A critical evaluation of oral baclofen in pediatric patients with cerebral palsy
1Introduction of cerebral palsy and spasticity
Cerebral palsy (CP) is one of the most common childhood onset conditions that results in abnormal movement and posturing. One of the most common clinical manifestations of CP is spasticity [1]. Spasticity greatly affects function, quality of life, and caregiver-related challenges [2]; thus, decreasing spasticity to a reasonable level helps to improve overall quality of life. With increasing pharmacologic intervention for this manifestation of CP, multiple therapeutic options have become standard for children with this condition, namely oral medications, botulinum toxin and phenol injections, intrathecal baclofen pump placements, orthopedic surgery, and neurosurgery. The determination of which therapeutic option to utilize has become more controversial.
Understanding which of these treatment options is most beneficial for children with CP allows for more individualized care and an improvement in long-term outcomes. Oral baclofen has come into favor as an appropriate oral medical for children with CP and spasticity [3]. This directly led to oral baclofen being a primary medication studied for the Best Pharmaceuticals for Children Act (BPCA), which authorizes research to improve safety of medication use for children [4]. This act became law in 2002, and it also encourages pharmaceutical companies to increase labeling for patented drug products for children by granting an additional six months of exclusivity. Furthermore, it allows for prioritization testing of off-patent pediatric medications and sponsored clinical trials and other research to provide data for labeling changes. Despite these national efforts, significant challenges remain for providers and families with this oral baclofen (See Box 1).
2History of oral baclofen development and utilization
Oral baclofen was initially synthesized in 1962 by Heinrich Keberle as an antiepileptic medication, but it was found to be a poor treatment option for this condition [5]. Although initially developed for epilepsy, many of the patients who took this medication noted decreased muscle tone, which led to the concept of using baclofen to manage centrally mediated spasticity. In the years that have followed, its use has increased, and it is now a commonly utilized oral agent for treatment of spasticity in the pediatric population [6, 7]. It was first approved for medical use in the United States in 1977 and currently ranks among the top 125 most prescribed medications in the United States [8]. Unfortunately, the rates of baclofen misuse, toxicity, and use and suicide attempts among adults in the United States have also increased more recently [9]. In the adult population over a five-year time frame (2013–2017), rates of baclofen exposures increased by 35% while abuse/misuse increased by 31.7%. Admissions to a healthcare facility occurred for 52.1%, which may highlight the need for careful consideration and the risk-benefit of misuse with this medication [9].
Baclofen is thought to bind to gamma-aminobutyric acid B (GABA-B) receptors that are present in both the brain and spinal cord. By binding these receptors and presynaptic neurons, it inhibits excitatory neurotransmitters that are implicated in causing spasticity and increased tone (Fig. 1) [10]. In order for this process to occur, enough of the medication needs to be able to reach available GABA-B target receptor sites. For orally administered formulations of baclofen, this process requires several steps of absorption, distribution, metabolism, and elimination before providing a clinically meaningful reduction in tone.
Fig. 1
Proposed mechanism of baclofen action serving as a competitive antagonist at the GABA-B receptor sites.
