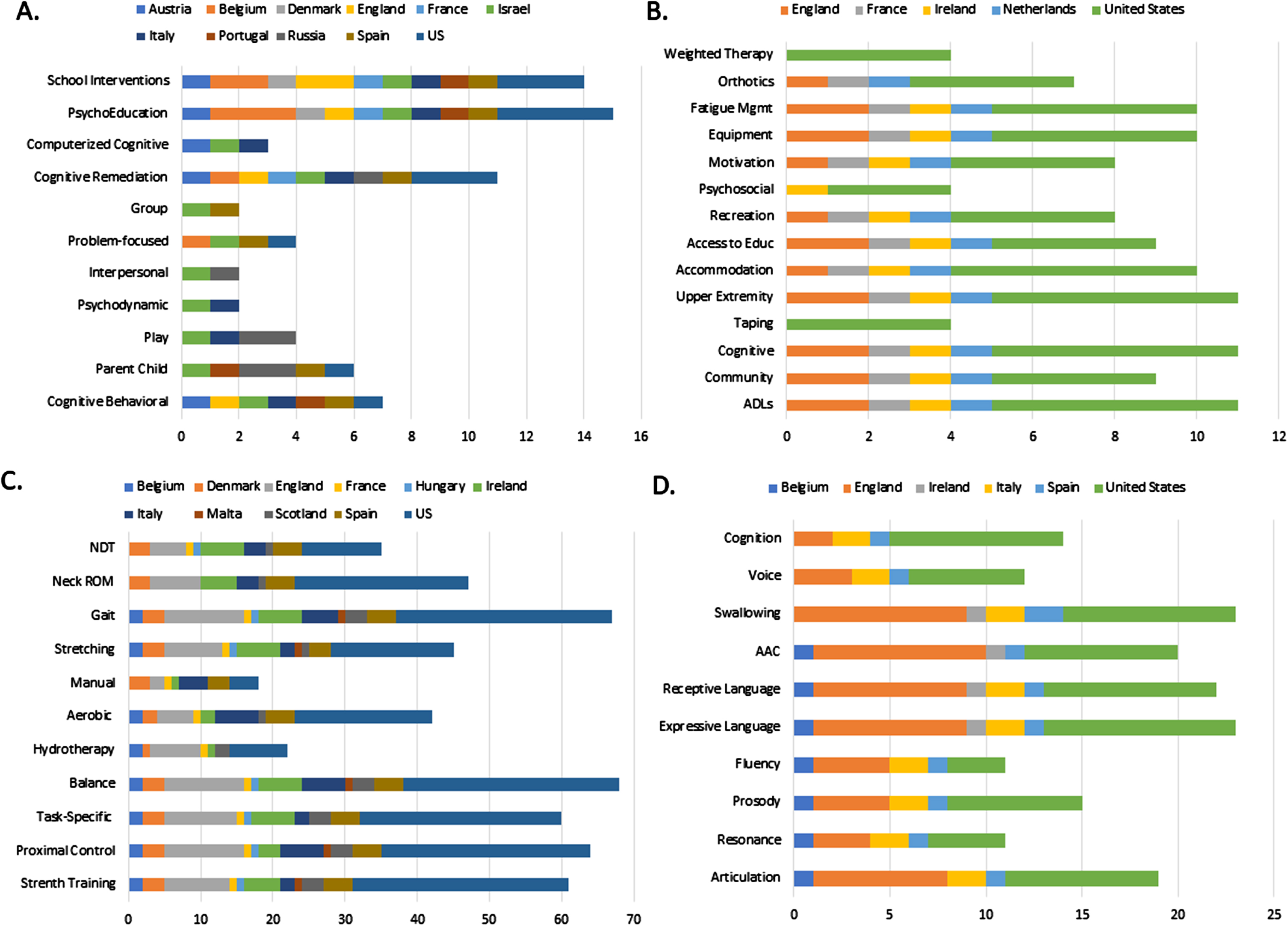
Table 5
Top ranked interventions believed to be the most effective in children with cerebellar mutism syndrome reported across the disciplines of neuropsychology (NP), occupational therapy (OT), physical therapy/physiotherapy (PT), and speech-language pathology (SLP)
| Ranking | NP | OT | PT | SLP |
| 1 | School Interventions | Upper Extremity Function | Gait Re-Training | Swallowing Intervention |
| 2 | Cognitive Behavioral | Adaptive Equipment | Balance | Augmentative/ Alternative Communication (AAC) |
| 3 | Psychoeducation | Activities of Daily Living | Proximal Control Activities | Articulation Intervention |
3.1.2OT
Types of interventions reported to be used with children with CMS included access to education (81%), accommodations (90%), activities of daily living (ADLs; 100%), adaptive equipment (90%), cognitive rehabilitation (100%), community re-integration (82%), fatigue management (90%), motivation (72%), orthotics (63%), psychosocial adaptation (36%), recreation (72%), taping (36%), upper extremity function (100%), and weighted therapy (36% ; Fig. 1). Yoga, virtual training, and biofeedback were each reported once. When asked to identify the one intervention they used most often in this population, 72% reported ADLs and 27% reported upper extremity activities. The top three interventions identified as the most effective in this population were upper extremity function followed by adaptive equipment and ADLs (Table 5). With a small, unbalanced sample, comparisons of types of interventions used across geographic regions were unable to be conducted.
3.1.3Physiotherapy/PT
The results indicated physiotherapists used a range of interventions including aerobic (54%), balance (90%), gait retraining (89%), hydrotherapy (32%), manual therapy (22%), neck range of motion (61%), neurodevelopment treatment (48%), proximal control activities (86%), strength training (81%), stretching (60%), and task-specific activities (80%). A few respondents added a text response to include other intervention types: bracing, wheelchair training/management, caregiver education, sensory integration, play therapy, and gross motor activities (Fig. 1). The three intervention types reportedly used most often were task-specific training (24%), balance exercises (21%), and proximal control activities (22% ; Fig. 2). When asked to rank the top three interventions perceived to be the most effective in this population, results revealed gait retraining to be ranked as the most effective intervention followed by balance and proximal control activities (Table 5).
Fig. 2
Interventions used “most often” with children with CMS reported by occupational theraposts (top), physical therapists (middle) and speech-language pathologists (bottom) in the UK (n = 9), US (n = 10), Spain (n = 3), Belgium (n = 1), Ireland (n = 1) and Italy (n = 2). Range of Motion (ROM).
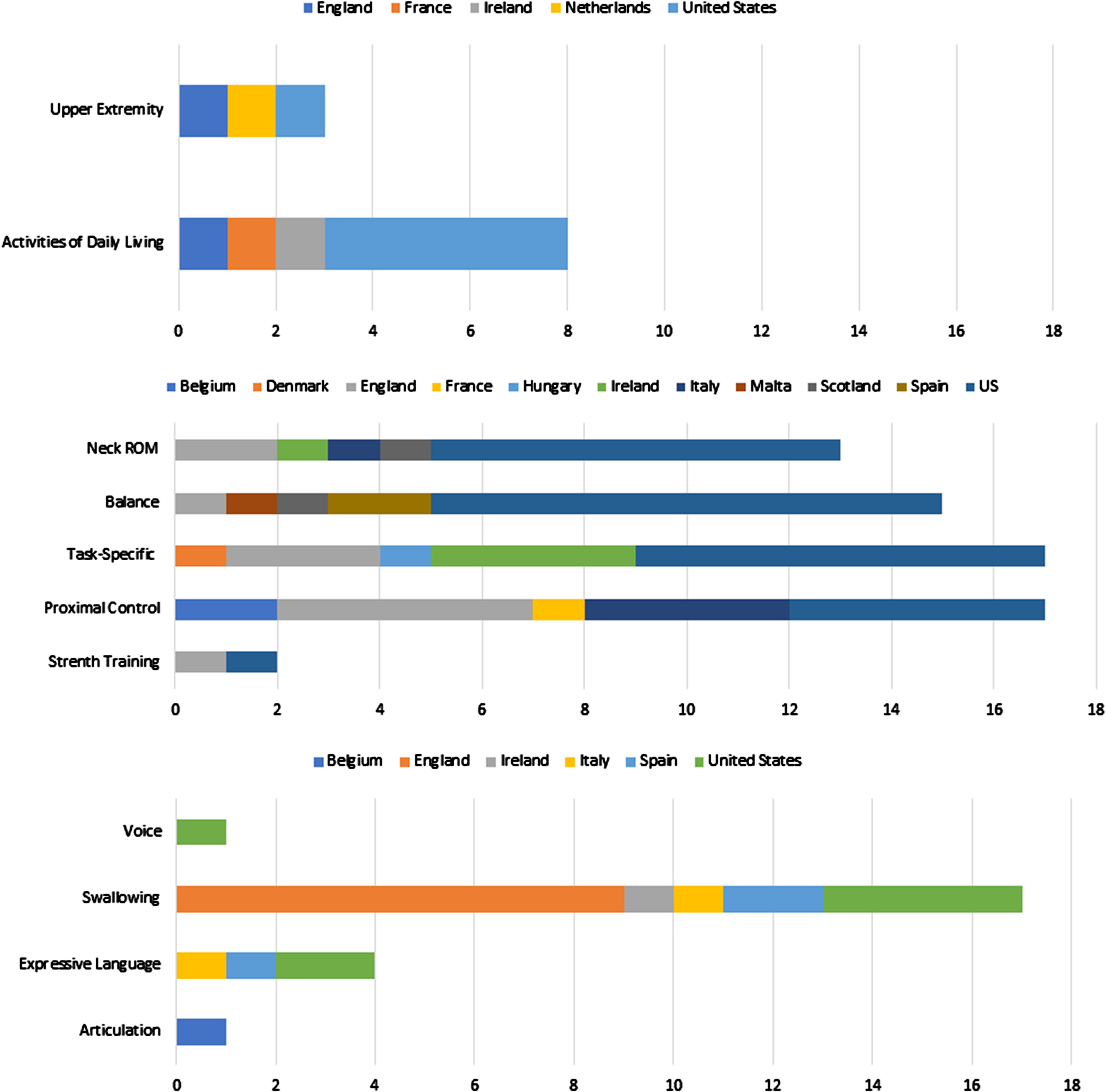
Since the sample size for physiotherapists was higher, practices in the UK (n = 21) and the US (n = 36) were compared. Proximal control activities were more commonly reported in the UK while balance exercises were more commonly reported by physiotherapists in the US.
From a predetermined list of “adjuncts to therapy,” respondents then selected which types they used in this population (multiple responses were possible). The adjunct therapy methods most often used were walking/mobility aids (42%) followed by treadmill training (12%) and weighted therapy (5%). Virtual training and Lycra garments were each chosen by one participant.
3.1.4SLP
When asked to identify the type(s) of interventions used for children with CMS, respondents reported articulation (73%), augmentative/alternative communication (AAC; 76%), cognition (53%), expressive language (84%), fluency (42%), prosody (57%), receptive language (84%), resonance (42%), swallowing (88%), and voice (46% ; Fig. 1). When asked to identify the intervention used most often, 65% reported swallowing therapy and 15% reported expressive language therapy (Fig. 2); articulation therapy and voice therapy each also received one selection. Finally, when asked to rank the top three interventions they perceived to be the most effective in children with CMS, swallowing therapy was ranked number one, followed by AAC and finally articulation therapy (Table 5).