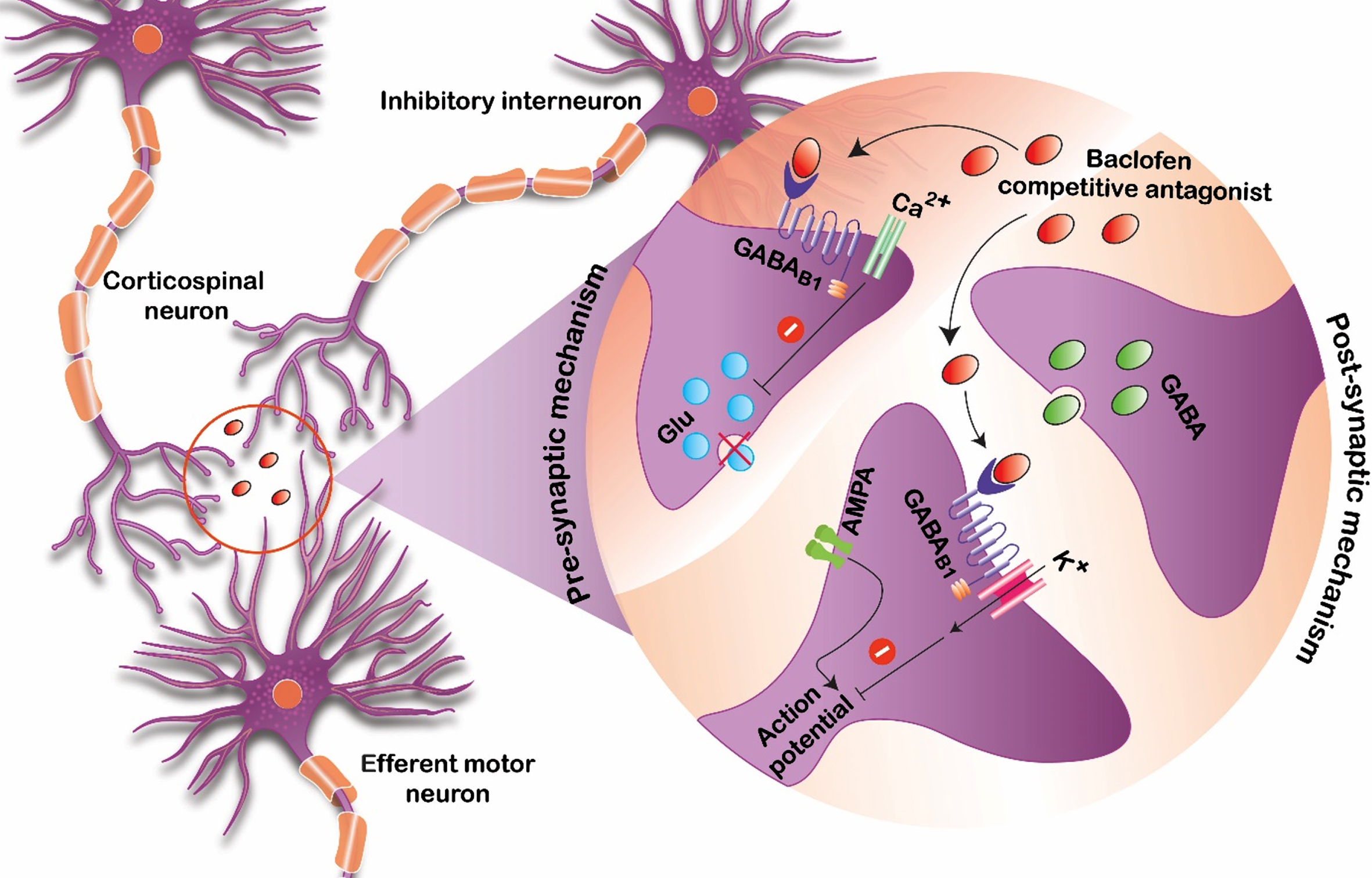
Although this medication is not approved by the Food and Drug Administration (FDA) for pediatric oral use for spasticity or dystonia management, recommendations for the adult population can be found in the product monograph [11]. Generally, adults are started at 5 mg orally three times daily, and the amount of baclofen given is titrated upward if more therapeutic effects are needed or downward if toxicities develop. It may take months to years to find a stable dose of oral baclofen for the adult population, and the same process occurs in children as well.
Some prior large-scale studies have started pediatric-aged patients at half of the starting dose (2.5 mg orally three times daily) of their adult counterparts [4]. In one double blind crossover clinical trial with baclofen, adverse events were reported to be as high at 25% [12]. The authors noted that side effects actually decreased when the dose of the medication was reduced; however, considering only the dose of a medication may not be representative of the entire clinical picture. Notably, this highlights that at ‘normal’ doses, patients may have responses that are sub- or supra-therapeutic and this should even be expected across a heterogenous population. Therefore, a consideration of the dose, the concentrations of the medication in different compartments in the body, and the ultimate clinical response (dose-exposure-response) should be thought of as a continuum of one decision point.