When asked what adjunctive therapy was used most often, respondents could choose one from a predetermined list. The most common response was “other” (61%) with write-in answers including sensory integration, music therapy, co-treating with OT and PT, electrical stimulation, and “no adjuncts used/available.” Auditory discrimination was chosen as the most common adjunct therapy by 22% of respondents, with virtual reality training chosen by two and biofeedback and aquatic therapy chosen by one each. Differences in interventions used across geographic regions could not be formally analyzed due to a small and unbalanced sample.
3.2Impact of experience on intervention
No significant relationship was found between total years of experience and 1) number of interventions used (r = -0.013, p = 0.88) or 2) number of adjuncts used (0.007, p = 0.94). There was also no significant relationship found between years of experience providing service to children with brain tumors and 1) number of interventions used (r = 0.043, p = 0.62) or 2) number of adjuncts used (0.106, p = 0.29).
3.3Dose of intervention and delivery method
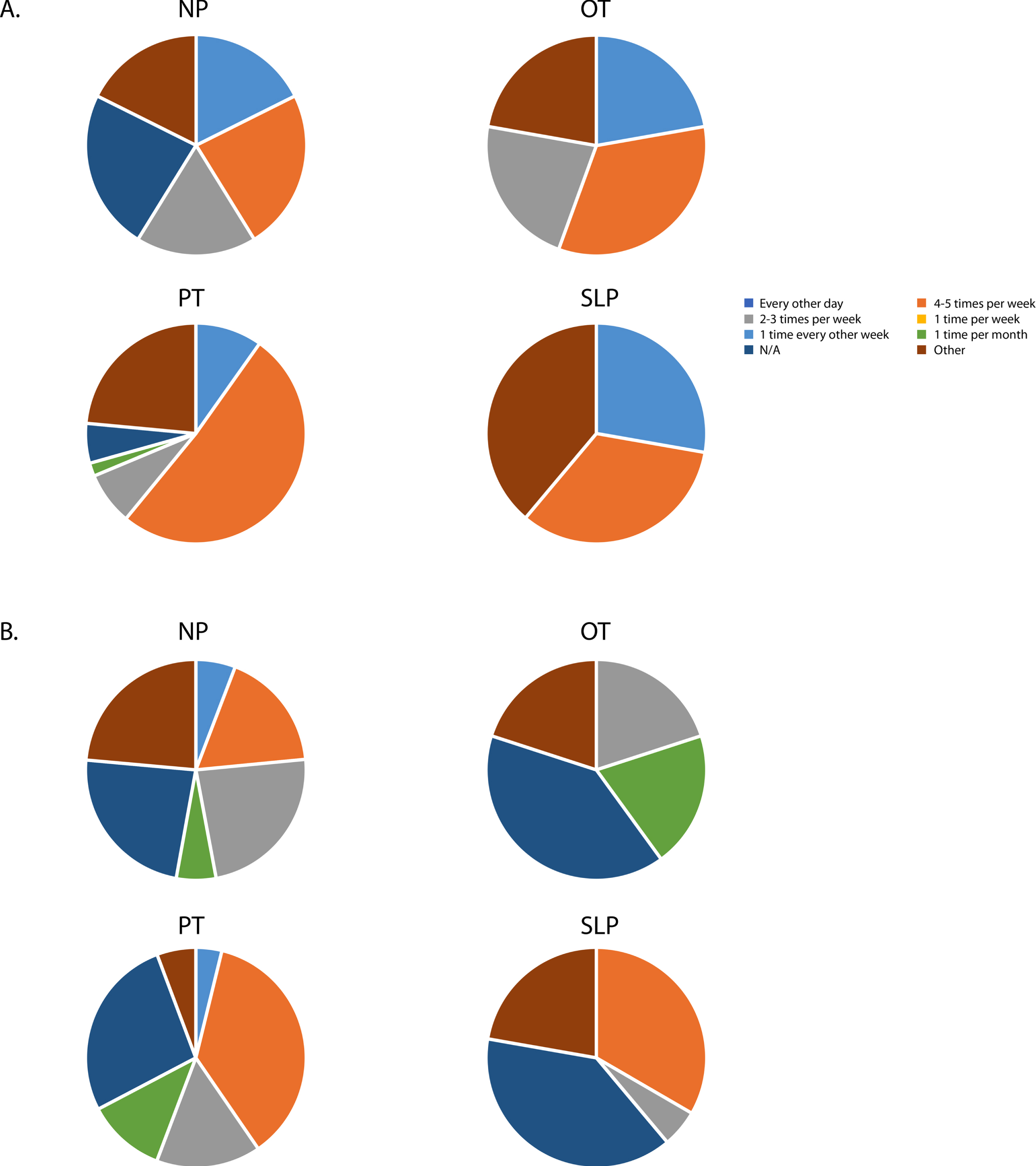
As expected, the frequency of intervention visits was higher in inpatient settings across disciplines with the majority of respondents reporting a frequency of 4–5 times per week and “other;” the latter category included typed responses which ranged from 6–7 days per week to twice per day 5–6 days per week. In outpatient settings, NP, PT, and SLP commonly reported 4–5 visits per week and every other day while OT in outpatient settings more commonly reported every other day and one time per month (Fig. 3). With such a small sample for NP, OT, and SLP, differences in dose across geographic regions were unable to be determined.