3Dose-exposure-response
Traditionally, physicians and other prescribers of medications have been focused on the dose of the medication that needs to be administered. While not entirely inappropriate, it is a limited way to think about the processes that occur in the human body. First, by doing this, physicians are limited to knowing only the dose that was given and having to wait for desired outcomes to appear, which sometimes takes days to months. This is termed a dose-response relationship.
Second, most of the dosing recommendations are for the ‘average’ patient. By limiting dosing to what would be best for the ‘average’ patient, some patients may benefit while others have no benefit. Even worse, a subset of those patients may develop unnecessary toxicities. This is because all that is known about the amount of medication in the system is the dose that was given. Without knowledge of what is occurring with the medication prior to reaching the target receptor site (pharmacokinetics), the ultimate clinical response (pharmacodynamics) becomes a guessing game of trying to interpret the degree of spasticity response observed without knowing the concentration of the drug that is present. By incorporating the known dose of the medication, the concentrations of the drug that are present throughout different compartments, and the ultimate clinical response, a complete dose-exposure-response relationship can be obtained. By manipulating the dose, the subsequent portions of this relationship change in parallel.
4Baclofen exposure
4.1Absorption
For any orally administered medication like baclofen to get into the bloodstream, it first requires liberation and transport from its administered form. Because of the chemical structure and properties of baclofen, it requires transport across the intestines and into the bloodstream (absorption). This process of absorption can be affected in several ways, notably by the co-administration of food. However, baclofen did not appear to be affected in a significant way by the co-administration of food in a small cohort of individuals [13]. Because of this and to minimize side effects, baclofen is frequently administered with food or milk to potentially decrease gastrointestinal side effects such as nausea, with reported rates as high as 12% [11].
4.2Distribution
Baclofen has many different formulations (i.e., pill, liquid, intravenous, and intrathecal). Contrasting the differences between those given orally and those given intrathecally is the best way to elucidate the distribution of baclofen. Distribution usually refers to the movement of a medication through the body, and oral baclofen concentrations can vary between individuals significantly. Therefore, intrathecal baclofen was created to eliminate both the absorption and distribution steps of pharmacokinetics by placing baclofen in cerebrospinal fluid (CSF), which is very close to where baclofen ultimately acts (GABA-B receptors) [14]. Since challenges related to absorption and elimination from the plasma/blood compartment are no longer a threat, this means that smaller doses of this medication are needed to obtain similar or higher therapeutic effects. Despite significant challenges with an implantable system [15], this type of drug delivery system has shown significant patient satisfaction over a long period of time [16]. One of the reasons is the elimination of many of the gastrointestinal side effects that occur when a medication is not given enterally.
The main challenge of orally administered medications is getting the drug to the site of action. For a medication that acts centrally, like baclofen, understanding the way the medication moves from the plasma and into the CSF is a significant key toward precision medicine and individualized, tailored drug dosing regimens. The central compartment is inaccessible to repetitive sampling to determine drug levels, so finding the proportion of baclofen that crosses the blood-brain barrier and how quickly that medication is cleared from CSF would be a key researchfinding.
4.3Clearance
The concentration, or amount, of the drug that is available as it moves through the body can be impacted in a variety of ways. With oral baclofen, there are many different areas where baclofen concentration can vary between individuals.
Oral baclofen is primarily cleared through the kidney, which occurs by renal excretion in an unchanged form (renal clearance: 10–17 L/h) [7]. Within the drug package insert, there are recommendations to consider precaution when prescribing this medication to patients that have a level of impaired renal function [11]; however, there are no clear recommendations on how cautious a provider should be and what doses would be recommended based on the severity of renal impairment other than for patients who are on dialysis [11]. In contrast, oral baclofen is minimally metabolized by the liver, so there are no recommendations to change the dose of oral baclofen based on liver function [11].
The largest oral baclofen study in pediatrics was done by He et al. in 2014. This study showed that there were variations in oral baclofen clearance, which was dependent on size and age [4]. Additionally, an add-on pharmacogenomic study showed that there was a genetic difference in the rate at which some children cleared oral baclofen (Fig. 2) [17]. This study was the first to identify a single-nucleotide polymorphism in ABCC9 that may double the rate of oral baclofen clearance when present. This would mean that patients may have significantly lower concentrations of oral baclofen in their plasma compared to others after just one half-life. Likely, these patients would be sub-therapeutic and would be considered ‘non-responders’ to oral baclofen. These patients would benefit from earlier discussions about and earlier implantation of intrathecal baclofen delivery systems to reach the highest probability of therapeutic response. These clearance differences remained even after allometric scaling factors were implemented (Fig. 3).
Fig. 2
Oral baclofen weight-corrected clearance differences between wild-type (grey dots) and heterogenous (white dots) children with cerebral palsy. This figure is reproduced with permission from the author per Elsevier’s retained author rights [17].
![Oral baclofen weight-corrected clearance differences between wild-type (grey dots) and heterogenous (white dots) children with cerebral palsy. This figure is reproduced with permission from the author per Elsevier’s retained author rights [17].](https://content.iospress.com:443/media/prm/2023/16-1/prm-16-1-prm230003/prm-16-prm230003-g002.jpg)
Fig. 3
Allometric scaling to approximate organ size comparing the wild-type (grey dots) to the heterogenous (white dots) patients with cerebral palsy. This figure is reproduced with permission from the author per Elsevier’s retained author rights [17].
![Allometric scaling to approximate organ size comparing the wild-type (grey dots) to the heterogenous (white dots) patients with cerebral palsy. This figure is reproduced with permission from the author per Elsevier’s retained author rights [17].](https://content.iospress.com:443/media/prm/2023/16-1/prm-16-1-prm230003/prm-16-prm230003-g003.jpg)
5Baclofen response
Baclofen has been associated with several different clinical outcomes that have indicated improvements in function, notably reducing spasticity better than placebo, allowing both active and passive limb movements to be carried out, and a decrease in scissoring [12, 17]. The findings of oral baclofen are corroborated by many of the studies done on intrathecal administration of baclofen, with long-term patient satisfaction in reduction of tone [16]. The challenge of baclofen is trying to anticipate or predict which patient may benefit and at what dose and concentration.
In the case of oral baclofen, clinical response is a function of a GABA-B target receptor binding ability and the relative number of baclofen molecules available to bind the target receptor. For oral baclofen to work, it needs to cross the blood-brain barrier and circulate through the CSF for it to ultimately act on the brain and spinal cord. At this point, the relative concentrations in the blood compared to the concentrations in the CSF are not well elucidated. Once this information is available, more modeling and simulations may be able to be performed to sample from an easily accessible compartment (blood/plasma) and correlate that with the proposed concentration within the CSF.
In cases in which toxicity occurs, it is clear that a supra-therapeutic clinical response due to excessive exposure is the primary cause. This usually occurs when a provider continues to increase the dose, as the ultimate concentration that causes toxicity in each patient is not known or measured since dose and exposure are the only known parts of the equation. For patients with supra-therapeutic responses (i.e., toxicity, adverse events), the most common findings are lethargy, feeling weak or sleepy, nausea or vomiting, headaches, or notable weakness or hypotonia [18]. In cases in which there has been no clinical response, it remains even more challenging when patients are non-responders or respond less than expected. In this situation, is the lack of clinical response a failure of the medication to reach the target receptors or is it a lack of receptors that are available for baclofen to bind due to the severity of CP?
Complicating the question of clinical response is an increasing understanding of target receptor concentrations. Due to the opioid crisis, providers now recognize that opioid receptors are downregulated and occur at a lower concentration in chronic opioid use. The same mechanism is hypothesized in the case of baclofen, as some investigators have noted that patients require more intrathecal baclofen over time [19]. Some of these patients exhibited signs of true tolerance, as a drug holiday helped in reducing the daily required baclofen dose upon resumption of administration. The authors concluded that tolerance occurs in approximately one in five patients receiving intrathecal baclofen [19].