Responses for the typical length of therapy sessions were aggregated across OT and PT disciplines. This question was not asked of NP respondents, and the SLP responses were not included in the exported data, possibly due to an error in REDCap. Participants reported the duration of intervention sessions to be equally divided between 30–45 minutes (40.9%) and 45–60 minutes (40.9%) with another 4.5% reporting > 60 minutes and two participants < 30 minutes.
Fig. 3
Frequency of intervention visits across disciplines in an A) inpatient setting and B) outpatient setting. Neuropsychology (NP); Occuapational Therapy (OT); Physical Therapy (PT); Speech-Langauge Pathology (SLP).
Raw data was aggregated across disciplines to report the delivery method of intervention. The majority of respondents chose individual intervention with therapist (86%), with 8.7% reporting multidisciplinary intervention and 1.2% reporting individual intervention with a therapy assistant.
3.4Decision-making
The majority of respondents across disciplines stated they did not base clinical decisions on clinical guidelines (NP = 81.3%, OT = 90.9%, PT = 77.3%, and SLP = 69.2%) because there are no clinical guidelines specific to children with CMS. Although a lesser percentage reported they did not base their intervention on scientific evidence (NP = 36.4%, OT = 45.5%, PT = 33.3% and SLP = 42.3%), many respondents stated they used research findings from related areas including pediatric brain tumor, cerebral palsy, cognitive rehabilitation, pediatric cancer, neuroplasticity, and ataxia literature to guide decision-making, and some reported utilizing a “treat by symptom approach.”
3.5Perceived benefit of intervention
Respondents were asked to rate their perception of the benefit of intervention in this population as exceptionally beneficial (12.5% NP, 24% PT, and 29% SLP), moderately beneficial (75% NP, 100% OT, 74% PT, and 71% SLP), or minimally beneficial (12.5% NP and 2% PT). To better understand the relationship between perceived benefit of intervention and experience, responses across disciplines were aggregated and a Spearman’s rho correlation was conducted. Results revealed no relationship between perceived benefit and 1) total years of experience (r = -0.031, p = 0.78), 2) total years of experience providing services to children with brain tumors (r = 0.020, p = 0.858) or 3) cases of pCMS per year (r = -0.032, p = 0.73). Results from a factorial ANOVA revealed no main effect of number of interventions used (F = 0.85, p = 0.57) or number of adjunct interventions used (F = 1.90, p = 0.11) and their perceived benefit, and no interaction between number of interventions and number of adjunct therapies used (F = 0.80, p = 0.67). Thus, results indicated that the number of interventions and adjunct therapies did not have an impact on the perceived benefit.
3.6Challenges to rehabilitation
Responses to the question “What are frequent problems/challenges you encounter when treating this population?” were categorized (Table 6) and later coded for thematic analysis. Three overarching themes emerged across all disciplines: 1) timing of rehabilitation, 2) standards of care including training and mentorship, and 3) resources including staffing, time, and space (Fig. 4). Regarding the timing of rehabilitation, 28% of all respondents across disciplines reported fatigue, adverse behaviors, and difficulty engaging the child in intervention as a barrier to progress. Thirty-eight percent of all respondents reported the behavioral impacts of cancer therapies (specifically, chemotherapy and radiation) to be a barrier to functional recovery. Some reported that timing of rehabilitation sessions around chemotherapy and/or radiation schedules was a primary challenge and rehabilitation sessions at their facility often got canceled due to fatigue following radiation. Regarding the second theme, 40.2% of all respondents reported the need for standards of care, training, and mentorship in providing rehabilitation services to children with pCMS and their families.
Table 6
Categorized responses to qualitative questions regarding challenges and resources needed in the provision of services in pediatric cerebellar mutism syndrome (CMS) across disciplines and geographic regions
| Question | Categories |
| Reported challenges providing | Scheduling |
| services to children with CMS | Patient behaviors and variability of symptoms associated with CMS |
| Access to rehabilitation and/or equipment | |
| Behavioral and cognitive impact of chemotherapy and radiation therapy | |
| Availability of services | |
| Lack of intervention evidence, practice guidelines, and professional resources including training | |
| Communication | |
| Awareness/Support | |
| Reported resources needed to improve | Multidisciplinary approach |
| service provision for children with CMS | More evidence, research, education |
| More funding, access to resources | |
| Increased staffing | |
| Standardized therapy approaches/strategies, rating scales for diagnosis | |
| More time | |
| Family and patient education and community education; increased awareness | |
| More rehabilitation services | |
| Specialized rehabilitation spaces | |
| Transition/Continuity of care |
Fig. 4
Thematic interpretation of responses identifying challenges to providing rehabilitation to children with Cerebellar Mutism syndrome.