A recently published article related to the genetic polymorphisms associated with an improvement in response to oral baclofen recently highlighted the need to further investigate areas of the oral baclofen pathway [17]. With the ability to perform whole exome sequencing and whole genome investigations, many genes may provide potential clues into the interaction between the dose-exposure-response relationship. Ideally, this would allow physicians to understand whether baclofen would be an appropriate medication and determine a priori whether a certain patient would likely be a responder or non-responder. By understanding which of these two categories apply to each individual patient, providers could then guide treatment options to other medications, other forms of baclofen (intrathecal baclofen delivery systems), consideration of chemodenervation procedures, or even to discuss surgical options earlier. The time-value of improving the time to optimize a treatment may have significant long-term impact and implications on ensuring preservation of function for as long as possible.
6Conclusion
Despite its widespread use, oral baclofen remains a challenging medication to prescribe because of its varied success in treating patients with CP and tone issues. Part of this may be due to an incomplete understanding of the impact the body has on baclofen (pharmacokinetics) and the interplay between pharmacokinetics and the ultimate pharmacodynamics (clinical response). The promise of understanding the clinical pharmacology of oral baclofen is an opportunity to tailor dosing to optimize clinical responses.
Conflict of interest
Dr. McLaughlin receives funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (1R03HD107642-01).
Box 1: Clinical Correlation
As with all medications, families must be appropriately educated about the risks and potential side effects of baclofen. This education must also consider healthcare literacy, and techniques such as teach back have been shown to be valuable in medication education [20]. Baclofen medication considerations may be conceptualized in three main ideas: 1. goals and intended effect of the medication; 2. costs/potential risks of administration; and 3. risks of withdrawal.
1. Goals and intended effect of the medication: Compliance and understanding of the role of medicine is best achieved when specific shared goals are discussed. It is also the authors’ anecdotal experience that families are more satisfied with medication outcomes if they understand the degree of improvement in spasticity expected by the provider.
2. Costs/potential risk of administration: Depending on the formulation of baclofen prescribed, families should made aware of the financial and time cost of obtaining the medication which may require insurance prior authorization or specialty pharmacies if compounding is required. There is a time and organizational cost of administering a medication three times per day. The side effects of administration, most commonly including sedation, dizziness, and cognitive dysfunction, should be discussed. Families should be counselled on the likelihood of observing some side effects with dose changes and guidelines for continuing the medication, to allow for patient adaptation, versus guidelines for seeking additional provider instructions. While mainly associated with withdrawal and not considered by most as an absolute contraindication in patients with a seizure history, baclofen has been purported to lower the seizure threshold [21]. In this context, the provider may consider discussing baclofen initiation with the child’s neurologist.
3. Risks of withdrawal: Families need to be intentionally counselled on the risks of abrupt cessation or sequentially missed doses. The risks associated with baclofen withdrawal can be severe and life threatening and include pruritis, altered mental status, sometimes severe increases in hypertonia, and seizures. Through intentional education of these withdrawal risks, the provider/patient/caregiver triad may decide that baclofen dosing consistency requirements are too high to safely avoid withdrawal and alternate management should be pursued.