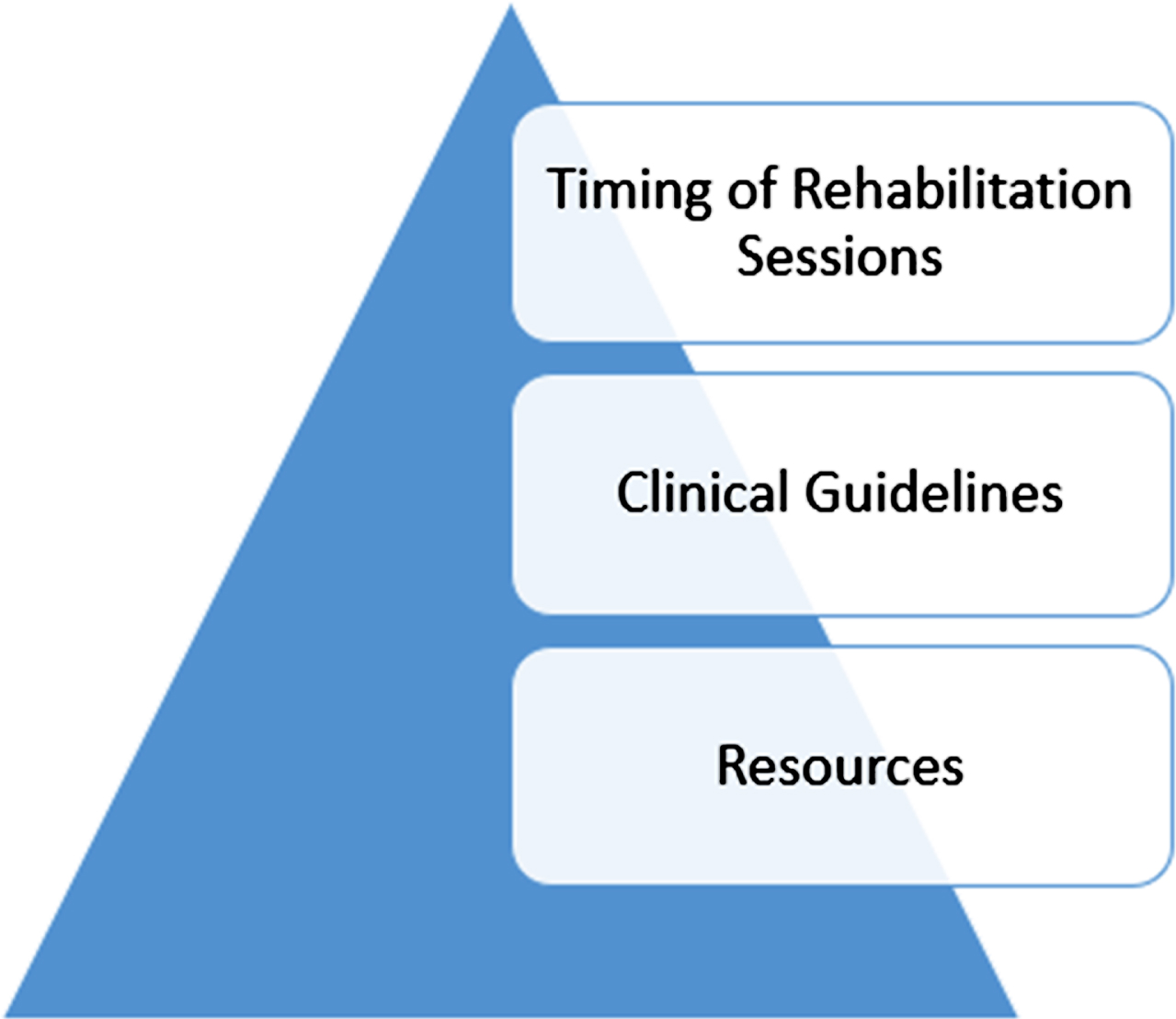
When asked “What is needed to improve the efficacy of rehabilitation in children with CMS?” the majority of responses fit into the categories of 1) clinical guidelines, 2) standard intervention approaches, and 3) evidence. Additional responses included the need for clinician training, more resources (staff, time, space), continuity of services from hospitals and clinics into the communities and schools, pathways for pre-and post-operative services, and finally requests for a standardized rating scale to measure progress.
4Discussion
The need for provision of rehabilitation services to individuals with a diagnosis of brain tumor is well-established in adults [5–8] and pediatric patients [4, 9–13]. In a recent review of brain tumor aftercare in adults, Sierpowska and colleagues reported more institutions across Europe made referrals for speech therapy and neuropsychological rehabilitation than physiotherapy and OT, although a high proportion identified physiotherapy to be essential [14]. However, details regarding the type, intensity, and frequency of rehabilitation have yet to be established. In addition, children with CMS present a unique challenge due to the constellation of complex symptoms that accompany the diagnosis. The REhabilitation Approaches in CHildren (REACH) survey was created to document current rehabilitation practices across disciplines and receive feedback about challenges they face when providing services to this population.
4.1Type of intervention
Rehabilitation does lead to improved outcomes following brain tumor resection in adults [15–17] and pediatric patients [18]. Although functional outcomes following rehabilitation were not the purpose of this survey study, it is important to note interventions reported by respondents were consistent with those reported as beneficial in the literature. There was agreement among respondents that there was a lack of evidence in intervention approaches specific to children with CMS. Although evidence is limited, range-of-motion exercise, progressive resistive exercise, balance training, endurance training, gait training, cognitive rehabilitation, upper extremity proprioceptive neuromuscular facilitation, and ADL training appear to be efficacious in adults post brain tumor resection [19] and children with ataxia following a traumatic brain injury [20, 21]. Marsden has suggested that intensive rehabilitation including pharmacological interventions and noninvasive electric stimulation of the cerebellum may be in future rehabilitation plans for children who present with cerebellar ataxia [22]. Dysphagia treatment has been shown to be an effective intervention for adults following brain tumor resection [23]. Although reportedly used by few NP respondents, computer-based cognitive rehabilitation programs have been gaining recent attention due to the reported benefits in children following brain tumor resection [24, 25].