References
[1] | Vadivelu S , Stratton A , Pierce W Pediatric tone management, Phys Med Rehabil Clin N Am (2015) ;26: (1):69–78. 10.1016/j.pmr.2014.09.008. |
[2] | Akodu AK , Oluwale OAT , Adegoke ZO , Ahmed UA , Akinola TO Relationship between spasticity and health related quality of life in individuals with cerebral palsy, Nig Q J Hosp Med (2012) ;22: (2):99–102. |
[3] | McLaughlin MJ , Abdel-Rahman S , Leeder JS Examining the role of precision medicine with oral baclofen in pediatric patients with cerebral palsy, Curr Phys Med Rehabil Rep (2019) ;7: (1):40–45. |
[4] | He Y , Brunstrom-Hernandez JE , Thio LL , et al. Population pharmacokinetics of oral baclofen in pediatric patients with cerebral palsy, J Pediatr (2014) ;164: (5):1188–1188-e8. doi: 10.1016/j.jpeds.2014.01.029. |
[5] | Froestl W , Mickel SJ , Hall RG , et al. Phosphinic acid analogues of GABA 1. New potent and selective GABAB agonists, J Med Chem (1995) ;38: (17):3297–312. 10.1021/jm00017a015. |
[6] | Groves L , Shellenberger MK , Davis CS Tizanidine treatment of spasticity: a meta-analysis of controlled, double-blind, comparative studies with baclofen and diazepam, Adv Ther (1998) ;15: (4):241–51. |
[7] | Wuis EW , Dirks MJ , Termond EF , Vree TB , Van der Kleijn E Plasma and urinary excretion kinetics of oral baclofen in healthy subjects, Eur J Clin Pharmacol (1989) ;37: (2):181–4. 10.1007/BF00558228. |
[8] | The Top 300 of 2020. ClinCalc LLC; 2022 [cited 2 February 2023]. Available from: https://clincalc.com/DrugStats/Top300Drugs.aspx |
[9] | Reynolds K , Kaufman R , Korenoski A , Fennimore L , Shulman J , Lynch M Trends in gabapentin and baclofen exposures reported to U S. poison centers, Clin Toxicol (Phila) (2020) ;58: (7):763–72. doi: 10.1080/15563650.2019.1687902. |
[10] | Deon LL , Gaebler-Spira D Assessment and treatment of movement disorders in children with cerebral palsy, Orthop Clin North Am (2010) ;41: (4):507–17. doi: 10.1016/j.ocl.2010.06.001. |
[11] | Baclofen [package insert]. Concord, NC: Piramal Enterprises Limited, McKesson Packaging Services; 2014. |
[12] | Milla PJ , Jackson AD A controlled trial of baclofen in children with cerebral palsy, J Int Med Res (1977) ;5: (6):398–404. 10.1177/030006057300100203. |
[13] | Peterson GM , McLean S , Millingen KS Food does not affect the bioavailability of baclofen, Med J Aust (1985) ;142: (13):689–90. doi: 10.5694/j.1326-5377.1985.tb113595.x. |
[14] | Gilmartin R , Bruce D , Storrs BB , et al. Intrathecal baclofen for management of spastic cerebral palsy: multicenter trial, J Child Neuro (2000) ;15: (2):71–7. doi: 10.1177/088307380001500201. |
[15] | Ghosh D , Mainali G , Khera J , Luciano M Complications of intrathecal baclofen pumps in children: experience from a tertiary care center, Pediatr Neurosurg (2013) ;49: (3):138–44. doi: 10.1159/000358307. |
[16] | Krach LE , Nettleton A , Klempka B Satisfaction of individuals treated long-term with continuous infusion of intrathecal baclofen by implanted programmable pum, Pediatr Rehabil (2006) ;9: (3):210–8. doi: 10.1080/13638490500138678. |
[17] | McLaughlin MJ , He Y , Brunstrom-Hernandez J , et al. Pharmacogenomic Variability of Oral Baclofen Clearance and Clinical Response in Children With Cerebral Palsy, PMR (2018) ;10: (3):235–43. doi: 10.1016/j.pmrj.2017.08.441. |
[18] | Baclofen Price of 37 Brands. Medindia [updated 31 January 2023; cited 3 November 2017]. Available from: https://www.medindia.net/drug-price/baclofen.htm |
[19] | Heetla HW , Staal MJ , Kliphuis C , van Laar T The incidence and management of tolerance in intrathecal baclofen therapy, Spinal Cord (2009) ;47: (10):751–6. doi: 10.1038/sc.2009.34. |
[20] | Yen PH , Leasure AR Use and Effectiveness of the Teach-Back Method in Patient Education and Health Outcomes, Fed Pract (2019) ;36: (6):284–9. |
[21] | Oh CY , Bainbridge J Lowering the seizure threshold associated with antidepressants, stimulants, antipsychotics, and others, Mental Health Clinician (2012) ;2: (5):127–8. doi: 10.9740/mhc.n127568. |