Interventions reported to be used most often were not always consistent with those thought to be “most effective.” In NP, although CBT was reported as one of the top three most effective modalities, it was reportedly used less than cognitive remediation. In physiotherapy, gait retraining was ranked the most effective intervention for children with CMS; however, only 18% of clinicians reported it as the most commonly used intervention. This discrepancy could be due to the high number of clinicians reporting from inpatient facilities in which the children may not yet be ready for gait training. In SLP, although AAC and articulation therapy were reported as two of the most effective interventions in pCMS, neither were reportedly “used most often.” In fact, only one respondent identified articulation therapy to be used most often and no respondents reported AAC to be used most often. Access to AAC technology/devices and expertise could be a factor in this finding. Also, clinicians reported using expressive language intervention often. However, there was no opportunity for them to specify if low-tech AAC approaches were used, which are common in early stages of recovery. In other words, AAC could have been used but grouped in with expressive language therapy by respondents. Although articulation therapy was ranked the third most effective intervention, only one clinician reported using this intervention most often. Interestingly, this respondent reported working in an outpatient facility where, more than likely, patients are in the subacute to chronic/late stages of recovery. This may explain the discrepancy between the high ranking of effectiveness of articulation therapy and the lack of reported use. Typically, during inpatient rehabilitation, SLP prioritizes swallowing and basic communication due to urgency. In addition, children with CMS often do not have the endurance, self-awareness, attention, and auditory/visual perception skills needed for articulation therapy in acute and even subacute stages of recovery. Therefore, articulation therapy may not have been reported as “used most often” by SLP respondents working in inpatient facilities. Interestingly, years of experience did not have an influence on number of interventions or adjuncts. Clinicians with different experience levels chose similar number(s) of intervention types and adjunct therapies for children with CMS.
Across all disciplines, not only the complex nature of pCMS, but also the number of different intervention approaches (and assessment areas in NP) revealed the challenge faced by rehabilitation professionals in prioritizing functional targets. In lieu of clinical guidelines and standards of practice in intervention for pCMS, clinicians reported using a symptom-based approach, choosing interventions that have been shown to be efficacious for other pediatric and adult populations.
4.2Dose of intervention
Dose of intervention is often dictated by clinical models, facility resources, and third-party reimbursement rather than evidence-based practice. A standardized dose of behavioral neurorehabilitation following brain tumor resection has not been identified. In the current study, respondents across OT, PT, and SLP reported similar dose of intervention sessions across disciplines, between 30–60 minutes per session, 4–5 times per week or more during inpatient care and 4–5 times per week or less during outpatient care. Session duration was in agreement with that reported across European institutions in adults after brain tumor surgery [14]. This dose is similar to past reports of positive outcomes. For example, “high” intensity rehabilitation, defined as one hour each of PT and OT five days per week, was found to be beneficial in the recovery of adults following brain tumor resection [19]. In the adult stroke literature, 30–60 minutes, 6–7 days per week has been deemed an “effective dose” of physiotherapy [26].
Another factor of rehabilitation is the timing of assessment points and intervention sessions, not only in relation to post-surgery, but also around chemotherapy/radiation and across disciplines. The survey conducted by Sierpowska and colleagues offers insight to current assessment timepoints being most frequently reported within 10 days of surgery and again between 2–5 months post-surgery and one year post-surgery. Fewer institutions reported assessments during the acute phase (defined as 11–60 days) and late phase (defined as 5–12 months) [14]. These authors also reported timing of the initiation of rehabilitation within 1–2 weeks post-surgery across disciplines. Optimal timing of rehabilitation, specifically cognitive rehabilitation has been identified as a need in adults following brain tumor resection [27]. Because rehabilitation progress can be negatively impacted by the cognitive-behavioral effects of chemotherapy [28, 29] and radiation therapy [30–32], determining when intervention sessions should be scheduled around these therapies is critical. The current state of the literature and results from this survey indicated that identifying optimal timing of rehabilitation sessions during and beyond cancer treatment should be considered a research priority in rehabilitation planning following brain tumor resection. In addition, no studies investigating the sequence of discipline-specific interventions on recovery were found for any population. For example, do individuals perform better in speech-language therapy sessions if they are preceded by PT? In other words, can one intervention session act as a “primer” for neuroplastic change? The principles of neuroplasticity need to be applied to this population to ensure types of intervention, dose, and timing of intervention promote true neuroplastic recovery versus maladaptive neuroplasticity [33].
4.3Challenges and perceived benefit
Establishing efficacy of intervention approaches across disciplines and dose and timing of intervention sessions across recovery stages is a critical step in creating standards of care and clinical guidelines for children with CMS. Results from open-ended questions regarding challenges and resources needed revealed a clear agreement of the need for clinical guidelines for the rehabilitation of children with CMS. While there are clinical guidelines for the management of brain tumors, the few that include rehabilitation are lacking the level of detail needed for clinicians to utilize them [34]. Previously established guidelines for rehabilitation in pediatric cancer [35] and speech-language services in pediatric brain tumor and leukemia [36] could serve as a framework for creating guidelines across all rehabilitation disciplines providing services for children with CMS. Without standards of care and clinical guidelines, formal training and mentorship programs cannot be established. Responses in this survey were in agreement with the current literature regarding the need for additional education and training to serve this population [37]. While some multidisciplinary education programs have been established for rehabilitation for children with cancer, with a portion of training dedicated to content around children with CMS [38], there are no known formal rehabilitation training programs specific to children with CMS at this time. It could be hypothesized that the development of such frameworks and training would improve clinicians’ perception of rehabilitation benefits.
5Conclusion
The array of complex symptoms of CMS combined with the impact(s) of cancer treatments pose a unique challenge to rehabilitation professionals charged with creating effective, evidence-based, individualized treatment plans. At the 2018 Posterior Fossa Society Consensus Meeting, a multidisciplinary subcommittee was formed to create a research agenda to address the rehabilitation needs of children with CMS. The survey results reported here will be used to drive future work of the subcommittee, including designing intervention studies that will provide much needed outcome data for standards of care and clinical guidelines. Once established, structured, specific training and mentorship programs can be created across disciplines.
Functional recovery of cognition, communication, and motor skills following posterior fossa tumor resection resulting in pCMS can be negatively impacted by obstacles to the rehabilitation process such as the surgically induced injury, cranial radiation, and the behavioral sequelae that accompany both. The long-term goal of the rehabilitation working group of the Posterior Fossa Society is to create clinical practice guidelines for the rehabilitation of children with CMS by identifying efficacious interventions or combinations of interventions including optimal timing.
Acknowledgments
Members of the Posterior Fossa Society.
Conflict of interest
The authors have no conflicts of interest.
Ethical considerations
This study, as an anonymous survey, is exempt from IRB approval.
All participants completed an e-consent process within the survey through REDCap.
References
[1] | Catsman-Berrevoets CE . Cerebellar mutism syndrome: cause and rehabilitation. Curr Opin Neurol. (2017) ;30: (2):133–9. doi: 10.1007/s00381-011-1509-7. |
[2] | Gudrunardottir T , Morgan AT , Lux AL , et al. Consensus paper on post-operative pediatric cerebellar mutism syndrome: the Iceland Delphi results. Childs Nerv Syst. (2016) ;32: (7):1195–203. doi: 10.1007/s00381-016-3093-3. |
[3] | Paquier PF , Walsh KS , Docking KM , Hartley H , Kumar R , Catsman-Berrevoets CE . Post-operative cerebellar mutism syndrome: rehabilitation issues. Childs Nerv Syst. (2020) ;36: (6):1215–22. doi: 10.1007/s00381-019-04229-6. |
[4] | Pruitt DW , Bolikal PD , Bolger AK . Rehabilitation Considerations in Pediatric Brain Tumors. Curr Phys Med Rehabil Rep. (2019) ;7: (2):81–8. doi: 10.1007/s40141-019-00218-7. |
[5] | Thakkar P , Greenwald B , Patel P . Rehabilitation of Adult Patients with Primary Brain Tumors: A Narrative Review. Brain Sci. (2020) ;10: (8):492. doi: 10.3390/brainsci10080492. |
[6] | Kushner DS , Amidei C . Rehabilitation of motor dysfunction in primary brain tumor patients†. Neuro-Oncol Pract. (2015) ;2: (4):185–91. doi: 10.1093/nop/npv019. |
[7] | Bell KR , O’Dell MW , Barr K , Yablon SA . Rehabilitation of the patient with brain tumor. Arch Phys Med Rehabil. (1998) ;79: (3):S37–46. doi: 10.1016/S0003-9993(98)90120-4. |
[8] | Vargo M . Brain Tumor Rehabilitation. Am J Phys Med Rehabil. (2011) ;90: (5):S50–62. doi: 10.1097/PHM.0b013e31820be31f. |
[9] | Pruitt DW , Ayyangar R , Craig K , White A , Neufeld JA . Pediatric brain tumor rehabilitation. J Pediatr Rehabil Med. (2011) ;4: (1):59–70. doi: 10.3233/PRM-2011-0154. |
[10] | Gragert MN , Ris MD . Neuropsychological late effects and rehabilitation following pediatric brain tumor. J Pediatr Rehabil Med. (2011) ;4: (1):47–58. doi: 10.3233/PRM-2011-0153. |
[11] | Fountain DM , Burke GAA . Multidisciplinary rehabilitation for children with brain tumors: A systematic review. Dev Neurorehabilitation. (2017) ;20: (2):68–75. doi: 10.3109/17518423.2015.1065017. |
[12] | Lim AN , Lange BJ , King AA . Rehabilitation for survivors of pediatric brain tumors: our work has just begun. Future Neurol. (2010) ;5: (1):135–46. doi: 10.2217/fnl.09.65. |
[13] | Nazemi KJ , Butler RW . Neuropsychological rehabilitation for survivors of childhood and adolescent brain tumors: A view of the past and a vision for a promising future. J Pediatr Rehabil Med. (2011) ;4: (1):37–46. doi: 10.3233/PRM-2011-0151. |
[14] | Sierpowska J , Rofes A , Dahlslätt K , et al. The Aftercare Survey: Assessment and intervention practices after brain tumor surgery in Europe. Neuro-Oncol Pract.. (2022) ;9: (4):328–37. doi: 10.1093/nop/npac029. |
[15] | Mukand JA , Blackinton D , Crincoli M , Lee J , Santos B . Incidence of neurologic deficits and rehabilitation of patients with brain tumors. Am J Phys Med Rehabil. (2001) ;80: (5):346–50. doi: 10.1097/00002060-200105000-00005. |
[16] | O’Dell MW , Barr K , Spanier D , Warnick RE . Functional outcome of inpatient rehabilitation in persons with brain tumors. Arch Phys Med Rehabil. (1998) ;79: (12):1530–4. doi: 10.1016/S0003-9993(98)90414-2. |
[17] | Greenberg E , Treger I , Ring H . Rehabilitation Outcomes in Patients with Brain Tumors and Acute Stroke: Comparative Study of Inpatient Rehabilitation. Am J Phys Med Rehabil. (2006) ;85: (7):568–73. doi: 10.1097/01.phm.0000223218.38152.53. |
[18] | Day AM , Slomine BS , Salama C , Quinton TL , Suskauer SJ , Salorio CF . Functional Gains in Children Receiving Inpatient Rehabilitation After Brain Tumor Resection. Arch Phys Med Rehabil. (2134) ;102: (11):2134–40. doi: 10.1016/j.apmr.2021.05.001. |
[19] | Yu J , Jung Y , Park J , et al. Intensive Rehabilitation Therapy Following Brain Tumor Surgery: A Pilot Study of Effectiveness and Long-Term Satisfaction. Ann Rehabil Med. (2019) ;43: (2):129–41. doi: 10.5535/arm.2019.43.2.129. |
[20] | Sartor-Glittenberg C , Brickner L . A multidimensional physical therapy program for individuals with cerebellar ataxia secondary to traumatic brain injury: a case series. Physiother Theory Pract. (2014) Feb;30: (2):138–48. doi: 10.3109/09593985.2013.819952. |
[21] | Katz-Leurer M , Eisenstein E , Liebermann DG . Feasibility of motor capability training at home in children with acquired brain injury. Physiotherapy. (2008) ;94: (1):71–7. doi: 10.1016/j.physio.2007.04.003. |
[22] | Marsden JF Cerebellar ataxia. In: Handbook of Clinical Neurology. Amsterdam: Elsevier; 2018. |
[23] | Nickell M , Statkus D , Escobar N , Wesling M , Brady S , Jensen M . Dysphagia Outcomes in Patients with Brain Tumors Undergoing Inpatient Rehabilitation. Dysphagia. (2003) ;18: (3):203–10. doi: 10.1007/s00455-002-0098-8. |
[24] | Hardy KK , Willard VW , Allen TM , Bonner MJ . Working memory training in survivors of pediatric cancer: a randomized pilot study: Working memory training in survivors. Psychooncology. (2013) ;22: (8):1856–65. doi: 10.1002/pon.3222. |
[25] | Cox LE , Ashford JM , Clark KN , et al. Feasibility and acceptability of a remotely administered computerized intervention to address cognitive late effects among childhood cancer survivors. Neuro-Oncol Pract. (2015) ;2: (2):78–87. doi: 10.1093/nop/npu036. |
[26] | Pollock A , Baer G , Campbell P , et al. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Stroke Group, editor. Cochrane Database Syst Rev. (2014) ;2014: (4):CD001920. doi: 10.1002/14651858.CD001920.pub3. |
[27] | Weyer-Jamora C , Brie MS , Luks TL , Smith EM , Hervey-Jumper SL , Taylor JW . Postacute Cognitive Rehabilitation for Adult Brain Tumor Patients. Neurosurgery. (2021) ;89: (6):945–53. doi: 10.1093/neuros/nyaa552. |
[28] | Jansen NCAJ , Kingma A , Schuitema A , Bouma A , Veerman AJP , Kamps WA . Neuropsychological Outcome in Chemotherapy-Only-Treated Children With Acute Lymphoblastic Leukemia. J Clin Oncol. (2008) ;26: (18):3025–30. doi: 10.1200/JCO.2007.12.4149. |
[29] | Mennes M , Stiers P , Vandenbussche E , et al. Attention and information processing in survivors of childhood acute lymphoblastic leukemia treated with chemotherapy only. Pediatr Blood Cancer. (2005) ;44: (5):479–86. doi: 10.1002/pbc.20147. |
[30] | Mulhern RK , Kepner JL , Thomas PR , Armstrong FD , Friedman HS , Kun LE . Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive conventional or reduced-dose craniospinal irradiation: a Pediatric Oncology Group study. J Clin Oncol. (1998) ;16: (5):1723–8. doi: 10.1200/JCO.1998.16.5.1723. |
[31] | Raghubar KP , Mahone EM , Yeates KO , Cecil KM , Makola M , Ris MD . Working memory and attention in pediatric brain tumor patients treated with and without radiation therapy. Child Neuropsychol. (2017) ;23: (6):642–54. doi: 10.1080/09297049.2016.1183608. |
[32] | Spiegler BJ , Bouffet E , Greenberg ML , Rutka JT , Mabbott DJ . Change in Neurocognitive Functioning After Treatment With Cranial Radiation in Childhood. J Clin Oncol. (2004) ;22: (4):706–13. doi: 10.1200/JCO.2004.05.186. |
[33] | Kleim J , Jones T . Principles of experience-dependent neural plasticity: Implications for rehabiltiation after brain damage. J Speech Lang Hear Res. (2008) ;51: (1):S225–39. doi: 10.1044/1092-4388(2008/018). |
[34] | Kim W , Novotna K , Amatya B , Khan F . Clinical practice guidelines for the management of brain tumours: A rehabilitation perspective. J Rehabil Med. (2019) ;51: (2):89–96. doi: 10.2340/16501977-2509. |
[35] | Tanner L , Keppner K , Lesmeister D , Lyons K , Rock K , Sparrow J . Cancer Rehabilitation in the Pediatric and Adolescent/Young Adult Population. Semin Oncol Nurs. (2020) ;36: (1):150984. doi: 10.1016/j.soncn.2019.150984. |
[36] | Docking K , Campbell E , Campbell L , et al. Clinical practice guideline for the management of communication and swallowing in children diagnosed with childhood brain tumour and leukaemia. Sydney, NSW: The University of Sydney; 2020. |
[37] | Houdeshell MJ , Thomas KM , King AA , L’Hotta AJ . Limitations of Current Rehabilitation Practices in Pediatric Oncology: Implications for Improving Comprehensive Clinical Care. Arch Phys Med Rehabil. (2021) ;102: (12):2353–61. doi: 10.1016/j.apmr.2021.05.021. |
[38] | Tanner LR , Sencer S , Gossai N , Watson D , Hooke MC . CREATE Childhood Cancer Rehabilitation Program development: Increase access through interprofessional collaboration. Pediatr Blood Cancer. (2022) ;69: (11):e29912. doi: 10.1002/pbc.29912. |




